Targeted Alpha Therapy: All We Need to Know about 225Ac’s Physical Characteristics and Production as a Potential Theranostic Radionuclide
Abstract
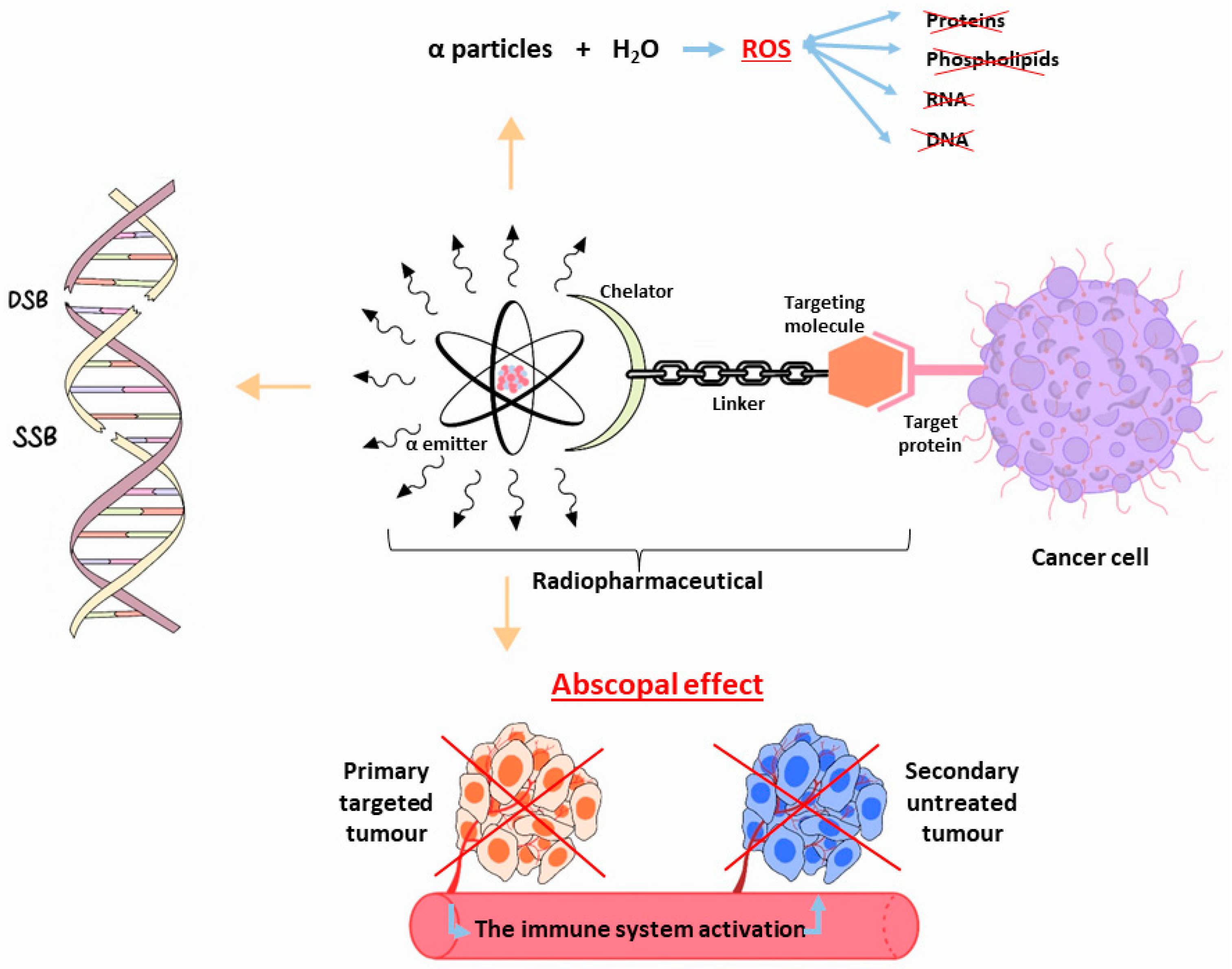
1. Introduction
2. 225Ac: Physical Characteristics
3. 225Ac and Its Potential Theranostic Use
4. Radiochemistry
5. 225Ac Radiopharmaceuticals and Clinical Applications
6. The Production Routes of 225Ac
6.1. Radiochemical Extraction from 229Th
6.2. Accelerator-Based Routes
6.2.1. The Spallation of 232Th
6.2.2. The Irradiations of 226Ra
The Proton Irradiation of 226Ra
The Deuterons’ Irradiation of 226Ra
The photonuclear reaction 226Ra(γ,n)225Ra
6.3. 225Ac/213Bi Radionuclide Generators
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
References
- McDevitt, M.R.; Sgouros, G.; Sofou, S. Targeted and Nontargeted α-Particle Therapies. Annu. Rev. Biomed. Eng. 2018, 20, 73–93. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Jurcic, J.G.; Ravandi, F.; Pagel, J.M.; Park, J.H.; Smith, B.D.; Douer, D.; Estey, E.H.; Kantarjian, H.M.; Wahl, R.L.; Earle, D.; et al. Phase I Trial of Targeted Alpha-Particle Therapy Using Actinium-225 (225Ac)-Lintuzumab (Anti-CD33) in Combination with Low-Dose Cytarabine (LDAC) for Older Patients with Untreated Acute Myeloid Leukemia (AML). Blood 2014, 124, 5293. [Google Scholar] [CrossRef]
- Johnson, J.D.; Heines, M.; Bruchertseifer, F.; Chevallay, E.; Cocolios, T.E.; Dockx, K.; Duchemin, C.; Heinitz, S.; Heinke, R.; Hurier, S.; et al. Resonant Laser Ionization and Mass Separation of 225Ac. Sci. Rep. 2023, 13, 1347. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G.; et al. MIRD Pamphlet No. 22 (Abridged): Radiobiology and Dosimetry of Alpha-Particle Emitters for Targeted Radionuclide Therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Wulbrand, C.; Seidl, C.; Gaertner, F.C.; Bruchertseifer, F.; Morgenstern, A.; Essler, M.; Senekowitsch-Schmidtke, R. Alpha-Particle Emitting 213Bi-Anti-EGFR Immunoconjugates Eradicate Tumor Cells Independent of Oxygenation. PLoS ONE 2013, 8, e64730. [Google Scholar] [CrossRef]
- Elgqvist, J.; Frost, S.; Pouget, J.-P.; Albertsson, P. The Potential and Hurdles of Targeted Alpha Therapy—Clinical Trials and Beyond. Front. Oncol. 2014, 3, 324. [Google Scholar] [CrossRef] [PubMed]
- Friesen, C.; Glatting, G.; Koop, B.; Schwarz, K.; Morgenstern, A.; Apostolidis, C.; Debatin, K.-M.; Reske, S.N. Breaking Chemoresistance and Radioresistance with [213Bi]Anti-CD45 Antibodies in Leukemia Cells. Cancer Res. 2007, 67, 1950–1958. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC Receptor-Targeted Alpha-Radionuclide Therapy Induces Remission in Neuroendocrine Tumours Refractory to Beta Radiation: A First-in-Human Experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Humm, J.L.; Cobb, L.M. Nonuniformity of Tumor Dose in Radioimmunotherapy. J. Nucl. Med. 1990, 31, 75–83. [Google Scholar] [PubMed]
- Guerra Liberal, F.D.C.; O’Sullivan, J.M.; McMahon, S.J.; Prise, K.M. Targeted Alpha Therapy: Current Clinical Applications. Cancer Biother. Radiopharm. 2020, 35, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Ahenkorah, S.; Cassells, I.; Deroose, C.M.; Cardinaels, T.; Burgoyne, A.R.; Bormans, G.; Ooms, M.; Cleeren, F. Bismuth-213 for Targeted Radionuclide Therapy: From Atom to Bedside. Pharmaceutics 2021, 13, 599. [Google Scholar] [CrossRef]
- Vermeulen, K.; Vandamme, M.; Bormans, G.; Cleeren, F. Design and Challenges of Radiopharmaceuticals. Semin. Nucl. Med. 2019, 49, 339–356. [Google Scholar] [CrossRef]
- Beyls, C.; Haustermans, K.; Deroose, C.M.; Pans, S.; Vanbeckevoort, D.; Verslype, C.; Dekervel, J. Could Autoimmune Disease Contribute to the Abscopal Effect in Metastatic Hepatocellular Carcinoma? Hepatology 2020, 72, 1152–1154. [Google Scholar] [CrossRef] [PubMed]
- Seidl, C. Radioimmunotherapy with α-Particle-Emitting Radionuclides. Immunotherapy 2014, 6, 431–458. [Google Scholar] [CrossRef]
- Zimmermann, R. Is Actinium Really Happening? J. Nucl. Med. 2023, 64, 1516–1518. [Google Scholar] [CrossRef]
- Engle, J.W. The Production of Ac-225. Curr. Radiopharm. 2018, 11, 173–179. [Google Scholar] [CrossRef]
- Hatcher-Lamarre, J.L.; Sanders, V.A.; Rahman, M.; Cutler, C.S.; Francesconi, L.C. Alpha Emitting Nuclides for Targeted Therapy. Nucl. Med. Biol. 2021, 92, 228–240. [Google Scholar] [CrossRef]
- Eychenne, R.; Chérel, M.; Haddad, F.; Guérard, F.; Gestin, J.-F. Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight”. Pharmaceutics 2021, 13, 906. [Google Scholar] [CrossRef]
- Pommé, S.; Marouli, M.; Suliman, G.; Dikmen, H.; Van Ammel, R.; Jobbágy, V.; Dirican, A.; Stroh, H.; Paepen, J.; Bruchertseifer, F.; et al. Measurement of the 225Ac Half-Life. Appl. Radiat. Isot. 2012, 70, 2608–2614. [Google Scholar] [CrossRef] [PubMed]
- Suliman, G.; Pommé, S.; Marouli, M.; Van Ammel, R.; Stroh, H.; Jobbágy, V.; Paepen, J.; Dirican, A.; Bruchertseifer, F.; Apostolidis, C.; et al. Half-Lives of 221Fr, 217At, 213Bi, 213Po and 209Pb from the 225Ac Decay Series. Appl. Radiat. Isot. 2013, 77, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics 2020, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Scheinberg, D.A.; McDevitt, M.R. Actinium-225 in Targeted Alpha-Particle Therapeutic Applications. Curr. Radiopharm. 2011, 4, 306–320. [Google Scholar] [CrossRef]
- Muslimov, A.R.; Antuganov, D.; Tarakanchikova, Y.V.; Karpov, T.E.; Zhukov, M.V.; Zyuzin, M.V.; Timin, A.S. An Investigation of Calcium Carbonate Core-Shell Particles for Incorporation of 225Ac and Sequester of Daughter Radionuclides: In Vitro and in Vivo Studies. J. Control Release 2021, 330, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.J.B.; Wilson, J.; Andersson, J.D.; Wuest, F. Theranostic Imaging Surrogates for Targeted Alpha Therapy: Progress in Production, Purification, and Applications. Pharmaceuticals 2023, 16, 1622. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Bartels, J.L.; Appiah, J.-P.K.; Rider, J.H.; Baumhover, N.; Schultz, M.K.; Lapi, S.E. Optimized Methods for the Production of High-Purity 203Pb Using Electroplated Thallium Targets. J. Nucl. Med. 2023, 64, 1791–1797. [Google Scholar] [CrossRef]
- Bobba, K.N.; Bidkar, A.P.; Meher, N.; Fong, C.; Wadhwa, A.; Dhrona, S.; Sorlin, A.; Bidlingmaier, S.; Shuere, B.; He, J.; et al. Evaluation of 134Ce/134La as a PET Imaging Theranostic Pair for 225Ac α-Radiotherapeutics. J. Nucl. Med. 2023, 64, 1076–1082. [Google Scholar] [CrossRef]
- Aluicio-Sarduy, E.; Barnhart, T.E.; Weichert, J.; Hernandez, R.; Engle, J.W. Cyclotron-Produced 132La as a PET Imaging Surrogate for Therapeutic 225Ac. J. Nucl. Med. 2021, 62, 1012–1015. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Ferguson, S.; Wuest, M.; Wilson, J.; Duke, M.J.M.; Richter, S.; Soenke-Jans, H.; Andersson, J.D.; Juengling, F.; Wuest, F. First In Vivo and Phantom Imaging of Cyclotron-Produced 133La as a Theranostic Radionuclide for 225Ac and 135La. J. Nucl. Med. 2022, 63, 584–590. [Google Scholar] [CrossRef]
- Bailey, T.A.; Mocko, V.; Shield, K.M.; An, D.D.; Akin, A.C.; Birnbaum, E.R.; Brugh, M.; Cooley, J.C.; Engle, J.W.; Fassbender, M.E.; et al. Developing the 134Ce and 134La Pair as Companion Positron Emission Tomography Diagnostic Isotopes for 225Ac and 227Th Radiotherapeutics. Nat. Chem. 2021, 13, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.A.; Wacker, J.N.; An, D.D.; Carter, K.P.; Davis, R.C.; Mocko, V.; Larrabee, J.; Shield, K.M.; Lam, M.N.; Booth, C.H.; et al. Evaluation of 134Ce as a PET Imaging Surrogate for Antibody Drug Conjugates Incorporating 225Ac. Nucl. Med. Biol. 2022, 110–111, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Aluicio-Sarduy, E.; Brown, V.; MacMillan, S.N.; Becker, K.V.; Barnhart, T.E.; Radchenko, V.; Ramogida, C.F.; Engle, J.W.; Wilson, J.J. Py-Macrodipa: A Janus Chelator Capable of Binding Medicinally Relevant Rare-Earth Radiometals of Disparate Sizes. J. Am. Chem. Soc. 2021, 143, 10429–10440. [Google Scholar] [CrossRef]
- Thiele, N.A.; Brown, V.; Kelly, J.M.; Amor-Coarasa, A.; Jermilova, U.; MacMillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Ramogida, C.F.; Robertson, A.K.H.; et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem. Int. Ed. Engl. 2017, 56, 14712–14717. [Google Scholar] [CrossRef]
- Rizk, H.E.; Breky, M.M.E.; Attallah, M.F. Development of Purification of No-Carrier-Added 47Sc of Theranostic Interest: Selective Separation Study from the natTi(n,p) Process. Radiochim. Acta 2023, 111, 273–282. [Google Scholar] [CrossRef]
- Mousa, A.M.; Abdel Aziz, O.A.; Al-Hagar, O.E.A.; Gizawy, M.A.; Allan, K.F.; Attallah, M.F. Biosynthetic New Composite Material Containing CuO Nanoparticles Produced by Aspergillus Terreus for 47Sc Separation of Cancer Theranostics Application from Irradiated Ca Target. Appl. Radiat. Isot. 2020, 166, 109389. [Google Scholar] [CrossRef] [PubMed]
- Attallah, M.F.; Rizk, S.E.; Shady, S.A. Separation of 152+154Eu, 90Sr from Radioactive Waste Effluent Using Liquid–Liquid Extraction by Polyglycerol Phthalate. Nucl. Sci. Tech. 2018, 29, 84. [Google Scholar] [CrossRef]
- Hooijman, E.L.; Ntihabose, C.M.; Reuvers, T.G.A.; Nonnekens, J.; Aalbersberg, E.A.; van de Merbel, J.R.J.P.; Huijmans, J.E.; Koolen, S.L.W.; Hendrikx, J.J.M.A.; de Blois, E. Radiolabeling and Quality Control of Therapeutic Radiopharmaceuticals: Optimization, Clinical Implementation and Comparison of Radio-TLC/HPLC Analysis, Demonstrated by [177Lu]Lu-PSMA. EJNMMI Radiopharm. Chem. 2022, 7, 29. [Google Scholar] [CrossRef]
- Mdanda, S.; Ngema, L.M.; Mdlophane, A.; Sathekge, M.M.; Zeevaart, J.R. Recent Innovations and Nano-Delivery of Actinium-225: A Narrative Review. Pharmaceutics 2023, 15, 1719. [Google Scholar] [CrossRef]
- Abou, D.S.; Zerkel, P.; Robben, J.; McLaughlin, M.; Hazlehurst, T.; Morse, D.; Wadas, T.J.; Pandya, D.N.; Oyama, R.; Gaehle, G.; et al. Radiopharmaceutical Quality Control Considerations for Accelerator-Produced Actinium Therapies. Cancer Biother. Radiopharm. 2022, 37, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Dumond, A.R.S.; Rodnick, M.E.; Piert, M.R.; Scott, P.J.H. Synthesis of 225Ac-PSMA-617 for Preclinical Use. Curr. Radiopharm. 2022, 15, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Thakral, P.; Simecek, J.; Marx, S.; Kumari, J.; Pant, V.; Sen, I.B. In-House Preparation and Quality Control of Ac-225 Prostate-Specific Membrane Antigen-617 for the Targeted Alpha Therapy of Castration-Resistant Prostate Carcinoma. Indian. J. Nucl. Med. 2021, 36, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Amor-Coarasa, A.; Sweeney, E.; Wilson, J.J.; Causey, P.W.; Babich, J.W. A Suitable Time Point for Quantifying the Radiochemical Purity of 225Ac-Labeled Radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2021, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Hooijman, E.L.; Chalashkan, Y.; Ling, S.W.; Kahyargil, F.F.; Segbers, M.; Bruchertseifer, F.; Morgenstern, A.; Seimbille, Y.; Koolen, S.L.W.; Brabander, T.; et al. Development of [225Ac]Ac-PSMA-I&T for Targeted Alpha Therapy According to GMP Guidelines for Treatment of mCRPC. Pharmaceutics 2021, 13, 715. [Google Scholar] [CrossRef] [PubMed]
- Busslinger, S.D.; Tschan, V.J.; Richard, O.K.; Talip, Z.; Schibli, R.; Müller, C. [225Ac]Ac-SibuDAB for Targeted Alpha Therapy of Prostate Cancer: Preclinical Evaluation and Comparison with [225Ac]Ac-PSMA-617. Cancers 2022, 14, 5651. [Google Scholar] [CrossRef] [PubMed]
- King, A.P.; Gutsche, N.T.; Raju, N.; Fayn, S.; Baidoo, K.E.; Bell, M.M.; Olkowski, C.S.; Swenson, R.E.; Lin, F.I.; Sadowski, S.M.; et al. 225Ac-MACROPATATE: A Novel α-Particle Peptide Receptor Radionuclide Therapy for Neuroendocrine Tumors. J. Nucl. Med. 2023, 64, 549–554. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Bal, C. Efficacy and Safety of 225Ac-DOTATATE Targeted Alpha Therapy in Metastatic Paragangliomas: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1595–1606. [Google Scholar] [CrossRef]
- Rathke, H.; Bruchertseifer, F.; Kratochwil, C.; Keller, H.; Giesel, F.L.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. First Patient Exceeding 5-Year Complete Remission after 225Ac-PSMA-TAT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 311–312. [Google Scholar] [CrossRef]
- Zacherl, M.J.; Gildehaus, F.J.; Mittlmeier, L.; Böning, G.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Schmidt-Hegemann, N.; Belka, C.; Kretschmer, A.; et al. First Clinical Results for PSMA-Targeted α-Therapy Using 225Ac-PSMA-I&T in Advanced-mCRPC Patients. J. Nucl. Med. 2021, 62, 669–674. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.O.; Knoesen, O.; Mahapane, J.; Davis, C.; Reyneke, F.; Maes, A.; Kratochwil, C.; et al. Predictors of Overall and Disease-Free Survival in Metastatic Castration-Resistant Prostate Cancer Patients Receiving 225Ac-PSMA-617 Radioligand Therapy. J. Nucl. Med. 2020, 61, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Camacaro, J.F.; Dunckley, C.P.; Harman, S.E.; Fitzgerald, H.A.; Lakes, A.L.; Liao, Z.; Ludwig, R.C.; McBride, K.M.; Yalcintas Bethune, E.; Younes, A.; et al. Development of 225Ac Production from Low Isotopic Dilution 229Th. ACS Omega 2023, 8, 38822–38827. [Google Scholar] [CrossRef]
- Parida, G.K.; Panda, R.A.; Bishnoi, K.; Agrawal, K. Efficacy and Safety of Actinium-225 Prostate-Specific Membrane Antigen Radioligand Therapy in Metastatic Prostate Cancer: A Systematic Review and Metanalysis. Med. Princ. Pract. 2023, 32, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, L.; Liao, T.; Gong, W.; Zhang, C. Efficacy and Safety of 225Ac-PSMA-617-Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 796657. [Google Scholar] [CrossRef]
- Sanli, Y.; Kuyumcu, S.; Simsek, D.H.; Büyükkaya, F.; Civan, C.; Isik, E.G.; Ozkan, Z.G.; Basaran, M.; Sanli, O. 225Ac-Prostate-Specific Membrane Antigen Therapy for Castration-Resistant Prostate Cancer: A Single-Center Experience. Clin. Nucl. Med. 2021, 46, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Sen, I.; Thakral, P.; Tiwari, P.; Pant, V.; Das, S.S.; Manda, D.; Raina, V. Therapeutic Efficacy of 225Ac-PSMA-617 Targeted Alpha Therapy in Patients of Metastatic Castrate Resistant Prostate Cancer after Taxane-Based Chemotherapy. Ann. Nucl. Med. 2021, 35, 794–810. [Google Scholar] [CrossRef]
- Feuerecker, B.; Tauber, R.; Knorr, K.; Heck, M.; Beheshti, A.; Seidl, C.; Bruchertseifer, F.; Pickhard, A.; Gafita, A.; Kratochwil, C.; et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-Resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur. Urol. 2021, 79, 343–350. [Google Scholar] [CrossRef]
- Van der Doelen, M.J.; Mehra, N.; van Oort, I.M.; Looijen-Salamon, M.G.; Janssen, M.J.R.; Custers, J.A.E.; Slootbeek, P.H.J.; Kroeze, L.I.; Bruchertseifer, F.; Morgenstern, A.; et al. Clinical Outcomes and Molecular Profiling of Advanced Metastatic Castration-Resistant Prostate Cancer Patients Treated with 225Ac-PSMA-617 Targeted Alpha-Radiation Therapy. Urol. Oncol. 2021, 39, 729.e7–729.e16. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Tripathi, M.; Seth, A.; Bal, C. Efficacy and Safety of 225Ac-PSMA-617 Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer Patients. Theranostics 2020, 10, 9364–9377. [Google Scholar] [CrossRef]
- Satapathy, S.; Mittal, B.R.; Sood, A.; Das, C.K.; Singh, S.K.; Mavuduru, R.S.; Bora, G.S. Health-Related Quality-of-Life Outcomes with Actinium-225-Prostate-Specific Membrane Antigen-617 Therapy in Patients with Heavily Pretreated Metastatic Castration-Resistant Prostate Cancer. Indian. J. Nucl. Med. 2020, 35, 299–304. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in Chemotherapy-Naive Patients with Advanced Prostate Cancer: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J. Nucl. Med. 2018, 59, 795–802. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Tripathi, M.; Sahoo, R.K.; Bal, C. Survival Outcomes in Metastatic Gastroenteropancreatic Neuroendocrine Tumor Patients Receiving Concomitant 225Ac-DOTATATE Targeted Alpha Therapy and Capecitabine: A Real-World Scenario Management Based Long-Term Outcome Study. J. Nucl. Med. 2022, 64, 211–218. [Google Scholar] [CrossRef]
- Kratochwil, C.; Apostolidis, L.; Rathke, H.; Apostolidis, C.; Bicu, F.; Bruchertseifer, F.; Choyke, P.L.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Dosing 225Ac-DOTATOC in Patients with Somatostatin-Receptor-Positive Solid Tumors: 5-Year Follow-up of Hematological and Renal Toxicity. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 54–63. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening Horizons with 225Ac-DOTATATE Targeted Alpha Therapy for Gastroenteropancreatic Neuroendocrine Tumour Patients Stable or Refractory to 177Lu-DOTATATE PRRT: First Clinical Experience on the Efficacy and Safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. Ac-225-DOTATOC—An Empiric Dose Finding for Alpha Particle Emitter Based Radionuclide Therapy of Neuroendocrine Tumors. J. Nucl. Med. 2015, 56, 1232. [Google Scholar]
- Rosenblat, T.L.; McDevitt, M.R.; Carrasquillo, J.A.; Pandit-Taskar, N.; Frattini, M.G.; Maslak, P.G.; Park, J.H.; Douer, D.; Cicic, D.; Larson, S.M.; et al. Treatment of Patients with Acute Myeloid Leukemia with the Targeted Alpha-Particle Nanogenerator Actinium-225-Lintuzumab. Clin. Cancer Res. 2022, 28, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G. Clinical Studies with Bismuth-213 and Actinium-225 for Hematologic Malignancies. Curr. Radiopharm. 2018, 11, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G.; Levy, M.Y.; Park, J.H.; Ravandi, F.; Perl, A.E.; Pagel, J.M.; Smith, B.D.; Estey, E.H.; Kantarjian, H.; Cicic, D.; et al. Phase I Trial of Targeted Alpha-Particle Therapy with Actinium-225 (225Ac)-Lintuzumab and Low-Dose Cytarabine (LDAC) in Patients Age 60 or Older with Untreated Acute Myeloid Leukemia (AML). Blood 2016, 128, 4050. [Google Scholar] [CrossRef]
- Jurcic, J.G.; Rosenblat, T.L.; McDevitt, M.R.; Pandit-Taskar, N.; Carrasquillo, J.A.; Chanel, S.M.; Zikaras, K.; Frattini, M.G.; Maslak, P.G.; Cicic, D.; et al. Phase I Trial of the Targeted Alpha-Particle Nano-Generator Actinium-225 (225Ac)-Lintuzumab (Anti-CD33; HuM195) in Acute Myeloid Leukemia (AML). Blood 2011, 118, 768. [Google Scholar] [CrossRef]
- Pretze, M.; Kunkel, F.; Runge, R.; Freudenberg, R.; Braune, A.; Hartmann, H.; Schwarz, U.; Brogsitter, C.; Kotzerke, J. Ac-EAZY! Towards GMP-Compliant Module Syntheses of 225Ac-Labeled Peptides for Clinical Application. Pharmaceuticals 2021, 14, 652. [Google Scholar] [CrossRef]
- Eryilmaz, K.; Kilbas, B. Fully-Automated Synthesis of 177Lu Labelled FAPI Derivatives on the Module Modular Lab-Eazy. EJNMMI Radiopharm. Chem. 2021, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R. Managing the Uranium-233 Stockpile of the United States. Sci. Glob. Secur. 2013, 21, 53–69. [Google Scholar] [CrossRef]
- Robertson, A.K.H.; Ramogida, C.F.; Schaffer, P.; Radchenko, V. Development of 225Ac Radiopharmaceuticals: TRIUMF Perspectives and Experiences. Curr. Radiopharm. 2018, 11, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Boll, R.A.; Malkemus, D.; Mirzadeh, S. Production of Actinium-225 for Alpha Particle Mediated Radioimmunotherapy. Appl. Radiat. Isot. 2005, 62, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, C.; Molinet, R.; Rasmussen, G.; Morgenstern, A. Production of Ac-225 from Th-229 for Targeted Alpha Therapy. Anal. Chem. 2005, 77, 6288–6291. [Google Scholar] [CrossRef]
- Kotovskii, A.A.; Nerozin, N.A.; Prokof’ev, I.V.; Shapovalov, V.V.; Yakovshchits, Y.A.; Bolonkin, A.S.; Dunin, A.V. Isolation of Actinium-225 for Medical Purposes. Radiochemistry 2015, 57, 285–291. [Google Scholar] [CrossRef]
- Morgenstern, A.; Apostolidis, C.; Bruchertseifer, F. Supply and Clinical Application of Actinium-225 and Bismuth-213. Semin. Nucl. Med. 2020, 50, 119–123. [Google Scholar] [CrossRef]
- Harvey, J.T.; Nolen, J.; Vandergrift, G.; Gomes, I.; Kroc, T.; Horwitz, P.; McAlister, D.; Bowers, D.; Sullivan, V.; Greene, J. Production of Actinium-225 via High Energy Proton Induced Spallation of Thorium-232; NorthStar Medical Radioisotopes, LLC.: Madison, WI, USA, 2011. [Google Scholar]
- Samsonov, M.D.; Nerozin, N.A.; Podsoblyaev, D.A.; Prokof’ev, I.V.; Tkachev, S.V.; Khamianov, S.V.; Shapovalov, V.V. Isolation of Alpha-Emitting Radionuclides for Nuclear Medicine in JSC “SSC RF–IPPE. In Proceedings of the 10th International Symposium on Targeted Alpha Therapy, Kanazawa, Japan, 30 May–1 June 2017. [Google Scholar]
- USDOE Office of Science (SC). Meeting Isotope Needs and Capturing Opportunities for the Future: The 2015 Long. Range Plan. for the DOE-NP Isotope Progarm, NSAC Isotopes Subcommitee, July 2015; USDOE Office of Science (SC): Bethesda, MD, USA, 2015. [Google Scholar]
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.E.; Simon, S.; Birnbaum, E.R.; Atcher, R.W.; John, K.D.; Rixe, O.; et al. Alpha-Emitters and Targeted Alpha Therapy in Oncology: From Basic Science to Clinical Investigations. Target. Oncol. 2018, 13, 189–203. [Google Scholar] [CrossRef]
- Morgenstern, A.; Bruchertseifer, F.; Apostolidis, C. Bismuth-213 and Actinium-225—Generator Performance and Evolving Therapeutic Applications of Two Generator-Derived Alpha-Emitting Radioisotopes. Curr. Radiopharm. 2012, 5, 221–227. [Google Scholar] [CrossRef]
- Hogle, S.; Boll, R.A.; Murphy, K.; Denton, D.; Owens, A.; Haverlock, T.J.; Garland, M.; Mirzadeh, S. Reactor Production of Thorium-229. Appl. Radiat. Isot. 2016, 114, 19–27. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Englert, M.; Krall, L.; Ewing, R.C. Is Nuclear Fission a Sustainable Source of Energy? MRS Bull. 2012, 37, 417–424. [Google Scholar] [CrossRef]
- Hoehr, C.; Bénard, F.; Buckley, K.; Crawford, J.; Gottberg, A.; Hanemaayer, V.; Kunz, P.; Ladouceur, K.; Radchenko, V.; Ramogida, C.; et al. Medical Isotope Production at TRIUMF—From Imaging to Treatment. Phys. Procedia 2017, 90, 200–208. [Google Scholar] [CrossRef]
- Griswold, J.R.; Medvedev, D.G.; Engle, J.W.; Copping, R.; Fitzsimmons, J.M.; Radchenko, V.; Cooley, J.C.; Fassbender, M.E.; Denton, D.L.; Murphy, K.E.; et al. Large Scale Accelerator Production of 225Ac: Effective Cross Sections for 78-192MeV Protons Incident on 232Th Targets. Appl. Radiat. Isot. 2016, 118, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Weidner, J.W.; Mashnik, S.G.; John, K.D.; Ballard, B.; Birnbaum, E.R.; Bitteker, L.J.; Couture, A.; Fassbender, M.E.; Goff, G.S.; Gritzo, R.; et al. 225Ac and 223Ra Production via 800 MeV Proton Irradiation of Natural Thorium Targets. Appl. Radiat. Isot. 2012, 70, 2590–2595. [Google Scholar] [CrossRef] [PubMed]
- Weidner, J.W.; Mashnik, S.G.; John, K.D.; Hemez, F.; Ballard, B.; Bach, H.; Birnbaum, E.R.; Bitteker, L.J.; Couture, A.; Dry, D.; et al. Proton-Induced Cross Sections Relevant to Production of 225Ac and 223Ra in Natural Thorium Targets below 200 MeV. Appl. Radiat. Isot. 2012, 70, 2602–2607. [Google Scholar] [CrossRef]
- Cutler, C.S. US DOE Tri-Lab Effort to Produce Ac-225; International Atomic Energy Agency (IAEA): Vienna, Austria, 2020. [Google Scholar]
- Aliev, R.A.; Ermolaev, S.V.; Vasiliev, A.N.; Ostapenko, V.S.; Lapshina, E.V.; Zhuikov, B.L.; Zakharov, N.V.; Pozdeev, V.V.; Kokhanyuk, V.M.; Myasoedov, B.F.; et al. Isolation of Medicine-Applicable Actinium-225 from Thorium Targets Irradiated by Medium-Energy Protons. Solvent Extr. Ion Exch. 2014, 32, 468–477. [Google Scholar] [CrossRef]
- Mastren, T.; Radchenko, V.; Owens, A.; Copping, R.; Boll, R.; Griswold, J.R.; Mirzadeh, S.; Wyant, L.E.; Brugh, M.; Engle, J.W.; et al. Simultaneous Separation of Actinium and Radium Isotopes from a Proton Irradiated Thorium Matrix. Sci. Rep. 2017, 7, 8216. [Google Scholar] [CrossRef]
- Radchenko, V.; Engle, J.W.; Wilson, J.J.; Maassen, J.R.; Nortier, F.M.; Taylor, W.A.; Birnbaum, E.R.; Hudston, L.A.; John, K.D.; Fassbender, M.E. Application of Ion Exchange and Extraction Chromatography to the Separation of Actinium from Proton-Irradiated Thorium Metal for Analytical Purposes. J. Chromatogr. A 2015, 1380, 55–63. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Robertson, A.K.H.; Jermilova, U.; Zhang, C.; Yang, H.; Kunz, P.; Lassen, J.; Bratanovic, I.; Brown, V.; Southcott, L.; et al. Evaluation of Polydentate Picolinic Acid Chelating Ligands and an α-Melanocyte-Stimulating Hormone Derivative for Targeted Alpha Therapy Using ISOL-Produced 225Ac. EJNMMI Radiopharm. Chem. 2019, 4, 21. [Google Scholar] [CrossRef]
- Robertson, A.K.H.; McNeil, B.L.; Yang, H.; Gendron, D.; Perron, R.; Radchenko, V.; Zeisler, S.; Causey, P.; Schaffer, P. 232Th-Spallation-Produced 225Ac with Reduced 227Ac Content. Inorg. Chem. 2020, 59, 12156–12165. [Google Scholar] [CrossRef] [PubMed]
- Augusto, R.S.; Smith, J.; Varah, S.; Paley, W.; Egoriti, L.; McEwen, S.; Goodacre, T.D.; Mildenberger, J.; Gottberg, A.; Trudel, A.; et al. Design and Radiological Study of the 225Ac Medical Target at the TRIUMF-ARIEL Proton-Target Station. Radiat. Phys. Chem. 2022, 201, 110491. [Google Scholar] [CrossRef]
- Apostolidis, C.; Molinet, R.; McGinley, J.; Abbas, K.; Möllenbeck, J.; Morgenstern, A. Cyclotron Production of Ac-225 for Targeted Alpha Therapy. Appl. Radiat. Isot. 2005, 62, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Nesteruk, K.P.; Ramseyer, L.; Carzaniga, T.S.; Braccini, S. Measurement of the Beam Energy Distribution of a Medical Cyclotron with a Multi-Leaf Faraday Cup. Instruments 2019, 3, 4. [Google Scholar] [CrossRef]
- Higashi, T.; Nagatsu, K.; Tsuji, A.B.; Zhang, M.-R. Research and Development for Cyclotron Production of 225Ac from 226Ra—The Challenges in a Country Lacking Natural Resources for Medical Applications. Processes 2022, 10, 1215. [Google Scholar] [CrossRef]
- Morgenstern, A.; Abbas, K.; Bruchertseifer, F.; Apostolidis, C. Production of Alpha Emitters for Targeted Alpha Therapy. Curr. Radiopharm. 2008, 1, 135–143. [Google Scholar] [CrossRef]
- Maslov, O.D.; Sabel’nikov, A.V.; Dmitriev, S.N. Preparation of 225Ac by 226Ra(γ, n) Photonuclear Reaction on an Electron Accelerator, MT-25 Microtron. Radiochemistry 2006, 48, 195–197. [Google Scholar] [CrossRef]
- Melville, G.; Meriarty, H.; Metcalfe, P.; Knittel, T.; Allen, B.J. Production of Ac-225 for Cancer Therapy by Photon-Induced Transmutation of Ra-226. Appl. Radiat. Isot. 2007, 65, 1014–1022. [Google Scholar] [CrossRef]
- Bruchertseifer, F.; Kellerbauer, A.; Malmbeck, R.; Morgenstern, A. Targeted Alpha Therapy with Bismuth-213 and Actinium-225: Meeting Future Demand. J. Labelled Comp. Radiopharm. 2019, 62, 794–802. [Google Scholar] [CrossRef]
- IBA and SCK CEN Join Forces to Enable Production of Actinium-225|SCK CEN. Available online: https://www.sckcen.be/en/news/iba-and-sck-cen-join-forces-enable-production-actinium-225 (accessed on 5 June 2023).
- Ermolaev, S.; Skasyrskaya, A.; Vasiliev, A. A Radionuclide Generator of High-Purity Bi-213 for Instant Labeling. Pharmaceutics 2021, 13, 914. [Google Scholar] [CrossRef] [PubMed]
- Bruchertseifer, F.; Apostolidis, C.; Mirzadeh, S.; Boll, R.; Murphy, K.; Morgenstern, A. Development of a High-Activity 225Ac/213Bi Radionuclide Generator for Synthesis of Clinical Doses of 213Bi-Labelled Biomolecules. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC82742 (accessed on 4 June 2023).



| Study | Preparation method | Radiopharmaceutical | RCY/RCP |
|---|---|---|---|
| Abou. et al., 2022 [41] |
| 225Ac-DOTA-conjugated peptide | >99%/>95% |
| Dumond. et al., 2022 [42] |
| 225Ac-PSMA-617 | >99%/98 ± 1% |
| Thakral. et al., 2021 [43] |
| 225Ac-PSMA-617 | 85–87%/97–99% |
| Kelly. et al., 2021 [44] |
| 225Ac-PSMA conjugated peptide/ 225Ac-DOTA conjugated peptide/ 225Ac-macropa conjugated peptide | 2.7 ± 0.55%–98.8 ± 0.09%/1.8–99.5% |
| Hooijman. et al., 2021 [45] |
| 225Ac-PSMA-I&T | >95%/>90% |
| Disease | Study | Radiopharmaceutical |
|---|---|---|
| Prostate cancer | Parida et al., 2023 [53] | 225Ac-PSMA RLT |
| Ma et al., 2022 [54] | 225Ac-PSMA-617 | |
| Sanli et al., 2021 [55] | 225Ac-PSMA-617 | |
| Sen et al., 2021 [56] | 225Ac-PSMA-617 | |
| Zacherl et al., 2021 [50] | 225Ac-PSMA-I&T | |
| Feuerecker et al., 2021 [57] | 225Ac-PSMA-617 | |
| Van Der Doelen et al., 2021 [58] | 225Ac-PSMA-617 | |
| Sathekge et al., 2020 [51] | 225Ac-PSMA-617 | |
| Yadav et al., 2020 [59] | 225Ac-PSMA-617 | |
| Satapathy et al., 2020 [60] | 225Ac-PSMA-617 | |
| Sathekge et al., 2019 [61] | 225Ac-PSMA-617 | |
| Kratochwil et al., 2018 [62] | 225Ac-PSMA-617 | |
| Neuroendocrine tumours | Ballal et al., 2022 [63] | 225Ac-DOTATATE |
| Yadav et al., 2022 [48] | 225Ac-DOTATATE | |
| Kratochwil et al., 2021 [64] | 225Ac-DOTATATE | |
| Ballal et al., 2020 [65] | 225Ac-DOTATATE | |
| Kratochwil et al., 2015 [66] | 225Ac-DOTATOC | |
| Acute myeloid leukaemia | Rosenblat et al., 2022 [67] | 225Ac-lintuzumab |
| Jurcic, 2018 [68] | 225Ac-lintuzumab | |
| Jurcic et al., 2016 [69] | 225Ac-lintuzumab | |
| Jurcic et al., 2011 [70] | 225Ac-lintuzumab |
| Production Methods | Advantages | Disadvantages |
|---|---|---|
| Radiochemical extraction of 225Ac from 229Th |
|
|
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| Spallation of 232Th |
|
|
|
| |
|
| |
|
| |
| ||
| ||
| Proton irradiation of 226Ra |
|
|
|
| |
| ||
| ||
| Deuterons’ irradiation of 226Ra |
|
|
| ||
| ||
| Photonuclear reaction 226Ra(γ,n) 225Ra |
|
|
| ||
| ||
| ||
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalloul, W.; Ghizdovat, V.; Stolniceanu, C.R.; Ionescu, T.; Grierosu, I.C.; Pavaleanu, I.; Moscalu, M.; Stefanescu, C. Targeted Alpha Therapy: All We Need to Know about 225Ac’s Physical Characteristics and Production as a Potential Theranostic Radionuclide. Pharmaceuticals 2023, 16, 1679. https://doi.org/10.3390/ph16121679
Jalloul W, Ghizdovat V, Stolniceanu CR, Ionescu T, Grierosu IC, Pavaleanu I, Moscalu M, Stefanescu C. Targeted Alpha Therapy: All We Need to Know about 225Ac’s Physical Characteristics and Production as a Potential Theranostic Radionuclide. Pharmaceuticals. 2023; 16(12):1679. https://doi.org/10.3390/ph16121679
Chicago/Turabian StyleJalloul, Wael, Vlad Ghizdovat, Cati Raluca Stolniceanu, Teodor Ionescu, Irena Cristina Grierosu, Ioana Pavaleanu, Mihaela Moscalu, and Cipriana Stefanescu. 2023. "Targeted Alpha Therapy: All We Need to Know about 225Ac’s Physical Characteristics and Production as a Potential Theranostic Radionuclide" Pharmaceuticals 16, no. 12: 1679. https://doi.org/10.3390/ph16121679
APA StyleJalloul, W., Ghizdovat, V., Stolniceanu, C. R., Ionescu, T., Grierosu, I. C., Pavaleanu, I., Moscalu, M., & Stefanescu, C. (2023). Targeted Alpha Therapy: All We Need to Know about 225Ac’s Physical Characteristics and Production as a Potential Theranostic Radionuclide. Pharmaceuticals, 16(12), 1679. https://doi.org/10.3390/ph16121679






