Empagliflozin Protects against Haloperidol Experimentally-Induced Ovarian Toxicity
Abstract
:1. Introduction
2. Results
2.1. Effect of EMPA on Oxidative Stress Parameters in HAL-Induced Ovarian Toxicity
2.2. Effect of EMPA on Hormonal Parameters in HAL-Induced Ovarian Toxicity
2.3. Effect of EMPA on Inflammatory and Apoptotic Parameters in HAL-Induced Ovarian Toxicity
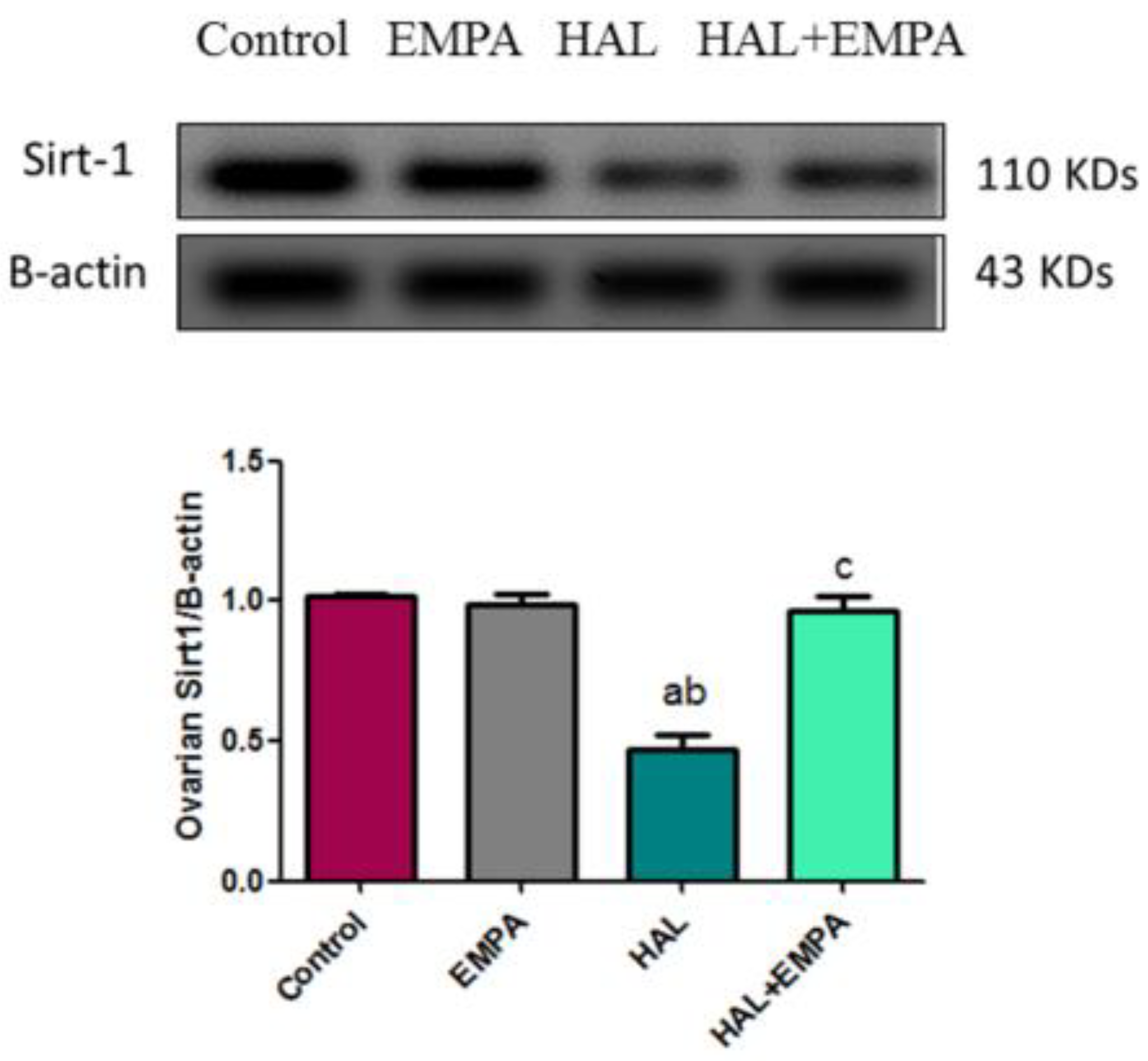
2.4. Effect of EMPA on Expression of Sirt-1 in HAL-Induced Ovarian Toxicity in Rats
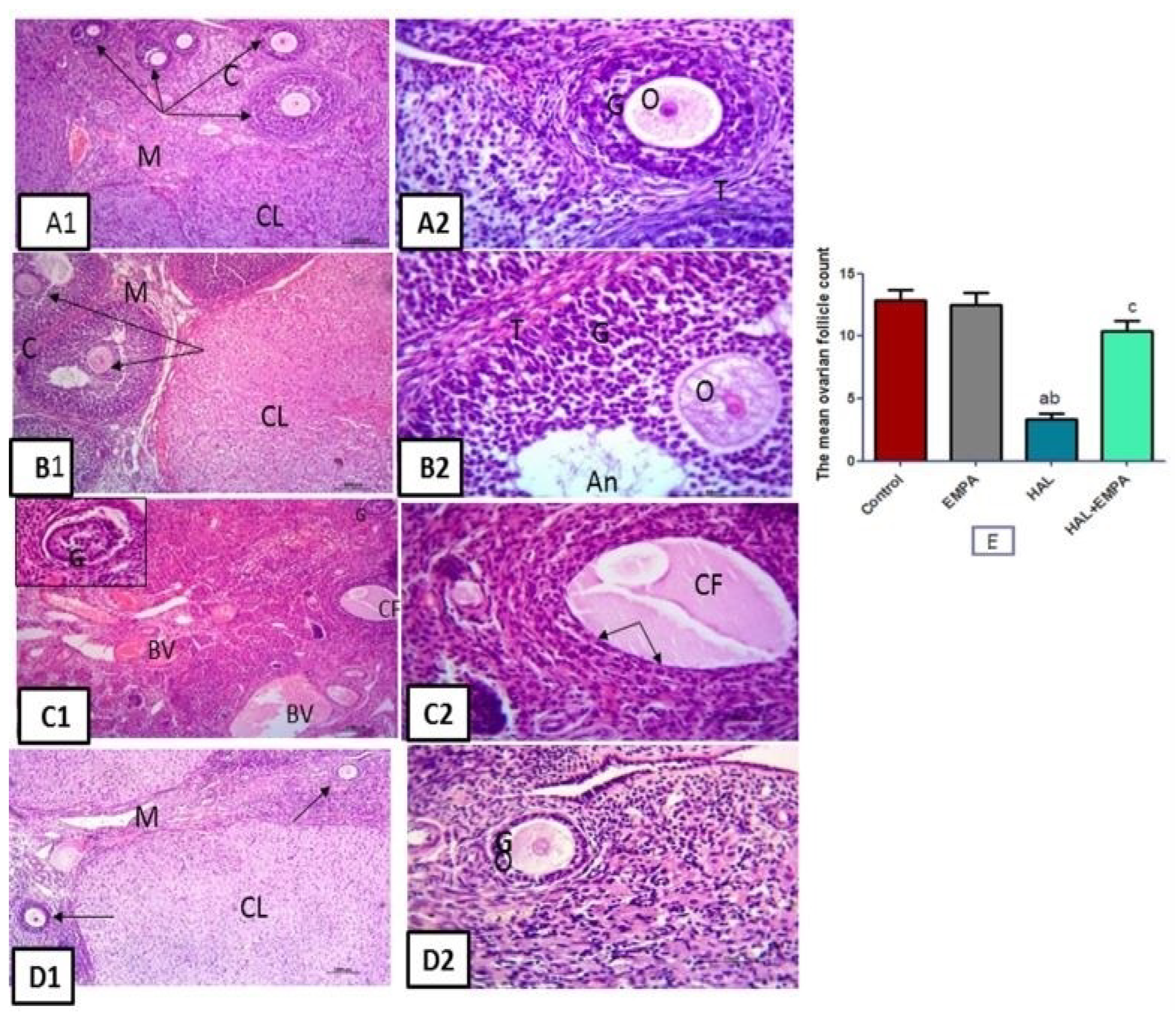
2.5. Histological Results
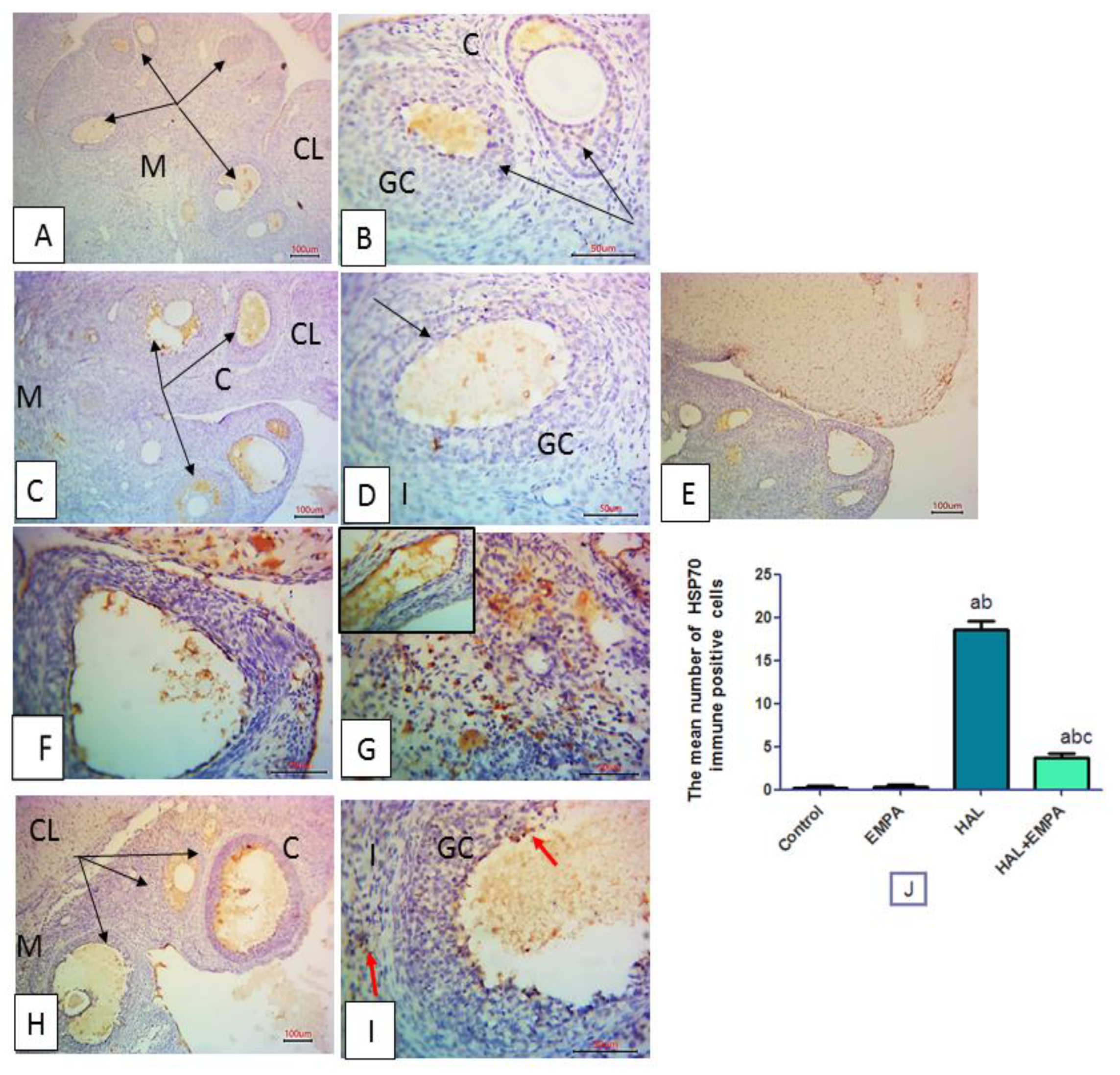
2.6. Immunohistochemical Results
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
- (1)
- Control group: only the vehicle was given.
- (2)
- (3)
- (4)
- HAL + EMPA group: HAL (2 mg/kg/day) PO for 28 days was combined with EMPA (10 mg/kg/day) PO for 28 days.
4.2. Chemicals and Drugs
4.3. Blood and Tissue Samples
4.4. Biochemical Determination
4.4.1. Estimation of the Oxidative Stress Indicators
4.4.2. Estimation of Hormonal Parameters
4.4.3. Estimation of Inflammatory and Apoptotic Parameters
4.5. Immunoblotting of Sirt1
4.6. Histological Study
4.6.1. Histological Procedures
4.6.2. Immunohistochemical Study
4.6.3. Image Capture
4.6.4. Morphometrical Measurement
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winship, I.R.; Dursun, S.M.; Baker, G.B.; Balista, P.A.; Kandratavicius, L.; Maia-de-Oliveira, J.P.; Hallak, J.; Howland, J.G. An Overview of Animal Models Related to Schizophrenia. Can. J. Psychiatry 2019, 64, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinterbuchinger, B.; König, D.; Gmeiner, A.; Listabarth, S.; Fellinger, M.; Thenius, C.; Baumgartner, J.S.; Vyssoki, S.; Waldhoer, T.; Vyssoki, B.; et al. Seasonality in schizophrenia—An analysis of a nationwide registry with 110,735 hospital admissions. Eur. Psychiatry 2020, 63, e55. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Abhishek, P.; Kumar, S.P.; Prashant, T. Study of oestrus cycle periodicity and oogenesis of adult albino rats: Response to hyperprolactinaemia induced by haloperidol. Asian Pac. J. Reprod. 2013, 2, 99–104. [Google Scholar] [CrossRef]
- Khalaf, H.A.; Elmorsy, E.; Mahmoud, E.-H.M.; Aggour, A.M.; Amer, S.A. The role of oxidative stress in ovarian toxicity induced by haloperidol and clozapine—A histological and biochemical study in albino rats. Cell Tissue Res. 2019, 378, 371–383. [Google Scholar] [CrossRef] [Green Version]
- Samadi, A.; Isikhan, S.Y.; Ansari, M.H.K.; Samadi, M.; Sabuncuoglu, S. Effects of clozapine and haloperidol treatment on plasma concentrations of androgen hormones and androgen dependent organ changes in rats. Indian J. Pharmacol. 2019, 51, 269–275. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.M.; Mohamed, A.S.M.; Abdelazem, O.; Okasha, A.M.M.; Kamel, M.Y. Cilostazol protects against cyclophosphamide-induced ovarian toxicity in female rats: Role of cAMP and HO-1. Toxicol. Mech. Methods 2020, 30, 526–535. [Google Scholar] [CrossRef]
- Abdelzaher, W.Y.; Abdel-Hafez, S.M.N.; Rofaeil, R.R.; Ali, A.S.A.; Hegazy, A.; Bahaa, H.A. The protective effect of fenofibrate, triptorelin, and their combination against premature ovarian failure in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 137–149. [Google Scholar] [CrossRef]
- Zhao, F.; Li, K.; Zhao, L.; Liu, J.; Suo, Q.; Zhao, J.; Wang, H.; Zhao, S. Effect of Nrf2 on rat ovarian tissues against atrazine-induced anti-oxidative response. Int. J. Clin. Exp. Pathol. 2014, 7, 2780–2789. [Google Scholar]
- Tao, X.; Zhang, X.; Ge, S.Q.; Zhang, E.H.; Zhang, B. Expression of SIRT1 in the ovaries of rats with polycystic ovary syndrome before and after therapeutic intervention with exenatide. Int. J. Clin. Exp. Pathol. 2015, 8, 8276–8283. [Google Scholar]
- Simes, B.C.; MacGregor, G.G. Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors: A Clinician’s Guide. Diabetes Metab. Syndr. Obes. 2019, 12, 2125–2136. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Wang, S.; Zhu, P.; Hu, S.; Chen, Y.; Ren, J. Empagliflozin Rescues Diabetic Myocardial Microvascular Injury via AMPK-Mediated Inhibition of Mitochondrial Fission. Redox Biol. 2018, 15, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Heo, Y.J.; Choi, S.E.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kang, Y.; Lee, K.W.; Kim, H.J. Anti-inflammatory Effects of Empagliflozin and Gemigliptin on LPS-Stimulated Macrophage via the IKK/NF-κB, MKK7/JNK, and JAK2/STAT1 Signalling Pathways. J. Immunol. Res. 2021, 2021, 9944880. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.J.; Lee, N.J.; Slack, K.; Karl, T.; Duffy, L.; O’brien, E.; Matsumoto, I.; Dedova, I.; Herzog, H.; Sainsbury, A. Distinct endocrine effect of chronic haloperidol or risperidone administration in male rats. Neuropharmacology 2006, 51, 1129–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Elmorsy, E.; Smith, P.A. Bioenergetic disruption of human micro-vascular endothelial cells by antipsychotics. Biochem. Biophys. Res. Commun. 2015, 460, 857–862. [Google Scholar] [CrossRef]
- Miyamoto, K.; Sato, E.F.; Kasahara, E.; Jikumaru, M.; Hiramoto, K.; Tabata, H.; Katsuragi, M.; Odo, S.; Utsumi, K.; Inoue, M. Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free Radic. Biol. Med. 2010, 49, 674–681. [Google Scholar] [CrossRef]
- Nakamura, B.N.; Fielder, T.J.; Hoang, Y.D.; Lim, J.; McConnachie, L.A.; Kavanagh, T.J.; Luderer, U. Lack of maternal glutamate cysteine ligase modifier subunit (Gclm) decreases oocyte glutathione concentrations and disrupts preimplantation development in mice. Endocrinology 2011, 152, 2806–2815. [Google Scholar] [CrossRef]
- Kunimatsu, T.; Kimura, J.; Funabashi, H.; Inoue, T.; Seki, T. The antipsychotics haloperidol and chlorpromazine increase bone metabolism and induce osteopenia in female rats. Regul. Toxicol. Pharmacol. 2010, 58, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Winters, S.J.; Ghooray, D.T.; Yang, R.Q.; Holmes, J.B.; O’Brien, A.R.; Morgan, J.; Moore, J.P., Jr. Dopamine-2 receptor activation suppresses PACAP expression in gonadotrophs. Endocrinology 2014, 155, 2647–2657. [Google Scholar] [CrossRef] [Green Version]
- Khedr, N.F. Protective effect of mirtazapine and hesperidin on cyclophosphamide-induced oxidative damage and infertility in rat ovaries. Exp. Biol. Med. 2015, 240, 1682–1689. [Google Scholar] [CrossRef] [Green Version]
- Ince, S.; Ozer, M.; Kadioglu, B.G.; Kuzucu, M.; Ozkaraca, M.; Gezer, A.; Suleyman, H.; Cetin, N. The effect of taxifolin on oxidative ovarian damage and reproductive dysfunctions induced by antipsychotic drugs in female rats. J. Obstet. Gynaecol. Res. 2021, 47, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Suemasu, S.; Tanaka, K.; Namba, T.; Ishihara, T.; Katsu, T.; Fujimoto, M.; Adachi, H.; Sobue, G.; Takeuchi, K.; Nakai, A.; et al. A role for HSP70 in protecting against indomethacin-induced gastric lesions. J. Biol. Chem. 2009, 284, 19705–19715. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, S.; Ambade, A.; Fulham, M.A.; Deshpande, J.; Catalano, D.; Mandrekar, P. Moderate alcohol induces stress proteins HSF1 and hsp70 and inhibits proinflammatory cytokines resulting in endotoxin tolerance. J. Immunol. 2014, 193, 1975–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevtsov, M.; Multhoff, G. Heat shock protein peptide and HSP-based immunotherapies for the treatment of cancer. Front. Immunol. 2016, 7, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezzomo, N.F.; da Silva Schmitz, I.; de Lima, V.B.; Dorneles, G.P.; Schaffer, L.F.; Boeck, C.R.; Romao, P.R.T.; Peroza, L.R. Reversal of haloperidol-induced orofacial dyskinesia and neuroinflammation by isoflavones. Mol. Biol. Rep. 2022, 49, 1917–1923. [Google Scholar] [CrossRef]
- Chowdhury, B.; Luu, A.Z.; Luu, V.Z.; Kabir, M.G.; Pan, Y.; Teoh, H.; Quan, A.; Sabongui, S.; Al-Omran, M.; Bhatt, D.L.; et al. The SGLT2 inhibitor empagliflozin reduces mortality and prevents progression in experimental pulmonary hypertension. Biochem. Biophys. Res. Commun. 2020, 524, 50–56. [Google Scholar] [CrossRef]
- Lin, B.; Koibuchi, N.; Hasegawa, Y.; Sueta, D.; Toyama, K.; Uekawa, K.; Ma, M.; Nakagawa, T.; Kusaka, H.; Kim-Mitsuyama, S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 2014, 13, 148. [Google Scholar] [CrossRef] [Green Version]
- Sa-Nguanmoo, P.; Tanajak, P.; Kerdphoo, S.; Jaiwongkam, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol. Appl. Pharmacol. 2017, 333, 43–50. [Google Scholar] [CrossRef]
- Kim, S.; Jo, C.H. Kim, GH. Effects of empagliflozin on nondiabetic salt-sensitive hypertension in uninephrectomized rats. Hypertens. Res. 2019, 42, 1905–1915. [Google Scholar] [CrossRef] [Green Version]
- Kolijn, D.; Pabel, S.; Tian, Y.; Lódi, M.; Herwig, M.; Carrizzo, A.; Zhazykbayeva, S.; Kovács, Á.; Fülöp, G.Á.; Falcão-Pires, I.; et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc. Res. 2021, 117, 495–507. [Google Scholar] [CrossRef]
- Xu, B.; Hu, Y.; Luo, Y.T.; Xu, H.; Qi, S.H.; Zhang, Y.Y.; Zhao, D.; Yuan, D.Z. Changes in Expression of Follicular Glucose Transporters May Be Involved in Ovarian Function Impairment during Diabetic Hyperglycemia. Ann. Clin. Lab. Sci. 2019, 49, 785–793. [Google Scholar] [PubMed]
- Amin, E.F.; Rifaai, R.A.; Abdel-Latif, R.G. Empagliflozin attenuates transient cerebral ischemia/reperfusion injury in hyperglycemic rats via repressing oxidative-inflammatory-apoptotic pathway. Fundam. Clin. Pharmacol. 2020, 34, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malleo, G.; Mazzon, E.; Siriwardena, A.K.; Cuzzocrea, S. Role of tumor necrosis factor-alpha in acute pancreatitis: From biological basis to clinical evidence. Shock 2007, 28, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Lian, P.; Wu, X.; Shi, B.; Zhuang, M.; Zhou, R.; Zhao, R.; Zhao, Z.; Guo, S.; Ji, Z.; et al. Abate Cytochrome C induced apoptosome to protect donor liver against ischemiareperfusion injury on rat liver transplantation model. Am. J. Transl. Res. 2016, 8, 1738–1747. [Google Scholar]
- Lee, H.C.; Shiou, Y.L.; Jhuo, S.J.; Chang, C.Y.; Liu, P.L.; Jhuang, W.J.; Dai, Z.K.; Chen, W.Y.; Chen, Y.F.; Lee, A.S. The sodium-glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc. Diabetol. 2019, 18, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trang, N.N.; Chung, C.C.; Lee, T.W.; Cheng, W.L.; Kao, Y.H.; Huang, S.Y.; Lee, T.I.; Chen, Y.J. Empagliflozin and Liraglutide Differentially Modulate Cardiac Metabolism in Diabetic Cardiomyopathy in Rats. Int. J. Mol. Sci. 2021, 22, 1177. [Google Scholar] [CrossRef]
- Kostakis, I.D.; Zavras, N.; Damaskos, C.; Sakellariou, S.; Korkolopoulou, P.; Misiakos, E.P.; Tsaparas, P.; Vaos, G.; Karatzas, T. Erythropoietin and sildenafil protect against ischemia/reperfusion injury following testiculartorsion in adult rats. Exp. Ther. Med. 2017, 13, 3341–3347. [Google Scholar] [CrossRef] [Green Version]
- Botros, S.R.; Matouk, A.I.; Anter, A.; Khalifa, M.M.A.; Heeba, G.H. Protective effect of empagliflozin on gentamicin-induced acute renal injury via regulation of SIRT1/NF-κB signaling pathway. Environ. Toxicol. Pharmacol. 2022, 94, 103907. [Google Scholar] [CrossRef]
- Ojima, A.; Matsui, T.; Nishino, Y.; Nakamura, N.; Yamagishi, S. Empagliflozin, an Inhibitor of Sodium-Glucose Cotransporter 2 Exerts Anti-Inflammatory and Antifibrotic Effects on Experimental Diabetic Nephropathy Partly by Suppressing AGEs-Receptor Axis. Horm. Metab. Res. 2015, 47, 686–692. [Google Scholar] [CrossRef] [Green Version]
- Ashrafi Jigheh, Z.; Ghorbani Haghjo, A.; Argani, H.; Roshangar, L.; Rashtchizadeh, N.; Sanajou, D.; Nazari Soltan Ahmad, S.; Rashedi, J.; Dastmalchi, S.; Mesgari Abbasi, M. Empagliflozin Attenuates Renal and Urinary Markers of Tubular Epithelial Cell Injury in Streptozotocin-induced Diabetic Rats. Indian J. Clin. Biochem. 2020, 35, 109–114. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Sastry, K.V.; Moudgal, R.P.; Mohan, J.; Tyagi, J.S.; Rao, G.S. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal. Biochem. 2002, 306, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Côté, S. Current protocol for light microscopy immunocytochemistry. Immunohistochemistry 1993, 2, 148–167. [Google Scholar]
| Group | Ovarian MDA (nmol/g Tissue) | Ovarian SOD (U/g Tissue) | Ovarian NOx (nmol/g Tissue) |
|---|---|---|---|
| Control | 13.41± 0.91 | 256.00 ± 6.38 | 52.34 ± 2.28 |
| EMPA | 13.10 ± 0.90 | 257.50 ± 6.52 | 53.88 ± 2.74 |
| HAL | 50.92 ± 3.17 ab | 95.78 ± 4.67 ab | 127.20 ± 3.15 ab |
| HAL + EMPA | 18.71 ± 1.65 c | 241.7 ± 4.54 c | 64.77 ± 3.32 ac |
| Group | FSH (IU/L) | LH (IU/L) | AMH (ng/mL) |
|---|---|---|---|
| Control | 7.68 ± 0.62 | 5.52 ± 0.42 | 7.84 ± 0.59 |
| EMPA | 7.16 ± 0.61 | 7.41 ± 0.67 | 7.70 ± 0.69 |
| HAL | 15.48 ± 1.14 ab | 16.89 ± 1.54 ab | 2.17 ± 0.15 ab |
| HAL + EMPA | 8.344 ± 0.75 c | 8.78 ± 0.58 c | 6.70 ± 0.59 c |
| Group | Ovarian Nrf2 (pg/g Tissue) | Ovarian TNF-α (pg/g Tissue) | Ovarian IL-6 (pg/g Tissue) | Ovarian Caspase-3 (ng/g Tissue) |
|---|---|---|---|---|
| Control | 87.60 ± 4.94 | 86.17 ± 5.88 | 97.75 ± 4.24 | 8.845 ± 0.64 |
| EMPA | 86.05 ± 4.37 | 85.10 ± 7.70 | 104.9 ± 4.17 | 9.27 ± 0.66 |
| HAL | 46.75 ± 4.22 ab | 180.00 ± 6.90 ab | 182.40 ± 6.57 ab | 23.35 ± 1.57 ab |
| HAL + EMPA | 81.49 ± 6.60 c | 94.43 ± 8.50 c | 107.90 ± 2.98 c | 10.19 ± 0.60 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelzaher, W.Y.; De Waard, M.; Abdelmonaem, A.A.; Ali, D.M.; El-Tahawy, N.F.G.; Rifaai, R.A.; Mohamed, H.A.; Shaheen, K.; Zeen El-Din, M.A.; Welson, N.N.; et al. Empagliflozin Protects against Haloperidol Experimentally-Induced Ovarian Toxicity. Pharmaceuticals 2023, 16, 168. https://doi.org/10.3390/ph16020168
Abdelzaher WY, De Waard M, Abdelmonaem AA, Ali DM, El-Tahawy NFG, Rifaai RA, Mohamed HA, Shaheen K, Zeen El-Din MA, Welson NN, et al. Empagliflozin Protects against Haloperidol Experimentally-Induced Ovarian Toxicity. Pharmaceuticals. 2023; 16(2):168. https://doi.org/10.3390/ph16020168
Chicago/Turabian StyleAbdelzaher, Walaa Yehia, Michel De Waard, Alyaa Abdelfattah Abdelmonaem, Dalia Mohamed Ali, Nashwa Fathy Gamal El-Tahawy, Rehab Ahmed Rifaai, Hatem A. Mohamed, Kareem Shaheen, Mohamed Ahmed Zeen El-Din, Nermeen N. Welson, and et al. 2023. "Empagliflozin Protects against Haloperidol Experimentally-Induced Ovarian Toxicity" Pharmaceuticals 16, no. 2: 168. https://doi.org/10.3390/ph16020168
APA StyleAbdelzaher, W. Y., De Waard, M., Abdelmonaem, A. A., Ali, D. M., El-Tahawy, N. F. G., Rifaai, R. A., Mohamed, H. A., Shaheen, K., Zeen El-Din, M. A., Welson, N. N., Tawfeek, S. E., Batiha, G. E.-S., & Abdel-Aziz, A. M. (2023). Empagliflozin Protects against Haloperidol Experimentally-Induced Ovarian Toxicity. Pharmaceuticals, 16(2), 168. https://doi.org/10.3390/ph16020168








