Isoxazole/Isoxazoline Skeleton in the Structural Modification of Natural Products: A Review
Abstract
1. Introduction
2. Biological Effects of Natural Products Containing the Isoxazole/Isoxazoline Moiety
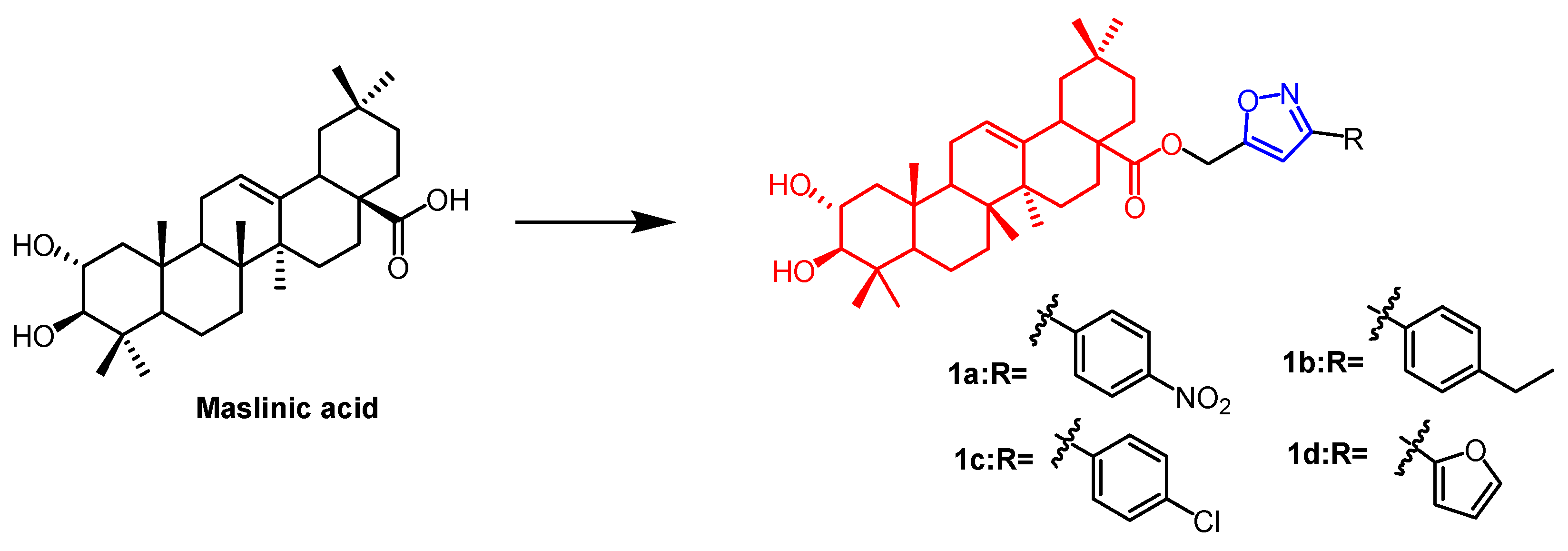
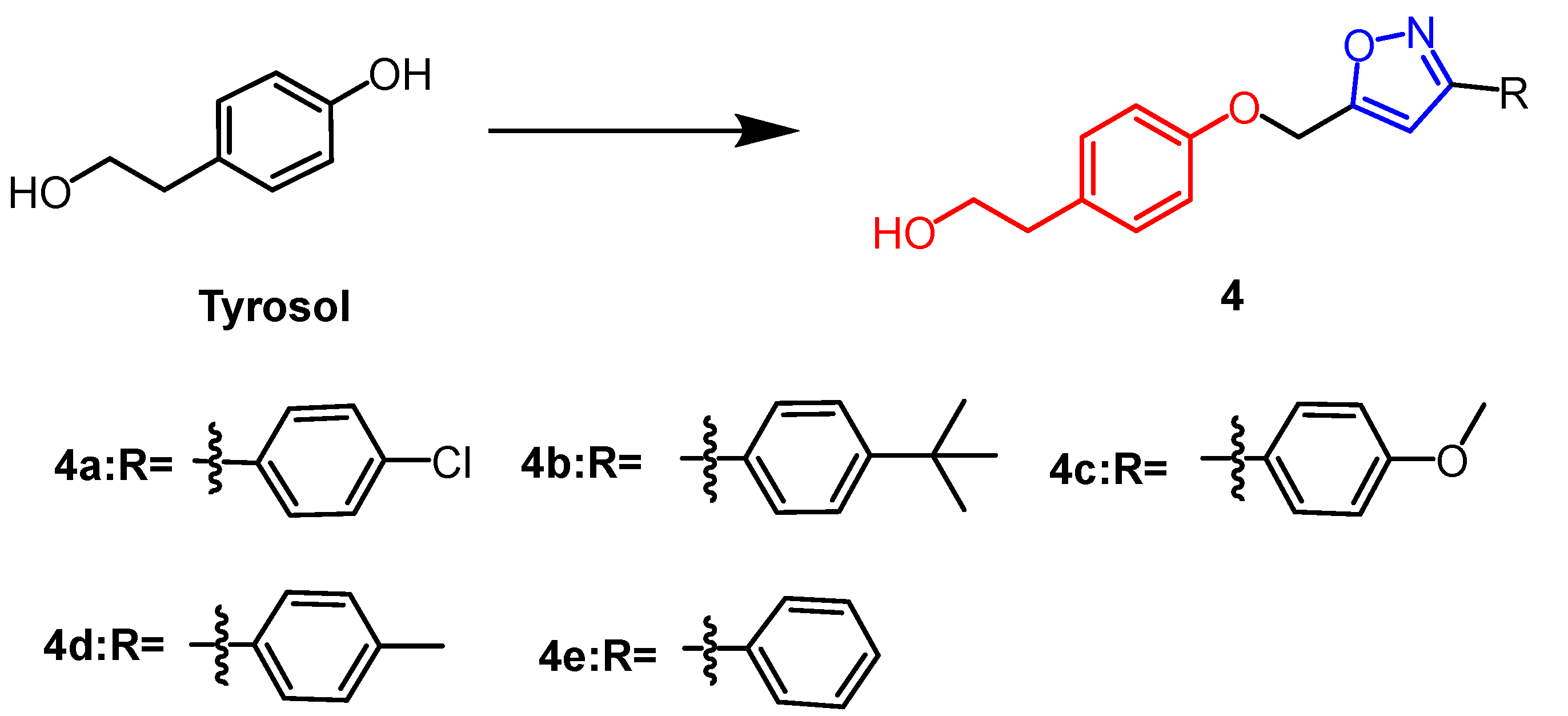
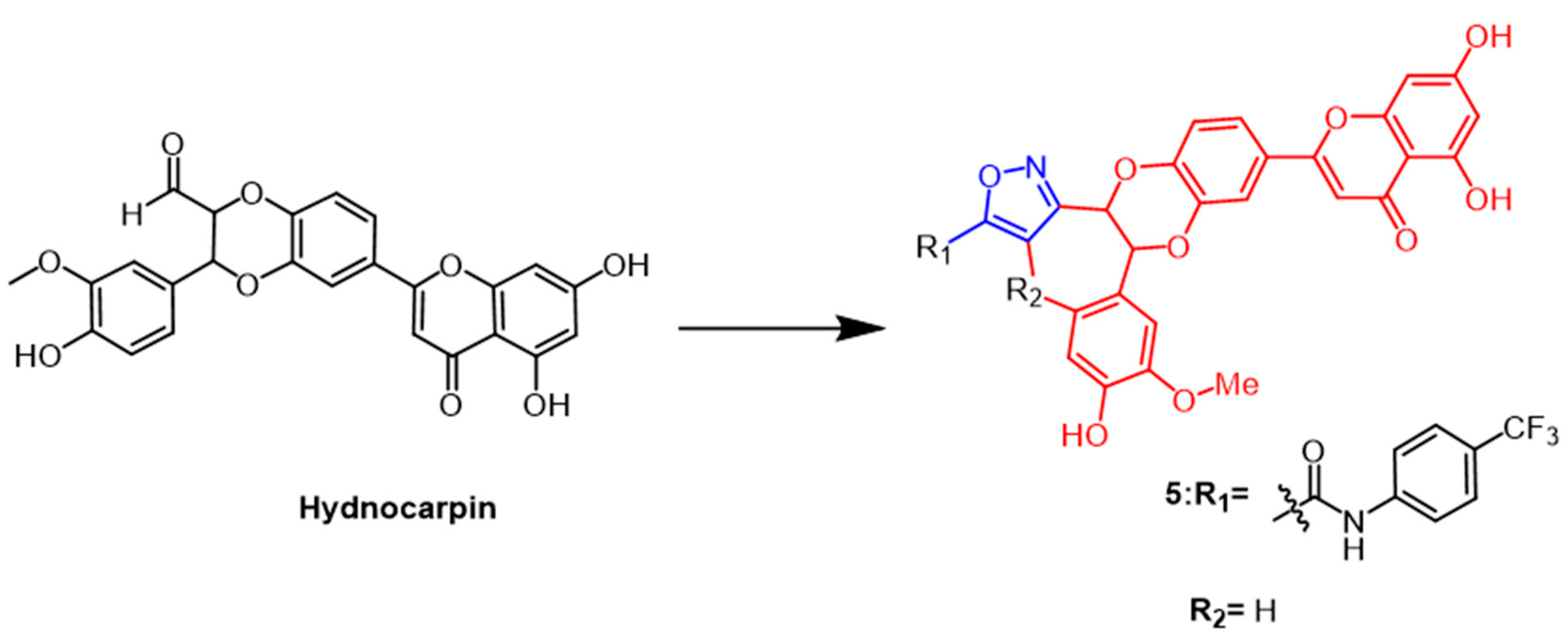
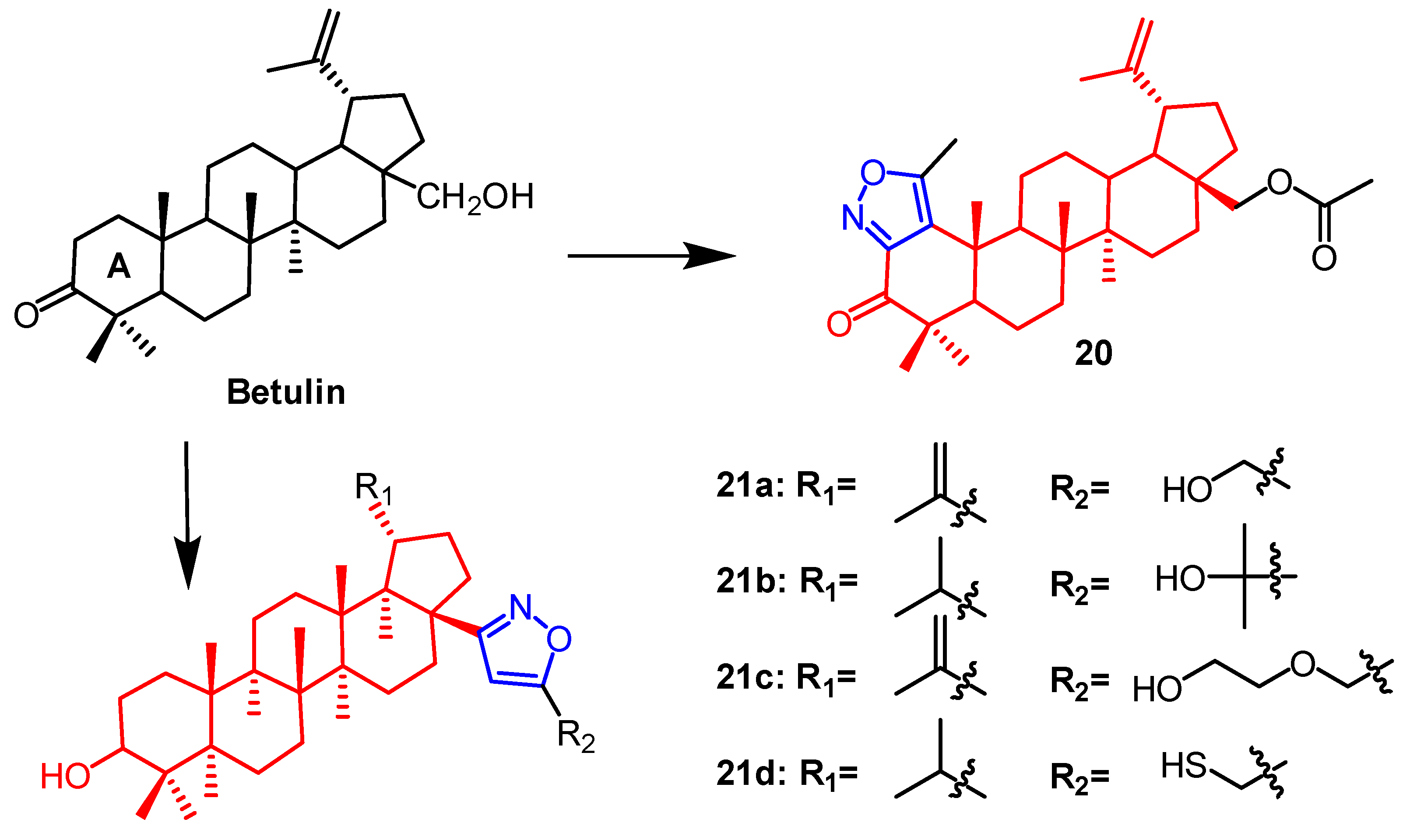
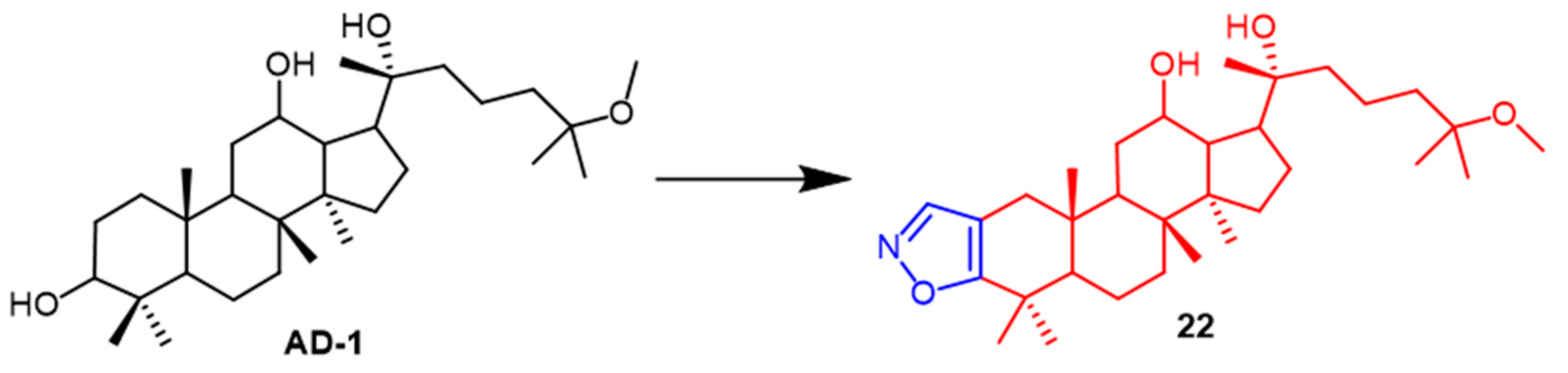
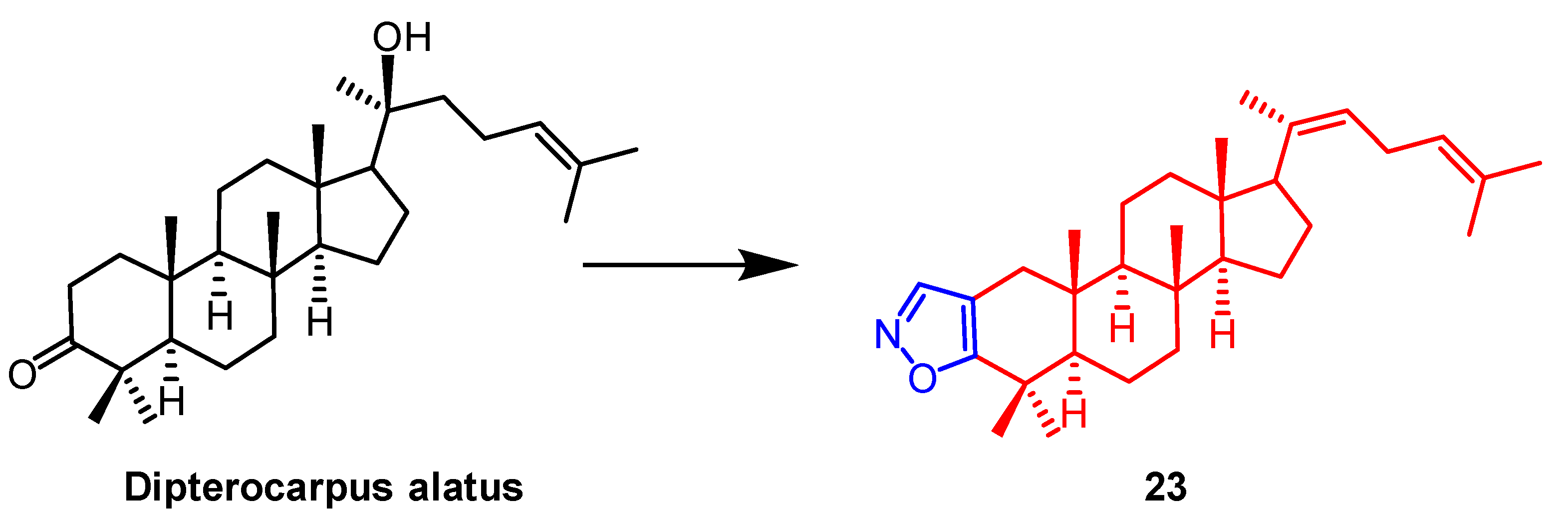
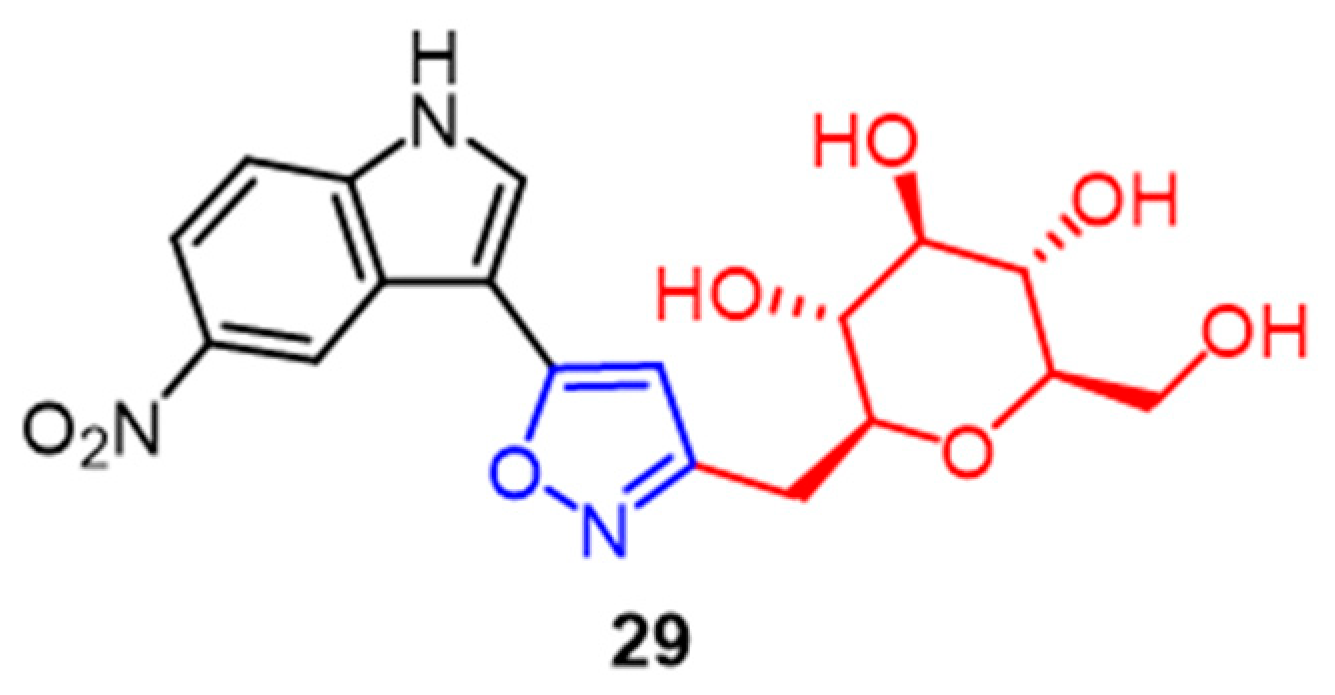
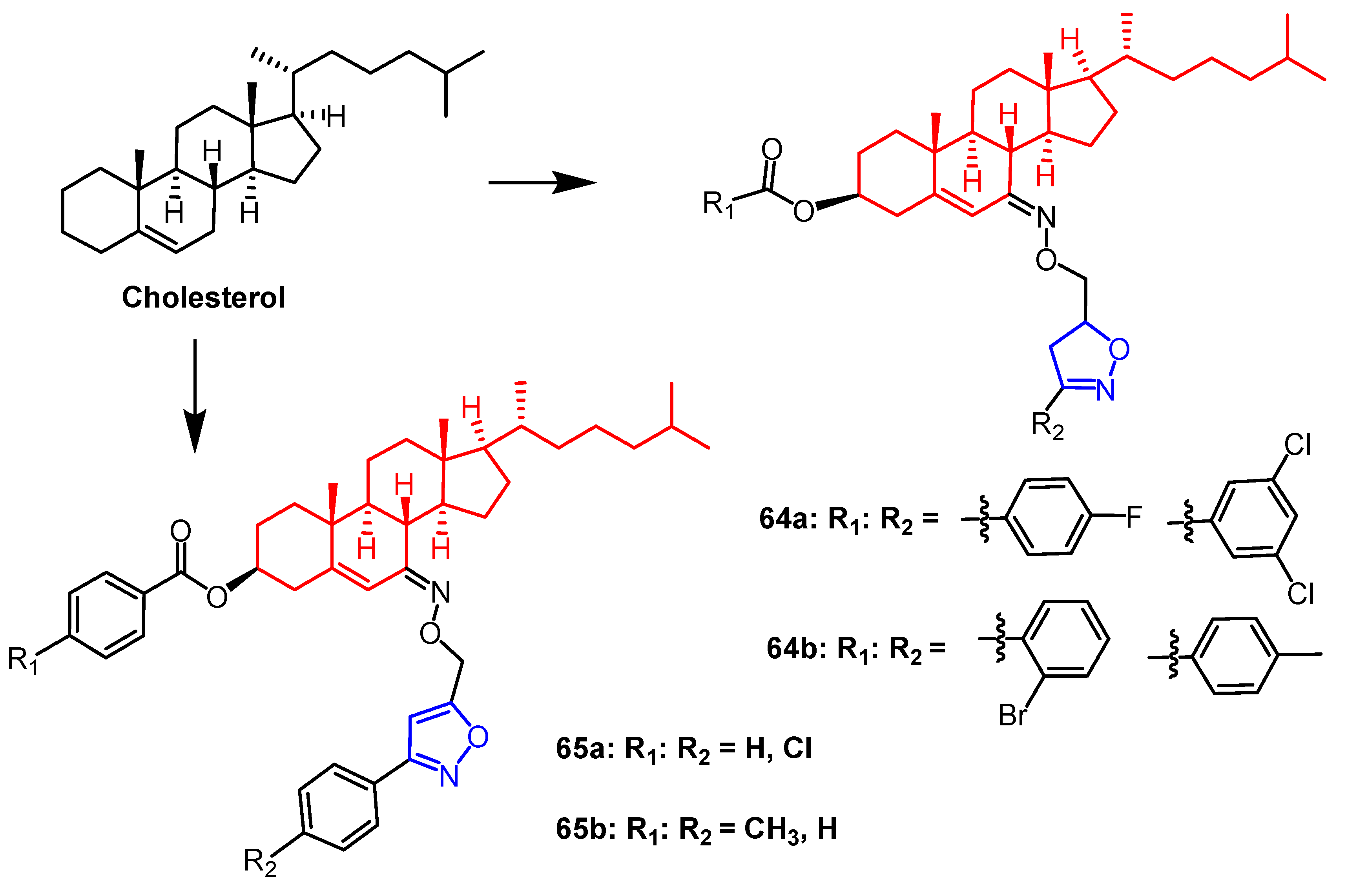
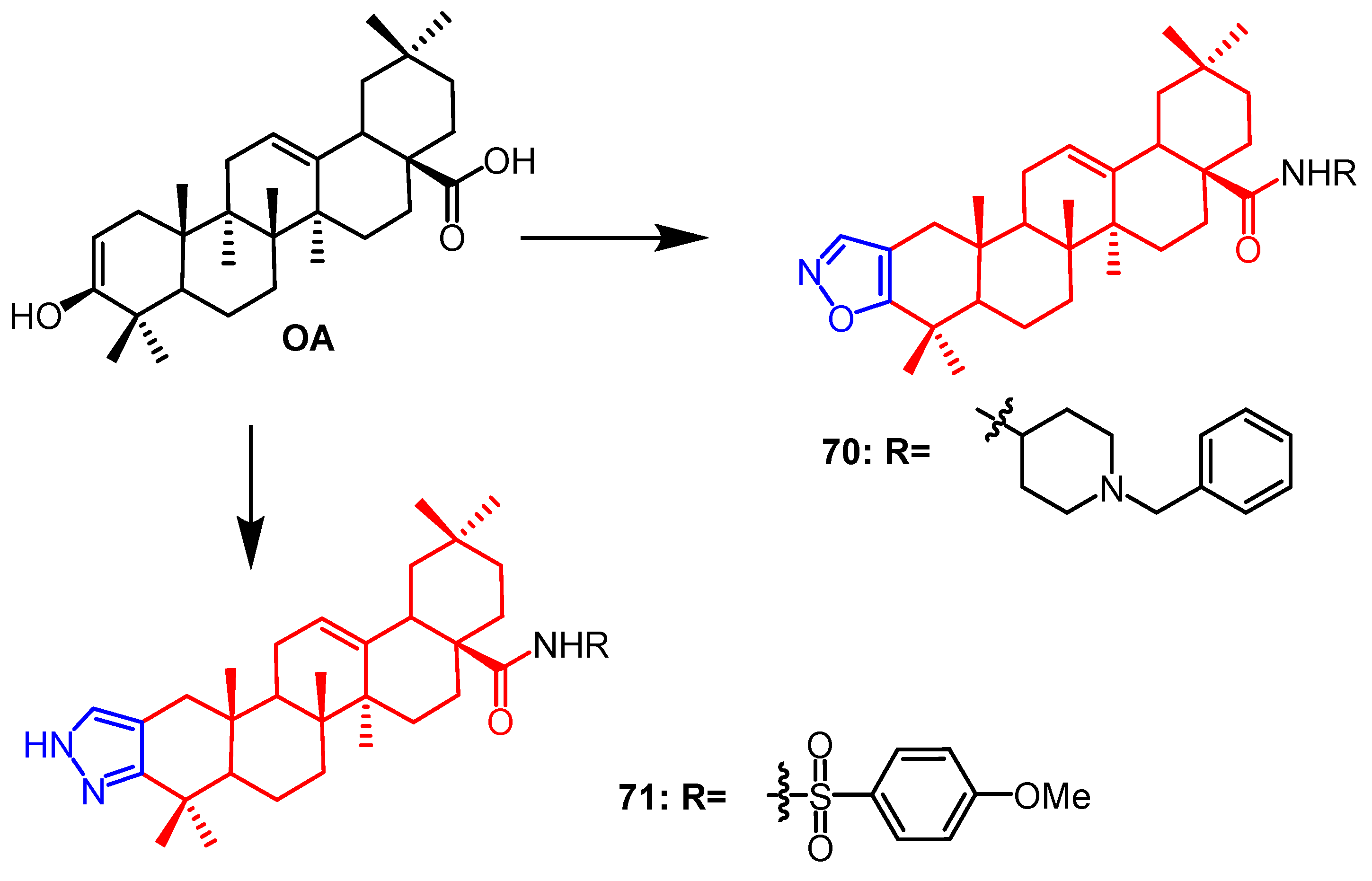
2.1. Antitumour Activity
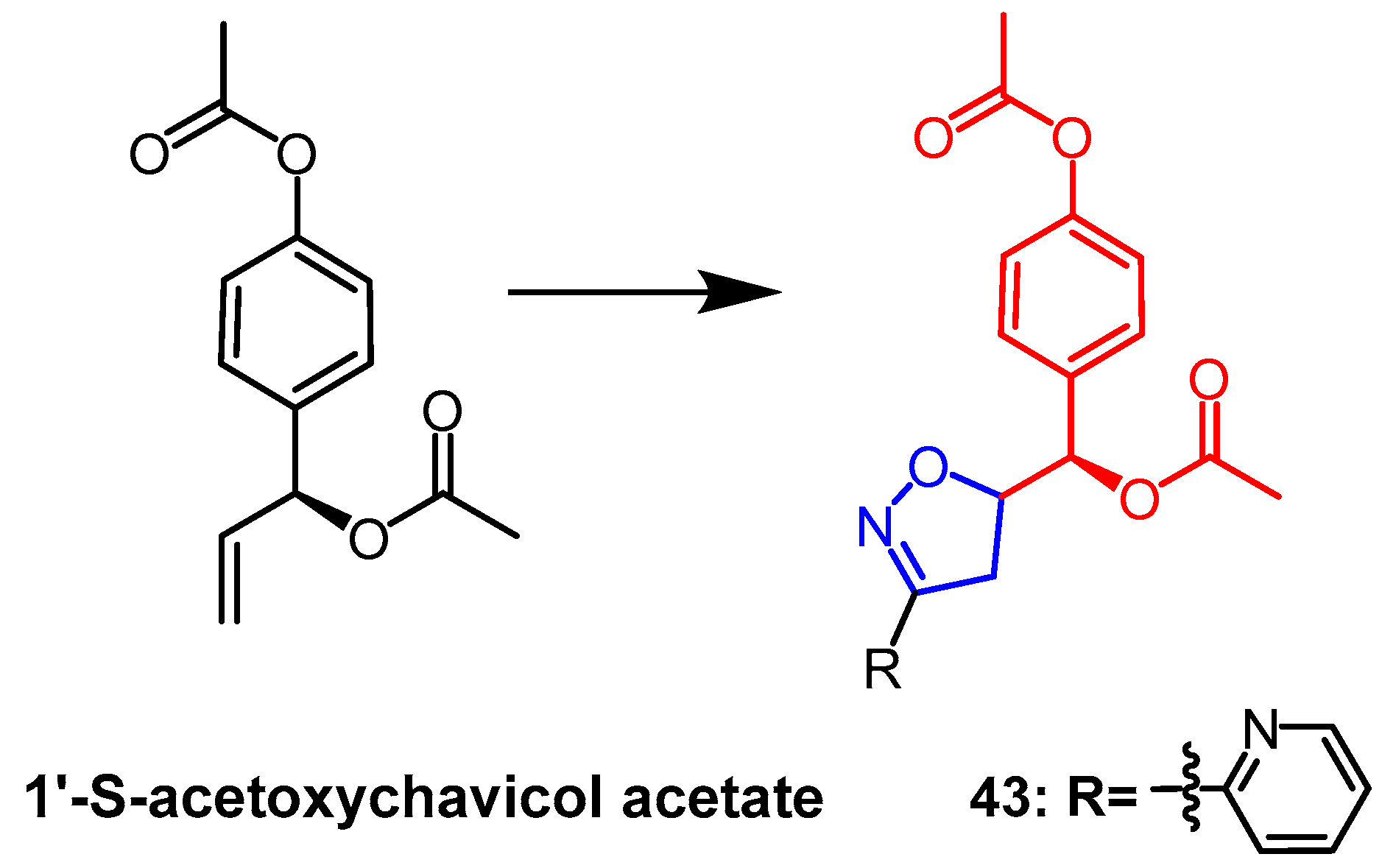
2.2. Antibacterial Activity
2.3. Anti-Diabetic Activity
2.4. Anti-Inflammatory Activity
2.5. Insecticidal Activity
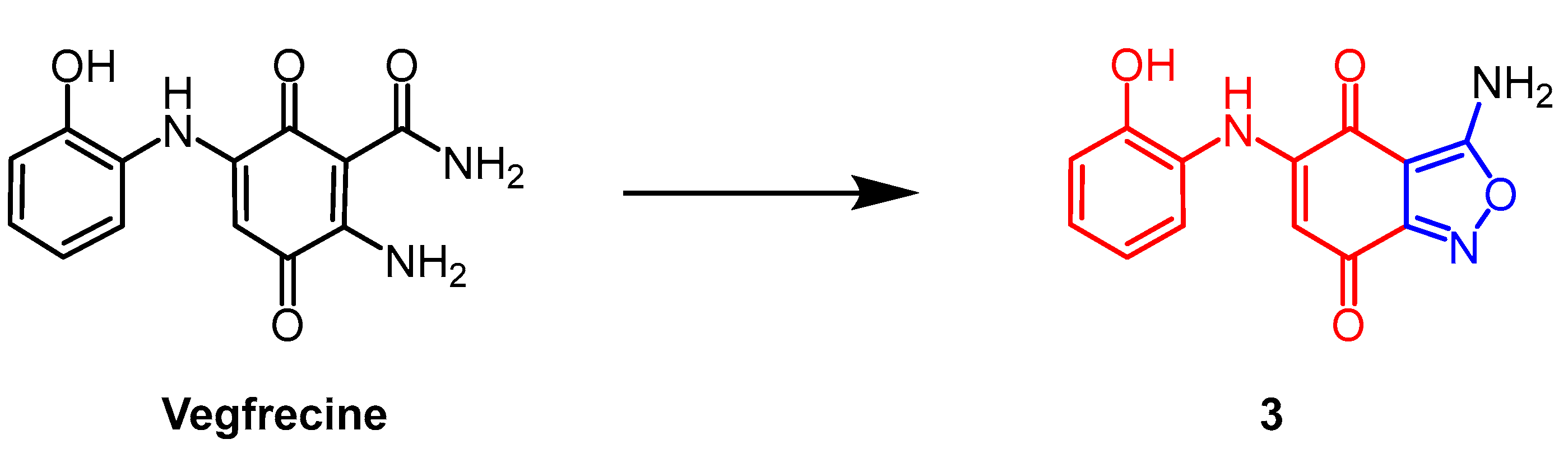
2.6. Other Biological Activity
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, G.; Shankar, R. 2-Isoxazolines: A Synthetic and Medicinal Overview. Chemmedchem 2021, 16, 430–447. [Google Scholar] [CrossRef]
- Pandhurnekar, C.P.; Pandhurnekar, H.C.; Mungole, A.J.; Butoliya, S.S.; Yadao, B.G. A review of recent synthetic strategies and biological activities of isoxazole. J. Heterocycl. Chem. 2022, 60, 1–29. [Google Scholar] [CrossRef]
- Tilvi, S.; Singh, K.S. Synthesis of Oxazole, Oxazoline and Isoxazoline Derived Marine Natural Products: A Review. Curr. Org. Chem. 2016, 20, 898–929. [Google Scholar] [CrossRef]
- Arya, G.C.; Kaur, K.; Jaitak, V. Isoxazole derivatives as anticancer agent: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2021, 221, 113511. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, V.; Sharma, A.K.; Gupta, G.K. Isoxazoline containing natural products as anticancer agents: A review. Eur. J. Med. Chem. 2014, 77, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A.; El-Shazly, M. Synthesis of New Isoxazole-, Pyridazine-, Pyrimidopyrazines and their Anti-Inflammatory and Analgesic Activity. Med. Chem. 2018, 14, 356–371. [Google Scholar] [CrossRef]
- Mota, F.V.B.; Neta, M.S.D.; Franco, E.D.; Bastos, I.; da Araujo, L.C.C.; da Silva, S.C.; de Oliveira, T.B.; Souza, E.K.; de Almeida, V.M.; Ximenes, R.M.; et al. Evaluation of anti-inflammatory activity and molecular docking study of new aza-bicyclic isoxazoline acylhydrazone derivatives. Medchemcomm 2019, 10, 1916–1925. [Google Scholar] [CrossRef]
- Aarjane, M.; Slassi, S.; Ghaleb, A.; Tazi, B.; Amine, A. Synthesis, biological evaluation, molecular docking and in silico ADMET screening studies of novel isoxazoline derivatives from acridone. Arab. J. Chem. 2021, 14, 103057. [Google Scholar] [CrossRef]
- Shaik, A.; Bhandare, R.R.; Palleapati, K.; Nissankararao, S.; Kancharlapalli, V.; Shaik, S. Antimicrobial, Antioxidant, and Anticancer Activities of Some Novel Isoxazole Ring Containing Chalcone and Dihydropyrazole Derivatives. Molecules 2020, 25, 1047. [Google Scholar] [CrossRef]
- Rastegari, A.; Safavi, M.; Vafadarnejad, F.; Najafi, Z.; Hariri, R.; Bukhari, S.N.A.; Iraji, A.; Edraki, N.; Firuzi, O.; Saeedi, M.; et al. Synthesis and evaluation of novel arylisoxazoles linked to tacrine moiety: In vitro in vivo biological activities against Alzheimer’s disease. Mol. Divers. 2022, 26, 409–428. [Google Scholar] [CrossRef]
- Patil, P.; Thakur, A.; Sharma, A.; Flora, S.J.S. Natural products and their derivatives as multifunctional ligands against Alzheimer’s disease. Drug Dev. Res. 2020, 81, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Eryilmaz, S. Synthesis, Antioxidant Activity and Theoretical Investigation of Isoxazolines Derivatives of Monoterpenoids. Lett. Org. Chem. 2019, 16, 501–510. [Google Scholar] [CrossRef]
- Pothuri, V.V.; Machiraju, P.V.S.; Rao, V.S.S. Synthesis and Biological Activity of Some Novel Derivatives of 4-[5-(2,3-Dihydrobenzo[b][1,4]dioxin-7-yl)isoxazole-3-yl]benzoic Acid. Russ. J. Gen. Chem. 2020, 90, 889–894. [Google Scholar] [CrossRef]
- Huang, S.S.; Zhu, B.B.; Wang, K.H.; Yu, M.; Wang, Z.W.; Li, Y.Q.; Liu, Y.X.; Zhang, P.L.; Li, S.J.; Li, Y.L.; et al. Design, synthesis, and insecticidal and fungicidal activities of quaternary ammonium salt derivatives of a triazolyphenyl isoxazoline insecticide. Pest Manag. Sci. 2022, 78, 2011–2021. [Google Scholar] [CrossRef]
- Trefzger, O.S.; Barbosa, N.V.; Scapolatempo, R.L.; das Neves, A.R.; Ortale, M.; Carvalho, D.B.; Honorato, A.M.; Fragoso, M.R.; Shuiguemoto, C.Y.K.; Perdomo, R.T.; et al. Design, synthesis, antileishmanial, and antifungal biological evaluation of novel 3,5-disubstituted isoxazole compounds based on 5-nitrofuran scaffolds. Arch. Der Pharm. 2020, 353, 1900241. [Google Scholar] [CrossRef]
- Zhang, T.; Dong, M.Y.; Zhao, J.J.; Zhang, X.F.; Mei, X.D. Synthesis and antifungal activity of novel pyrazolines and isoxazolines derived from cuminaldehyde. J. Pestic. Sci. 2019, 44, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, C.X.; Shi, W.; Cai, X.G.; Dai, Y.X.; Liao, C.; Huang, W.L.; Qian, H. Identification of highly potent and orally available free fatty acid receptor 1 agonists bearing isoxazole scaffold. Bioorganic Med. Chem. 2018, 26, 703–711. [Google Scholar] [CrossRef]
- Fettach, S.; Thari, F.Z.; Hafidi, Z.; Karrouchi, K.; Bouathmany, K.; Cherrah, Y.; El Achouri, M.; Benbacer, L.; El Mzibri, M.; Sefrioui, H.; et al. Biological, toxicological and molecular docking evaluations of isoxazoline-thiazolidine-2,4-dione analogues as new class of anti-hyperglycemic agents. J. Biomol. Struct. Dyn. 2021, 12, 1072–1084. [Google Scholar] [CrossRef]
- Sysak, A.; Obminska-Mrukowicz, B. Isoxazole ring as a useful scaffold in a search for new therapeutic agents. Eur. J. Med. Chem. 2017, 137, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Tugrak, M.; Gul, H.I.; Bandow, K.; Sakagami, H.; Gulcin, I.; Ozkay, Y.; Supuran, C.T. Synthesis and biological evaluation of some new mono Mannich bases with piperazines as possible anticancer agents and carbonic anhydrase inhibitors. Bioorganic Chem. 2019, 90, 103095. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Bendi, A.; Singh, L. A Study on Synthesis of Chalcone Derived-5-Membered Isoxazoline and Isoxazole Scaffolds. Curr. Org. Synth. 2022, 19, 643–663. [Google Scholar] [PubMed]
- Chopra, B.; Dhingra, A.K. Natural products: A lead for drug discovery and development. Phytother. Res. 2021, 35, 4660–4702. [Google Scholar] [CrossRef]
- Yao, H.; Liu, J.K.; Xu, S.T.; Zhu, Z.Y.; Xu, J.Y. The structural modification of natural products for novel drug discovery. Expert Opin. Drug Discov. 2017, 12, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Ngaini, Z. Synthesis of Benzalacetophenone-based Isoxazoline and Isoxazole Derivatives. Curr. Org. Chem. 2022, 26, 679–692. [Google Scholar] [CrossRef]
- Wang, H.B.; He, Y.; Jian, M.L.; Fu, X.G.; Cheng, Y.H.; He, Y.J.; Fang, J.; Li, L.; Zhang, D. Breaking the Bottleneck in Anticancer Drug Development: Efficient Utilization of Synthetic Biology. Molecules 2022, 27, 7480. [Google Scholar] [CrossRef]
- Chouiab, K.; Romdhane, A.; Delemasure, S.; Dutartre, P.; Elie, N.; Touboul, D.; Ben Jannet, H.; Hamza, M.A. Regiospecific synthesis, anti-inflammatory and anticancer evaluation of novel 3,5-disubstituted isoxazoles from the natural maslinic and oleanolic acids. Ind. Crops Prod. 2016, 85, 287–299. [Google Scholar] [CrossRef]
- Mallavadhani, U.V.; Vanga, N.R.; Jeengar, M.K.; Naidu, V.G.M. Synthesis of novel ring-A fused hybrids of oleanolic acid with capabilities to arrest cell cycle and induce apoptosis in breast cancer cells. Eur. J. Med. Chem. 2014, 74, 398–404. [Google Scholar] [CrossRef]
- Adachi, H.; Nosaka, C.; Atsumi, S.; Nakae, K.; Umezawa, Y.; Sawa, R.; Kubota, Y.; Nakane, C.; Shibuya, M.; Nishimura, Y. Structure-activity relationships of natural quinone vegfrecine analogs with potent activity against VEGFR-1 and-2 tyrosine kinases. J. Antibiot. 2021, 74, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Aissa, I.; Abdelkafi-Koubaa, Z.; Chouaib, K.; Jalouli, M.; Assel, A.; Romdhane, A.; Harrath, A.H.; Marrakchi, N.; Ben Jannet, H. Glioblastoma-specific anticancer activity of newly synthetized 3,5-disubstituted isoxazole and 1,4-disubstituted triazole-linked tyrosol conjugates. Bioorg. Chem. 2021, 114, 105071. [Google Scholar] [CrossRef]
- Mathai, B.M.; Joseph, M.M.; Maniganda, S.; Nair, J.B.; Arya, J.S.; Karunakaran, V.; Radhakrishnan, K.V.; Maiti, K.K. Guanidinium rich dendron-appended hydnocarpin exhibits superior anti-neoplastic effects through caspase mediated apoptosis. RSC Adv. 2016, 6, 52772–52780. [Google Scholar] [CrossRef]
- Arya, J.S.; Joseph, M.M.; Sherin, D.R.; Nair, J.B.; Manojkumar, T.K.; Maiti, K.K. Exploring Mitochondria-Mediated Intrinsic Apoptosis by New Phytochemical Entities: An Explicit Observation of Cytochrome c Dynamics on Lung and Melanoma Cancer Cells. J. Med. Chem. 2019, 62, 8311–8329. [Google Scholar] [CrossRef]
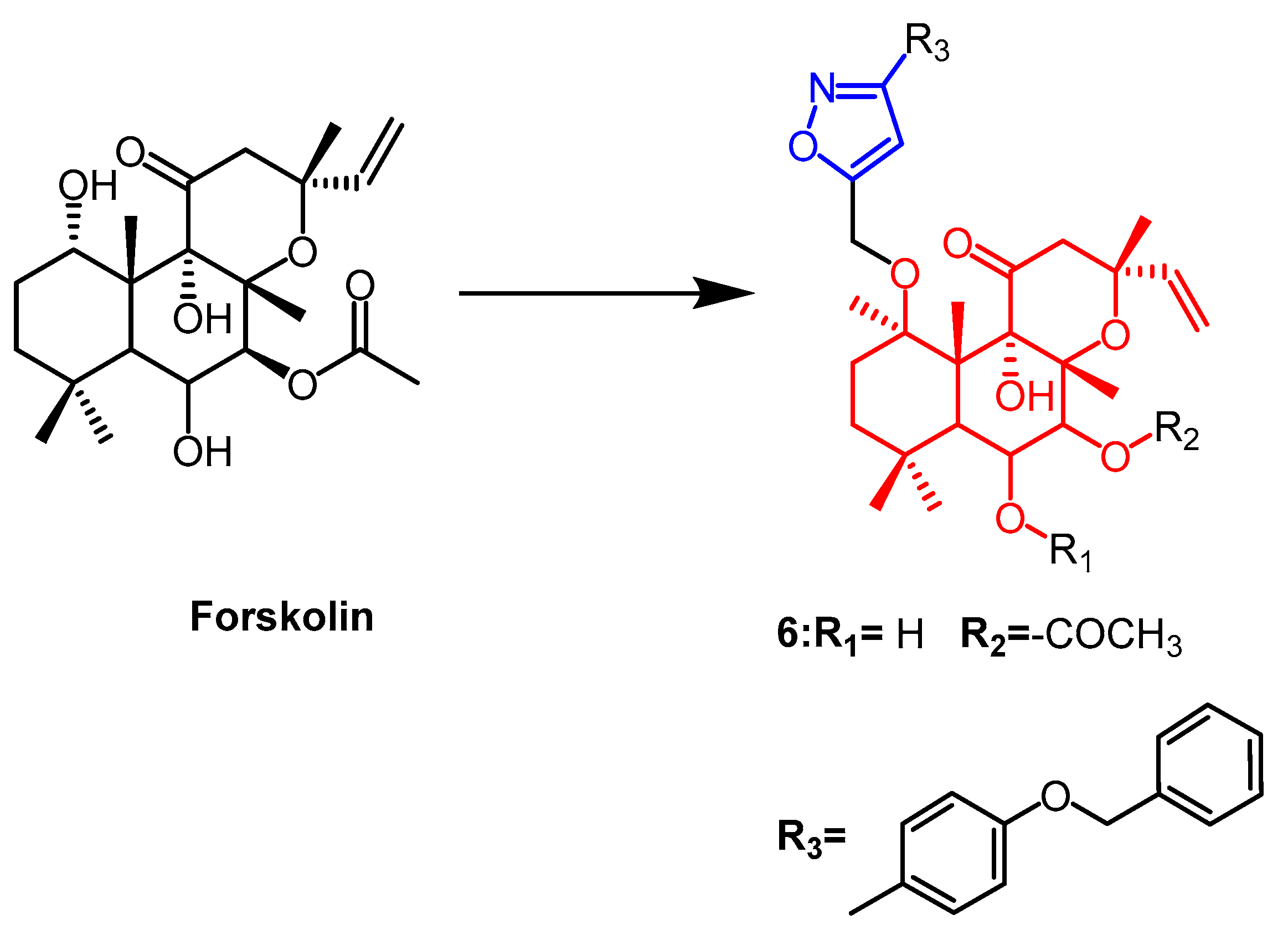
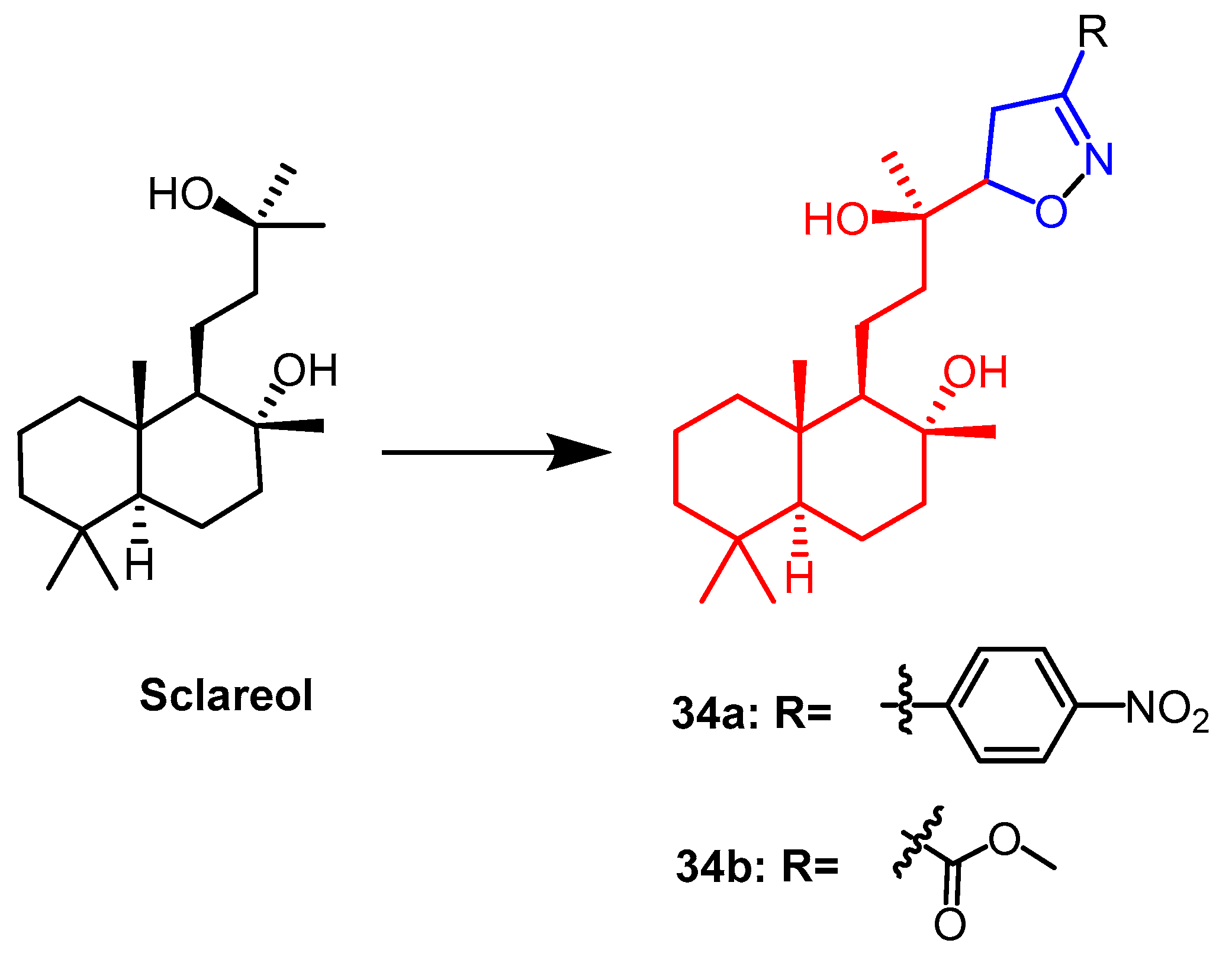
- Burra, S.; Voora, V.; Rao, C.P.; Vijay Kumar, P.; Kancha, R.K.; David Krupadanam, G.L. Synthesis of novel forskolin isoxazole derivatives with potent anti-cancer activity against breast cancer cell lines. Bioorg. Med. Chem. Lett. 2017, 27, 4314–4318. [Google Scholar] [CrossRef] [PubMed]
- Chernysheva, N.B.; Maksimenko, A.S.; Andreyanov, F.A.; Kislyi, V.P.; Strelenko, Y.A.; Khrustalev, V.N.; Semenova, M.N.; Semenov, V.V. Regioselective synthesis of 3,4-diaryl-5-unsubstituted isoxazoles, analogues of natural cytostatic combretastatin A4. Eur. J. Med. Chem. 2018, 146, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Semenova, M.N.; Demchuk, D.V.; Tsyganov, D.V.; Chernysheya, N.B.; Samet, A.V.; Silyanova, E.A.; Kislyi, V.P.; Maksimenko, A.S.; Varakutin, A.E.; Konyushkin, L.D.; et al. Sea Urchin Embryo Model As a Reliable in Vivo Phenotypic Screen to Characterize Selective Antimitotic Molecules. Comparative evaluation of Combretapyrazoles, -isoxazoles,-1,2,3-triazoles, and -pyrroles as Tubulin-Binding Agents. ACS Comb. Sci. 2018, 20, 700–721. [Google Scholar] [CrossRef]
- Silyanova, E.A.; Ushkarov, V.I.; Samet, A.V.; Maksimenko, A.S.; Koblov, I.A.; Kislyi, V.P.; Semenova, M.N.; Semenov, V.V. A comparative evaluation of monomethoxy substituted o-diarylazoles as antiproliferative microtubule destabilizing agents. Mendeleev Commun. 2022, 32, 120–122. [Google Scholar] [CrossRef]
- Thiriveedhi, A.; Nadh, R.V.; Srinivasu, N.; Kaushal, K. Novel Hybrid Molecules of Isoxazole Chalcone Derivatives: Synthesis and Study of In Vitro Cytotoxic Activities. Lett. Drug Des. Discov. 2018, 15, 576–582. [Google Scholar] [CrossRef]
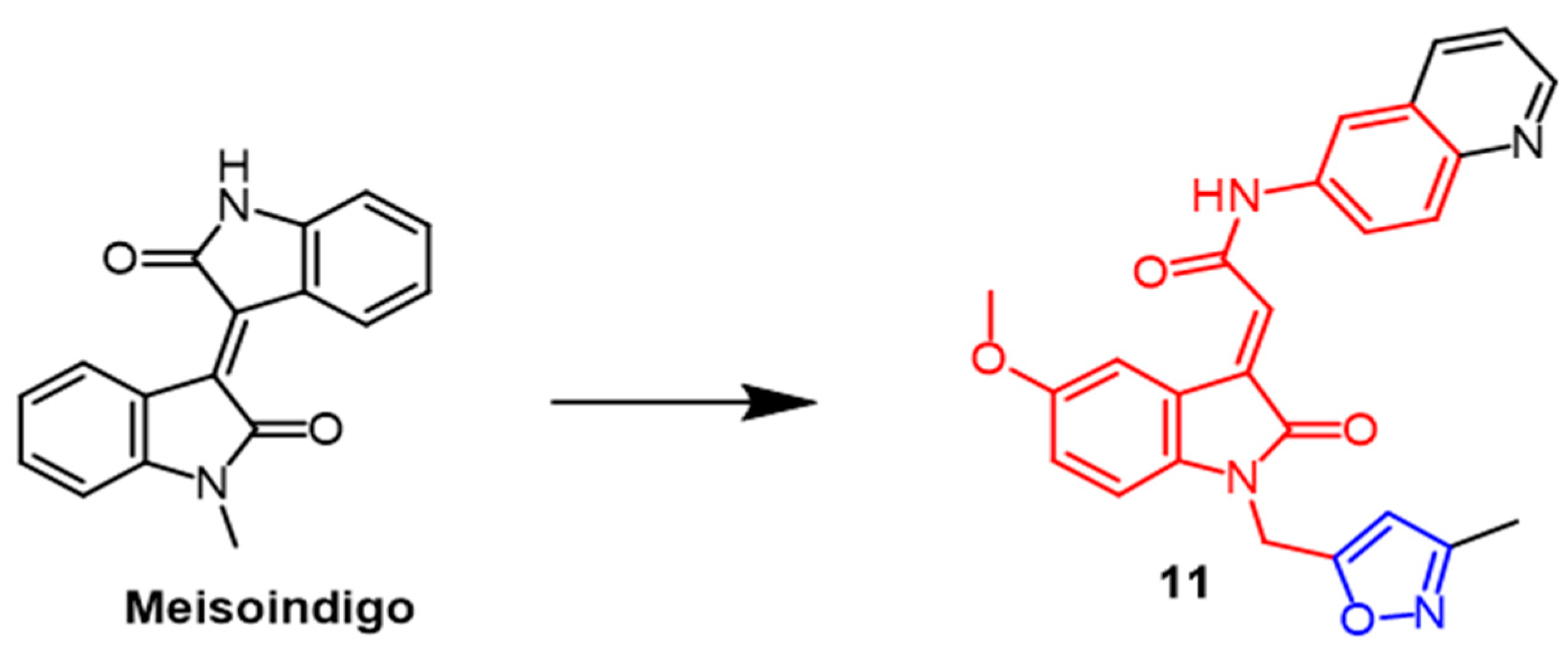
- Chiou, C.-T.; Lee, W.-C.; Liao, J.-H.; Cheng, J.-J.; Lin, L.-C.; Chen, C.-Y.; Song, J.-S.; Wu, M.-H.; Shia, K.-S.; Li, W.-T. Synthesis and evaluation of 3-ylideneoxindole acetamides as potent anticancer agents. Eur. J. Med. Chem. 2015, 98, 1–12. [Google Scholar] [CrossRef]
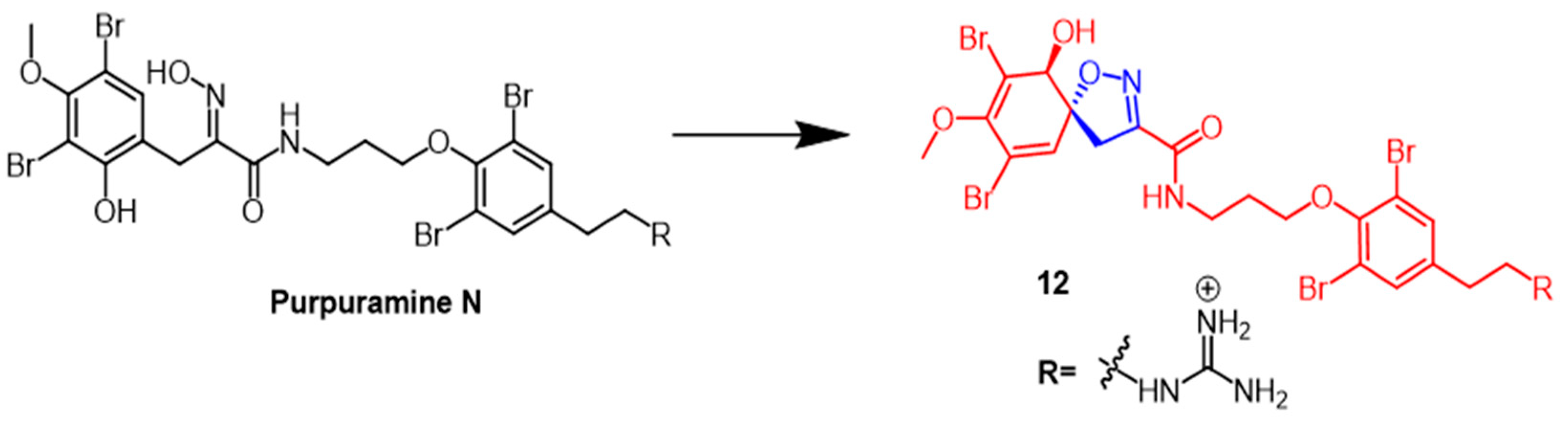
- Dai, J.; Parrish, S.M.; Yoshida, W.Y.; Yip, M.L.R.; Turkson, J.; Kelly, M.; Williams, P. Bromotyrosine-derived metabolites from an Indonesian marine sponge in the family Aplysinellidae (Order Verongiida). Bioorg. Med. Chem. Lett. 2016, 26, 499–504. [Google Scholar] [CrossRef]
- Diana, P.; Carbone, A.; Barraja, P.; Kelter, G.; Fiebig, H.-H.; Cirrincione, G. Synthesis and antitumor activity of 2,5-bis(3′-indolyl)-furans and 3,5-bis(3′-indolyl)-isoxazoles, nortopsentin analogues. Bioorg. Med. Chem. 2010, 18, 4524–4529. [Google Scholar] [CrossRef]
- Fawzi, M.; Oubella, A.; Bimoussa, A.; Bamou, F.Z.; Khdar, Z.A.; Auhmani, A.; Riahi, A.; Robert, A.; Morjani, H.; Itto, M.Y.A. Design, synthesis, evaluation of new 3-acetylisoxazolines and their hybrid analogous as anticancer agents: In vitro and in silico analysis. Comput. Biol. Chem. 2022, 98, 107666. [Google Scholar] [CrossRef]
- Oubella, A.; Ait Itto, M.Y.; Auhmani, A.; Riahi, A.; Robert, A.; Daran, J.-C.; Morjani, H.; Parish, C.A.; Esseffar, M.H. Diastereoselective synthesis and cytotoxic evaluation of new isoxazoles and pyrazoles with monoterpenic skeleton. J. Mol. Struct. 2019, 1198, 126924. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Wang, C.C.; Huang, M.Z.; Tao, Z.H.; Yan, W.J.; Du, Y.Q. Naturally Occurring Sesquiterpene Lactone-Santonin, Exerts Anticancer Effects in Multi-Drug Resistant Breast Cancer Cells by Inducing Mitochondrial Mediated Apoptosis, Caspase Activation, Cell Cycle Arrest, and by Targeting Ras/Raf/MEK/ERK Signaling Pathway. Med. Sci. Monit. 2019, 25, 3676–3682. [Google Scholar]
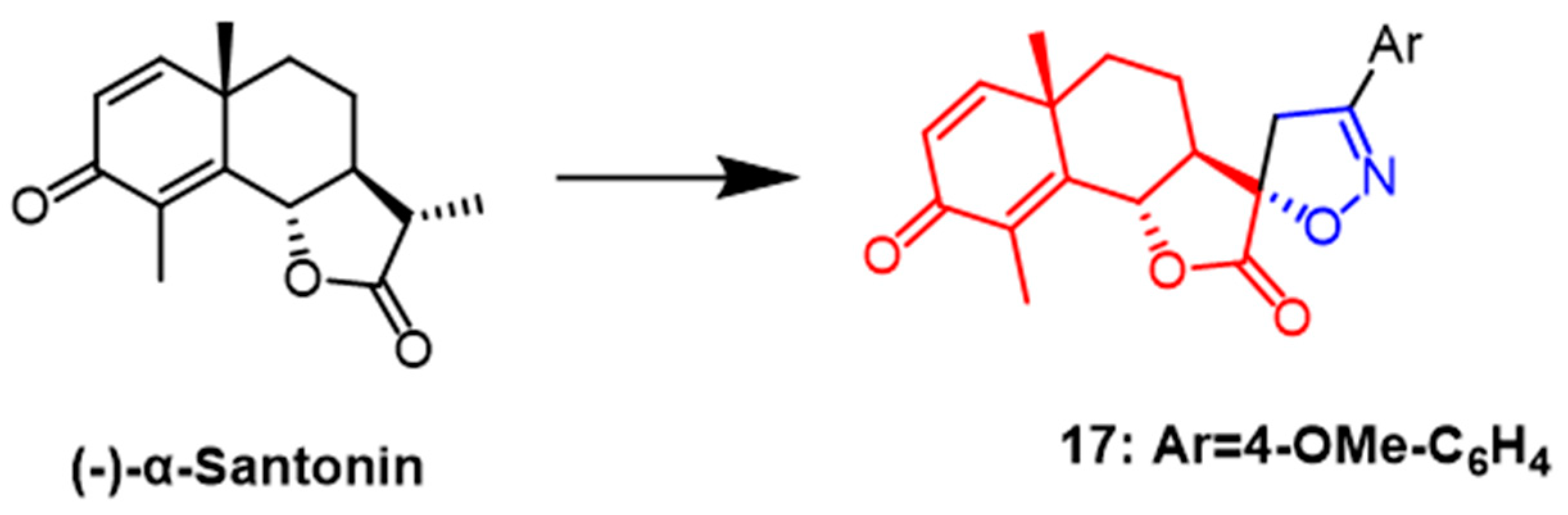
- Khazir, J.; Singh, P.P.; Reddy, D.M.; Hyder, I.; Shafi, S.; Sawant, S.D.; Chashoo, G.; Mahajan, A.; Alam, M.S.; Saxena, A.K.; et al. Synthesis and anticancer activity of novel spiro-isoxazoline and spiro-isoxazolidine derivatives of α-santonin. Eur. J. Med. Chem. 2013, 63, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Ock, C.W.; Kim, G.D. Harmine Hydrochloride Mediates the Induction of G2/M Cell Cycle Arrest in Breast Cancer Cells by Regulating the MAPKs and AKT/FOXO3a Signaling Pathways. Molecules 2021, 26, 6714. [Google Scholar] [CrossRef]
- Filali, I.; Bouajila, J.; Znati, M.; Bousejra-El Garah, F.; Ben Jannet, H. Synthesis of new isoxazoline derivatives from harmine and evaluation of their anti-Alzheimer, anti-cancer and anti-inflammatory activities. J. Enzyme Inhib. Med. Chem. 2015, 30, 371–376. [Google Scholar] [CrossRef]
- Filali, I.; Romdhane, A.; Znati, M.; Jannet, H.B.; Bouajila, J. Synthesis of New Harmine Isoxazoles and Evaluation of their Potential Anti-Alzheimer, Anti-inflammatory, and Anticancer Activities. Med. Chem. 2016, 12, 184–190. [Google Scholar] [CrossRef]
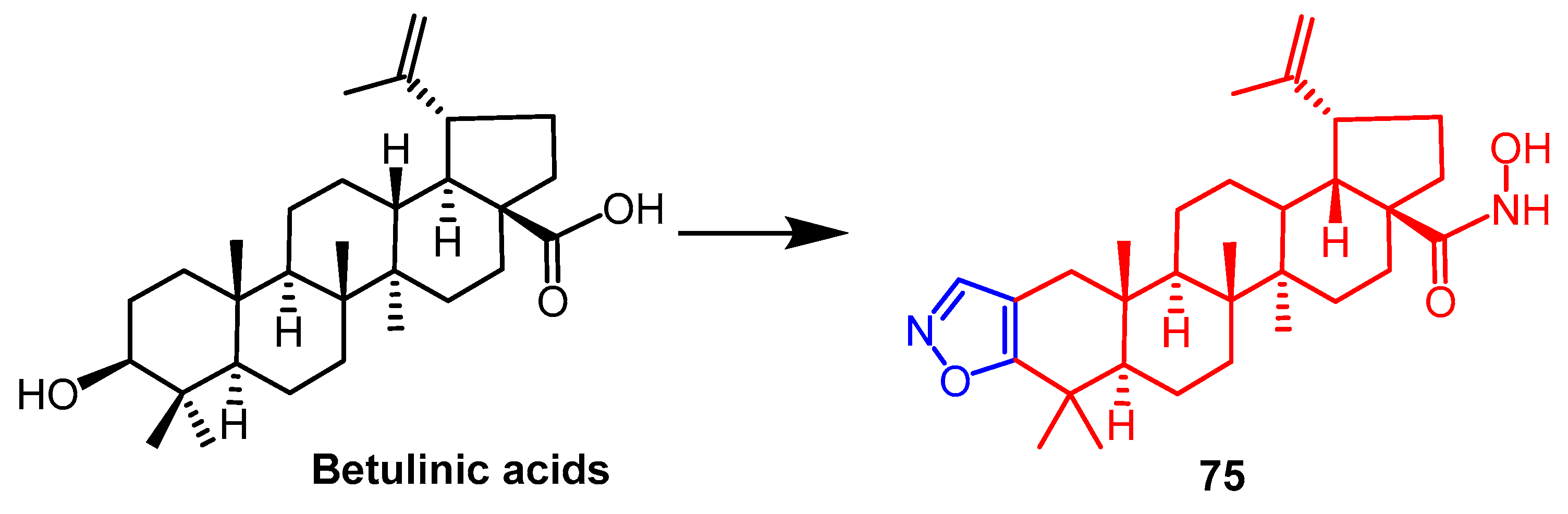
- Luginina, J.; Linden, M.; Bazulis, M.; Kumpins, V.; Mishnev, A.; Popov, S.A.; Golubeva, T.S.; Waldvogel, S.R.; Shults, E.E.; Turks, M. Electrosynthesis of Stable Betulin-Derived Nitrile Oxides and their Application in Synthesis of Cytostatic Lupane-Type Triterpenoid-Isoxazole Conjugates. Eur. J. Org. Chem. 2021, 2021, 2557–2577. [Google Scholar] [CrossRef]
- Ma, L.; Miao, D.Y.; Lee, J.J.; Li, T.; Chen, Y.; Su, G.Y.; Zhao, Y.Q. Synthesis and biological evaluation of heterocyclic ring-fused dammarane-type ginsenoside derivatives as potential anti-tumor agents. Bioorganic Chem. 2021, 116, 105365. [Google Scholar] [CrossRef]
- Smirnova, I.E.; Kazakova, O.B.; Loesche, A.; Hoenke, S.; Csuk, R. Evaluation of cholinesterase inhibitory activity and cytotoxicity of synthetic derivatives of di- and triterpene metabolites from Pinus silvestris and Dipterocarpus alatus resins. Med. Chem. Res. 2020, 29, 1478–1485. [Google Scholar] [CrossRef]
- Erdagi, S.I.; Yildiz, U. Synthesis, Structural Analysis and Antiproliferative Activity of Nitrogen-Containing Hetero Spirostan Derivatives: Oximes, Heterocyclic Ring-Fused and Furostanes. Chemistryselect 2022, 7, e202200439. [Google Scholar] [CrossRef]
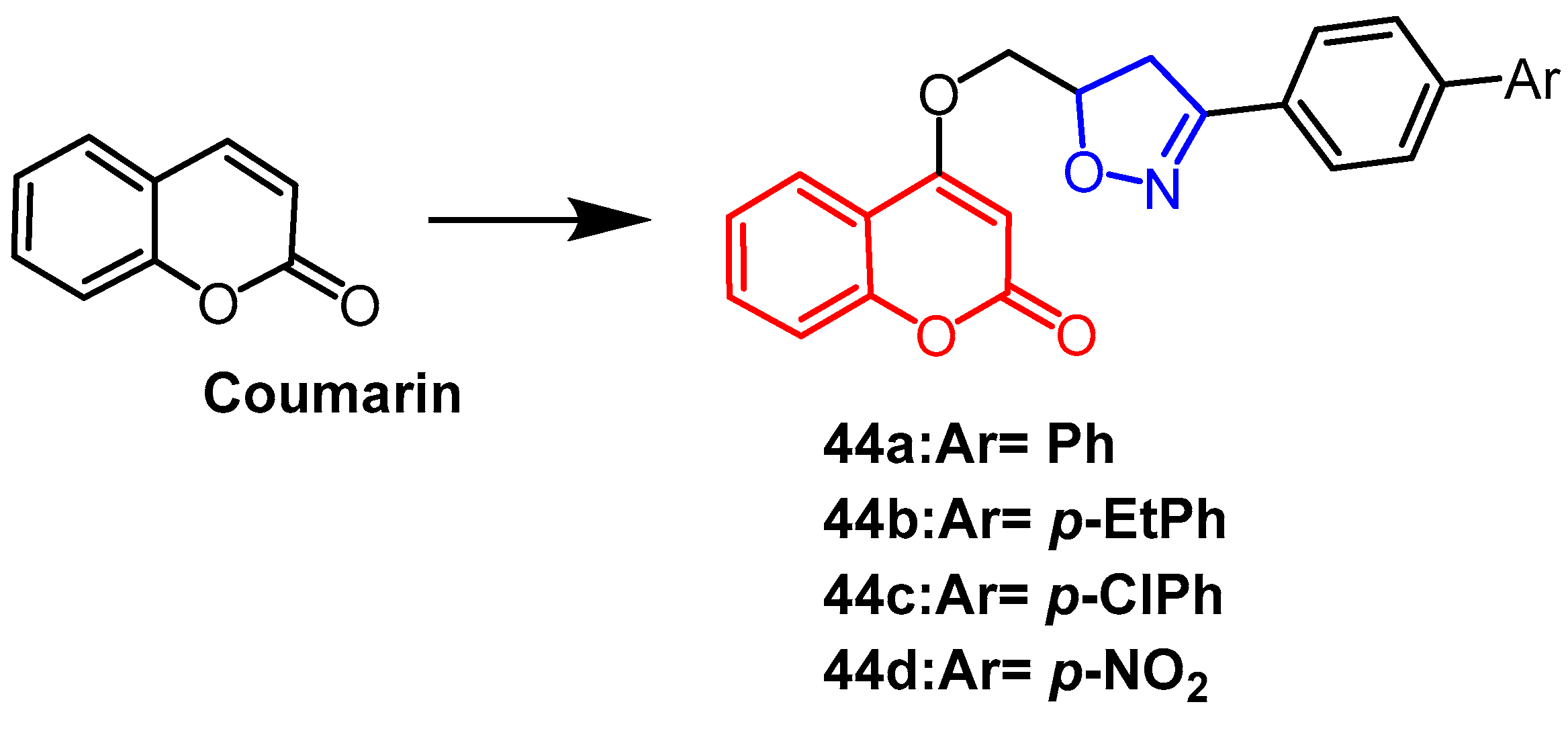
- Lingaraju, G.S.; Balaji, K.S.; Jayarama, S.; Anil, S.M.; Kiran, K.R.; Sadashiva, M.P. Synthesis of new coumarin tethered isoxazolines as potential anticancer agents. Bioorg. Med. Chem. Lett. 2018, 28, 3606–3612. [Google Scholar] [CrossRef]
- Krishna, C.; Bhargavi, M.V.; Rao, Y.J.; Krupadanam, G.L.D. Synthesis of pyrano isoxazoline/isoxazole annulated coumarins via intramolecular nitrile oxide cycloaddition and their cytotoxicity. Russ. J. Gen. Chem. 2017, 87, 1857–1863. [Google Scholar] [CrossRef]
- Znati, M.; Debbabi, M.; Romdhane, A.; Ben Jannet, H.; Bouajila, J. Synthesis of new anticancer and anti-inflammatory isoxazolines and aziridines from the natural (-)-deltoin. J. Pharm. Pharmacol. 2018, 70, 1700–1712. [Google Scholar] [CrossRef]
- Kumari, P.; Mishra, V.S.; Narayana, C.; Khanna, A.; Chakrabarty, A.; Sagar, R. Publisher Correction: Design and efficient synthesis of pyrazoline and isoxazole bridged indole C-glycoside hybrids as potential anticancer agents. Sci. Rep. 2020, 10, 10095. [Google Scholar] [CrossRef]
- Liu, X.-W.; Yao, Z.; Yang, J.; Chen, Z.-Y.; Liu, X.-L.; Zhao, Z.; Lu, Y.; Zhou, Y.; Cao, Y. 1,3-Dipolar cycloaddition enabled isoxazole-fused spiropyrrolidine oxindoles syntheses from 3-methyl-4-nitro-5-alkenyl-isoxazoles and azomethine ylides. Tetrahedron 2016, 72, 1364–1374. [Google Scholar] [CrossRef]
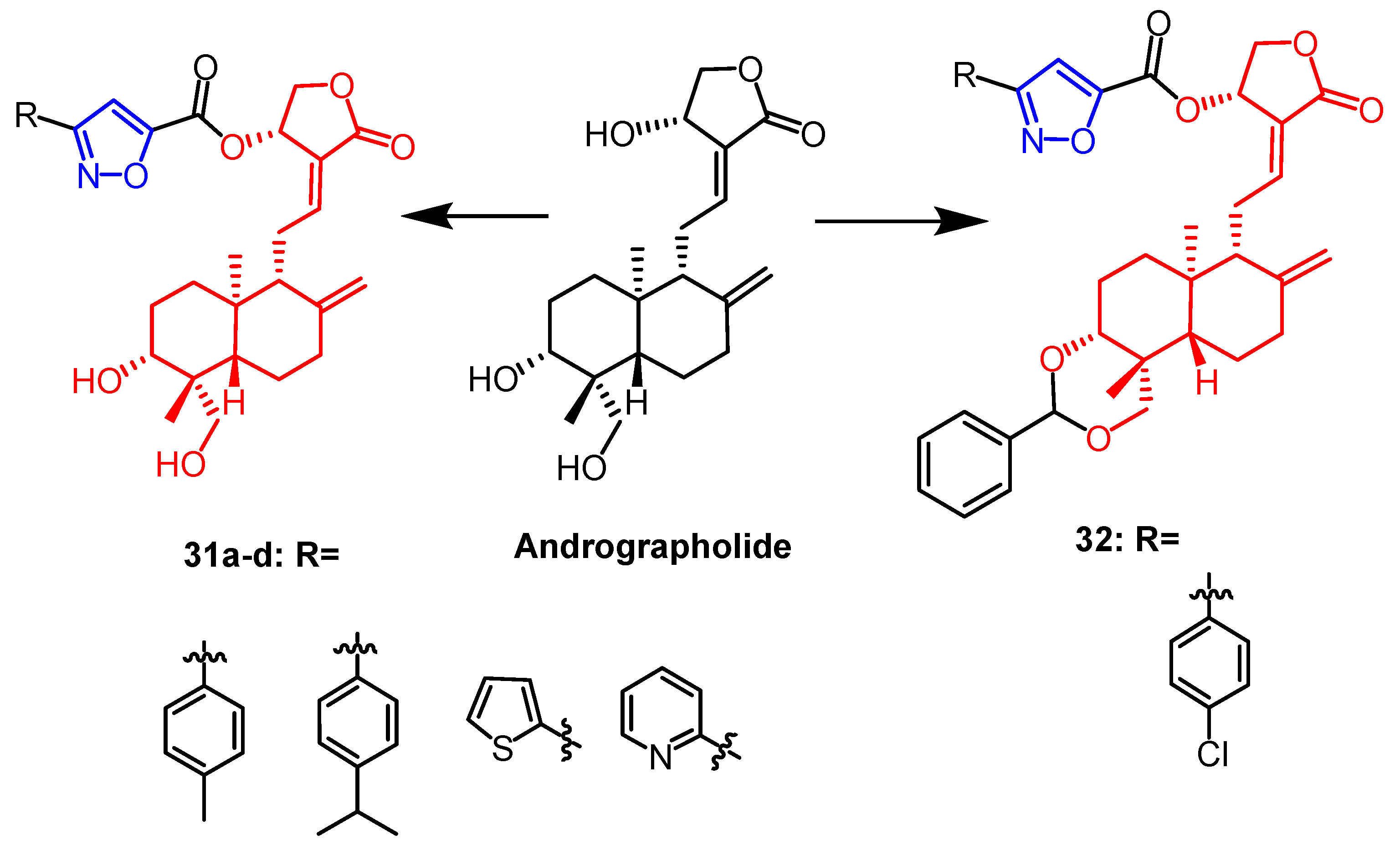
- Mokenapelli, S.; Yerrabelli, J.R.; Das, N.; Roy, P.; Chitneni, P.R. Synthesis and cytotoxicity of novel 14α-O-(andrographolide-3-subsitutedisoxazole-5-carboxylate) derivatives. Nat. Prod. Res. 2021, 35, 3738–3744. [Google Scholar] [CrossRef]
- Oubella, A.; Taia, A.; Byadi, S.; Ait Lahcen, M.; Bimoussa, A.; Essaber, M.; Podlipnik, C.; Morjani, H.; Ait Itto, M.Y.; Aatif, A. Chemical profiling, cytotoxic activities through apoptosis induction in human fibrosarcoma and carcinoma cells, and molecular docking of some 1,2,3-triazole-isoxazoline hybrids using the eugenol as a precursors. J. Biomol. Struct. Dyn. 2022, 40, 1–13. [Google Scholar] [CrossRef]
- Phanumartwiwath, A.; Kesornpun, C.; Sureram, S.; Hongmanee, P.; Pungpo, P.; Kamsri, P.; Punkvang, A.; Eurtivong, C.; Kittakoop, P.; Ruchirawat, S. Antitubercular and antibacterial activities of isoxazolines derived from natural products: Isoxazolines as inhibitors of Mycobacterium tuberculosis InhA. J. Chem. Res. 2021, 45, 1003–1015. [Google Scholar] [CrossRef]
- Pratap, S.; Naaz, F.; Reddy, S.; Jha, K.K.; Sharma, K.; Sahal, D.; Akhter, M.; Nayakanti, D.; Kumar, H.M.S.; Kumari, V.; et al. Anti-proliferative and anti-malarial activities of spiroisoxazoline analogues of artemisinin. Arch. Pharm. 2019, 352, 1800192. [Google Scholar] [CrossRef]
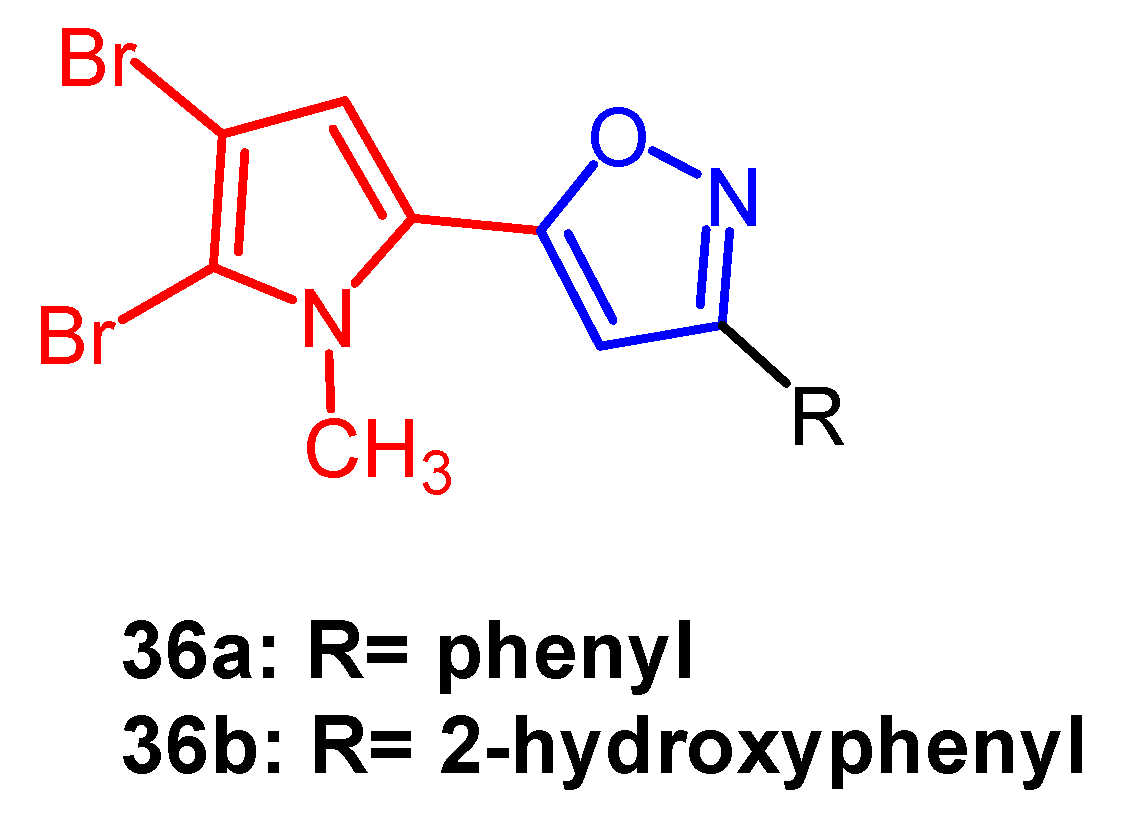
- Rane, R.A.; Sahu, N.U.; Gutte, S.D.; Mahajan, A.A.; Shah, C.P.; Bangalore, P. Synthesis and evaluation of novel marine bromopyrrole alkaloid-based hybrids as anticancer agents. Eur. J. Med. Chem. 2013, 63, 793–799. [Google Scholar] [CrossRef]
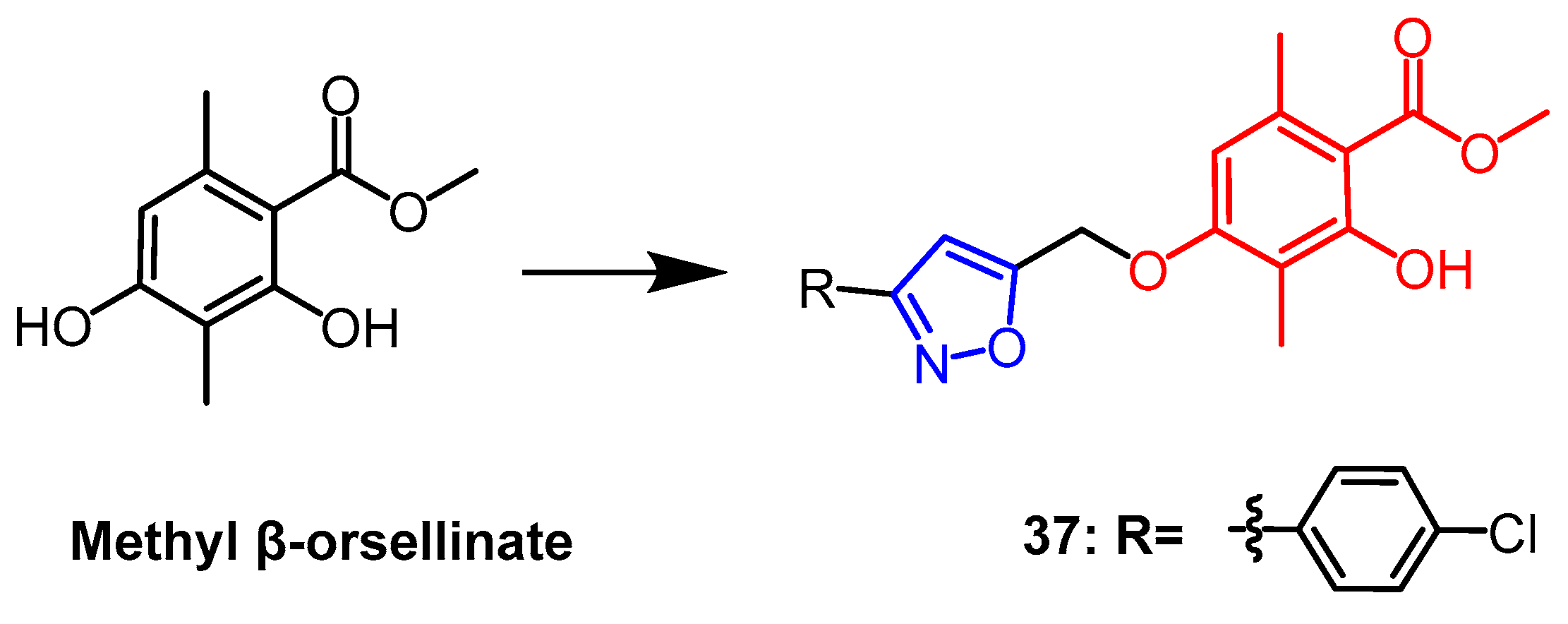
- Reddy, S.T.; Mendonza, J.J.; Makani, V.K.K.; Bhadra, M.P.; Uppuluri, V.M. Synthesis of some novel methyl β-orsellinate based 3, 5-disubstituted isoxazoles and their anti-proliferative activity: Identification of potent leads active against MCF-7 breast cancer cell. Bioorg. Chem. 2020, 105, 104374. [Google Scholar] [CrossRef]
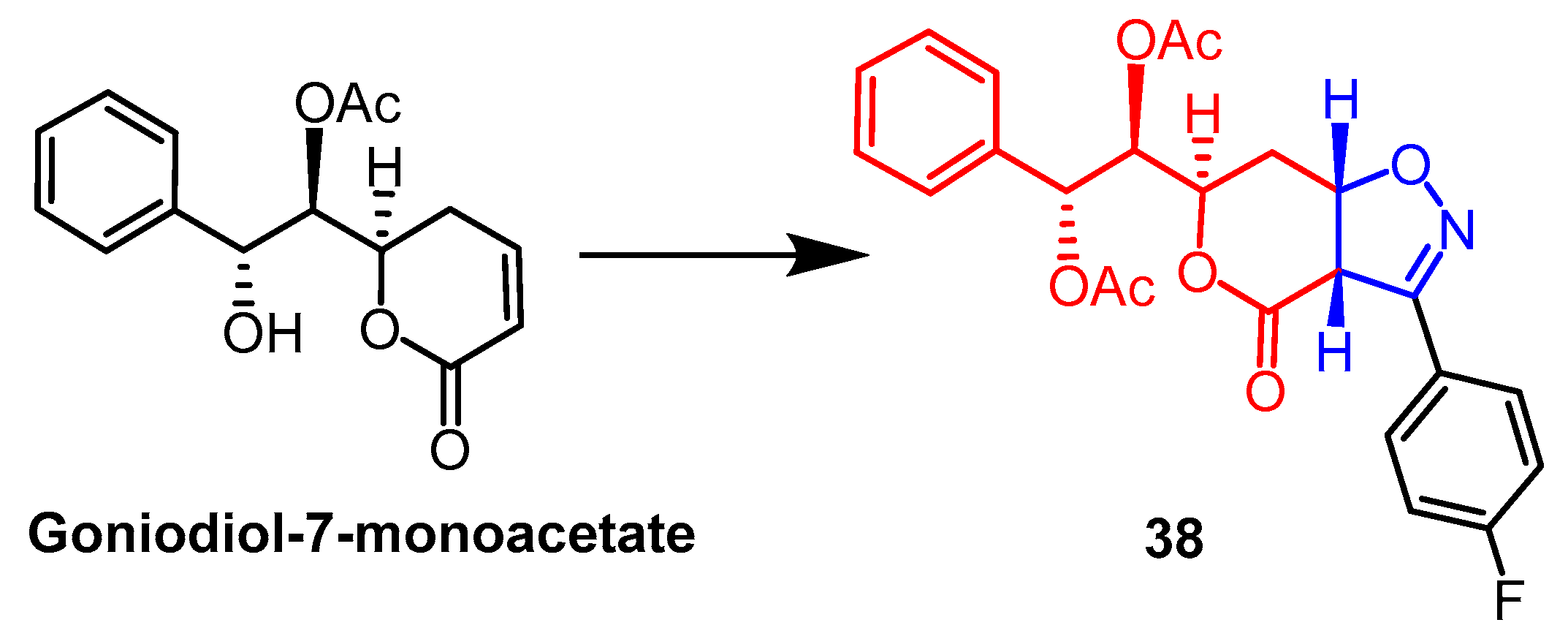
- Talimarada, D.; Sharma, A.; Wakhradkar, M.G.; Dhuri, S.N.; Gunturu, K.C.; Sundaram, V.N.N.; Holla, H. Synthesis, DFT analysis and in-vitro anti-cancer study of novel fused bicyclic pyranone isoxazoline derivatives of Goniodiol-diacetate-a natural product derivative. Fitoterapia 2022, 163, 105316. [Google Scholar] [CrossRef]
- Tang, J.-J.; He, Q.-R.; Dong, S.; Guo, X.; Wang, Y.-G.; Lei, B.-L.; Tian, J.-M.; Gao, J.-M. Diversity Modification and Structure-Activity Relationships of Two Natural Products 1β-hydroxy Alantolactone and Ivangustin as Potent Cytotoxic Agents. Sci. Rep. 2018, 8, 1722. [Google Scholar] [CrossRef]
- Rodrigues, F.C.; Kumar, N.V.A.; Hari, G.; Pai, K.S.R.; Thakur, G. The inhibitory potency of isoxazole-curcumin analogue for the management of breast cancer: A comparative in vitro and molecular modeling investigation. Chem. Pap. 2021, 75, 5995–6008. [Google Scholar] [CrossRef]
- Kudryavtseva, T.N.; Lamanov, A.Y.; Sysoev, P.I.; Klimova, L.G. Synthesis and Antibacterial Activity of New Acridone Derivatives Containing an Isoxazoline Fragment. Russ. J. Gen. Chem. 2020, 90, 45–49. [Google Scholar] [CrossRef]
- Li, Z.; Liu, N.; Tu, J.; Ji, C.J.; Han, G.Y.; Wang, Y.; Sheng, C.Q. Discovery of novel simplified isoxazole derivatives of sampangine as potent anti-cryptococcal agents. Bioorganic Med. Chem. 2019, 27, 832–840. [Google Scholar] [CrossRef]
- Zghab, I.; Trimeche, B.; Ben Mansour, M.; Hassine, M.; Touboul, D.; Ben Jannet, H. Regiospecific synthesis, antibacterial and anticoagulant activities of novel isoxazoline chromene derivatives. Arab. J. Chem. 2017, 10, S2651–S2658. [Google Scholar] [CrossRef]
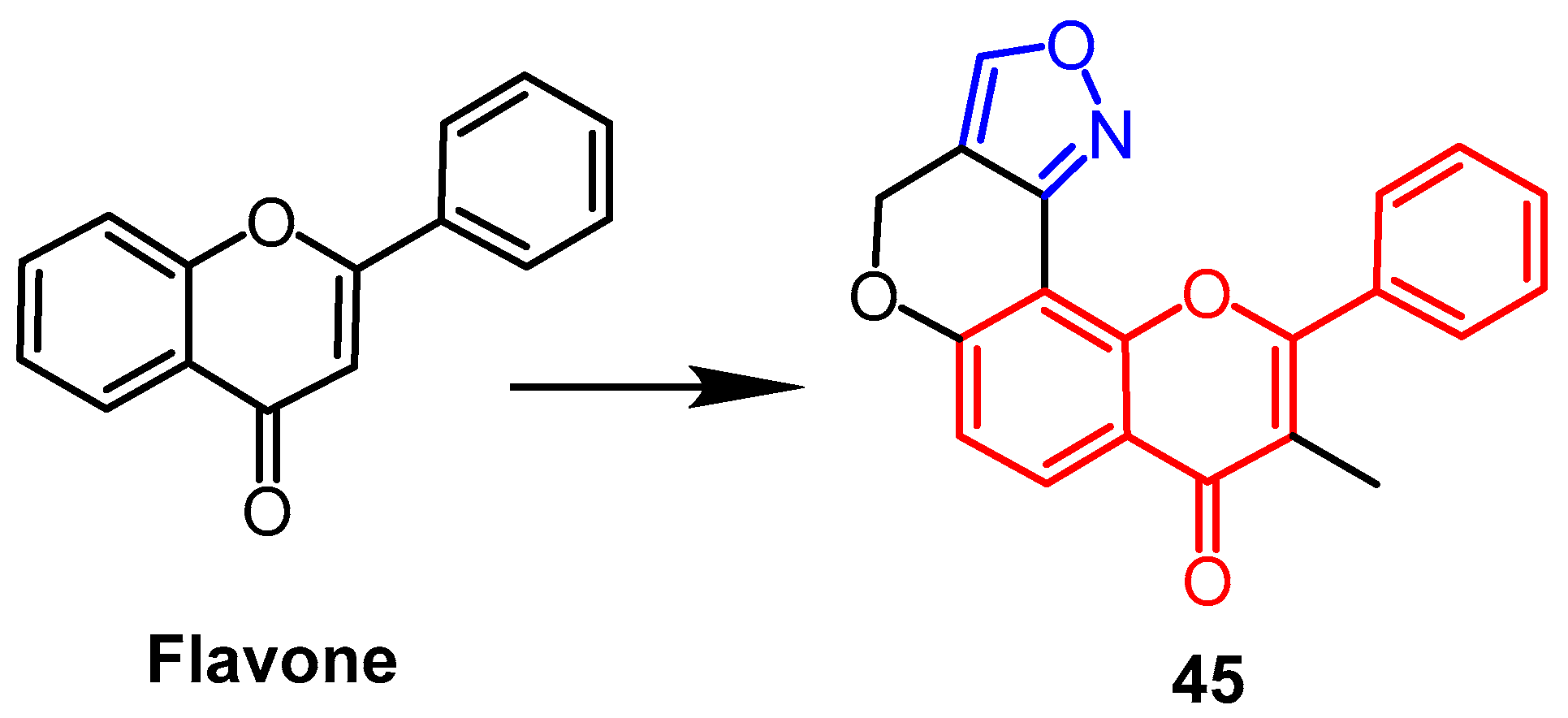
- Rao, Y.J.; Sowjanya, T.; Thirupathi, G.; Murthy, N.Y.S.; Kotapalli, S.S. Synthesis and biological evaluation of novel flavone/triazole/benzimidazole hybrids and flavone/isoxazole-annulated heterocycles as antiproliferative and antimycobacterial agents. Mol. Divers. 2018, 22, 803–814. [Google Scholar] [CrossRef]
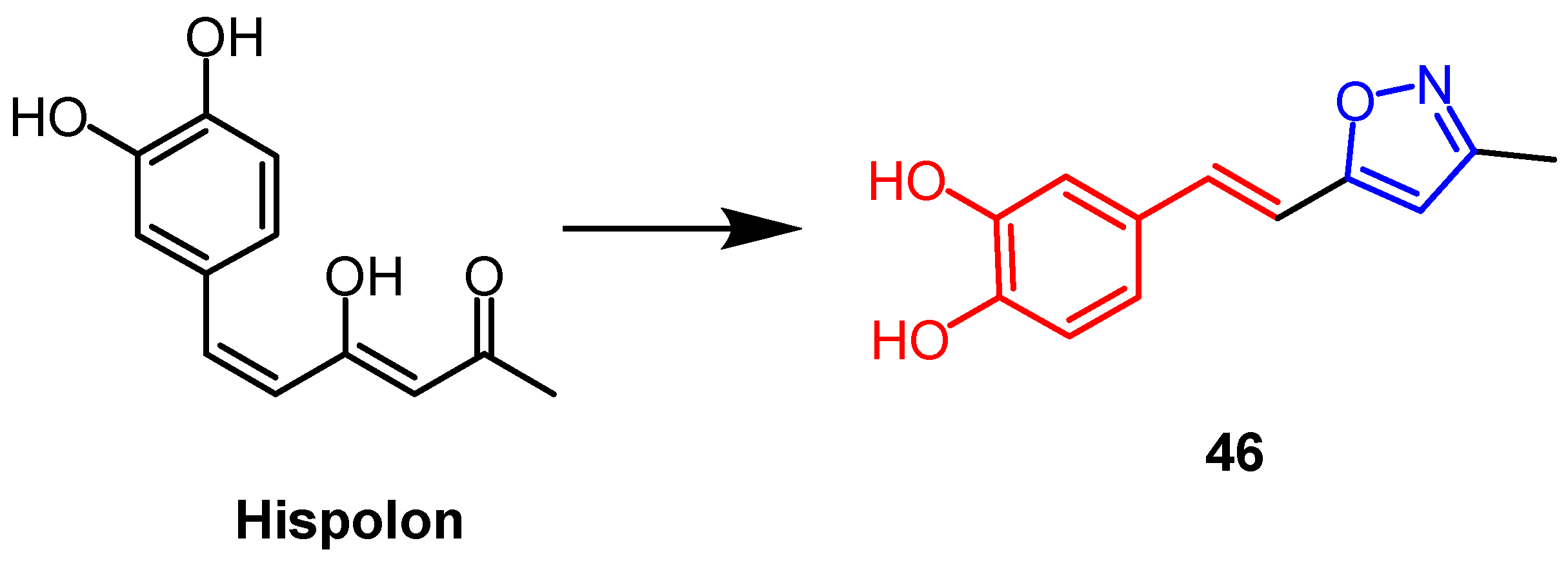
- Balaji, N.V.; HariBabu, B.; Rao, V.U.; Subbaraju, G.V.; Nagasree, K.P.; Kumar, M.M.K. Synthesis, Screening and Docking Analysis of Hispolon Pyrazoles and Isoxazoles as Potential Antitubercular AgentsHispolon. Curr. Top. Med. Chem. 2019, 19, 662–682. [Google Scholar] [CrossRef]
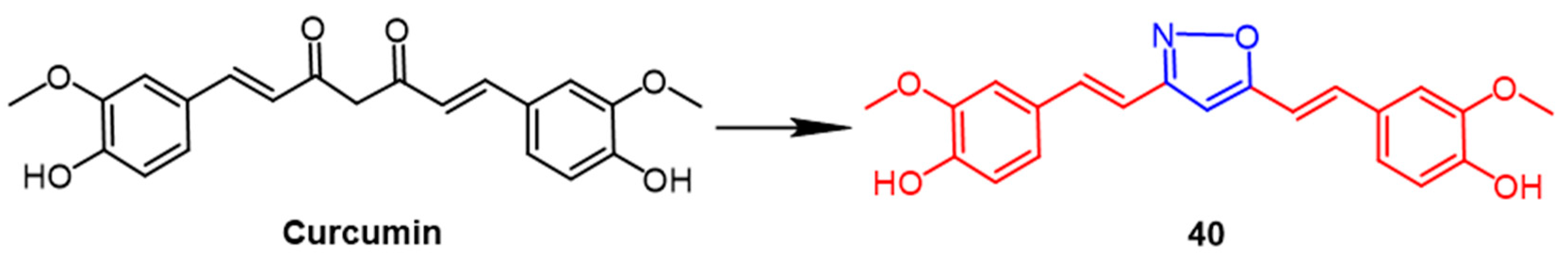
- Changtam, C.; Hongmanee, P.; Suksamrarn, A. Isoxazole analogs of curcuminoids with highly potent multidrug-resistant antimycobacterial activity. Eur. J. Med. Chem. 2010, 45, 4446–4457. [Google Scholar] [CrossRef]
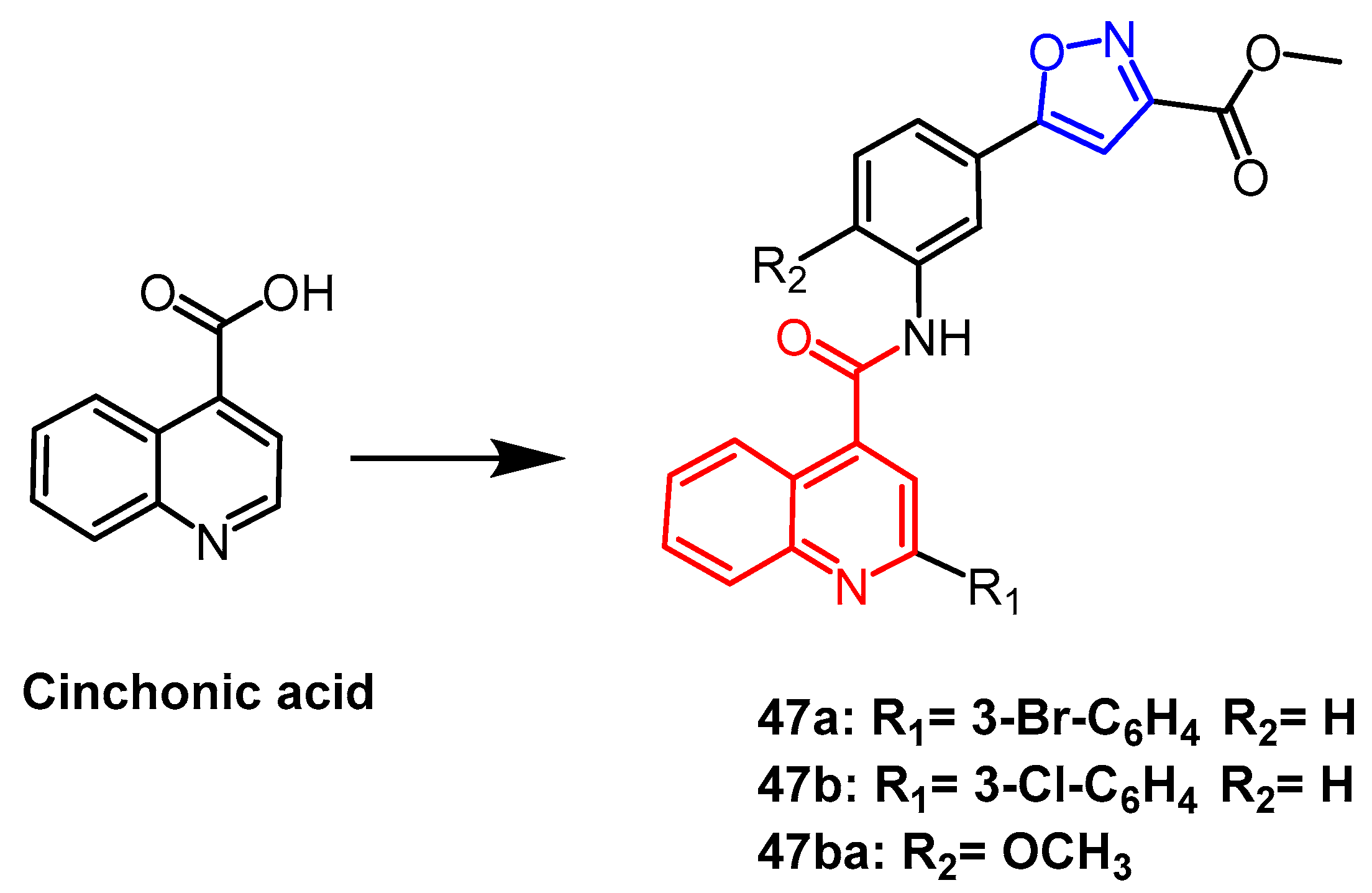
- Sahoo, S.K.; Ahmad, M.N.; Kaul, G.; Nanduri, S.; Dasgupta, A.; Chopra, S.; Yaddanapudi, V.M. Exploration of Isoxazole-Carboxylic Acid Methyl Ester Based 2-Substituted Quinoline Derivatives as Promising Antitubercular Agents. Chem. Biodivers. 2022, 19, e202200324. [Google Scholar]
- Sahoo, S.K.; Rani, B.; Gaikwad, N.B.; Ahmad, M.N.; Kaul, G.; Shukla, M.; Nanduri, S.; Dasgupta, A.; Chopra, S.; Yaddanapudi, V.M. Synthesis and structure-activity relationship of new chalcone linked 5-phenyl-3-isoxazolecarboxylic acid methyl esters potentially active against drug resistant Mycobacterium tuberculosis. Eur. J. Med. Chem. 2021, 222, 113580. [Google Scholar] [CrossRef] [PubMed]
- das Neves, A.R.; Trefzger, O.S.; Barbosa, N.V.; Honorato, A.M.; Carvalho, D.B.; Moslaves, I.S.; Kadri, M.C.T.; Yoshida, N.C.; Kato, M.J.; Arruda, C.C.P.; et al. Effect of isoxazole derivatives of tetrahydrofuran neolignans on intracellular amastigotes of Leishmania (Leishmania) amazonensis: A structure-activity relationship comparative study with triazole-neolignan-based compounds. Chem. Biol. Drug Des. 2019, 94, 2004–2012. [Google Scholar] [CrossRef]
- Trefzger, O.S.; das Neves, A.R.; Barbosa, N.V.; Carvalho, D.B.; Pereira, I.C.; Perdomo, R.T.; Matos, M.F.C.; Yoshida, N.C.; Kato, M.J.; de Albuquerque, S.; et al. Design, synthesis and antitrypanosomatid activities of 3,5-diaryl-isoxazole analogues based on neolignans veraguensin, grandisin and machilin G. Chem. Biol. Drug Des. 2019, 93, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Algethami, F.K.; Saidi, I.; Abdelhamid, H.N.; Elamin, M.R.; Abdulkhair, B.Y.; Chrouda, A.; Ben Jannet, H. Trifluoromethylated Flavonoid-Based Isoxazoles as Antidiabetic and Anti-Obesity Agents: Synthesis, In Vitro α-Amylase Inhibitory Activity, Molecular Docking and Structure-Activity Relationship Analysis. Molecules 2021, 26, 5214. [Google Scholar] [CrossRef] [PubMed]
- Saidi, I.; Manachou, M.; Znati, M.; Bouajila, J.; Ben Jannet, H. Synthesis of new halogenated flavonoid-based isoxazoles: In vitro and in silico evaluation of alpha-amylase inhibitory potential, a SAR analysis and DFT studies. J. Mol. Struct. 2022, 1247, 131379. [Google Scholar] [CrossRef]
- Goyard, D.; Konya, B.; Chajistamatiou, A.S.; Chrysina, E.D.; Leroy, J.; Balzarin, S.; Tournier, M.; Tousch, D.; Petit, P.; Duret, C.; et al. Glucose-derived spiro-isoxazolines are anti-hyperglycemic agents against type 2 diabetes through glycogen phosphorylase inhibition. Eur. J. Med. Chem. 2016, 108, 444–454. [Google Scholar] [CrossRef]
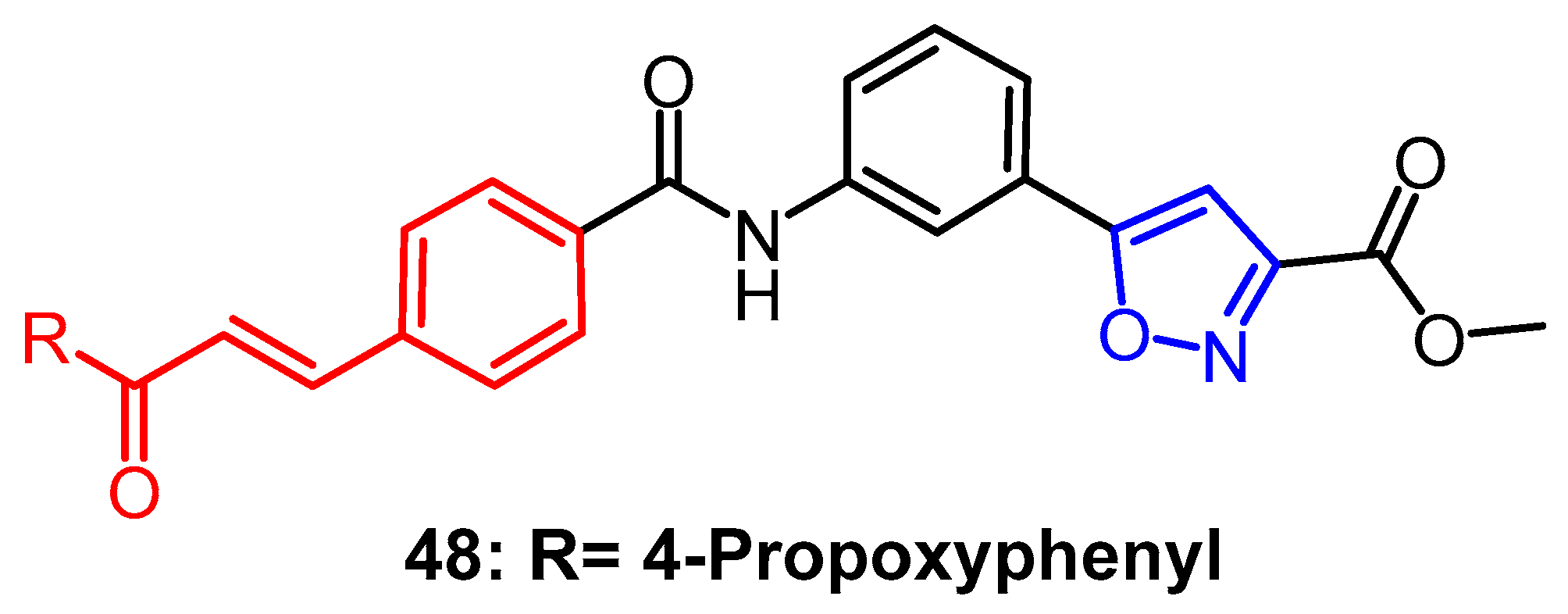
- He, H.; Ge, Y.; Dai, H.; Cui, S.; Ye, F.; Jin, J.; Shi, Y. Design, synthesis and biological evaluation of stilbene derivatives as novel inhibitors of protein Tyrosine Phosphatase 1B. Molecules 2016, 21, 1722. [Google Scholar] [CrossRef]
- Olanipekun, B.E.; Ponnapalli, M.G.; Patel, H.K.; Munipalle, K.; Shaik, K. Design, synthesis of new phenyl acetylene and isoxazole analogues of arjunolic acid as potent tyrosinase and alpha glucosidase inhibitors. Nat. Prod. Res. 2021, 35, 1–6. [Google Scholar] [CrossRef]
- Nie, J.-P.; Qu, Z.-N.; Chen, Y.; Chen, J.-H.; Jiang, Y.; Jin, M.-N.; Yu, Y.; Niu, W.-Y.; Duan, H.-Q.; Qin, N. Discovery and anti-diabetic effects of novel isoxazole based flavonoid derivatives. Fitoterapia 2020, 142, 104499. [Google Scholar] [CrossRef]
- Ghidini, E.; Capelli, A.M.; Carnini, C.; Cenacchi, V.; Marchini, G.; Virdis, A.; Italia, A.; Facchinetti, F. Discovery of a novel isoxazoline derivative of prednisolone endowed with a robust anti-inflammatory profile and suitable for topical pulmonary administration. Steroids 2015, 95, 88–95. [Google Scholar] [CrossRef]
- Jin, J.; Teng, P.; Liu, H.-L.; Wu, J.; Liu, Y.-M.; Xu, Q.; Li, J.-X. Microfluidics assisted synthesis and bioevaluation of sinomenine derivatives as antiinflammatory agents. Eur. J. Med. Chem. 2013, 62, 280–288. [Google Scholar] [CrossRef]
- Pan, H.; Lu, T.; Wu, X.; Gu, C.; Tao, N.; Zhang, B.; Wang, A.; Chen, G.; Zhang, K.; Cheng, J.; et al. Design and synthesis of sinomenine isoxazole derivatives via 1,3-dipolar cycloaddition reaction. Nat. Prod. Res. 2021, 35, 2360–2364. [Google Scholar] [CrossRef] [PubMed]
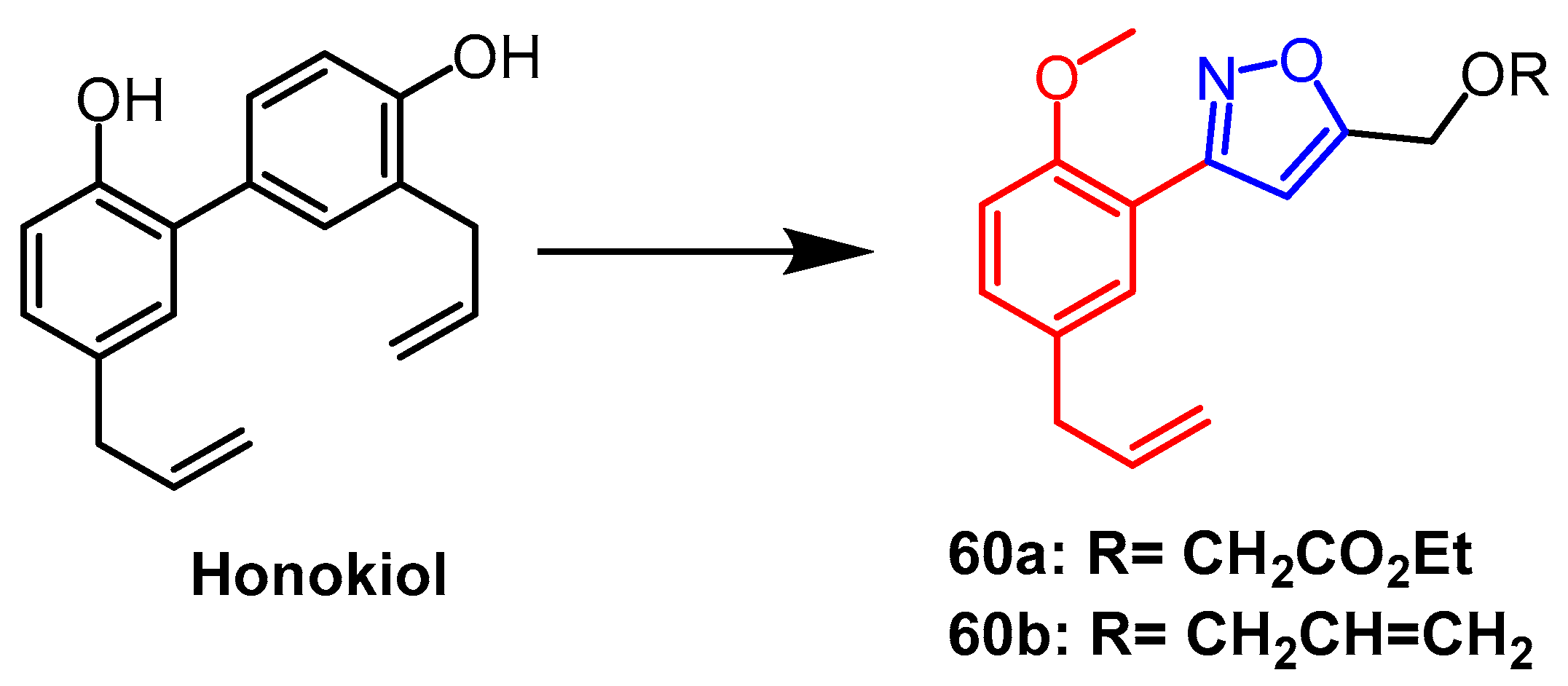
- Lee, S.; Yuan, Y.; Kwon, S.-I.; Lee, J.; Seo, S.-Y. Synthesis and Anti-Neuroinflammatory Activity of N-Heterocyclic Analogs Based on Natural Biphenyl-Neolignan Honokiol; American Chemical Society: Washington, WA, USA, 2019. [Google Scholar]
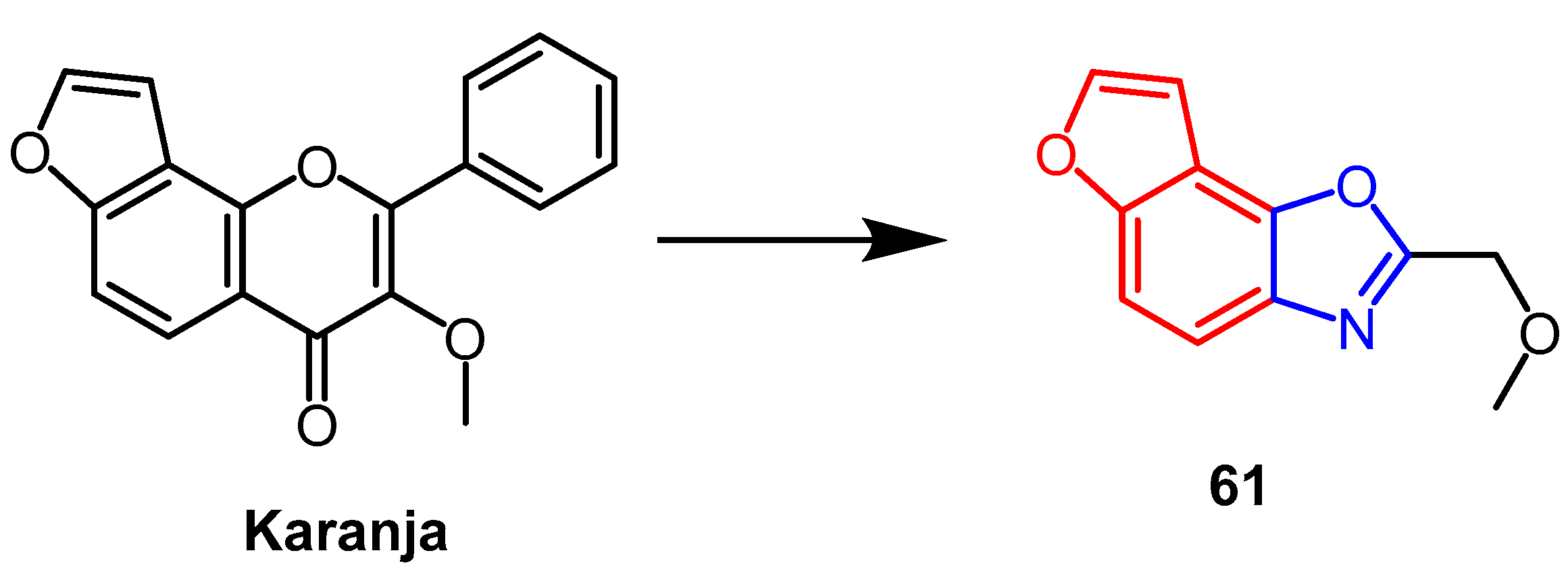
- Rekha, M.J.; Bettadaiah, B.K.; Muthukumar, S.P.; Govindaraju, K. Synthesis, characterization and anti-inflammatory properties of karanjin (Pongamia pinnata seed) and its derivatives. Bioorganic Chem. 2021, 106, 104471. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Abdul Qadir, M.; Hameed, A.; Imran, M.; Muddassar, M. Screening of curcumin-derived isoxazole, pyrazoles, and pyrimidines for their ‘, antinociceptive, and cyclooxygenase-2 inhibition. Chem. Biol. Drug Des. 2018, 91, 338–343. [Google Scholar] [CrossRef]
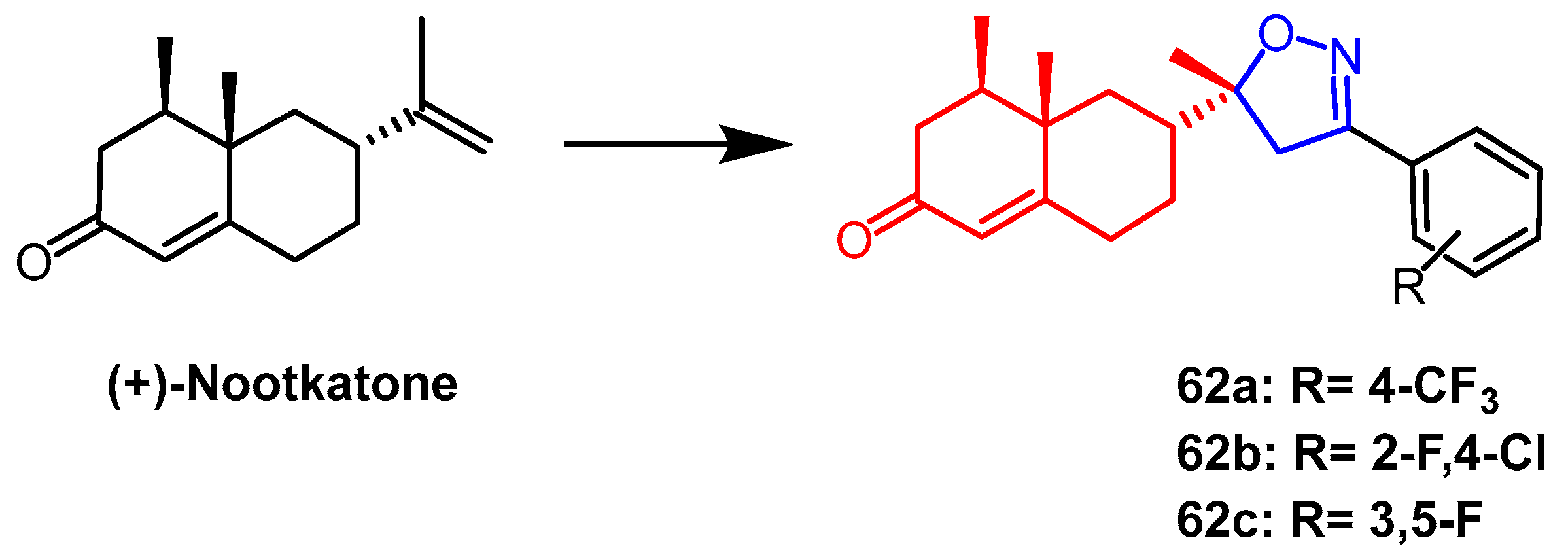
- Guo, Y.; Zhang, Q.; Liu, Z.; Bao, C.; Fan, J.; Yang, R. Non-food bioactive products: Design and semisynthesis of novel (+)-nootkatone derivatives containing isoxazoline moiety as insecticide. Ind. Crops Prod. 2019, 140, 111706. [Google Scholar] [CrossRef]
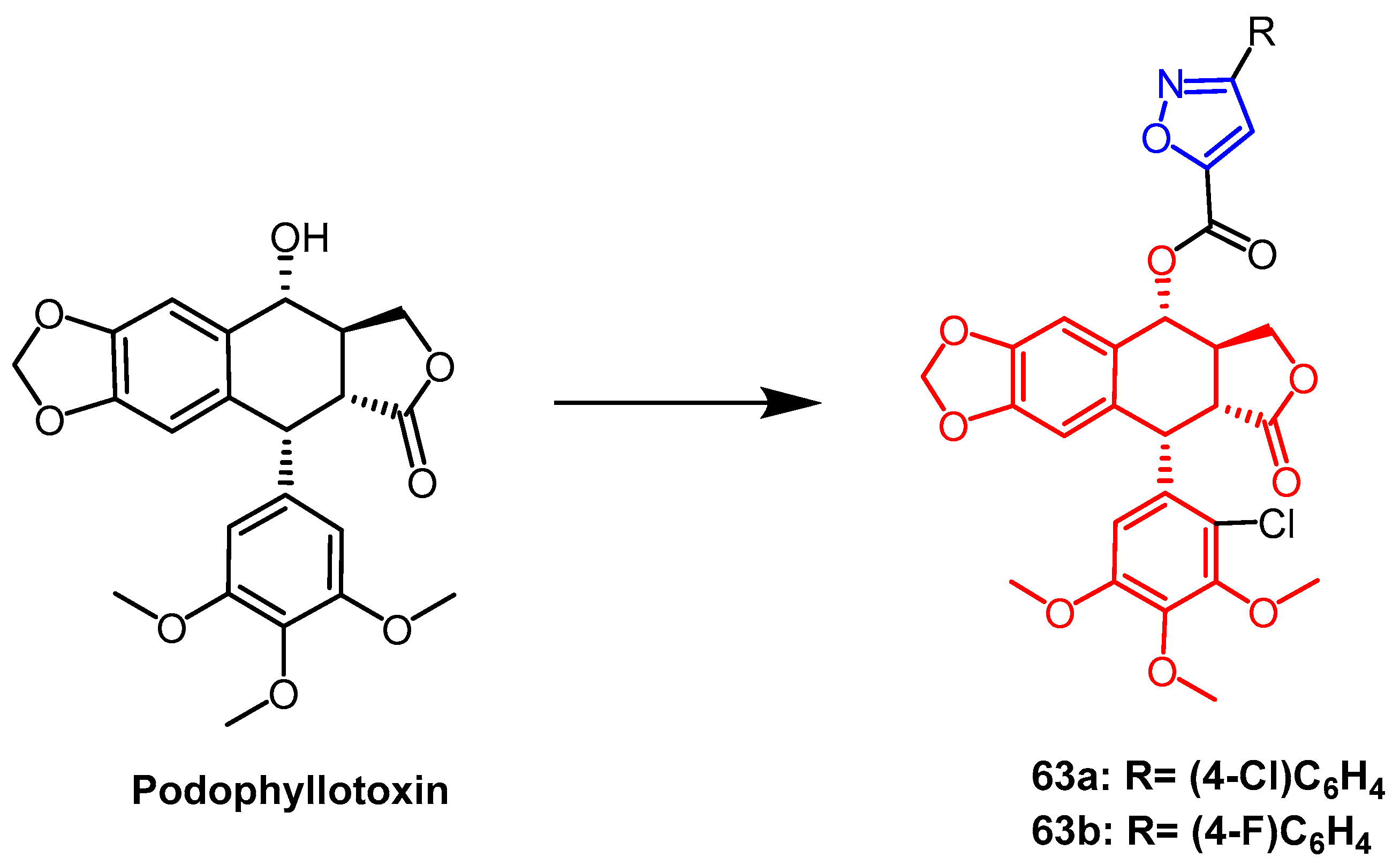
- Yang, R.G.; Ma, M.F.; Lv, M.; Zhang, S.Y.; Xu, H. Non-food bioactive products for pesticides candidates (III): Agricultural properties of isoxazole esters from the plant product podophyllotoxin as botanical pesticides. Ind. Crops Prod. 2021, 174, 114181. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, K.; Lv, M.; Hao, M. Construction of Cholesterol Oxime Ether Derivatives Containing Isoxazoline/Isoxazole Fragments and Their Agricultural Bioactive Properties/Control Efficiency. J. Agric. Food Chem. 2021, 69, 8098–8109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Han, M.Y.; Yan, X.T.; Cheng, W.Q.; Tang, Z.S.; Cui, L.P.; Yang, R.G.; Guo, Y. Design, Synthesis, and Biological Evaluation of Novel Osthole-Based Isoxazoline Derivatives as Insecticide Candidates. J. Agric. Food Chem. 2022, 70, 7921–7928. [Google Scholar] [CrossRef]
- Shan, X.J.; Lv, M.; Wang, J.R.; Qin, Y.J.; Xu, H. Acaricidal and insecticidal efficacy of new esters derivatives of a natural coumarin osthole. Ind. Crops Prod. 2022, 182, 114855. [Google Scholar] [CrossRef]
- Yin, L.; Niu, C.; Liao, L.-X.; Dou, J.; Habasi, M.; Aisa, H.A. An isoxazole chalcone derivative enhances melanogenesis in B16 melanoma cells via the Akt/GSK3β/β-catenin signaling pathways. Molecules 2017, 22, 2077. [Google Scholar] [CrossRef]
- Pang, G.X.; Niu, C.; Mamat, N.; Aisa, H.A. Synthesis and in vitro biological evaluation of novel coumarin derivatives containing isoxazole moieties on melanin synthesis in B16 cells and inhibition on bacteria. Bioorg. Med. Chem. Lett. 2017, 27, 2674–2677. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Bao, B.H.; Shen, Q.; Zhang, Y.C.; Jiang, Q.; Li, J.X. Novel heterocyclic ring-fused oleanolic acid derivatives as osteoclast inhibitors for osteoporosis. Medchemcomm 2016, 7, 371–377. [Google Scholar] [CrossRef]
- Helal, M.H.M.; Ahmed, N.S.; Elwessaly, M.S.; Ammar, Y.A. Synthesis, Characterization, and Antioxidant and Bleomycin-Dependent DNA Damage Evaluation of Curcumin Analogs. Arch. Pharm. 2014, 347, 123–133. [Google Scholar] [CrossRef]
- Sherin, D.R.; Rajasekharan, K.N. Mechanochemical Synthesis and Antioxidant Activity of Curcumin-Templated Azoles. Arch. Pharm. 2015, 348, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Qadir, M.A.; Hameed, A.; Arshad, M.N.; Asiri, A.M.; Muddassar, M. Sulfonamides containing curcumin scaffold: Synthesis, characterization, carbonic anhydrase inhibition and molecular docking studies. Bioorganic Chem. 2018, 76, 218–227. [Google Scholar] [CrossRef]
- Minassi, A.; Rogati, F.; Cruz, C.; Prados, M.E.; Galera, N.; Jinenez, C.; Appendino, G.; Bellido, M.L.; Calzado, M.A.; Caprioglio, D.; et al. Triterpenoid Hydroxamates as HIF Prolyl Hydrolase Inhibitors. J. Nat. Prod. 2018, 81, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Luo, G.; Li, X.; Zheng, F.; Li, H.; Zhang, J.; You, Q.; Xiang, H. Lipid accumulation inhibitory activities of novel isoxazole-based chenodeoxycholic acids: Design, synthesis and preliminary mechanism study. Bioorg. Med. Chem. Lett. 2018, 28, 2879–2884. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, R.; Shi, Y.; Li, W.; Li, M.; Chen, P.; Pan, B.; Wang, Q.; Li, C.; Wang, J.; et al. Synthesis and biological evaluation of panaxatriol derivatives against myocardial ischemia/reperfusion injury in the rat. Eur. J. Med. Chem. 2020, 185, 111729. [Google Scholar] [CrossRef]









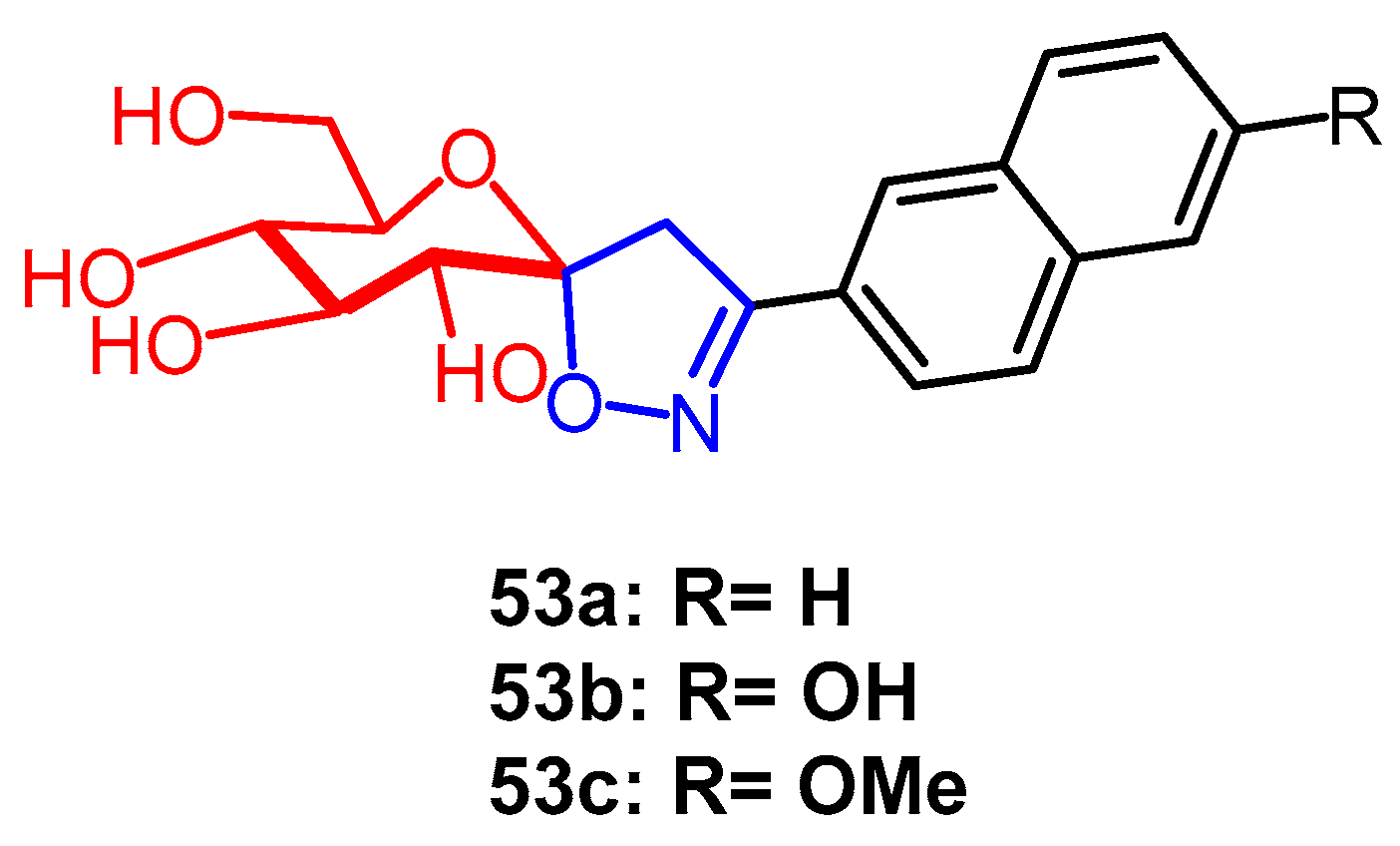
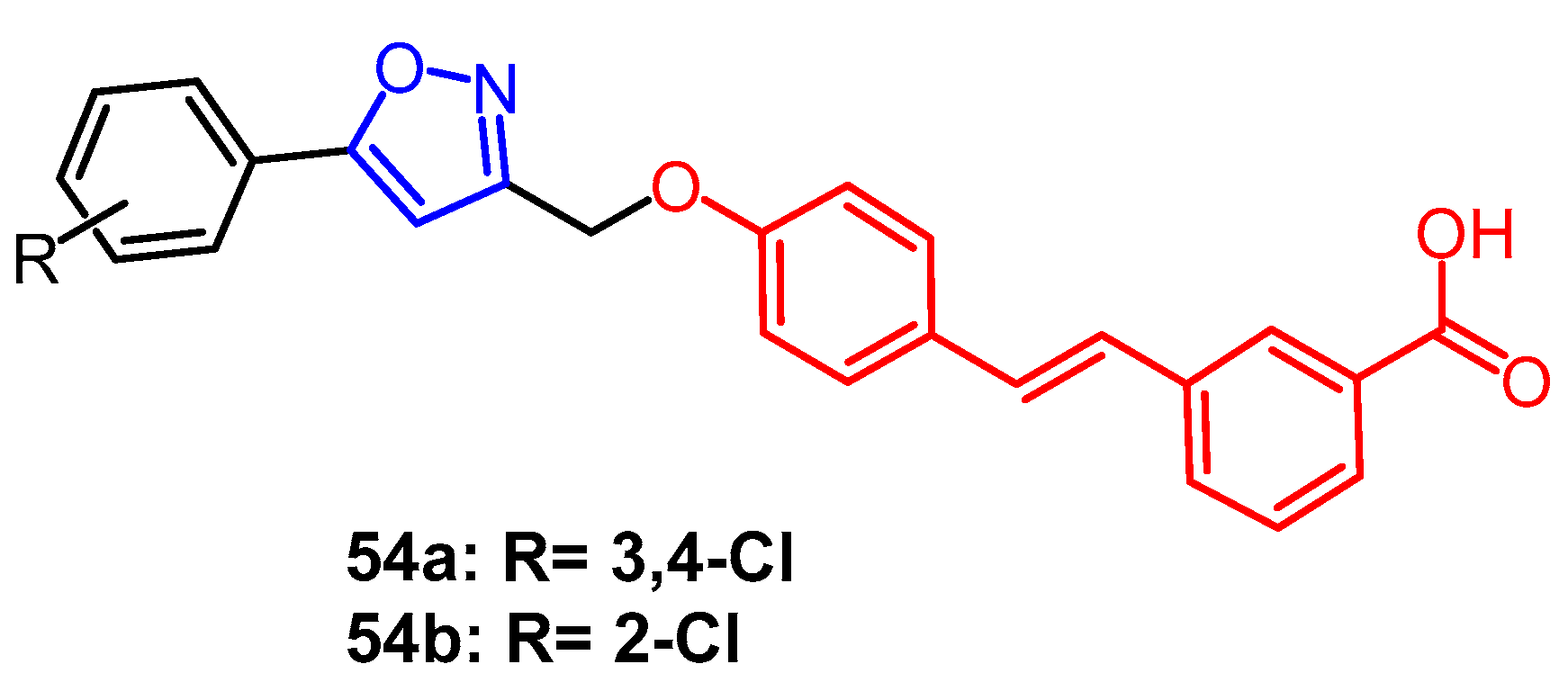
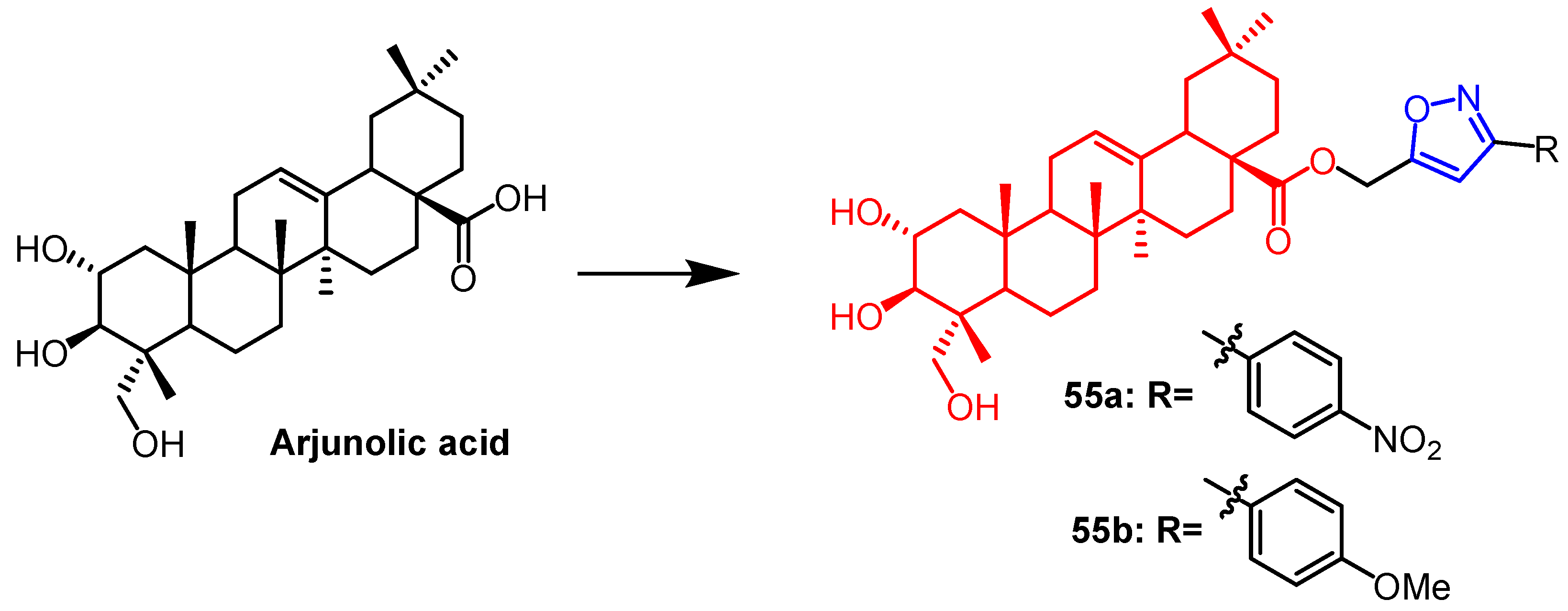
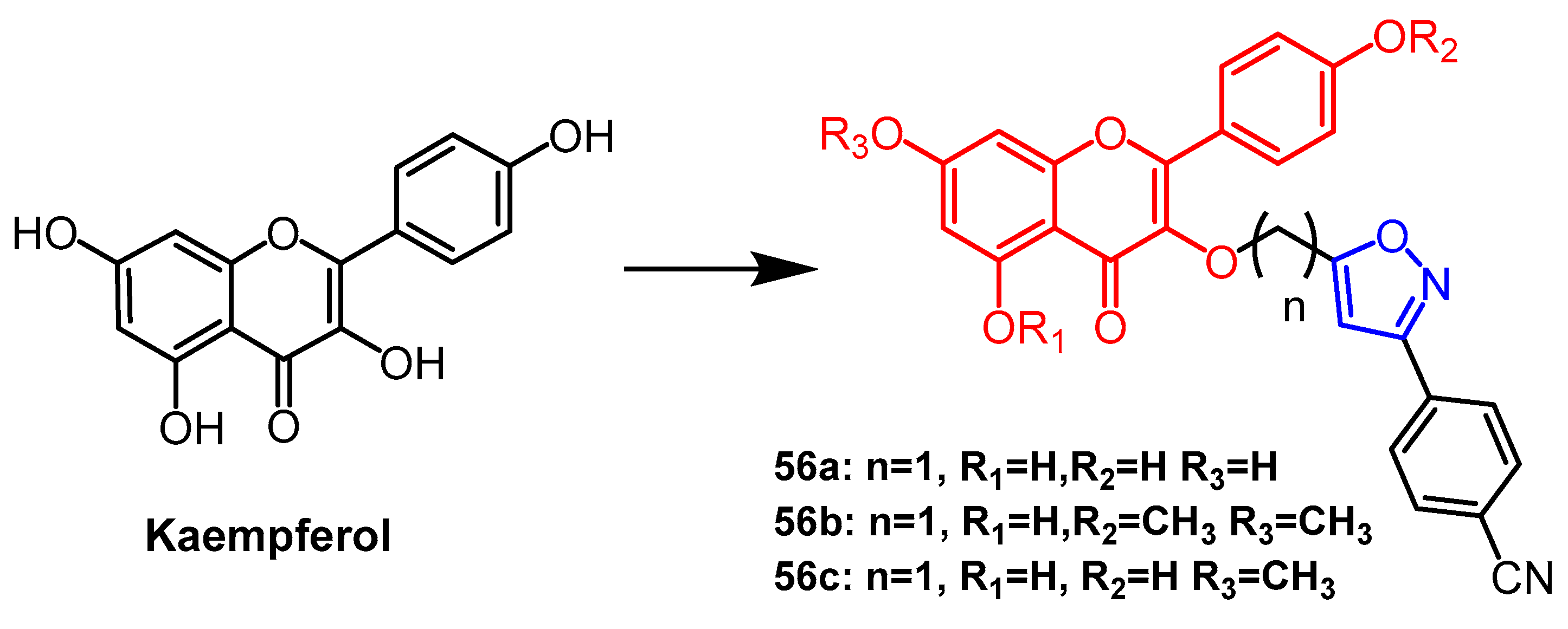





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Hu, Q.; Tang, H.; Pan, X. Isoxazole/Isoxazoline Skeleton in the Structural Modification of Natural Products: A Review. Pharmaceuticals 2023, 16, 228. https://doi.org/10.3390/ph16020228
Wang X, Hu Q, Tang H, Pan X. Isoxazole/Isoxazoline Skeleton in the Structural Modification of Natural Products: A Review. Pharmaceuticals. 2023; 16(2):228. https://doi.org/10.3390/ph16020228
Chicago/Turabian StyleWang, Xiyue, Qingyun Hu, Hui Tang, and Xinhui Pan. 2023. "Isoxazole/Isoxazoline Skeleton in the Structural Modification of Natural Products: A Review" Pharmaceuticals 16, no. 2: 228. https://doi.org/10.3390/ph16020228
APA StyleWang, X., Hu, Q., Tang, H., & Pan, X. (2023). Isoxazole/Isoxazoline Skeleton in the Structural Modification of Natural Products: A Review. Pharmaceuticals, 16(2), 228. https://doi.org/10.3390/ph16020228















