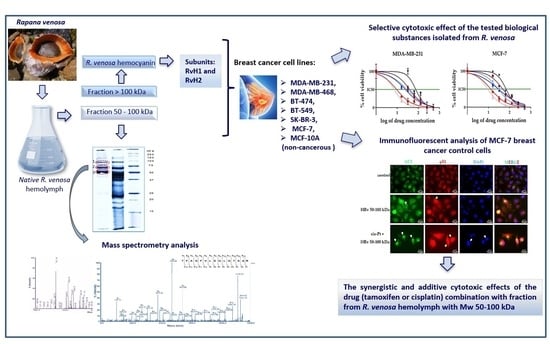
Antitumor Activity of Bioactive Compounds from Rapana venosa against Human Breast Cell Lines
Abstract
1. Introduction
2. Results
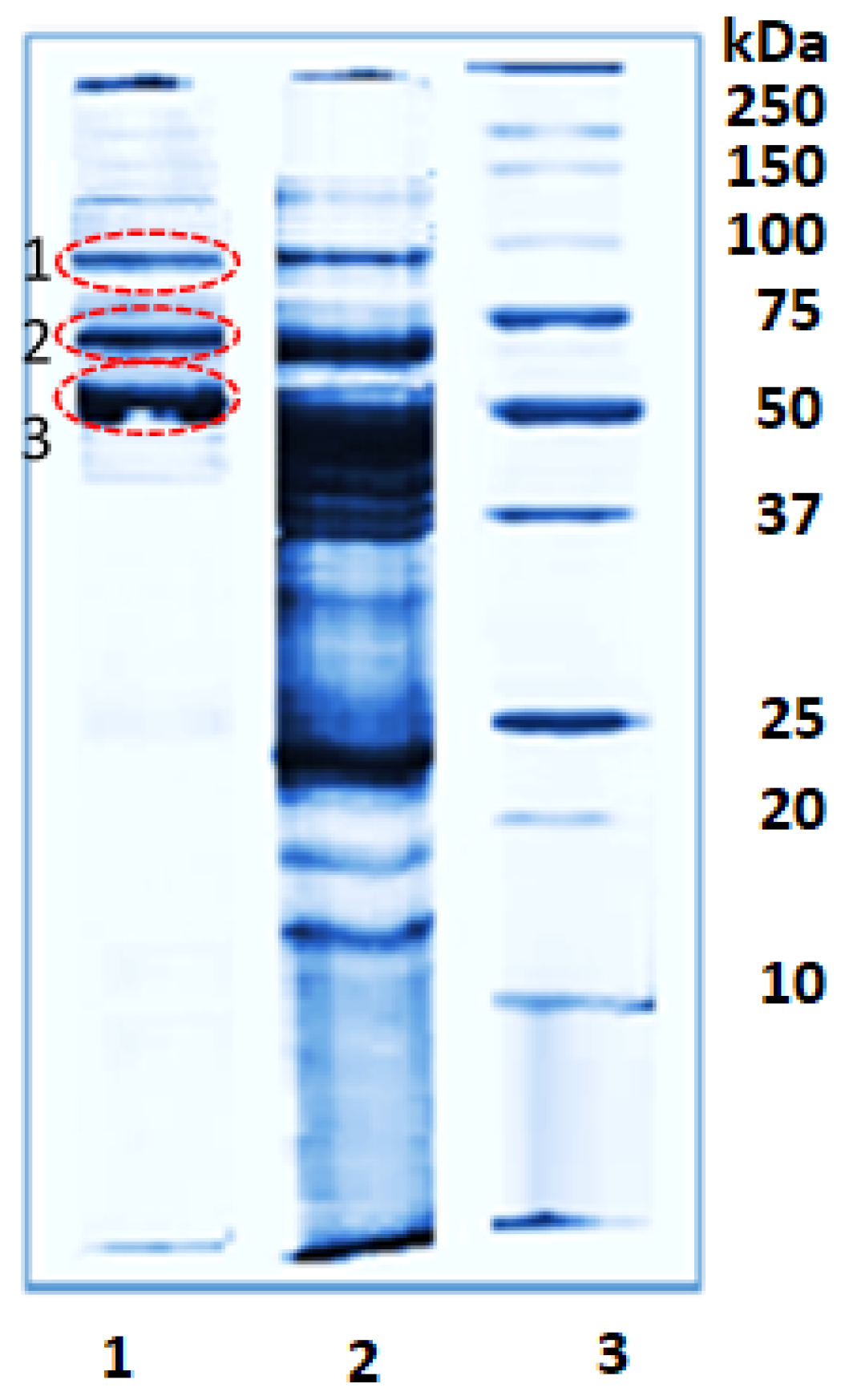
2.1. Isolation of Bioactive Compounds from the Hemolymph of the Marine Snail Rapana venosa
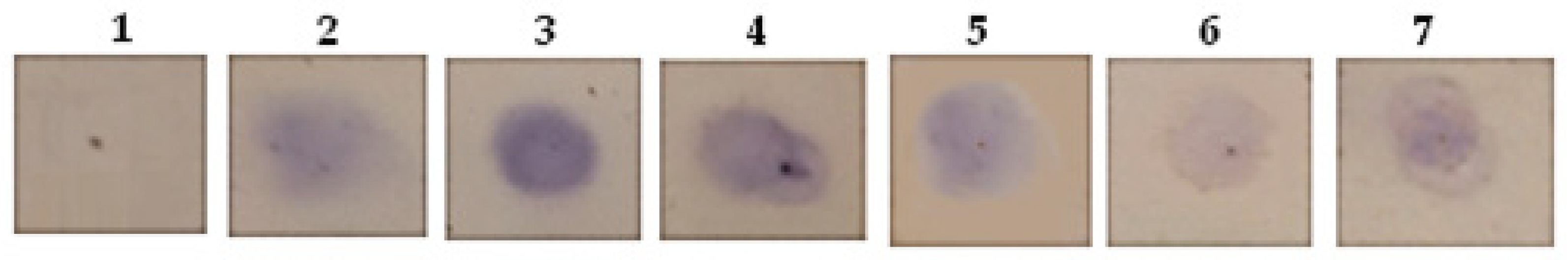
2.2. A Glycosylation Screening
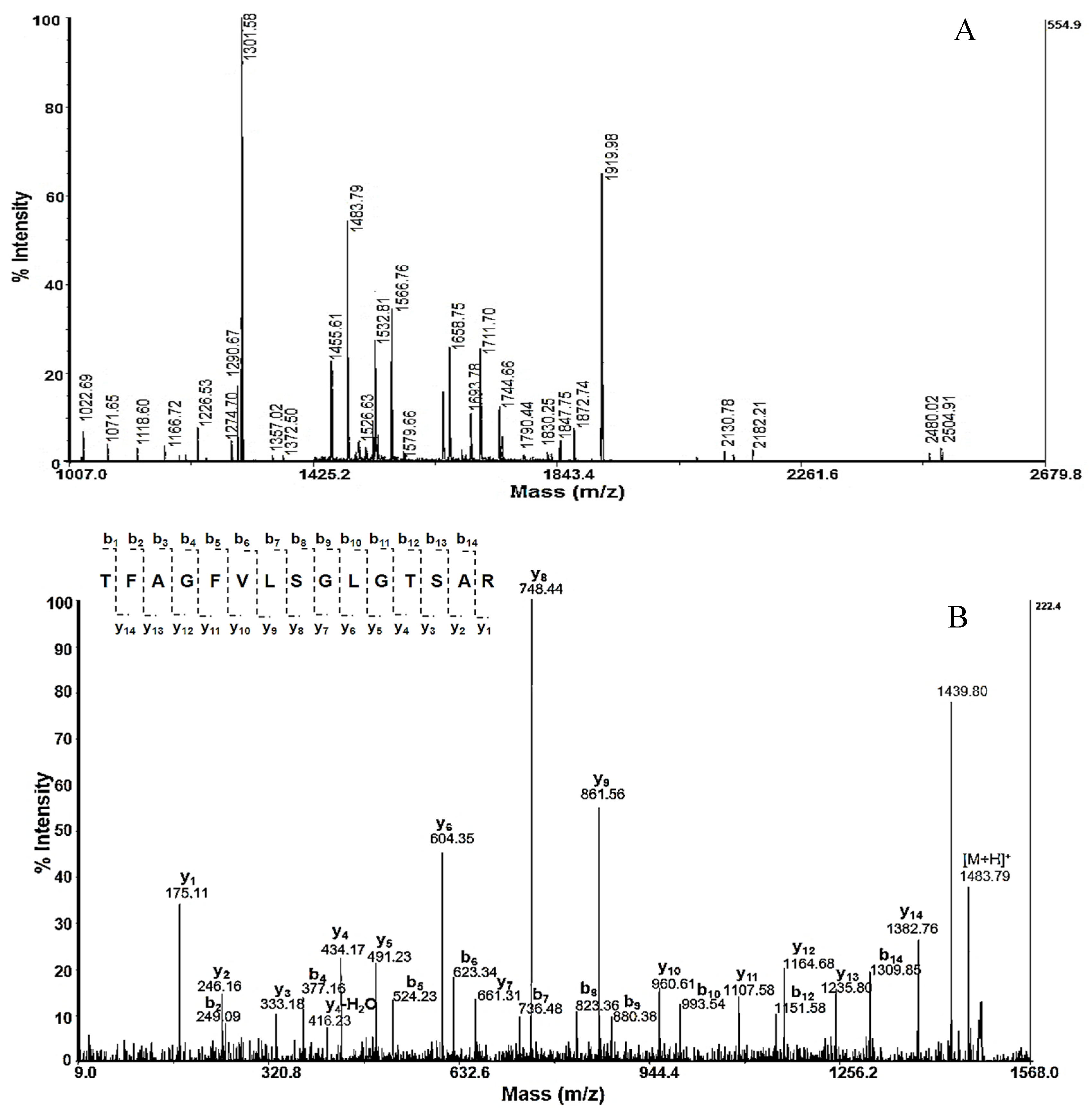
2.3. Identification of Proteins in Active Fraction with Mw 50–100 kDa from R. venosa Hemolymph
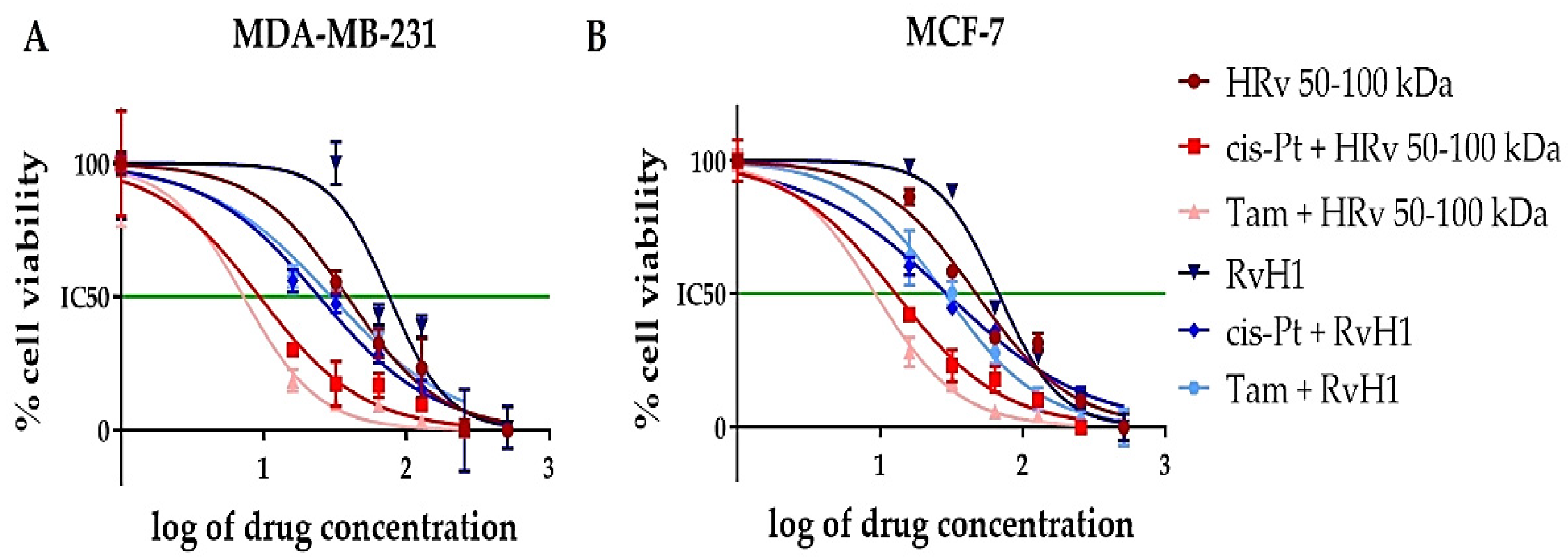
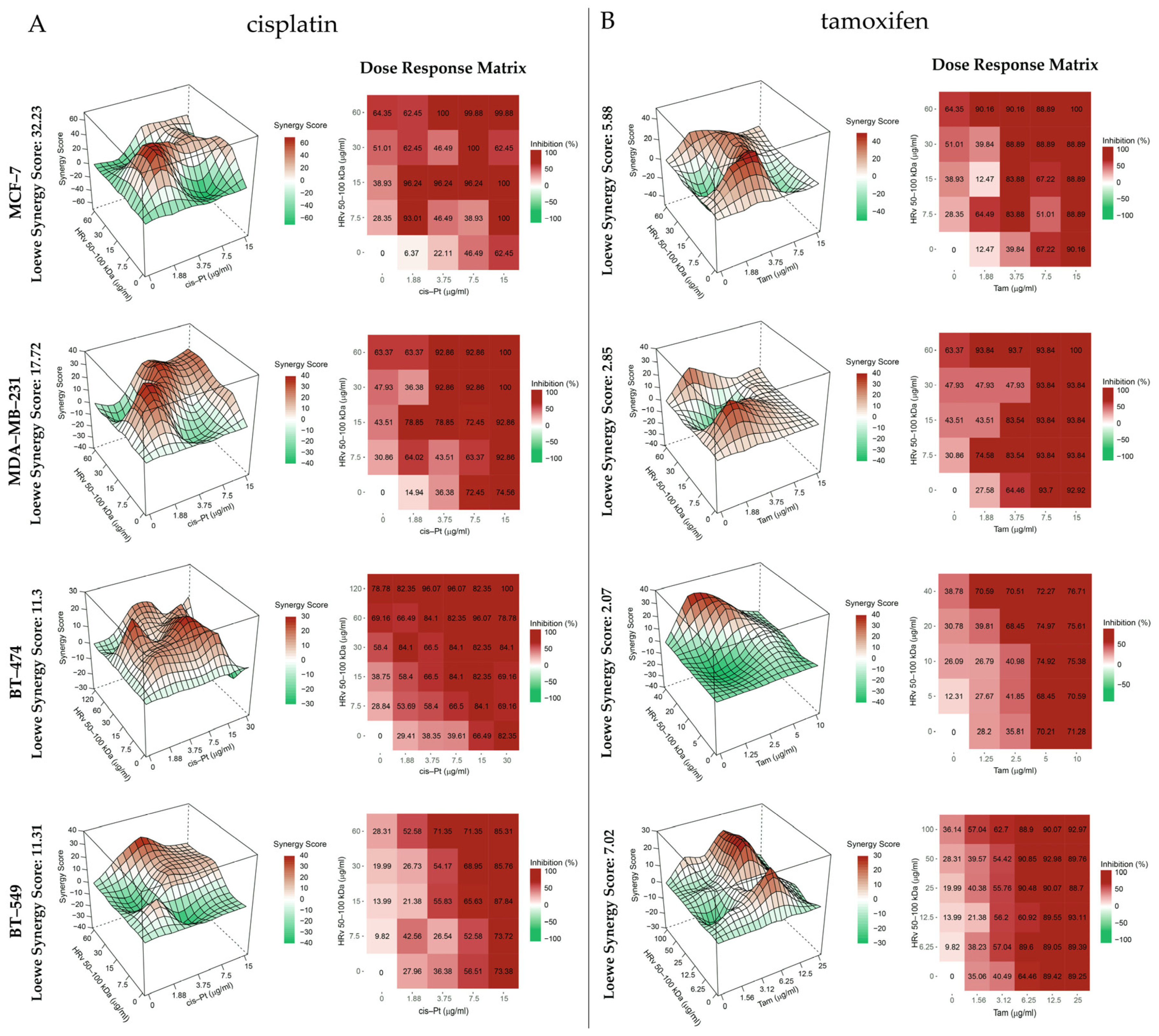
2.4. Cytotoxic Effect of the Tested Biological Substances Isolated from R. venosa
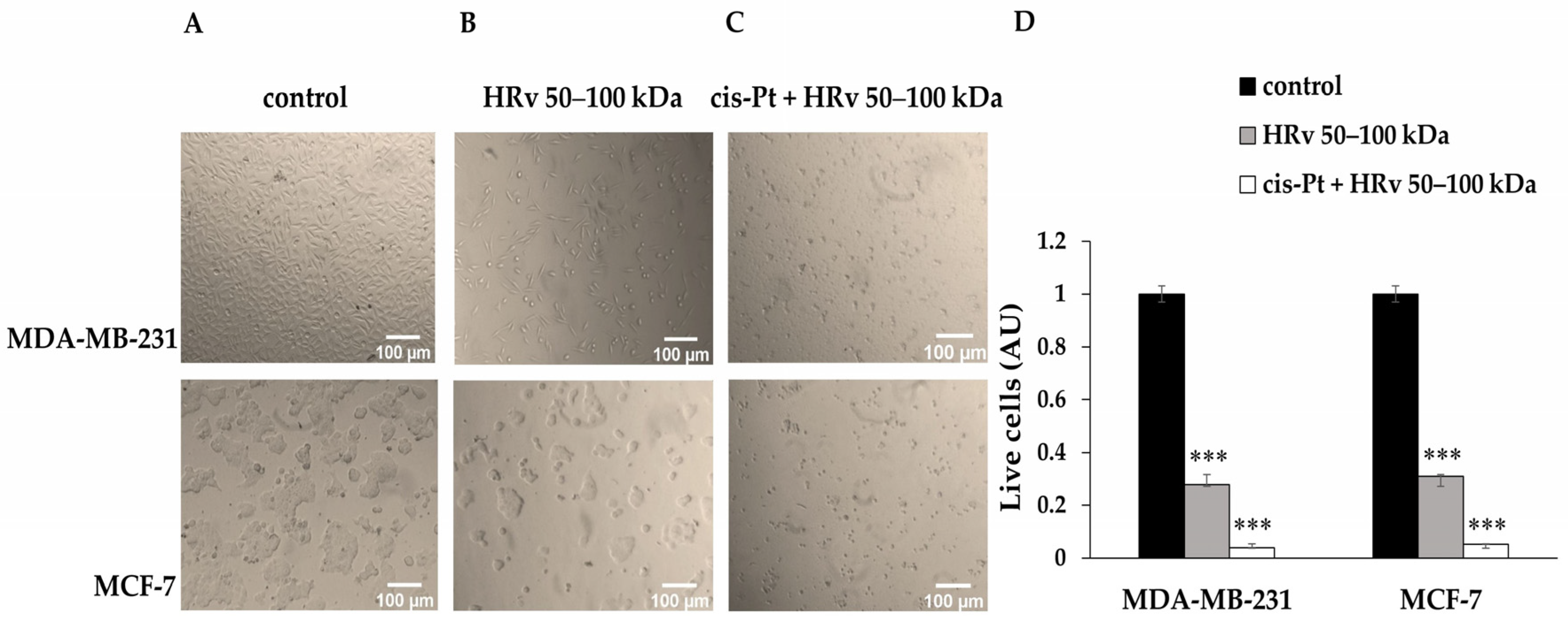
2.5. Microscopic Observation and Analysis of the Morphological Changes and Cell Viability Testing by Trypan Blue
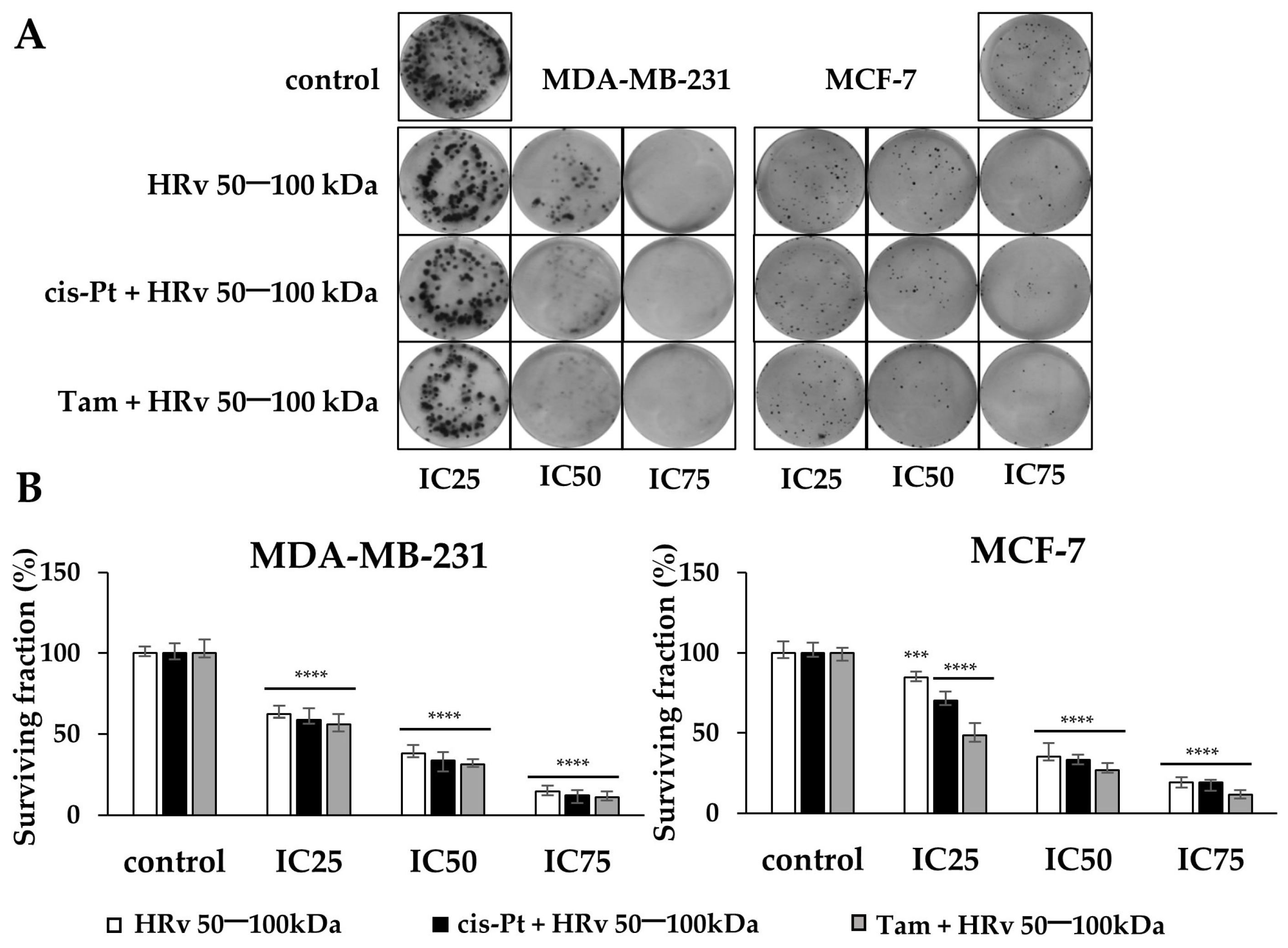
2.6. The Effects of the Hemolymph Fraction of R. venosa with Mw 50–100 kDa on the Proliferation of Breast Cancer Cells, Tested by a Colony-Forming Assay
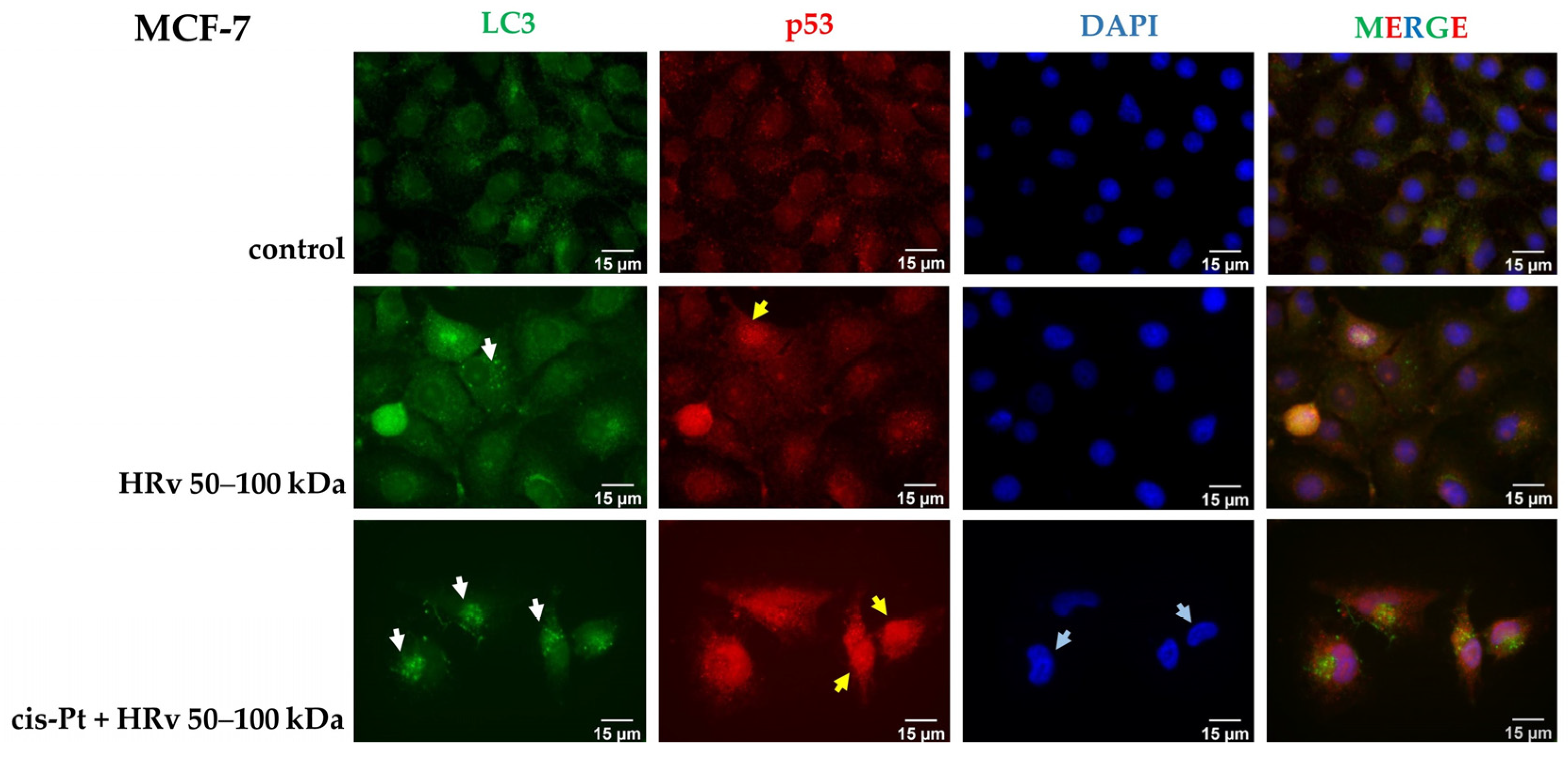
2.7. Immunofluorescent Analysis of p53 Localization and Autophagy Marker LC3 Expression in MCF-7 Cells Treated with Hemolymph from R. venosa 50–100 kDa Alone and in Combination with Cisplatin
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Isolation of Bioactive Compounds from the Hemolymph of Marine Snail R. venosa
4.2.1. Isolation of R. venosa Hemocyanin and Its Structural Subunits
4.2.2. Isolation of Bioactive Fraction from Hemolymph of R. venosa
4.3. SDS-PAGE Electrophoresis
4.4. A Glycosylation Screening
4.5. Analyses of Proteins after Tryptic Digestion
4.6. Mass Spectrometry Analysis
4.7. Cytotoxic Assay
4.8. Colony Formation Assay
4.9. Microscopic Observations
4.10. Immunofluorescent Microscopy
4.11. Chou–Talalay Method for Determination of the Combination Index (CI)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Partridge, A.H.; Burstein, H.J.; Winer, E.P. Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. JNCI Monogr. 2001, 2001, 135–142. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- De Melo Gagliato, D.; Jardim, D.L.; Marchesi, M.S.; Hortobagyi, G.N. Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget 2016, 7, 64431–64446. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.; Ingarra, A.M.; Raimondi, M.V.; Spanò, V.; Piccionello, A.P.; De Franco, M.; Menilli, L.; Gandin, V.; Miolo, G.; Barraja, P.; et al. New tricyclic systems as photosensitizers towards triple negative breast cancer cells. Arch. Pharm. Res. 2022, 45, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.; Ingarra, A.M.; Raimondi, M.V.; Spanò, V.; De Franco, M.; Menilli, L.; Gandin, V.; Miolo, G.; Barraja, P.; Montalbano, A. Insight on pyrimido[5,4-g]indolizine and pyrimido[4,5-c]pyrrolo[1,2-a]azepine systems as promising photosensitizers on malignant cells. Eur. J. Med. Chem. 2022, 237, 114399. [Google Scholar] [CrossRef]
- Wang, L.; Dong, C.; Li, X.; Han, W.; Su, X. Anticancer potential of bioactive peptides from animal sources (Review). Oncol. Rep. 2017, 38, 637–651. [Google Scholar] [CrossRef]
- Antonova, O.; Dolashka, P.; Toncheva, D.; Rammense, H.G.; Floetenmeyer, M.; Stevanovic, S. In Vitro Antiproliferative Effect of Helix aspersa Hemocyanin on Multiple Malignant Cell Lines. Z. Naturforsch. C 2014, 69, 325–334. [Google Scholar] [CrossRef]
- Dolashki, A.; Dolashka, P.; Stenzl, A.; Stevanovic, S.; Aicher, W.K.; Velkova, L.; Velikova, R.; Voelter, W. Antitumour activity of Helix hemocyanin against bladder carcinoma permanent cell lines. Biotechnol. Biotechnol. Equip. 2019, 33, 20–32. [Google Scholar] [CrossRef]
- Georgieva, A.; Todorova, K.; Iliev, I.; Dilcheva, V.; Vladov, I.; Petkova, S.; Toshkova, R.; Velkova, L.; Dolashki, A.; Dolashka, P. Hemocyanins from Helix and Rapana Snails Exhibit In Vitro Antitumor Effects in Human Colorectal Adenocarcinoma. Biomedicines 2020, 8, 194. [Google Scholar] [CrossRef]
- Pizarro-Bauerle, J.; Maldonado, I.; Sosoniuk-Roche, E.; Vallejos, G.; Lopez, M.N.; Salazar-Onfray, F.; Aguilar-Guzman, L.; Valck, C.; Ferreira, A.; Becker, M.I. Molluskan Hemocyanins Activate the Classical Pathway of the Human Complement System through Natural Antibodies. Front. Immunol. 2017, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Markl, J. Evolution of molluscan hemocyanin structures. Biochim. Biophys. Acta 2013, 1834, 1840–1852. [Google Scholar] [CrossRef] [PubMed]
- Lamm, D.L.; Dehaven, J.I.; Riggs, D.R. Keyhole limpet hemocyanin immunotherapy of bladder cancer: Laboratory and clinical studies. Eur. Urol. 2000, 37, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Krug, L.M.; Ragupathi, G.; Hood, C.; Kris, M.G.; Miller, V.A.; Allen, J.R.; Keding, S.J.; Danishefsky, S.J.; Gomez, J.; Tyson, L.; et al. Vaccination of patients with small-cell lung cancer with synthetic fucosyl GM-1 conjugated to keyhole limpet hemocyanin. Clin. Cancer Res. 2004, 10 Pt 1, 6094–6100. [Google Scholar] [CrossRef]
- Lammers, R.J.; Witjes, W.P.; Janzing-Pastors, M.H.; Caris, C.T.; Witjes, J.A. Intracutaneous and intravesical immunotherapy with keyhole limpet hemocyanin compared with intravesical mitomycin in patients with non-muscle-invasive bladder cancer: Results from a prospective randomized phase III trial. J. Clin. Oncol. 2012, 30, 2273–2279. [Google Scholar] [CrossRef]
- Moltedo, B.; Faunes, F.; Haussmann, D.; De Ioannes, P.; De Ioannes, A.E.; Puente, J.; Becker, M.I. Immunotherapeutic effect of Concholepas hemocyanin in the murine bladder cancer model: Evidence for conserved antitumor properties among hemocyanins. J. Urol. 2006, 176 Pt 1, 2690–2695. [Google Scholar] [CrossRef]
- Palacios, M.; Tampe, R.; Del Campo, M.; Zhong, T.Y.; Lopez, M.N.; Salazar-Onfray, F.; Becker, M.I. Antitumor activity and carrier properties of novel hemocyanins coupled to a mimotope of GD2 ganglioside. Eur. J. Med. Chem. 2018, 150, 74–86. [Google Scholar] [CrossRef]
- Arancibia, S.; Espinoza, C.; Salazar, F.; Del Campo, M.; Tampe, R.; Zhong, T.Y.; De Ioannes, P.; Moltedo, B.; Ferreira, J.; Lavelle, E.C.; et al. A novel immunomodulatory hemocyanin from the Limpet Fissurella latimarginata promotes potent anti-tumor activity in melanoma. PLoS ONE 2014, 9, e87240. [Google Scholar] [CrossRef]
- Arancibia, S.; Salazar, F.; Becker, M.I. Hemocyanins in the immunotherapy of superficial bladder cancer. In Bladder Cancer—From Basic Science to Robotic Surgery; Canda, A.E., Ed.; InTech: Rijeka, Croatia, 2012; pp. 221–242. [Google Scholar] [CrossRef]
- McFadden, D.W.; Riggs, D.R.; Jackson, B.J.; Vona-Davis, L. Keyhole limpet hemocyanin, a novel immune stimulant with promising anticancer activity in Barrett’s esophageal adenocarcinoma. Am. J. Surg. 2003, 186, 552–555. [Google Scholar] [CrossRef]
- Riggs, D.R.; Jackson, B.J.; Vona-Davis, L.; Nigam, A.; McFadden, D.W. In vitro effects of keyhole limpet hemocyanin in breast and pancreatic cancer in regards to cell growth, cytokine production, and apoptosis. Am. J. Surg. 2005, 189, 680–684. [Google Scholar] [CrossRef]
- Dolashka-Angelova, P.; Stefanova, T.; Livaniou, E.; Velkova, L.; Klimentzou, P.; Stevanovic, S.; Salvato, B.; Neychev, H.; Voelter, W. Immunological potential of Helix vulgaris and Rapana venosa hemocyanins. Immunol. Investig. 2008, 37, 822–840. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.Y.; Arancibia, S.; Born, R.; Tampe, R.; Villar, J.; Del Campo, M.; Manubens, A.; Becker, M.I. Hemocyanins Stimulate Innate Immunity by Inducing Different Temporal Patterns of Proinflammatory Cytokine Expression in Macrophages. J. Immunol. 2016, 196, 4650–4662. [Google Scholar] [CrossRef] [PubMed]
- Dolashka-Angelova, P.; Schwarz, H.; Dolashki, A.; Stevanovic, S.; Fecker, M.; Saeed, M.; Voelter, W. Oligomeric stability of Rapana venosa hemocyanin (RvH) and its structural subunits. Biochim. Biophys. Acta 2003, 1646, 77–85. [Google Scholar] [CrossRef]
- Dolashka-Angelova, P.; Beck, A.; Dolashki, A.; Stevanovic, S.; Beltramini, M.; Salvato, B.; Hristova, R.; Velkova, L.; Voelter, W. Carbohydrate moieties of molluscan Rapana venosa hemocyanin. Micron 2004, 35, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Dolashka-Angelova, P.; Beck, A.; Dolashki, A.; Beltramini, M.; Stevanovic, S.; Salvato, B.; Voelter, W. Characterization of the carbohydrate moieties of the functional unit RvH(1)-a of Rapana venosa haemocyanin using HPLC/electrospray ionization MS and glycosidase digestion. Biochem. J. 2003, 374 Pt 1, 185–192. [Google Scholar] [CrossRef]
- Sandra, K.; Dolashka-Angelova, P.; Devreese, B.; Van Beeumen, J. New insights in Rapana venosa hemocyanin N-glycosylation resulting from on-line mass spectrometric analyses. Glycobiology 2007, 17, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Dolashka, P.; Velkova, L.; Shishkov, S.; Kostova, K.; Dolashki, A.; Dimitrov, I.; Atanasov, B.; Devreese, B.; Voelter, W.; Van Beeumen, J. Glycan structures and antiviral effect of the structural subunit RvH2 of Rapana hemocyanin. Carbohydr. Res. 2010, 345, 2361–2367. [Google Scholar] [CrossRef]
- Dalziel, M.; Crispin, M.; Scanlan, C.N.; Zitzmann, N.; Dwek, R.A. Emerging principles for the therapeutic exploitation of glycosylation. Science 2014, 343, 1235681. [Google Scholar] [CrossRef]
- Dolashka, P.; Dolashki, A.; Van Beeumen, J.; Floetenmeyer, M.; Velkova, L.; Stevanovic, S.; Voelter, W. Antimicrobial Activity of Molluscan Hemocyanins from Helix and Rapana Snails. Curr. Pharm. Biotechnol. 2016, 17, 263–270. [Google Scholar] [CrossRef]
- Dolashka, P.; Velkova, L.; Iliev, I.; Beck, A.; Dolashki, A.; Yossifova, L.; Toshkova, R.; Voelter, W.; Zacharieva, S. Antitumor activity of glycosylated molluscan hemocyanins via Guerin ascites tumor. Immunol. Investig. 2011, 40, 130–149. [Google Scholar] [CrossRef]
- Dolashka-Angelova, P.; Lieb, B.; Velkova, L.; Heilen, N.; Sandra, K.; Nikolaeva-Glomb, L.; Dolashki, A.; Galabov, A.S.; Van Beeumen, J.; Stevanovic, S.; et al. Identification of Glycosylated Sites in Rapana Hemocyanin by Mass Spectrometry and Gene Sequence, and Their Antiviral Effect. Bioconjug. Chem. 2009, 20, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Dolashka, P.; Moshtanska, V.; Borisova, V.; Dolashki, A.; Stevanovic, S.; Dimanov, T.; Voelter, W. Antimicrobial proline-rich peptides from the hemolymph of marine snail Rapana venosa. Peptides 2011, 32, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Todorovska, E.; Radkova, M.; Georgiev, O.; Dolashki, A.; Dolashka, P. Molecular cloning, characterization and phylogenetic analysis of an actin gene from the marine mollusk Rapana venosa (class Gastropoda). Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 687–700. [Google Scholar]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Loewe, S. Die quantitativen Probleme der Pharmakologie. Ergeb. Physiol. 1928, 27, 47–187. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, W.; Aldahdooh, J.; Malyutina, A.; Shadbahr, T.; Tanoli, Z.; Pessia, A.; Tang, J. Synergy Finder Plus: Toward Better Interpretation and Annotation of Drug Combination Screening Datasets. Genom. Proteom. Bioinform. 2022, 20, 587–596. [Google Scholar] [CrossRef]
- Chen, L.; Liu, S.; Tao, Y. Regulating tumor suppressor genes: Post-translational modifications. Signal Transduct. Target. Ther. 2020, 5, 90. [Google Scholar] [CrossRef]
- Prives, C.; Hall, P.A. The p53 pathway. J. Pathol. 1999, 187, 112–126. [Google Scholar] [CrossRef]
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the p53 network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef]
- Wang, W.; Zafar, A.; Rajaei, M.; Zhang, R. Two Birds with One Stone: NFAT1-MDM2 Dual Inhibitors for Cancer Therapy. Cells 2020, 9, 1176. [Google Scholar] [CrossRef]
- Lane, D. Awakening angels. Nature 1998, 394, 616–617. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Lain, S.; Verma, C.S.; Fersht, A.R.; Lane, D.P. Awakening guardian angels: Drugging the p53 pathway. Nat. Rev. Cancer 2009, 9, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Gorina, S.; Jeffrey, P.D.; Pavletich, N.P. Crystal structure of a p53 tumor suppressor-DNA complex: Understanding tumorigenic mutations. Science 1994, 265, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [CrossRef]
- Crighton, D.; Wilkinson, S.; O’Prey, J.; Syed, N.; Smith, P.; Harrison, P.R.; Gasco, M.; Garrone, O.; Crook, T.; Ryan, K.M. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 2006, 126, 121–134. [Google Scholar] [CrossRef]
- Liu, Y.; Levine, B. Autosis and autophagic cell death: The dark side of autophagy. Cell Death Differ. 2015, 22, 367–376. [Google Scholar] [CrossRef]
- Mora Roman, J.J.; Del Campo, M.; Villar, J.; Paolini, F.; Curzio, G.; Venuti, A.; Jara, L.; Ferreira, J.; Murgas, P.; Lladser, A.; et al. Immunotherapeutic Potential of Mollusk Hemocyanins in Combination with Human Vaccine Adjuvants in Murine Models of Oral Cancer. J. Immunol. Res. 2019, 2019, 7076942. [Google Scholar] [CrossRef]
- Yamazaki, M. Antitumor and antimicrobial glycoproteins from sea hares. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1993, 105, 141–146. [Google Scholar] [CrossRef]
- Antonova, O.; Yossifova, L.; Staneva, R.; Stevanovic, S.; Dolashka, P.; Toncheva, D. Changes in the gene expression profile of the bladder cancer cell lines after treatment with Helix lucorum and Rapana venosa hemocyanin. J. Buon. 2015, 20, 180–187. [Google Scholar]
- Stenzl, A.; Dolashki, A.; Stevanovic, S.; Voelter, W.; Aicher, W.; Dolashka, P. Cytotoxic effects of Rapana venosa hemocyanin on bladder cancer permanent cell lines. J. US China Med. Sci. 2016, 13, 79–188. [Google Scholar] [CrossRef]
- Dolashki, A.; Antonova, O.; Velkova, L.; Kaynarov, D.; Voelter, W.; Dolashka, P. Selective cytotoxicity and changes in protein expression of T24 bladder carcinoma permanent cell line after treatment with hemocyanins. Curr. Med. Chem. 2022, 29, 6479–6498. [Google Scholar] [CrossRef] [PubMed]
- Bevara, G.B.; Kumar, A.D.N.; Koteswramma, K.L.; Badana, A.K.; Kumari, S.; Yarla, N.S.; Malla, R.R. C-glycosyl Flavone from Urginea indica Inhibits Growth and Dissemination of Ehrlich Ascites Carcinoma Cells in Mice. Anticancer Agents Med. Chem. 2017, 17, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.A.; Pomin, V.H. Marine Carbohydrate-Based Compounds with Medicinal Properties. Mar. Drugs 2018, 16, 233. [Google Scholar] [CrossRef] [PubMed]
- Hew, C.S.; Khoo, B.Y.; Gam, L.H. The anti-cancer property of proteins extracted from Gynura procumbens (Lour.) Merr. PLoS ONE 2013, 8, e68524. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, H.; Muramoto, K.; Yamazaki, M. Aplysianin-A, an antibacterial and antineoplastic glycoprotein in the albumen gland of a sea hare, Aplysia kurodai. Experientia 1986, 42, 1065–1067. [Google Scholar] [CrossRef]
- Izidoro, L.F.M.; Sobrinho, J.C.; Mendes, M.M.; Costa, T.R.; Grabner, A.N.; Rodrigues, V.M.; da Silva, S.L.; Zanchi, F.B.; Zuliani, J.P.; Fernandes, C.F.C.; et al. Snake venom L-amino acid oxidases: Trends in pharmacology and biochemistry. Biomed. Res. Int. 2014, 2014, 196754. [Google Scholar] [CrossRef]
- Marie, B.; Jackson, D.J.; Ramos-Silva, P.; Zanella-Cleon, I.; Guichard, N.; Marin, F. The shell-forming proteome of Lottia gigantea reveals both deep conservations and lineage-specific novelties. FEBS J. 2013, 280, 214–232. [Google Scholar] [CrossRef]
- Staudacher, E.; Stepan, H.; Gutternigg, M. Protein N-Glycosylation of Gastropods. Curr. Top. Biochem. Res. 2009, 11, 29–39. [Google Scholar]
- Wang, L.; Zhao, X.; Fu, J.; Xu, W.; Yuan, J. The Role of Tumour Metabolism in Cisplatin Resistance. Front. Mol. Biosci. 2021, 8, 691795. [Google Scholar] [CrossRef]
- Geyer, H.; Wuhrer, M.; Resemann, A.; Geyer, R. Identification and characterization of keyhole limpet hemocyanin N-glycans mediating cross-reactivity with Schistosoma mansoni. J. Biol. Chem. 2005, 280, 40731–40748. [Google Scholar] [CrossRef]
- Nikolova, M.; Konstantinov, S.; Velkova, L.; Kaynarov, D.; Dolashki, A.; Dolashka, P. Antitumour Activity of Different Bioactive Compounds from Hemolymph and Mucus of Mollusca against Human Urinary Bladder Cancer Cell Lines. C. R. Acad. Bulg. Sci. 2022, 75, 726–736. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, X.; Zhang, P.; Chen, C.; Liu, S.; Huang, R.; Zhong, M.; Wei, C.; Zhang, Y. Hemocyanin from Shrimp Litopenaeus vannamei has antiproliferative effect against HeLa cell in vitro. PLoS ONE 2016, 11, e90972. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, J.; Capdevielle, J.; Guillemot, J.C.; Ferrara, P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal. Biochem. 1992, 203, 173–179. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Dobrikov, G.M.; Valcheva, V.; Nikolova, Y.; Ugrinova, I.; Pasheva, E.; Dimitrov, V. Efficient synthesis of new (R)-2-amino-1-butanol derived ureas, thioureas and acylthioureas and in vitro evaluation of their antimycobacterial activity. Eur. J. Med. Chem. 2013, 63, 468–473. [Google Scholar] [CrossRef]
- Kamenova-Nacheva, M.; Schroder, M.; Pasheva, E.; Slavchev, I.; Dimitrov, V.; Momekov, G.; Nikolova, R.; Shivachev, B.; Ugrinova, I.; Dobrikov, G.M. Synthesis of ferrocenylmethylidene and arylidene substituted camphane based compounds as potential anticancer agents. New J. Chem. 2017, 41, 9103–9112. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Ugrinova, I.; Monier, K.; Ivaldi, C.; Thiry, M.; Storck, S.; Mongelard, F.; Bouvet, P. Inactivation of nucleolin leads to nucleolar disruption, cell cycle arrest and defects in centrosome duplication. BMC Mol. Biol. 2007, 8, 66. [Google Scholar] [CrossRef]
| Band | AAS of Peptide | Mass Exp. [M+H]+ | Protein Name | UniProt ID | Identities |
|---|---|---|---|---|---|
| 1 | HGDDCCDMDMR | 1297.41 | Peroxidase-like protein 2 [L. gigantea] | B3A0P3 | 100%, E = 0.20 |
| DHGEPPYDDFR | 1347.56 | Peroxidase-like protein 2 [L. gigantea] | B3A0P3 | 73%, E = 0.035 | |
| LPGAFTGPTFNCIAR | 1635.83 | Peroxidase-like protein 3 [L. gigantea] | B3A0Q8 | 63%, E = 0.001 | |
| Peroxidase-like protein 2 [L. gigantea] | B3A0P3 | 63%, E = 0.015 | |||
| Peroxidase-like protein [P. margaritifera] | H2A0M7 | 67%, E = 0.12 | |||
| 2.1 | MPAQPVAGLFDR | 1301.58 | Peroxidase-like protein 2 [L. gigantea] | B3A0P3 | 100%, E = 0.021 |
| LDWPVLFNDR | 1274.7 | Aplysianin-A [Aplysia kurodai] | Q17043 | 83%, E = 0.97 | |
| KLFWHMDWK | 1290.67 | L-amino-acid oxidase LAAO [A. califonica] | Q6IWZ0 | 63%, E = 1.0 | |
| MFHFDELLDLPR | 1532.81 | L-amino-acid oxidase LAAO [A. californica] | Q6IWZ0 | 86%, E = 0.35 | |
| Aplysianin-A [A. kurodai] | Q17043 | 86%, E = 0.35 | |||
| DYHFDELLDLMR | 1566.76 | Aplysianin-A [A. kurodai] | Q17043 | 55%, E = 0.085 | |
| L-amino-acid oxidase LAAO [A. californica] | Q6IWZ0 | 55%, E = 0.17 | |||
| YDRWDVPEPEFVVLR | 1919.98 | Aplysianin-A [A. kurodai] | Q17043 | 63%, E = 14 | |
| 2.2 | TFAGFVLSGLGTSAR | 1483.79 | Hemocyanin type 2 unit-e; RvH2-e [R. venosa]; | P83040 | 77%, E = 5e-04 |
| Hemocyanin G-type, units OdHa-g [E.dofleini] | O61363 | 77%, E = 4e-04 | |||
| EYRYYWDWQER | 1693.78 | Hemocyanin 1; Keyhole limpet hemocyanin A (KLH-A) [M. crenulata] | Q10583 | 83%, E = 0.034 | |
| Hemocyanin G-type, units OdHa-g [E.dofleini] | |||||
| Hemocyanin, units g and h [Sepia officinalis] | O61363 | 100%, E = 0.099 | |||
| Hemocyanin 2-c chain; KLH2-c [M. crenulata] | P56826 | 100%, E = 0.100 | |||
| P81732 | 100%, E = 0.100 | ||||
| 3 | GHKKRLRK | 1022.68 | Hemocyanin 2-c chain; KLH2-c [M. crenulata], | P81732 | 63%, E = 3.7 |
| DEVVPNPFVR | 1171.61 | Hemocyanin 1; KLH-A [M. crenulata] | Q10583 | 75%, E = 0.057 | |
| Hemocyanin type 2 unit a; RvH2-a [R. venosa] | P80960 | 100%, E = 0.33 | |||
| Hemocyanin 2; KLH-B [M. crenulata] | Q10584 | 75%, E = 0.48 | |||
| VEITKALHKLGLR | 1477.92 | Hemocyanin 2-c, KLH2-c [M. crenulata] | P81732 | 64%, E = 0.29 | |
| YHRQEHRRWWKD | 1796.9 | Hemocyanin 1; KLH-A [M. crenulata] | Q10583 | 83%, E = 1.0 |
| BAS/Cells | MCF-10A | MCF-7 | BT-474 | SK-BR-3 | MDA-MB-468 | BT-549 | MDA-MB-231 |
|---|---|---|---|---|---|---|---|
| cis-Pt [µM] | 28 ± 1.8 | 19.6±2.5 | 19.3 ± 4.4 | 20.5 ± 2.1 | 3 ± 3.3 | 13.1±3.7 | 24 ± 3.1 |
| Tam [µM] | 26 ± 2.6 | 18±3.4 | 2.9 ± 4.4 | 9.3 ± 1.2 | 13.8 ± 4.1 | 9.8 ± 2.2 | 21 ± 2.4 |
| RvH1 [μg/mL] | 68 ± 3.9 | 63±4.1 | 67 ± 3.2 | 96 ± 2.3 | 79 ± 2.6 | 92 ± 3.6 | 72 ± 4.9 |
| RvH2 [μg/mL] | 204 ± 4.7 | 198±3.7 | 207 ± 3.4 | 210 ± 1.7 | 213 ± 3.7 | 218± 1.8 | 215 ± 5.1 |
| HRv 50–100kDa [μg/mL] | 47 ± 2.8 | 32 ±3.3 | 26.2 ± 2.7 | 74.5 ± 2.5 | 41.2 ± 4.6 | 80.4±4.1 | 38 ± 2.9 |
| BAS/Cells | MCF-10A | MCF-7 | MDA-MB-231 |
|---|---|---|---|
| cis-Pt [μM]+ RvH1 [IC25 = 23 μg/mL] | 29 ± 3.4 | 22 ± 4.3 | 24 ± 3.5 |
| cis-Pt [μM] + RvH2 [IC25 = 70 μg/mL] | 28 ± 2.8 | 32 ± 3.6 | 26 ± 3.5 |
| cis-Pt [μM] + HRv 50–100 kDa [IC25 = 13 μg/mL] | 13 ± 2.6 | 10 ± 3.3 | 9 ± 2.1 |
| Tam [μM]+ RvH1 [IC25 = 23 μg/mL] | 28 ± 3.7 | 25 ± 2.4 | 33 ± 1.8 |
| Tam [μM] + RvH2 [IC25 = 70 μg/mL] | 42 ± 3.8 | 48 ± 5.2 | 30 ± 4.4 |
| Tam [μM] + HRv 50–100 kDa [IC25 = 13 μg/mL] | 9 ± 2.4 | 6 ± 1.6 | 7 ± 2.2 |
| MDA-MB-231 | |||||||||
| Dose HRv 50–100 kDa (µg/mL) | Dose cis-Pt (µg/mL) | Fa | CI | Combined Effect | Dose HRv 50–100 kDa (µg/mL) | Dose Tam (µg/mL) | Fa | CI | Combined Effect |
| 12 | 1.5 | 0.30 | 0.92 | ± | 12 | 1.2 | 0.06 | 2.16 | --- |
| 12 | 3 | 0.48 | 0.86 | + | 12 | 2.4 | 0.26 | 1.32 | -- |
| 12 | 6 | 0.61 | 0.84 | ++ | 12 | 4.8 | 0.70 | 0.83 | ++ |
| 12 | 12 | 0.5 | 0.88 | + | 12 | 9.8 | 0.83 | 1 | ± |
| 12 | 24 | 0.86 | 1.67 | --- | 12 | 19.2 | 0.83 | 1.77 | --- |
| 12 | 48 | 0.96 | 1.72 | --- | 12 | 38 | 0.90 | 2.59 | --- |
| 12 | 95 | 1.00 | 0.62 | +++ | 12 | 77 | 1.00 | 0.59 | +++ |
| MCF-7 | |||||||||
| Dose HRv 50–100 kDa (µg/mL) | Dose cis-Pt (µg/mL) | Fa | CI | Combined Effect | Dose HRv 50–100 kDa (µg/mL) | Dose Tam (µg/mL) | Fa | CI | Combined Effect |
| 12 | 1.5 | 0.16 | 2.13 | --- | 12 | 1.2 | 0.26 | 1.36 | -- |
| 12 | 3 | 0.59 | 0.79 | ++ | 12 | 2.4 | 0.44 | 0.95 | ± |
| 12 | 6 | 0.79 | 0.69 | +++ | 12 | 4.8 | 0.60 | 0.83 | ++ |
| 12 | 12 | 0.98 | 0.33 | ++++ | 12 | 9.8 | 0.74 | 0.84 | ++ |
| 12 | 24 | 0.99 | 0.39 | +++ | 12 | 19.2 | 0.80 | 1.12 | - |
| 12 | 48 | 1.00 | 0.38 | +++ | 12 | 38 | 0.84 | 1.74 | --- |
| 12 | 95 | 1.00 | 0.71 | ++ | 12 | 77 | 0.96 | 1.09 | - |
| 12 | 190 | 1.00 | 0.93 | ++ | 12 | 154 | 1.00 | 0.15 | ++++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, M.; Vlahova, Z.; Schröder, M.; Todorova, J.; Tzintzarov, A.; Gospodinov, A.; Velkova, L.; Kaynarov, D.; Dolashki, A.; Dolashka, P.; et al. Antitumor Activity of Bioactive Compounds from Rapana venosa against Human Breast Cell Lines. Pharmaceuticals 2023, 16, 181. https://doi.org/10.3390/ph16020181
Petrova M, Vlahova Z, Schröder M, Todorova J, Tzintzarov A, Gospodinov A, Velkova L, Kaynarov D, Dolashki A, Dolashka P, et al. Antitumor Activity of Bioactive Compounds from Rapana venosa against Human Breast Cell Lines. Pharmaceuticals. 2023; 16(2):181. https://doi.org/10.3390/ph16020181
Chicago/Turabian StylePetrova, Maria, Zlatina Vlahova, Maria Schröder, Jordana Todorova, Alexander Tzintzarov, Anastas Gospodinov, Lyudmila Velkova, Dimitar Kaynarov, Aleksandar Dolashki, Pavlina Dolashka, and et al. 2023. "Antitumor Activity of Bioactive Compounds from Rapana venosa against Human Breast Cell Lines" Pharmaceuticals 16, no. 2: 181. https://doi.org/10.3390/ph16020181
APA StylePetrova, M., Vlahova, Z., Schröder, M., Todorova, J., Tzintzarov, A., Gospodinov, A., Velkova, L., Kaynarov, D., Dolashki, A., Dolashka, P., & Ugrinova, I. (2023). Antitumor Activity of Bioactive Compounds from Rapana venosa against Human Breast Cell Lines. Pharmaceuticals, 16(2), 181. https://doi.org/10.3390/ph16020181