2022 FDA TIDES (Peptides and Oligonucleotides) Harvest
Abstract
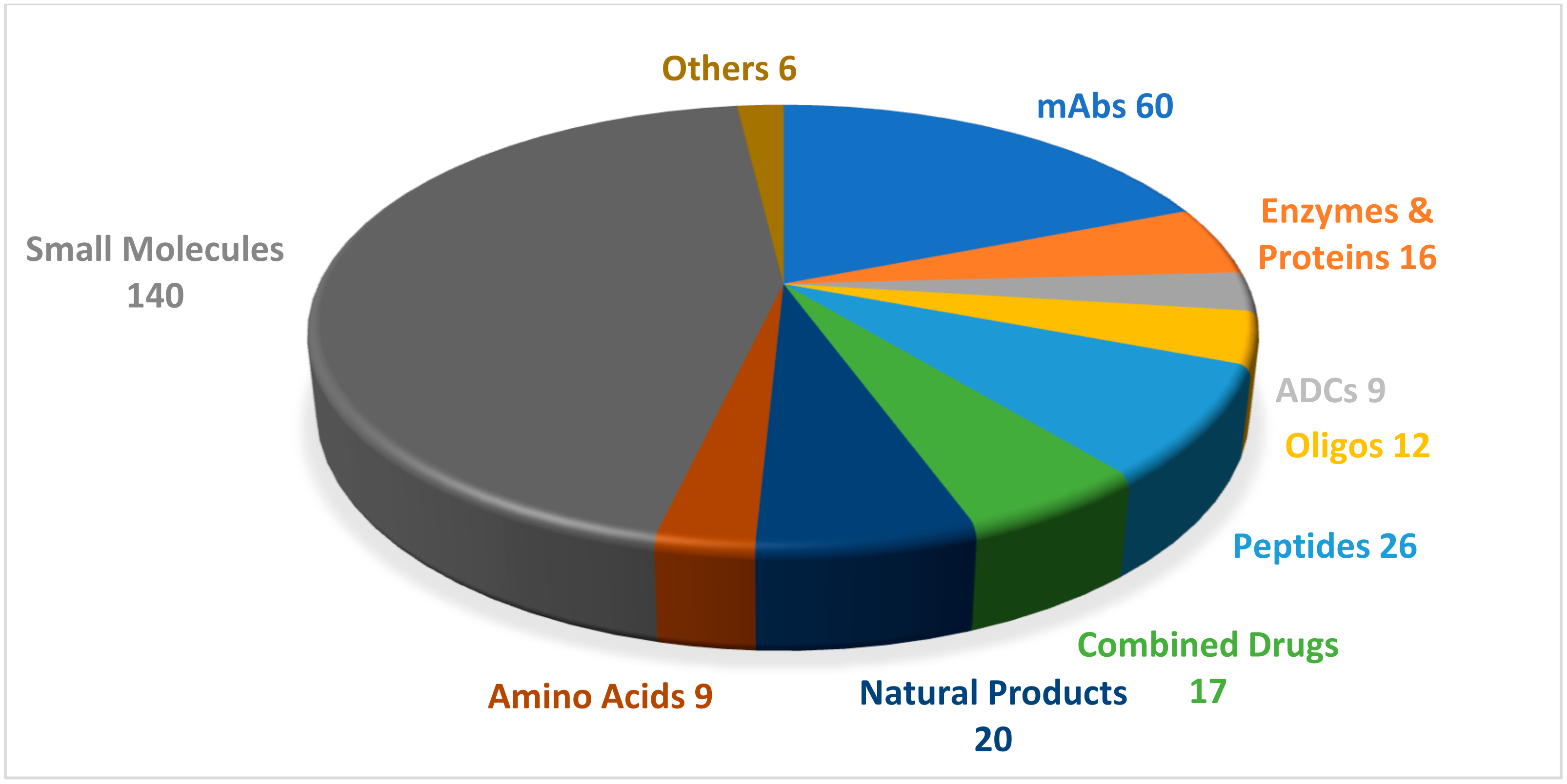
:1. Introduction
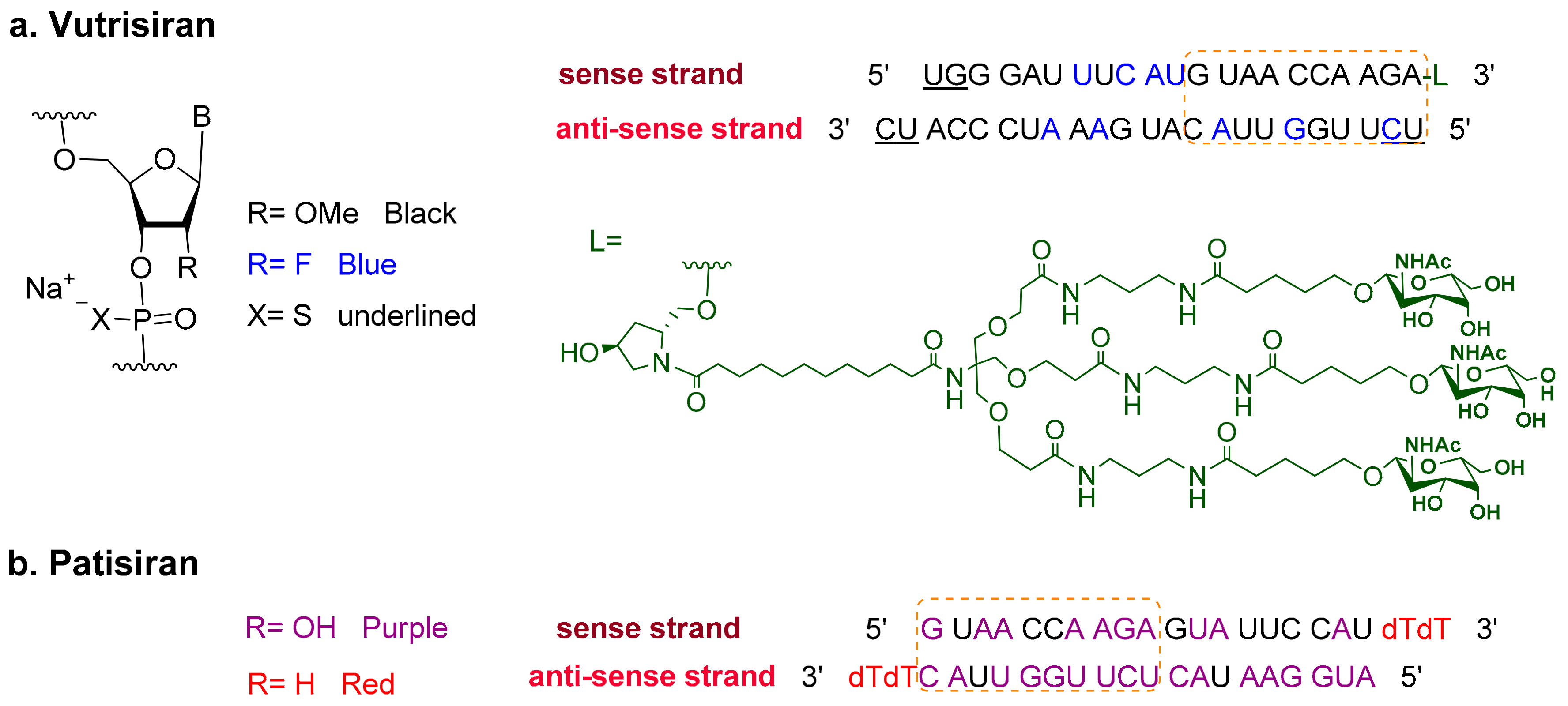
2. Oligonucleotides
Vutrisiran (AmvuttraTM)
3. Peptides
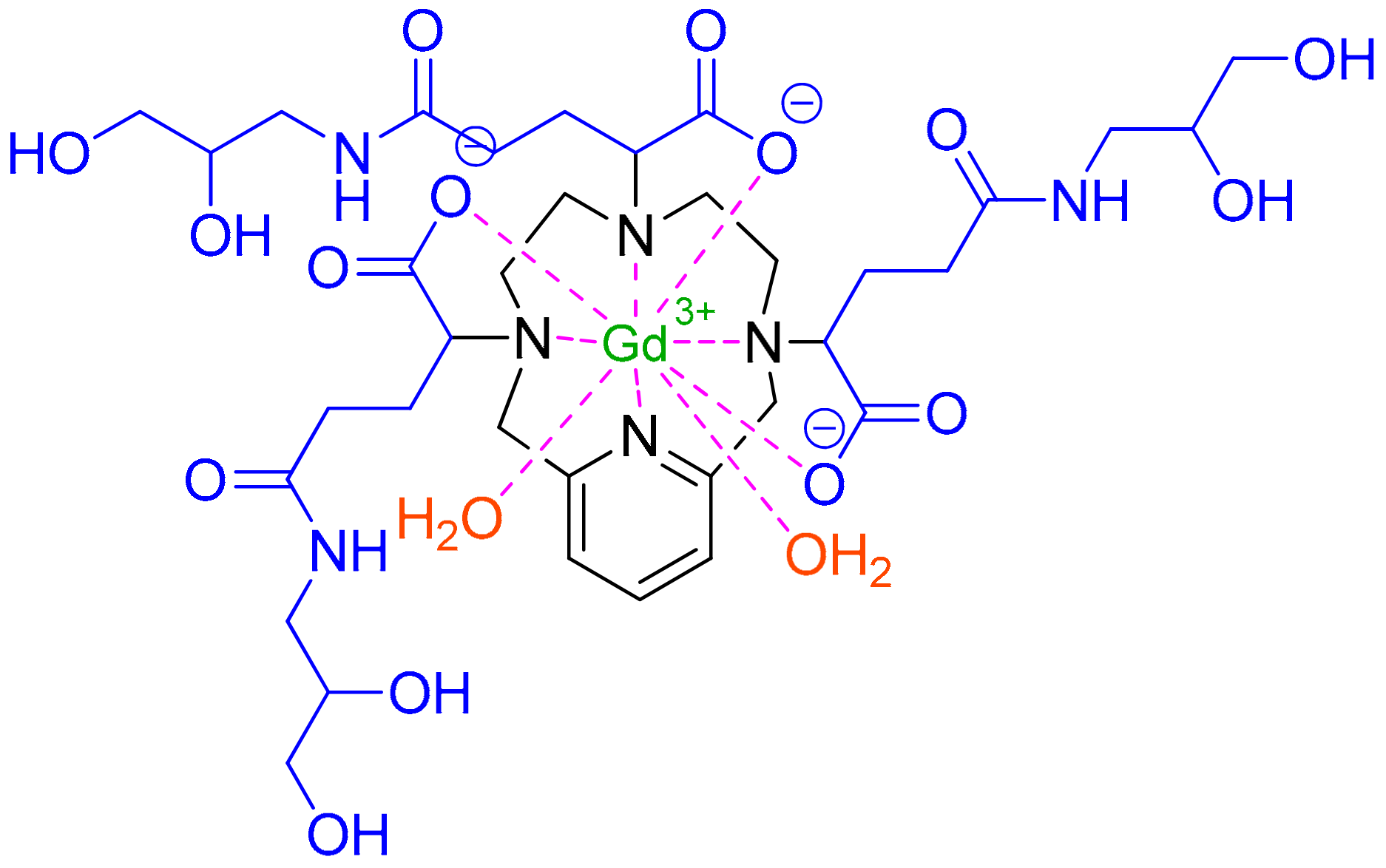
3.1. Gadopiclenol (EluciremTM)
3.2. Tirzepatide (MounjaroTM)
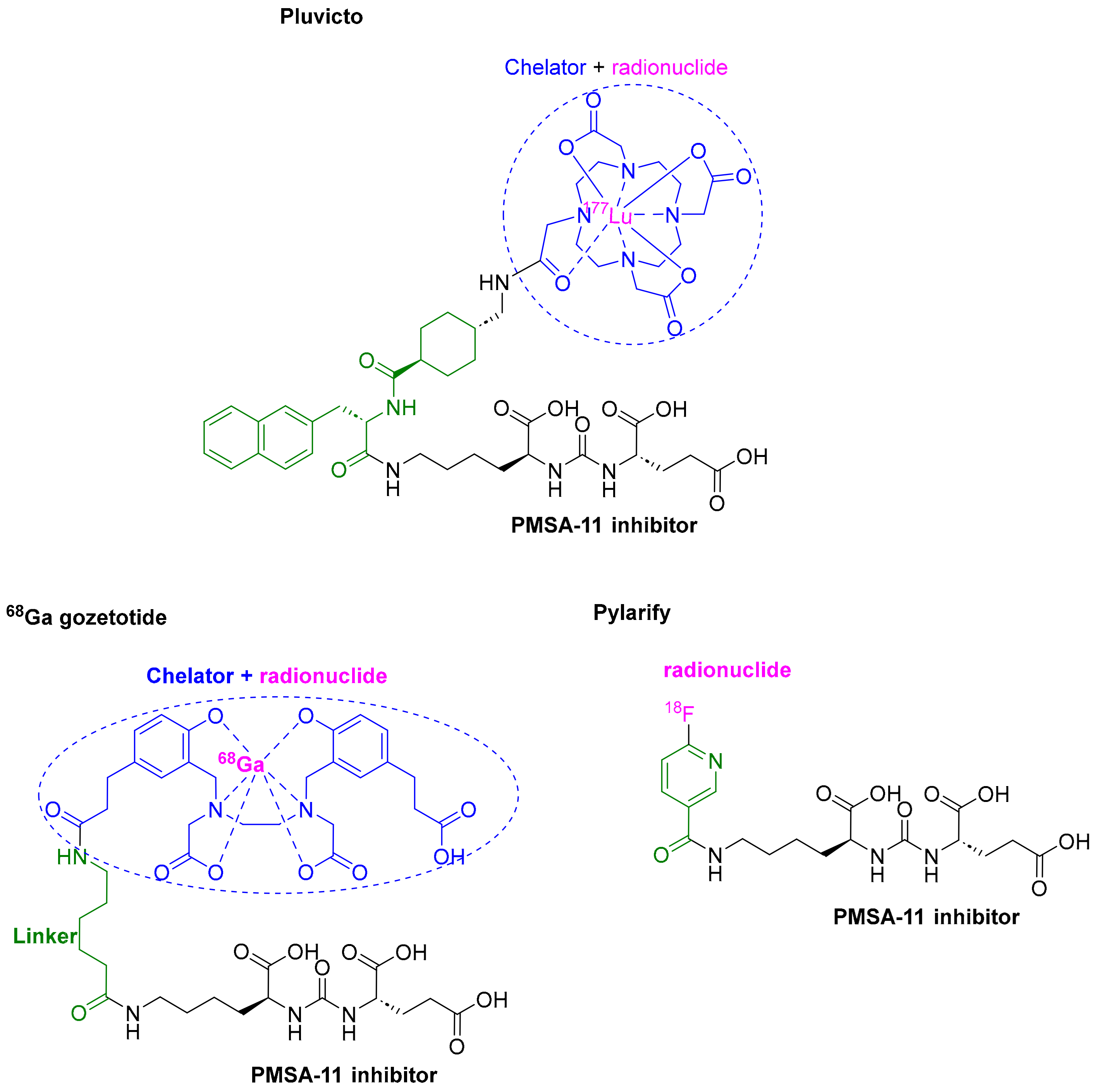
3.3. Lutetium 177Lu Vipivotide Tetraxetan (PluvictoTM)
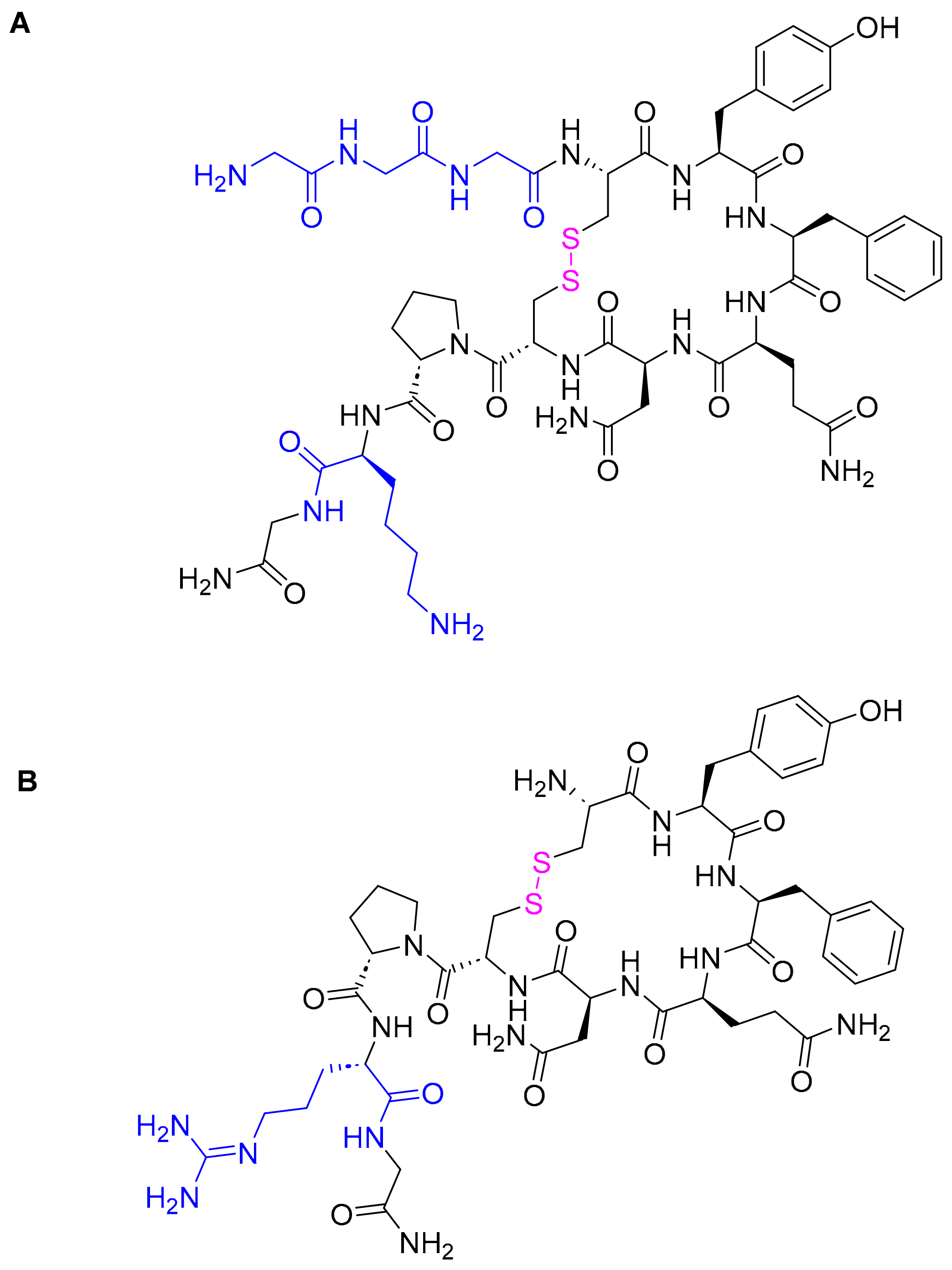
3.4. Terlipressin (TerlivazTM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2022: An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2023, 28, 1038. [Google Scholar] [CrossRef]
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2021. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2022, 27, 1075. [Google Scholar] [CrossRef]
- Mullard, A. 2022 FDA approvals. Nat. Rev. Drug Discov. 2023. [Google Scholar] [CrossRef]
- Keam, S.J. Vutrisiran: First Approval. Drugs 2022, 82, 1419–1425. [Google Scholar] [CrossRef]
- Habtemariam, B.A.; Karsten, V.; Attarwala, H.; Goel, V.; Melch, M.; Clausen, V.A.; Garg, P.; Vaishnaw, A.K.; Sweetser, M.T.; Robbie, G.J.; et al. Single-Dose Pharmacokinetics and Pharmacodynamics of Transthyretin Targeting N-acetylgalactosamine-Small Interfering Ribonucleic Acid Conjugate, Vutrisiran, in Healthy Subjects. Clin. Pharmacol. Ther. 2021, 109, 372–382. [Google Scholar] [CrossRef]
- Amvuttra Drug Label. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215515s000lbl.pdf (accessed on 24 January 2023).
- Vutrisiran: An Investigational RNAi Therapeutic for ATTR Amyloidosis. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.alnylam.com/sites/default/files/pdfs/Vutrisiran-Fact-Sheet.pdf (accessed on 24 January 2023).
- Al Shaer, D.; Al Musaimi, O.; Albericio, F.; de la Torre, B.G. 2019 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2020, 13, 40. [Google Scholar] [CrossRef] [Green Version]
- Al Musaimi, O.; Al Shaer, D.; Albericio, F.; de la Torre, B.G. 2020 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2021, 14, 145. [Google Scholar] [CrossRef]
- Al Shaer, D.; Al Musaimi, O.; Albericio, F.; de la Torre, B.G. 2021 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2022, 15, 222. [Google Scholar] [CrossRef]
- Onpattro Drug Label. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210922s000lbl.pdf (accessed on 24 January 2023).
- Hoy, S.M. Patisiran: First Global Approval. Drugs 2018, 78, 1625–1631. [Google Scholar] [CrossRef]
- Al Shaer, D.; Al Musaimi, O.; Albericio, F.; de la Torre, B.G. 2018 FDA Tides Harvest. Pharmaceuticals 2019, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Tschöpe, C.; Elsanhoury, A. Treatment of Transthyretin Amyloid Cardiomyopathy: The Current Options, the Future, and the Challenges. J. Clin. Med. 2022, 11, 2148. [Google Scholar] [CrossRef]
- HELIOS-B: A Study to Evaluate Vutrisiran in Patients with Transthyretin Amyloidosis with Cardiomyopathy. Available online: https://clinicaltrials.gov/ct2/show/NCT04153149 (accessed on 3 February 2023).
- Adams, D.; Tournev, I.L.; Taylor, M.S.; Coelho, T.; Planté-Bordeneuve, V.; Berk, J.L.; González-Duarte, A.; Gillmore, J.D.; Low, S.-C.; Sekijima, Y.; et al. Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: A randomized clinical trial. Amyloid 2022, 23, 1–9. [Google Scholar] [CrossRef]
- Robic, C.; Port, M.; Rousseaux, O.; Louguet, S.; Fretellier, N.; Catoen, S.; Factor, C.; Le Greneur, S.; Medina, C.; Bourrinet, P.; et al. Physicochemical and Pharmacokinetic Profiles of Gadopiclenol: A New Macrocyclic Gadolinium Chelate with High T1 Relaxivity. Investig. Radiol. 2019, 54, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Dioury, F.; Callewaert, M.; Cadiou, C.; Henoumont, C.; Molinari, M.; Laurent, S.; Portefaix, C.; Port, M.; Chuburu, F. Pyclen-based Gd complex with ionisable side-chain as a contrastophore for the design of hypersensitive MRI nanoprobes: Synthesis and relaxation studies. Results Chem. 2021, 3, 100237. [Google Scholar] [CrossRef]
- Elucirem Drug Label. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/216986s000lbl.pdf (accessed on 24 January 2023).
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Lancelot, E.; Raynaud, J.S.; Desché, P. Current and Future MR Contrast Agents: Seeking a Better Chemical Stability and Relaxivity for Optimal Safety and Efficacy. Investig. Radiol. 2020, 55, 578–588. [Google Scholar] [CrossRef]
- Hao, J.; Bourrinet, P.; Desché, P. Assessment of Pharmacokinetic, Pharmacodynamic Profile, and Tolerance of Gadopiclenol, A New High Relaxivity GBCA, in Healthy Subjects and Patients with Brain Lesions (Phase I/IIa Study). Investig. Radiol. 2019, 54, 396–402. [Google Scholar] [CrossRef]
- Loevner, L.A.; Kolumban, B.; Hutóczki, G.; Dziadziuszko, K.; Bereczki, D.; Bago, A.; Pichiecchio, A. Efficacy and Safety of Gadopiclenol for Contrast-Enhanced MRI of the Central Nervous System: The PICTURE Randomized Clinical Trial. Investig. Radiol. 2022, 19. [Google Scholar] [CrossRef]
- Gadopiclenol: Positive Results for Phase III Clinical Trials. Available online: https://www.guerbet.com/news/gadopiclenol-positive-results-for-phase-iii-clinical-trials (accessed on 24 January 2023).
- Min, T.; Bain, S.C. The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type2 Diabetes: The SURPASS Clinical Trials. Diabetes Ther. 2020, 12, 143–157. [Google Scholar] [CrossRef]
- Mounjaro Drug Label. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215866s000lbl.pdf (accessed on 24 January 2023).
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2016. An Analysis of FDA Drug Approvals from a Perspective of the Molecule Type. Molecules 2017, 22, 368. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.; Nikooienejad, A.; Bray, R.; Cui, X.; Wilson, J.; Duffin, K.; Milicevic, Z.; Haupt, A.; Robins, D. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2022, 106, 388–396. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Al Shaer, D.; de la Torre, B.G.; Albericio, F. 2017 FDA Peptide Harvest. Pharmaceuticals 2018, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Manghi, F.C.P.; Landó, L.F.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Mounjaro Approval Letter. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215866Orig1s000ltr.pdf (accessed on 24 January 2023).
- Keam, S.J. Lutetium Lu 177 Vipivotide Tetraxetan: First Approval. Mol. Diagn. Ther. 2022, 26, 467–475. [Google Scholar] [CrossRef]
- Rosar, F.; Kochems, N.; Bartholomä, M.; Maus, S.; Stemler, T.; Linxweiler, J.; Khreish, F.; Ezziddin, S. Renal Safety of [(177)Lu]Lu-PSMA-617 Radioligand Therapy in Patients with Compromised Baseline Kidney Function. Cancers 2021, 13, 95. [Google Scholar] [CrossRef]
- Pluvicto Drug Label. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215833s000lbl.pdf (accessed on 24 January 2023).
- Fallah, J.; Agrawal, S.; Gittleman, H.; Fiero, M.H.; Subramaniam, S.; John, C.; Chen, W.; Ricks, T.K.; Niu, G.; Fotenos, A.; et al. FDA Approval Summary: Lutetium Lu 177 Vipivotide Tetraxetan for Patients with Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2023, OF1–OF7. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Shah, H.; Ravi, P.; Sonpavde, G.; Jacene, H. Lutetium Lu 177 vipivotide tetraxetan for metastatic castration-resistant prostate cancer. Expert Rev. Anticancer. Ther. 2022, 22, 1163–1175. [Google Scholar] [CrossRef]
- Pluvicto Approval Letter. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215833Orig1s000ltr.pdf (accessed on 24 January 2023).
- Terlivaz Drug Label. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/022231s000lbl.pdf (accessed on 24 January 2023).
- Pesaturo, A.B.; Jennings, H.R.; Voils, S.A. Terlipressin: Vasopressin analog and novel drug for septic shock. Ann. Pharmacother. 2006, 40, 2170–2177. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Doust, J.; Rockey, D.C. Terlipressin in acute oesophageal variceal haemorrhage. Aliment Pharmacol. Ther. 2003, 17, 53–64. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Boyer, T.; Garcia-Tsao, G.; Regenstein, F.; Rossaro, L.; Appenrodt, B.; Blei, A.; Gülberg, V.; Sigal, S.; Teuber, P. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology 2008, 134, 1360–1368. [Google Scholar] [CrossRef] [Green Version]
- Ioannou, G.N.; Doust, J.; Rockey, D.C. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database Syst. Rev. 2003. [Google Scholar] [CrossRef]
- Fabrizi, F.; Dixit, V.; Martin, P. Meta-analysis: Terlipressin therapy for the hepatorenal syndrome. Aliment Pharmacol. Ther. 2006, 24, 935–944. [Google Scholar] [CrossRef]
- Terlivaz Approval Letter. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-improve-kidney-function-adults-hepatorenal-syndrome (accessed on 24 January 2023).

| No. | Active Ingredient (Trade Name) | Indication | Therapeutic Target | Administration Route |
|---|---|---|---|---|
| Oligonucleotides | ||||
| 1 | Vutrisiran (AmvuttraTM) | To treat polyneuropathy of hereditary transthyretin-mediated amyloidosis. | TTR mRNA | Subcutaneous |
| Peptides | ||||
| 2 | Gadopiclenol (EluciremTM) | To detect and visualize lesions, together with MRI, with abnormal vascularity in the central nervous system and the body. | Extracellular fluids in tissues with abnormal vascularity | Intravenous |
| 3 | Tirzepatide (MounjaroTM) | To improve blood sugar control in diabetes, in addition to diet and exercise. | GIP and GLP-1 receptors | Subcutaneous |
| 4 | Lutetium (177Lu) vipivotide tetraxetan (PluvictoTM) | To treat prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer after other therapies. | PSMA | Intravenous |
| 5 | Terlipressin (TerlivazTM) | To improve kidney function in adults with hepatorenal syndrome with rapid reduction in kidney function. | V1 and V2 receptors | Intravenous |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Musaimi, O.; Al Shaer, D.; Albericio, F.; de la Torre, B.G. 2022 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2023, 16, 336. https://doi.org/10.3390/ph16030336
Al Musaimi O, Al Shaer D, Albericio F, de la Torre BG. 2022 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals. 2023; 16(3):336. https://doi.org/10.3390/ph16030336
Chicago/Turabian StyleAl Musaimi, Othman, Danah Al Shaer, Fernando Albericio, and Beatriz G. de la Torre. 2023. "2022 FDA TIDES (Peptides and Oligonucleotides) Harvest" Pharmaceuticals 16, no. 3: 336. https://doi.org/10.3390/ph16030336
APA StyleAl Musaimi, O., Al Shaer, D., Albericio, F., & de la Torre, B. G. (2023). 2022 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals, 16(3), 336. https://doi.org/10.3390/ph16030336










