Evaluation of ctDNA in the Prediction of Response to Neoadjuvant Therapy and Prognosis in Locally Advanced Rectal Cancer Patients: A Prospective Study
Abstract
:1. Introduction
2. Results
2.1. Study Enrollment
2.2. Clinicopathological Features
2.3. Detection of Somatic Mutations in Plasma
2.4. Association between Response to Preoperative Therapy and ctDNA
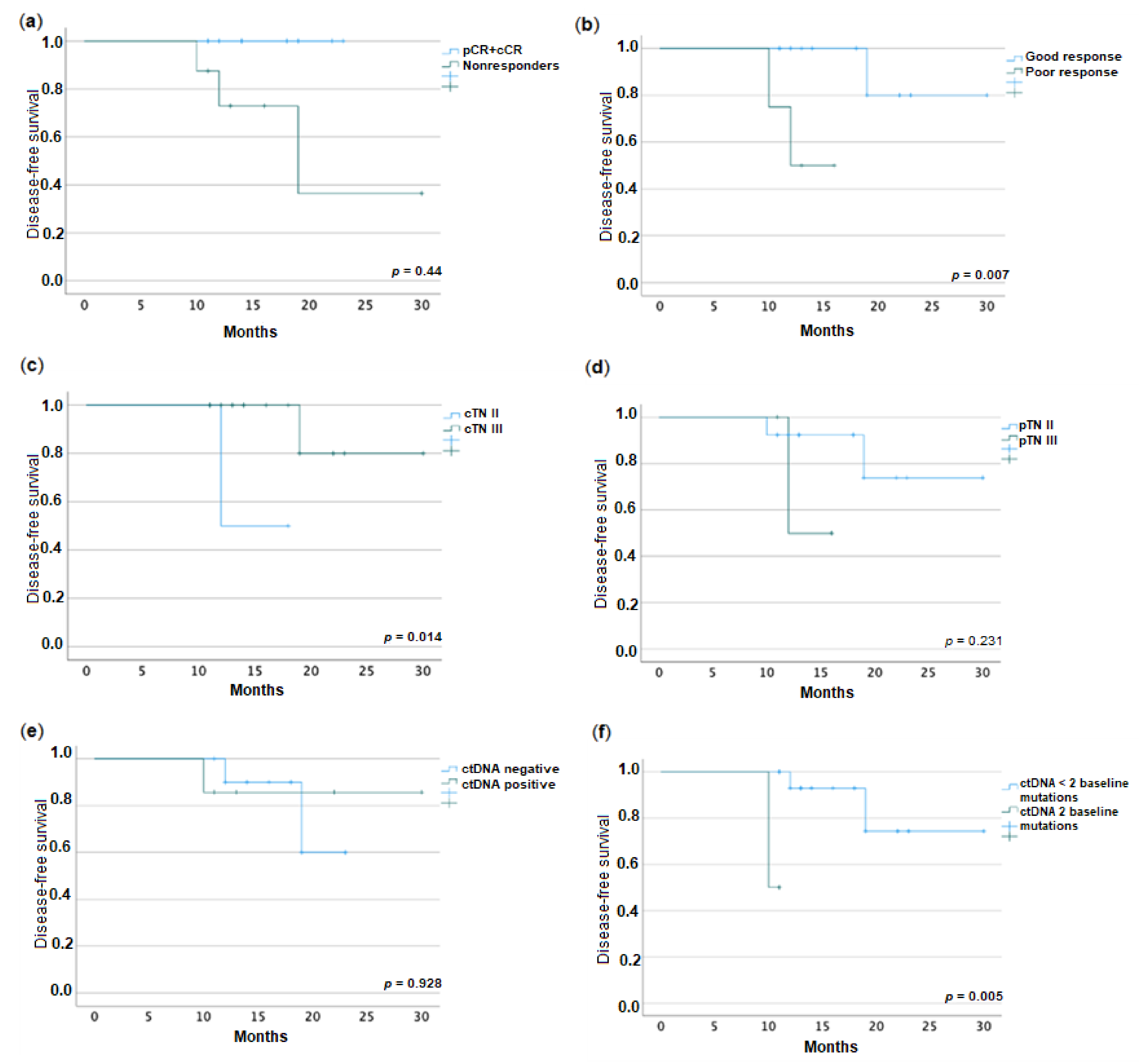
2.5. Association between Disease-Free Survival and ctDNA
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Treatment
4.3. Response Assessment
4.4. Blood Sampling, Cell-Free DNA Isolation and Sequencing
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, A.B., 3rd; Venook, A.P.; Bekaii-Saab, T.; Chan, E.; Chen, Y.J.; Cooper, H.S.; Engstrom, P.F.; Enzinger, P.C.; Fenton, M.J.; Fuchs, C.S.; et al. Rectal Cancer, Version 1.2022. J. Natl. Compr. Cancer Netw. JNCCN 2022, 13, 719–728, quiz 728. [Google Scholar] [CrossRef] [Green Version]
- Crane, C.H.; Skibber, J.M.; Feig, B.W.; Vauthey, J.N.; Thames, H.D.; Curley, S.A.; Rodriguez-Bigas, M.A.; Wolff, R.A.; Ellis, L.M.; Delclos, M.E.; et al. Response to preoperative chemoradiation increases the use of sphincter-preserving surgery in patients with locally advanced low rectal carcinoma. Cancer 2003, 97, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Bujko, K.; Rutkowski, A.; Chang, G.J.; Michalski, W.; Chmielik, E.; Kusnierz, J. Is the 1-cm Rule of Distal Bowel Resection Margin in Rectal Cancer Based on Clinical Evidence? A Systematic Review. Indian J. Surg. Oncol. 2012, 3, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Silberfein, E.J.; Kattepogu, K.M.; Hu, C.Y.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Feig, B.; Das, P.; Krishnan, S.; Crane, C.; Kopetz, S.; et al. Long-term survival and recurrence outcomes following surgery for distal rectal cancer. Ann. Surg. Oncol. 2010, 17, 2863–2869. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.F.; Etienne, P.L.; Rio, E.; François, É.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef]
- Schou, J.V.; Larsen, F.O.; Rasch, L.; Linnemann, D.; Langhoff, J.; Hogdall, E.; Nielsen, D.L.; Vistisen, K.; Fromm, A.; Jensen, B.V. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann. Oncol. 2012, 23, 2627–2633. [Google Scholar] [CrossRef]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rodel, C.; Kuo, L.J.; Calvo, F.A.; Garcia-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U., Jr.; e Sousa, A.H.S., Jr.; Campos, F.G.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711–717; discussion 717–718. [Google Scholar] [CrossRef]
- Wiltink, L.M.; Chen, T.Y.; Nout, R.A.; Kranenbarg, E.M.; Fiocco, M.; Laurberg, S.; van de Velde, C.J.; Marijnen, C.A. Health-related quality of life 14 years after preoperative short-term radiotherapy and total mesorectal excision for rectal cancer: Report of a multicenter randomised trial. Eur. J. Cancer 2014, 50, 2390–2398. [Google Scholar] [CrossRef] [Green Version]
- Osseis, M.; Nehmeh, W.A.; Rassy, N.; Derienne, J.; Noun, R.; Salloum, C.; Rassy, E.; Boussios, S.; Azoulay, D. Surgery for T4 Colorectal Cancer in Older Patients: Determinants of Outcomes. J. Pers. Med. 2022, 12, 1534. [Google Scholar] [CrossRef]
- Liu, S.; Zhong, G.X.; Zhou, W.X.; Xue, H.D.; Pan, W.D.; Xu, L.; Lu, J.Y.; Wu, B.; Lin, G.L.; Qiu, H.Z.; et al. Can Endorectal Ultrasound, MRI, and Mucosa Integrity Accurately Predict the Complete Response for Mid-Low Rectal Cancer after Preoperative Chemoradiation? A Prospective Observational Study from a Single Medical Center. Dis. Colon Rectum 2018, 61, 903–910. [Google Scholar] [CrossRef]
- Osumi, H.; Shinozaki, E.; Takeda, Y.; Wakatsuki, T.; Ichimura, T.; Saiura, A.; Yamaguchi, K.; Takahashi, S.; Noda, T.; Zembutsu, H. Clinical relevance of circulating tumor DNA assessed through deep sequencing in patients with metastatic colorectal cancer. Cancer Med. 2019, 8, 408–417. [Google Scholar] [CrossRef]
- Husain, H.; Velculescu, V.E. Cancer DNA in the Circulation: The Liquid Biopsy. JAMA 2017, 318, 1272–1274. [Google Scholar] [CrossRef] [Green Version]
- Dasari, A.; Morris, V.K.; Allegra, C.J.; Atreya, C.; Benson, A.B., 3rd; Boland, P.; Chung, K.; Copur, M.S.; Corcoran, R.B.; Deming, D.A.; et al. ctDNA applications and integration in colorectal cancer: An NCI Colon and Rectal-Anal Task Forces whitepaper. Nat. Rev. Clin. Oncol. 2020, 17, 757–770. [Google Scholar] [CrossRef]
- Tie, J.; Kinde, I.; Wang, Y.; Wong, H.L.; Roebert, J.; Christie, M.; Tacey, M.; Wong, R.; Singh, M.; Karapetis, C.S.; et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann. Oncol. 2015, 26, 1715–1722. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yoshino, T. Clinical Utility of Analyzing Circulating Tumor DNA in Patients with Metastatic Colorectal Cancer. Oncologist 2018, 23, 1310–1318. [Google Scholar] [CrossRef] [Green Version]
- Schou, J.V.; Larsen, F.O.; Sorensen, B.S.; Abrantes, R.; Boysen, A.K.; Johansen, J.S.; Jensen, B.V.; Nielsen, D.L.; Spindler, K.L. Circulating cell-free DNA as predictor of treatment failure after neoadjuvant chemo-radiotherapy before surgery in patients with locally advanced rectal cancer. Ann. Oncol. 2018, 29, 610–615. [Google Scholar] [CrossRef]
- Forshew, T.; Murtaza, M.; Parkinson, C.; Gale, D.; Tsui, D.W.; Kaper, F.; Dawson, S.J.; Piskorz, A.M.; Jimenez-Linan, M.; Bentley, D.; et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci. Transl. Med. 2012, 4, 136ra168. [Google Scholar] [CrossRef]
- Thierry, A.R.; Mouliere, F.; Gongora, C.; Ollier, J.; Robert, B.; Ychou, M.; Del Rio, M.; Molina, F. Origin and quantification of circulating DNA in mice with human colorectal cancer xenografts. Nucleic Acids Res. 2010, 38, 6159–6175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignatiadis, M.; Lee, M.; Jeffrey, S.S. Circulating Tumor Cells and Circulating Tumor DNA: Challenges and Opportunities on the Path to Clinical Utility. Clin. Cancer Res. 2015, 21, 4786–4800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpinetti, P.; Donnard, E.; Bettoni, F.; Asprino, P.; Koyama, F.; Rozanski, A.; Sabbaga, J.; Habr-Gama, A.; Parmigiani, R.B.; Galante, P.A.; et al. The use of personalized biomarkers and liquid biopsies to monitor treatment response and disease recurrence in locally advanced rectal cancer after neoadjuvant chemoradiation. Oncotarget 2015, 6, 38360–38371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Huang, T.; Cheng, F.; Huang, K.; Liu, M.; He, W.; Li, M.; Zhang, X.; Xu, M.; Chen, S.; et al. Monitoring colorectal cancer following surgery using plasma circulating tumor DNA. Oncol. Lett. 2018, 15, 4365–4375. [Google Scholar] [CrossRef] [PubMed]
- Burrell, R.A.; Swanton, C. The evolution of the unstable cancer genome. Curr. Opin. Genet. Dev. 2014, 24, 61–67. [Google Scholar] [CrossRef]
- Morais, M.; Pinto, D.M.; Machado, J.C.; Carneiro, S. ctDNA on liquid biopsy for predicting response and prognosis in locally advanced rectal cancer: A systematic review. Eur. J. Surg. Oncol. 2022, 48, 218–227. [Google Scholar] [CrossRef]
- Bitterman, D.S.; Resende Salgado, L.; Moore, H.G.; Sanfilippo, N.J.; Gu, P.; Hatzaras, I.; Du, K.L. Predictors of Complete Response and Disease Recurrence Following Chemoradiation for Rectal Cancer. Front. Oncol. 2015, 5, 286. [Google Scholar] [CrossRef] [Green Version]
- Restivo, A.; Zorcolo, L.; Cocco, I.M.; Manunza, R.; Margiani, C.; Marongiu, L.; Casula, G. Elevated CEA levels and low distance of the tumor from the anal verge are predictors of incomplete response to chemoradiation in patients with rectal cancer. Ann. Surg. Oncol. 2013, 20, 864–871. [Google Scholar] [CrossRef]
- Garland, M.L.; Vather, R.; Bunkley, N.; Pearse, M.; Bissett, I.P. Clinical tumour size and nodal status predict pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Int. J. Color. Dis. 2014, 29, 301–307. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Wynn, G.; Marks, J.; Kessler, H.; Gama-Rodrigues, J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: Characterization of clinical and endoscopic findings for standardization. Dis. Colon Rectum 2010, 53, 1692–1698. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wallace, M.; Livingstone, J.I.; Meyrick-Thomas, J. Complete clinical response after preoperative chemoradiation in rectal cancer: Is a “wait and see” policy justified? Dis Colon Rectum 2008, 51, 10–19; discussion 19–20. [Google Scholar] [CrossRef]
- Van der Valk, M.J.M.; Hilling, D.E.; Bastiaannet, E.; Meershoek-Klein Kranenbarg, E.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.O.; Renehan, A.G.; van de Velde, C.J.H.; et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018, 391, 2537–2545. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, L.; Bao, H.; Fan, X.; Xia, F.; Wan, J.; Shen, L.; Guan, Y.; Bao, H.; Wu, X.; et al. Utility of ctDNA in predicting response to neoadjuvant chemoradiotherapy and prognosis assessment in locally advanced rectal cancer: A prospective cohort study. PLoS Med. 2021, 18, e1003741. [Google Scholar] [CrossRef]
- Smith, J.J.; Strombom, P.; Chow, O.S.; Roxburgh, C.S.; Lynn, P.; Eaton, A.; Widmar, M.; Ganesh, K.; Yaeger, R.; Cercek, A.; et al. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients with a Complete Response after Neoadjuvant Therapy. JAMA Oncol. 2019, 5, e185896. [Google Scholar] [CrossRef]
- Reinert, T.; Scholer, L.V.; Thomsen, R.; Tobiasen, H.; Vang, S.; Nordentoft, I.; Lamy, P.; Kannerup, A.S.; Mortensen, F.V.; Stribolt, K.; et al. Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut 2016, 65, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Tie, J.; Cohen, J.D.; Wang, Y.; Li, L.; Christie, M.; Simons, K.; Elsaleh, H.; Kosmider, S.; Wong, R.; Yip, D.; et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: A prospective biomarker study. Gut 2019, 68, 663–671. [Google Scholar] [CrossRef]
- Murahashi, S.; Akiyoshi, T.; Sano, T.; Fukunaga, Y.; Noda, T.; Ueno, M.; Zembutsu, H. Serial circulating tumour DNA analysis for locally advanced rectal cancer treated with preoperative therapy: Prediction of pathological response and postoperative recurrence. Br. J. Cancer 2020, 123, 803–810. [Google Scholar] [CrossRef]
- Zhou, P.; Goffredo, P.; Ginader, T.; Thompson, D.; Hrabe, J.; Gribovskaja-Rupp, I.; Kapadia, M.; Hassan, I. Impact of KRAS status on tumor response and survival after neoadjuvant treatment of locally advanced rectal cancer. J. Surg. Oncol. 2021, 123, 278–285. [Google Scholar] [CrossRef]
- Vidal, J.; Casadevall, D.; Bellosillo, B.; Pericay, C.; Garcia-Carbonero, R.; Losa, F.; Layos, L.; Alonso, V.; Capdevila, J.; Gallego, J.; et al. Clinical Impact of Presurgery Circulating Tumor DNA after Total Neoadjuvant Treatment in Locally Advanced Rectal Cancer: A Biomarker Study from the GEMCAD 1402 Trial. Clin. Cancer Res. 2021, 27, 2890–2898. [Google Scholar] [CrossRef]
- Pazdirek, F.; Minarik, M.; Benesova, L.; Halkova, T.; Belsanova, B.; Macek, M.; Stepanek, L.; Hoch, J. Monitoring of Early Changes of Circulating Tumor DNA in the Plasma of Rectal Cancer Patients Receiving Neoadjuvant Concomitant Chemoradiotherapy: Evaluation for Prognosis and Prediction of Therapeutic Response. Front. Oncol. 2020, 10, 1028. [Google Scholar] [CrossRef]
- Guo, Z.W.; Xiao, W.W.; Yang, X.X.; Yang, X.; Cai, G.X.; Wang, X.J.; Han, B.W.; Li, K.; Zhai, X.M.; Li, F.X.; et al. Noninvasive prediction of response to cancer therapy using promoter profiling of circulating cell-free DNA. Clin. Transl. Med. 2020, 10, e174. [Google Scholar] [CrossRef] [PubMed]
- Benesova, L.; Belsanova, B.; Suchanek, S.; Kopeckova, M.; Minarikova, P.; Lipska, L.; Levy, M.; Visokai, V.; Zavoral, M.; Minarik, M. Mutation-based detection and monitoring of cell-free tumor DNA in peripheral blood of cancer patients. Anal. Biochem. 2013, 433, 227–234. [Google Scholar] [CrossRef] [PubMed]
- McDuff, S.G.R.; Hardiman, K.M.; Ulintz, P.J.; Parikh, A.R.; Zheng, H.; Kim, D.W.; Lennerz, J.K.; Hazar-Rethinam, M.; Van Seventer, E.E.; Fetter, I.J.; et al. Circulating Tumor DNA Predicts Pathologic and Clinical Outcomes Following Neoadjuvant Chemoradiation and Surgery for Patients with Locally Advanced Rectal Cancer. JCO Precis. Oncol. 2021, 5, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef] [Green Version]
- Roesel, R.; Epistolio, S.; Molinari, F.; Saletti, P.; De Dosso, S.; Valli, M.; Franzetti-Pellanda, A.; Deantonio, L.; Biggiogero, M.; Spina, P.; et al. A Pilot, Prospective, Observational Study to Investigate the Value of NGS in Liquid Biopsies to Predict Tumor Response after Neoadjuvant Chemo-Radiotherapy in Patients with Locally Advanced Rectal Cancer: The LiBReCa Study. Front. Oncol. 2022, 12, 900945. [Google Scholar] [CrossRef]
- Spitz, F.R.; Nguyen, D.; Skibber, J.M.; Meyn, R.E.; Cristiano, R.J.; Roth, J.A. Adenoviral-mediated wild-type p53 gene expression sensitizes colorectal cancer cells to ionizing radiation. Clin. Cancer Res. 1996, 2, 1665–1671. [Google Scholar]
- Sakai, K.; Kazama, S.; Nagai, Y.; Murono, K.; Tanaka, T.; Ishihara, S.; Sunami, E.; Tomida, S.; Nishio, K.; Watanabe, T. Chemoradiation provides a physiological selective pressure that increases the expansion of aberrant TP53 tumor variants in residual rectal cancerous regions. Oncotarget 2014, 5, 9641–9649. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.B.; Wu, X.Y.; Yu, R.; Li, C.; Wang, L.Q.; Shen, W.; Lu, P.H. P53 status as a predictive biomarker for patients receiving neoadjuvant radiation-based treatment: A meta-analysis in rectal cancer. PLoS ONE 2012, 7, e45388. [Google Scholar] [CrossRef] [Green Version]
- Boussios, S.; Ozturk, M.A.; Moschetta, M.; Karathanasi, A.; Zakynthinakis-Kyriakou, N.; Katsanos, K.H.; Christodoulou, D.K.; Pavlidis, N. The Developing Story of Predictive Biomarkers in Colorectal Cancer. J. Pers. Med. 2019, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Beganton, B.; Coyaud, E.; Laurent, E.M.N.; Mange, A.; Jacquemetton, J.; Le Romancer, M.; Raught, B.; Solassol, J. Proximal Protein Interaction Landscape of RAS Paralogs. Cancers 2020, 12, 3326. [Google Scholar] [CrossRef]
- O’Connell, E.; Reynolds, I.S.; McNamara, D.A.; Burke, J.P.; Prehn, J.H.M. Resistance to Cell Death in Mucinous Colorectal Cancer—A Review. Cancers 2021, 13, 1389. [Google Scholar] [CrossRef]
- Chow, O.S.; Kuk, D.; Keskin, M.; Smith, J.J.; Camacho, N.; Pelossof, R.; Chen, C.T.; Chen, Z.; Avila, K.; Weiser, M.R.; et al. KRAS and Combined KRAS/TP53 Mutations in Locally Advanced Rectal Cancer are Independently Associated with Decreased Response to Neoadjuvant Therapy. Ann. Surg. Oncol. 2016, 23, 2548–2555. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Aguilar, J.; Chen, Z.; Smith, D.D.; Li, W.; Madoff, R.D.; Cataldo, P.; Marcet, J.; Pastor, C. Identification of a biomarker profile associated with resistance to neoadjuvant chemoradiation therapy in rectal cancer. Ann. Surg. 2011, 254, 486–492; discussion 492–493. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.M.; Trembath, D.; Deal, A.M.; Funkhouser, W.K.; Calvo, B.F.; Finnegan, T.; Weck, K.E.; Tepper, J.E.; O’Neil, B.H. Phospho-ERK and AKT status, but not KRAS mutation status, are associated with outcomes in rectal cancer treated with chemoradiotherapy. Radiat. Oncol. 2011, 6, 114. [Google Scholar] [CrossRef] [Green Version]
- Adeleke, S.; Haslam, A.; Choy, A.; Diaz-Cano, S.; Galante, J.R.; Mikropoulos, C.; Boussios, S. Microsatellite instability testing in colorectal patients with Lynch syndrome: Lessons learned from a case report and how to avoid such pitfalls. Pers. Med. 2022, 19, 277–286. [Google Scholar] [CrossRef]
- Khakoo, S.; Carter, P.D.; Brown, G.; Valeri, N.; Picchia, S.; Bali, M.A.; Shaikh, R.; Jones, T.; Begum, R.; Rana, I.; et al. MRI Tumor Regression Grade and Circulating Tumor DNA as Complementary Tools to Assess Response and Guide Therapy Adaptation in Rectal Cancer. Clin. Cancer Res. 2020, 26, 183–192. [Google Scholar] [CrossRef]
- Capirci, C.; Valentini, V.; Cionini, L.; De Paoli, A.; Rodel, C.; Glynne-Jones, R.; Coco, C.; Romano, M.; Mantello, G.; Palazzi, S.; et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: Long-term analysis of 566 ypCR patients. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 99–107. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeong, S.Y.; Choi, S.J.; Ryoo, S.B.; Park, J.W.; Park, K.J.; Oh, J.H.; Kang, S.B.; Park, H.C.; Heo, S.C.; et al. Survival paradox between stage IIB/C (T4N0) and stage IIIA (T1-2N1) colon cancer. Ann. Surg. Oncol. 2015, 22, 505–512. [Google Scholar] [CrossRef]
- Ji, D.; Zhang, D.; Zhan, T.; Jia, J.; Han, W.; Li, Z.; Li, M.; Song, C.; Wang, J.; Gu, J. Tumor mutation burden in blood predicts benefit from neoadjuvant chemo/radiotherapy in locally advanced rectal cancer. Genomics 2021, 113, 957–966. [Google Scholar] [CrossRef]
- Jessup, J.M.; Goldberg, R.M.; Asare, E.A.; Benson, A.B.; Brierley, J.D.; Chang, G.J.; Chen, V.; Compton, C.C.; De Nardi, P.; Goodman, K.A.; et al. Colon and Rectum. In AJCC Cancer Staging Manual; Amin, M.B., Edge, S.B., Greene, F.L., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 252–274. [Google Scholar]
- Patel, U.B.; Blomqvist, L.K.; Taylor, F.; George, C.; Guthrie, A.; Bees, N.; Brown, G. MRI after treatment of locally advanced rectal cancer: How to report tumor response--the MERCURY experience. AJR Am. J. Roentgenol. 2012, 199, W486–W495. [Google Scholar] [CrossRef]
- Ryan, R.; Gibbons, D.; Hyland, J.M.; Treanor, D.; White, A.; Mulcahy, H.E.; O’Donoghue, D.P.; Moriarty, M.; Fennelly, D.; Sheahan, K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005, 47, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Xu, C. A review of somatic single nucleotide variant calling algorithms for next-generation sequencing data. Comput. Struct. Biotechnol. J. 2018, 16, 15–24. [Google Scholar] [CrossRef] [PubMed]


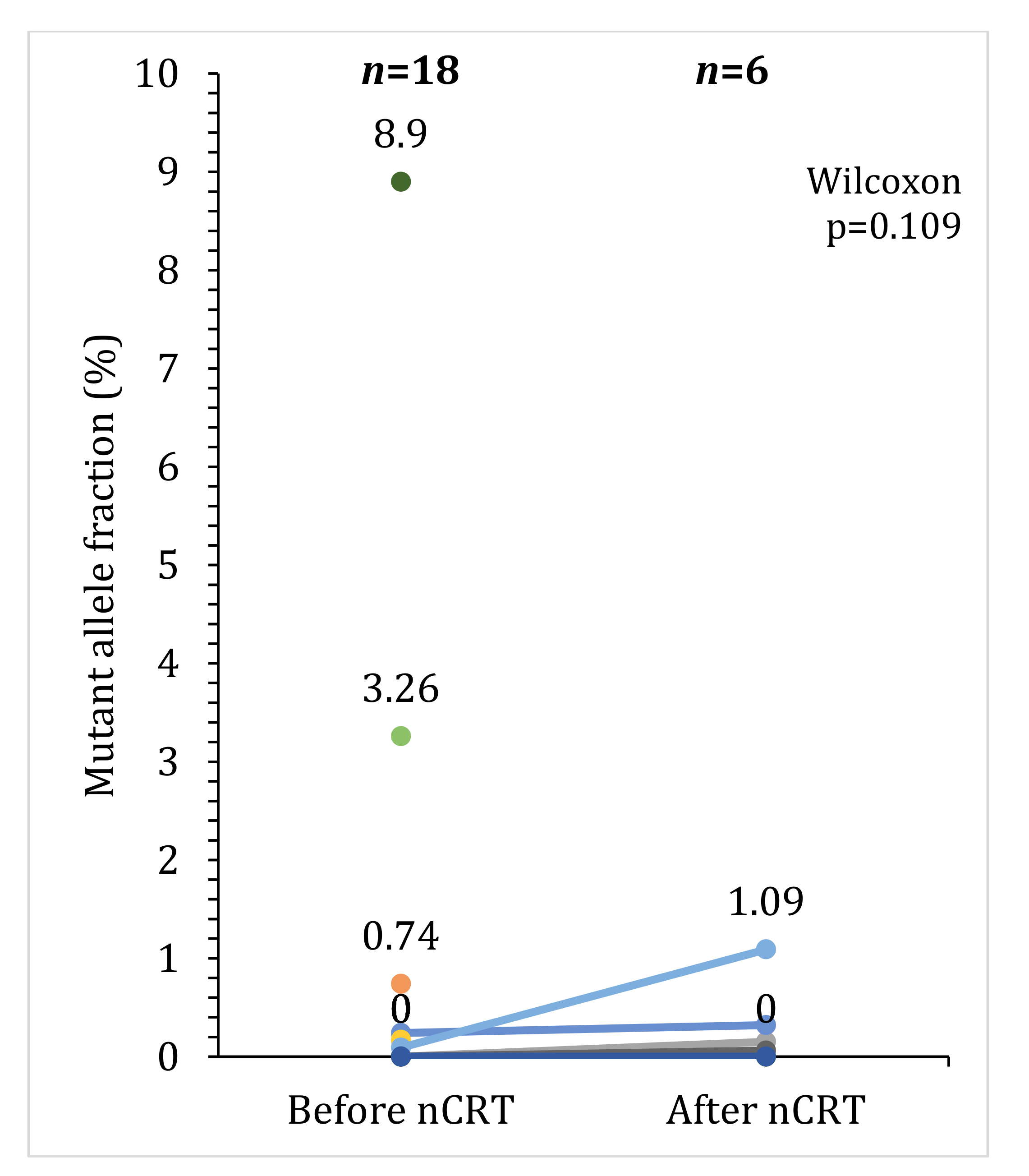
| N | % | |
| Age, years, median | 65 (38–86) | |
| Sex | ||
| Male | 8 | 44.4 |
| Female | 10 | 55.6 |
| BMI, kg/m2, median | 25.6 (16.9–30.7) | |
| Smokers | 6 | 33.3% |
| Tumor location from the anal verge, median cm | 6 (2–12) | |
| Pretreatment CEA, median ng/mL | 2.6 (1–21) | |
| Pretreatment positive ctDNA | 7 | 38.9 |
| Post-treatment positive ctDNA | 4 | 66.7 |
| Clinical T | ||
| cT3 | 16 | 88.9 |
| cT4 | 2 | 11.1 |
| Clinical stage | ||
| II | 3 | 16.7 |
| III | 14 | 77.8 |
| mrEMVI positive | 9 | 50.0 |
| Neoadjuvant treatment | ||
| nCRT | 12 | 66.7 |
| SCRT+TNT | 6 | 33.3 |
| Interval from therapy to surgery, median weeks | 10 (8–26) | |
| MRI response | ||
| Complete (mrTRG1) | 2 | 11.1 |
| Good (mrTRG2) | 9 | 50.0 |
| Poor (mrTRG3-5) | 5 | 27.8 |
| Surgery | ||
| Anterior resection | 12 | 66.7 |
| APR | 4 | 22.2 |
| Pathological T | ||
| ypT0-2 | 10 | 55.6 |
| ypT3-4 | 6 | 33.3 |
| Pathological N | ||
| ypN0 | 13 | 72.2 |
| ypN1-2 | 3 | 16.7 |
| Mucinous histology | 2 | 12.5 |
| Venous invasion | 3 | 16.7 |
| Perineural invasion | 1 | 5.6 |
| Adjuvant therapy | 5 | 27.8 |
| Follow-up (months) | 14 (11–30) | |
| Recurrence (systemic) | 3 | 16.7 |
| Nonresponders vs. pCR + cCR | p | Poor Responders vs. Good Responders | p | |
|---|---|---|---|---|
| cTN III | 85.7% vs. 80.0% | 1.000 a | 66.7% vs. 85.7% | 0.465 a |
| mrEMVI+ | 100% vs. 40.0% | 0.044 a | 100% vs. 50.0% | 0.229 a |
| NAC vs. nCRT | 37.5% vs. 30.0% | 1.000 a | 50.0% vs. 28.6% | 0.569 a |
| Interval therapy to surgery (weeks) | 11 vs. 9.5 | 0.161 b | 11 vs. 14 | 0.684 b |
| mrTRG 3-5 | 42.9% vs. 22.2% | 0.596 a | 66.7% vs. 23.1% | 0.214 a |
| Baseline ctDNA+ | 50.0% vs. 30.0% | 0.630 a | 50% vs. 35.7% | 1.000 a |
| Baseline MAF | 0.044 vs. 0.0 | 0.479 b | 0.044 vs. 0.0 | 0.904 b |
| Either mrEMVI or ctDNA+ | 2.000 (0.260–15.381) | 0.289 c | 1.667 (0.135–20.578) | 0.021 c |
| Post-treatment ctDNA+ | 60.0% vs. 100% | 1.000 a | 50.0% vs. 100% | 0.467 a |
| Post-treatment MAF | 0.057 vs. NA | 0.776 a | 0.159 vs. 0.105 | 1.000 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, M.; Fonseca, T.; Melo-Pinto, D.; Prieto, I.; Vilares, A.T.; Duarte, A.L.; Leitão, P.; Cirnes, L.; Machado, J.C.; Carneiro, S. Evaluation of ctDNA in the Prediction of Response to Neoadjuvant Therapy and Prognosis in Locally Advanced Rectal Cancer Patients: A Prospective Study. Pharmaceuticals 2023, 16, 427. https://doi.org/10.3390/ph16030427
Morais M, Fonseca T, Melo-Pinto D, Prieto I, Vilares AT, Duarte AL, Leitão P, Cirnes L, Machado JC, Carneiro S. Evaluation of ctDNA in the Prediction of Response to Neoadjuvant Therapy and Prognosis in Locally Advanced Rectal Cancer Patients: A Prospective Study. Pharmaceuticals. 2023; 16(3):427. https://doi.org/10.3390/ph16030427
Chicago/Turabian StyleMorais, Marina, Telma Fonseca, Diogo Melo-Pinto, Isabel Prieto, Ana Teresa Vilares, Ana Luísa Duarte, Patrícia Leitão, Luís Cirnes, José Carlos Machado, and Silvestre Carneiro. 2023. "Evaluation of ctDNA in the Prediction of Response to Neoadjuvant Therapy and Prognosis in Locally Advanced Rectal Cancer Patients: A Prospective Study" Pharmaceuticals 16, no. 3: 427. https://doi.org/10.3390/ph16030427





