Abstract
The high energy of α emitters, and the strong linear energy transfer that goes along with it, lead to very efficient cell killing through DNA damage. Moreover, the degree of oxygenation and the cell cycle state have no impact on these effects. Therefore, α radioisotopes can offer a treatment choice to individuals who are not responding to β− or gamma-radiation therapy or chemotherapy drugs. Only a few α-particle emitters are suitable for targeted alpha therapy (TAT) and clinical applications. The majority of available clinical research involves 225Ac and its daughter nuclide 213Bi. Additionally, the 225Ac disintegration cascade generates γ decays that can be used in single-photon emission computed tomography (SPECT) imaging, expanding the potential theranostic applications in nuclear medicine. Despite the growing interest in applying 225Ac, the restricted global accessibility of this radioisotope makes it difficult to conduct extensive clinical trials for many radiopharmaceutical candidates. To boost the availability of 225Ac, along with its clinical and potential theranostic applications, this review attempts to highlight the fundamental physical properties of this α-particle-emitting isotope, as well as its existing and possible production methods.
1. Introduction
At the end of the 1800s, Pierre and Marie Curie, along with Alexander Graham Bell in the early 1900s, conducted research linked to cancer-targeted α therapy (TAT), which represented one of the earliest non-surgical cancer treatments [1]. Furthermore, α-particle emitters have significant curative effects, particularly in patients with limited therapeutic options and metastatic spread [2,3,4]. They can target very small clusters of metastatic cancer cells.
There are many benefits of using these radioisotopes in cancer therapy over common methods. α particles can selectively destroy tumour cells while preserving adjacent normal tissues due to their narrow extent in human tissue, corresponding to less than 0.1 mm [5]. Meanwhile, highly efficient cell destruction through DNA double-strand and DNA cluster damage is caused by the high energy of α emitters, in addition to the strong linear energy transfer (LET) (80 keV/µm) that goes along with it. These effects are mainly unaffected by the state of the cell cycle and oxygenation [6,7,8]. Thus, α radioisotopes can provide a therapeutic option for patients who are resistant to therapy with β− or gamma radiation or chemotherapeutic medications [9,10,11]. According to research estimations, tens of thousands of β− particles are needed to reach a single-cell killing rate of 99.99%, whereas only a few α decays are needed to accomplish a similar killing potential [4,12].
The high-LET radiation’s biological efficacy is explained by its tendency to cause complex multiple clusters and double-strand or single-strand breaks in a target cells’ DNA, rendering cellular repair mechanisms ineffective [4,13]. Additionally, reactive oxygen species (ROS), which are produced when emitted particles interact with water, can react with biomolecules such as proteins, phospholipids, RNA, and DNA, leading to permanent cell deterioration [14]. Moreover, during this type of therapy, the primary tumour and any additional cancerous lesions in the body that the radiation did not directly target may decrease as a result of “the abscopal effect” [14]. It is thought that the immune system is a key player in this process, even though the precise biological mechanisms underlying the phenomenon are as yet unknown [4,15,16] (Figure 1).
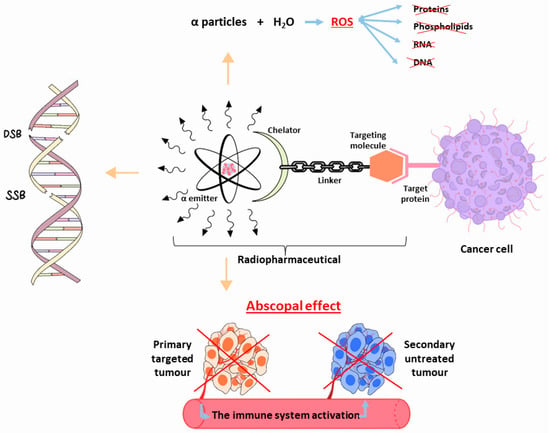
Figure 1.
Schematic representation of the biological effects following the use of α-particle emitter radiopharmaceutical for cancer therapy. SSD = Single-Strand Break, DSB = Double-Strand Break, ROS = Reactive Oxygen Species.
Considering the clinical application of TAT, only a limited number of α-particle emitters are appropriate [17]. The use of 225Ac and its short-lived daughter nuclide 213Bi represents the vast majority of available experience in clinical research [5]. Furthermore, applying γ decays, which are produced during the radioactive 225Ac cascade [5] in SPECT imaging, raises the possibility of theranostic nuclear medicine applications.
Although interest in using 225Ac as an α-emitting radiolabel has been steadily increasing [18], substantial clinical investigations of many radiopharmaceutical candidates cannot be supported due to 225Ac’s limited worldwide accessibility [19]. Notwithstanding the significant financial investments made by numerous laboratories to establish production pathways, the widespread use of 225Ac-labeled radiopharmaceuticals in human patients is still not achievable [19]. This ongoing shortage in 225Ac supply can be explained by the practical production techniques that need difficult logistical tasks, such as using controlled nuclear materials or highly irradiating radioactive accelerator targets [19].
In order to increase the availability of 225Ac and thus boosting the clinical use of α-particle-emitter therapeutics and potential theranostic applications, this review aims to outline the fundamental physical characteristics of 225Ac in addition to its existing and potential production routes.
2. 225Ac: Physical Characteristics
Actinium is a radioactive component with atomic number 89 [20]. Only two of its 32 isotopes, 228Ac and 227Ac, are naturally produced as a result of the disintegration of 232Th and 235U, respectively [20,21]. With its long half-life of 21.7 years and predominant β− emissions decay, 227Ac represents the most common actinium isotope. However, 228Ac, which is also a β− emitter, is highly uncommon [20,21].
225Ac is the initial element in the actinide family, and its radioactive parents are parts of the now-extinct “neptunium series” [19,21]. This α-emitter isotope has a long half-life of 9.9 days [5,22].
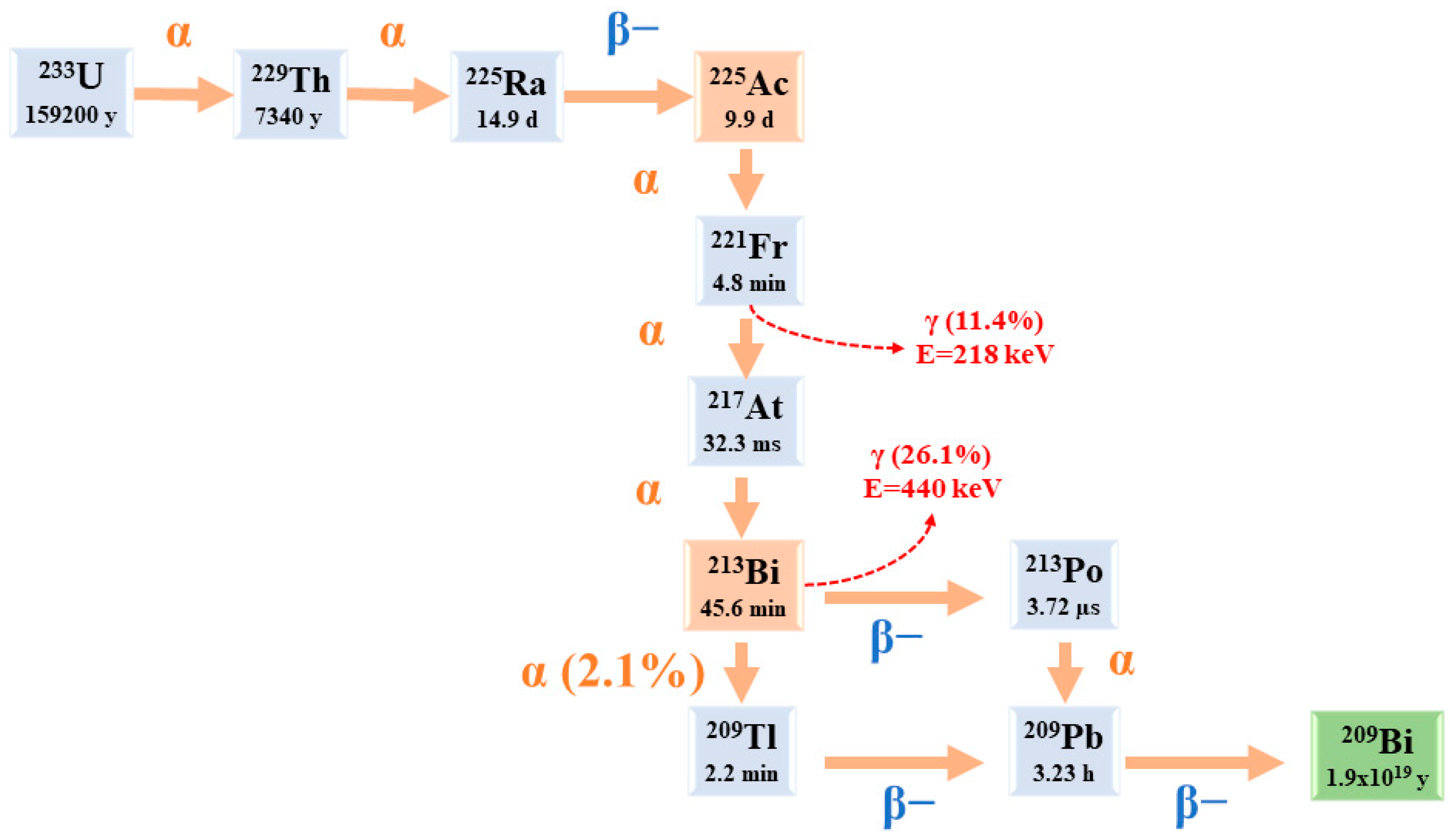
Starting from 225Ac to reach 209Bi (T1/2 = 1.9 × 1019 y), the decay series includes six short-lived radionuclide daughters [5,23].
This radioactive cascade is represented by 221Fr (T1/2 = 4.8 min; 6.3 MeV α particle and 218 keV γ emission), 217At (T1/2 = 32.3 ms; 7.1 MeV α particle), 213Bi (T1/2 = 45.6 min; 5.9 MeV α particle, 492 keV β− particle and 440 keV γ emission), 213Po (T1/2 = 3.72 µs; 8.4 MeV α particle), 209Tl (T1/2 = 2.2 min; 178 keV β− particle), 209Pb (T1/2 = 3.23 h; 198 keV β− particle) [24] (Figure 2) [14].
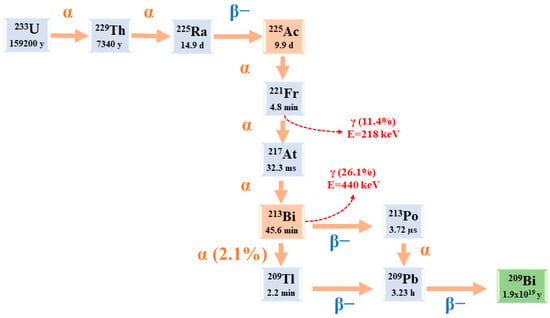
Figure 2.
The decay chain of 233U to 225Ac and 213Bi.
3. 225Ac and Its Potential Theranostic Use
225Ac is considered a “nanogenerator”, since one decay of this element produces a total of four α and three β particles, in addition to two γ emissions [24]. Taking into account its α particle emissions, along with the fact that the non-tumour binding activity can be eliminated before most of its dose is deposited in organs, 225Ac is considered an appealing choice for TAT [24,25]. However, it is important to give attention to the notable 225Ac cytotoxicity, including renal toxicity [26], due to its extended half-life and the various α particles produced throughout its decay chain [5].
A theranostic-based approach, characterised by the imaging–therapeutic duality, is the process of obtaining positron emission tomography (PET) and SPECT scans by exchanging the therapeutic α-emitting radionuclide with a positron or gamma diagnostic imaging radionuclide. Significant information on dosimetry and TAT reactions is obtained from these relevant nuclear medicine images.
Chemical characteristics, half-life, radioactive emission type and intensity, related dosimetry, ease and scalability of production, radionuclidic purity, economics, and radionuclide progeny considerations are the factors that determine “the ideal” imaging surrogates for targeted alpha therapy [27,28].
Therapeutic use of 225Ac is often paired with imperfect PET imaging surrogates, such as 68Ga, 89Zr, or 111In, despite significant differences in their half-lives or chelation chemistry [29]. Studies are being conducted to address the limitations of imaging radionuclides by utilising lanthanum (La) as a potential alternative, especially 132La (T1/2 = 4.8 h, 42% β+) and 133La (T1/2 = 3.9 h, 7% β+) [30,31]. However, the half-lives of these isotopes are much shorter than that of 225Ac, limiting their applicability in PET imaging [29]. In this regard, the production of 134Ce (T1/2 = 3.2 d) has recently been started by the U.S. Department of Energy (DOE) Isotope Program [32]. The long 134Ce T1/2 and the similar chemical properties of 225Ac and 134Ce were considered potential benefits for monitoring in vivo pharmacokinetics. For PET imaging of the chelate and the antibody trastuzumab, 134Ce has been demonstrated to bind with diethylenetriamine pentaacetate (DTPA) [32] and dodecane tetraacetic acid (DOTA) [33]. On the other hand, greater molar ratios and higher temperatures are needed for isotope combinations with DOTA and DTPA [29]. In contrast, N, N′-bis[(6-carboxy-2-pyridyl)methyl]-4,13-diaza-18-crown-6 (macropa) has shown great stability for nonradioactive cerium and better chelate characteristics for 225Ac [34], indicating that it might be useful for the theranostic development of 134Ce/225Ac [35].
The potential use of γ disintegrations, obtained by the decay of the intermediate 221Fr (218 keV, 11.6% emission probability) and 213Bi (440 keV, 26.1% emission probability) [5], in SPECT in vivo imaging could lead the 225Ac radioactive cascade to a possible theranostic prospective in nuclear medicine applications. Nonetheless, planar SPECT imaging would be challenging because of the effectiveness of 225Ac, which results in modest administered doses (~50–200 kBq/kg [5]), along with low γ emissions [24,25]. As a possible solution to this limitation, we can notice the suitable use of 213Bi, which can be isolated from the 225Ac decay cascades [24]. Nevertheless, it is mandatory to consider the short half-life of 213Bi (45.6 min), which poses difficulties for processing, radiolabelling, and radiopharmaceutical delivery [24]. In addition, it is necessary to point out that these radiations make reaction monitoring complicated. Moreover, the secular equilibrium must be attained (for at least 6 h) before measuring a trustworthy radiochemical yield (RCY) [21]. Actinium’s chemistry lacks advancement because of its restricted availability; all Ac isotopes need specific management and facilities [20].
4. Radiochemistry
During the production of radionuclides, it is mandatory to take into consideration a set of important aspects, such as safety, the co-generation of a few long-lived radionuclidic impurities, and adjustability, to enable delivery through clinical sites [27]. Once the target material has been irradiated, potent chemical purification methods are required to isolate the radioisotope [27,36,37,38]. Furthermore, the alpha particle may radiolytically damage the radiopharmaceutical itself, reducing in vivo targeting and producing more radioactive deposits in nontarget tissue. [27].
Since radiopharmaceuticals are considered typical pharmaceuticals, special manuals have been developed in the European Pharmacopoeia to deal with quality control issues [39]. Additionally, optimised protocols for preparing 225Ac agents in therapeutic doses have been established [40] (Table 1).

Table 1.
Research on 225Ac chemistry. RCY = Radiochemical yield, RCP = Radiochemical purity, TLC = Thin-layer chromatography, ITLC = Instant thin-layer chromatography.
5. 225Ac Radiopharmaceuticals and Clinical Applications
The delivery of the radiopharmaceutical via the circulatory system enables the targeting of both the main tumour and its metastases. Whether a radiopharmaceutical is intended for therapeutic or diagnostic purposes depends on the decay properties of the linked radioisotope. For the purpose of curing, controlling, or palliating symptoms, TAT aims to provide an adequate amount of ionising radiation to intended malignities areas [27]. This means that any TAT agent must have a thorough understanding of its stability, pharmacokinetics, and dosimetry.
Investigations on 225Ac have shown potential in treating neuroendocrine tumours, acute myeloid leukaemia, and metastatic prostate cancer, and more radiopharmaceuticals are being developed for other cancer types [46,47,48,49,50,51,52] (Table 2).

Table 2.
Clinical research based on 225Ac.
The use of 225Ac in clinical practice is limited by its low availability. Breaking through this barrier would allow 225Ac therapy to spread widely. Automated synthesis and consistent patient doses are essential, regardless of the production route chosen for this α-isotope acquisition. 225Ac can be adapted for the commonly accessible DOTA-conjugated peptides for therapy [41], which are already capable of labelling 177Lu or 90Y. Marc Pretze et al. [71] studied the effectiveness and consistency of the radiosynthesis process for creating 225Ac-labelled DOTA-conjugated peptides. Additionally, the research aimed to establish whether this process could be adapted for clinical production purposes through an automated synthesis platform (cassette-based module—Modular-Lab EAZY, Eckert & Ziegler) [72]. After comparing two purification methods, the researchers obtained 225Ac-labelled peptides in an RCY of 80–90% for tumour therapy in patients [71]. Thus, the whole process was meticulously validated in accordance with the regulations of the German Pharmaceuticals Act §13.2b, knowing that the estimated costs for the automated synthesis of 1 MBq 225Ac is around EUR 300–390, taking into account that the peptides would cost EUR 600–1000, the cassettes would cost EUR 180–200, and the ML EAZY would cost EUR ~30,000 [71].
6. The Production Routes of 225Ac
As already mentioned, 225Ac is part of the 237Np disintegration family that has vanished in nature. This radioactive element could be artificially reproduced [21]. In addition to direct production paths, 225Ac is conveniently reachable at numerous points along the decay chain, in particular via 233U (T1/2 =159200 y, 100% α), 229Th (T1/2 = 7340 y, 100% α), and 225Ra (T1/2 = 14.9 d, 100% β−) [19]. 225Ac possesses many fewer nucleons than other actinide nuclei that are more stable to be employed as production targets, such as 232Th and 226Ra [19]. Thus, production methods should, with rare exceptions, rely on radioactive decay or greater energy bombardments.
The available production routes of 225Ac and its parents are listed below (Figure 3) [14]:
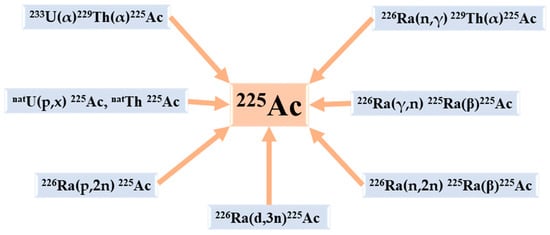
Figure 3.
The principal production routes for 225Ac.
6.1. Radiochemical Extraction from 229Th
For more than two decades, the main source of 225Ac has been the accumulation of 229Th (T1/2 = 7340 y) from the disintegration of 233U (T1/2 = 160,000 y) reserves. At this time, all clinical trials and a large number of pre-clinical studies involving 225Ac and 213Bi have so far used this type of generation route [5].
A large portion of 233U was created between 1954 and 1970 by neutron irradiating 232Th when it was being researched for use in nuclear weapons and reactors that were never completely implemented [73,74]. A significant stockpile of 233U was kept after the thorium fuel cycle was abandoned in favour of fast reactors powered by plutonium at the end of the 1970s [21]. From supplies kept at the Oak Ridge National Laboratory (ORNL, Oak Ridge, TN, USA), 229Th produced via 233U disintegration was recovered between 1995 and 2005 [74]. Currently, there are three principal sources for this 229Th: at ORNL (5.55 GBq (150 mCi), or 704 mg) [74,75], at the Directorate for Nuclear Safety and Security of the Joint Research Centre (JRC) of the European Commission (JRC, Karlsruhe, Germany) (1.7 GBq (46 mCi), or 215 mg), formerly known as the Institute for Transuranium Elements (ITU) [74,76], and at the Leipunskii Institute for Physics and Power Engineering (IPPE, Obninsk, Russia) (5.55 GBq (150 mCi), or 704 mg) [74,77]. Canadian Nuclear Laboratories (CNL) has more recently announced the isolation of an important 229Th source [5]. Very pure sources of 229Th were also discovered, prepared, and used for pre-clinical research at the Belgian Nuclear Research Centre (SCK CEN) in Mol, Belgium [14].
By producing approximately 33 GBq (893,23 mCi) (ORNL) [78] and 13.1 GBq (350 mCi) (JRC) [74,76] of 225Ac annually, the ORNL and JRC represent, up to now, the principal worldwide providers of 225Ac and its parent 225Ra (T1/2 = 14.9 d). Anion exchange and extraction chromatography are combined to produce 225Ac from 229Th at JRC Karlsruhe, whereas anion [52] and cation exchange are used in the process at ORNL [78]. Even though the IPPE source has the same amount of 229Th as the ORNL source, the recorded values show that the IPPE source intermittently produces 225Ac [74,77,79]. According to Samsonov MD et al., IPPE 225Ac production could reach 22 GBq per year [80].
Additionally, it has been noted that starting from 2019, a very considerable rise in the availability of 229Th will be produced through the extraction of 229Th from historical wastes kept by the US DOE [4,52,78]. According to estimations, there could be up to 45 g of total 229Th available, which could result in a 40-fold boost in the supply of 225Ac above current levels [78].
The 225Ac developed at JRC Karlsruhe and ORNL is considered safe for human use and has been significantly utilised for patient treatment [5], although there have been no reports to date about the direct clinical application of 225Ac made at IPPE [5].
Approximately 68 GBq of 225Ac from 229Th are generated per year on a global scale [5]. Knowing that the 225Ac-labelled ligands’ given activities typically range from 4 to 50 MBq per therapeutic dosage [5], the amount of this isotope’s supply is sufficient to treat several hundred patients annually and permits the performance of pre-clinical research. Although a major benefit of this production method is that the resulting 225Ac is free of other actinium isotopes, the globally generated 229Th is not enough to satisfy the extensive use and implementation in healthcare applications across the world [74,81]. Therefore, the development of 225Ac radiopharmaceuticals is hindered by the limited supply and high cost that make 225Ac inaccessible to many researchers [74]. In addition, the production of 233U (T1/2 = 160,000 y) is not viewed as a realistic solution for addressing expected short-term 225Ac demand, because decades of steady growth are necessary to boost 229Th (T1/2 = 7340 y) supply [19,82,83]. As a result, numerous other techniques for generating 225Ac on a wide scale have been researched.
Exposing radium targets to high fluxes of thermal neutrons is considered an effective procedure to induce 229Th production [19]. This approach has been carefully investigated by ORNL researchers with access to the High Flux Isotope Reactor’s (HFIR) > 1015 n cm−2 s thermal fluxes, noticing the production of 229Th from 226Ra, 228Ra, and 227Ac [19]. An HFIR cycle of 26 days generated 229Th yields at 74 ± 7.4 MBq g−1 from 226Ra, 260 ± 10 Bq g−1 229Th from 228Ra, and 1200 ± 50 MBq g−1 from 227Ac [19,84].
226Ra(n,γ)227Ra(β−)227Ac(n,γ)228Ac(β−)228Th(n,γ)229Th is the predominant generation pathway from 226Ra targets and is driven by a combination of neutron capture probability and decay kinetics [19]. The short half-lives of 227Ra (T1/2 = 42.2 min, 100% β−) and 228Ac (T1/2 = 6.15 h, 100% β−) represent the important restrictions for these possible 229Th generation routes [19]. The magnitude of the 226Ra(n, γ) 229Th cross section has the biggest impact on the amount of 229Th that can be produced [19]. Unfortunately, this predominant pathway passes through 228Th. This Th isotope is a dosimetrically undesirable contaminant that can only be eliminated from 229Th by mass isolation or burnup and lowers the yield of 229Th that may be produced [19]. The handling of the radium target and the generation of 228Th (T1/2 = 1.9 y) intermediate represent important challenges of this process [14,52,85]. In addition, there is still a sizable gap between the theoretically predicted yields and the measured ones. In HFIR, ideal 5-cycle activations are expected to provide approximately 0.8 GBq (20 mCi g−1) of 229Th for every gram of 226Ra [19].
Whereas pure 227Ac or 228Ra targets are projected to generate somewhat more 229Th, the current supply of these radionuclides is substantially less than that of 226Ra [19]. Although improving the cost effectiveness of centralised recovery and distribution from 229Th stocks, the dedication of even relatively small quantities of 226Ra to such irradiations will significantly help to ease the current 225Ac shortages. Yet, the full scope of the predicted need cannot be promptly met using this production technique. Thus, other production methods will undoubtedly be pursued simultaneously.
6.2. Accelerator-Based Routes
6.2.1. The Spallation of 232Th
This method is based on the spallation of 232Th to produce 225Ac. As a target material, 232Th (4.1103 Bq/g, 110 nCi/g) is widely accessible, not excessively radioactive, and presents fewer radiation risks [74]. Many countries are known to have stocks of tens of kilograms of thorium metal and hundreds of tonnes of thorium oxide or thorium nitrate, which are created every year as a byproduct of rare-earth mining and used to make more thorium metal in large amounts [74,86].
Waste recycling of the irradiated 232Th target material might not be necessary because of its important accessibility [74].
The irradiation of 232Th with highly energetic protons (0.6–2 GeV) accessible at large accelerators has been shown to produce considerable amounts of 225Ac [5,87,88]. Production yields of several GBq have been recorded for 10-day irradiations utilising highly energetic proton beams [5,89,90]. From the irradiations of 5 g cm−2 targets throughout their roughly 8-month annual running durations, Los Alamos National Laboratory (LANL) can create between 40 and 80 GBq (1–2 Ci) every 10 days. This method is considered to be the most developed production procedure [78] and was validated at the Institute for Nuclear Research (INR), Russian Academy of Sciences (RAS) in Troitsk, Russia, and LANL in the US [78]. Furthermore, the routine use of this technique was introduced by the US DOE Tri-Lab (ORNL, Brookhaven National Laboratory (BNL), LANL) [78]. Once the targets are being handled and the completed product is delivered from ORNL, irradiations can be carried out at BNL (200 MeV at 165 mA) and LANL (100 MeV at 275 mA) [78,91].
The co-production of long-lived 227Ac (T1/2 = 21.8 y) is the process’ primary constraint [27,78]. A large amount of these radionuclidic impurities is simultaneously produced by the spallation of 232Th and needs to be eliminated using the proper multi-step chemical separation methods [5,92,93,94]. The effects of the isotopic impurity on the therapeutic application of the produced 225Ac need to be taken into account because 225Ac and 227Ac cannot be totally chemically separated (0.1–0.2% of the relative activity of 225Ac) [21,88]. Even with this limitation, the 225Ac produced from high-energy accelerators may still be perfectly suitable for the manufacturing of 225Ac/213Bi generators, as all actinium daughters will be kept on the generator [14]. According to preliminary research, the 227Ac impurity will not significantly affect patient dosimetry [78]. Recently, new purifying techniques that enable a reduction in the 227Ac level and the recovery of 225Ac with better purity, such as isotope separation (isotope separation on-line (ISOL) at Canada’s particle accelerator centre (TRIUMF)) or a manufacturing method using 225Ra produced after the proton irradiation of 232Th, have been developed [4,21,95,96,97]. Nonetheless, there are still challenges to be resolved regarding long-lived 227Ac licensing and accessibility in medical applications. In addition, due to the 21.8-year half-life, waste management is still a serious issue and will necessitate measures with possibly high related costs.
6.2.2. The Irradiations of 226Ra
The Proton Irradiation of 226Ra
Compared with the 232Th spallation reaction, the generation of 225Ac from 226Ra targets by proton irradiation in a cyclotron has several benefits. This method is based on the reaction 226Ra(p,2n)225Ac. In medium-sized cyclotrons, at proton energies below 20 MeV (around 16 MeV), this procedure can be carried out with excellent results and at a reasonable cost [5,78,98]. About 5 GBq 225Ac, which is comparable to 500 patient doses of 10 MBq 225Ac, should be produced after a 24 h exposure of 50 mg 226Ra to the highest excitation function at 15–16 MeV with a current of 100 mA protons [78]. It is noteworthy that research, both fundamental and applied, is believed to have relevance to medical cyclotrons that produce radioisotopes at energies between 15 and 25 MeV [14,99].
Since no other long-lived actinium isotopes, such as 227Ac, are created during the chemical purification of the irradiation targets, 225Ac with high isotopic purity is obtained. By choosing the right proton energies, it is possible to reduce the co-production of the short-lived 226Ac (T1/2 = 29 h) and 224Ac (T1/2 = 2.9 h) impurities produced by the reactions 226Ra(p,n)226Ac and 226Ra(p,3n)224Ac [5,78]. Furthermore, during the time needed for target cooling and reprocessing, their activity will continue to decrease to low levels. Handling targets that contain milligram amounts of radioactive 226Ra (T1/2 = 1600 y) and controlling its highly radiotoxic gaseous decay product 222Rn (T1/2 = 3.8 d) [5,14,98,100] pose significant challenges in the production, processing, and control procedures [5,78]. In addition, due to the limited availability of the target material, it is necessary to consider its recycling process [20]. Currently, facilities in North and South America, Europe, and Asia are researching how to utilise this production strategy. For instance, work on the investigation and development of 225Ac generation using 226Ra (stored as radioactive waste) has started at the National Institutes for Quantum Science and Technology (QST), Chiba, Japan [100]. These amounts of 226Ra have previously been used as a sealed source for brachytherapy. Even in this resource-constrained country, some 226Ra was accessible as a target for proton irradiation thanks to the national waste management program [100].
The Deuterons’ Irradiation of 226Ra
An improved method for producing 225Ac, which involves irradiating 226Ra with deuterons through the reaction 226Ra(d,3n)225Ac, has been proposed [101]. Although experimental measurements of the reaction’s cross sections are still in development, simulations indicate that the process will have a greater production yield than the 226Ra(p,2n)225Ac reaction and a maximum cross section of 864 mb at 18.5 MeV [78]. It is important to consider the prolonged cooling period by the 226Ac decay, since deuteron irradiation might result in an increased co-production of 226Ac (T1/2 = 29 h) [78]. Moreover, there are only a few accelerators that can produce deuteron beams with enough energy.
The photonuclear reaction 226Ra(γ,n)225Ra
The photonuclear reaction 226Ra(γ,n)225Ra, followed by the beta decay of 225Ra to 225Ac is a different method for producing 225Ac by irradiating 226Ra. It is noticed that the photon energy cutoff for the reaction is 6.4 MeV. However, experimentally established cross-section data are not yet available [78]. Modelling data predict modest reaction yields and high-intensity electron beams from modern accelerators are required for commercially viable production. At JRC Karlsruhe, the process’s fundamentals have been experimentally verified [78]. A zircaloy capsule containing 1 mg of 226Ra embedded in 800 mg of a BaCl2 matrix underwent 3.5 h of 52 MeV betatron irradiation to generate 0.24 mCi of 225Ac [78]. At the INR in Dubna, Russia [102], as well as the Illawarra Cancer Centre (ICC) in Wollongong, Australia [103], the procedure’s viability has also been effectively validated. At a maximum photon energy of 24 MeV, a radiation yield of 550 Bq/(mAh mg 226Ra) was recorded [102]. For a more precise estimate of production yields, it is extremely important to quantify the cross-section data in detail in this reaction.
The main challenges in this method are the recycling requirement of the 226Ra target and some handling issues with the 222Rn daughter [20]. However, large-scale 225Ac manufacturing using this procedure is already being implemented at several plants [104]. It was reported that SCK CEN is capable of generating high-grade GMP-grade 225Ac and also continually supplying it using a backup system [18,100]. During the creation of GMP-grade 225Ac, SCK CEN has been collaborating with the Institute of Radioelements Environment & Lifescience Technology (IRE Elit) and Global Morpho Pharma (GMP) (France) [100]. Starting in 2019, SCK-CEN began irradiating their stock of several hundred grammes of 226Ra. This Belgian research centre is also equipped with a BR2 reactor and an accelerator-driven subcritical reactor named Multi-purpose hYbrid Research Reactor for High-tech Application (MYRRHA) that are used in this approach [100]. Additionally, utilising an IBA (Ion Beam Applications S.A., EURONEXT) Rhodotron, SCK CEN could produce GMP-grade 225Ac at a weekly rate of 37 GBq (1000 mCi) by irradiating with 40 MeV electrons at 125 kW [100]. The prospects should be kept an eye on, as SCK CEN and IBA established a research and development partnership agreement for the joint production of 225Ac in 2021 [105] (Table 3).

Table 3.
Advantages and disadvantages of the potential 225Ac production methods.
6.3. 225Ac/213Bi Radionuclide Generators
In the middle of the 1990s, the JRC was the first laboratory to offer 225Ac/213Bi to clinical partners [5]. Ever since, the JRC has produced these radioisotopes on an annual basis for preclinical research and clinical testing carried out at JRC Karlsruhe or in partnership with a large network of healthcare partners.
In order to produce the short-lived 213Bi (T1/2 = 45.6 min) on-site, 225Ac can either be utilised directly as a therapeutic nuclide [50,106] or set onto 225Ac/213Bi generators [78,83]. All patient investigations with 213Bi up to now have utilised 225Ac/213Bi generators.
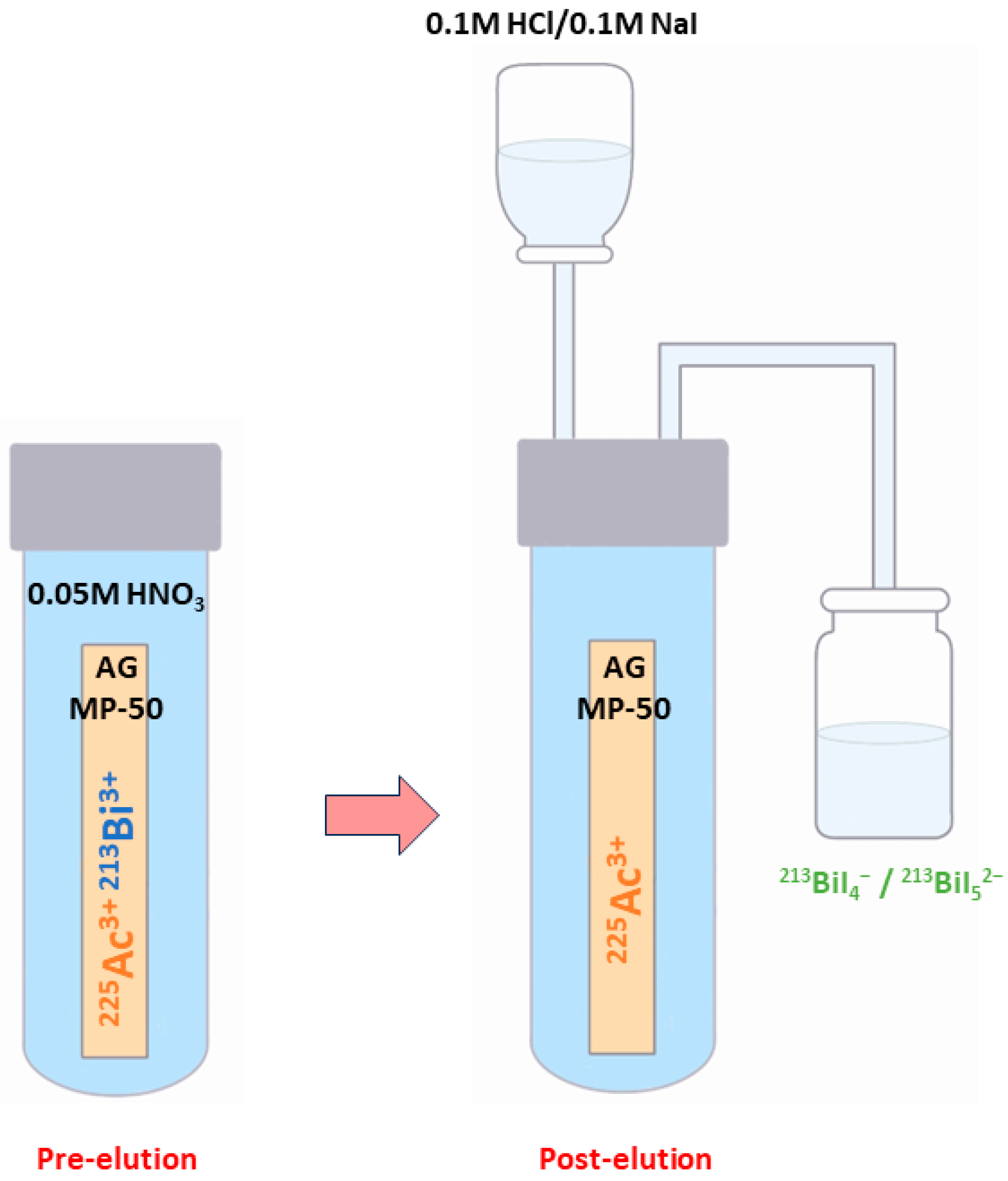
There are numerous generator types available, including those based on ion exchange, extraction chromatography, and inorganic sorbents [106]. The most widely used type is a single-column “direct” generator that was invented at the ITU and based on the strongly acidic cation-exchange sorbent AG MP-50 [106].
In this well-known approach, 213Bi is obtained starting from 225Ac, which is tightly bound to the sorbent and drowned in 0.05M HNO3 solution [14,78,83]. At roughly every 3 h [14,78], 213Bi (213BiI4- and 213BiI52-) is obtained for immediate use through elution with a mixture of 0.1 M HCl/0.1 M NaI [104] (Figure 4) [14].
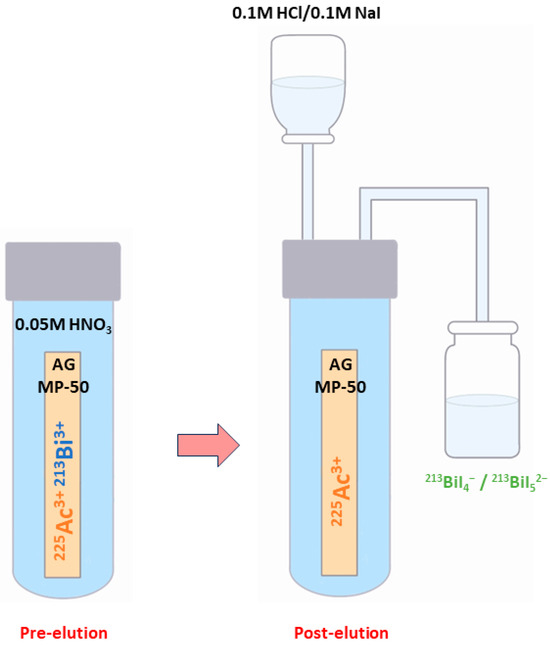
Figure 4.
Schematic representation of the 213Bi elution using the single-column “direct” 225Ac/213Bi generator.
The high-activity generator technology created at JRC Karlsruhe enables the generator to function reliably even when supplied with up to 4 GBq 225Ac of activities [5,78]. Although the penetration of 225Ac is less than 0.2 ppm, the yields of 213Bi elution may be more than 80% [107]. The process of distributing 225Ac activity uniformly over about two-thirds of the generator resin ensures stable performance over several weeks and minimises radiolytic degradation of the organic resin [5,78].
Injection-ready therapeutic dosages of 213Bi-labeled peptides with activities of up to 2.3 GBq have been successfully prepared using the generator for clinical applications [78] including the locoregional therapy of brain tumours [5,13]. Due to the relatively long parent half-life, which enables the transport of the generator to radiopharmacy facilities over vast distances, these generators may be employed clinically.
7. Conclusions
Taking into account its α-particle emissions, along with the ability to eliminate the non-tumour binding activity before most of its dose is deposited in organs, 225Ac is considered an appealing choice for TAT. Nevertheless, because of its long half-life and the different α particles created throughout its decay chain, it is crucial to pay attention to the considerable cytotoxicity of 225Ac. Additionally, the γ disintegrations that result from the intermediate 221Fr and 213Bi disintegration may be used in SPECT clinical imaging. Thus, the radioactive cascade of 225Ac could be used in nuclear medicine, especially in theranostic applications. However, the small 225Ac doses given lead to low γ emissions, which makes planar SPECT imaging difficult. A potential alternative for this constraint is to make appropriate use of 213Bi, which can be isolated from the decay cascades of 225Ac. However, the brief half-life of 213Bi must be taken into account since it presents challenges for radiopharmaceutical distribution, processing, and radiolabelling.
Apart from direct production pathways, 225Ac can be easily accessed at many points in the decay chain, especially through 233U, 229Th, and 225Ra. Compared with other actinide nuclei, including 232Th and 226Ra, which are more stable to use as production targets, 225Ac has many fewer nucleons. As a result, production techniques must, for the most part, rely on radioactive decay or higher energy bombardments.
All the production techniques discussed in this paper are expensive and will all struggle to satisfy demand at the expected level if they are used separately.
It is necessary to readjust the facilities that are accessible throughout the world, to use suitable production methods that are adapted to the available infrastructure, and take into consideration the advantages and disadvantages of every used production modality. In addition, fruitful collaboration between the different centres and experienced scientific staff will pave the way for the widespread clinical use of actinium-based radiopharmaceuticals as a new standard of care.
The European medical isotope programme: Production of High-Purity Isotopes by Mass Separation Project (PRISMAP) represents an important initiative of this type of collaboration. Coordinated by the European Laboratory for Nuclear Research (CERN), the project partners come from thirteen nations: Austria, Belgium, Denmark, France, Germany, Italy, Latvia, Norway, Portugal, Poland, Sweden, Switzerland, and the United Kingdom. Nine significant EU, national, or regional infrastructures, four developing infrastructures, leader research institutes, medical facilities, the European Joint Research Centre, and one small and midsize enterprise (SME) are among the twenty-three partners that make up the PRISMAP Consortium. With the help of these considerable facilities, the programme goal is to create a sustainable source of high-purity-grade new radionuclides for medical use. It also aims to offer an accessible point of entry for all researchers working in this field, including those from SMEs, global pharmaceutical companies, nuclear centres, hospitals, and universities, by implementing standardised access procedures.
Several PRISMAP partners, including JRC Belgium, Narodowe Centrum Badań Jądrowych (NCBJ), Poland, Institut Max von Laue—Paul Langevin (ILL), France, and SCK CEN, Belgium, are additionally implicated in another promising project in the field of the sustainability of medical isotope production and its safe application in Europe, named the Strengthening the European Chain of sUpply for next-generation medical RadionuclidEs (SECURE). The project focuses on encouraging advancements in the creation of irradiation targets and manufacturing processes for both new and existing isotopes used in nuclear medicine and diagnostics. A list of crucial alpha-emitting radioisotopes in nuclear medicine was created, and 225Ac was selected at the top of this list. The research aims to overcome the primary challenges to ensure the future availability of these isotopes by: (1) creating a framework of guidelines and recommendations that enable investigating the full clinical potential of alpha and beta particle therapy and its safe application; (2) offering significant insights that serve as a model for resolving challenges with upscaling and continuous isotope production; (3) removing critical obstacles along the production of specific alpha- and beta-emitting isotopes that restrict a sustainable production.
Author Contributions
Conceptualization, W.J., V.G., C.R.S. and C.S.; methodology, W.J., I.C.G. and C.S.; software, V.G., M.M., T.I. and I.C.G.; validation, W.J., T.I. and C.S.; analysis, C.R.S., I.P. and I.C.G.; data curation, V.G., C.R.S. and T.I.; writing—original draft preparation, W.J.; writing—review and editing, W.J., M.M. and C.S.; visualization, V.G., I.P. and I.C.G.; supervision, M.M., C.S. and I.P. administration, C.R.S., T.I. and I.C.G. All authors have contributed equally to this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by project SECURE (Strengthening the European Chain of sUpply for next generation medical RadionuclidEs), GA no. 101061230, a HORIZON EURATOM funded project (https://enen.eu/index.php/portfolio/secure-project/).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks to the team of the North East Regional Innovative Cluster for Structural and Molecular Imaging (Imago-Mol) for the professional support.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Funding statement. This change does not affect the scientific content of the article.
Abbreviations
Targeted Alpha Therapy (TAT), Single-Photon Emission Computed Tomography (SPECT), Linear Energy Transfer (LET), Reactive Oxygen Species (ROS), Single-Strand Break (SSD), Double-Strand Break (DSB), Positron Emission Tomography (PET), Department of Energy (DOE), Diethylenetriamine Pentaacetate (DTPA), Dodecane Tetraacetic Acid (DOTA), N,N′-Bis[(6-carboxy-2-pyridyl)methyl]-4,13-diaza-18-crown-6 (macropa), Radiochemical Yield (RCY), Radiochemical Purity (RCP), Thin-Layer Chromatography (TLC), Instant Thin-Layer Chromatography (ITLC), Oak Ridge National Laboratory (ORNL), Directorate for Nuclear Safety and Security of the Joint Research Centre (JRC), Institute for Transuranium Elements (ITU), Leipunskii Institute for Physics and Power Engineering (IPPE), Canadian Nuclear Laboratories (CNL), Belgian Nuclear Research Centre (SCK CEN), High Flux Isotope Reactor (HFIR), Los Alamos National Laboratory (LANL), Institute for Nuclear Research (INR), Russian Academy of Sciences (RAS), Brookhaven National Laboratory (BNL), Isotope Separation On-Line (ISOL), Canada’s Particle Accelerator Centre (TRIUMF), National Institutes for Quantum Science and Technology (QST), Illawarra Cancer Centre (ICC), Institute of Radioelements Environment & Lifescience Technology (IRE Elit), Global Morpho Pharma (GMP), Multi-purpose hYbrid Research Reactor for High-tech Application (MYRRHA), Ion Beam Applications S.A. (IBA), The European Medical Isotope Programme: Production of High-Purity Isotopes by Mass Separation (PRISMAP), European Laboratory for Nuclear Research (CERN), Small and Midsize Enterprise (SME), Narodowe Centrum Badań Jądrowych (NCBJ), Institut Max von Laue—Paul Langevin (ILL), Strengthening the European Chain of sUpply for next generation medical RadionuclidEs (SECURE).
References
- McDevitt, M.R.; Sgouros, G.; Sofou, S. Targeted and Nontargeted α-Particle Therapies. Annu. Rev. Biomed. Eng. 2018, 20, 73–93. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Jurcic, J.G.; Ravandi, F.; Pagel, J.M.; Park, J.H.; Smith, B.D.; Douer, D.; Estey, E.H.; Kantarjian, H.M.; Wahl, R.L.; Earle, D.; et al. Phase I Trial of Targeted Alpha-Particle Therapy Using Actinium-225 (225Ac)-Lintuzumab (Anti-CD33) in Combination with Low-Dose Cytarabine (LDAC) for Older Patients with Untreated Acute Myeloid Leukemia (AML). Blood 2014, 124, 5293. [Google Scholar] [CrossRef]
- Johnson, J.D.; Heines, M.; Bruchertseifer, F.; Chevallay, E.; Cocolios, T.E.; Dockx, K.; Duchemin, C.; Heinitz, S.; Heinke, R.; Hurier, S.; et al. Resonant Laser Ionization and Mass Separation of 225Ac. Sci. Rep. 2023, 13, 1347. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G.; et al. MIRD Pamphlet No. 22 (Abridged): Radiobiology and Dosimetry of Alpha-Particle Emitters for Targeted Radionuclide Therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Wulbrand, C.; Seidl, C.; Gaertner, F.C.; Bruchertseifer, F.; Morgenstern, A.; Essler, M.; Senekowitsch-Schmidtke, R. Alpha-Particle Emitting 213Bi-Anti-EGFR Immunoconjugates Eradicate Tumor Cells Independent of Oxygenation. PLoS ONE 2013, 8, e64730. [Google Scholar] [CrossRef]
- Elgqvist, J.; Frost, S.; Pouget, J.-P.; Albertsson, P. The Potential and Hurdles of Targeted Alpha Therapy—Clinical Trials and Beyond. Front. Oncol. 2014, 3, 324. [Google Scholar] [CrossRef] [PubMed]
- Friesen, C.; Glatting, G.; Koop, B.; Schwarz, K.; Morgenstern, A.; Apostolidis, C.; Debatin, K.-M.; Reske, S.N. Breaking Chemoresistance and Radioresistance with [213Bi]Anti-CD45 Antibodies in Leukemia Cells. Cancer Res. 2007, 67, 1950–1958. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC Receptor-Targeted Alpha-Radionuclide Therapy Induces Remission in Neuroendocrine Tumours Refractory to Beta Radiation: A First-in-Human Experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Humm, J.L.; Cobb, L.M. Nonuniformity of Tumor Dose in Radioimmunotherapy. J. Nucl. Med. 1990, 31, 75–83. [Google Scholar] [PubMed]
- Guerra Liberal, F.D.C.; O’Sullivan, J.M.; McMahon, S.J.; Prise, K.M. Targeted Alpha Therapy: Current Clinical Applications. Cancer Biother. Radiopharm. 2020, 35, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Ahenkorah, S.; Cassells, I.; Deroose, C.M.; Cardinaels, T.; Burgoyne, A.R.; Bormans, G.; Ooms, M.; Cleeren, F. Bismuth-213 for Targeted Radionuclide Therapy: From Atom to Bedside. Pharmaceutics 2021, 13, 599. [Google Scholar] [CrossRef]
- Vermeulen, K.; Vandamme, M.; Bormans, G.; Cleeren, F. Design and Challenges of Radiopharmaceuticals. Semin. Nucl. Med. 2019, 49, 339–356. [Google Scholar] [CrossRef]
- Beyls, C.; Haustermans, K.; Deroose, C.M.; Pans, S.; Vanbeckevoort, D.; Verslype, C.; Dekervel, J. Could Autoimmune Disease Contribute to the Abscopal Effect in Metastatic Hepatocellular Carcinoma? Hepatology 2020, 72, 1152–1154. [Google Scholar] [CrossRef] [PubMed]
- Seidl, C. Radioimmunotherapy with α-Particle-Emitting Radionuclides. Immunotherapy 2014, 6, 431–458. [Google Scholar] [CrossRef]
- Zimmermann, R. Is Actinium Really Happening? J. Nucl. Med. 2023, 64, 1516–1518. [Google Scholar] [CrossRef]
- Engle, J.W. The Production of Ac-225. Curr. Radiopharm. 2018, 11, 173–179. [Google Scholar] [CrossRef]
- Hatcher-Lamarre, J.L.; Sanders, V.A.; Rahman, M.; Cutler, C.S.; Francesconi, L.C. Alpha Emitting Nuclides for Targeted Therapy. Nucl. Med. Biol. 2021, 92, 228–240. [Google Scholar] [CrossRef]
- Eychenne, R.; Chérel, M.; Haddad, F.; Guérard, F.; Gestin, J.-F. Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight”. Pharmaceutics 2021, 13, 906. [Google Scholar] [CrossRef]
- Pommé, S.; Marouli, M.; Suliman, G.; Dikmen, H.; Van Ammel, R.; Jobbágy, V.; Dirican, A.; Stroh, H.; Paepen, J.; Bruchertseifer, F.; et al. Measurement of the 225Ac Half-Life. Appl. Radiat. Isot. 2012, 70, 2608–2614. [Google Scholar] [CrossRef] [PubMed]
- Suliman, G.; Pommé, S.; Marouli, M.; Van Ammel, R.; Stroh, H.; Jobbágy, V.; Paepen, J.; Dirican, A.; Bruchertseifer, F.; Apostolidis, C.; et al. Half-Lives of 221Fr, 217At, 213Bi, 213Po and 209Pb from the 225Ac Decay Series. Appl. Radiat. Isot. 2013, 77, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics 2020, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Scheinberg, D.A.; McDevitt, M.R. Actinium-225 in Targeted Alpha-Particle Therapeutic Applications. Curr. Radiopharm. 2011, 4, 306–320. [Google Scholar] [CrossRef]
- Muslimov, A.R.; Antuganov, D.; Tarakanchikova, Y.V.; Karpov, T.E.; Zhukov, M.V.; Zyuzin, M.V.; Timin, A.S. An Investigation of Calcium Carbonate Core-Shell Particles for Incorporation of 225Ac and Sequester of Daughter Radionuclides: In Vitro and in Vivo Studies. J. Control Release 2021, 330, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.J.B.; Wilson, J.; Andersson, J.D.; Wuest, F. Theranostic Imaging Surrogates for Targeted Alpha Therapy: Progress in Production, Purification, and Applications. Pharmaceuticals 2023, 16, 1622. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Bartels, J.L.; Appiah, J.-P.K.; Rider, J.H.; Baumhover, N.; Schultz, M.K.; Lapi, S.E. Optimized Methods for the Production of High-Purity 203Pb Using Electroplated Thallium Targets. J. Nucl. Med. 2023, 64, 1791–1797. [Google Scholar] [CrossRef]
- Bobba, K.N.; Bidkar, A.P.; Meher, N.; Fong, C.; Wadhwa, A.; Dhrona, S.; Sorlin, A.; Bidlingmaier, S.; Shuere, B.; He, J.; et al. Evaluation of 134Ce/134La as a PET Imaging Theranostic Pair for 225Ac α-Radiotherapeutics. J. Nucl. Med. 2023, 64, 1076–1082. [Google Scholar] [CrossRef]
- Aluicio-Sarduy, E.; Barnhart, T.E.; Weichert, J.; Hernandez, R.; Engle, J.W. Cyclotron-Produced 132La as a PET Imaging Surrogate for Therapeutic 225Ac. J. Nucl. Med. 2021, 62, 1012–1015. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Ferguson, S.; Wuest, M.; Wilson, J.; Duke, M.J.M.; Richter, S.; Soenke-Jans, H.; Andersson, J.D.; Juengling, F.; Wuest, F. First In Vivo and Phantom Imaging of Cyclotron-Produced 133La as a Theranostic Radionuclide for 225Ac and 135La. J. Nucl. Med. 2022, 63, 584–590. [Google Scholar] [CrossRef]
- Bailey, T.A.; Mocko, V.; Shield, K.M.; An, D.D.; Akin, A.C.; Birnbaum, E.R.; Brugh, M.; Cooley, J.C.; Engle, J.W.; Fassbender, M.E.; et al. Developing the 134Ce and 134La Pair as Companion Positron Emission Tomography Diagnostic Isotopes for 225Ac and 227Th Radiotherapeutics. Nat. Chem. 2021, 13, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.A.; Wacker, J.N.; An, D.D.; Carter, K.P.; Davis, R.C.; Mocko, V.; Larrabee, J.; Shield, K.M.; Lam, M.N.; Booth, C.H.; et al. Evaluation of 134Ce as a PET Imaging Surrogate for Antibody Drug Conjugates Incorporating 225Ac. Nucl. Med. Biol. 2022, 110–111, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Aluicio-Sarduy, E.; Brown, V.; MacMillan, S.N.; Becker, K.V.; Barnhart, T.E.; Radchenko, V.; Ramogida, C.F.; Engle, J.W.; Wilson, J.J. Py-Macrodipa: A Janus Chelator Capable of Binding Medicinally Relevant Rare-Earth Radiometals of Disparate Sizes. J. Am. Chem. Soc. 2021, 143, 10429–10440. [Google Scholar] [CrossRef]
- Thiele, N.A.; Brown, V.; Kelly, J.M.; Amor-Coarasa, A.; Jermilova, U.; MacMillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Ramogida, C.F.; Robertson, A.K.H.; et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem. Int. Ed. Engl. 2017, 56, 14712–14717. [Google Scholar] [CrossRef]
- Rizk, H.E.; Breky, M.M.E.; Attallah, M.F. Development of Purification of No-Carrier-Added 47Sc of Theranostic Interest: Selective Separation Study from the natTi(n,p) Process. Radiochim. Acta 2023, 111, 273–282. [Google Scholar] [CrossRef]
- Mousa, A.M.; Abdel Aziz, O.A.; Al-Hagar, O.E.A.; Gizawy, M.A.; Allan, K.F.; Attallah, M.F. Biosynthetic New Composite Material Containing CuO Nanoparticles Produced by Aspergillus Terreus for 47Sc Separation of Cancer Theranostics Application from Irradiated Ca Target. Appl. Radiat. Isot. 2020, 166, 109389. [Google Scholar] [CrossRef] [PubMed]
- Attallah, M.F.; Rizk, S.E.; Shady, S.A. Separation of 152+154Eu, 90Sr from Radioactive Waste Effluent Using Liquid–Liquid Extraction by Polyglycerol Phthalate. Nucl. Sci. Tech. 2018, 29, 84. [Google Scholar] [CrossRef]
- Hooijman, E.L.; Ntihabose, C.M.; Reuvers, T.G.A.; Nonnekens, J.; Aalbersberg, E.A.; van de Merbel, J.R.J.P.; Huijmans, J.E.; Koolen, S.L.W.; Hendrikx, J.J.M.A.; de Blois, E. Radiolabeling and Quality Control of Therapeutic Radiopharmaceuticals: Optimization, Clinical Implementation and Comparison of Radio-TLC/HPLC Analysis, Demonstrated by [177Lu]Lu-PSMA. EJNMMI Radiopharm. Chem. 2022, 7, 29. [Google Scholar] [CrossRef]
- Mdanda, S.; Ngema, L.M.; Mdlophane, A.; Sathekge, M.M.; Zeevaart, J.R. Recent Innovations and Nano-Delivery of Actinium-225: A Narrative Review. Pharmaceutics 2023, 15, 1719. [Google Scholar] [CrossRef]
- Abou, D.S.; Zerkel, P.; Robben, J.; McLaughlin, M.; Hazlehurst, T.; Morse, D.; Wadas, T.J.; Pandya, D.N.; Oyama, R.; Gaehle, G.; et al. Radiopharmaceutical Quality Control Considerations for Accelerator-Produced Actinium Therapies. Cancer Biother. Radiopharm. 2022, 37, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Dumond, A.R.S.; Rodnick, M.E.; Piert, M.R.; Scott, P.J.H. Synthesis of 225Ac-PSMA-617 for Preclinical Use. Curr. Radiopharm. 2022, 15, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Thakral, P.; Simecek, J.; Marx, S.; Kumari, J.; Pant, V.; Sen, I.B. In-House Preparation and Quality Control of Ac-225 Prostate-Specific Membrane Antigen-617 for the Targeted Alpha Therapy of Castration-Resistant Prostate Carcinoma. Indian. J. Nucl. Med. 2021, 36, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Amor-Coarasa, A.; Sweeney, E.; Wilson, J.J.; Causey, P.W.; Babich, J.W. A Suitable Time Point for Quantifying the Radiochemical Purity of 225Ac-Labeled Radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2021, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Hooijman, E.L.; Chalashkan, Y.; Ling, S.W.; Kahyargil, F.F.; Segbers, M.; Bruchertseifer, F.; Morgenstern, A.; Seimbille, Y.; Koolen, S.L.W.; Brabander, T.; et al. Development of [225Ac]Ac-PSMA-I&T for Targeted Alpha Therapy According to GMP Guidelines for Treatment of mCRPC. Pharmaceutics 2021, 13, 715. [Google Scholar] [CrossRef] [PubMed]
- Busslinger, S.D.; Tschan, V.J.; Richard, O.K.; Talip, Z.; Schibli, R.; Müller, C. [225Ac]Ac-SibuDAB for Targeted Alpha Therapy of Prostate Cancer: Preclinical Evaluation and Comparison with [225Ac]Ac-PSMA-617. Cancers 2022, 14, 5651. [Google Scholar] [CrossRef] [PubMed]
- King, A.P.; Gutsche, N.T.; Raju, N.; Fayn, S.; Baidoo, K.E.; Bell, M.M.; Olkowski, C.S.; Swenson, R.E.; Lin, F.I.; Sadowski, S.M.; et al. 225Ac-MACROPATATE: A Novel α-Particle Peptide Receptor Radionuclide Therapy for Neuroendocrine Tumors. J. Nucl. Med. 2023, 64, 549–554. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Bal, C. Efficacy and Safety of 225Ac-DOTATATE Targeted Alpha Therapy in Metastatic Paragangliomas: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1595–1606. [Google Scholar] [CrossRef]
- Rathke, H.; Bruchertseifer, F.; Kratochwil, C.; Keller, H.; Giesel, F.L.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. First Patient Exceeding 5-Year Complete Remission after 225Ac-PSMA-TAT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 311–312. [Google Scholar] [CrossRef]
- Zacherl, M.J.; Gildehaus, F.J.; Mittlmeier, L.; Böning, G.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Schmidt-Hegemann, N.; Belka, C.; Kretschmer, A.; et al. First Clinical Results for PSMA-Targeted α-Therapy Using 225Ac-PSMA-I&T in Advanced-mCRPC Patients. J. Nucl. Med. 2021, 62, 669–674. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.O.; Knoesen, O.; Mahapane, J.; Davis, C.; Reyneke, F.; Maes, A.; Kratochwil, C.; et al. Predictors of Overall and Disease-Free Survival in Metastatic Castration-Resistant Prostate Cancer Patients Receiving 225Ac-PSMA-617 Radioligand Therapy. J. Nucl. Med. 2020, 61, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Camacaro, J.F.; Dunckley, C.P.; Harman, S.E.; Fitzgerald, H.A.; Lakes, A.L.; Liao, Z.; Ludwig, R.C.; McBride, K.M.; Yalcintas Bethune, E.; Younes, A.; et al. Development of 225Ac Production from Low Isotopic Dilution 229Th. ACS Omega 2023, 8, 38822–38827. [Google Scholar] [CrossRef]
- Parida, G.K.; Panda, R.A.; Bishnoi, K.; Agrawal, K. Efficacy and Safety of Actinium-225 Prostate-Specific Membrane Antigen Radioligand Therapy in Metastatic Prostate Cancer: A Systematic Review and Metanalysis. Med. Princ. Pract. 2023, 32, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, L.; Liao, T.; Gong, W.; Zhang, C. Efficacy and Safety of 225Ac-PSMA-617-Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 796657. [Google Scholar] [CrossRef]
- Sanli, Y.; Kuyumcu, S.; Simsek, D.H.; Büyükkaya, F.; Civan, C.; Isik, E.G.; Ozkan, Z.G.; Basaran, M.; Sanli, O. 225Ac-Prostate-Specific Membrane Antigen Therapy for Castration-Resistant Prostate Cancer: A Single-Center Experience. Clin. Nucl. Med. 2021, 46, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Sen, I.; Thakral, P.; Tiwari, P.; Pant, V.; Das, S.S.; Manda, D.; Raina, V. Therapeutic Efficacy of 225Ac-PSMA-617 Targeted Alpha Therapy in Patients of Metastatic Castrate Resistant Prostate Cancer after Taxane-Based Chemotherapy. Ann. Nucl. Med. 2021, 35, 794–810. [Google Scholar] [CrossRef]
- Feuerecker, B.; Tauber, R.; Knorr, K.; Heck, M.; Beheshti, A.; Seidl, C.; Bruchertseifer, F.; Pickhard, A.; Gafita, A.; Kratochwil, C.; et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-Resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur. Urol. 2021, 79, 343–350. [Google Scholar] [CrossRef]
- Van der Doelen, M.J.; Mehra, N.; van Oort, I.M.; Looijen-Salamon, M.G.; Janssen, M.J.R.; Custers, J.A.E.; Slootbeek, P.H.J.; Kroeze, L.I.; Bruchertseifer, F.; Morgenstern, A.; et al. Clinical Outcomes and Molecular Profiling of Advanced Metastatic Castration-Resistant Prostate Cancer Patients Treated with 225Ac-PSMA-617 Targeted Alpha-Radiation Therapy. Urol. Oncol. 2021, 39, 729.e7–729.e16. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Tripathi, M.; Seth, A.; Bal, C. Efficacy and Safety of 225Ac-PSMA-617 Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer Patients. Theranostics 2020, 10, 9364–9377. [Google Scholar] [CrossRef]
- Satapathy, S.; Mittal, B.R.; Sood, A.; Das, C.K.; Singh, S.K.; Mavuduru, R.S.; Bora, G.S. Health-Related Quality-of-Life Outcomes with Actinium-225-Prostate-Specific Membrane Antigen-617 Therapy in Patients with Heavily Pretreated Metastatic Castration-Resistant Prostate Cancer. Indian. J. Nucl. Med. 2020, 35, 299–304. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in Chemotherapy-Naive Patients with Advanced Prostate Cancer: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J. Nucl. Med. 2018, 59, 795–802. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Tripathi, M.; Sahoo, R.K.; Bal, C. Survival Outcomes in Metastatic Gastroenteropancreatic Neuroendocrine Tumor Patients Receiving Concomitant 225Ac-DOTATATE Targeted Alpha Therapy and Capecitabine: A Real-World Scenario Management Based Long-Term Outcome Study. J. Nucl. Med. 2022, 64, 211–218. [Google Scholar] [CrossRef]
- Kratochwil, C.; Apostolidis, L.; Rathke, H.; Apostolidis, C.; Bicu, F.; Bruchertseifer, F.; Choyke, P.L.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Dosing 225Ac-DOTATOC in Patients with Somatostatin-Receptor-Positive Solid Tumors: 5-Year Follow-up of Hematological and Renal Toxicity. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 54–63. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening Horizons with 225Ac-DOTATATE Targeted Alpha Therapy for Gastroenteropancreatic Neuroendocrine Tumour Patients Stable or Refractory to 177Lu-DOTATATE PRRT: First Clinical Experience on the Efficacy and Safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. Ac-225-DOTATOC—An Empiric Dose Finding for Alpha Particle Emitter Based Radionuclide Therapy of Neuroendocrine Tumors. J. Nucl. Med. 2015, 56, 1232. [Google Scholar]
- Rosenblat, T.L.; McDevitt, M.R.; Carrasquillo, J.A.; Pandit-Taskar, N.; Frattini, M.G.; Maslak, P.G.; Park, J.H.; Douer, D.; Cicic, D.; Larson, S.M.; et al. Treatment of Patients with Acute Myeloid Leukemia with the Targeted Alpha-Particle Nanogenerator Actinium-225-Lintuzumab. Clin. Cancer Res. 2022, 28, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G. Clinical Studies with Bismuth-213 and Actinium-225 for Hematologic Malignancies. Curr. Radiopharm. 2018, 11, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G.; Levy, M.Y.; Park, J.H.; Ravandi, F.; Perl, A.E.; Pagel, J.M.; Smith, B.D.; Estey, E.H.; Kantarjian, H.; Cicic, D.; et al. Phase I Trial of Targeted Alpha-Particle Therapy with Actinium-225 (225Ac)-Lintuzumab and Low-Dose Cytarabine (LDAC) in Patients Age 60 or Older with Untreated Acute Myeloid Leukemia (AML). Blood 2016, 128, 4050. [Google Scholar] [CrossRef]
- Jurcic, J.G.; Rosenblat, T.L.; McDevitt, M.R.; Pandit-Taskar, N.; Carrasquillo, J.A.; Chanel, S.M.; Zikaras, K.; Frattini, M.G.; Maslak, P.G.; Cicic, D.; et al. Phase I Trial of the Targeted Alpha-Particle Nano-Generator Actinium-225 (225Ac)-Lintuzumab (Anti-CD33; HuM195) in Acute Myeloid Leukemia (AML). Blood 2011, 118, 768. [Google Scholar] [CrossRef]
- Pretze, M.; Kunkel, F.; Runge, R.; Freudenberg, R.; Braune, A.; Hartmann, H.; Schwarz, U.; Brogsitter, C.; Kotzerke, J. Ac-EAZY! Towards GMP-Compliant Module Syntheses of 225Ac-Labeled Peptides for Clinical Application. Pharmaceuticals 2021, 14, 652. [Google Scholar] [CrossRef]
- Eryilmaz, K.; Kilbas, B. Fully-Automated Synthesis of 177Lu Labelled FAPI Derivatives on the Module Modular Lab-Eazy. EJNMMI Radiopharm. Chem. 2021, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R. Managing the Uranium-233 Stockpile of the United States. Sci. Glob. Secur. 2013, 21, 53–69. [Google Scholar] [CrossRef]
- Robertson, A.K.H.; Ramogida, C.F.; Schaffer, P.; Radchenko, V. Development of 225Ac Radiopharmaceuticals: TRIUMF Perspectives and Experiences. Curr. Radiopharm. 2018, 11, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Boll, R.A.; Malkemus, D.; Mirzadeh, S. Production of Actinium-225 for Alpha Particle Mediated Radioimmunotherapy. Appl. Radiat. Isot. 2005, 62, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, C.; Molinet, R.; Rasmussen, G.; Morgenstern, A. Production of Ac-225 from Th-229 for Targeted Alpha Therapy. Anal. Chem. 2005, 77, 6288–6291. [Google Scholar] [CrossRef]
- Kotovskii, A.A.; Nerozin, N.A.; Prokof’ev, I.V.; Shapovalov, V.V.; Yakovshchits, Y.A.; Bolonkin, A.S.; Dunin, A.V. Isolation of Actinium-225 for Medical Purposes. Radiochemistry 2015, 57, 285–291. [Google Scholar] [CrossRef]
- Morgenstern, A.; Apostolidis, C.; Bruchertseifer, F. Supply and Clinical Application of Actinium-225 and Bismuth-213. Semin. Nucl. Med. 2020, 50, 119–123. [Google Scholar] [CrossRef]
- Harvey, J.T.; Nolen, J.; Vandergrift, G.; Gomes, I.; Kroc, T.; Horwitz, P.; McAlister, D.; Bowers, D.; Sullivan, V.; Greene, J. Production of Actinium-225 via High Energy Proton Induced Spallation of Thorium-232; NorthStar Medical Radioisotopes, LLC.: Madison, WI, USA, 2011. [Google Scholar]
- Samsonov, M.D.; Nerozin, N.A.; Podsoblyaev, D.A.; Prokof’ev, I.V.; Tkachev, S.V.; Khamianov, S.V.; Shapovalov, V.V. Isolation of Alpha-Emitting Radionuclides for Nuclear Medicine in JSC “SSC RF–IPPE. In Proceedings of the 10th International Symposium on Targeted Alpha Therapy, Kanazawa, Japan, 30 May–1 June 2017. [Google Scholar]
- USDOE Office of Science (SC). Meeting Isotope Needs and Capturing Opportunities for the Future: The 2015 Long. Range Plan. for the DOE-NP Isotope Progarm, NSAC Isotopes Subcommitee, July 2015; USDOE Office of Science (SC): Bethesda, MD, USA, 2015. [Google Scholar]
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.E.; Simon, S.; Birnbaum, E.R.; Atcher, R.W.; John, K.D.; Rixe, O.; et al. Alpha-Emitters and Targeted Alpha Therapy in Oncology: From Basic Science to Clinical Investigations. Target. Oncol. 2018, 13, 189–203. [Google Scholar] [CrossRef]
- Morgenstern, A.; Bruchertseifer, F.; Apostolidis, C. Bismuth-213 and Actinium-225—Generator Performance and Evolving Therapeutic Applications of Two Generator-Derived Alpha-Emitting Radioisotopes. Curr. Radiopharm. 2012, 5, 221–227. [Google Scholar] [CrossRef]
- Hogle, S.; Boll, R.A.; Murphy, K.; Denton, D.; Owens, A.; Haverlock, T.J.; Garland, M.; Mirzadeh, S. Reactor Production of Thorium-229. Appl. Radiat. Isot. 2016, 114, 19–27. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Englert, M.; Krall, L.; Ewing, R.C. Is Nuclear Fission a Sustainable Source of Energy? MRS Bull. 2012, 37, 417–424. [Google Scholar] [CrossRef]
- Hoehr, C.; Bénard, F.; Buckley, K.; Crawford, J.; Gottberg, A.; Hanemaayer, V.; Kunz, P.; Ladouceur, K.; Radchenko, V.; Ramogida, C.; et al. Medical Isotope Production at TRIUMF—From Imaging to Treatment. Phys. Procedia 2017, 90, 200–208. [Google Scholar] [CrossRef]
- Griswold, J.R.; Medvedev, D.G.; Engle, J.W.; Copping, R.; Fitzsimmons, J.M.; Radchenko, V.; Cooley, J.C.; Fassbender, M.E.; Denton, D.L.; Murphy, K.E.; et al. Large Scale Accelerator Production of 225Ac: Effective Cross Sections for 78-192MeV Protons Incident on 232Th Targets. Appl. Radiat. Isot. 2016, 118, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Weidner, J.W.; Mashnik, S.G.; John, K.D.; Ballard, B.; Birnbaum, E.R.; Bitteker, L.J.; Couture, A.; Fassbender, M.E.; Goff, G.S.; Gritzo, R.; et al. 225Ac and 223Ra Production via 800 MeV Proton Irradiation of Natural Thorium Targets. Appl. Radiat. Isot. 2012, 70, 2590–2595. [Google Scholar] [CrossRef] [PubMed]
- Weidner, J.W.; Mashnik, S.G.; John, K.D.; Hemez, F.; Ballard, B.; Bach, H.; Birnbaum, E.R.; Bitteker, L.J.; Couture, A.; Dry, D.; et al. Proton-Induced Cross Sections Relevant to Production of 225Ac and 223Ra in Natural Thorium Targets below 200 MeV. Appl. Radiat. Isot. 2012, 70, 2602–2607. [Google Scholar] [CrossRef]
- Cutler, C.S. US DOE Tri-Lab Effort to Produce Ac-225; International Atomic Energy Agency (IAEA): Vienna, Austria, 2020. [Google Scholar]
- Aliev, R.A.; Ermolaev, S.V.; Vasiliev, A.N.; Ostapenko, V.S.; Lapshina, E.V.; Zhuikov, B.L.; Zakharov, N.V.; Pozdeev, V.V.; Kokhanyuk, V.M.; Myasoedov, B.F.; et al. Isolation of Medicine-Applicable Actinium-225 from Thorium Targets Irradiated by Medium-Energy Protons. Solvent Extr. Ion Exch. 2014, 32, 468–477. [Google Scholar] [CrossRef]
- Mastren, T.; Radchenko, V.; Owens, A.; Copping, R.; Boll, R.; Griswold, J.R.; Mirzadeh, S.; Wyant, L.E.; Brugh, M.; Engle, J.W.; et al. Simultaneous Separation of Actinium and Radium Isotopes from a Proton Irradiated Thorium Matrix. Sci. Rep. 2017, 7, 8216. [Google Scholar] [CrossRef]
- Radchenko, V.; Engle, J.W.; Wilson, J.J.; Maassen, J.R.; Nortier, F.M.; Taylor, W.A.; Birnbaum, E.R.; Hudston, L.A.; John, K.D.; Fassbender, M.E. Application of Ion Exchange and Extraction Chromatography to the Separation of Actinium from Proton-Irradiated Thorium Metal for Analytical Purposes. J. Chromatogr. A 2015, 1380, 55–63. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Robertson, A.K.H.; Jermilova, U.; Zhang, C.; Yang, H.; Kunz, P.; Lassen, J.; Bratanovic, I.; Brown, V.; Southcott, L.; et al. Evaluation of Polydentate Picolinic Acid Chelating Ligands and an α-Melanocyte-Stimulating Hormone Derivative for Targeted Alpha Therapy Using ISOL-Produced 225Ac. EJNMMI Radiopharm. Chem. 2019, 4, 21. [Google Scholar] [CrossRef]
- Robertson, A.K.H.; McNeil, B.L.; Yang, H.; Gendron, D.; Perron, R.; Radchenko, V.; Zeisler, S.; Causey, P.; Schaffer, P. 232Th-Spallation-Produced 225Ac with Reduced 227Ac Content. Inorg. Chem. 2020, 59, 12156–12165. [Google Scholar] [CrossRef] [PubMed]
- Augusto, R.S.; Smith, J.; Varah, S.; Paley, W.; Egoriti, L.; McEwen, S.; Goodacre, T.D.; Mildenberger, J.; Gottberg, A.; Trudel, A.; et al. Design and Radiological Study of the 225Ac Medical Target at the TRIUMF-ARIEL Proton-Target Station. Radiat. Phys. Chem. 2022, 201, 110491. [Google Scholar] [CrossRef]
- Apostolidis, C.; Molinet, R.; McGinley, J.; Abbas, K.; Möllenbeck, J.; Morgenstern, A. Cyclotron Production of Ac-225 for Targeted Alpha Therapy. Appl. Radiat. Isot. 2005, 62, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Nesteruk, K.P.; Ramseyer, L.; Carzaniga, T.S.; Braccini, S. Measurement of the Beam Energy Distribution of a Medical Cyclotron with a Multi-Leaf Faraday Cup. Instruments 2019, 3, 4. [Google Scholar] [CrossRef]
- Higashi, T.; Nagatsu, K.; Tsuji, A.B.; Zhang, M.-R. Research and Development for Cyclotron Production of 225Ac from 226Ra—The Challenges in a Country Lacking Natural Resources for Medical Applications. Processes 2022, 10, 1215. [Google Scholar] [CrossRef]
- Morgenstern, A.; Abbas, K.; Bruchertseifer, F.; Apostolidis, C. Production of Alpha Emitters for Targeted Alpha Therapy. Curr. Radiopharm. 2008, 1, 135–143. [Google Scholar] [CrossRef]
- Maslov, O.D.; Sabel’nikov, A.V.; Dmitriev, S.N. Preparation of 225Ac by 226Ra(γ, n) Photonuclear Reaction on an Electron Accelerator, MT-25 Microtron. Radiochemistry 2006, 48, 195–197. [Google Scholar] [CrossRef]
- Melville, G.; Meriarty, H.; Metcalfe, P.; Knittel, T.; Allen, B.J. Production of Ac-225 for Cancer Therapy by Photon-Induced Transmutation of Ra-226. Appl. Radiat. Isot. 2007, 65, 1014–1022. [Google Scholar] [CrossRef]
- Bruchertseifer, F.; Kellerbauer, A.; Malmbeck, R.; Morgenstern, A. Targeted Alpha Therapy with Bismuth-213 and Actinium-225: Meeting Future Demand. J. Labelled Comp. Radiopharm. 2019, 62, 794–802. [Google Scholar] [CrossRef]
- IBA and SCK CEN Join Forces to Enable Production of Actinium-225|SCK CEN. Available online: https://www.sckcen.be/en/news/iba-and-sck-cen-join-forces-enable-production-actinium-225 (accessed on 5 June 2023).
- Ermolaev, S.; Skasyrskaya, A.; Vasiliev, A. A Radionuclide Generator of High-Purity Bi-213 for Instant Labeling. Pharmaceutics 2021, 13, 914. [Google Scholar] [CrossRef] [PubMed]
- Bruchertseifer, F.; Apostolidis, C.; Mirzadeh, S.; Boll, R.; Murphy, K.; Morgenstern, A. Development of a High-Activity 225Ac/213Bi Radionuclide Generator for Synthesis of Clinical Doses of 213Bi-Labelled Biomolecules. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC82742 (accessed on 4 June 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).