Synthetic Methodologies and Therapeutic Potential of Indole-3-Carbinol (I3C) and Its Derivatives
Abstract
1. Introduction
2. I3C Natural and Exogenous Synthesis, In Vitro and In Vivo Activity, and Mechanism of Action
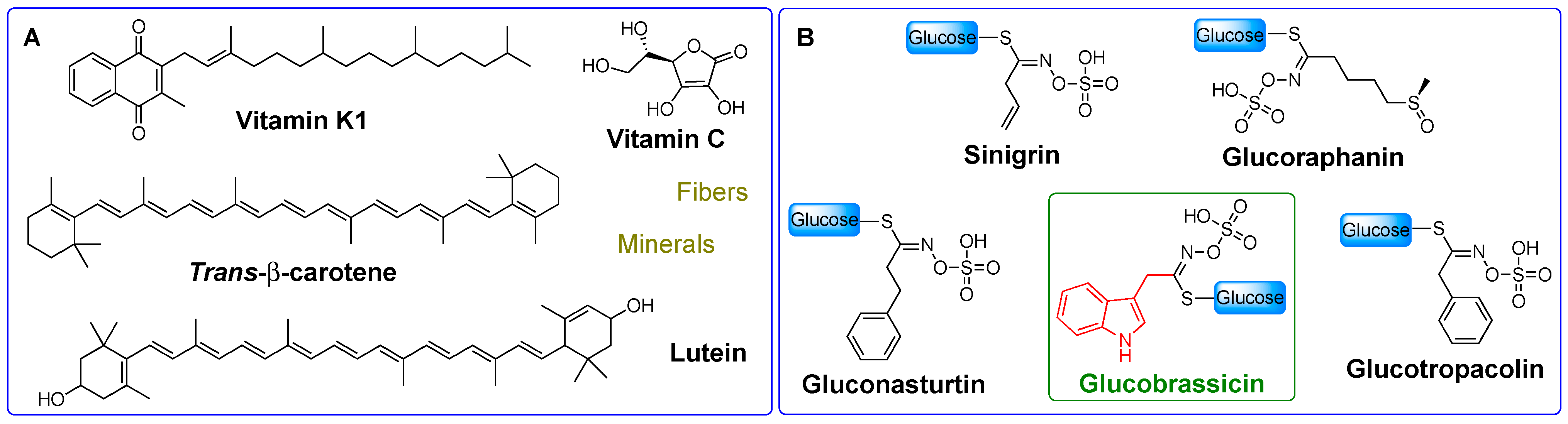
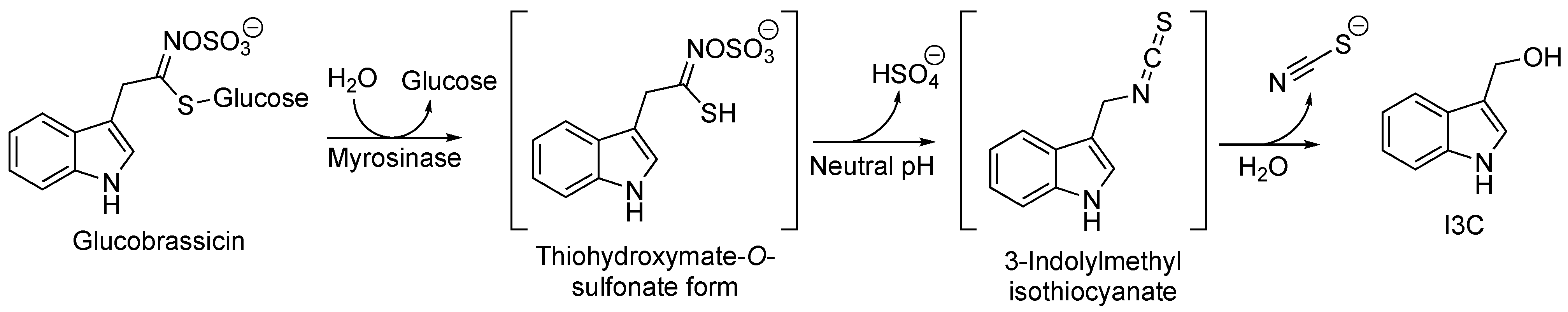
2.1. I3C Biosynthesis
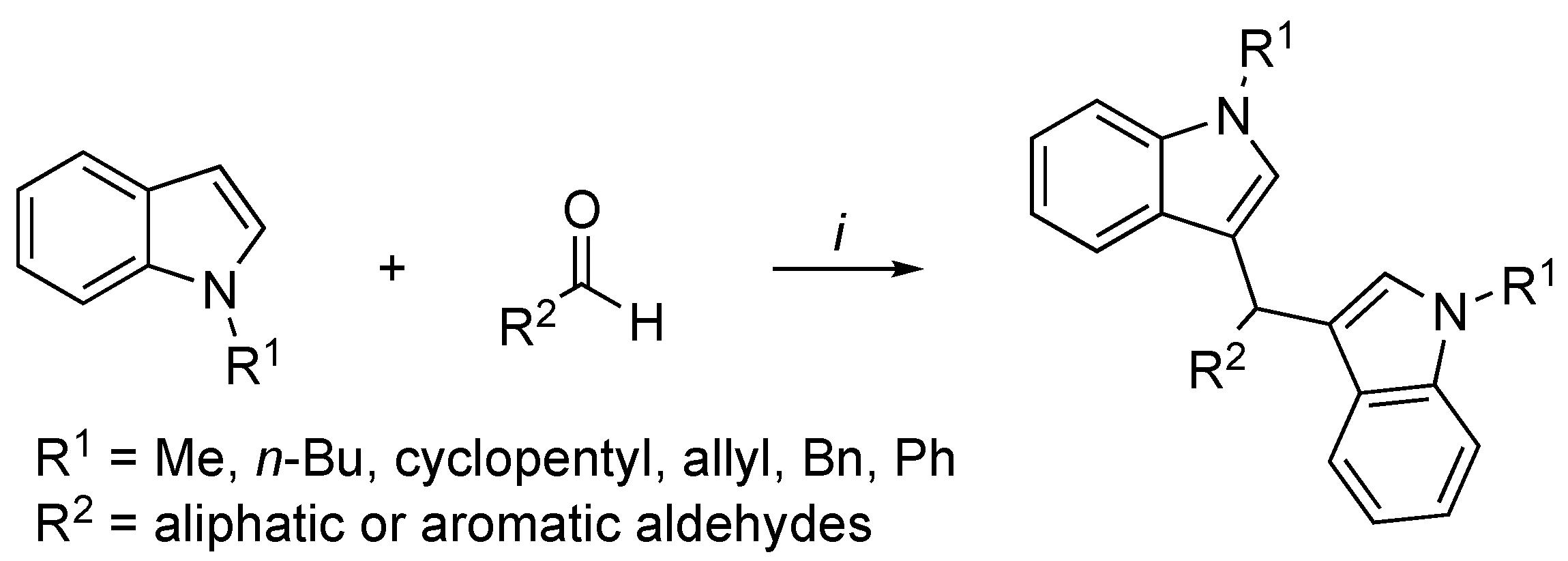
2.2. I3C Synthetic Methodologies
2.3. I3C Antitumor Activity: In Vitro, In Vivo, and Clinical Studies
2.4. I3C Anti-Inflammatory Activity: In Vitro, In Vivo, and Clinical Trials
2.5. I3C Antiviral Activity and Potential Application on COVID-19 Therapy
2.6. I3C Antimicrobial Activity
2.7. I3C Antioxidant Activity
2.8. I3C Antiobesity Activity and Chronic Diseases
2.9. Other I3C Activities
3. Natural and Synthetic Derivatives of I3C: (Bio)Synthesis, In Vitro, and In Vivo Activity, and Mechanisms of Action
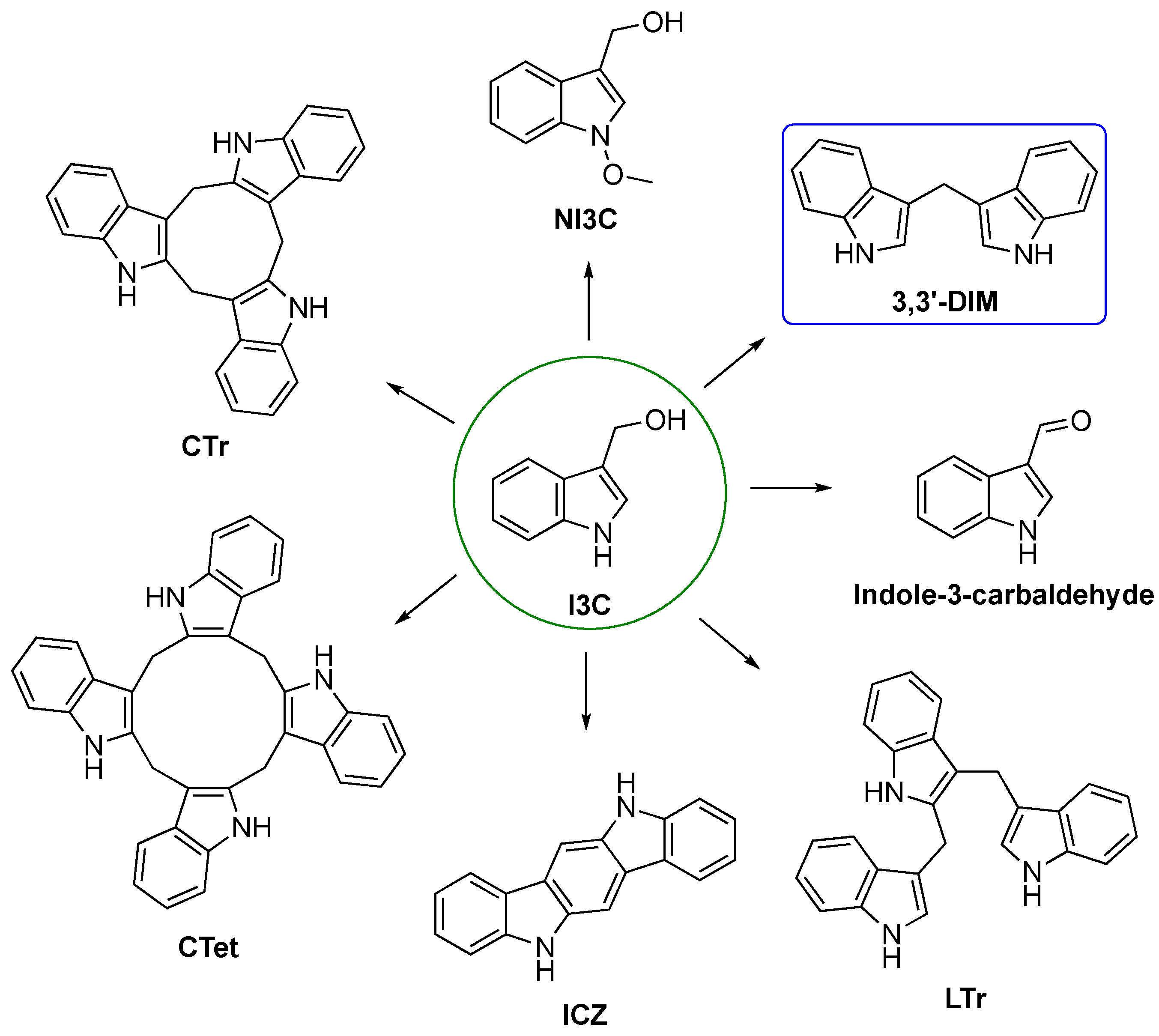
3.1. Biosynthesis of I3C Endogenous Derivatives
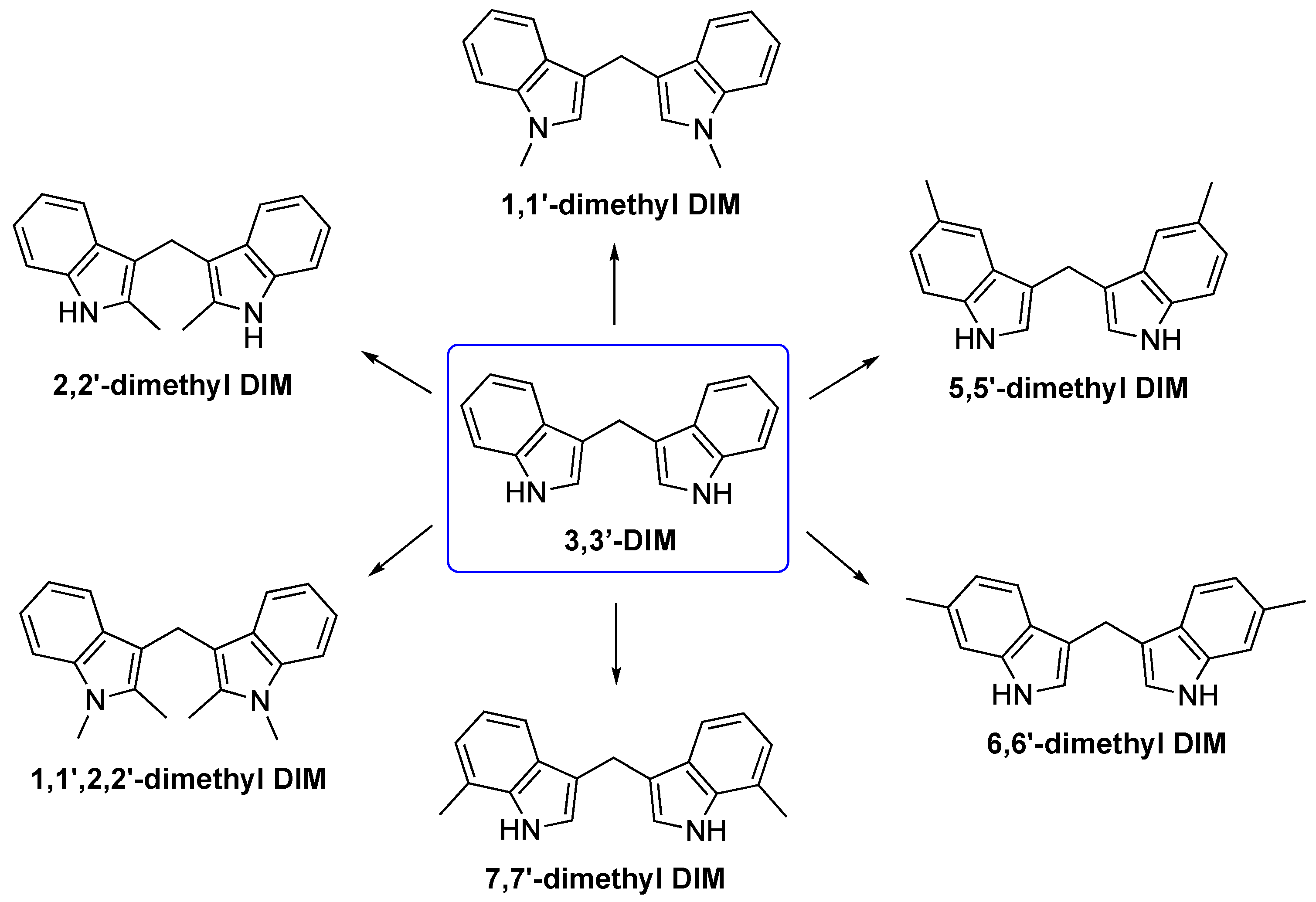
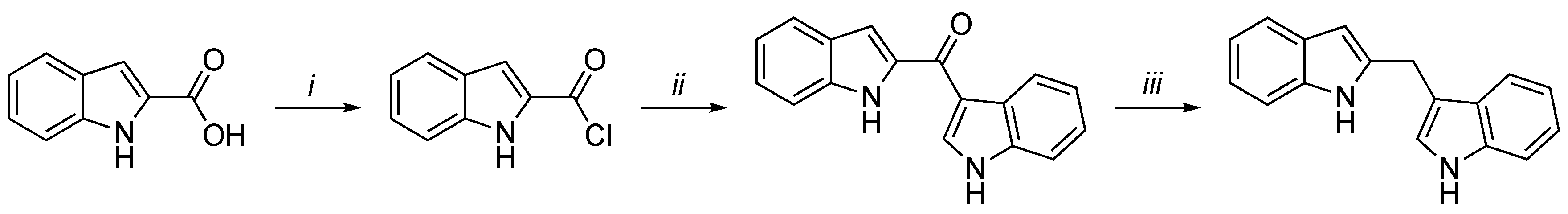
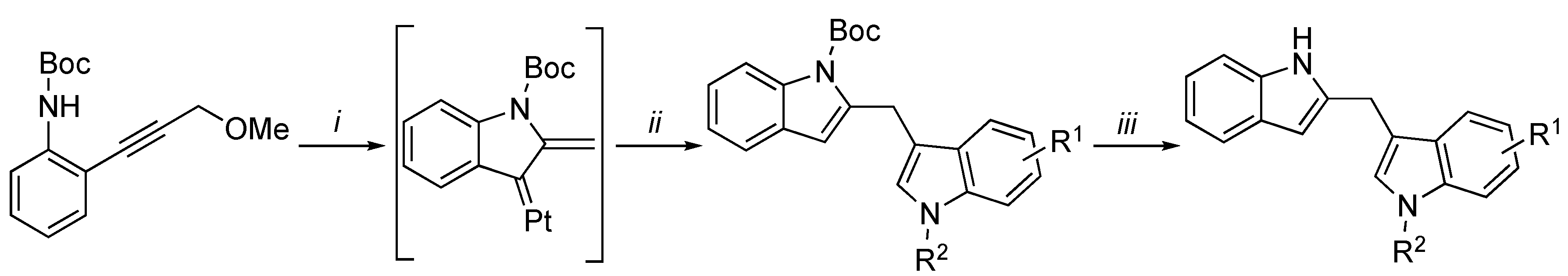
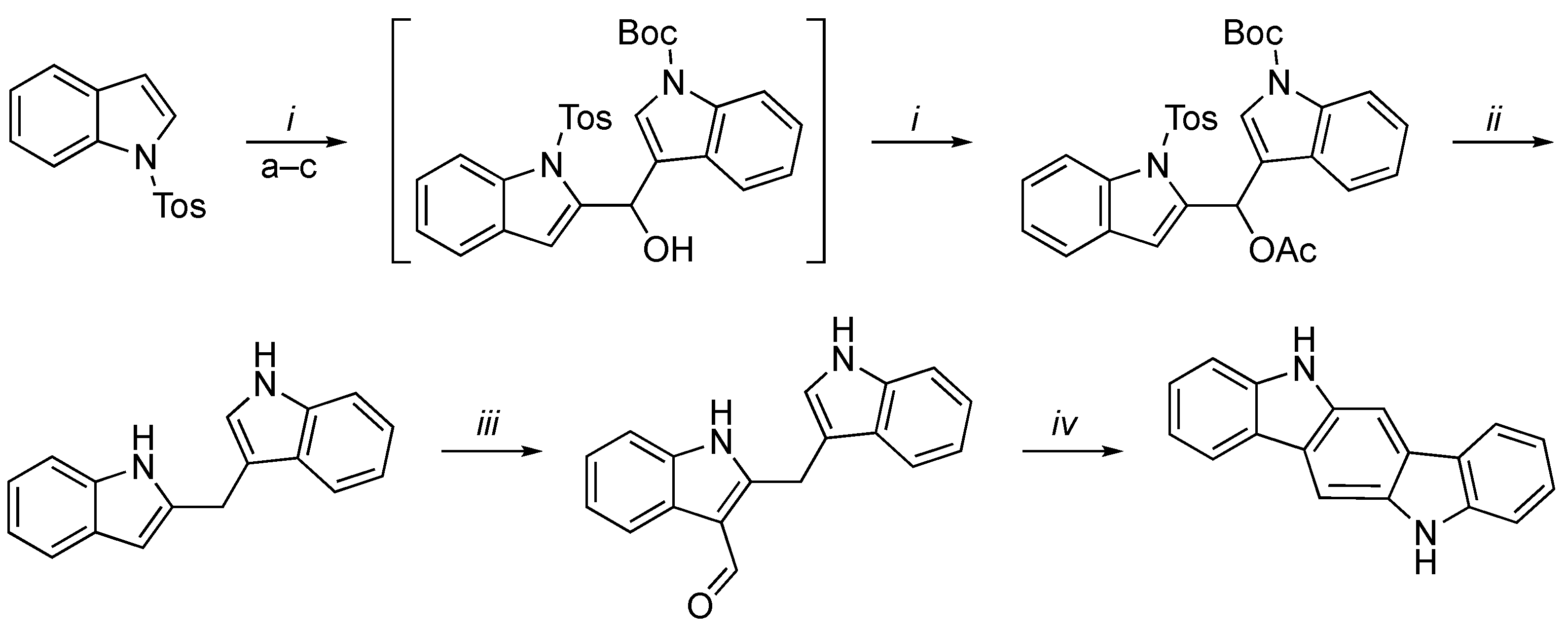
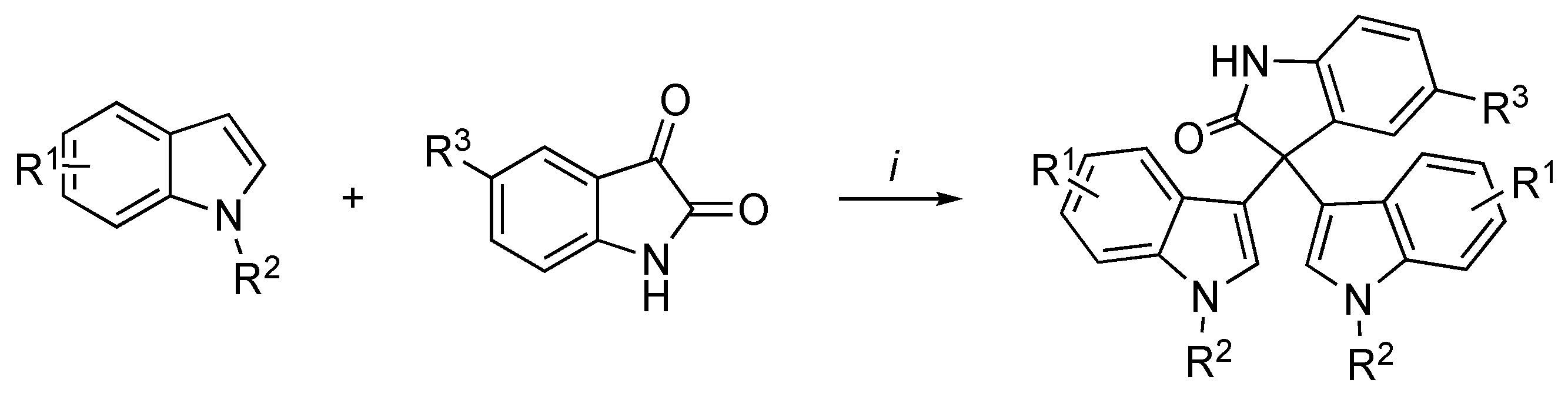
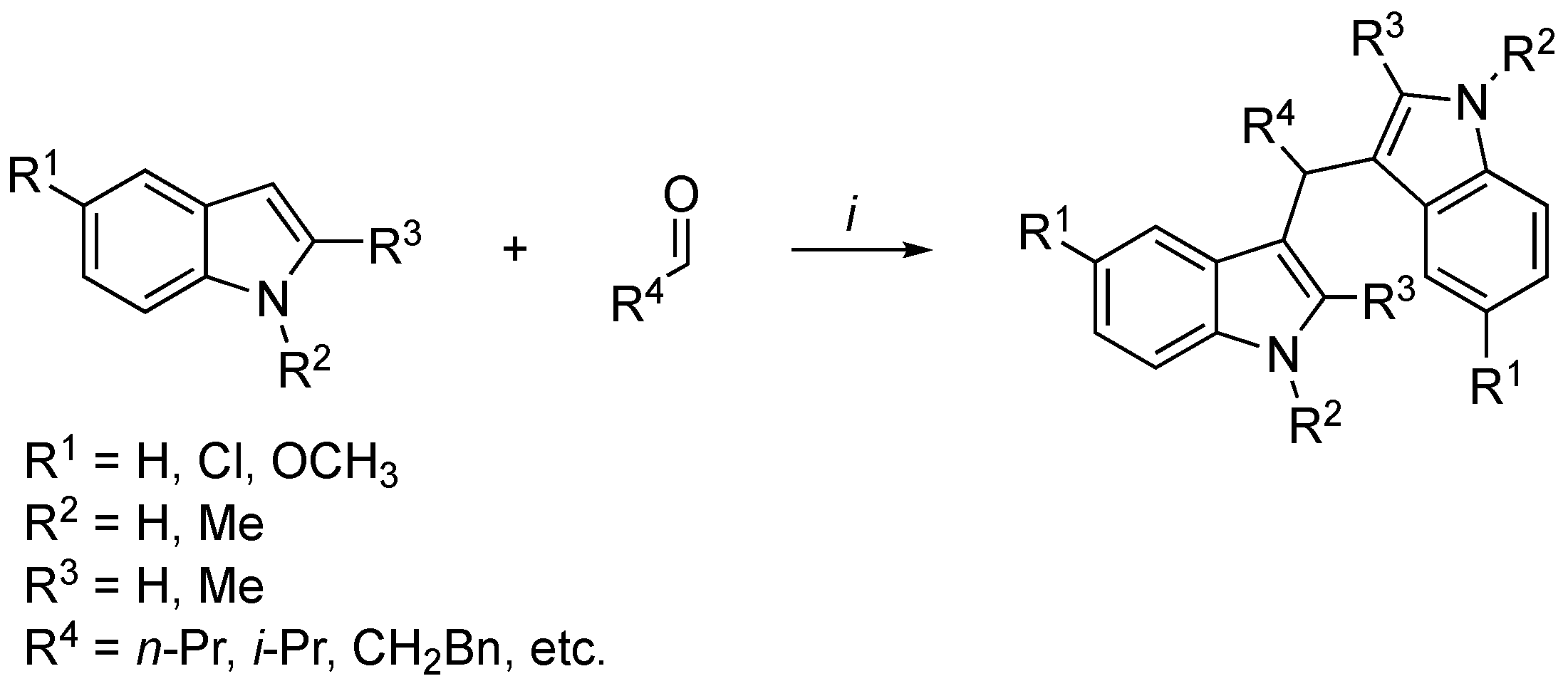
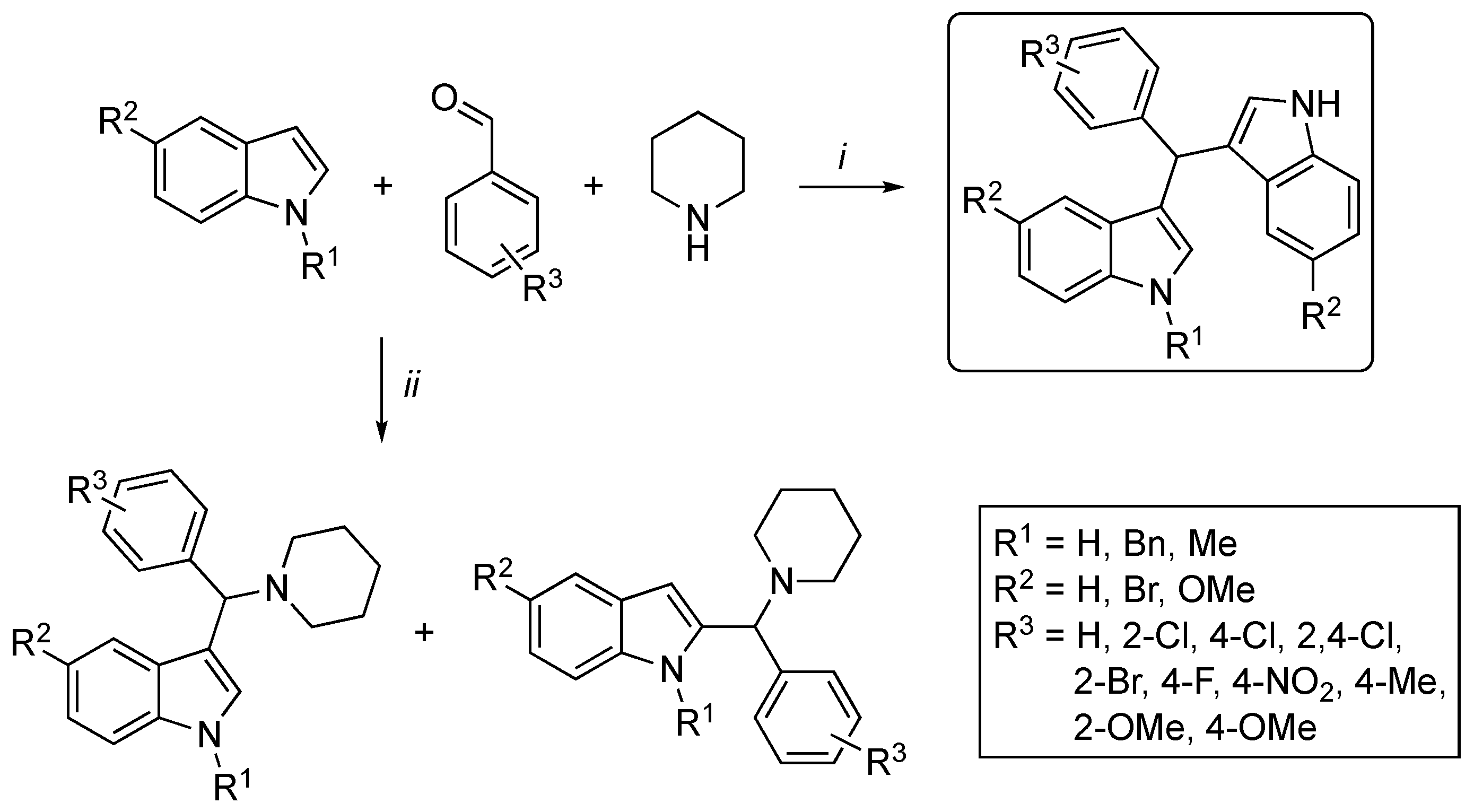
3.2. Synthetic Methodologies of I3C Exogenous Derivatives
3.3. In Vitro and In Vivo Activity and Mechanism of Action of I3C Derivatives
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. Glucosinolates from cruciferous vegetables and their potential role in chronic disease: Investigating the preclinical and clinical evidence. Front. Pharmacol. 2021, 12, 767975. [Google Scholar] [CrossRef] [PubMed]
- Ağagündüz, D.; Şahin, T.Ö.; Yılmaz, B.; Ekenci, K.D.; Duyar, Ö.Ş.; Capasso, R. Cruciferous vegetables and their bioactive metabolites: From prevention to novel therapies of colorectal cancer. Evid. Based Complement. Alternat. Med. 2022, 11, 1534083. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, L.F.M.F.; Alvarenga, L.A.; Ribeiro, M.; Dai, L.; Shiels, P.G.; Stenvinkel, P.; Lindholm, B.; Mafra, D. Cruciferous vegetables: Rationale for exploring potential salutary effects of sulforaphane-rich foods in patients with chronic kidney disease. Nutr Rev. 2021, 11, 1204–1224. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.J.; Ramirez, D.; Mazzei, L.; Parra, M.; Casarotto, M.; Calvo, J.P.; Cuello, C.D.; Ponce, Z.A.Z.; Diez, E.R.; Camargo, A.; et al. Anti-inflammatory, antioxidant, antihypertensive, and antiarrhythmic effect of indole-3-carbinol, a phytochemical derived from cruciferous vegetables. Heliyon 2022, 17, 08989. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Emran, T.B.; Chandran, D.; Zidan, B.M.R.M.; Das, R.; Mamada, S.S.; Masyita, A.; Salampe, M.; Nainu, F.; Khandaker, M.U.; et al. Cruciferous vegetables as a treasure of functional foods bioactive compounds: Targeting p53 family in gastrointestinal tract and associated cancers. Front Nutr. 2022, 9, 951935. [Google Scholar] [CrossRef]
- Johnson, I.T. Cruciferous vegetables and risk of cancers of the gastrointestinal tract. Mol. Nutr. Food Res. 2018, 62, e1701000. [Google Scholar] [CrossRef]
- Virtanen, A.I. Studies on organic sulphur compounds and other labile substances in plants. Pythochemistry 1965, 4, 207–228. [Google Scholar] [CrossRef]
- Wattenberg, L.W.; Loub, W.D. Inhibition of polycyclic aromatic hydrocarbon-induced neoplasia by naturally occurring indoles. Cancer Res. 1978, 38, 1410–1413. [Google Scholar]
- Li, Q.; Xia, B.; Wu, J.; Yuan, X.; Lu, X.; Huang, C.; Gu, H.; Zheng, K.; You, Q.; Liu, K. Indole-3-carbinol (I3C) protects the heart from ischemia/reperfusion injury by inhibiting oxidative stress, inflammation, and cellular apoptosis in mice. Front. Pharmacol. 2022, 13, 924174. [Google Scholar] [CrossRef]
- Novelli, G.; Liu, J.; Biancolella, M.; Alonzi, T.; Novelli, A.; Patten, J.J.; Cocciadiferro, D.; Agolini, E.; Colona, V.L.; Rizzacasa, B.; et al. Inhibition of HECT E3 ligases as potential therapy for COVID-19. Cell Death Dis. 2021, 12, 310. [Google Scholar] [CrossRef]
- Centofanti, F.; Alonzi, T.; Latini, A.; Spitaleri, P.; Murdocca, M.; Chen, X.; Cui, W.; Shang, Q.; Goletti, D.; Shi, Y.; et al. Indole-3-carbinol in vitro antiviral activity against SARS-CoV-2 virus and in vivo toxicity. Cell Death Discov. 2022, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Wattenberg, L.W. Effects of dietary constituents on the metabolism of chemical carcinogens. Cancer Res. 1975, 35, 3326–3331. [Google Scholar] [PubMed]
- Loub, W.D.; Wattenberg, L.W.; Davis, D.W. Aryl hydrocarbon hydroxylase induction in rat tissues by naturally occurring indoles of cruciferous plants. J. Natl. Cancer Inst. 1975, 54, 985–988. [Google Scholar] [PubMed]
- Bradlow, H.L.; Michnovicz, J.; Telang, N.T.; Osborne, M.P. Effects of dietary indole-3-carbinol on estradiol metabolism and spontaneous mammary tumors in mice. Carcinogenesis 1991, 12, 1571–1574. [Google Scholar] [CrossRef] [PubMed]
- Scholar, E.M.; Wolterman, K.; Birt, D.F.; Bresnick, E. The effect of dietary fat on metastasis of the Lewis lung carcinoma and the BALB/c mammary carcinoma. Nutr. Cancer 1989, 12, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Brandi, G.; Fraternale, A.; Lucarini, S.; Paiardini, M.; De Santi, M.; Cervasi, B.; Paoletti, M.F.; Galluzzi, L.; Duranti, A.; Magnani, M. Antitumoral activity of indole-3-carbinol cyclic tri- and tetrameric derivatives mixture in human breast cancer cells: In vitro and in vivo studies. Anticancer Agents Med. Chem. 2013, 13, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Kroymann, J.; Mitchell-Olds, T. The glucosinolate–myrosinase system in an ecological and evolutionary context. Curr. Opin. Plant Biol. 2005, 8, 264–271. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Liu, Y.; Miao, H.; Cai, C.; Shao, Z.; Guo, R.; Sun, B.; Jia, C.; Zhang, L.; et al. Classic myrosinase-dependent degradation of indole glucosinolate attenuates fumonisin B1-induced programmed cell death in Arabidopsis. Plant J. 2015, 81, 920–933. [Google Scholar] [CrossRef]
- Chen, X.; Fan, H.; Zhang, S.; Yu, C.; Wang, W. Facile installation of 2-reverse prenyl functionality into indoles by a tandem n-alkylation-aza-cope rearrangement reaction and its application in synthesis. Chem. Eur. J. 2016, 22, 716–723. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, D.; Wang, J.; Kang, C. Efficient synthesis and biological activity of novel indole derivatives as VEGFR-2 tyrosine kinase inhibitors. Russ. J. Gen. Chem. 2017, 87, 3006–3016. [Google Scholar] [CrossRef]
- Downey, C.W.; Poff, C.D.; Nizinski, A.N. Friedel-Crafts hydroxyalkylation of indoles mediated by trimethylsilyl trifluoromethanesulfonate. J. Org. Chem. 2015, 80, 10364–10369. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, D.T.; Goldbohm, R.A.; van Poppel, G.; Verhagen, H.; van den Brandt, P.A. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol. Biomarkers Prev. 1996, 5, 733–748. [Google Scholar] [PubMed]
- Firestone, G.L.; Bjeldanes, L.F. Indole-3-carbinol and 3-3’-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions. J. Nutr. 2003, 133, 2448S–2455S. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.-R.; Tsai, C.-H.; Kulp, S.K.; Chen, C.-S. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008, 262, 153–163. [Google Scholar] [CrossRef]
- Katz, E.; Nisani, S.; Chamovitz, D.A. Indole-3-carbinol: A plant hormone combatting cancer. F1000Research 2018, 7, 689. [Google Scholar] [CrossRef]
- Quirit, J.G.; Lavrenov, S.N.; Poindexter, K.; Xu, J.; Kyauk, C.; Durkin, K.A.; Aronchik, I.; Tomasiak, T.; Solomatin, Y.A.; Preobrazhenskaya, M.N.; et al. Indole-3-carbinol (I3C) analogues are potent small molecule inhibitors of NEDD4-1 ubiquitin ligase activity that disrupt proliferation of human melanoma cells. Biochem. Pharmacol. 2017, 127, 13–27. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Chen, M.; Lee, J.D.; Zhang, J.; Lin, S.-Y.; Fu, T.-M.; Chen, H.; Ishikawa, T.; Chiang, S.-Y.; Katon, J.; et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science 2019, 364, eaau0159. [Google Scholar] [CrossRef]
- Kishikawa, T.; Higuchi, H.; Wang, L.; Panch, N.; Maymi, V.; Best, S.; Lee, S.; Notoya, G.; Toker, A.; Matesic, L.E.; et al. WWP1 inactivation enhances efficacy of PI3K inhibitors while suppressing their toxicities in breast cancer models. J. Clin. Investig. 2021, 131, e140436. [Google Scholar] [CrossRef]
- Song, M.S.; Pandolfi, P.P. The HECT family of E3 ubiquitin ligases and PTEN. Semin. Cancer Biol. 2022, 85, 43–51. [Google Scholar] [CrossRef]
- Del Priore, G.; Gudipudi, D.K.; Montemarano, N.; Restivo, A.M.; Malanowska-Stega, J.; Arslan, A.A. Oral diindolylmethane (DIM): Pilot evaluation of a nonsurgical treatment for cervical dysplasia. Gynecol. Oncol. 2010, 116, 464–467. [Google Scholar] [CrossRef]
- Rajoria, S.; Suriano, R.; Parmar, P.S.; Wilson, Y.L.; Megwalu, U.; Moscatello, A.; Bradlow, H.L.; Sepkovic, D.W.; Geliebter, J.; Schantz, S.P.; et al. 3,3’-diindolylmethane modulates estrogen metabolism in patients with thyroid proliferative disease: A pilot study. Thyroid 2011, 21, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Sepkovic, D.W.; Raucci, L.; Stein, J.; Carlisle, A.D.; Auborn, K.; Ksieski, H.B.; Nyirenda, T.; Bradlow, H.L. 3,3’-Diindolylmethane increases serum interferon-γ levels in the K14-HPV16 transgenic mouse model for cervical cancer. In Vivo 2012, 26, 207–211. [Google Scholar] [PubMed]
- Fan, S.; Meng, Q.; Xu, J.; Jiao, Y.; Zhao, L.; Zhang, X.; Sarkar, F.H.; Brown, M.L.; Dritschilo, A.; Rosen, E.M. DIM (3,3’-diindolylmethane) confers protection against ionizing radiation by a unique mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18650–18655. [Google Scholar] [CrossRef] [PubMed]
- Perez-Chacon, G.; de Los Rios, C.; Zapata, J.M. Indole-3-carbinol induces cMYC and IAP-family downmodulation and promotes apoptosis of Epstein-Barr virus (EBV)-positive but not of EBV-negative Burkitt’s lymphoma cell lines. Pharmacol. Res. 2014, 89, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, L.; Sukhikh, G.; Kiselev, V.; Paltsev, M.; Drukh, V.; Kuznetsov, I.; Muyzhnek, E.; Apolikhina, I.; Andrianova, E. Double-blind randomized placebo-controlled multicenter clinical trial (phase IIa) on diindolylmethane’s efficacy and safety in the treatment of CIN: Implications for cervical cancer prevention. EPMA J. 2015, 6, 25. [Google Scholar] [CrossRef]
- Morales-Prieto, D.M.; Herrmann, J.; Osterwald, H.; Kochhar, P.S.; Schleussner, E.; Markert, U.R.; Oettel, M. Comparison of dienogest effects upon 3,3’-diindolylmethane supplementation in models of endometriosis and clinical cases. Reprod. Biol. 2018, 18, 252–258. [Google Scholar] [CrossRef]
- Rzemieniec, J.; Wnuk, A.; Lasoń, W.; Bilecki, W.; Kajta, M. The neuroprotective action of 3,3’-diindolylmethane against ischemia involves an inhibition of apoptosis and autophagy that depends on HDAC and AhR/CYP1A1 but not ERα/CYP19A1 signaling. Apoptosis 2019, 24, 435–452. [Google Scholar] [CrossRef]
- Esteve, M. Mechanisms underlying biological effects of cruciferous glucosinolate-derived isothiocyanates/indoles: A focus on metabolic syndrome. Front. Nutr. 2020, 7, 111. [Google Scholar] [CrossRef]
- NIH ClinicalTrials.gov. Available online: https://beta.clinicaltrials.gov/search?distance=50&term=indole-3-carbinol&viewType=Table&limit=25 (accessed on 20 December 2022).
- Karimabad, M.N.; Mahmoodi, M.; Jafarzadeh, A.; Darekordi, A.; Hajizadeh, M.R.; Hassanshahi, G. Molecular targets, anti-cancer properties and potency of synthetic indole-3-carbinol derivatives. Mini Rev. Med. Chem. 2019, 19, 540–554. [Google Scholar] [CrossRef]
- Mazurakova, A.; Koklesova, L.; Samec, M.; Kudela, E.; Kajo, K.; Skuciova, V.; Csizmár, S.H.; Mestanova, V.; Pec, M.; Adamkov, M.; et al. Anti-breast cancer effects of phytochemicals: Primary, secondary, and tertiary care. EPMA J. 2022, 13, 315–334. [Google Scholar] [CrossRef]
- Biancolella, M.; Testa, B.; Baghernajad Salehi, L.; D’Apice, M.R.; Novelli, G. Genetics and genomics of breast cancer: Update and translational perspectives. Semin. Cancer Biol. 2021, 72, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.M.N.; Araújo, C.B.B.; Barros, A.L.S.; Sato, M.R.; Oshiro-Júnior, J.A. Nanostructured lipid carrier as a strategy for the treatment of breast cancer. In Interdisciplinary Cancer Research; Springer: Cham, Switzerland, 2022; pp. 1–27. [Google Scholar] [CrossRef]
- Firestone, G.L.; Sundar, S.N. Minireview: Modulation of hormone receptor signaling by dietary anticancer indoles. Mol. Endocrinol. 2009, 23, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Sakr, W.A.; Rahman, K.M.W. Anticancer properties of indole compounds: Mechanism of apoptosis induction and role in chemotherapy. Curr. Drug Targets 2010, 11, 652–666. [Google Scholar] [CrossRef]
- Ahmad, A.; Sakr, W.A.; Rahman, K.M.W. Novel targets for detection of cancer and their modulation by chemopreventive natural compounds. Front. Biosci. 2012, 4, 410–425. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.H.; Li, Y.; Wang, Z.; Kong, D. Cellular signaling perturbation by natural products. Cell. Signal. 2009, 21, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.-Y.; Weng, J.-R.; Chiu, C.-F.; Wu, C.-Y.; Yeh, S.-P.; Sargeant, A.M.; Lin, P.-H.; Liao, Y.-M. OSU-A9, an indole-3-carbinol derivative, induces cytotoxicity in acute myeloid leukemia through reactive oxygen species-mediated apoptosis. Biochem. Pharmacol. 2013, 86, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Aronchik, I.; Kundu, A.; Quirit, J.G.; Firestone, G.L. The antiproliferative response of indole-3-carbinol in human melanoma cells is triggered by an interaction with NEDD4-1 and disruption of wild-type PTEN degradation. Mol. Cancer Res. 2014, 12, 1621–1634. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Aronchik, I.; Brar, G.A.; Nguyen, D.H.H.; Bjeldanes, L.F.; Firestone, G.L. The dietary phytochemical indole-3-carbinol is a natural elastase enzymatic inhibitor that disrupts cyclin E protein processing. Proc. Natl. Acad. Sci. USA 2008, 105, 19750–19755. [Google Scholar] [CrossRef]
- Aronchik, I.; Bjeldanes, L.F.; Firestone, G.L. Direct inhibition of elastase activity by indole-3-carbinol triggers a CD40-TRAF regulatory cascade that disrupts NF-kappaB transcriptional activity in human breast cancer cells. Cancer Res. 2010, 70, 4961–4971. [Google Scholar] [CrossRef]
- Aronchik, I.; Chen, T.; Durkin, K.A.; Horwitz, M.S.; Preobrazhenskaya, M.N.; Bjeldanes, L.F.; Firestone, G.L. Target protein interactions of indole-3-carbinol and the highly potent derivative 1-benzyl-I3C with the C-terminal domain of human elastase uncouples cell cycle arrest from apoptotic signaling. Mol. Carcinog. 2012, 51, 881–894. [Google Scholar] [CrossRef]
- Scully, R.; Chen, J.; Plug, A.; Xiao, Y.; Weaver, D.; Feunteun, J.; Ashley, T.; Livingston, D.M. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell 1997, 88, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.A.; Peterson, K.S.; Smith, H.J.; Gray, J.C.; Sullivan, D.K.; Mayo, M.S.; Crowell, J.A.; Hurwitz, A. A phase I study of indole-3-carbinol in women: Tolerability and effects. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1953–1960. [Google Scholar] [CrossRef]
- McMenamin, M.E.; Soung, P.; Perera, S.; Kaplan, I.; Loda, M.; Sellers, W.R. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999, 59, 4291–4296. [Google Scholar]
- Vincent, E.E.; Elder, D.J.E.; Thomas, E.C.; Phillips, L.; Morgan, C.; Pawade, J.; Sohail, M.; May, M.T.; Hetzel, M.R.; Tavaré, J.M. Akt phosphorylation on Thr308 but not on Ser473 correlates with Akt protein kinase activity in human non-small cell lung cancer. Br. J. Cancer 2011, 104, 1755–1761. [Google Scholar] [CrossRef]
- Cover, C.M.; Hsieh, S.J.; Tran, S.H.; Hallden, G.; Kim, G.S.; Bjeldanes, L.F.; Firestone, G.L. Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. J. Biol. Chem. 1998, 273, 3838–3847. [Google Scholar] [CrossRef]
- Kim, Y.S.; Milner, J.A. Targets for indole-3-carbinol in cancer prevention. J. Nutr. Biochem. 2005, 16, 65–73. [Google Scholar] [CrossRef]
- Watson, G.W.; Beaver, L.M.; Williams, D.E.; Dashwood, R.H.; Ho, E. Phytochemicals from cruciferous vegetables, epigenetics, and prostate cancer prevention. AAPS J. 2013, 15, 951–961. [Google Scholar] [CrossRef]
- Kaur, P.; Shorey, L.E.; Ho, E.; Dashwood, R.H.; Williams, D.E. The epigenome as a potential mediator of cancer and disease prevention in prenatal development. Nutr. Rev. 2013, 71, 441–457. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Khor, T.O.; Su, Z.-Y.; Saw, C.L.-L.; Shu, L.; Cheung, K.-L.; Huang, Y.; Yu, S.; Kong, A.-N.T. Epigenetic modifications of Nrf2 by 3,3’-diindolylmethane in vitro in TRAMP C1 cell line and in vivo TRAMP prostate tumors. AAPS J. 2013, 15, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.P.; Hsu, A.; Buchanan, A.; Palomera-Sanchez, Z.; Beaver, L.M.; Houseman, E.A.; Williams, D.E.; Dashwood, R.H.; Ho, E. Effects of sulforaphane and 3,3’-diindolylmethane on genome-wide promoter methylation in normal prostate epithelial cells and prostate cancer cells. PLoS ONE 2014, 9, e86787. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.A.; Patil, M.P.; Kang, M.-J.; Niyonizigiye, I.; Kim, G.-D. Biomedical application of Indole-3-carbinol: A mini-review. Phytochem. Lett. 2021, 41, 49–54. [Google Scholar] [CrossRef]
- Shorey, L.E.; Hagman, A.M.; Williams, D.E.; Ho, E.; Dashwood, R.H.; Benninghoff, A.D. 3,3’-Diindolylmethane induces G1 arrest and apoptosis in human acute T-cell lymphoblastic leukemia cells. PLoS ONE 2012, 7, e34975. [Google Scholar] [CrossRef]
- Kiselev, V.I.; Drukh, V.M.; Muyzhnek, E.L.; Kuznetsov, I.N.; Pchelintseva, O.I.; Paltsev, M.A. Preclinical antitumor activity of the diindolylmethane formulation in xenograft mouse model of prostate cancer. Exp. Oncol. 2014, 36, 90–93. [Google Scholar]
- Wu, Y.; Li, R.W.; Huang, H.; Fletcher, A.; Yu, L.; Pham, Q.; Yu, L.; He, Q.; Wang, T.T.Y. Inhibition of Tumor Growth by Dietary Indole-3-Carbinol in a Prostate Cancer Xenograft Model May Be Associated with Disrupted Gut Microbial Interactions. Nutrients 2019, 11, 467. [Google Scholar] [CrossRef]
- Kim, D.J.; Han, B.S.; Ahn, B.; Hasegawa, R.; Shirai, T.; Ito, N.; Tsuda, H. Enhancement by indole-3-carbinol of liver and thyroid gland neoplastic development in a rat medium-term multiorgan carcinogenesis model. Carcinogenesis 1997, 18, 377–381. [Google Scholar] [CrossRef]
- Shimamoto, K.; Hayashi, H.; Taniai, E.; Morita, R.; Imaoka, M.; Ishii, Y.; Suzuki, K.; Shibutani, M.; Mitsumori, K. Antioxidant N-acetyl-L-cysteine (NAC) supplementation reduces reactive oxygen species (ROS)-mediated hepatocellular tumor promotion of indole-3-carbinol (I3C) in rats. J. Toxicol. Sci. 2011, 36, 775–786. [Google Scholar] [CrossRef]
- Oganesian, A.; Hendricks, J.D.; Williams, D.E. Long term dietary indole-3-carbinol inhibits diethylnitrosamine-initiated hepatocarcinogenesis in the infant mouse model. Cancer Lett. 1997, 118, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kawajiri, K.; Kobayashi, Y.; Ohtake, F.; Ikuta, T.; Matsushima, Y.; Mimura, J.; Pettersson, S.; Pollenz, R.S.; Sakaki, T.; Hirokawa, T.; et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc. Natl. Acad. Sci. USA 2009, 106, 13481–13486. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kwon, H.-S.; Kim, D.H.; Shin, E.K.; Kang, Y.-H.; Park, J.H.Y.; Shin, H.-K.; Kim, J.-K. 3,3’-diindolylmethane attenuates colonic inflammation and tumorigenesis in mice. Inflamm. Bowel Dis. 2009, 15, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Lubet, R.A.; Heckman, B.M.; De Flora, S.L.; Steele, V.E.; Crowell, J.A.; Juliana, M.M.; Grubbs, C.J. Effects of 5,6-benzoflavone, indole-3-carbinol (I3C) and diindolylmethane (DIM) on chemically-induced mammary carcinogenesis: Is DIM a substitute for I3C? Oncol. Rep. 2011, 26, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Benninghoff, A.D.; Williams, D.E. The role of estrogen receptor β in transplacental cancer prevention by indole-3-carbinol. Cancer Prev. Res. 2013, 6, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Staub, R.E.; Feng, C.; Onisko, B.; Bailey, G.S.; Firestone, G.L.; Bjeldanes, L.F. Fate of indole-3-carbinol in cultured human breast tumor cells. Chem. Res. Toxicol. 2002, 15, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.Y.; Milner, M.J.; Milner, J.A.; Kim, Y.S. Estrogen receptor alpha as a target for indole-3-carbinol. J. Nutr. Biochem. 2006, 17, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Staub, R.E.; Onisko, B.; Bjeldanes, L.F. Fate of 3,3’-diindolylmethane in cultured MCF-7 human breast cancer cells. Chem. Res. Toxicol. 2006, 19, 436–442. [Google Scholar] [CrossRef]
- Bradlow, H.L. Review. Indole-3-carbinol as a chemoprotective agent in breast and prostate cancer. In Vivo 2008, 22, 441–445. [Google Scholar]
- Saati, G.E.; Archer, M.C. Inhibition of fatty acid synthase and Sp1 expression by 3,3’-diindolylmethane in human breast cancer cells. Nutr. Cancer 2011, 63, 790–794. [Google Scholar] [CrossRef]
- Tin, A.S.; Park, A.H.; Sundar, S.N.; Firestone, G.L. Essential role of the cancer stem/progenitor cell marker nucleostemin for indole-3-carbinol anti-proliferative responsiveness in human breast cancer cells. BMC Biol. 2014, 12, 72. [Google Scholar] [CrossRef]
- Thomson, C.A.; Ho, E.; Strom, M.B. Chemopreventive properties of 3,3’-diindolylmethane in breast cancer: Evidence from experimental and human studies. Nutr. Rev. 2016, 74, 432–443. [Google Scholar] [CrossRef]
- Lee, J. 3,3’-Diindolylmethane inhibits TNF-α- and TGF-β-induced epithelial- mesenchymal transition in breast cancer cells. Nutr. Cancer 2019, 71, 992–1006. [Google Scholar] [CrossRef]
- Maruthanila, V.L.; Poornima, J.; Mirunalini, S. Attenuation of carcinogenesis and the mechanism underlying by the influence of indole-3-carbinol and its metabolite 3,3’-diindolylmethane: A therapeutic marvel. Adv. Pharmacol. Sci. 2014, 2014, 832161. [Google Scholar] [CrossRef]
- Fujioka, N.; Fritz, V.; Upadhyaya, P.; Kassie, F.; Hecht, S.S. Research on cruciferous vegetables, indole-3-carbinol, and cancer prevention: A tribute to Lee W. Wattenberg. Mol. Nutr. Food Res. 2016, 60, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Kassie, F.; Anderson, L.B.; Scherber, R.; Yu, N.; Lahti, D.; Upadhyaya, P.; Hecht, S.S. Indole-3-carbinol inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo(a)pyrene-induced lung tumorigenesis in A/J mice and modulates carcinogen-induced alterations in protein levels. Cancer Res. 2007, 67, 6502–6511. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.Y.; Bradlow, L.; Sepkovic, D.; Mehl, S.; Mailman, J.; Osborne, M.P. Dose-ranging study of indole-3-carbinol for breast cancer prevention. J. Cell. Biochem. Suppl. 1997, 28–29, 111–116. [Google Scholar] [CrossRef]
- Cashman, J.R.; Zhang, J. Human flavin-containing monooxygenases. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 65–100. [Google Scholar] [CrossRef] [PubMed]
- Krueger, S.K.; Williams, D.E. Mammalian flavin-containing monooxygenases: Structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol. Ther. 2005, 106, 357–387. [Google Scholar] [CrossRef]
- Larsen-Su, S.; Williams, D.E. Dietary indole-3-carbinol inhibits FMO activity and the expression of flavin-containing monooxygenase form 1 in rat liver and intestine. Drug Metab. Dispos. 1996, 24, 927–931. [Google Scholar] [PubMed]
- Cashman, J.R.; Xiong, Y.; Lin, J.; Verhagen, H.; van Poppel, G.; van Bladeren, P.J.; Larsen-Su, S.; Williams, D.E. In vitro and in vivo inhibition of human flavin-containing monooxygenase form 3 (FMO3) in the presence of dietary indoles. Biochem. Pharmacol. 1999, 58, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Katchamart, S.; Stresser, D.M.; Dehal, S.S.; Kupfer, D.; Williams, D.E. Concurrent flavin-containing monooxygenase down-regulation and cytochrome P-450 induction by dietary indoles in rat: Implications for drug-drug interaction. Drug Metab. Dispos. 2000, 28, 930–936. [Google Scholar]
- Wang, T.; Shankar, K.; Ronis, M.J.; Mehendale, H.M. Potentiation of thioacetamide liver injury in diabetic rats is due to induced CYP2E1. J. Pharmacol. Exp. Ther. 2000, 294, 473–479. [Google Scholar]
- Katchamart, S.; Williams, D.E. Indole-3-carbinol modulation of hepatic monooxygenases CYP1A1, CYP1A2 and FMO1 in guinea pig, mouse and rabbit. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2001, 129, 377–384. [Google Scholar] [CrossRef]
- Vogel, V.G. Reducing the risk of breast cancer with tamoxifen in women at increased risk. J. Clin. Oncol. 2001, 19, 87S–92S. [Google Scholar] [PubMed]
- Poon, G.K.; Walter, B.; Lønning, P.E.; Horton, M.N.; McCague, R. Identification of tamoxifen metabolites in human Hep G2 cell line, human liver homogenate, and patients on long-term therapy for breast cancer. Drug Metab. Dispos. 1995, 23, 377–382. [Google Scholar] [PubMed]
- Bradlow, H.L.; Zeligs, M.A. Diindolylmethane (DIM) spontaneously forms from indole-3-carbinol (I3C) during cell culture experiments. In Vivo 2010, 24, 387–391. [Google Scholar] [PubMed]
- Maier, M.L.V.; Siddens, L.K.; Uesugi, S.L.; Choi, J.; Leonard, S.W.; Pennington, J.M.; Tilton, S.C.; Smith, J.N.; Ho, E.; Chow, H.H.S.; et al. 3,3’-Diindolylmethane Exhibits Significant Metabolism after Oral Dosing in Humans. Drug Metab. Dispos. 2021, 49, 694–705. [Google Scholar] [CrossRef]
- Dalessandri, K.M.; Firestone, G.L.; Fitch, M.D.; Bradlow, H.L.; Bjeldanes, L.F. Pilot study: Effect of 3,3’-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr. Cancer 2004, 50, 161–167. [Google Scholar] [CrossRef]
- Heath, E.I.; Heilbrun, L.K.; Li, J.; Vaishampayan, U.; Harper, F.; Pemberton, P.; Sarkar, F.H. A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3’- Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. Am. J. Transl. Res. 2010, 2, 402–411. [Google Scholar]
- Li, Y.; Sarkar, F.H. Role of BioResponse 3,3’-diindolylmethane in the treatment of human prostate cancer: Clinical experience. Med. Princ. Pract. 2016, 25 (Suppl. S2), 11–17. [Google Scholar] [CrossRef]
- Hwang, C.; Sethi, S.; Heilbrun, L.K.; Gupta, N.S.; Chitale, D.A.; Sakr, W.A.; Menon, M.; Peabody, J.O.; Smith, D.W.; Sarkar, F.H.; et al. Anti-androgenic activity of absorption-enhanced 3, 3’-diindolylmethane in prostatectomy patients. Am. J. Transl. Res. 2016, 8, 166–176. [Google Scholar]
- Paltsev, M.; Kiselev, V.; Drukh, V.; Muyzhnek, E.; Kuznetsov, I.; Andrianova, E.; Baranovskiy, P. First results of the double-blind randomized placebo-controlled multicenter clinical trial of DIM-based therapy designed as personalized approach to reverse prostatic intraepithelial neoplasia (PIN). EPMA J. 2016, 7, 5. [Google Scholar] [CrossRef]
- Thomson, C.A.; Chow, H.H.S.; Wertheim, B.C.; Roe, D.J.; Stopeck, A.; Maskarinec, G.; Altbach, M.; Chalasani, P.; Huang, C.; Strom, M.B.; et al. A randomized, placebo-controlled trial of diindolylmethane for breast cancer biomarker modulation in patients taking tamoxifen. Breast Cancer Res. Treat. 2017, 165, 97–107. [Google Scholar] [CrossRef]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008, 47 (Suppl. S2), 73–88. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Tang, L. Cruciferous vegetable intake and cancer prevention: Role of nutrigenetics. Cancer Prev. Res. 2009, 2, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Park, J.H.Y. Cruciferous vegetable intake and the risk of human cancer: Epidemiological evidence. Proc. Nutr. Soc. 2009, 68, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, E.; Lachnitt, N.; Rudzitis-Auth, J.; Schmitt, B.M.; Menger, M.D.; Laschke, M.W. Indole-3-carbinol is a potent inhibitor of ischemia-reperfusion-induced inflammation. J. Surg. Res. 2017, 215, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, J.; Liang, Y.; Li, J.; Ding, S.; Wang, Y.; Chen, Y.; Liu, J. Advances in drug therapy for Systemic Lupus Erythematosus. Curr. Med. Chem. 2021, 28, 1251–1268. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Y.; Ming, B.; Wu, X.; Chen, Y.; Dong, L. Regulatory T-cell levels in systemic lupus erythematosus patients: A meta-analysis. Lupus 2019, 28, 445–454. [Google Scholar] [CrossRef]
- Mohammadi, S.; Memarian, A.; Sedighi, S.; Behnampour, N.; Yazdani, Y. Immunoregulatory effects of indole-3-carbinol on monocyte-derived macrophages in systemic lupus erythematosus: A crucial role for aryl hydrocarbon receptor. Autoimmunity 2018, 51, 199–209. [Google Scholar] [CrossRef]
- Yan, X.-J.; Qi, M.; Telusma, G.; Yancopoulos, S.; Madaio, M.; Satoh, M.; Reeves, W.H.; Teichberg, S.; Kohn, N.; Auborn, K.; et al. Indole-3-carbinol improves survival in lupus-prone mice by inducing tandem B- and T-cell differentiation blockades. Clin. Immunol. 2009, 131, 481–494. [Google Scholar] [CrossRef]
- Auborn, K.J.; Qi, M.; Yan, X.J.; Teichberg, S.; Chen, D.; Madaio, M.P.; Chiorazzi, N. Lifespan is prolonged in autoimmune-prone (NZB/NZW) F1 mice fed a diet supplemented with indole-3-carbinol. J. Nutr. 2003, 133, 3610–3613. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Gulin, J.; Chen, T.; Klug, T.; Lahita, R.; Nuite, M. Indole-3-carbinol in women with SLE: Effect on estrogen metabolism and disease activity. Lupus 2001, 10, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.; Gustafsson, J.-A. Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nucl. Recept. Signal. 2006, 4, e016. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Food, immunity, and the microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical importance of indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef] [PubMed]
- Megna, B.W.; Carney, P.R.; Kennedy, G.D. Intestinal inflammation and the diet: Is food friend or foe? World J. Gastrointest. Surg. 2016, 8, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wiatrak, B.J. Overview of recurrent respiratory papillomatosis. Curr. Opin. Otolaryngol. Head Neck Surg. 2003, 11, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Essman, E.J.; Abramson, A. Estrogen binding sites on membranes from human laryngeal papilloma. Int. J. Cancer 1984, 33, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Coll, D.A.; Rosen, C.A.; Auborn, K.; Potsic, W.P.; Bradlow, H.L. Treatment of recurrent respiratory papillomatosis with indole-3-carbinol. Am. J. Otolaryngol. 1997, 18, 283–285. [Google Scholar] [CrossRef]
- Tsalik, E.L.; Fiorino, C.; Aqeel, A.; Liu, Y.; Henao, R.; Ko, E.R.; Burke, T.W.; Reller, M.E.; Bodinayake, C.K.; Nagahawatte, A.; et al. The host response to viral infections reveals common and virus-specific Signatures in the peripheral blood. Front. Immunol. 2021, 12, 741837. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Peterson, D.R.; Falsey, A.R. Human metapneumovirus infections in adults: Another piece of the puzzle. Arch. Intern. Med. 2008, 168, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.L.; Starita, L.; Kiniry, E.; Phillips, C.H.; Wellwood, S.; Cho, S.; Kiavand, A.; Truong, M.; Han, P.; Richardson, M.; et al. Incidence of medically attended acute respiratory illnesses due to respiratory viruses across the life course during the 2018/19 influenza season. Clin. Infect. Dis. 2021, 73, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Spitalieri, P.; Centofanti, F.; Murdocca, M.; Scioli, M.G.; Latini, A.; Cesare, S.D.; Citro, G.; Rossi, A.; Orlandi, A.; Miersch, S.; et al. Two different therapeutic approaches for SARS-CoV-2 in hiPSCs-derived lung organoids. Cells 2022, 11, 1235. [Google Scholar] [CrossRef]
- Murdocca, M.; Citro, G.; Romeo, I.; Lupia, A.; Miersch, S.; Amadio, B.; Bonomo, A.; Rossi, A.; Sidhu, S.S.; Pandolfi, P.P.; et al. Peptide platform as a powerful tool in the fight against COVID-19. Viruses 2021, 13, 1667. [Google Scholar] [CrossRef]
- COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 20 December 2022).
- Olliaro, P.; Torreele, E.; Vaillant, M. COVID-19 vaccines: Effectiveness and number needed to treat—Authors’ reply. Lancet. Microbe 2021, 2, e282. [Google Scholar] [CrossRef]
- Feikin, D.R.; Abu-Raddad, L.J.; Andrews, N.; Davies, M.-A.; Higdon, M.M.; Orenstein, W.A.; Patel, M.K. Assessing vaccine effectiveness against severe COVID-19 disease caused by omicron variant. Report from a meeting of the World Health Organization. Vaccine 2022, 40, 3516–3527. [Google Scholar] [CrossRef]
- Kuhlmann, C.; Mayer, C.K.; Claassen, M.; Maponga, T.; Burgers, W.A.; Keeton, R.; Riou, C.; Sutherland, A.D.; Suliman, T.; Shaw, M.L.; et al. Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. Lancet 2022, 399, 625–626. [Google Scholar] [CrossRef]
- Parums, D. V Editorial: SARS-CoV-2 vaccine responses and breakthrough COVID-19. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e935624. [Google Scholar] [CrossRef]
- Elmorsy, M.A.; El-Baz, A.M.; Mohamed, N.H.; Almeer, R.; Abdel-Daim, M.M.; Yahya, G. In silico screening of potent inhibitors against COVID-19 key targets from a library of FDA-approved drugs. Environ. Sci. Pollut. Res. Int. 2022, 29, 12336–12346. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Joyce, R.; Tan, H.; Hu, Y.; Wang, J. SARS-CoV-2 main protease drug design, assay development, and drug resistance studies. Acc. Chem. Res. 2023, 56, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Vegivinti, C.T.R.; Evanson, K.W.; Lyons, H.; Akosman, I.; Barrett, A.; Hardy, N.; Kane, B.; Keesari, P.R.; Pulakurthi, Y.S.; Sheffels, E.; et al. Efficacy of antiviral therapies for COVID-19: A systematic review of randomized controlled trials. BMC Infect. Dis. 2022, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Lovato, E.C.W.; Barboza, L.N.; Wietzikoski, S.; de Souza, A.N.V.; Auth, P.A.; Junior, A.G.; Dos Reis Lívero, F.A. Repurposing drugs for the management of patients with confirmed coronavirus disease 2019 (COVID-19). Curr. Pharm. Des. 2021, 27, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Jiang, D.; Zhang, Y.; Zhang, J.; Xu, T.; Sun, Y.; Fan, J.; Wang, J.; Chang, N.; Wu, Y.; et al. Elevated ubiquitination contributes to protective immunity against severe SARS-CoV-2 infection. Clin. Transl. Med. 2022, 12, e1103. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, V.J.E.; Huynh, N.; Palà, J.; Patel, A.; Singer, A.; Slater, C.; Chung, J.; van Huizen, M.; Teyra, J.; Miersch, S.; et al. Ubiquitin variants potently inhibit SARS-CoV-2 PLpro and viral replication via a novel site distal to the protease active site. PloS Pathog. 2022, 18, e1011065. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.S.; Lee, D.G. In vitro antimicrobial activity and the mode of action of indole-3-carbinol against human pathogenic microorganisms. Biol. Pharm. Bull. 2007, 30, 1865–1869. [Google Scholar] [CrossRef]
- Sung, W.S.; Lee, D.G. Mechanism of decreased susceptibility for Gram-negative bacteria and synergistic effect with ampicillin of indole-3-carbinol. Biol. Pharm. Bull. 2008, 31, 1798–1801. [Google Scholar] [CrossRef]
- Sung, W.S.; Lee, D.G. The candidacidal activity of indole-3-carbinol that binds with DNA. IUBMB Life 2007, 59, 408–412. [Google Scholar] [CrossRef]
- Julliard, W.; De Wolfe, T.J.; Fechner, J.H.; Safdar, N.; Agni, R.; Mezrich, J.D. Amelioration of Clostridium difficile Infection in Mice by Dietary Supplementation with Indole-3-carbinol. Ann. Surg. 2017, 265, 1183–1191. [Google Scholar] [CrossRef]
- Ismail, A.; Marjan, Z.M.; Foong, C.W. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Shertzer, H.G.; Berger, M.L.; Tabor, M.W. Intervention in free radical mediated hepatotoxicity and lipid peroxidation by indole-3-carbinol. Biochem. Pharmacol. 1988, 37, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Nho, C.W.; Jeffery, E. Crambene, a bioactive nitrile derived from glucosinolate hydrolysis, acts via the antioxidant response element to upregulate quinone reductase alone or synergistically with indole-3-carbinol. Toxicol. Appl. Pharmacol. 2004, 198, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Sanchez-Bravo, J.; Acosta, M. Indole-3-carbinol as a scavenger of free radicals. Biochem. Mol. Biol. Int. 1996, 39, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, Y.; Park, S.; Lee, K.W.; Park, T. Indole-3-carbinol prevents diet-induced obesity through modulation of multiple genes related to adipogenesis, thermogenesis or inflammation in the visceral adipose tissue of mice. J. Nutr. Biochem. 2012, 23, 1732–1739. [Google Scholar] [CrossRef]
- Trujillo, M.E.; Scherer, P.E. Adipose tissue-derived factors: Impact on health and disease. Endocr. Rev. 2006, 27, 762–778. [Google Scholar] [CrossRef]
- Wang, M.L.; Lin, S.H.; Hou, Y.Y.; Chen, Y.H. Suppression of lipid accumulation by indole-3-carbinol is associated with increased expression of the aryl hydrocarbon receptor and cyp1b1 proteins in adipocytes and with decreased adipocyte-stimulated endothelial tube formation. Int. J. Mol. Sci. 2016, 17, 1256. [Google Scholar] [CrossRef]
- Chang, H.-P.; Wang, M.-L.; Hsu, C.-Y.; Liu, M.-E.; Chan, M.-H.; Chen, Y.-H. Suppression of inflammation-associated factors by indole-3-carbinol in mice fed high-fat diets and in isolated, co-cultured macrophages and adipocytes. Int. J. Obes. 2011, 35, 1530–1538. [Google Scholar] [CrossRef]
- Mohamad, K.A.; El-Naga, R.N.; Wahdan, S.A. Neuroprotective effects of indole-3-carbinol on the rotenone rat model of Parkinson’s disease: Impact of the SIRT1-AMPK signaling pathway. Toxicol. Appl. Pharmacol. 2022, 435, 115853. [Google Scholar] [CrossRef]
- Grose, K.R.; Bjeldanes, L.F. Oligomerization of indole-3-carbinol in aqueous acid. Chem Res Toxicol. 1992, 5, 188–193. [Google Scholar] [CrossRef]
- Williams, D.E. Indoles derived from glucobrassicin: Cancer chemoprevention by indole-3-carbinol and 3,3′-diindolylmethane. Front Nutr. 2021, 8, 734334. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Ichikawa, H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 2005, 4, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Bjeldanes, L.F.; Kim, J.-Y.; Grose, K.R.; Bartholomew, J.C.; Bradfield, C.A. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: Comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc. Natl. Acad. Sci. USA 1991, 88, 9543–9547. [Google Scholar] [CrossRef] [PubMed]
- De Kruif, C.A.; Marsman, J.W.; Venekamp, J.C.; Falke, H.E.; Noordhoek, J.; Blaauboer, B.J.; Wortelboer, H.M. Structure elucidation of acid reaction products of indole-3-carbinol: Detection in vivo and enzyme induction in vitro. Chem.-Biol. Interact. 1991, 80, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Riby, J.E.; Feng, C.; Chang, Y.-C.; Schaldach, C.M.; Firestone, G.L.; Bjeldanes, L.F. The major cyclic trimeric product of indole-3-carbinol is a strong agonist of the estrogen receptor signaling pathway. Biochemistry 2000, 39, 910–918. [Google Scholar] [CrossRef]
- Brandi, G.; Paiardini, M.; Cervasi, B.; Fiorucci, C.; Filippone, P.; De Marco, C.; Zaffaroni, N.; Magnani, M. A new indole-3-carbinol tetrameric derivative inhibits cyclin-dependent kinase 6 expression, and induces G1 cell cycle arrest in both estrogen-dependent and estrogen-independent breast cancer cell lines. Cancer Res. 2003, 63, 4028–4036. [Google Scholar]
- d’Argy, R.; Bergman, J.; Dencker, L. Effects of immunosuppressive chemicals on lymphoid development in fetal thymus organ cultures. Pharmacol. Toxicol. 1989, 64, 33–38. [Google Scholar] [CrossRef]
- Wortelboer, H.M.; Van der Linden, E.C.; de Kruif, C.A.; Noordhoek, J.; Blaauboer, B.J.; van Bladeren, P.J.; Falke, H.E. Effects of indole-3-carbinol on biotransformation enzymes in the rat: In vivo changes in liver and small intestinal mucosa in comparison with primary hepatocyte cultures. Food Chem. Toxicol. 1992, 30, 589–599. [Google Scholar] [CrossRef]
- Neave, A.S.; Sarup, S.M.; Seidelin, M.; Duus, F.; Vang, O. Characterization of the N-methoxyindole-3-carbinol (NI3C)—Induced cell cycle arrest in human colon cancer cell lines. Toxicol. Sci. 2005, 83, 126–135. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Riby, J.; Chang, G.H.-F.; Peng, B.C.; Firestone, G.; Bjeldanes, L.F. Cytostatic and antiestrogenic effects of 2-(indol-3-ylmethyl)-3,3’-diindolylmethane, a major in vivo product of dietary indole-3-carbinol. Biochem. Pharmacol. 1999, 58, 825–834. [Google Scholar] [CrossRef]
- Wahlström, N.; Stensland, B.; Bergman, J. Synthesis of 2,3′-diindolylmethanes and substituted indolo[3,2-b]carbazoles. Synthesis 2004, 8, 1187–1194. [Google Scholar] [CrossRef]
- Winston-McPherson, G.N.; Shu, D.; Tang, W. Synthesis and biological evaluation of 2,3’-diindolylmethanes as agonists of aryl hydrocarbon receptor. Bioorg. Med. Chem. 2014, 27, 1561–1565. [Google Scholar] [CrossRef]
- Wille, G.; Mayser, P.; Thoma, W.; Monsees, T.; Baumgart, A.; Schmitz, H.J.; Schrenk, D.; Polborn, K.; Steglich, W. Malassezin—A novel agonist of the arylhydrocarbon receptor from the yeast Malassezia furfur. Bioorg. Med. Chem. 2001, 9, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Staub, R.E.; Bjeldanes, L.F. Convenient synthesis of 5,6,11,12,17,18-hexahydrocyclononal[1,2-b:4,5-b′:7,8-b″]triindole, a novel phytoestrogen. J. Org. Chem. 2003, 68, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.; Högberg, S.; Lindström, J.-O. Macrocyclic condensation products of indole and simple aldehydes. Tetrahedron 1970, 26, 3347–3352. [Google Scholar] [CrossRef]
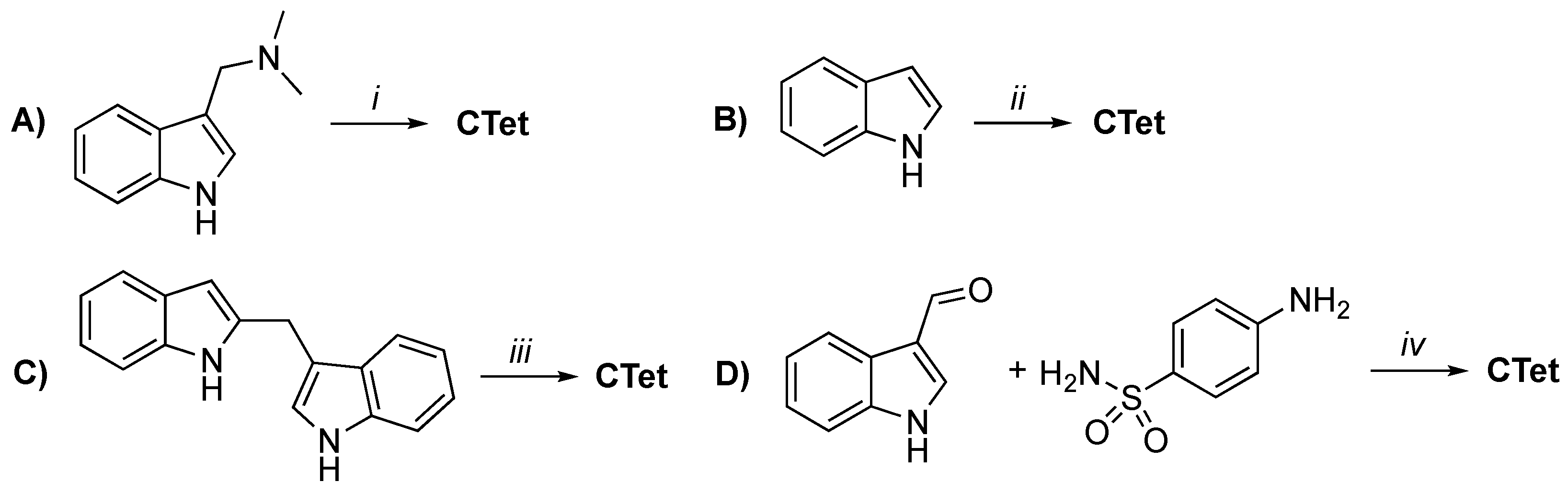
- Lucarini, S.; De Santi, M.; Antonietti, F.; Brandi, G.; Diamantini, G.; Fraternale, A.; Paoletti, M.F.; Tontini, A.; Magnani, M.; Duranti, A. Synthesis and Biological Evaluation of a γ-Cyclodextrin-based Formulation of the Anticancer Agent 5,6,11,12,17,18,23,24- Octahydrocyclododeca[1,2-b:4,5-b′:7,8-b″:10,11-b‴]tetraindole (CTet). Molecules 2010, 15, 4085–4093. [Google Scholar] [CrossRef]
- De Santi, M.; Galluzzi, L.; Lucarini, S.; Paoletti, M.F.; Fraternale, A.; Duranti, A.; De Marco, C.; Fanelli, M.; Zaffaroni, N.; Brandi, G.; et al. The indole-3-carbinol cyclic tetrameric derivative CTet inhibits cell proliferation via overexpression of p21/CDKN1A in both estrogen receptorpositive and triple-negative breast cancer cell lines. Breast Cancer Res. 2011, 13, R33. [Google Scholar] [CrossRef]
- Lucarini, S.; Antonietti, F.; Tontini, A.; Mestichelli, P.; Magnani, M.; Duranti, A. A practical and expeditious method for the preparation of the potential anticancer agent 5,6,11,12,17,18,23,24-octahydrocyclododeca[1,2-b:4,5-b′:7,8-b″:10,11-b‴]tetraindole (CTet). Tetrahedron Lett. 2011, 52, 2812–2814. [Google Scholar] [CrossRef]
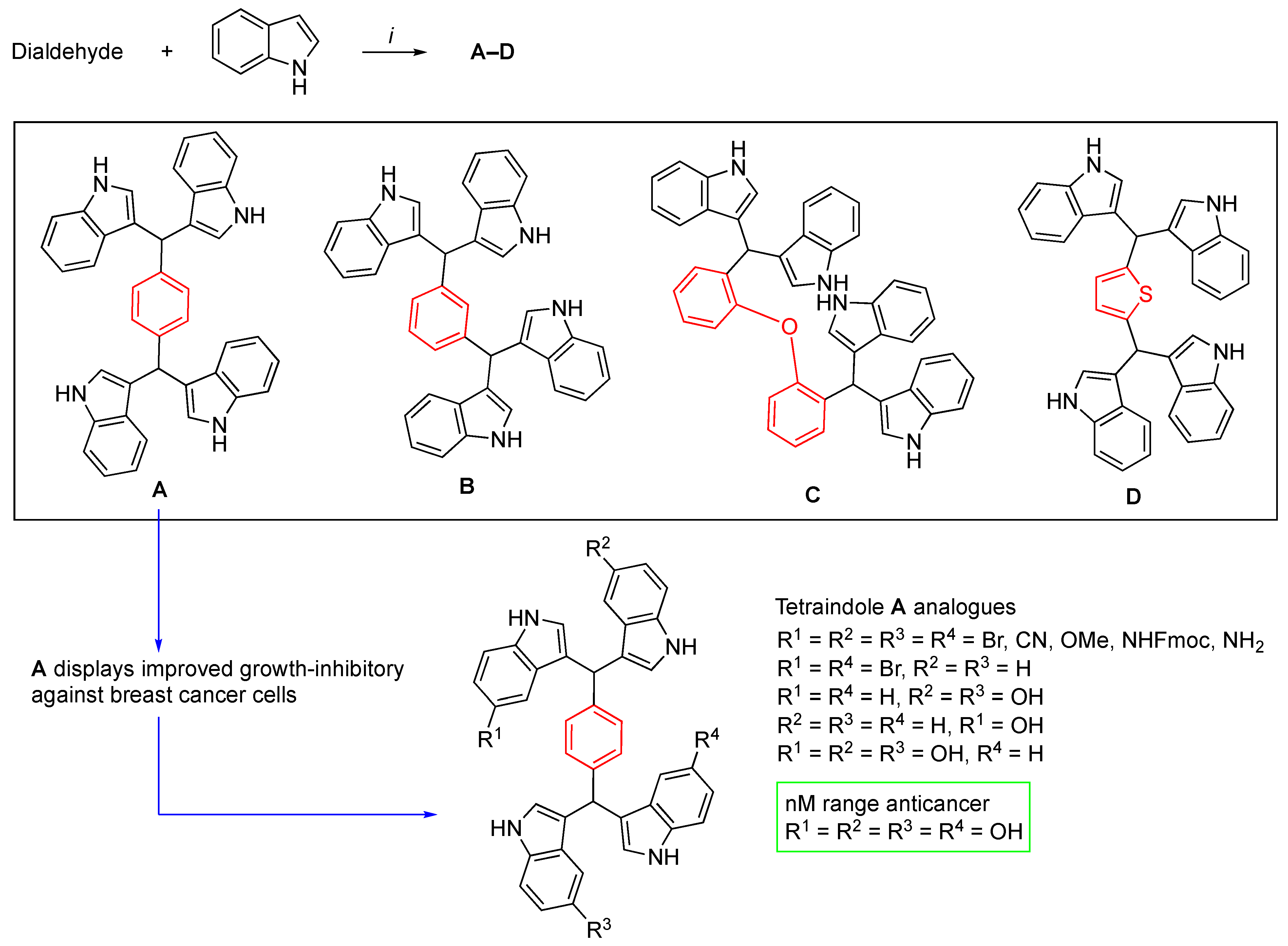
- Li, W.S.; Wang, C.H.; Ko, S.; Chang, T.T.; Jen, Y.C.; Yao, C.F.; More, S.V.; Jao, S.C. Synthesis and evaluation of the cytotoxicities of tetraindoles: Observation that the 5-hydroxy tetraindole (SK228) induces G2 arrest and apoptosis in human breast cancer cells. J. Med. Chem. 2012, 55, 1583–1592. [Google Scholar] [CrossRef]
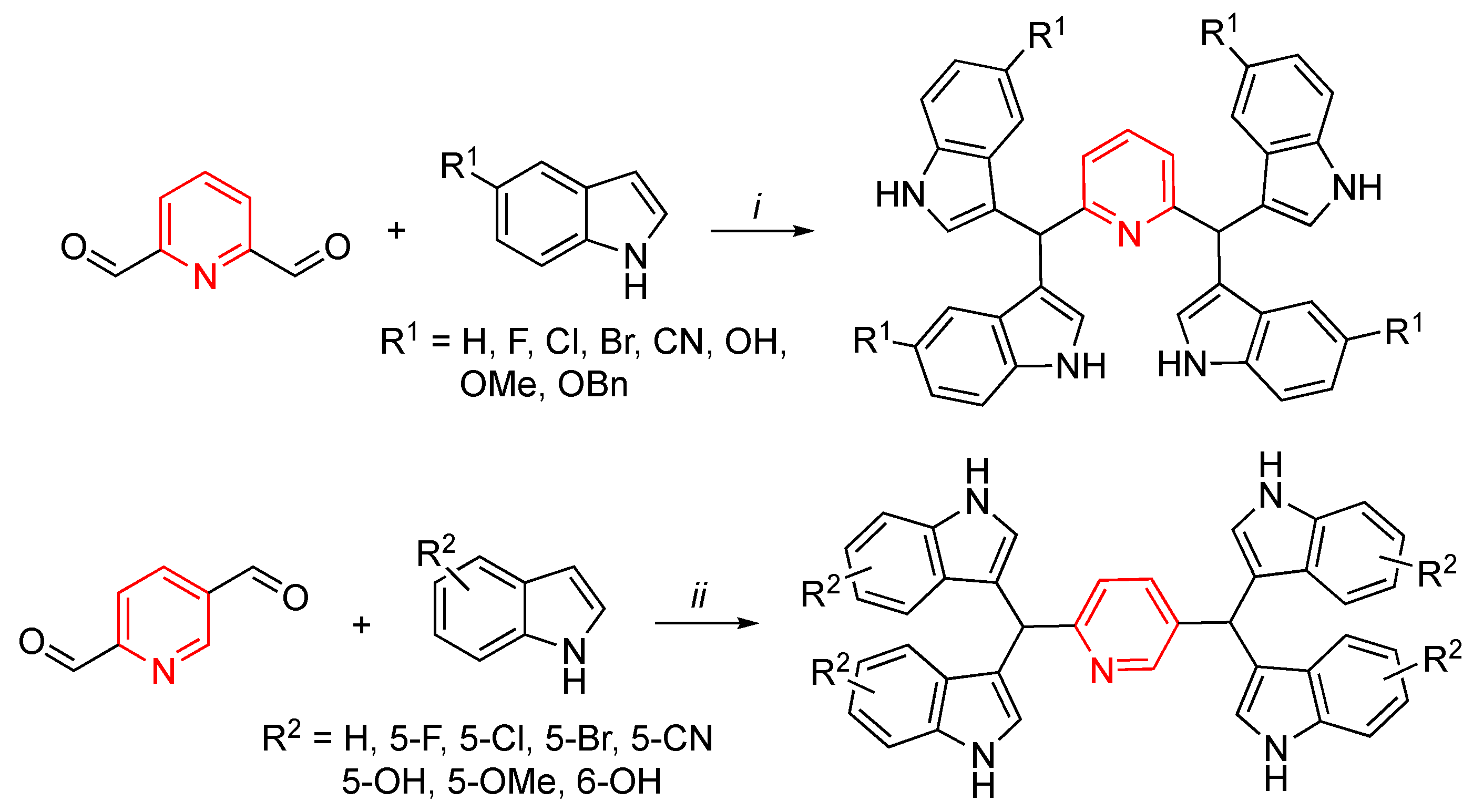
- Fu, C.W.; Hsieh, Y.J.; Chang, T.T.; Chen, C.L.; Yang, C.Y.; Liao, A.; Hsiao, P.W.; Li, W.S. Anticancer efficacy of unique pyridine-based tetraindoles. Eur. J. Med. Chem. 2015, 104, 165–176. [Google Scholar] [CrossRef]
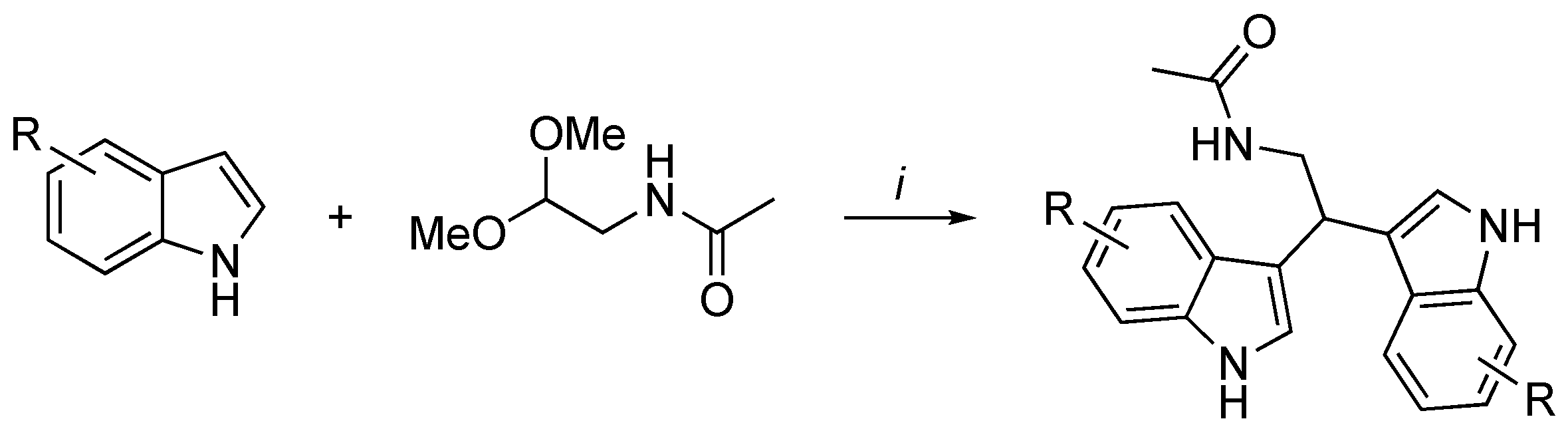
- Mari, M.; Tassoni, A.; Lucarini, S.; Fanelli, M.; Piersanti, G.; Spadoni, G. Brønsted acid catalyzed bisindolization of α-amido acetals: Synthesis and anticancer activity of bis(indolyl)ethanamino derivatives. Eur. J. Org. Chem. 2014, 3822–3830. [Google Scholar] [CrossRef]
- Grosso, C.; Cardoso, A.L.; Lemos, A.; Varela, J.; Rodrigues, M.J.; Custodio, L.; Barreira, L.; Pinho e Melo, T.M.V.D. Novel approach to bis(indolyl)methanes: De novo synthesis of 1-hydroxyiminomethyl derivatives with anti-cancer properties. Eur. J. Med. Chem. 2015, 93, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Srikanth, Y.V.V.; Naseer, M.; Khan, A.; Shaik, T.B.; Ashraf, M. Synthesis of 3,3-diindolyl oxyindoles efficiently catalysed by FeCl3 and their in vitro evaluation for anticancer activity. Bioorg. Med. Chem. Lett. 2010, 20, 5229–5231. [Google Scholar] [CrossRef] [PubMed]
- Hikawa, H.; Suzuki, H.; Yokoyama, Y.; Azumaya, I. Mechanistic studies for synthesis of bis(indolyl)methanes: Pd-Catalyzed C–H activation of indole–carboxylic acids with benzyl alcohols in water. Catalysts 2013, 3, 486–500. [Google Scholar] [CrossRef]
- Beltrá, J.; Gimeno, M.C.; Herrera, R.P. A new approach for the synthesis of bisindoles through AgOTf as catalyst. J. Org. Chem. 2014, 10, 2206–2214. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, M.K.; Kumar, M.; Saxena, D. Micelle promoted multicomponent synthesis of 3-amino alkylated indoles via a Mannich-type reaction in water. RSC Adv. 2013, 3, 1673. [Google Scholar] [CrossRef]
- Galathri, E.M.; Di Terlizzi, L.; Fagnoni, M.; Protti, S.; Kokotos, C.G. Friedel-Crafts arylation of aldehydes with indoles utilizing arylazo sulfones as the photoacid generator. Org. Biomol. Chem. 2022, 10, 1039. [Google Scholar] [CrossRef]
- Biersack, B. 3,3′-Diindolylmethane and its derivatives: Nature-inspired strategies tackling drug resistant tumors by regulation of signal transduction, transcription factors and microRNAs. Cancer Drug Resist. 2020, 3, 867–878. [Google Scholar] [CrossRef]
- Chen, I.; McDougal, A.; Wang, F.; Safe, S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis 1998, 19, 1631–1639. [Google Scholar] [CrossRef]
- Auborn, K.J.; Fan, S.; Rosen, E.M.; Goodwin, L.; Chandraskaren, A.; Williams, D.E.; Chen, D.; Carter, T.H. Indole-3-carbinol is a negative regulator of estrogen. J. Nutr. 2003, 133, 2470S–2475S. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Harnessing the fruits of nature for the development of multi-targeted cancer therapeutics. Cancer Treat. Rev. 2009, 35, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Guo, B. Chemopreventive agent 3,3′-diindolylmethane selectively induces proteasomal degradation of class I histone deacetylases. Cancer Res. 2010, 70, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Beaver, L.M.; Yu, T.-W.; Sokolowski, E.I.; Williams, D.E.; Dashwood, R.H.; Ho, E. 3,3′-Diindolylmethane, but not indole-3-carbinol, inhibits histone deacetylase activity in prostate cancer cells. Toxicol. Appl. Pharmacol. 2012, 263, 345–351. [Google Scholar] [CrossRef]
- Jin, U.-H.; Cheng, Y.; Park, H.; Davidson, L.A.; Callaway, E.S.; Chapkin, R.S.; Jayaraman, A.; Asante, A.; Allred, C.; Weaver, E.A.; et al. Short Chain Fatty Acids Enhance Aryl Hydrocarbon (Ah) Responsiveness in Mouse Colonocytes and Caco-2 Human Colon Cancer Cells. Sci. Rep. 2017, 7, 10163. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.L.; Spink, D.C.; Spink, B.C.; Cao, J.Q.; Walker, N.J.; Sutter, T.R. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc. Natl. Acad. Sci. USA 1996, 93, 9776–9781. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, L.; Liu, Y.; Wang, J.; Jiang, W. Indole-3-carbinol (I3C) and its major derivatives: Their pharmacokinetics and important roles in hepatic protection. Curr. Drug Metabol. 2016, 17, 401–409. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, J.H.; Park, S.; Lee, J. The anticancer agent 3,3′-diindolylmethane inhibits multispecies biofilm formation by acne-causing bacteria and Candida albicans. Microbiol. Spectr. 2022, 10, 0205621. [Google Scholar] [CrossRef]
- Jayakumar, P.; Pugalendi, K.V.; Sankaran, M. Attenuation of hyperglycemia-mediated oxidative stress by indole-3-carbinol and its metabolite 3, 3′-diindolylmethane in C57BL/6J mice. J. Physiol. Biochem. 2014, 70, 525–534. [Google Scholar] [CrossRef]
- Guan, H.; Zhu, L.; Fu, M.; Yang, D.; Tian, S.; Guo, Y.; Cui, C.; Wang, L.; Jiang, H. 3,3′-Diindolylmethane suppresses vascular smooth muscle cell phenotypic modulation and inhibits neointima formation after carotid injury. PLoS ONE 2012, 7, 34957. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Ahmad, A.; Bao, B.; Sarkar, F.H. Antioxidant function of isoflavone and 3,3’-diindolylmethane: Are they important for cancer prevention and therapy? Antioxid. Redox Signal. 2013, 19, 139–150. [Google Scholar] [CrossRef]
- Kong, D.; Heath, E.; Chen, W.; Cher, M.L.; Powell, I.; Heilbrun, L.; Li, Y.; Ali, S.; Sethi, S.; Hassan, O.; et al. Loss of let-7 upregulates EZH2 in prostate cancer consistent with the acquisition of cancer stem cell signatures that are attenuated by BR-DIM. PLoS ONE. 2012, 7, 33729. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Schaldach, C.M.; Janosik, T.; Bergman, J.; Bjeldanes, L.F. Effects of analogs of indole-3-carbinol cyclic trimerization product in human breast cancer cells. Chem.-Biol. Interact. 2005, 152, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; De Santi, M.; Crinelli, R.; De Marco, C.; Zaffaroni, N.; Duranti, A.; Brandi, G.; Magnani, M. Induction of endoplasmic reticulum stress response by the indole-3-carbinol cyclic tetrameric derivative CTet in human breast cancer cell lines. PLoS ONE 2012, 7, 43249. [Google Scholar] [CrossRef] [PubMed]
- De Santi, M.; Galluzzi, L.; Duranti, A.; Magnani, M.; Brandi, G. The indole-3-carbinol cyclic tetrameric derivative CTet synergizes with cisplatin and doxorubicin in triple-negative breast cancer cell lines. Anticancer Res. 2013, 33, 1867–1872. [Google Scholar]
- De Santi, M.; Carloni, E.; Galluzzi, L.; Diotallevi, A.; Lucarini, S.; Magnani, M.; Brandi, G. Inhibition of testosterone aromatization by the indole-3-carbinol derivative CTet in CYP19A1-overexpressing MCF-7 breast cancer cells. Anti-Cancer Agents. Med. Chem. 2015, 15, 894–902. [Google Scholar] [CrossRef]


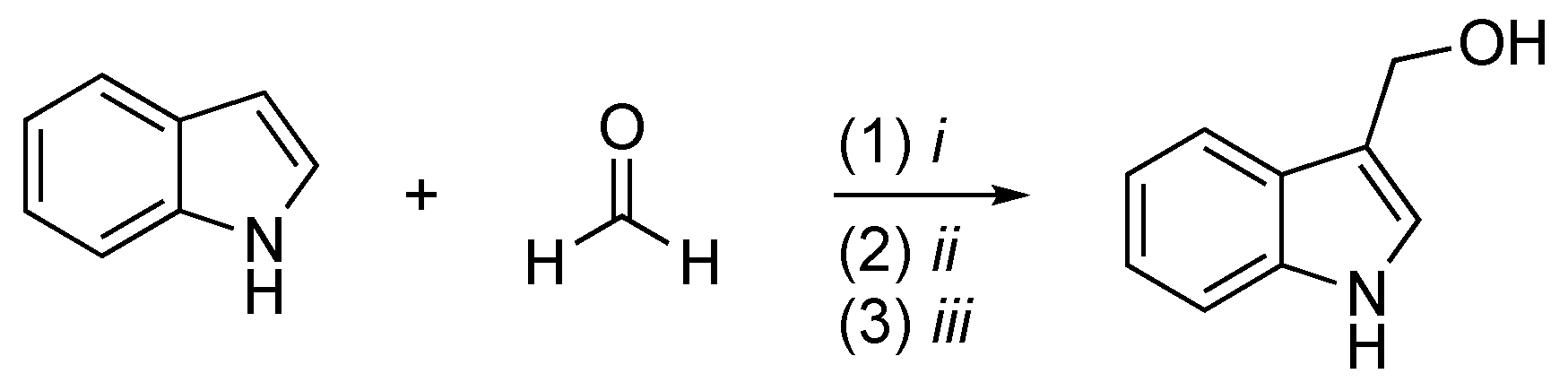



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Centofanti, F.; Buono, A.; Verboni, M.; Tomino, C.; Lucarini, S.; Duranti, A.; Pandolfi, P.P.; Novelli, G. Synthetic Methodologies and Therapeutic Potential of Indole-3-Carbinol (I3C) and Its Derivatives. Pharmaceuticals 2023, 16, 240. https://doi.org/10.3390/ph16020240
Centofanti F, Buono A, Verboni M, Tomino C, Lucarini S, Duranti A, Pandolfi PP, Novelli G. Synthetic Methodologies and Therapeutic Potential of Indole-3-Carbinol (I3C) and Its Derivatives. Pharmaceuticals. 2023; 16(2):240. https://doi.org/10.3390/ph16020240
Chicago/Turabian StyleCentofanti, Federica, Alessandro Buono, Michele Verboni, Carlo Tomino, Simone Lucarini, Andrea Duranti, Pier Paolo Pandolfi, and Giuseppe Novelli. 2023. "Synthetic Methodologies and Therapeutic Potential of Indole-3-Carbinol (I3C) and Its Derivatives" Pharmaceuticals 16, no. 2: 240. https://doi.org/10.3390/ph16020240
APA StyleCentofanti, F., Buono, A., Verboni, M., Tomino, C., Lucarini, S., Duranti, A., Pandolfi, P. P., & Novelli, G. (2023). Synthetic Methodologies and Therapeutic Potential of Indole-3-Carbinol (I3C) and Its Derivatives. Pharmaceuticals, 16(2), 240. https://doi.org/10.3390/ph16020240








