Disulfiram Enhances the Antineoplastic Activity and Sensitivity of Murine Hepatocellular Carcinoma to 5-FU via Redox Management
Abstract
:1. Introduction
2. Results
2.1. Effect on Cancer Markers
2.1.1. AFP
2.1.2. GOLPH 3
2.2. Effect on Oxidative Stress Parameters
2.2.1. SOD
2.2.2. CAT
2.2.3. GR
2.2.4. MDA Levels
2.2.5. Total Carbonyl Content
2.2.6. 8-OHdG
2.3. Effect on Apoptotic Markers
2.3.1. p-53
2.3.2. Cleaved PARP
2.3.3. Cleaved Caspase-3
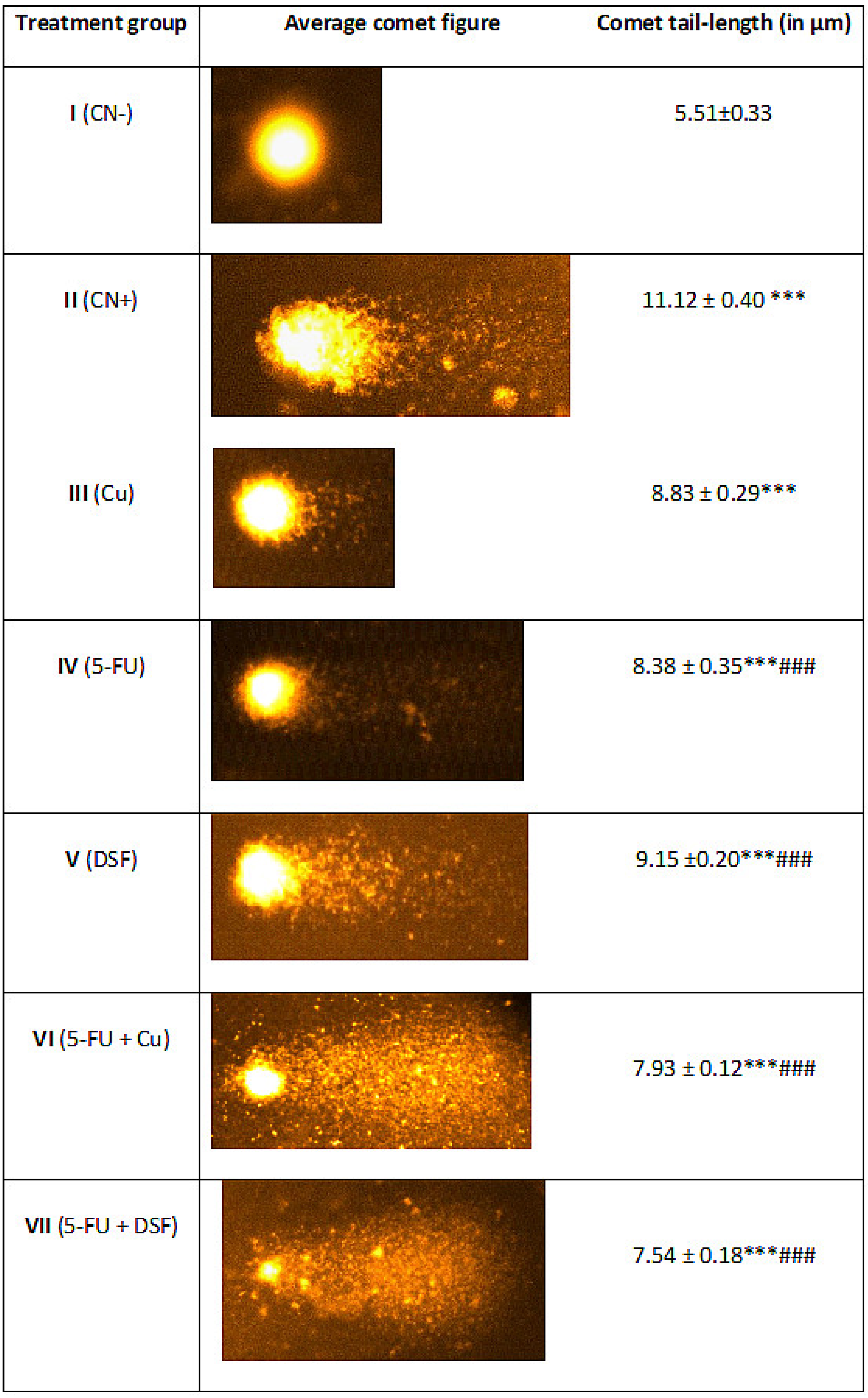
2.4. Effect on Nuclear DNA of the Liver Cells
2.5. Assessment of Necrosis by LDH Activity
2.6. Histopathological Evaluation
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conniot, J.; Silva, J.M.; Fernandes, J.G.; Silva, L.C.; Gaspar, R.; Brocchini, S.; Florindo, H.F.; Barata, T.S. Cancer immunotherapy: Nanodelivery approaches for immune cell targeting and tracking. Front. Chem. 2014, 2, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, I.; Khan, A.A.; Aman, S.; Qamar, W.; Ebaid, H.; Al-Tamimi, J.; Alhazza, I.M.; Rady, A.M. Restrained management of copper level enhances the antineoplastic activity of imatinib in vitro and in vivo. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Gao, Y.; Qi, Y.; Chen, L.; Ma, Y.; Li, Y. Peptide-based cancer therapy: Opportunity and challenge. Cancer Lett. 2014, 351, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, J.; Semlali, A.; Hassan, I.; Ebaid, H.; Alhazza, I.M.; Mehdi, S.H.; Al-Khalifa, M.; Alanazi, M.S. Samsum ant venom exerts anticancer activity through immunomodulation in vitro and in vivo. Cancer Biother. Radiopharm. 2018, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Araya, M.; Olivares, M.; Pizarro, F.; Mendez, M.A.; Gonzalez, M.; Uauy, R. Supplementing copper at the upper level of the adult dietary recommended intake induces detectable but transient changes in healthy adults. J. Nutr. 2005, 135, 2367–2371. [Google Scholar] [CrossRef] [Green Version]
- Shakil, S.; Baig, M.H.; Tabrez, S.; Rizvi, S.M.D.; Zaidi, S.K.; Ashraf, G.M.; Ansari, S.A.; Khan, A.A.P.; Al-Qahtani, M.H.; Abuzenadah, A.M. Molecular and enzoinformatics perspectives of targeting Polo-like kinase 1 in cancer therapy. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2019; pp. 47–55. [Google Scholar]
- Jabir, N.R.; Ahmad, S.; Tabrez, S. An insight on the association of glycation with hepatocellular carcinoma. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2018; pp. 56–63. [Google Scholar]
- Camakaris, J.; Voskoboinik, I.; Mercer, J. Molecular mechanisms of copper homeostasis. Biochem. Biophys. Res. Commun. 1999, 261, 225–232. [Google Scholar] [CrossRef]
- Ogra, Y.; Tejima, A.; Hatakeyama, N.; Shiraiwa, M.; Wu, S.; Ishikawa, T.; Yawata, A.; Anan, Y.; Suzuki, N. Changes in intracellular copper concentration and copper-regulating gene expression after PC12 differentiation into neurons. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Schlecht, U.; Suresh, S.; Xu, W.; Aparicio, A.M.; Chu, A.; Proctor, M.J.; Davis, R.W.; Scharfe, C.; St Onge, R.P. A functional screen for copper homeostasis genes identifies a pharmacologically tractable cellular system. BMC Genom. 2014, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zubair, H.; Khan, H.; Sohail, A.; Azim, S.; Ullah, M.; Ahmad, A.; Sarkar, F.; Hadi, S. Redox cycling of endogenous copper by thymoquinone leads to ROS-mediated DNA breakage and consequent cell death: Putative anticancer mechanism of antioxidants. Cell Death Dis. 2013, 4, e660. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.-F.; Sudhahar, V.; Youn, S.-W.; Das, A.; Cho, J.; Kamiya, T.; Urao, N.; McKinney, R.D.; Surenkhuu, B.; Hamakubo, T. Copper transport protein antioxidant-1 promotes inflammatory neovascularization via chaperone and transcription factor function. Sci. Rep. 2015, 5, 1–20. [Google Scholar] [CrossRef]
- Turski, M.L.; Thiele, D.J. New roles for copper metabolism in cell proliferation, signaling, and disease. J. Biol. Chem. 2009, 284, 717–721. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cao, X. Cellular and molecular regulation of innate inflammatory responses. Cell. Mol. Immunol. 2016, 13, 711–721. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, H.; Nair, J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: Role of lipid peroxidation, DNA damage, and repair. Langenbeck’s Arch. Surg. 2006, 391, 499–510. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Hassan, I.; Chibber, S.; Naseem, I. Vitamin B2: A promising adjuvant in cisplatin based chemoradiotherapy by cellular redox management. Food Chem. Toxicol. 2013, 59, 715–723. [Google Scholar] [CrossRef]
- Schetter, A.J.; Heegaard, N.H.; Harris, C.C. Inflammation and cancer: Interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis 2010, 31, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Alhazza, I.M.; Ebaid, H.; Omar, M.S.; Hassan, I.; Habila, M.A.; Al-Tamimi, J.; Sheikh, M. Supplementation with selenium nanoparticles alleviates diabetic nephropathy during pregnancy in the diabetic female rats. Environ. Sci. Pollut. Res. 2022, 29, 5517–5525. [Google Scholar] [CrossRef]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA: A Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef]
- Sugawara, N.; Sugawara, C.; Katakura, M.; Takahashi, H.; Mori, M. Copper metabolism in the LEC rat: Involvement of induction of metallothionein and disposition of zinc and iron. Experientia 1991, 47, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Eagon, P.K.; Teepe, A.G.; Elm, M.S.; Tadic, S.D.; Epley, M.J.; Beiler, B.E.; Shinozuka, H.; Rao, K.N. Hepatic hyperplasia and cancer in rats: Alterations in copper metabolism. Carcinogenesis 1999, 20, 1091–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Dou, Q.P. New uses for old copper-binding drugs: Converting the pro-angiogenic copper to a specific cancer cell death inducer. Expert Opin. Ther. Targets 2008, 12, 739–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Johansson, B. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr. Scand. 1992, 86, 15–26. [Google Scholar] [CrossRef]
- Meysken, F.L., Jr.; McNulty, S.E.; Buckmeier, J.A.; Tohidian, N.B.; Spillane, T.J.; Kahlon, R.S.; Gonzalez, R.I. Aberrant redox regulation in human metastatic melanoma cells compared to normal melanocytes. Free Radic. Biol. Med. 2001, 31, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Cui, Q.C.; Yang, H.; Dou, Q.P. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006, 66, 10425–10433. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, L.H.; Zhang, H.T.; Wang, Y.T.; Liu, S.; Zhou, W.L.; Yuan, X.Z.; Li, T.Y.; Wu, C.F.; Yang, J.Y. Disulfiram combined with copper inhibits metastasis and epithelial-mesenchymal transition in hepatocellular carcinoma through the NF-κB and TGF-β pathways. J. Cell Mol. Med. 2018, 22, 439–451. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, L.; Zhang, B.; Liu, S.; Zhang, K.; Yan, J.; Zhang, W.; Yu, M.; Chen, Z.; Xu, Y.; et al. Disulfiram combined with copper induces immunosuppression via PD-L1 stabilization in hepatocellular carcinoma. Am. J. Cancer Res. 2019, 9, 2442–2455. [Google Scholar]
- Khan, M.S.; Alomari, A.; Tabrez, S.; Hassan, I.; Wahab, R.; Bhat, S.A.; Alafaleq, N.O.; Altwaijry, N.; Shaik, G.M.; Zaidi, S.K. Anticancer potential of biogenic silver nanoparticles: A mechanistic study. Pharmaceutics 2021, 13, 707. [Google Scholar] [CrossRef]
- Kelley, K.C.; Grossman,, K.F.; Brittain-Blankenship, M.; Thorne, K.M.; Akerley, W.L.; Terrazas, M.C.; Kosak, K.M.; Boucher, K.M.; Buys, S.S.; McGregor, K.A.; et al. A Phase 1 dose-escalation study of disulfiram and copper gluconate in patients with advanced solid tumors involving the liver using S-glutathionylation as a biomarker. BMC Cancer 2021, 21, 510. [Google Scholar]
- Shah O’Brien, P.; Xi, Y.; Miller, J.R.; Brownell, A.L.; Zeng, Q.; Yoo, G.H.; Garshott, D.M.; O’Brien, M.B.; Galinato, A.E.; Cai, P.; et al. Disulfiram (Antabuse) Activates ROS-Dependent ER Stress and Apoptosis in Oral Cavity Squamous Cell Carcinoma. J. Clin. Med. 2019, 8, 611. [Google Scholar] [CrossRef]
- Hassan, I.; Khan, R.A.; Al-Tamimi, J.; Ebaid, H.; Husain, F.M.; Alhazza, I.M. Comparative efficacy of ternary Cu (II) complex and Zn (II)-complex in amelioration of carbon tetrachloride-induced hepatotoxicity in vivo. J. King Saud University – Sci. 2023, 35, 102420. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, I.; Ebaid, H.; Alhazza, I.M.; Al-Tamimi, J.; Rady, A.M. Disulfiram Enhances the Antineoplastic Activity and Sensitivity of Murine Hepatocellular Carcinoma to 5-FU via Redox Management. Pharmaceuticals 2023, 16, 169. https://doi.org/10.3390/ph16020169
Hassan I, Ebaid H, Alhazza IM, Al-Tamimi J, Rady AM. Disulfiram Enhances the Antineoplastic Activity and Sensitivity of Murine Hepatocellular Carcinoma to 5-FU via Redox Management. Pharmaceuticals. 2023; 16(2):169. https://doi.org/10.3390/ph16020169
Chicago/Turabian StyleHassan, Iftekhar, Hossam Ebaid, Ibrahim M. Alhazza, Jameel Al-Tamimi, and Ahmed M. Rady. 2023. "Disulfiram Enhances the Antineoplastic Activity and Sensitivity of Murine Hepatocellular Carcinoma to 5-FU via Redox Management" Pharmaceuticals 16, no. 2: 169. https://doi.org/10.3390/ph16020169
APA StyleHassan, I., Ebaid, H., Alhazza, I. M., Al-Tamimi, J., & Rady, A. M. (2023). Disulfiram Enhances the Antineoplastic Activity and Sensitivity of Murine Hepatocellular Carcinoma to 5-FU via Redox Management. Pharmaceuticals, 16(2), 169. https://doi.org/10.3390/ph16020169





