Span 60/Cholesterol Niosomal Formulation as a Suitable Vehicle for Gallic Acid Delivery with Potent In Vitro Antibacterial, Antimelanoma, and Anti-Tyrosinase Activity
Abstract
:1. Introduction
2. Results
2.1. Niosomal Formulations Obtained and Their Physicochemical Characteristics
2.2. Morphological Characterization of GAN with F2 Formulation
2.3. FTIR Analysis of F2 Formulation
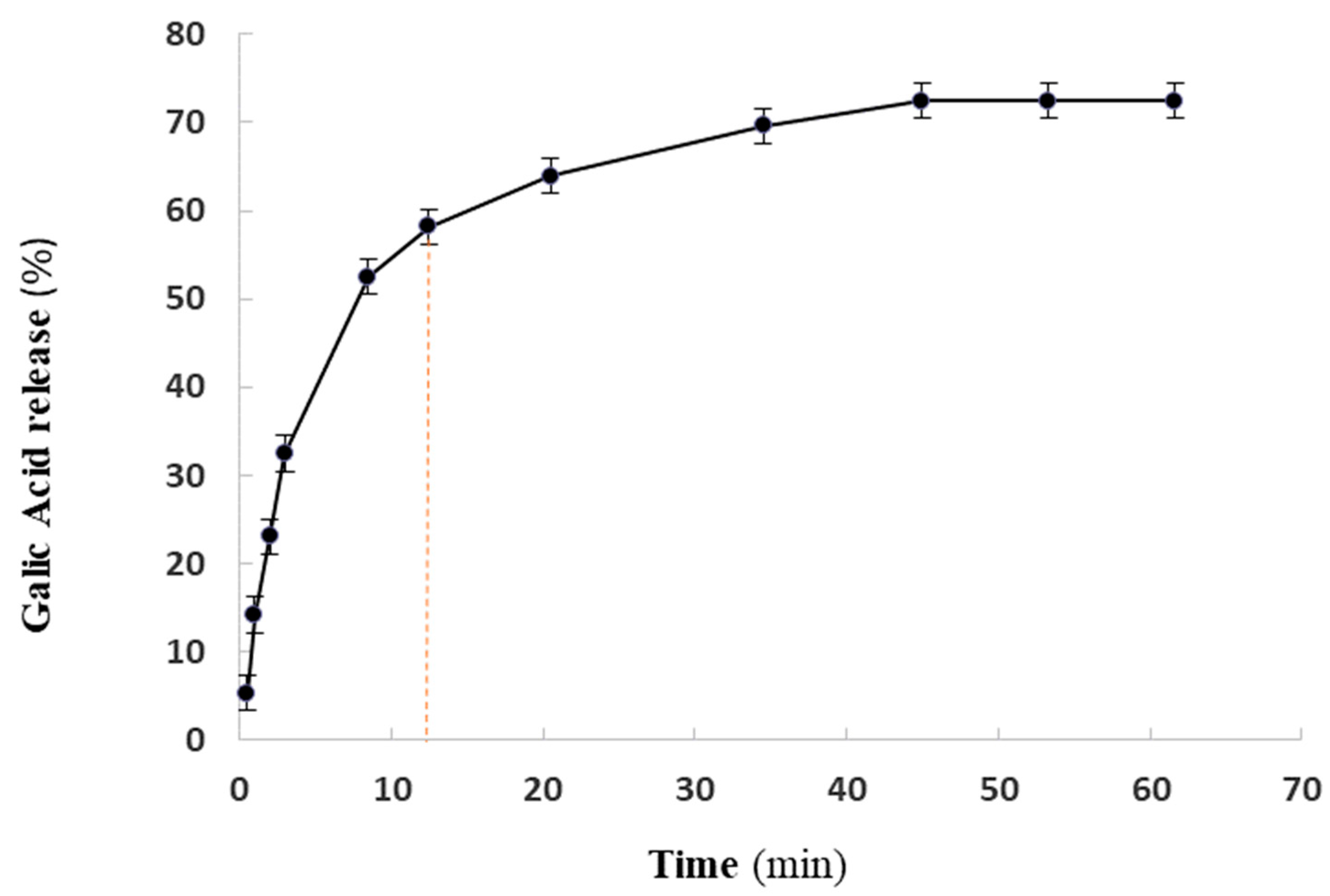
2.4. GA Release
2.5. Inhibitory and Bactericidal Concentration
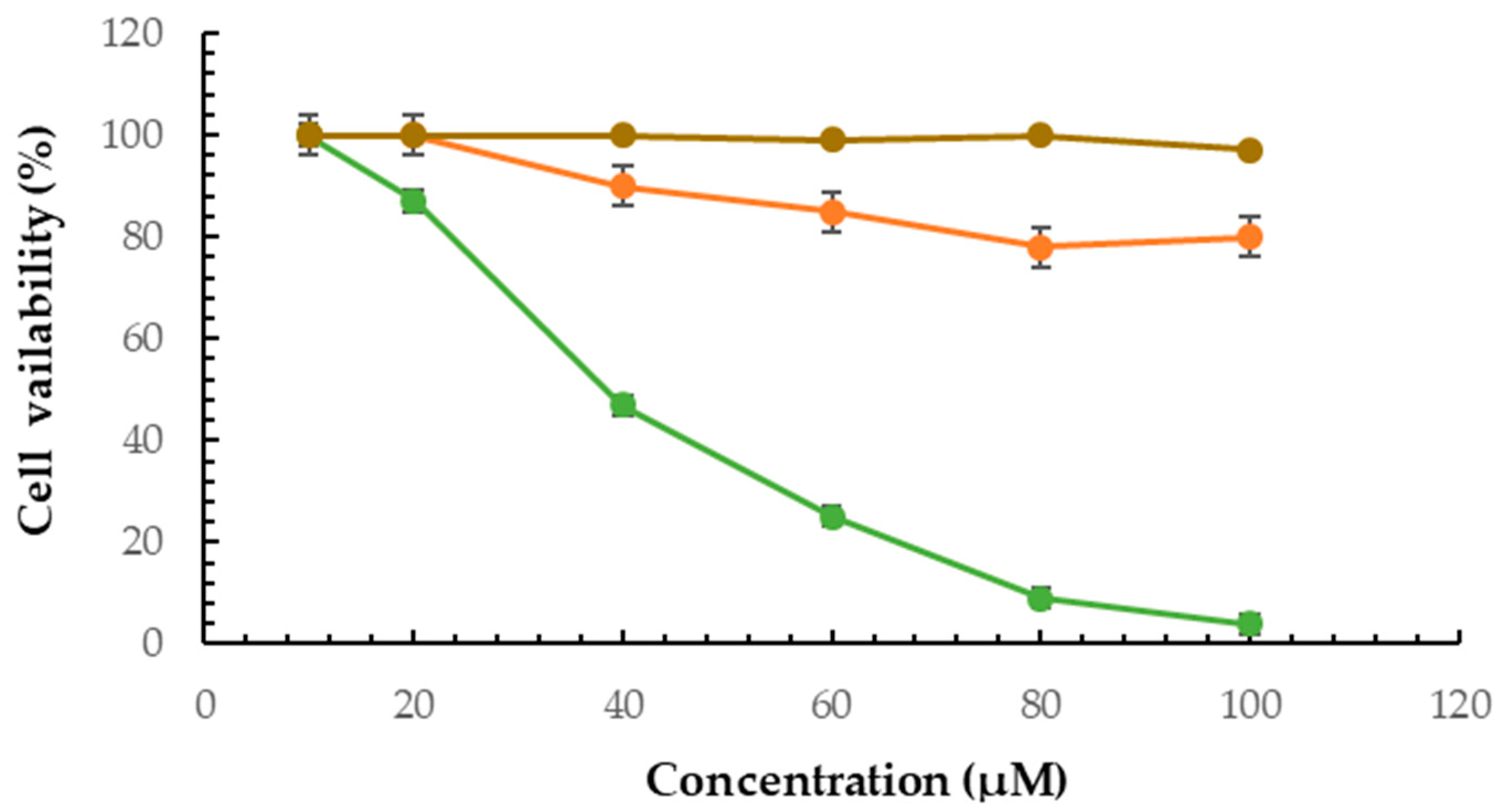
2.6. Cytotoxicity Effect of F2 Formulation
2.7. Melanin Assay
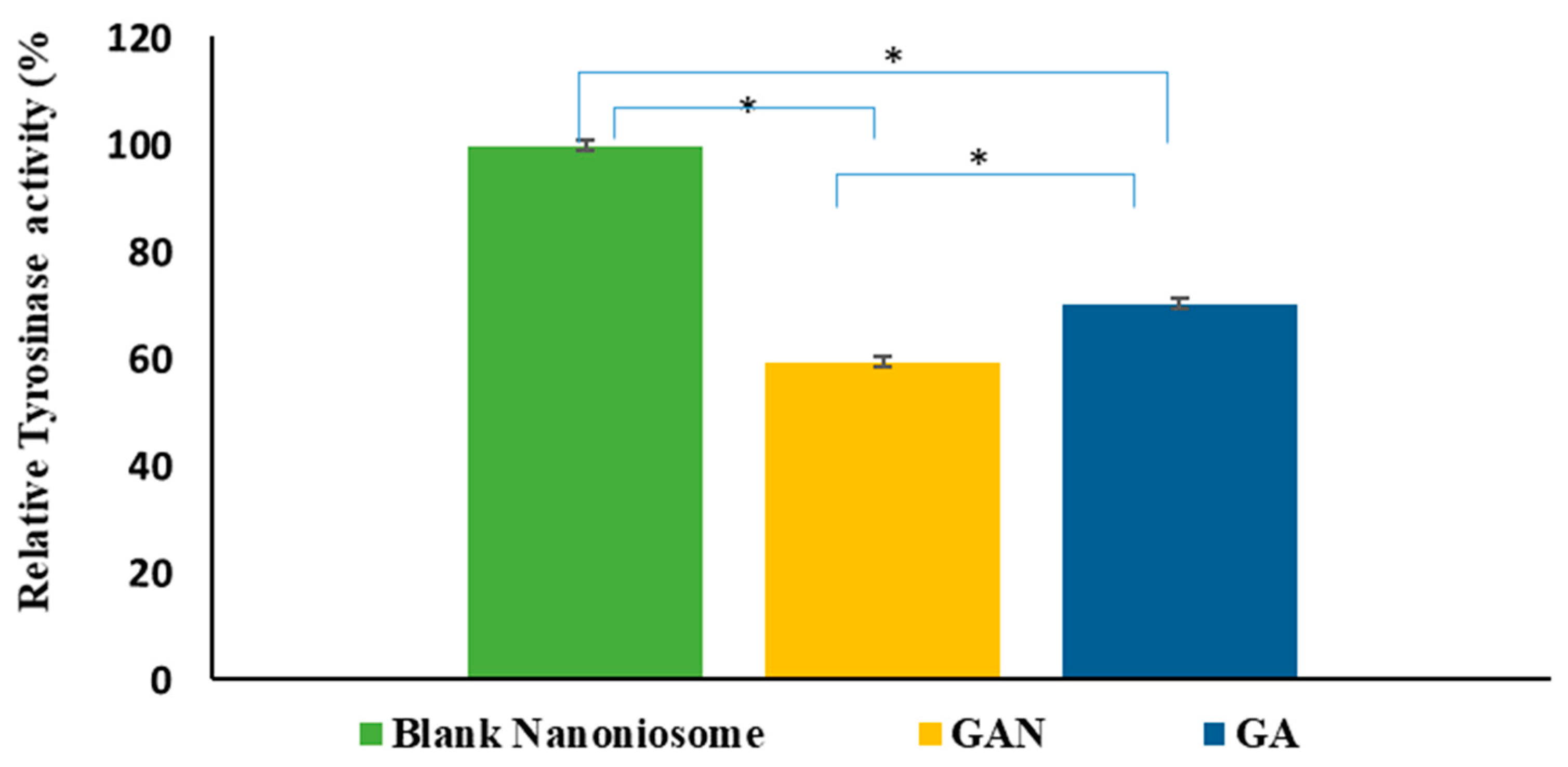
2.8. Intracellular TYR Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of GAN
4.3. Characterization of GAN
4.3.1. Dynamic Light Scattering (DLS) Measurement
4.3.2. Encapsulation Efficiency Evaluation
4.3.3. Scanning Electron Microscopy
4.3.4. Fourier-Transform Infrared (FTIR) Spectroscopy
4.4. In Vitro Kinetics Study of GA Releasing
- Zero-order model: Qt = k0 t,
- First-order model: logQt = kt/2.303,
- Korsmeyer–Peppas model: Mt/M∞ = k tn,
- Higuchi model: Qt = kH t1/2,
- Hixson–Crowell model: Wo1/3 − Wt1/3 = k t
4.5. Antimicrobial Activity Evaluation
4.6. Anti-melanoma Activity Measurement by MTT Assay
4.7. Melanin Assay
4.8. Intracellular TYR Activity
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedra, N.S.; Bona, N.P.; de Aguiar, M.S.S.; Spohr, L.; Alves, F.L.; Santos, F.d.S.d.; Saraiva, J.T.; Stefanello, F.M.; Braganhol, E.; Spanevello, R.M. Impact of gallic acid on tumor suppression: Modulation of redox homeostasis and purinergic response in in vitro and a preclinical glioblastoma model. J. Nutr. Biochem. 2022, 110, 109156. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Li, Y.; Tan, L.; Chen, L.; Shi, Q.; Zeng, Q.-H.; Liu, H.; Wang, J.J.; Zhao, Y. Anti-tyrosinase, antioxidant and antibacterial activities of gallic acid-benzylidenehydrazine hybrids and their application in preservation of fresh-cut apples and shrimps. Food Chem. 2022, 378, 132127. [Google Scholar] [CrossRef] [PubMed]
- Anwar, R.; Hajardhini, P. Antibacterial Activity of Gallic Acid from the Leaves of Altingia excelsa Noronha to Enterococcus faecalis. Open Access Maced. J. Med. Sci. 2022, 10, 10340. [Google Scholar] [CrossRef]
- Cai, L.; Wei, Z.; Zhao, X.; Li, Y.; Li, X.; Jiang, X. Gallic acid mitigates LPS-induced inflammatory response via suppressing NF-κB signalling pathway in IPEC-J2 cells. J. Anim. Physiol. Anim. Nutr. 2022, 106, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef] [PubMed]
- Gururaj, D.; Veerichetty, V. Comparative study on in vitro release kinetics of Gallic acid β Cyclodextrin complex and Gallic acid Pluronic loaded films. Mater. Today Proc. 2022, 59, 1155–1162. [Google Scholar] [CrossRef]
- Zolghadri, S.; Beygi, M.; Mohammad, T.F.; Alijanianzadeh, M.; Pillaiyar, T.; Garcia-Molina, P.; Garcia-Canovas, F.; Munoz-Munoz, J.; Saboury, A.A. Targeting tyrosinase in hyperpigmentation: Current status, limitations and future promises. Biochem. Pharmacol. 2023, 212, 115574. [Google Scholar] [CrossRef]
- Lee, S.Y.; Baek, N.; Nam, T.G. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2016, 31, 1004058. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. MDR 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Kang, J.; Liu, L.; Liu, M.; Wu, X.; Li, J. Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression. Food Control 2018, 94, 147–154. [Google Scholar] [CrossRef]
- Shukla, S.; Singh, B.; Singh, A.; Singh, C. Emerging and advanced drug delivery systems for improved biopharmaceutical attributes of gallic acid: A review. Phytomed. Plus 2022, 2, 100369. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure–function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Keyvani-Ghamsari, S.; Rahimi, M.; Khorsandi, K. An update on the potential mechanism of gallic acid as an antibacterial and anticancer agent. Food Sci. Nutr. 2023, 11, 5856–5872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pu, C.; Tang, W.; Wang, S.; Sun, Q. Gallic acid liposomes decorated with lactoferrin: Characterization, in vitro digestion and antibacterial activity. Food Chem. 2019, 293, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.; Codină, G.G.; Héjja, M.; András, C.D.; Chetrariu, A.; Dabija, A. Study of Antioxidant Activity of Garden Blackberries (Rubus fruticosus L.) Extracts Obtained with Different Extraction Solvents. Appl. Sci. 2022, 12, 4004. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Silva, C.G.; Silva, A.M.T.; Vilar, V.J.P.; Boaventura, R.A.R.; Faria, J.L. Photocatalytic activity of TiO2-coated glass raschig rings on the degradation of phenolic derivatives under simulated solar light irradiation. J. Chem. Eng. 2013, 224, 32–38. [Google Scholar] [CrossRef]
- Mortazavi, S.M.R.; Vaezi, Z.; Mahdavian, R.; Barzegar, M.; Naderi-Manesh, H. A novel cerasomal gallic acid as a non-ulcerogenic agent with an improved anti-inflammatory potential. J. Drug Deliv. Sci. Technol. 2023, 86, 104610. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Luo, L.-J. Antioxidant Gallic Acid-Functionalized Biodegradable in Situ Gelling Copolymers for Cytoprotective Antiglaucoma Drug Delivery Systems. Biomacromolecules 2015, 16, 2950–2963. [Google Scholar] [CrossRef]
- Azarmi, M.; Maleki, H.; Nikkam, N.; Malekinejad, H. Novel neurolisteriosis therapy using SPION as a drivable nanocarrier in gallic acid delivery to CNS. J. Controlled Release 2023, 353, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahhab, M.A.; Aljawish, A.; Kenawy, A.M.; El-Nekeety, A.A.; Hamed, H.S.; Abdel-Aziem, S.H. Grafting of gallic acid onto chitosan nano particles enhances antioxidant activities in vitro and protects against ochratoxin A toxicity in catfish (Clarias gariepinus). Environ. Toxicol. Pharmacol. 2016, 41, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; An, D.; Zhang, R.; Huang, Y.; Liu, Z. Preparation of carbon nanotubes and polyhedral oligomeric-reinforced molecularly imprinted polymer composites for drug delivery of gallic acid. Int. J. Pharm. 2022, 615, 121476. [Google Scholar] [CrossRef] [PubMed]
- Sunil Gowda, S.N.; Rajasowmiya, S.; Vadivel, V.; Banu Devi, S.; Celestin Jerald, A.; Marimuthu, S.; Devipriya, N. Gallic acid-coated sliver nanoparticle alters the expression of radiation-induced epithelial-mesenchymal transition in non-small lung cancer cells. In Vitro Toxicol. 2018, 52, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Vitonyte, J.; Manca, M.L.; Caddeo, C.; Valenti, D.; Peris, J.E.; Usach, I.; Nacher, A.; Matos, M.; Gutiérrez, G.; Orrù, G.; et al. Bifunctional viscous nanovesicles co-loaded with resveratrol and gallic acid for skin protection against microbial and oxidative injuries. Eur. J. Pharm. Biopharm. 2017, 114, 278–287. [Google Scholar] [CrossRef]
- Yasamineh, S.; Yasamineh, P.; Kalajahi, H.G.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Kheirkhah, A.H.; Taghizadeh, M.; Yazdani, Y. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm. 2022, 624, 121878. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Saleh, A.; Pirouzifard, M.; Alizadeh Khaledabad, M.; Almasi, H. Optimization and Characterization of Lippia citriodora Essential Oil Loaded Niosomes: A Novel Plant-based Food Nano Preservative. Colloids Surf. A Physicochem. Eng. 2022, 650, 129480. [Google Scholar] [CrossRef]
- Nsairat, H.; Ibrahim, A.A.; Jaber, A.M.; Abdelghany, S.; Atwan, R.; Shalan, N.; Abdelnabi, H.; Odeh, F.; El-Tanani, M.; Alshaer, W. Liposome bilayer stability: Emphasis on cholesterol and its alternatives. J. Liposome Res. 2023, 1–25. [Google Scholar] [CrossRef]
- Talebi, V.; Ghanbarzadeh, B.; Hamishehkar, H.; Pezeshki, A.; Ostadrahimi, A. Effects of different stabilizers on colloidal properties and encapsulation efficiency of vitamin D3 loaded nano-niosomes. J. Drug Deliv. Sci. Technol. 2021, 61, 101284. [Google Scholar] [CrossRef]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Witika, B.A.; Bassey, K.E.; Demana, P.H.; Siwe-Noundou, X.; Poka, M.S. Current Advances in Specialised Niosomal Drug Delivery: Manufacture, Characterization and Drug Delivery Applications. Int. J. Mol. Sci. 2022, 23, 9668. [Google Scholar] [CrossRef] [PubMed]
- Gorjian, H.; Amiri, Z.R.; Milani, J.M.; Khaligh, N.G. Preparation and characterization of the encapsulated myrtle extract nanoliposome and nanoniosome without using cholesterol and toxic organic solvents: A comparative study. Food Chem. 2021, 342, 128342. [Google Scholar] [CrossRef]
- Rajizadeh, M.A.; Motamedy, S.; Mir, Y.; Akhgarandouz, F.; Nematollahi, M.H.; Nezhadi, A. A comprehensive and updated review on the applications of vesicular drug delivery systems in treatment of brain disorders: A shelter against storms. J. Drug Deliv. Sci. Technol. 2023, 89, 105011. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Sedaghatnia, K.; Bourbour, M.; Fatemizadeh, M.; Moghaddam, Z.S.; Hejabi, F.; Heidari, F.; Quazi, S.; Far, B.F. Niosomes: A novel targeted drug delivery system for cancer. Med. Oncol. 2022, 39, 240. [Google Scholar] [CrossRef]
- Bozó, T.; Mészáros, T.; Mihály, J.; Bóta, A.; Kellermayer, M.S.Z.; Szebeni, J.; Kálmán, B. Aggregation of PEGylated liposomes driven by hydrophobic forces. Colloids Surf. B 2016, 147, 467–474. [Google Scholar] [CrossRef]
- Saddik, M.; Mohamed, E.-E.; El-Mahdy, M. Preparation and Characterization of Niosomal Carrier System of Hydrophilic Drug (Methylene Blue) for Photodynamic Therapy. Lat. Am. J. Pharm. 2020, 39, 561–569. [Google Scholar]
- El-Mahdy, M.M.; Mohamed, E.-E.M.; Saddik, M.S.; Ali, M.F.; El-Sayed, A.M. Formulation and clinical evaluation of niosomal methylene blue for successful treatment of acne. Int. J. Adv. Biol. Biomed. Res. 2020, 3, 116. [Google Scholar] [CrossRef]
- Khan, S.; Akhtar, M.U.; Khan, S.; Javed, F.; Khan, A.A. Nanoniosome-encapsulated levoflaxicin as an antibacterial agent against Brucella. J. Basic Microbiol. 2020, 60, 281–290. [Google Scholar] [CrossRef]
- Radmard, A.; Saeedi, M.; Morteza-Semnani, K.; Hashemi, S.M.H.; Nokhodchi, A. An eco-friendly and green formulation in lipid nanotechnology for delivery of a hydrophilic agent to the skin in the treatment and management of hyperpigmentation complaints: Arbutin niosome (Arbusome). Colloids Surf. B 2021, 201, 111616. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.A.; Salem, H.A.; Eladawy, S.A.; Elsamad, Z.; Kohaf, N.A. Development and clinical evaluation of topical hydroquinone niosomal gel formulation for the treatment of melasma. Int. J. App Pharm. 2020, 12, 228–236. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Ramzy, A.; Sawy, A.M.; Nabil, M.; Gad, M.Z.; El-Shazly, M.; Aboul-Soud, M.A.; Azzazy, H.M.E.-S. Ozonated Olive Oil: Enhanced Cutaneous Delivery via Niosomal Nanovesicles for Melanoma Treatment. Antioxidants 2022, 11, 1318. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, A.; Mazumder, A.; Du Plessis, L.; Du Preez, J.L.; Haynes, R.K.; Du Plessis, J. In vitro anti-cancer effects of artemisone nano-vesicular formulations on melanoma cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 2041–2050. [Google Scholar] [CrossRef]
- Obeid, M.A.; Alyamani, H.; Amawi, H.; Aljabali, A.A.; Rezigue, M.; Abdeljaber, S.N.; Ferro, V.A. Sirna delivery to melanoma cells with cationic niosomes. Methods Mol. Biol. 2021, 2265, 621–634. [Google Scholar] [PubMed]
- Kashani-Asadi-Jafari, F.; Hadjizadeh, A. Niosome-Encapsulated Doxycycline Hyclate for Potentiation of Acne Therapy: Formulation and Characterization. Pharm. Nanotechnol. 2022, 10, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Fan, C.; Stepanskiy, L.; Uitto, J.; Papazoglou, E. Effect of size at the nanoscale and bilayer rigidity on skin diffusion of liposomes. J. Biomed. Mater. Res. A 2009, 91, 140–148. [Google Scholar] [CrossRef]
- Abdelbary, G.; El-Gendy, N. Niosome-encapsulated gentamicin for ophthalmic controlled delivery. AAPS Pharmscitech 2008, 9, 740–747. [Google Scholar] [CrossRef]
- Noronha, C.M.; Granada, A.F.; de Carvalho, S.M.; Lino, R.C.; de Maciel, M.V.O.B.; Barreto, P.L.M. Optimization of α-tocopherol loaded nanocapsules by the nanoprecipitation method. Ind. Crops Prod. 2013, 50, 896–903. [Google Scholar] [CrossRef]
- Nazari-Vanani, R.; Karimian, K.; Azarpira, N.; Heli, H. Capecitabine-loaded nanoniosomes and evaluation of anticancer efficacy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 420–426. [Google Scholar] [CrossRef]
- Pando, D.; Beltrán, M.; Gerone, I.; Matos, M.; Pazos, C. Resveratrol entrapped niosomes as yoghurt additive. Food Chem. 2015, 170, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Shatalebi, M.A.; Mostafavi, S.A.; Moghaddas, A. Niosome as a drug carrier for topical delivery of N-acetyl glucosamine. Res. Pharm. Sci. 2010, 5, 107–117. [Google Scholar] [PubMed]
- Su, T.-R.; Lin, J.-J.; Tsai, C.-C.; Huang, T.-K.; Yang, Z.-Y.; Wu, M.-O.; Zheng, Y.-Q.; Su, C.-C.; Wu, Y.-J. Inhibition of melanogenesis by gallic acid: Possible involvement of the PI3K/Akt, MEK/ERK and Wnt/β-catenin signaling pathways in B16F10 cells. Int. J. Mol. Sci. 2013, 14, 20443–20458. [Google Scholar] [CrossRef] [PubMed]
- Chaikul, P.; Khat-Udomkiri, N.; Iangthanarat, K.; Manosroi, J.; Manosroi, A. Characteristics and in vitro anti-skin aging activity of gallic acid loaded in cationic CTAB niosome. Eur. J. Pharm. Sci. 2019, 131, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Ravalika, V.; Sailaja, A.K. Formulation and evaluation of etoricoxib niosomes by thin film hydration technique and ether injection method. Nano Biomed. Eng. 2017, 9, 242–248. [Google Scholar] [CrossRef]
- Thabet, Y.; Elsabahy, M.; Eissa, N.G. Methods for preparation of niosomes: A focus on thin-film hydration method. Methods 2022, 199, 9–15. [Google Scholar] [CrossRef]
- Manuela, I.; Dobreci, D.; Irimiciuc, S.; Agop, M.; Petrescu, T.; Doroftei, B. A theoretical mathematical model for assessing diclofenac release from chitosan-based formulations. Drug Deliv. 2020, 27, 1125–1133. [Google Scholar]
- Bazsefidpar, P.; Eftekhar, E.; Jahromi, M.Z.; Nikpoor, A.R.; Moghadam, M.E.; Zolghadri, S. In-vitro cytotoxicity and in-vivo antitumor activity of two platinum complexes with 1,3-dimethyl pentyl glycine ligand against breast cancer. J. Inorg. Biochem. 2023, 241, 112144. [Google Scholar] [CrossRef]
- Bazsefidpar, P.; Zolghadri, S.; Nikpoor, A.R.; Eftekhar, E.; Jahromi, M.Z. Anti-proliferative impact of three Schiff base platinum (II) complexes against human breast cancer cell line. J. Res. Pharm. 2022, 26, 1665–1675. [Google Scholar] [CrossRef]
- Eftekhar, E.; Nikpoor, A.R.; Zolghadri, S.; Zareian Jahromi, M. Toxicity effect of synthesized platinum Schiff bases on SKBR3 breast cancer cell line. Iran. J. Breast Dis. 2023, 16, 49–64. [Google Scholar]
- Eslami Moghadam, M.; Hasanzadeh Esfahani, M.; Behzad, M.; Zolghadri, S.; Ramezani, N.; Azadi, Y. New platinum (II) complexes based on Schiff bases: Synthesis, specification, X-ray structure, ADMET, DFT, molecular docking, and anticancer activity against breast cancer. J. Biol. Inorg. Chem. 2023, 28, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, N.; Eslami Moghadam, M.; Behzad, M.; Zolghadri, S. Two new oral candidates as anticancer platinum complexes of 1,3-dimethyl pentyl glycine ligand as doping agents against breast cancer. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 251, 119415. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Ghanbariasad, A.; Fallahian, F.; Rahban, M.; Kalavani, M.; Bahman Jahromi, E.; Asadzadeh, A.; Hajiani, M. Anticancer activity of N-heteroaryl acetic acid salts against breast cancer; in silico and in vitro investigation. Mol. Biol. Rep. 2022, 49, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Chung, Y.C.; Hyun, C.G. Induction of Melanogenesis by Fosfomycin in B16F10 Cells Through the Upregulation of P-JNK and P-p38 Signaling Pathways. Antibiotics 2020, 9, 172. [Google Scholar] [CrossRef]
- Tayarani-Najaran, Z.; Eghbali-Feriz, S.; Taleghani, A.; Al-Najjar, H.; Emami, S.A.; Rahimi, H.; Asili, J.; Hasanzadeh, S. Anti-melanogenesis and anti-tyrosinase properties of Pistacia atlantica subsp. mutica extracts on B16F10 murine melanoma cells. Res. Pharm. Sci. 2018, 13, 533–545. [Google Scholar]



| Formulation | Cholesterol/ Surfactant (Molar Ratio) | Drug (Mm) | Cholesterol: Span 60 to Tween 60 | Zeta Potential (mv) ± SD | Size ± SD | PDI ± SD | EE% ± SD |
|---|---|---|---|---|---|---|---|
| F1 | 1-1 | 1.5 | 1:0.5:0.5 | −24.96 ± 2.1 | 169.8 ± 4.1 | 0.22 ± 0.1 | 80 ± 4 |
| F2 | 1-1 | 1.5 | 1:1:0 | −44.78 ± 2.3 | 80.2 ± 5.7 | 0.26 ± 0.01 | 75 ± 3 |
| F3 | 1-1 | 1.5 | 1:0:1 | −9.78 ± 1.1 | 276.8 ± 7.4 | 0.34 ± 0.04 | 96 ± 6 |
| F01 (blank) | 1-1 | 0 | 1:0.5:0.5 | −3.4 ± 1.5 | 118.3 ± 4.3 | 0.23 ± 0.08 | --- |
| F02 (blank) | 1-1 | 0 | 1:1:0 | −31.2 ± 3.6 | 64.2 ± 3.1 | 0.21 ± 0.02 | --- |
| F03 (blank) | 1-1 | 0 | 1:0:1 | −8.65 ± 2.0 | 180 ± 1.2 | 0.3 ± 0.01 | --- |
| Mathematical Model for Releasing | First Stage (Up to 60% Release) | ||
|---|---|---|---|
| R2 | k | n | |
| Korsmeyer–Peppas | 0.98 | 0.025 | 0.63 |
| Higuchi | 0.94 | 15.6 | |
| Hixson–Crowell | 0.85 | 0.06 | |
| First-order | 0.96 | 0.02 | |
| Zero-order | 0.91 | 2.7 | |
| Test Groups | MBC (µM) | MIC (µM) | ||||
|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | K. pneumonia | E. coli | P. aeruginosa | K. pneumonia | |
| GA | 750 | 750 | >750 | 375 | 375 | 750 |
| GAN (F2 formulation) | 46 | 375 | 750 | 23 | 187 | 750 |
| Gentamycin | 131 | 524 | 262 | 131 | 262 | 131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolghadri, S.; Asad, A.G.; Farzi, F.; Ghajarzadeh, F.; Habibi, Z.; Rahban, M.; Zolghadri, S.; Stanek, A. Span 60/Cholesterol Niosomal Formulation as a Suitable Vehicle for Gallic Acid Delivery with Potent In Vitro Antibacterial, Antimelanoma, and Anti-Tyrosinase Activity. Pharmaceuticals 2023, 16, 1680. https://doi.org/10.3390/ph16121680
Zolghadri S, Asad AG, Farzi F, Ghajarzadeh F, Habibi Z, Rahban M, Zolghadri S, Stanek A. Span 60/Cholesterol Niosomal Formulation as a Suitable Vehicle for Gallic Acid Delivery with Potent In Vitro Antibacterial, Antimelanoma, and Anti-Tyrosinase Activity. Pharmaceuticals. 2023; 16(12):1680. https://doi.org/10.3390/ph16121680
Chicago/Turabian StyleZolghadri, Sara, Ali Ghanbari Asad, Fatemeh Farzi, Fatemeh Ghajarzadeh, Zeinab Habibi, Mahdie Rahban, Samaneh Zolghadri, and Agata Stanek. 2023. "Span 60/Cholesterol Niosomal Formulation as a Suitable Vehicle for Gallic Acid Delivery with Potent In Vitro Antibacterial, Antimelanoma, and Anti-Tyrosinase Activity" Pharmaceuticals 16, no. 12: 1680. https://doi.org/10.3390/ph16121680
APA StyleZolghadri, S., Asad, A. G., Farzi, F., Ghajarzadeh, F., Habibi, Z., Rahban, M., Zolghadri, S., & Stanek, A. (2023). Span 60/Cholesterol Niosomal Formulation as a Suitable Vehicle for Gallic Acid Delivery with Potent In Vitro Antibacterial, Antimelanoma, and Anti-Tyrosinase Activity. Pharmaceuticals, 16(12), 1680. https://doi.org/10.3390/ph16121680












