Mast Cells in Regeneration of the Skin in Burn Wound with Special Emphasis on Molecular Hydrogen Effect
Abstract
:1. Introduction
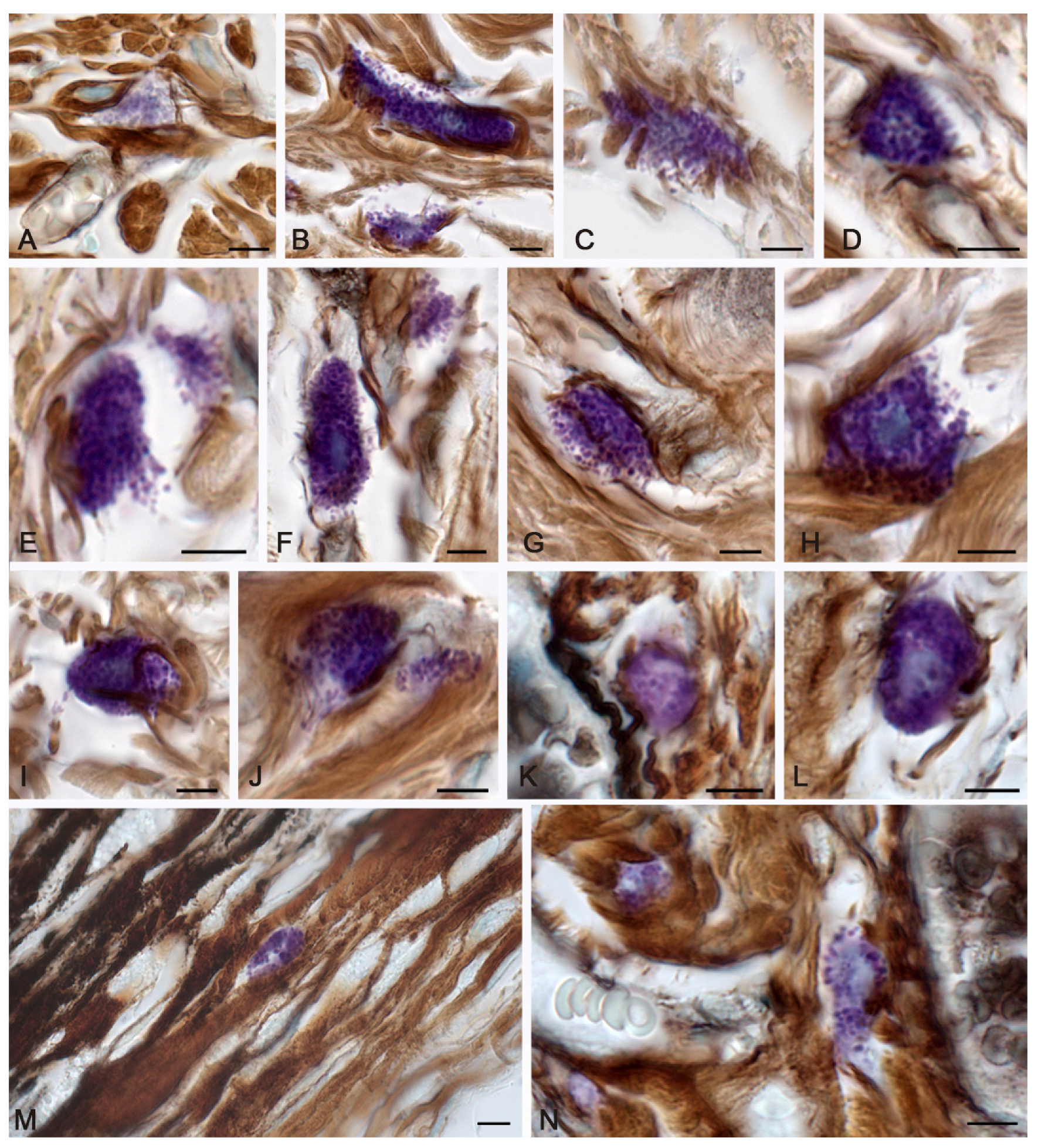
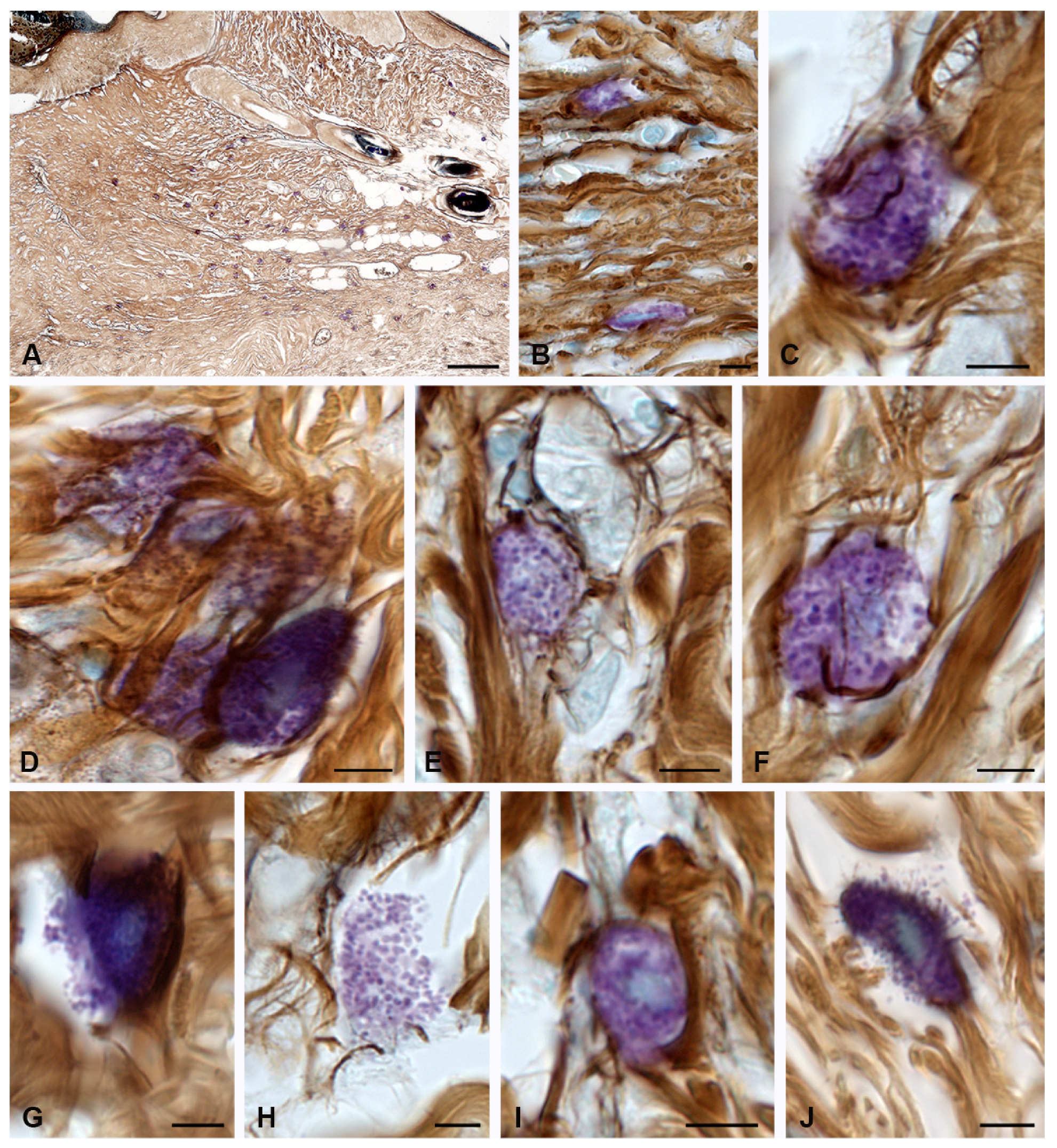
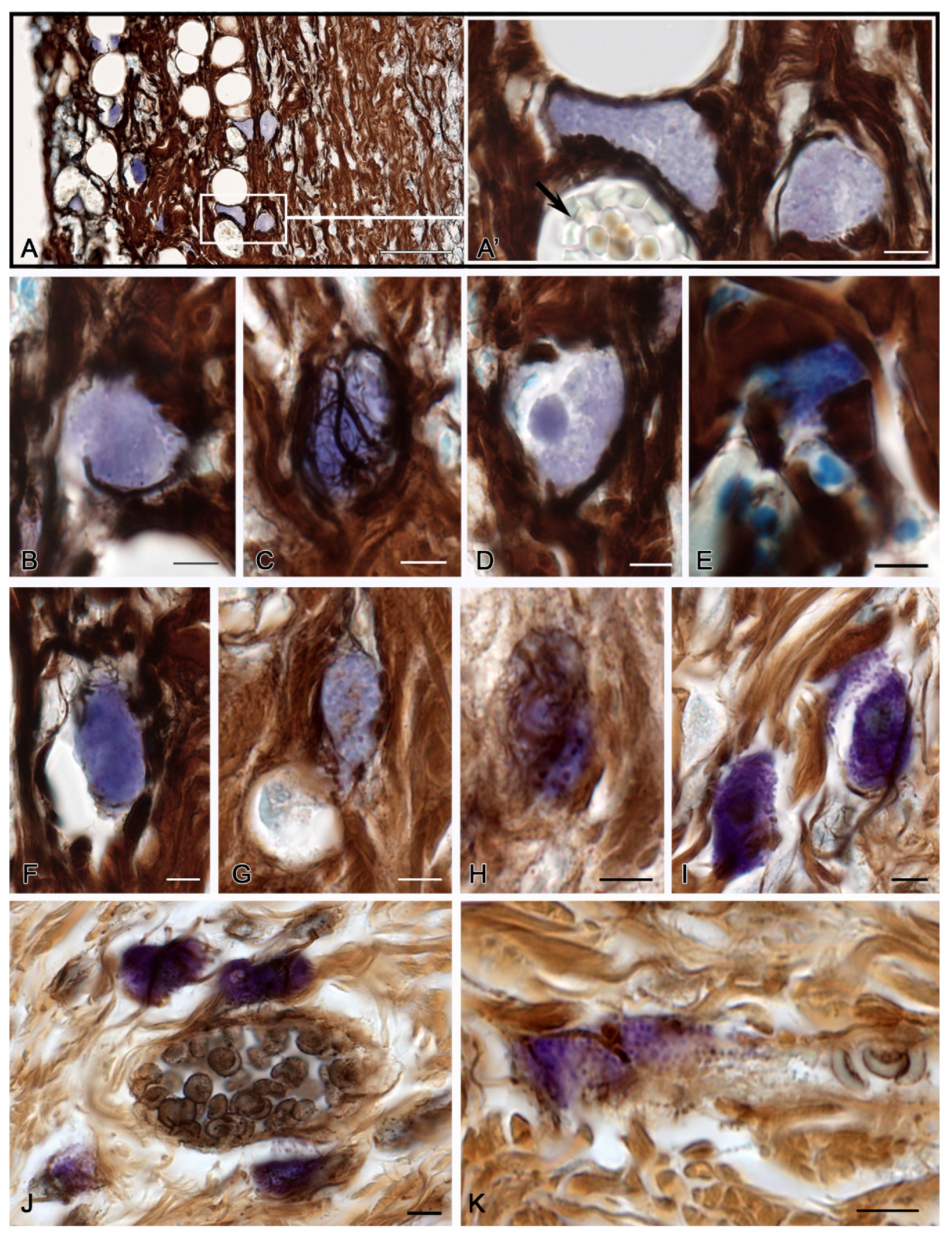
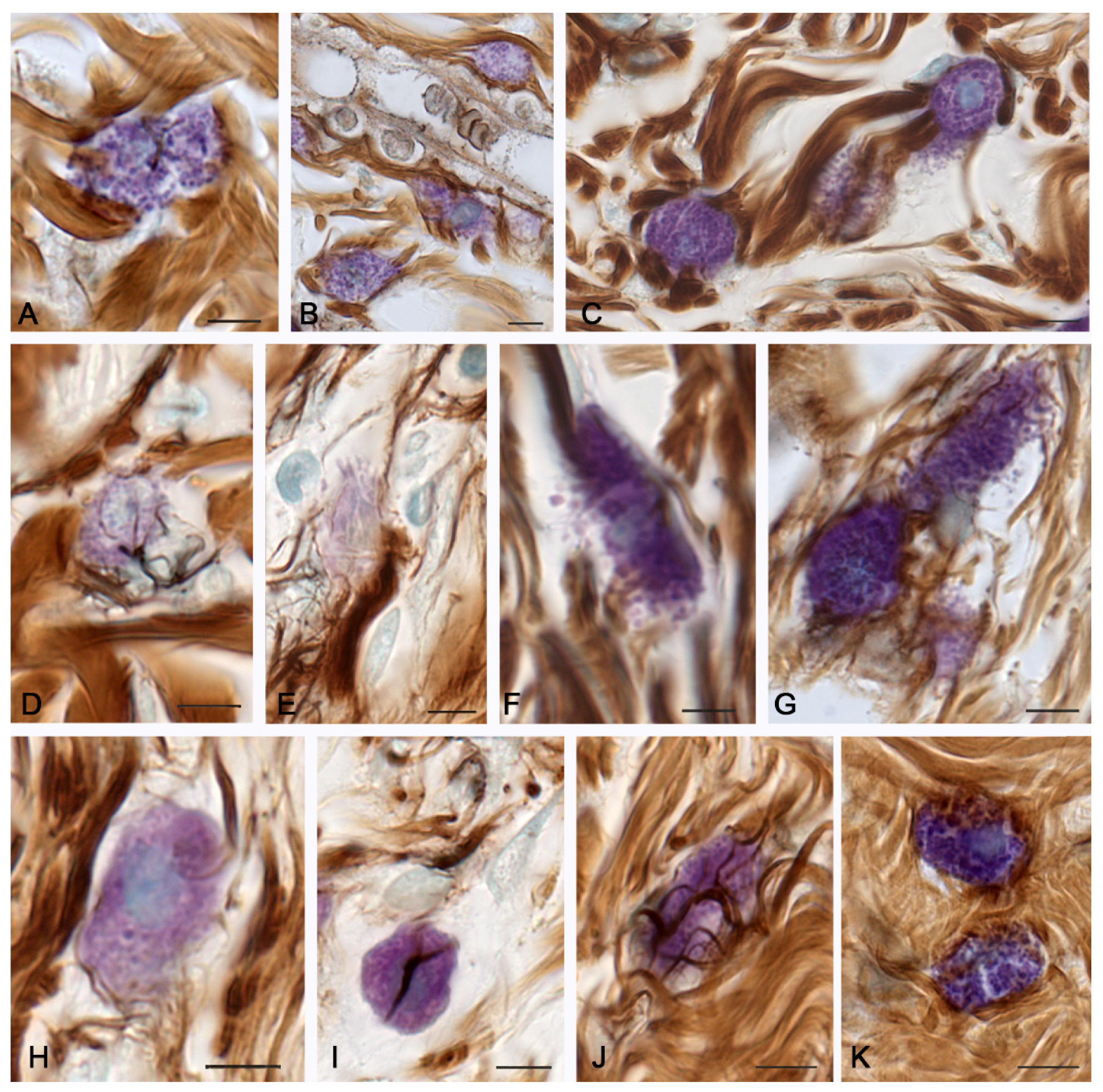
2. Results
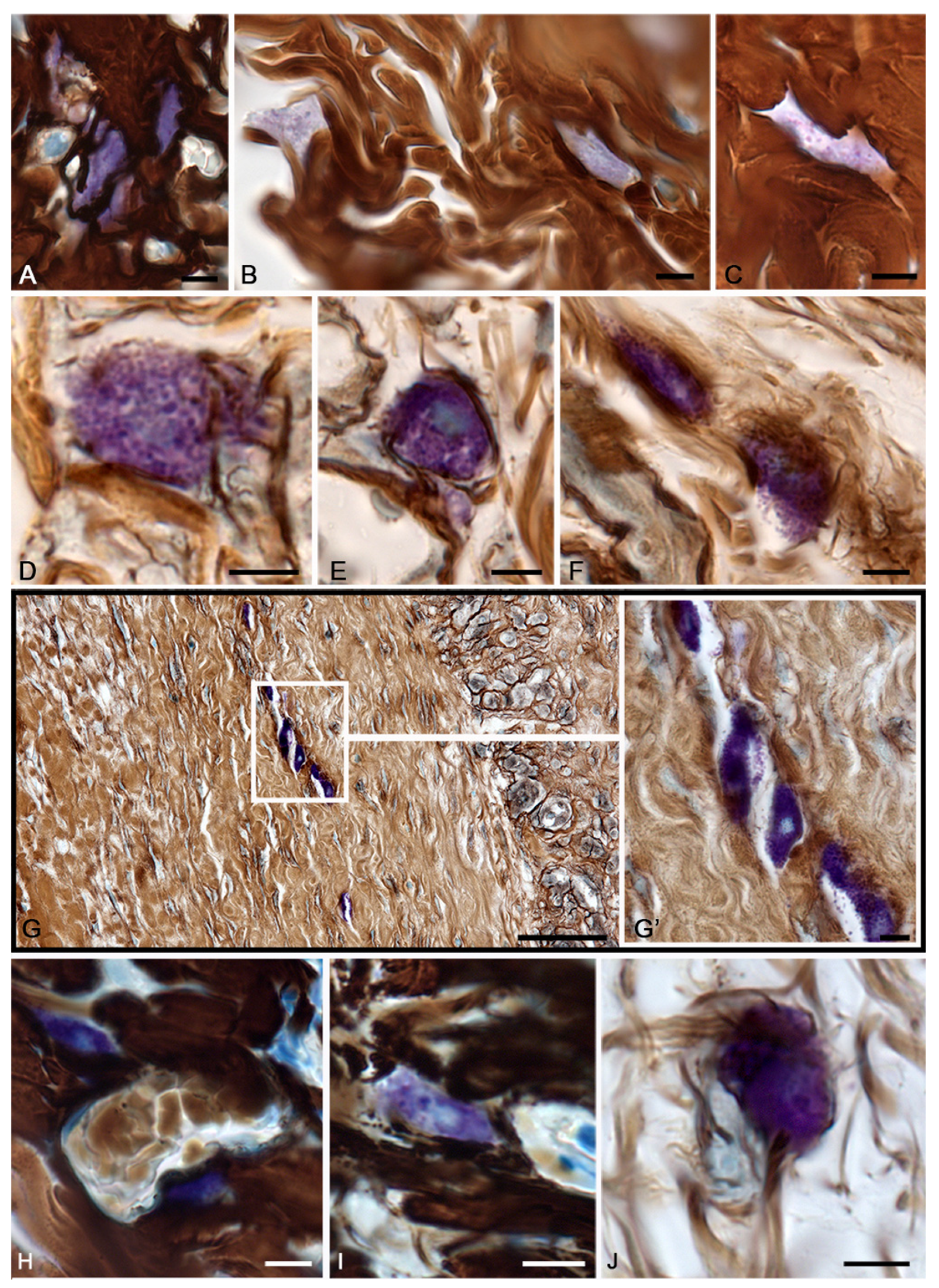
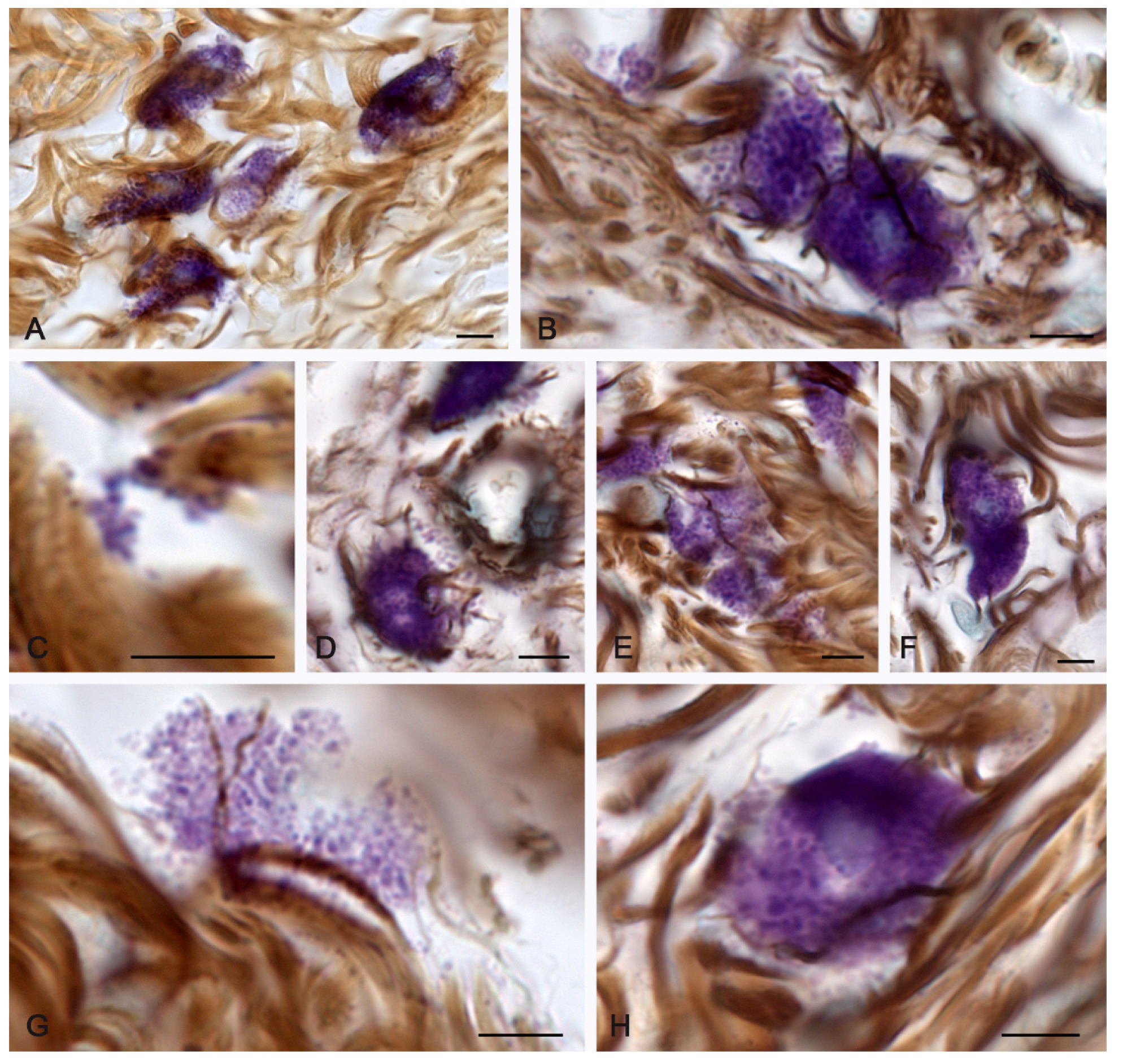
2.1. Spontaneous Healing of the Skin after a Burn
2.2. Fibrillogenesis of Collagen under Therapeutic Ointment Application
2.3. Molecular Hydrogen Application in the Burn Management
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Histoprocessing
4.3. Tissue Probe Staining
4.4. Image Acquisition
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burgess, M.; Valdera, F.; Varon, D.; Kankuri, E.; Nuutila, K. The Immune and Regenerative Response to Burn Injury. Cells 2022, 11, 3073. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, P.M.; Tahmasebi, S.; Esmaeilzadeh, A. Cellular and biological factors involved in healing wounds and burns and treatment options in tissue engineering. Regen. Med. 2022, 17, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Botting, R.A.; Haniffa, M. The developing immune network in human prenatal skin. Immunology 2020, 160, 149–156. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Plum, T.; Wang, X.; Rettel, M.; Krijgsveld, J.; Feyerabend, T.B.; Rodewald, H.-R. Human Mast Cell Proteome Reveals Unique Lineage, Putative Functions, and Structural Basis for Cell Ablation. Immunity 2020, 52, 404–416.e5. [Google Scholar] [CrossRef]
- Akula, S.; Paivandy, A.; Fu, Z.; Thorpe, M.; Pejler, G.; Hellman, L. Quantitative In-Depth Analysis of the Mouse Mast Cell Transcriptome Reveals Organ-Specific Mast Cell Heterogeneity. Cells 2020, 9, 211. [Google Scholar] [CrossRef] [Green Version]
- Wulff, B.C.; Wilgus, T.A. Examining the role of mast cells in fetal wound healing using cultured cells in vitro. Methods Mol. Biol. 2013, 1037, 495–506. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef]
- Ozpinar, E.W.; Frey, M.A.L.; Arthur, G.K.; Mora-Navarro, C.; Biehl, M.A.; Snider, D.B.; Cruse, G.; Freytes, D.O. Dermal Extracellular Matrix-Derived Hydrogels as an In Vitro Substrate to Study Mast Cell Maturation. Tissue Eng. Part A 2021, 27, 1008–1022. [Google Scholar] [CrossRef]
- Bacci, S. Fine Regulation during Wound Healing by Mast Cells, a Physiological Role Not Yet Clarified. Int. J. Mol. Sci. 2022, 23, 1820. [Google Scholar] [CrossRef]
- Ng, M.F. The role of mast cells in wound healing. Int. Wound J. 2010, 7, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Norrby, K. Do mast cells contribute to the continued survival of vertebrates? APMIS 2022, 130, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Douaiher, J.; Succar, J.; Lancerotto, L.; Gurish, M.F.; Orgill, D.P.; Hamilton, M.J.; Krilis, S.A.; Stevens, R.L. Development of Mast Cells and Importance of Their Tryptase and Chymase Serine Proteases in Inflammation and Wound Healing. Adv. Immunol. 2014, 122, 211–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atiakshin, D.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Tryptase as a polyfunctional component of mast cells. Histochem. Cell Biol. 2018, 149, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cells and collagen fibrillogenesis. Histochem. Cell Biol. 2020, 154, 21–40. [Google Scholar] [CrossRef]
- Atiakshin, D.; Kostin, A.; Trotsenko, I.; Samoilova, V.; Buchwalow, I.; Tiemann, M. Carboxypeptidase A3—A Key Component of the Protease Phenotype of Mast Cells. Cells 2022, 11, 570. [Google Scholar] [CrossRef]
- Qi, D.-D.; Ding, M.-Y.; Wang, T.; Hayat, M.A.; Liu, T.; Zhang, J.-T. The Therapeutic Effects of Oral Intake of Hydrogen Rich Water on Cutaneous Wound Healing in Dogs. Veter. Sci. 2021, 8, 264. [Google Scholar] [CrossRef]
- Fan, S.-Q.; Cai, J.-L.; Qin, L.-Y.; Wang, Z.-H.; Liu, Z.-Z.; Sun, M.-L. Effect of Heparin on Production of Transforming Growth Factor (TGF)-β1 and TGF-β1 mRNA Expression by Human Normal Skin and Hyperplastic Scar Fibroblasts. Ann. Plast. Surg. 2008, 60, 299–305. [Google Scholar] [CrossRef]
- Fan, S.-Q.; Qin, L.-Y.; Cai, J.-L.; Zhu, G.-Y.; Bin, X.; Yan, H.-S. Effect of Heparin on Production of Basic Fibroblast Growth Factor and Transforming Growth Factor-Beta1 by Human Normal Skin and Hyperplastic Scar Fibroblasts. J. Burn. Care Res. 2007, 28, 734–741. [Google Scholar] [CrossRef]
- Kulke, M.; Geist, N.; Friedrichs, W.; Langel, W. Molecular dynamics simulations on networks of heparin and collagen. Proteins 2017, 85, 1119–1130. [Google Scholar] [CrossRef]
- Atiakshin, D.; Buchwalow, I.; Horny, P.; Tiemann, M. Protease profile of normal and neoplastic mast cells in the human bone marrow with special emphasis on systemic mastocytosis. Histochem. Cell Biol. 2021, 155, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Shishkina, V.; Gerasimova, O.; Meshkova, V.; Samodurova, N.; Samoilenko, T.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Combined histochemical approach in assessing tryptase expression in the mast cell population. Acta Histochem. 2021, 123, 151711. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Kostin, A.; Buchwalow, I.; Morozow, D.; Samoilova, V.; Tiemann, M. Chloroacetate esterase reaction combined with immunohistochemical characterization of the specific tissue microenvironment of mast cells. Histochem. Cell Biol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Welle, M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J. Leukoc. Biol. 1997, 61, 233–245. [Google Scholar] [CrossRef]
- Hallgren, J.; Pejler, G. Biology of mast cell tryptase. An inflammatory mediator. FEBS J. 2006, 273, 1871–1895. [Google Scholar] [CrossRef]
- Pejler, G.; Åbrink, M.; Ringvall, M.; Wernersson, S. Mast cell proteases. Adv. Immunol. 2007, 95, 167–255. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 2007, 217, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Caughey, G.H. Mast cell proteases as protective and inflammatory mediators. Adv. Exp. Med. Biol. 2011, 716, 212–234. [Google Scholar] [CrossRef] [Green Version]
- Caughey, G.H. Mast cell proteases as pharmacological targets. Eur. J. Pharmacol. 2015, 778, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Robida, P.A.; Puzzovio, P.G.; Pahima, H.; Levi-Schaffer, F.; Bochner, B.S. Human eosinophils and mast cells: Birds of a feather flock together. Immunol. Rev. 2018, 282, 151–167. [Google Scholar] [CrossRef]
- de Souza, D.A.; Toso, V.D.; Campos, M.R.D.C.; Lara, V.S.; Oliver, C.; Jamur, M.C. Expression of Mast Cell Proteases Correlates with Mast Cell Maturation and Angiogenesis during Tumor Progression. PLoS ONE 2012, 7, e40790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Tsai, M.; Tam, S.-Y.; Jones, C.; Zehnder, J.; Galli, S.J. Mast cells can promote the development of multiple features of chronic asthma in mice. J. Clin. Investig. 2006, 116, 1633–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribatti, D. Mast cells in lymphomas. Crit. Rev. Oncol. Hematol. 2016, 101, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Magnúsdóttir, E.I.; Grujic, M.; Bergman, J.; Pejler, G.; Lagerström, M.C. Mouse connective tissue mast cell proteases tryptase and carboxypeptidase A3 play protective roles in itch induced by endothelin-1. J. Neuroinflammation 2020, 17, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komi, D.E.A.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Levi-Schaffer, F.; Piliponsky, A.M. Tryptase, a novel link between allergic inflammation and fibrosis. Trends Immunol. 2003, 24, 158–161. [Google Scholar] [CrossRef]
- Ui, H.; Andoh, T.; Lee, J.-B.; Nojima, H.; Kuraishi, Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur. J. Pharmacol. 2006, 530, 172–178. [Google Scholar] [CrossRef]
- Chen, L.; Gao, B.; Zhang, Y.; Lu, H.; Li, X.; Pan, L.; Yin, L.; Zhi, X. PAR2 promotes M1 macrophage polarization and inflammation via FOXO1 pathway. J. Cell. Biochem. 2019, 120, 9799–9809. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; He, M.; Xie, G.; Liu, Y.; Zhao, Y.; Ye, X.; Li, X.; Zhang, M. The release of tryptase from mast cells promote tumor cell metastasis via exosomes. BMC Cancer 2019, 19, 1015. [Google Scholar] [CrossRef] [Green Version]
- Lojda, Z.; Gossrau, R.; Schiebler, T. Enzyme Histochemistry. In A Laboratory Manual; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1979. [Google Scholar]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Experimental Groups | Relative Content of Fibers Adjacent to Mast Cells (in %, M ± m) | ||||
|---|---|---|---|---|---|
| Absence of Impregnated Fibers in the Pericellular Zone | Fibrous Structures Diameter 0.2–0.5 µm | Fibrous Structures Diameter 0.5–1 µm | Fibrous Structures with a Diameter of More than 1 µm | ||
| The control group | 24.2 ± 2.2 +,Δ | 6.4 ±0.8 +,∞,Δ | 28.2 ± 2.1 +∞ Δ | 41.2 ± 3.4 +,∞,Δ | |
| Day 3 of the recovery period | Spontaneous healing | 32.7 ± 2.6% *,∞,Δ | 22.7 ± 2.9 *,∞,Δ | 13.2 ± 1.1 *,∞,Δ | 31.4 ± 0.9 *,∞,Δ |
| Application of ointment | 24.4 ± 1.8 +,Δ | 57.4 ± 4.3 *,+,Δ | 8.8 ± 0.9 *,+,Δ | 9.4 ± 1.1 *,+ | |
| Molecular hydrogen use | 15.6 ± 1.1 *,+,∞ | 73.4 ± 5.2 *,+,∞ | 3.4 ± 0.5 *,+,∞ | 7.6 ± 0.9 *,+ | |
| The control group | 24.2 ± 2.2 +,∞,Δ | 6.4 ± 0.8 +,∞,Δ | 28.2 ± 2.1 +,∞,Δ | 41.2 ± 3.4 +,∞,Δ | |
| Day 7 of the recovery period | Spontaneous healing | 22.7 ± 1.9 ∞ | 52.7 ± 3.8 *,∞ | 8.2 ± 0.8 *,∞ | 16.4 ± 1.4 * |
| Application of ointment | 14.2 ± 0.8 *,+ | 68.4 ± 4.5 *,+ | 5.0 ± 4.4 *,+ | 12.4 ± 0.9 *,+,Δ | |
| Molecular hydrogen use | 12.2 ± 1.1 *,+ | 67.4 ± 5.2 *,+ | 4.8 ± 0.4 *,+ | 15.6 ± 1.2 *,∞ | |
| The control group | 24.2 ± 2.2 +,∞,Δ | 6.4 ± 0.8 +,∞,Δ | 28.2 ± 2.1 +,∞,Δ | 41.2 ± 3.4 +,∞,Δ | |
| Day 14 of the recovery period | Spontaneous healing | 13.4 ± 1.1 *,Δ | 55.5 ± 3.4 *,Δ | 15.7 ± 1.1 * | 15.4 ± 1.3 *,Δ |
| Application of ointment | 14.2 ± 0.9 *,Δ | 54.7 ± 4.5 *,Δ | 16.4 ± 1.2 | 14.7 ± 1.2 *,+,Δ | |
| Molecular hydrogen use | 8.4 ± 0.7 *,+,∞ | 65.2 ± 4.1 *,+,∞ | 18.0 ± 1.4 *,+ | 8.4 ± 0.7 *,+,∞ | |
| Dyes | Catalogue Number | Provider | Dilution | Manufacturer |
|---|---|---|---|---|
| Toluidine blue | 07-002 | Biovitrum | Ready-to-use | ErgoProduction LLC, Russia |
| Giemsa solution, article number | 20-043/L | Biovitrum | Ready-to-use | ErgoProduction LLC, Russia |
| Silver impregnation | 21-026 | Biovitrum | Ready-to-use | ErgoProduction LLC, Russia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atiakshin, D.; Soboleva, M.; Nikityuk, D.; Alexeeva, N.; Klochkova, S.; Kostin, A.; Shishkina, V.; Buchwalow, I.; Tiemann, M. Mast Cells in Regeneration of the Skin in Burn Wound with Special Emphasis on Molecular Hydrogen Effect. Pharmaceuticals 2023, 16, 348. https://doi.org/10.3390/ph16030348
Atiakshin D, Soboleva M, Nikityuk D, Alexeeva N, Klochkova S, Kostin A, Shishkina V, Buchwalow I, Tiemann M. Mast Cells in Regeneration of the Skin in Burn Wound with Special Emphasis on Molecular Hydrogen Effect. Pharmaceuticals. 2023; 16(3):348. https://doi.org/10.3390/ph16030348
Chicago/Turabian StyleAtiakshin, Dmitri, Mariya Soboleva, Dmitry Nikityuk, Nataliya Alexeeva, Svetlana Klochkova, Andrey Kostin, Viktoriya Shishkina, Igor Buchwalow, and Markus Tiemann. 2023. "Mast Cells in Regeneration of the Skin in Burn Wound with Special Emphasis on Molecular Hydrogen Effect" Pharmaceuticals 16, no. 3: 348. https://doi.org/10.3390/ph16030348
APA StyleAtiakshin, D., Soboleva, M., Nikityuk, D., Alexeeva, N., Klochkova, S., Kostin, A., Shishkina, V., Buchwalow, I., & Tiemann, M. (2023). Mast Cells in Regeneration of the Skin in Burn Wound with Special Emphasis on Molecular Hydrogen Effect. Pharmaceuticals, 16(3), 348. https://doi.org/10.3390/ph16030348








