Wound Healing Performance in a Moist Environment of Crystalline Glucose/Mannose Film as a New Dressing Material Using a Rat Model: Comparing with Medical-Grade Wound Dressing and Alginate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of G/M
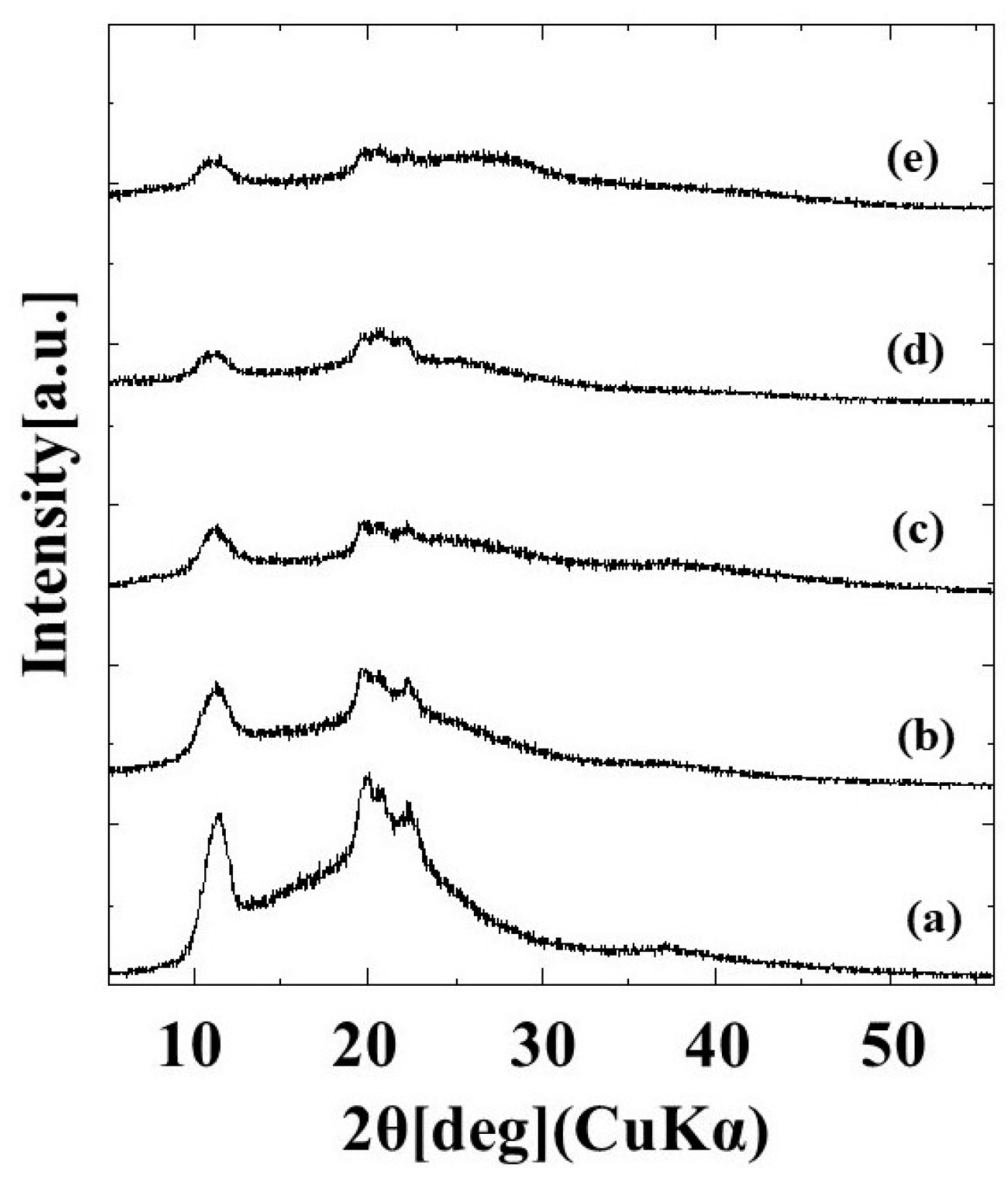
2.1.1. X-ray Diffraction Measurement
2.1.2. Mass Spectroscopy
2.1.3. Water Absorption Behavior
2.1.4. Water Vapor Transmission Rate
2.1.5. Surface Roughness
2.2. In Vivo Evaluation of Commercial Hydrocolloid Dressing (Duoactive ET®), Alginate, and G/M
2.2.1. Macroscopic Evaluation
2.2.2. Histological Evaluation
2.3. Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Glucose/Mannose (G/M) Film
3.3. Preparation of Alginate
3.4. Characterization of G/M Film
3.4.1. X-ray Diffraction Measurement (XRD)
3.4.2. Mass Spectroscopy
3.4.3. Behavior of Water Absorption in G/M Film
3.4.4. Water Vapor Transmission Rate
3.4.5. Surface Roughness
3.5. In Vivo Study of Full Thickness Wound
3.5.1. Animal Operation
3.5.2. Macroscopic Evaluation
3.5.3. Histological Evaluation
3.5.4. Angiogenesis Evaluation
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ji, Y.; Huang, M.; Li, T.; Liu, Y.; Lü, S.; Liu, M. Nucleobase-Inspired Self-Adhesive and Inherently Antibacterial Hydrogel for Wound Dressing. ACS Mater. Lett. 2020, 2, 1375–1380. [Google Scholar] [CrossRef]
- Lamke, L.O. Evaporative water loss from burns under different environmental conditions. Scand. J. Plast. Reconstr. Surg. 1971, 5, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Queen, D.; Gaylor, J.D.; Evans, J.H.; Courtney, J.M.; Reid, W.H. The preclinical evaluation of the water vapour transmission rate through burn wound dressings. Biomaterials 1987, 8, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, M.; Bajpai, S.K.; Gautam, D. Investigation of Regenerated Cellulose/Poly(acrylic acid) Composite Films for Potential Wound Healing Applications: A Preliminary Study. J. Appl. Chem. 2014, 2014, 325627. [Google Scholar] [CrossRef]
- Heyer, K.; Augustin, M.; Protz, K.; Herberger, K.; Spehr, C.; Rustenbach, S.J. Effectiveness of advanced versus conventional wound dressings on healing of chronic wounds: Systematic review and meta-analysis. Dermatology 2013, 226, 172–184. [Google Scholar] [CrossRef]
- Rosique, R.G.; Rosique, M.J.; Farina Junior, J.A. Curbing Inflammation in Skin Wound Healing: A Review. Int. J. Inflam. 2015, 2015, 316235. [Google Scholar] [CrossRef]
- Xu, R.; Xia, H.; He, W.; Li, Z.; Zhao, J.; Liu, B.; Wang, Y.; Lei, Q.; Kong, Y.; Bai, Y.; et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci. Rep. 2016, 6, 24596. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Takeuchi, T.; Ito, M.; Yamaguchi, S.; Watanabe, S.; Honda, M.; Imahashi, T.; Yamada, T.; Kokubo, T. Hydrocolloid dressing improves wound healing by increasing M2 macrophage polarization in mice with diabetes. Nagoya J. Med. Sci. 2020, 82, 487–498. [Google Scholar] [PubMed]
- Barnes, H.R. Wound care: Fact and fiction about hydrocolloid dressings. J. Gerontol. Nurs. 1993, 19, 23–26. [Google Scholar] [CrossRef]
- Aruan, N.M.; Sriyanti, I.; Edikresnha, D.; Suciati, T.; Munir, M.M.; Khairurrijal. Polyvinyl Alcohol/Soursop Leaves Extract Composite Nanofibers Synthesized Using Electrospinning Technique and their Potential as Antibacterial Wound Dressing. Procedia Eng. 2017, 170, 31–35. [Google Scholar] [CrossRef]
- Bowling, F.L.; Rashid, S.T.; Boulton, A.J. Preventing and treating foot complications associated with diabetes mellitus. Nat. Rev. Endocrinol. 2015, 11, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Bharat, S.; Rucha, G.; Rahul, H. A Comprehensive Review on Wound Dressings and Their Comparative Effectiveness on Healing of Contaminated Wounds and Ulcers. Arch. Anesthesiol. Crit. Care 2022, 8. [Google Scholar] [CrossRef]
- Bower, K.A.; Mulder, G.D.; Reineke, A.; Guide, S.V. Dermatologic Conditions and Symptom Control. In Textbook of Interdisciplinary Pediatric Palliative Care; Wolfe, J., Hinds, P.S., Sourkes, B.M., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 350–367. [Google Scholar]
- Lim, J.Z.; Ng, N.S.; Thomas, C. Prevention and treatment of diabetic foot ulcers. J. R. Soc. Med. 2017, 110, 104–109. [Google Scholar] [CrossRef]
- Wietlisbach, C.M. 21—Wound Care. In Fundamentals of Hand Therapy, 2nd ed.; Cooper, C., Ed.; Mosby: Maryland Heights, MI, USA, 2014; pp. 206–218. [Google Scholar]
- Zhao, Y.; Wang, X.; Qi, R.; Yuan, H. Recent Advances of Natural-Polymer-Based Hydrogels for Wound Antibacterial Therapeutics. Polymers 2023, 15, 3305. [Google Scholar] [CrossRef]
- Sheokand, B.; Vats, M.; Kumar, A.; Srivastava, C.M.; Bahadur, I.; Pathak, S.R. Natural polymers used in the dressing materials for wound healing: Past, present and future. J. Polym. Sci. 2023, 61, 1389–1414. [Google Scholar] [CrossRef]
- Hassan, M.A.; Tamer, T.M.; Valachová, K.; Omer, A.M.; El-Shafeey, M.; Mohy Eldin, M.S.; Šoltés, L. Antioxidant and antibacterial polyelectrolyte wound dressing based on chitosan/hyaluronan/phosphatidylcholine dihydroquercetin. Int. J. Biol. Macromol. 2021, 166, 18–31. [Google Scholar] [CrossRef]
- Borbolla-Jiménez, F.V.; Peña-Corona, S.I.; Farah, S.J.; Jiménez-Valdés, M.T.; Pineda-Pérez, E.; Romero-Montero, A.; Del Prado-Audelo, M.L.; Bernal-Chávez, S.A.; Magaña, J.J.; Leyva-Gómez, G. Films for Wound Healing Fabricated Using a Solvent Casting Technique. Pharmaceutics 2023, 15, 1914. [Google Scholar] [CrossRef]
- Weigelt, M.A.; Sanchez, D.P.; Lev-Tov, H. 20—Dressings and Wound Care Supplies for Hidradenitis Suppurativa. In A Comprehensive Guide to Hidradenitis Suppurativa; Shi, V.Y., Hsiao, J.L., Lowes, M.A., Hamzavi, I.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–207. [Google Scholar]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; McAnulty, J.F.; Schurr, M.J.; Murphy, C.J.; Abbott, N.L. Polymeric materials for chronic wound and burn dressings. In Advanced Wound Repair Therapies; Woodhead Publishing Limited: Sawston, UK, 2011; pp. 186–208. [Google Scholar]
- Weller, C.; Weller, C.; Team, V. 4—Interactive dressings and their role in moist wound management. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 105–134. [Google Scholar]
- Huang, Y.-C.; Yang, C.-Y.; Chu, H.-W.; Wu, W.-C.; Tsai, J.-S. Effect of alkali on konjac glucomannan film and its application on wound healing. Cellulose 2015, 22, 737–747. [Google Scholar] [CrossRef]
- Chen, H.; Lan, G.; Ran, L.; Xiao, Y.; Yu, K.; Lu, B.; Dai, F.; Wu, D.; Lu, F. A novel wound dressing based on a Konjac glucomannan/silver nanoparticle composite sponge effectively kills bacteria and accelerates wound healing. Carbohydr. Polym. 2018, 183, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, N.; Venkataraman, S.S.; Daniel, R.; Aravind, R.J.; Kumarakrishnan, V.B. Molecular biology of wound healing. J. Pharm. Bioallied Sci. 2012, 4 (Suppl. S2), S334–S337. [Google Scholar] [CrossRef]
- Wu, H.; Bu, N.; Chen, J.; Chen, Y.; Sun, R.; Wu, C.; Pang, J. Construction of Konjac Glucomannan/Oxidized Hyaluronic Acid Hydrogels for Controlled Drug Release. Polymers 2022, 14, 927. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Albrecht, S.; van Muiswinkel, G.C.J.; Schols, H.A.; Voragen, A.G.J.; Gruppen, H. Introducing Capillary Electrophoresis with Laser-Induced Fluorescence Detection (CE-LIF) for the Characterization of Konjac Glucomannan Oligosaccharides and Their in Vitro Fermentation Behavior. J. Agric. Food Chem. 2009, 57, 3867–3876. [Google Scholar] [CrossRef]
- Alves, A.; Miguel, S.P.; Araujo, A.; de Jesús Valle, M.J.; Sánchez Navarro, A.; Correia, I.J.; Ribeiro, M.P.; Coutinho, P. Xanthan Gum-Konjac Glucomannan Blend Hydrogel for Wound Healing. Polymers 2020, 12, 99. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, T.; Yan, J.; Li, X.; Xie, Y.; Chen, H. Fabrication and characterization of matrine-loaded konjac glucomannan/fish gelatin composite hydrogel as antimicrobial wound dressing. Food Hydrocoll. 2020, 104, 105702. [Google Scholar] [CrossRef]
- Doloff, J.C.; Veiseh, O.; de Mezerville, R.; Sforza, M.; Perry, T.A.; Haupt, J.; Jamiel, M.; Chambers, C.; Nash, A.; Aghlara-Fotovat, S.; et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nat. Biomed. Eng. 2021, 5, 1115–1130. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Ngoc Le, T.T.; Nguyen, A.T.; Thien Le, H.N.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, M.; Yuan, M.; Shi, X.; Song, J.; He, Y.; Mao, H.; Kong, D.; Gu, Z. Gallium(III)-Mediated Dual-Cross-Linked Alginate Hydrogels with Antibacterial Properties for Promoting Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 22426–22442. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Q.; Guo, Z.; Liu, Y.; Gao, W.; Pu, Y.; He, B. Konjac glucomannan hydrogel dressing and its combination with Chinese medicine for the wound treatment. New J. Chem. 2022, 46, 23077–23087. [Google Scholar] [CrossRef]
- Wardhani, D.H.; Puspitosari, D.; Ashidiq, M.A.; Aryanti, N.; Prasetyaningrum, A. Effect of deacetylation on functional properties of glucomannan. AIP Conf. Proc. 2017, 1855, 030020. [Google Scholar]
- Nunes, R.C.R. 13—Rubber nanocomposites with nanocellulose. In Progress in Rubber Nanocomposites; Thomas, S., Maria, H.J., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 463–494. [Google Scholar]
- Varshney, N.; Sahi, A.K.; Poddar, S.; Vishwakarma, N.K.; Kavimandan, G.; Prakash, A.; Mahto, S.K. Freeze–Thaw-Induced Physically Cross-linked Superabsorbent Polyvinyl Alcohol/Soy Protein Isolate Hydrogels for Skin Wound Dressing: In Vitro and In Vivo Characterization. ACS Appl. Mater. Interfaces 2022, 14, 14033–14048. [Google Scholar] [CrossRef]
- Yamamoto, O.; Nagashima, M.; Nakata, Y.; Udagawa, E. The Significant Potential ofSimonkolleite Powder for Deep Wound Healing under a Moist Environment: In Vivo Histological Evaluation Using a Rat Model. Bioengineering 2023, 10, 375. [Google Scholar] [CrossRef]
- Li, Y.; Wei, S.; Chu, H.; Jian, H.; Anand, A.; Nain, A.; Huang, Y.; Chang, H.; Huang, C.; Lai, J. Poly-quercetin-based nanoVelcro as a multifunctional wound dressing for effective treatment of chronic wound infections. Chem. Eng. J. 2022, 437, 135315. [Google Scholar] [CrossRef]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.C.Q.; Tomura, K.; Yamamoto, O. Wound Healing Performance in a Moist Environment of Crystalline Glucose/Mannose Film as a New Dressing Material Using a Rat Model: Comparing with Medical-Grade Wound Dressing and Alginate. Pharmaceuticals 2023, 16, 1532. https://doi.org/10.3390/ph16111532
Wong CCQ, Tomura K, Yamamoto O. Wound Healing Performance in a Moist Environment of Crystalline Glucose/Mannose Film as a New Dressing Material Using a Rat Model: Comparing with Medical-Grade Wound Dressing and Alginate. Pharmaceuticals. 2023; 16(11):1532. https://doi.org/10.3390/ph16111532
Chicago/Turabian StyleWong, Celine Chia Qi, Kanako Tomura, and Osamu Yamamoto. 2023. "Wound Healing Performance in a Moist Environment of Crystalline Glucose/Mannose Film as a New Dressing Material Using a Rat Model: Comparing with Medical-Grade Wound Dressing and Alginate" Pharmaceuticals 16, no. 11: 1532. https://doi.org/10.3390/ph16111532
APA StyleWong, C. C. Q., Tomura, K., & Yamamoto, O. (2023). Wound Healing Performance in a Moist Environment of Crystalline Glucose/Mannose Film as a New Dressing Material Using a Rat Model: Comparing with Medical-Grade Wound Dressing and Alginate. Pharmaceuticals, 16(11), 1532. https://doi.org/10.3390/ph16111532








