A Physiologically Based Pharmacokinetic Model to Predict Systemic Ondansetron Concentration in Liver Cirrhosis Patients
Abstract
:1. Introduction
2. Results
2.1. Healthy PBPK Model Development and Evaluation
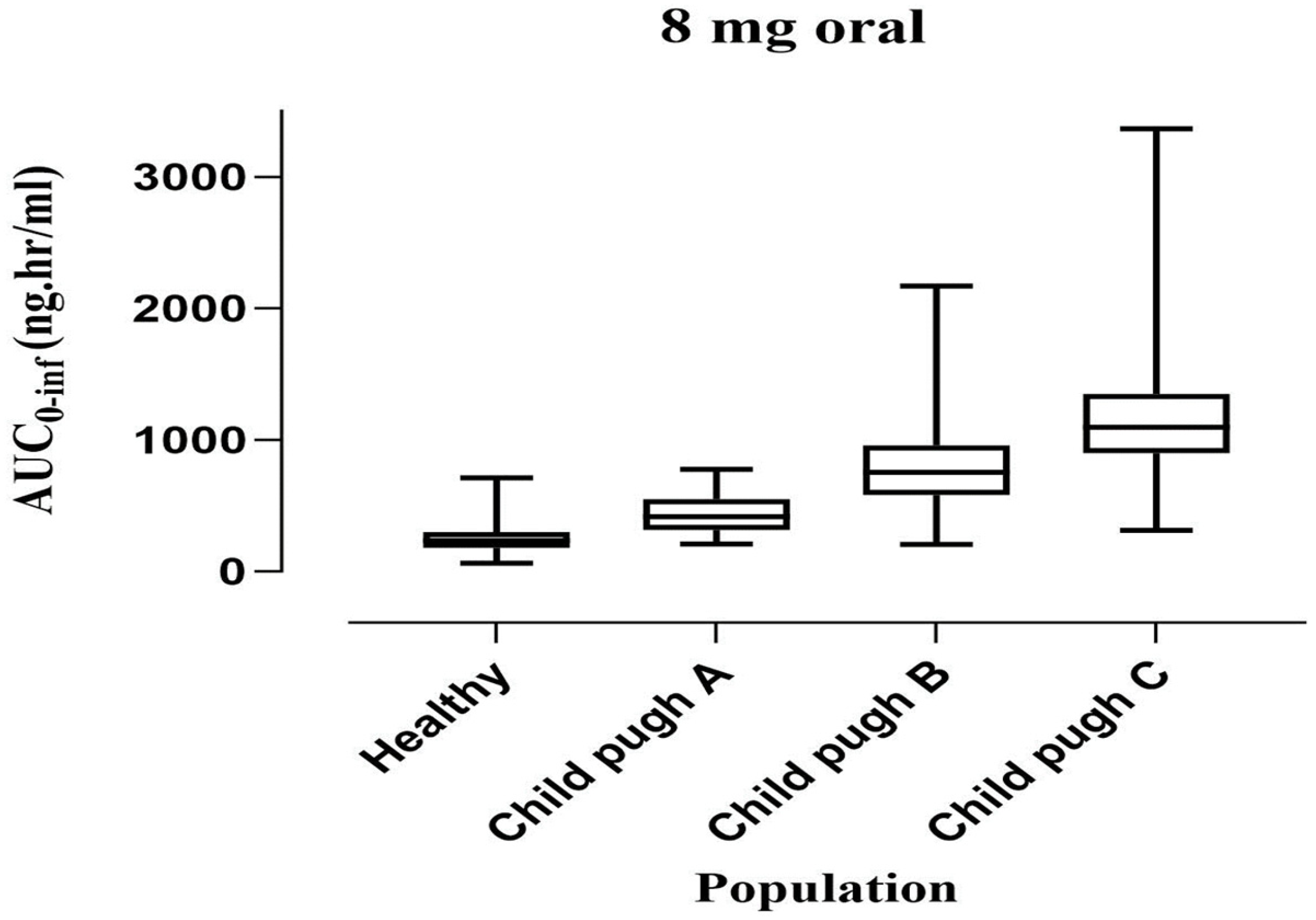
2.2. PBPK Model Development and Evaluation for Cirrhotic Patient Population
3. Discussion
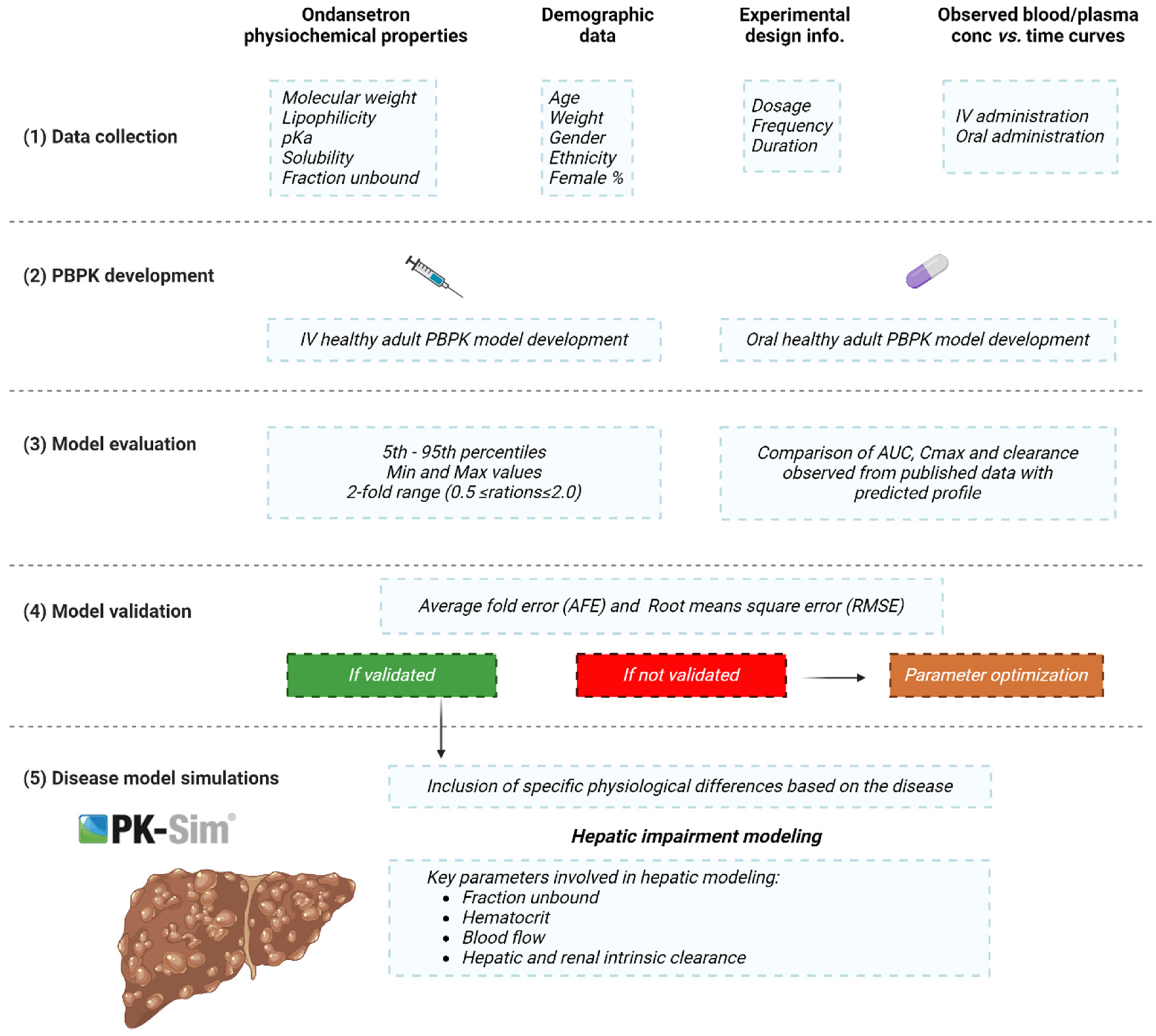
4. Materials and Methods
4.1. Clinical Pharmacokinetic Data
4.2. PBPK Modeling Software
4.3. Development of Building Blocks
4.4. Modeling Strategy
4.5. Model Parametrization
4.6. Model Structure in Cirrhotic Population
4.7. Model Appraisal and Verification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Medicines Agency. Updated Signal Assessment Report on Birth Defects Following In-Utero Exposure during the First Trimester of Pregnancy Arising from Recent Publications with Ondansetron 1. 2019, Volume 31. Available online: https://www.ema.europa.eu/en/documents/prac-recommendation/updated-signal-assessment-report-birth-defects-following-utero-exposure-during-first-trimester_en.pdf (accessed on 15 May 2023).
- Kovac, A.L. Comparative Pharmacology and Guide to the Use of the Serotonin 5-HT3 Receptor Antagonists for Postoperative Nausea and Vomiting. Drugs 2016, 76, 1719–1735. [Google Scholar] [CrossRef]
- Huddart, R.; Altman, R.B.; Klein, T.E. PharmGKB summary: Ondansetron and tropisetron pathways, pharmacokinetics and pharmacodynamics. Pharmacogenet. Genom. 2019, 29, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Patka, J.; Wu, D.T.; Abraham, P.; Sobel, R.M. Randomized Controlled Trial of Ondansetron vs. Prochlorperazine in Adults in the Emergency Department. West. J. Emerg. Med. 2011, 12, 1–5. [Google Scholar] [PubMed]
- Carstairs, S.D. Ondansetron Use in Pregnancy and Birth Defects. Obstet. Gynecol. 2016, 127, 878–883. [Google Scholar] [CrossRef]
- Goodin, S.; Cunningham, R. 5-HT3-Receptor Antagonists for the Treatment of Nausea and Vomiting: A Reappraisal of Their Side-Effect Profile. Oncologist 2002, 7, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Charbit, B.; Alvarez, J.C.; Dasque, E.; Abe, E.; Démolis, J.L.; Funck-Brentano, C. Droperidol and Ondansetron-Induced QT Interval Prolongation: A Clinical Drug Interaction Study. Anesthesiology 2008, 109, 206–212. [Google Scholar] [CrossRef]
- Roila, F.; Del Favero, A. Ondansetron Clinical Pharmacokinetics. Clin. Pharmacokinet. 1995, 29, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.H.; Hicks, F.M. Clinical Pharmacokinetics of Ondansetron. A Review. J. Pharm. Pharmacol. 1996, 48, 774–781. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Sultan, P.; Carvalho, B.; Peltz, G.; Wu, M.; Clavijo, C.; Galinkin, J.L.; Drover, D.R. Ondansetron Pharmacokinetics in Pregnant Women and Neonates: Towards a New Treatment for Neonatal Abstinence Syndrome. Clin. Pharmacol. Ther. 2015, 97, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Jamei, M. Recent Advances in Development and Application of Physiologically-Based Pharmacokinetic (PBPK) Models: A Transition from Academic Curiosity to Regulatory Acceptance. Curr. Pharmacol. Rep. 2016, 2, 161–169. [Google Scholar] [CrossRef]
- Kuepfer, L.; Niederalt, C.; Wendl, T.; Schlender, J.F.; Willmann, S.; Lippert, J.; Block, M.; Eissing, T.; Teutonico, D. Applied Concepts in PBPK Modeling: How to Build a PBPK/PD Model. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 516–531. [Google Scholar] [CrossRef]
- Martins, F.S.; Zhu, P.; Heinrichs, M.T.; Sy, S.K.B. Physiologically Based Pharmacokinetic-Pharmacodynamic Evaluation of Meropenem plus Fosfomycin in Paediatrics. Br. J. Clin. Pharmacol. 2021, 87, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.S.; Martins, J.E.S.; Severino, P.; Annaert, P.; Sy, S.K.B. Physiologically Based Pharmacokinetic Modelling to Inform Combination Dosing Regimens of Ceftaroline and Daptomycin in Special Populations. Br. J. Clin. Pharmacol. 2023, 89, 2726–2738. [Google Scholar] [CrossRef] [PubMed]
- Talha Zahid, M.; Zamir, A.; Majeed, A.; Imran, I.; Alsanea, S.; Ahmad, T.; Alqahtani, F.; Fawad Rasool, M. A Physiologically Based Pharmacokinetic Model of Cefepime to Predict Its Pharmacokinetics in Healthy, Pediatric and Disease Populations. Saudi Pharm. J. 2023, 31, 101675. [Google Scholar] [CrossRef]
- Rasool, M.F.; Khalid, S.; Majeed, A.; Saeed, H.; Imran, I.; Mohany, M.; Al-Rejaie, S.S.; Alqahtani, F. Development and Evaluation of Physiologically Based Pharmacokinetic Drug–Disease Models for Predicting Rifampicin Exposure in Tuberculosis and Cirrhosis Populations. Pharmaceutics 2019, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.F.; Khalil, F.; Läer, S. A Physiologically Based Pharmacokinetic Drug–Disease Model to Predict Carvedilol Exposure in Adult and Paediatric Heart Failure Patients by Incorporating Pathophysiological Changes in Hepatic and Renal Blood Flows. Clin. Pharmacokinet. 2015, 54, 943–962. [Google Scholar] [CrossRef]
- Johnson, T.N.; Boussery, K.; Rowland-Yeo, K.; Tucker, G.T.; Rostami-Hodjegan, A. A Semi-Mechanistic Model to Predict the Effects of Liver Cirrhosis on Drug Clearance. Clin. Pharmacokinet. 2010, 49, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Edginton, A.N.; Willmann, S. Physiology-Based Simulations of a Pathological Condition. Clin. Pharmacokinet. 2008, 47, 743–752. [Google Scholar] [CrossRef]
- Colthup, P.V.; Felgate, C.C.; Palmer, J.L.; Scully, N.L. Determination of Ondansetron in Plasma and Its Pharmacokinetics in the Young and Elderly. J. Pharm. Sci. 1991, 80, 868–871. [Google Scholar] [CrossRef]
- Hsyu, P.H.; Pritchard, J.F.; Bozigian, H.P.; Lloyd, T.L.; Griffin, R.H.; Shamburek, R.; Krishna, G.; Barr, W.H. Comparison of the Pharmacokinetics of an Ondansetron Solution (8 mg) When Administered Intravenously, Orally, to the Colon, and to the Rectum. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1994, 11, 156–159. [Google Scholar]
- Blake, J.; Palmer, J.; Minton, N.; Burroughs, A. The Pharmacokinetics of Intravenous Ondansetron in Patients with Hepatic Impairment. Br. J. Clin. Pharmacol. 1993, 35, 441–443. [Google Scholar] [CrossRef]
- Ashforth, E.; Palmer, J.; Bye, A.; Bedding, A. The Pharmacokinetics of Ondansetron after Intravenous Injection in Healthy Volunteers Phenotyped as Poor or Extensive Metabolisers of Debrisoquine. Br. J. Clin. Pharmacol. 1994, 37, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.F.; Bryson, J.C.; Kernodle, A.E.; Benedetti, T.L.; Powell, J.R. Age and Gender Effects on Ondansetron Pharmacokinetics: Evaluation of Healthy Aged Volunteers. Clin. Pharmacol. Ther. 1992, 51, 51–55. [Google Scholar] [CrossRef]
- Bozigian, H.P.; Pritchard, J.F.; Gooding, A.E.; Pakes, G.E. Ondansetron Absorption in Adults: Effect of Dosage Form, Food, and Antacids. J. Pharm. Sci. 1994, 83, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Cánovas, M.; Rios, J.; Domenech, G.; Cebrecos, J.; Pelagio, P.; Canals, M.; Polonio, F.; Cabré, F. Bioequivalence Study of 2 Orodispersible Formulations of Ondansetron 8 Mg in Healthy Volunteers. Arzneim.-Forsch./Drug Res. 2012, 62, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, C.M.; Kazmi, Y.; Stewart, J.; Weidler, D.J.; Tenjarla, S.N.; Ward, E.S.; Jann, M.W. Pharmacokinetics of Three Formulations of Ondansetron Hydrochloride in Healthy Volunteers: 24-Mg Oral Tablet, Rectal Suppository, and i.v. Infusion. Am. J. Health-Syst. Pharm. 2000, 57, 1046–1050. [Google Scholar] [CrossRef]
- Hsyu, P.-H.; Pritchard, J.F.; Bozigian, H.P.; Gooding, A.E.; Griffin, R.H.; Mitchell, R.; Bjurstrom, T.; Panella, T.L.; Huang, A.T.; Hansen, L.A. Oral Ondansetron Pharmacokinetics: The Effect of Chemotherapy. J. Clin. Pharmacol. 1994, 34, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Figg, W.D.; Dukes, G.E.; Pritchard, J.F.; Hermann, D.J.; Lesesne, H.R.; Carson, S.W.; Songer, S.S.; Powell, J.R.; Hak, L.J. Pharmacokinetics of Ondansetron in Patients with Hepatic Insufficiency. J. Clin. Pharmacol. 1996, 36, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Willmann, S.; Thelen, K.; Lippert, J. Integration of Dissolution into Physiologically-Based Pharmacokinetic Models III: PK-Sim Â. J. Pharm. Pharmacol. 2012, 64, 997–1007. [Google Scholar] [CrossRef]
- Somers, G.I.; Harris, A.J.; Bayliss, M.K.; Houston, J.B. The Metabolism of the 5HT3 Antagonists Ondansetron, Alosetron and GR87442 I: A Comparison of In Vitro and In Vivo Metabolism and In Vitro Enzyme Kinetics in Rat, Dog and Human Hepatocytes, Microsomes and Recombinant Human Enzymes. Xenobiotica 2007, 37, 832–854. [Google Scholar] [CrossRef]
- Joshi, A.S.; Patel, H.S.; Belgamwar, V.S.; Agrawal, A.; Tekade, A.R. Solid Lipid Nanoparticles of Ondansetron HCl for Intranasal Delivery: Development, Optimization and Evaluation. J. Mater. Sci. Mater. Med. 2012, 23, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Obach, R.S.; Lombardo, F.; Waters, N.J. Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 670 Drug Compounds. Drug Metab. Dispos. 2008, 36, 1385–1405. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.E.; Edginton, A.N. Model Qualification of the PK-Sim® Pediatric Module for Pediatric Exposure Assessment of CYP450 Metabolized Compounds. J. Toxicol. Environ. Health-Part A Curr. Issues 2019, 82, 789–814. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, C.P.; Harding, S.M. The Clinical Pharmacology of Ondansetron. Eur. J. Cancer Clin. Oncol. 1989, 25 (Suppl. 1), S21–S24. [Google Scholar]
- Kostewicz, E.S.; Aarons, L.; Bergstrand, M.; Bolger, M.B.; Galetin, A.; Hatley, O.; Jamei, M.; Lloyd, R.; Pepin, X.; Rostami-Hodjegan, A.; et al. PBPK Models for the Prediction of In Vivo Performance of Oral Dosage Forms. Eur. J. Pharm. Sci. 2014, 57, 300–321. [Google Scholar] [CrossRef]
- Zhou, W.; Johnson, T.N.; Bui, K.H.; Cheung, S.Y.A.; Li, J.; Xu, H.; Al-Huniti, N.; Zhou, D. Predictive Performance of Physiologically Based Pharmacokinetic (PBPK) Modeling of Drugs Extensively Metabolized by Major Cytochrome P450s in Children. Clin. Pharmacol. Ther. 2018, 104, 188–200. [Google Scholar] [CrossRef]
- Villikka, K.; Kivistö, K.T.; Neuvonen, P.J. The Effect of Rifampin on the Pharmaco-Kinetics of Oral and Intravenous Ondansetron. Clin. Pharmacol. Ther. 1999, 65, 377–381. [Google Scholar] [CrossRef]
- Li, R.; Barton, H.A.; Maurer, T.S. A Mechanistic Pharmacokinetic Model for Liver Transporter Substrates under Liver Cirrhosis Conditions. CPT Pharmacomet. Syst. Pharmacol. 2015, 4, 338–349. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An Add-in Program for Pharmacokinetic and Pharmacodynamic Data Analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- T’jollyn, H.; Vermeulen, A.; Van Bocxlaer, J. PBPK and Its Virtual Populations: The Impact of Physiology on Pediatric Pharmacokinetic Predictions of Tramadol. AAPS J. 2019, 21, 8. [Google Scholar] [CrossRef]
- Rasool, M.F.; Khalid, R.; Imran, I.; Majeed, A.; Saeed, H.; Alasmari, F.; Alanazi, M.M.; Alqahtani, F. Investigating the Role of Altered Systemic Albumin Concentration on the Disposition of Theophylline in Adult and Pediatric Patients with Asthma by Using the Physiologically Based Pharmacokinetic Approach. Drug Metab. Dispos. 2020, 48, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Pierrillas, P.B.; Henin, E.; Ball, K.; Ogier, J.; Amiel, M.; Kraus-Berthier, L.; Chenel, M.; Bouzom, F.; Tod, M. Prediction of Human Nonlinear Pharmacokinetics of a New Bcl-2 Inhibitor Using PBPK Modeling and Interspecies Extrapolation Strategy. Drug Metab. Dispos. 2019, 47, 648–656. [Google Scholar] [CrossRef] [PubMed]
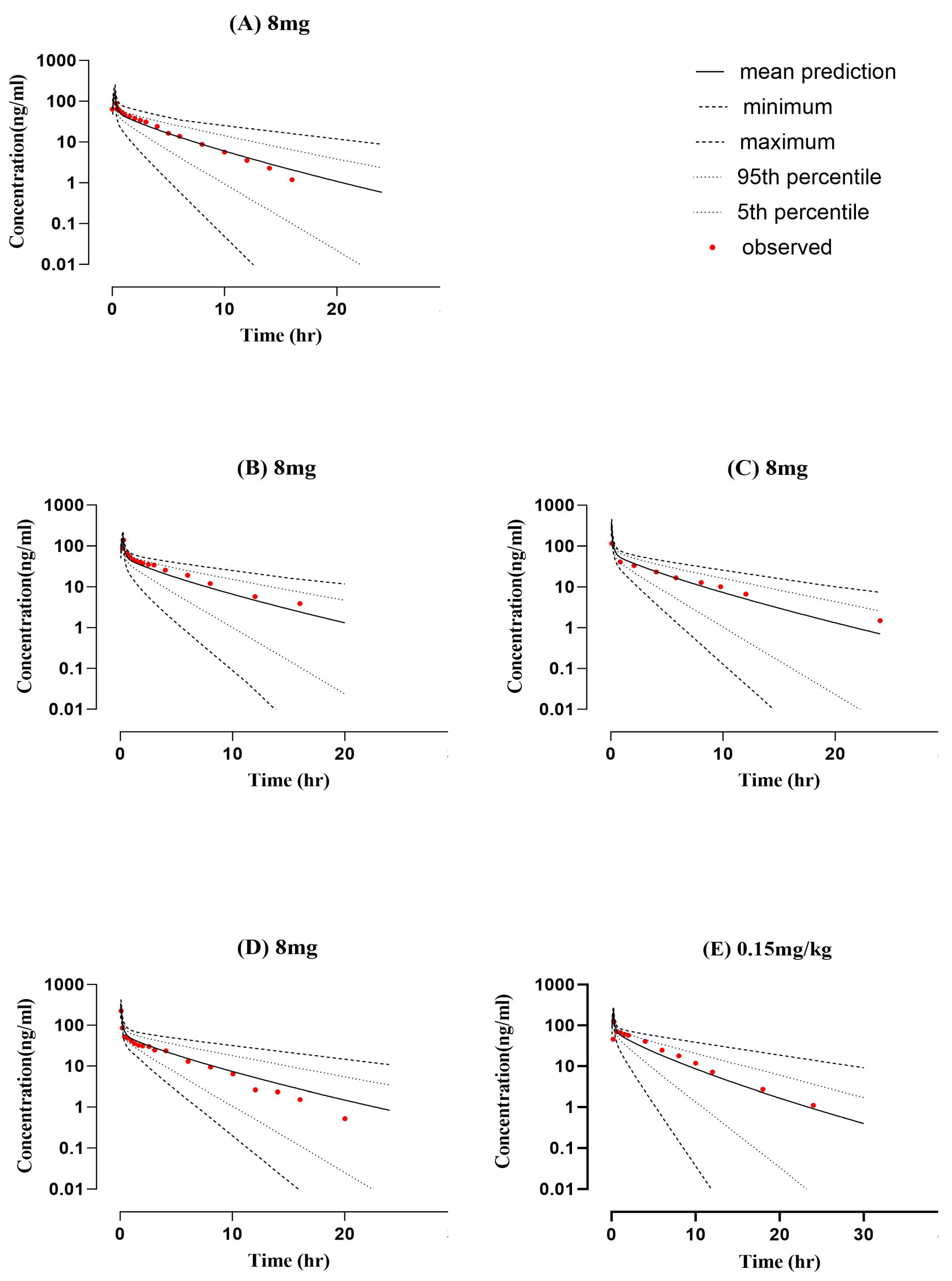
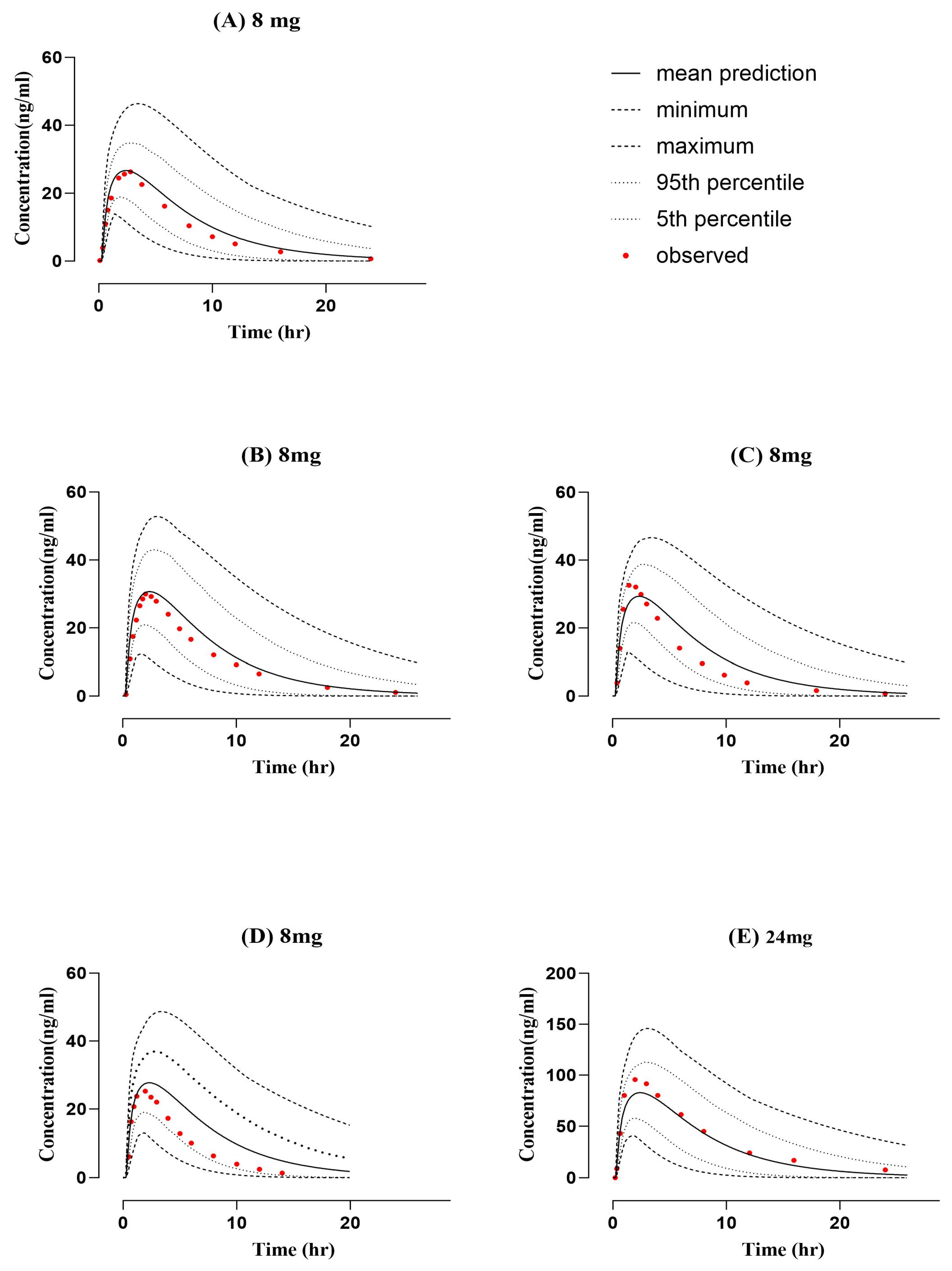
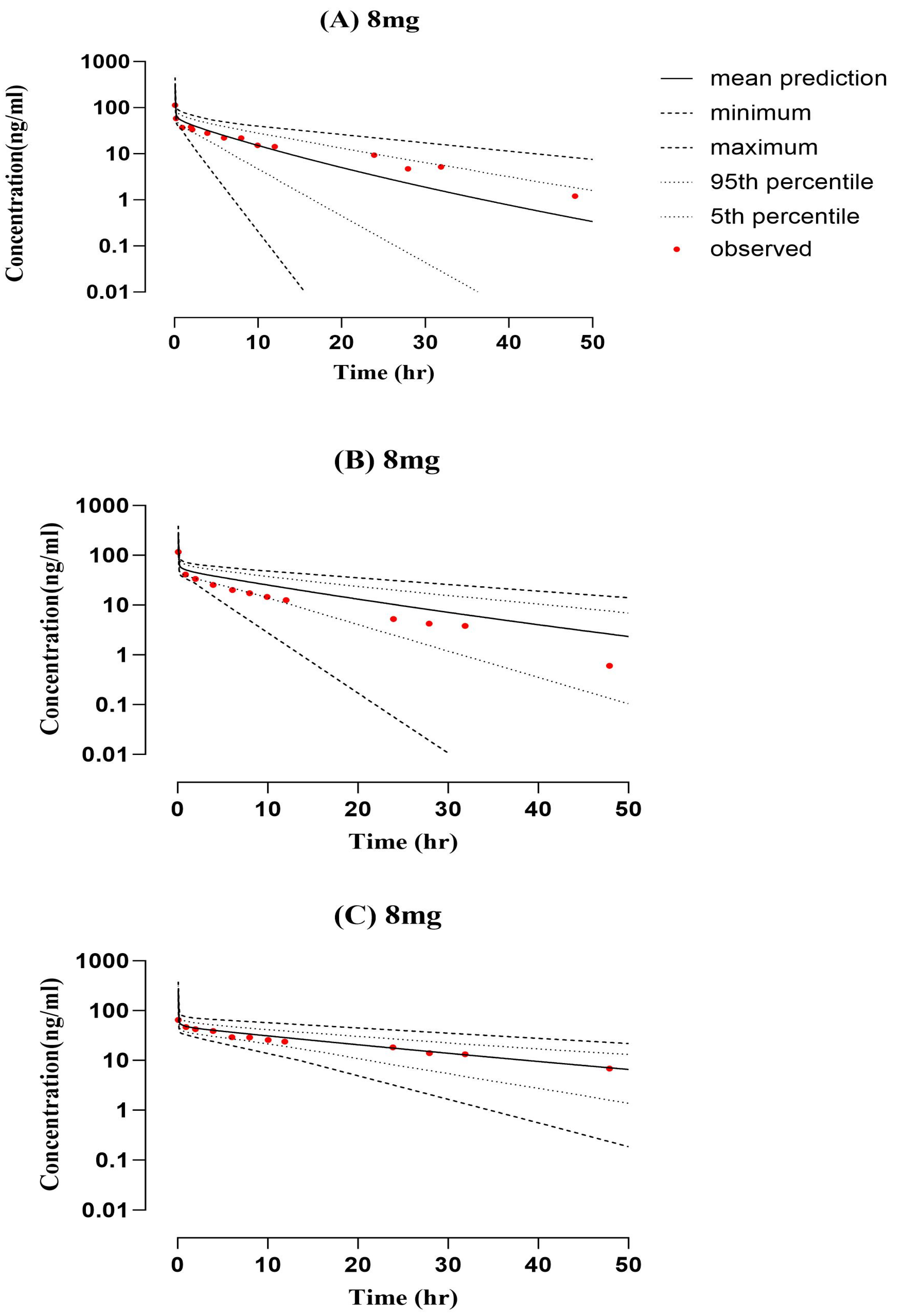

| PK Parameters (Unit) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose, Reference | AUC0–∞ (ng/mL·h) | Cmax (ng/mL) | CL (mL/min/kg) | ||||||
| Obs. | Pred. | Obs./Pred Ratio | Obs. | Pred. | Obs./Pred Ratio | Obs. | Pred. | Obs./Pred Ratio | |
| IV administration | |||||||||
| 8-mg [20] | 246.5 | 271.06 | 0.91 | 102.5 | 170.69 | 0.60 | 7.41 | 6.73 | 1.10 |
| 8 mg [22] | 279 | 324.87 | 0.86 | 161 | 344.15 | 0.47 | 6.54 | 5.70 | 1.15 |
| 8 mg [23] | 257 | 315.18 | 0.82 | 343 | 315.95 | 1.09 | 7.10 | 5.70 | 1.25 |
| 8 mg [21] | 313 | 278.54 | 1.12 | 159 | 166.97 | 0.95 | 6.7 | 6.52 | 1.03 |
| 0.15 mg/kg [24] | 435.46 | 372.41 | 1.17 | 170 | 225.86 | 0.75 | 5.81 | 6.76 | 0.86 |
| Oral administration | |||||||||
| 24 mg [27] | 805.9 | 777.09 | 1.04 | 94.6 | 82.81 | 1.14 | 8.64 | 7.02 | 1.23 |
| 8 mg [26] | 244.496 | 280.70 | 0.87 | 36.113 | 30.69 | 1.18 | 8.06 | 6.52 | 1.24 |
| 8 mg [25] | 246.1 | 247.22 | 1.00 | 26.3 | 26.68 | 0.99 | 7.42 | 7.31 | 1.01 |
| 8 mg [24] | 209.27 | 266.78 | 0.78 | 32.57 | 29.34 | 1.11 | 8.9 | 7.47 | 1.19 |
| 8-mg [20] | 168.8 | 246.96 | 0.68 | 26.4 | 27.73 | 0.95 | 10.8 | 7.28 | 1.48 |
| Parameters | AFE | RMSE |
|---|---|---|
| Intravenous | ||
| AUC | 0.98 | 47.44 |
| Cmax | 0.77 | 91.77 |
| CL | 1.08 | 0.91 |
| Oral | ||
| AUC | 0.87 | 50.90 |
| Cmax | 1.07 | 6.01 |
| CL | 1.23 | 1.94 |
| PK Parameters (Unit) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose, Reference | AUC0–∞ (ng/mL·h) | Cmax (ng/mL) | CL (mL/min/kg) | ||||||
| Obs. | Pred. | Obs./Pred Ratio | Obs. | Pred. | Obs./Pred Ratio | Obs. | Pred. | Obs./Pred Ratio | |
| IV Administration | |||||||||
| (8 mg) Child–Pugh-A [22] | 633 | 476.46 | 1.33 | 113.7 | 323.39 | 0.35 | 2.89 | 3.81 | 0.76 |
| (8 mg) Child–Pugh-B [22] | 641 | 801.90 | 0.80 | 174.6 | 274.22 | 0.64 | 2.84 | 2.28 | 1.25 |
| (8 mg) Child–Pugh-C [22] | 1383 | 1208.94 | 1.14 | 149.5 | 251.84 | 0.60 | 1.32 | 1.51 | 0.87 |
| Study | Dose | Infusion Time | n | Female [n] | Mean Age [Years] ± SD | Mean w.t [kg] ± SD | Population |
|---|---|---|---|---|---|---|---|
| Intravenous application in healthy population | |||||||
| [20] | 8 mg | 15 min | 32 | 0 | 18–40 | 58.3–95.8 | Healthy |
| [22] | 8 mg | 5 min | 6 | 2 | 19–23 | N/A | Healthy |
| [23] | 8 mg | 5 min | 6 | 2 | 32–43 | 50–80 | Healthy |
| [21] | 8 mg | 15 min | 6 | 0 | 19–35 | 55.5–90.5 | Healthy |
| [24] | 0.15 mg/kg | 15 min | 11 | 5 | 31 ± 7 | 66.1 ± 8.5 | Healthy |
| Oral application in healthy population | |||||||
| [27] | 24 mg | PO | 12 | 6 | N/A | N/A | Healthy |
| [26] | 8 mg | PO | 22 | 11 | 18–41 | 49–94 | Healthy |
| [25] | 8 mg | PO | 24 | 0 | 19–39 | 60–90 | Healthy |
| [24] | 8 mg | PO | 11 | 5 | 31 ± 7 | 66.1 ± 8.5 | Healthy |
| [20] | 8 mg | PO | 32 | 0 | (18–40) | 58.3–95.8 | Healthy |
| Intravenous application in disease population | |||||||
| [30] | 8 mg | 5 min | 19 | 8 | (20–69) | N/A | Disease |
| Parameter | Input Value | Reference |
|---|---|---|
| Physicochemical parameters | ||
| Molecular weight (g/mol) | 293.4 | Pubchem |
| Lipophilicity (log units) | 2.25 | [31,32] |
| Plasma protein binding | Albumin | [8] |
| Solubility(mg/L) | 0.36 | Drugbank |
| pKa(base) | 7.40 | [25] |
| Absorption | ||
| Specific intestinal permeability (cm/min) | 2.24 × 10−5 | Pk-Sim calculated |
| Distribution | ||
| Specific organ permeability (cm/min) | 8.02 × 10−3 | Pk-Sim calculated |
| Fraction unbound (Fu)% | 27 | [33] |
| Partition coefficient model | Poulin and Theil | Pk-Sim |
| Cellular permeability model | Pk-Sim standard | Pk-Sim |
| Metabolism | ||
| Intrinsic clearance CYP1A2 (L/min) | 0.21 | [34] |
| Intrinsic clearance CYP3A4 (L/min) | 0.03 | |
| Excretion | ||
| Renal clearance (mL/min/kg) | 0.13 | [8,35] |
| Parameters | Control (PK Sim) | Child–Pugh Score | |||
|---|---|---|---|---|---|
| CP-A | CP-B | CP-C | |||
| Functional liver mass b | 2.38 | 0.69 | 0.55 | 0.28 | |
| Hepatic enzymes fraction (cyp) pmol/mg. | 3A4 a,b | 4.32 | 0.589 a | 0.4 b | 0.4 b |
| 1A2 a | 1.8 | 0.63 a | 0.26 a | 0.12 a | |
| Albumin conc (g/L). c | 1 | 0.84 ± 0.15 | 0.69 ± 0.15 | 0.53 ± 0.15 | |
| Haematocrit Value (%) b | 0.47 | 0.39 | 0.37 | 0.35 | |
| Blood Flow b | Portal (mL/min) | 1.21 | 0.4 | 0.36 | 0.04 |
| Hepatic arterial (mL/min) | 17.94 | 1.3 | 2.3 | 3.4 | |
| Other organs (mL/min) | - | 1.75 | 2.25 | 2.75 | |
| Renal (mL/min) | 302.71 | 0.88 | 0.65 | 0.48 | |
| GFR (mL/min) a | 116 | 0.7 | 0.58 | 0.55 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, F.; Alruwaili, A.H.; Alasmari, M.S.; Almazroa, S.A.; Alsuhaibani, K.S.; Rasool, M.F.; Alruwaili, A.F.; Alsanea, S. A Physiologically Based Pharmacokinetic Model to Predict Systemic Ondansetron Concentration in Liver Cirrhosis Patients. Pharmaceuticals 2023, 16, 1693. https://doi.org/10.3390/ph16121693
Alqahtani F, Alruwaili AH, Alasmari MS, Almazroa SA, Alsuhaibani KS, Rasool MF, Alruwaili AF, Alsanea S. A Physiologically Based Pharmacokinetic Model to Predict Systemic Ondansetron Concentration in Liver Cirrhosis Patients. Pharmaceuticals. 2023; 16(12):1693. https://doi.org/10.3390/ph16121693
Chicago/Turabian StyleAlqahtani, Faleh, Abdullah H. Alruwaili, Mohammed S. Alasmari, Sultan A. Almazroa, Khaled S. Alsuhaibani, Muhammad F. Rasool, Abdulkarim F. Alruwaili, and Sary Alsanea. 2023. "A Physiologically Based Pharmacokinetic Model to Predict Systemic Ondansetron Concentration in Liver Cirrhosis Patients" Pharmaceuticals 16, no. 12: 1693. https://doi.org/10.3390/ph16121693
APA StyleAlqahtani, F., Alruwaili, A. H., Alasmari, M. S., Almazroa, S. A., Alsuhaibani, K. S., Rasool, M. F., Alruwaili, A. F., & Alsanea, S. (2023). A Physiologically Based Pharmacokinetic Model to Predict Systemic Ondansetron Concentration in Liver Cirrhosis Patients. Pharmaceuticals, 16(12), 1693. https://doi.org/10.3390/ph16121693









