Propolis: A Detailed Insight of Its Anticancer Molecular Mechanisms
Abstract
:1. Introduction
2. Chemical Constituents, Bioavailability, and Biological Activities of Propolis
2.1. Chemical Constituents of Propolis
2.2. Bioavailability of Propolis and Its Constituents
2.3. Biological Activities of Propolis
3. Review Studies on Propolis Anticancer Activity
Propolis Constituents Exhibiting Anticancer Activity
4. Molecular Mechanisms of Anticancer Activities of Propolis
4.1. Apoptosis Induction
4.2. Autophagy Induction
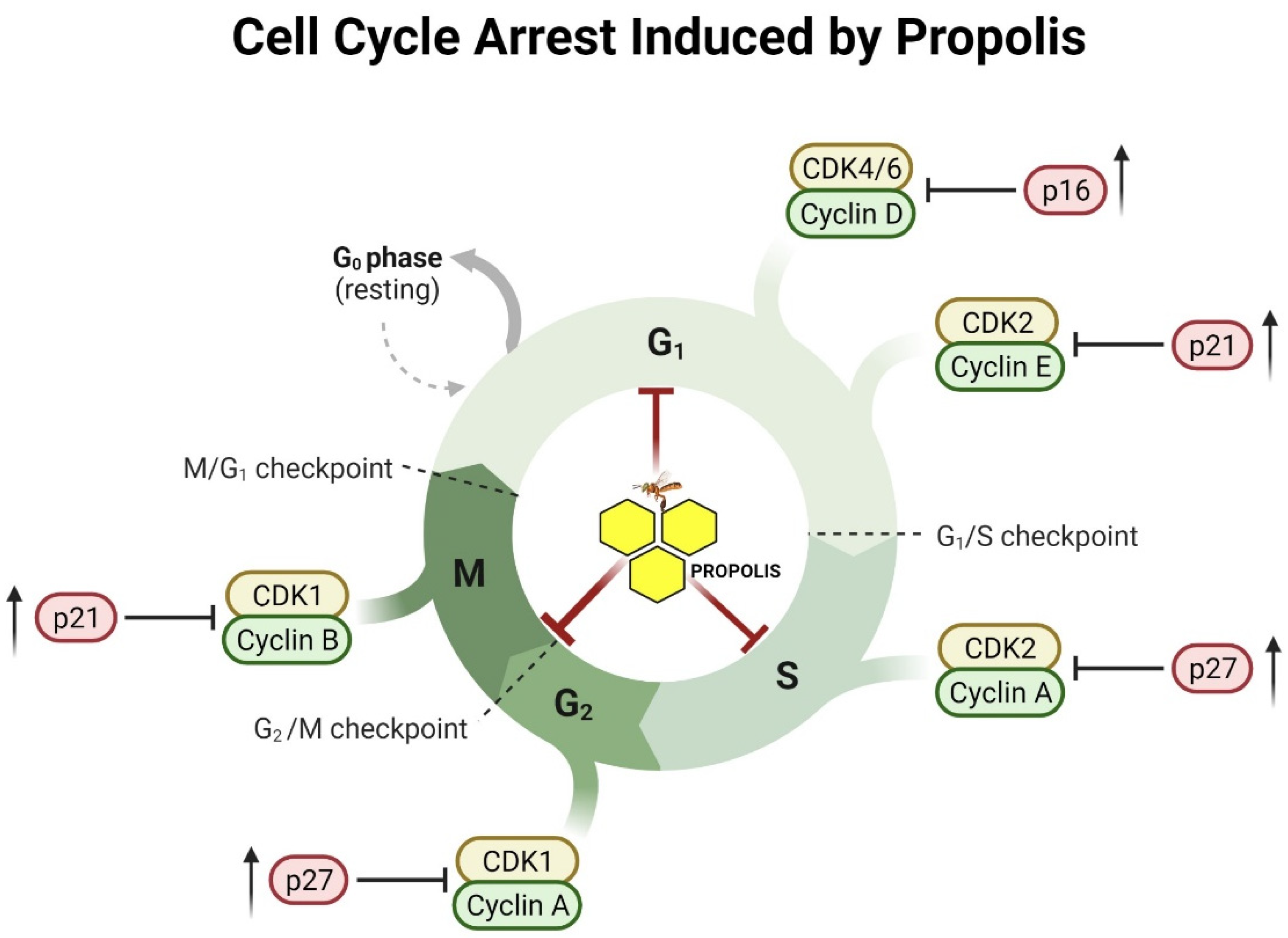
4.3. Anti-Proliferative and Cell Cycle Arrest
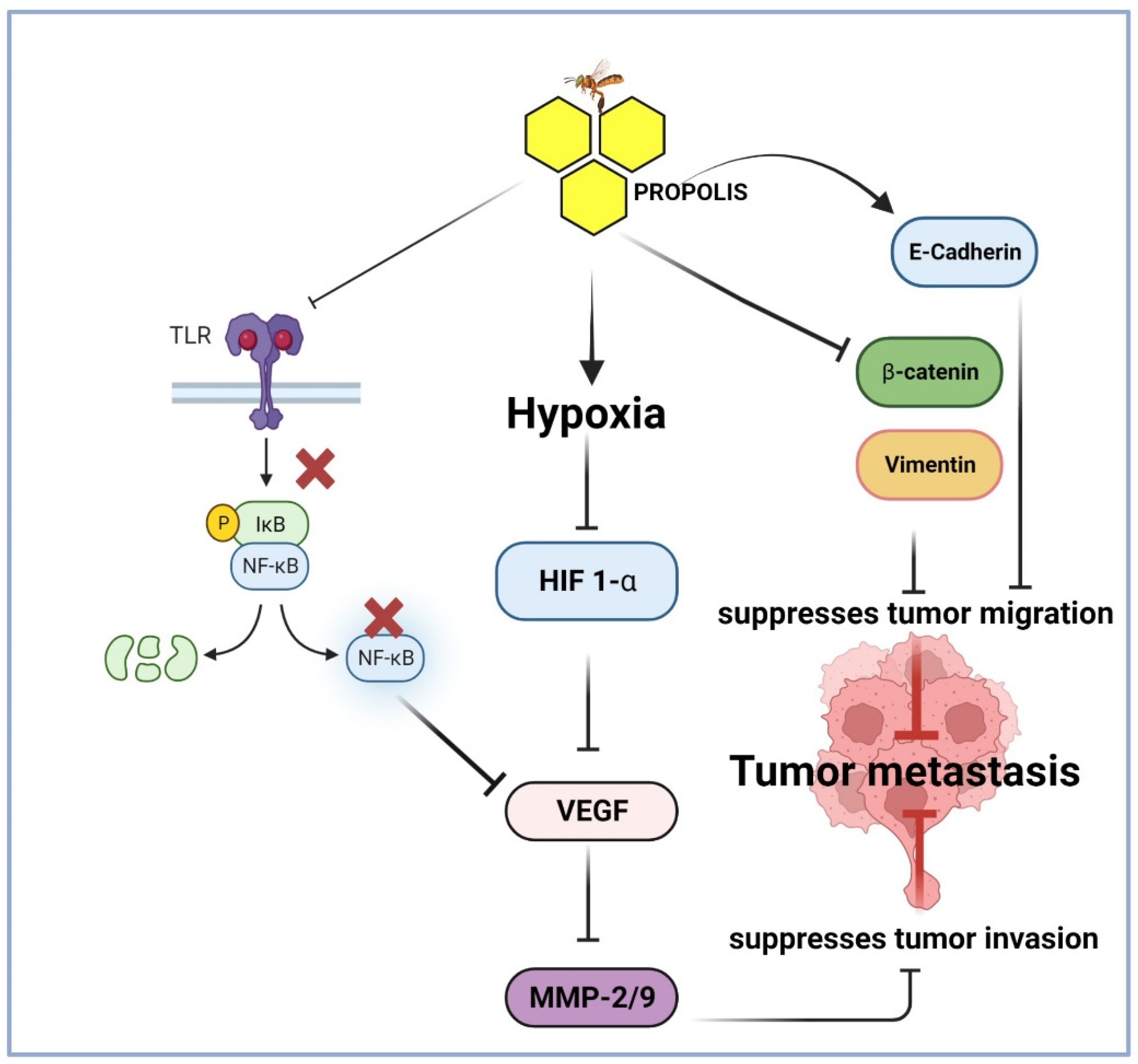
4.4. Anti-Metastatic and Anti-Angiogenesis Effects
4.5. Suppressing Inflammatory Pathway
4.6. Epigenetic Modulations
4.7. Telomerase Inhibition
5. Synergistic Effect of Propolis with Other Anticancer Agents
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.M.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018, 124, 2785–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal plants and cancer chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solowey, E.; Lichtenstein, M.; Sallon, S.; Paavilainen, H.; Solowey, E.; Lorberboum-Galski, H. Evaluating medicinal plants for anticancer activity. Sci. World J. 2014, 2014, 721402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwell, M.; Rahman, P. Medicinal plants: Their use in anticancer treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [PubMed]
- Roy, P.; Saikia, B. Cancer and cure: A critical analysis. Indian J. Cancer 2016, 53, 441–442. [Google Scholar] [PubMed]
- Zhang, Q.-Y.; Wang, F.-X.; Jia, K.-K.; Kong, L.-D. Natural product interventions for chemotherapy and radiotherapy-induced side effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef] [Green Version]
- Zwitter, M. Low-dose gemcitabine in long infusion: When less is more. Indian J. Cancer 2012, 49, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Luqmani, Y. Mechanisms of drug resistance in cancer chemotherapy. Med. Princ. Pract. 2005, 14, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.L.; Yabroff, K.R.; Meekins, A.; Topor, M.; Lamont, E.B.; Brown, M.L. Evaluation of trends in the cost of initial cancer treatment. J. Natl. Cancer Inst. 2008, 100, 888–897. [Google Scholar] [CrossRef] [Green Version]
- Kooti, W.; Servatyari, K.; Behzadifar, M.; Asadi-Samani, M.; Sadeghi, F.; Nouri, B.; Zare Marzouni, H. Effective medicinal plant in cancer treatment, part 2: Review study. J. Evid.-Based Complement. Altern. Med. 2017, 22, 982–995. [Google Scholar] [CrossRef] [Green Version]
- Taylor, W.F.; Moghadam, S.E.; Moridi Farimani, M.; Ebrahimi, S.N.; Tabefam, M.; Jabbarzadeh, E. A multi-targeting natural compound with growth inhibitory and anti-angiogenic properties re-sensitizes chemotherapy resistant cancer. PLoS ONE 2019, 14, e0218125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oršolić, N. A review of propolis antitumor action in vivo and in vitro. J. ApiProduct ApiMedical Sci. 2010, 2, 1–20. [Google Scholar] [CrossRef]
- Watanabe, M.A.E.; Amarante, M.K.; Conti, B.J.; Sforcin, J.M. Cytotoxic constituents of propolis inducing anticancer effects: A review. J. Pharm. Pharmacol. 2011, 63, 1378–1386. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Han, Y.-C.; Shen, Y.-C.; Golovinskaia, O.; Venkatakrishnan, K.; Wang, C.-K. Chemopreventive and chemotherapeutic effect of propolis and its constituents: A mini-review. J. Cancer Prev. 2020, 25, 70–78. [Google Scholar] [CrossRef]
- Turan, I.; Demir, S.; Misir, S.; Kilinc, K.; Mentese, A.; Aliyazicioglu, Y.; Deger, O. Cytotoxic effect of Turkish propolis on liver, colon, breast, cervix and prostate cancer cell lines. Trop. J. Pharm. Res. 2015, 14, 777–782. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.M.; Donia, T.; Abu-Khudir, R.; Ramadan, H.; Ali, E.M.; Mohamed, T.M. Propolis potentiates methotrexate anticancer mechanism and reduces its toxic effects. Nutr. Cancer 2020, 72, 460–480. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-J.; Shin, J.-A.; Yang, I.-H.; Won, D.-H.; Ahn, C.H.; Kwon, H.-J.; Lee, J.-S.; Cho, N.-P.; Kim, E.-C.; Yoon, H.-J. Apoptosis induced by caffeic acid phenethyl ester in human oral cancer cell lines: Involvement of Puma and Bax activation. Arch. Oral Biol. 2017, 84, 94–99. [Google Scholar] [CrossRef]
- Czyżewska, U.; Siemionow, K.; Zaręba, I.; Miltyk, W. Proapoptotic activity of propolis and their components on human tongue squamous cell carcinoma cell line (CAL-27). PLoS ONE 2016, 11, e0157091. [Google Scholar] [CrossRef] [Green Version]
- El-khawaga, O.-A.Y.; Salem, T.A.; Elshal, M.F. Protective role of Egyptian propolis against tumor in mice. Clin. Chim. Acta 2003, 338, 11–16. [Google Scholar] [CrossRef]
- Motomura, M.; Kwon, K.M.; Suh, S.J.; Lee, Y.C.; Kim, Y.K.; Lee, I.S.; Kim, M.S.; Kwon, D.Y.; Suzuki, I.; Kim, C.H. Propolis induces cell cycle arrest and apoptosis in human leukemic U937 cells through Bcl-2/Bax regulation. Environ. Toxicol. Pharmacol. 2008, 26, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.S.; Liao, C.H.; Chen, C.N.; Wu, C.L.; Lin, J.K. Propolin H from Taiwanese propolis induces G1 arrest in human lung carcinoma cells. J. Agric. Food Chem. 2007, 55, 5289–5298. [Google Scholar] [CrossRef] [PubMed]
- Hwu, Y.-J.; Lin, F.-Y. Effectiveness of propolis on oral health: A meta-analysis. J. Nurs. Res. 2014, 22, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-F.; Chen, Y.-Y.; Liu, J.-J.; Hsu, M.-L.; Shieh, H.-J.; Liao, H.-J.; Shieh, C.-J.; Shiao, M.-S.; Chen, Y.-J. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J. Agric. Food Chem. 2003, 51, 7907–7912. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Yang, C.-S.; Chi, H.-I.; Han, Y.-C.; Shen, Y.-C.; Venkatakrishnan, K.; Wang, C.-K. Cyclooxygenase-2 expression in oral precancerous and cancerous conditions and its inhibition by caffeic acid phenyl ester-enriched propolis in human oral epidermal carcinoma KB cells. Arch. Biol. Sci. 2017, 69, 83–91. [Google Scholar] [CrossRef]
- Doi, K.; Fujioka, M.; Sokuza, Y.; Ohnishi, M.; Gi, M.; Takeshita, M.; Kumada, K.; Kakehashi, A.; Wanibuchi, H. Chemopreventive action by ethanol-extracted Brazilian green propolis on post-initiation phase of inflammation-associated rat colon tumorigenesis. In Vivo 2017, 31, 187–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuesta-Rubio, O.; Piccinelli, A.L.; Campo Fernandez, M.; Marquez Hernandez, I.; Rosado, A.; Rastrelli, L. Chemical characterization of Cuban propolis by HPLC−PDA, HPLC−MS, and NMR: The brown, red, and yellow Cuban varieties of propolis. J. Agric. Food Chem. 2007, 55, 7502–7509. [Google Scholar] [CrossRef]
- Lotti, C.; Campo Fernandez, M.; Piccinelli, A.L.; Cuesta-Rubio, O.; Marquez Hernandez, I.; Rastrelli, L. Chemical constituents of red Mexican propolis. J. Agric. Food Chem. 2010, 58, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Barth, O.M. Pollen analysis of Brazilian propolis. Grana 1998, 37, 97–101. [Google Scholar] [CrossRef]
- Daugsch, A.; Moraes, C.S.; Fort, P.; Park, Y.K. Brazilian red propolis—Chemical composition and botanical origin. Evid.-Based Complement. Altern. Med. 2008, 5, 435–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thirugnanasampandan, R.; Raveendran, S.B.; Jayakumar, R. Analysis of chemical composition and bioactive property evaluation of Indian propolis. Asian Pac. J. Trop. Biomed. 2012, 2, 651–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldoni, T.L.C.; Cabral, I.S.; d’Arce, M.A.R.; Rosalen, P.L.; Ikegaki, M.; Nascimento, A.M.; Alencar, S.M. Isolation and analysis of bioactive isoflavonoids and chalcone from a new type of Brazilian propolis. Sep. Purif. Technol. 2011, 77, 208–213. [Google Scholar] [CrossRef]
- Uzel, A.; Önçağ, Ö.; Çoğulu, D.; Gençay, Ö. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol. Res. 2005, 160, 189–195. [Google Scholar] [CrossRef]
- Hernández, I.M.; Fernandez, M.C.; Cuesta-Rubio, O.; Piccinelli, A.L.; Rastrelli, L. Polyprenylated benzophenone derivatives from Cuban propolis. J. Nat. Prod. 2005, 68, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Piccinelli, A.L.; Campo Fernandez, M.; Cuesta-Rubio, O.; Márquez Hernández, I.; De Simone, F.; Rastrelli, L. Isoflavonoids isolated from Cuban propolis. J. Agric. Food Chem. 2005, 53, 9010–9016. [Google Scholar] [CrossRef]
- Bankova, V. Chemical diversity of propolis makes it a valuable source of new biologically active compounds. J. ApiProduct ApiMedical Sci. 2009, 1, 23–28. [Google Scholar] [CrossRef]
- Nie, P.; Xia, Z.; Sun, D.-W.; He, Y. Application of visible and near infrared spectroscopy for rapid analysis of chrysin and galangin in Chinese propolis. Sensors 2013, 13, 10539–10549. [Google Scholar] [CrossRef] [Green Version]
- Rufatto, L.C.; dos Santos, D.A.; Marinho, F.; Henriques, J.A.P.; Ely, M.R.; Moura, S. Red propolis: Chemical composition and pharmacological activity. Asian Pac. J. Trop. Biomed. 2017, 7, 591–598. [Google Scholar] [CrossRef]
- Coelho, J.; Falcão, S.I.; Vale, N.; Almeida-Muradian, L.B.; Vilas-Boas, M. Phenolic composition and antioxidant activity assessment of southeastern and south Brazilian propolis. J. Apic. Res. 2017, 56, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Franchin, M.; Rosalen, P.L.; da Silva Prado, D.; Paraluppi, M.C.; Silva, R.L.; Damasceno, L.E.A.; Morelo, D.F.C.; Napimoga, M.H.; Cunha, F.Q.; Alves-Filho, J.C. Cinnamoyloxy-mammeisin, a coumarin from propolis of stingless bees, attenuates Th17 cell differentiation and autoimmune inflammation via STAT3 inhibition. Eur. J. Pharmacol. 2022, 929, 175127. [Google Scholar] [CrossRef]
- Buchta, V.; Černý, J.; Opletalová, V. In vitro antifungal activity of propolis samples of Czech and Slovak origin. Open Life Sci. 2011, 6, 160–166. [Google Scholar] [CrossRef]
- Słotwiński, B.; Pączkowski, C.; Szakiel, A. Characteristics of the content of lipophilic compounds in propolis and selected types of honey. In Proceedings of the XIII International Scientific Agricultural Symposium “Agrosym 2022”, Jahorina, Bosnia and Herzegovina, 6–9 October 2022; p. 262. [Google Scholar]
- Tani, H.; Hikami, S.; Takahashi, S.; Kimura, Y.; Matsuura, N.; Nakamura, T.; Yamaga, M.; Koshino, H. Isolation, identification, and synthesis of a new prenylated cinnamic acid derivative from Brazilian green propolis and simultaneous quantification of bioactive components by LC-MS/MS. J. Agric. Food Chem. 2019, 67, 12303–12312. [Google Scholar] [CrossRef] [PubMed]
- Kegode, T.M.; Bargul, J.L.; Mokaya, H.O.; Lattorff, H.M.G. Phytochemical composition and bio-functional properties of Apis mellifera propolis from Kenya. R. Soc. Open Sci. 2022, 9, 211214. [Google Scholar] [CrossRef] [PubMed]
- Salleh, S.N.A.S.; Hanapiah, N.A.M.; Johari, W.L.W.; Ahmad, H.; Osman, N.H. Analysis of bioactive compounds and chemical composition of Malaysian stingless bee propolis water extracts. Saudi J. Biol. Sci. 2021, 28, 6705–6710. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, N.; Akkus, S.; Yaman, M.; Asci, B.; Silici, S. Amino acid and vitamin content of propolis collected by native caucasican honeybees. J. Apic. Sci. 2016, 60, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Rebiai, A.; Belfar, M.; Mesbahi, M.; Nani, S.; Tliba, A.; Amara, D.G.; Chouikh, A. Fatty acid composition of Algerian propolis. J. Fundam. Appl. Sci. 2017, 9, 1656–1671. [Google Scholar]
- Abdullah, N.A.; Zullkiflee, N.; Zaini, S.N.Z.; Taha, H.; Hashim, F.; Usman, A. Phytochemicals, mineral contents, antioxidants, and antimicrobial activities of propolis produced by Brunei stingless bees Geniotrigona thoracica, Heterotrigona itama, and Tetrigona binghami. Saudi J. Biol. Sci. 2020, 27, 2902–2911. [Google Scholar] [CrossRef]
- Porrini, M.; Riso, P. Factors influencing the bioavailability of antioxidants in foods: A critical appraisal. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 647–650. [Google Scholar] [CrossRef]
- Curti, V.; Zaccaria, V.; Tsetegho Sokeng, A.J.; Dacrema, M.; Masiello, I.; Mascaro, A.; D’Antona, G.; Daglia, M. Bioavailability and in vivo antioxidant activity of a standardized polyphenol mixture extracted from brown propolis. Int. J. Mol. Sci. 2019, 20, 1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Siddiqui, N.; Etim, I.; Du, T.; Zhang, Y.; Liang, D. Developing nutritional component chrysin as a therapeutic agent: Bioavailability and pharmacokinetics consideration, and ADME mechanisms. Biomed. Pharmacother. 2021, 142, 112080. [Google Scholar] [CrossRef] [PubMed]
- Mesbah, L.; Samia, A. Bioavailability and pharmacokinetic of the Algerian propolis constituent naringenin in rats after oral administration. Planta Med. 2011, 77, PA11. [Google Scholar] [CrossRef]
- Kumazawa, S.; Shimoi, K.; Hayashi, K.; Ishii, T.; Hamasaka, T.; Nakayama, T. Identification of metabolites in plasma and urine of Uruguayan propolis-treated rats. J. Agric. Food Chem. 2004, 52, 3083–3088. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Ashida, H.; Matsuura, Y.; Kanazawa, K. Antioxidative bioavailability of artepillin C in Brazilian propolis. Arch. Biochem. Biophys. 2004, 424, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Song, X.-M.; Qiu, Y.-L.; Zheng, H.-Q.; Hu, F.-L.; Li, H.-L. Unique dynamic mode between Artepillin C and human serum albumin implies the characteristics of Brazilian green propolis representative bioactive component. Sci. Rep. 2020, 10, 17277. [Google Scholar] [CrossRef]
- Paulino, N.; Abreu, S.R.L.; Uto, Y.; Koyama, D.; Nagasawa, H.; Hori, H.; Dirsch, V.M.; Vollmar, A.M.; Scremin, A.; Bretz, W.A. Anti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolis. Eur. J. Pharmacol. 2008, 587, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Boufadi, Y.M.; Van Antwerpen, P.; Chikh Alard, I.; Nève, J.; Djennas, N.; Riazi, A.; Soubhye, J. Antioxidant effects and bioavailability evaluation of propolis extract and its content of pure polyphenols. J. Food Biochem. 2018, 42, e12434. [Google Scholar] [CrossRef]
- Tan, M.I.; Rahayu, A.K. Synthesis of Chitosan-Folic Acid Nanoparticles as a Drug Delivery System for Propolis Compounds. Multifaceted Protoc. Biotechnol. 2021, 2, 145–159. [Google Scholar]
- Cavalu, S.; Bisboaca, S.; Mates, I.M.; Pasca, P.M.; Laslo, V.; Costea, T.; Fritea, L.; Vicas, S. Novel Formulation Based on Chitosan-Arabic Gum Nanoparticles Entrapping Propolis Extract. Rev. Chim. 2018, 69, 3756–3760. [Google Scholar] [CrossRef]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Christov, R.; Kujumgiev, A.; Marcucci, M.; Popov, S. Chemical composition and antibacterial activity of Brazilian propolis. Z. Nat. C 1995, 50, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Trusheva, B.; Todorov, I.; Ninova, M.; Najdenski, H.; Daneshmand, A.; Bankova, V. Antibacterial mono-and sesquiterpene esters of benzoic acids from Iranian propolis. Chem. Cent. J. 2010, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Silici, S.; Kutluca, S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J. Ethnopharmacol. 2005, 99, 69–73. [Google Scholar] [CrossRef]
- Kumar, N.; KK, M.A.; Dang, R.; Husain, A. Antioxidant and antimicrobial activity of propolis from Tamil Nadu zone. J. Med. Plants Res. 2008, 2, 361–364. [Google Scholar]
- Monzote, L.; Cuesta-Rubio, O.; Campo Fernandez, M.; Márquez Hernandez, I.; Fraga, J.; Pérez, K.; Kerstens, M.; Maes, L.; Cos, P. In vitro antimicrobial assessment of Cuban propolis extracts. Memórias Do Inst. Oswaldo Cruz 2012, 107, 978–984. [Google Scholar] [CrossRef] [Green Version]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharmacol. Pharm. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef] [Green Version]
- Farnesi, A.P.; Aquino-Ferreira, R.; Jong, D.D.; Bastos, J.K.; Soares, A.E.E. Effects of stingless bee and honey bee propolis on four species of bacteria. Genet. Mol. Res. 2009, 8, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Kai, H.; Obuchi, M.; Yoshida, H.; Watanabe, W.; Tsutsumi, S.; Park, Y.K.; Matsuno, K.; Yasukawa, K.; Kurokawa, M. In vitro and in vivo anti-influenza virus activities of flavonoids and related compounds as components of Brazilian propolis (AF-08). J. Funct. Foods 2014, 8, 214–223. [Google Scholar] [CrossRef]
- Gekker, G.; Hu, S.X.; Spivak, M.; Lokensgard, J.R.; Peterson, P.K. Anti-HIV-1 activity of propolis in CD4(+) lymphocyte and microglial cell cultures. J. Ethnopharmacol. 2005, 102, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother. Res. 2010, 24, S20–S28. [Google Scholar] [CrossRef]
- Refaat, H.; Mady, F.M.; Sarhan, H.A.; Rateb, H.S.; Alaaeldin, E. Optimization and evaluation of propolis liposomes as a promising therapeutic approach for COVID-19. Int. J. Pharm. 2021, 592, 120028. [Google Scholar] [CrossRef] [PubMed]
- Koc, A.N.; Silici, S.; Mutlu-Sariguzel, F.; Sagdic, O. Antifungal activity of propolis in four different fruit juices. Food Technol. Biotechnol. 2007, 45, 57–61. [Google Scholar]
- Koç, A.N.; Silici, S.; Kasap, F.; Hörmet-Öz, H.T.; Mavus-Buldu, H.; Ercal, B.D. Antifungal activity of the honeybee products against Candida spp. and Trichosporon spp. J. Med. Food 2011, 14, 128–134. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Alencar, S.M.; Koo, H.; Ikegaki, M.; Silva, G.V.; Napimoga, M.H.; Rosalen, P.L. Anti-inflammatory and antimicrobial evaluation of neovestitol and vestitol isolated from Brazilian red propolis. J. Agric. Food Chem. 2013, 61, 4546–4550. [Google Scholar] [CrossRef]
- Oliveira, A.C.P.; Shinobu, C.S.; Longhini, R.; Franco, S.L.; Svidzinski, T.I.E. Antifungal activity of propolis extract against yeasts isolated from onychomycosis lesions. Memórias Do Inst. Oswaldo Cruz 2006, 101, 493–497. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, E.; Menezes, H. Anti-inflammatory activity of propolis extracts: A review. J. Venom. Anim. Toxins 2002, 8, 191–212. [Google Scholar] [CrossRef]
- Mirzoeva, O.; Calder, P. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukot. Essent. Fat. Acids 1996, 55, 441–449. [Google Scholar] [CrossRef]
- Borrelli, F.; Maffia, P.; Pinto, L.; Ianaro, A.; Russo, A.; Capasso, F.; Ialenti, A. Phytochemical compounds involved in the anti-inflammatory effect of propolis extract. Fitoterapia 2002, 73, S53–S63. [Google Scholar] [CrossRef]
- Özcan, M. Use of propolis extract as a natural antioxidant for plant oils. Grasas Y Aceites 2000, 51, 251–253. [Google Scholar] [CrossRef] [Green Version]
- Ahn, M.-R.; Kumazawa, S.; Usui, Y.; Nakamura, J.; Matsuka, M.; Zhu, F.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 2007, 101, 1383–1392. [Google Scholar] [CrossRef]
- Silva, V.; Genta, G.; Möller, M.a.N.; Masner, M.; Thomson, L.; Romero, N.; Radi, R.; Fernandes, D.C.; Laurindo, F.R.; Heinzen, H. Antioxidant activity of Uruguayan propolis. In vitro and cellular assays. J. Agric. Food Chem. 2011, 59, 6430–6437. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols and antioxidant activity of hydro-alcoholic extracts of propolis from Algarve, South of Portugal. Food Chem. Toxicol. 2010, 48, 3418–3423. [Google Scholar] [CrossRef] [PubMed]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.-M.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar] [CrossRef]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its role and efficacy in human health and diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef] [PubMed]
- Desamero, M.J.; Kakuta, S.; Tang, Y.; Chambers, J.K.; Uchida, K.; Estacio, M.A.; Cervancia, C.; Kominami, Y.; Ushio, H.; Nakayama, J. Tumor-suppressing potential of stingless bee propolis in in vitro and in vivo models of differentiated-type gastric adenocarcinoma. Sci. Rep. 2019, 9, 19635. [Google Scholar] [CrossRef] [Green Version]
- Brihoum, H.; Maiza, M.; Sahali, H.; Boulmeltout, M.; Barratt, G.; Benguedouar, L.; Lahouel, M. Dual effect of Algerian propolis on lung cancer: Antitumor and chemopreventive effects involving antioxidant activity. Braz. J. Pharm. Sci. 2018, 54. [Google Scholar] [CrossRef]
- Choudhari, M.K.; Haghniaz, R.; Rajwade, J.M.; Paknikar, K.M. Anticancer activity of Indian stingless bee propolis: An in vitro study. Evid.-Based Complement. Altern. Med. 2013, 2013, 928280. [Google Scholar] [CrossRef] [Green Version]
- Calhelha, R.C.; Falcão, S.; Queiroz, M.J.R.; Vilas-Boas, M.; Ferreira, I.C. Cytotoxicity of Portuguese propolis: The proximity of the in vitro doses for tumor and normal cell lines. BioMed Res. Int. 2014, 2014, 897361. [Google Scholar] [CrossRef] [Green Version]
- Umthong, S.; Phuwapraisirisan, P.; Puthong, S.; Chanchao, C. In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines. BMC Complement. Altern. Med. 2011, 11, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amini-Sarteshnizi, N.; Mobini-Dehkordi, M.; Khosravi-Farsani, S.; Teimori, H. Anticancer activity of ethanolic extract of propolis on AGS cell line. J. Herbmed Pharmacol. 2015, 4, 29–34. [Google Scholar]
- De Mendonça, I.C.G.; de Moraes Porto, I.C.C.; do Nascimento, T.G.; de Souza, N.S.; dos Santos Oliveira, J.M.; dos Santos Arruda, R.E.; Mousinho, K.C.; dos Santos, A.F.; Basílio-Júnior, I.D.; Parolia, A. Brazilian red propolis: Phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complement. Altern. Med. 2015, 15, 357. [Google Scholar] [CrossRef] [Green Version]
- Waly, M.I.; Al Ajimi, H.; Al-Lawati, H.T.; Guizani, N.I.; Rahman, S.S. In vivo and In vitro evidence of anticancer effects of Omani propolis against colon cancer. FASEB J. 2017, 31, 790.22. [Google Scholar]
- Carvalho, A.A.; Finger, D.; Machado, C.S.; Schmidt, E.M.; da Costa, P.M.; Alves, A.P.N.N.; Morais, T.M.F.; de Queiroz, M.G.R.; Quináia, S.P.; da Rosa, M.R. In vivo antitumoural activity and composition of an oil extract of Brazilian propolis. Food Chem. 2011, 126, 1239–1245. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Awale, S.; Zhang, H.; Tezuka, Y.; Esumi, H.; Kadota, S. Chemical constituents of propolis from Myanmar and their preferential cytotoxicity against a human pancreatic cancer cell line. J. Nat. Prod. 2009, 72, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Awale, S.; Tezuka, Y.; Kadota, S. Cytotoxic constituents of propolis from Myanmar and their structure–activity relationship. Biol. Pharm. Bull. 2009, 32, 2075–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Kapur, A.; Yang, J.X.; Srivastava, S.; McLeod, D.G.; Paredes-Guzman, J.F.; Daugsch, A.; Park, Y.K.; Rhim, J.S. Antiproliferation of human prostate cancer cells by ethanolic extracts of Brazilian propolis and its botanical origin. Int. J. Oncol. 2007, 31, 601–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Huang, Q.; Ong, C.-N.; Yang, X.-F.; Shen, H.-M. Chrysin sensitizes tumor necrosis factor-α-induced apoptosis in human tumor cells via suppression of nuclear factor-kappaB. Cancer Lett. 2010, 293, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Utispan, K.; Chitkul, B.; Koontongkaew, S. Cytotoxic activity of propolis extracts from the stingless bee Trigona sirindhornae against primary and metastatic head and neck cancer cell lines. Asian Pac. J. Cancer Prev. 2017, 18, 1051–1055. [Google Scholar]
- Barlak, Y.; Değer, O.; Çolak, M.; Karataylı, S.C.; Bozdayı, A.M.; Yücesan, F. Effect of Turkish propolis extracts on proteome of prostate cancer cell line. Proteome Sci. 2011, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Khacha-Ananda, S.; Tragoolpua, K.; Chantawannakul, P.; Tragoolpua, Y. Antioxidant and anti-cancer cell proliferation activity of propolis extracts from two extraction methods. Asian Pac. J. Cancer Prev. 2013, 14, 6991–6995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oršolić, N.; Knežević, A.H.; Šver, L.; Terzić, S.; Bašić, I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J. Ethnopharmacol. 2004, 94, 307–315. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Liao, P.-H.; Chen, W.-K.; Yang, C.-C. Preferential cytotoxicity of caffeic acid phenethyl ester analogues on oral cancer cells. Cancer Lett. 2000, 153, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Shiao, M.-S.; Hsu, M.-L.; Tsai, T.-H.; Wang, S.-Y. Effect of caffeic acid phenethyl ester, an antioxidant from propolis, on inducing apoptosis in human leukemic HL-60 cells. J. Agric. Food Chem. 2001, 49, 5615–5619. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Kaji, A.; Ma, W.y.; Miyamoto, K.i.; Dong, Z. Suppression of cell transformation and induction of apoptosis by caffeic acid phenethyl ester. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Anderson Cancer Cent. 2001, 31, 83–89. [Google Scholar] [CrossRef]
- Hung, M.-W.; Shiao, M.-S.; Tsai, L.-C.; Chang, G.-G.; Chang, T.-C. Apoptotic effect of caffeic acid phenethyl ester and its ester and amide analogues in human cervical cancer ME180 cells. Anticancer Res. 2003, 23, 4773–4780. [Google Scholar]
- Jin, U.-H.; Song, K.-H.; Motomura, M.; Suzuki, I.; Gu, Y.-H.; Kang, Y.-J.; Moon, T.-C.; Kim, C.-H. Caffeic acid phenethyl ester induces mitochondria-mediated apoptosis in human myeloid leukemia U937 cells. Mol. Cell. Biochem. 2008, 310, 43–48. [Google Scholar] [CrossRef]
- Watabe, M.; Hishikawa, K.; Takayanagi, A.; Shimizu, N.; Nakaki, T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFκB and activation of Fas in human breast cancer MCF-7 cells. J. Biol. Chem. 2004, 279, 6017–6026. [Google Scholar] [CrossRef] [Green Version]
- Bulavin, D.V.; Saito, S.; Hollander, M.C.; Sakaguchi, K.; Anderson, C.W.; Appella, E.; Fornace, A.J. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999, 18, 6845–6854. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Prieto, R.; Rojas, J.M.; Taya, Y.; Gutkind, J.S. A role for the p38 mitogen-activated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res. 2000, 60, 2464–2472. [Google Scholar]
- Weng, M.-S.; Ho, Y.-S.; Lin, J.-K. Chrysin induces G1 phase cell cycle arrest in C6 glioma cells through inducing p21Waf1/Cip1 expression: Involvement of p38 mitogen-activated protein kinase. Biochem. Pharmacol. 2005, 69, 1815–1827. [Google Scholar] [CrossRef]
- Ishida, Y.; Gao, R.; Shah, N.; Bhargava, P.; Furune, T.; Kaul, S.C.; Terao, K.; Wadhwa, R. Anticancer activity in honeybee propolis: Functional insights to the role of caffeic acid phenethyl ester and its complex with γ-cyclodextrin. Integr. Cancer Ther. 2018, 17, 867–873. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, P.; Grover, A.; Nigam, N.; Kaul, A.; Ishida, Y.; Kakuta, H.; Kaul, S.C.; Terao, K.; Wadhwa, R. Anticancer activity of the supercritical extract of Brazilian green propolis and its active component, artepillin C: Bioinformatics and experimental analyses of its mechanisms of action. Int. J. Oncol. 2018, 52, 925–932. [Google Scholar]
- Kuo, H.-C.; Kuo, W.-H.; Lee, Y.-J.; Lin, W.-L.; Chou, F.-P.; Tseng, T.-H. Inhibitory effect of caffeic acid phenethyl ester on the growth of C6 glioma cells in vitro and in vivo. Cancer Lett. 2006, 234, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R.; Nigam, N.; Bhargava, P.; Dhanjal, J.K.; Goyal, S.; Grover, A.; Sundar, D.; Ishida, Y.; Terao, K.; Kaul, S.C. Molecular characterization and enhancement of anticancer activity of caffeic acid phenethyl ester by γ cyclodextrin. J. Cancer 2016, 7, 1755–1771. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-N.; Weng, M.-S.; Wu, C.-L.; Lin, J.-K. Comparison of radical scavenging activity, cytotoxic effects and apoptosis induction in human melanoma cells by Taiwanese propolis from different sources. Evid.-Based Complement. Altern. Med. 2004, 1, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-J.; Chang, W.-H.; Lin, C.-C.; Liu, C.-Y.; Wang, T.-E.; Chu, C.-H.; Shih, S.-C.; Chen, Y.-J. Caffeic acid phenethyl ester induces apoptosis of human pancreatic cancer cells involving caspase and mitochondrial dysfunction. Pancreatology 2008, 8, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Aliabadi, H.; Hamzeh, J.; Mirian, M. Investigation of Astragalus honey and propolis extract’s cytotoxic effect on two human cancer cell lines and their oncogen and proapoptotic gene expression profiles. Adv. Biomed. Res. 2015, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.J.; Jeong, Y.-J.; Park, J.-W.; Kwon, T.K. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem. Biophys. Res. Commun. 2004, 325, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Jafari-Ghahfarokhi, H.; Jazaeri, A.; Amini-Sarteshnizi, N.; Teimori, H. The impact of caffeic acid phenethyl ester, chrysin and ethanolic extracts of propolis on PLD1 gene expression in AGS cell line. J. Herbmed Pharmacol. 2019, 8, 308–313. [Google Scholar] [CrossRef] [Green Version]
- Touzani, S.; Embaslat, W.; Imtara, H.; Kmail, A.; Kadan, S.; Zaid, H.; ElArabi, I.; Badiaa, L.; Saad, B. In Vitro Evaluation of the Potential Use of Propolis as a Multitarget Therapeutic Product: Physicochemical Properties, Chemical Composition, and Immunomodulatory, Antibacterial, and Anticancer Properties. BioMed Res. Int. 2019, 2019, 4836378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samarghandian, S.; Afshari, J.T.; Davoodi, S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics 2011, 66, 1073–1079. [Google Scholar] [CrossRef] [Green Version]
- Celińska-Janowicz, K.; Zaręba, I.; Lazarek, U.; Teul, J.; Tomczyk, M.; Pałka, J.; Miltyk, W. Constituents of propolis: Chrysin, caffeic acid, p-coumaric acid, and ferulic acid induce PRODH/POX-dependent apoptosis in human tongue squamous cell carcinoma cell (CAL-27). Front. Pharmacol. 2018, 9, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubina, R.; Kabała-Dzik, A.; Dziedzic, A.; Bielec, B.; Wojtyczka, R.D.; Bułdak, R.J.; Wyszyńska, M.; Stawiarska-Pięta, B.; Szaflarska-Stojko, E. The ethanol extract of polish propolis exhibits anti-proliferative and/or pro-apoptotic effect on HCT 116 colon cancer and Me45 Malignant melanoma cells in vitro conditions. Adv. Clin. Exp. Med. 2015, 24, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kustiawan, P.M.; Lirdprapamongkol, K.; Palaga, T.; Puthong, S.; Phuwapraisirisan, P.; Svasti, J.; Chanchao, C. Molecular mechanism of cardol, isolated from Trigona incisa stingless bee propolis, induced apoptosis in the SW620 human colorectal cancer cell line. BMC Pharmacol. Toxicol. 2017, 18, 32. [Google Scholar] [CrossRef] [Green Version]
- Ha, T.K.; Kim, M.E.; Yoon, J.H.; Bae, S.J.; Yeom, J.; Lee, J.S. Galangin induces human colon cancer cell death via the mitochondrial dysfunction and caspase-dependent pathway. Exp. Biol. Med. 2013, 238, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lan, Y.; Huang, Q.; Hua, Z. Galangin induces B16F10 melanoma cell apoptosis via mitochondrial pathway and sustained activation of p38 MAPK. Cytotechnology 2013, 65, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Pichichero, E.; Cicconi, R.; Mattei, M.; Canini, A. Chrysin-induced apoptosis is mediated through p38 and Bax activation in B16-F1 and A375 melanoma cells. Int. J. Oncol. 2011, 38, 473–483. [Google Scholar]
- McEleny, K.; Coffey, R.; Morrissey, C.; Fitzpatrick, J.M.; Watson, R.W.G. Caffeic acid phenethyl ester-induced PC-3 cell apoptosis is caspase-dependent and mediated through the loss of inhibitors of apoptosis proteins. BJU Int. 2004, 94, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Frión-Herrera, Y.; Díaz-García, A.; Ruiz-Fuentes, J.; Rodríguez-Sánchez, H.; Sforcin, J.M. The cytotoxic effects of propolis on breast cancer cells involve PI3K/Akt and ERK1/2 pathways, mitochondrial membrane potential, and reactive oxygen species generation. Inflammopharmacology 2019, 27, 1081–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, S.; Lim, W.; Bazer, F.W.; Song, G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. J. Cell. Physiol. 2017, 232, 3786–3797. [Google Scholar] [CrossRef] [PubMed]
- Chuu, C.-P.; Lin, H.-P.; Ciaccio, M.F.; Kokontis, J.M.; Hause, R.J.; Hiipakka, R.A.; Liao, S.; Jones, R.B. Caffeic acid phenethyl ester suppresses the proliferation of human prostate cancer cells through inhibition of p70S6K and Akt signaling networks. Cancer Prev. Res. 2012, 5, 788–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szliszka, E.; Czuba, Z.P.; Bronikowska, J.; Mertas, A.; Paradysz, A.; Krol, W. Ethanolic extract of propolis augments TRAIL-induced apoptotic death in prostate cancer cells. Evid.-Based Complement. Altern. Med. 2011, 2011, 535172. [Google Scholar] [CrossRef] [Green Version]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic extract of propolis (EEP) enhances the apoptosis-inducing potential of TRAIL in cancer cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Sokół-Łętowska, A.; Kucharska, A.Z.; Jaworska, D.; Czuba, Z.P.; Król, W. Ethanolic extract of polish propolis: Chemical composition and TRAIL-R2 death receptor targeting apoptotic activity against prostate cancer cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 757628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szliszka, E.; Zydowicz, G.; Janoszka, B.; Dobosz, C.; Kowalczyk-Ziomek, G.; Krol, W. Ethanolic extract of Brazilian green propolis sensitizes prostate cancer cells to TRAIL-induced apoptosis. Int. J. Oncol. 2011, 38, 941–953. [Google Scholar]
- Ishihara, M.; Naoi, K.; Hashita, M.; Itoh, Y.; Suzui, M. Growth inhibitory activity of ethanol extracts of Chinese and Brazilian propolis in four human colon carcinoma cell lines. Oncol. Rep. 2009, 22, 349–354. [Google Scholar] [PubMed]
- Suk, F.-M.; Lien, G.-S.; Huang, W.-J.; Chen, C.-N.; Lu, S.-Y.; Yang, Y.-C.; Yan, M.-D.; Liang, Y.-C. A Taiwanese propolis derivative induces apoptosis through inducing endoplasmic reticular stress and activating transcription factor-3 in human hepatoma cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 658370. [Google Scholar] [CrossRef] [Green Version]
- Xuan, H.; Li, Z.; Yan, H.; Sang, Q.; Wang, K.; He, Q.; Wang, Y.; Hu, F. Antitumor activity of Chinese propolis in human breast cancer MCF-7 and MDA-MB-231 cells. Evid.-Based Complement. Altern. Med. 2014, 2014, 280120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, H.; Zhao, J.; Miao, J.; Li, Y.; Chu, Y.; Hu, F. Effect of Brazilian propolis on human umbilical vein endothelial cell apoptosis. Food Chem. Toxicol. 2011, 49, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Aso, K.; Kanno, S.-I.; Tadano, T.; Satoh, S.; Ishikawa, M. Inhibitory effect of propolis on the growth of human leukemia U937. Biol. Pharm. Bull. 2004, 27, 727–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, E. The role for autophagy in cancer. J. Clin. Investig. 2015, 125, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Wang, Y.; Yin, X.; Liu, X.; Xuan, H. Ethanol extract of propolis and its constituent caffeic acid phenethyl ester inhibit breast cancer cells proliferation in inflammatory microenvironment by inhibiting TLR4 signal pathway and inducing apoptosis and autophagy. BMC Complement. Altern. Med. 2017, 17, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Wu, Y.; Chen, X.; Jiang, X.; Wang, K.; Hu, F. Chinese propolis exerts anti-proliferation effects in human melanoma cells by targeting NLRP1 inflammatory pathway, inducing apoptosis, cell cycle arrest, and autophagy. Nutrients 2018, 10, 1170. [Google Scholar] [CrossRef]
- Endo, S.; Hoshi, M.; Matsunaga, T.; Inoue, T.; Ichihara, K.; Ikari, A. Autophagy inhibition enhances anticancer efficacy of artepillin C, a cinnamic acid derivative in Brazilian green propolis. Biochem. Biophys. Res. Commun. 2018, 497, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Peng, Y.H.; Wei, W.Y. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022, 32, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, C.; Biray, C.; Kosova, B.; Yilmaz, B.; Eroglu, Z.; Şahin, F.; Omay, S.B.; Cogulu, O. Evaluation of Manisa propolis effect on leukemia cell line by telomerase activity. Leuk. Res. 2005, 29, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-J.; Liu, B.-H.; Xiang, D.-B.; Qiao, Z.-Y.; Fu, T.; He, Y.-H. Inhibitory effect of caffeic acid phenethyl ester on the growth of SW480 colorectal tumor cells involves β-catenin associated signaling pathway down-regulation. World J. Gastroenterol. WJG 2006, 12, 4981–4985. [Google Scholar] [CrossRef]
- Xiang, D.; Wang, D.; He, Y.; Xie, J.; Zhong, Z.; Li, Z.; Xie, J. Caffeic acid phenethyl ester induces growth arrest and apoptosis of colon cancer cells via the β-catenin/T-cell factor signaling. Anti-Cancer Drugs 2006, 17, 753–762. [Google Scholar] [CrossRef]
- Saarem, W.; Wang, F.Y.; Farfel, E. Propolis or caffeic acid phenethyl ester (CAPE) inhibits growth and viability in multiple oral cancer cell lines. Int. J. Med. Biomed. Stud. 2019, 3, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, C.-Z.; Du, G.-J.; Qi, L.-W.; Calway, T.; He, T.-C.; Du, W.; Yuan, C.-S. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int. J. Oncol. 2013, 43, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Das, S.K.; Hashimoto, T.; Sowa, Y.; Yoshida, T.; Sakai, T.; Matsuura, Y.; Kanazawa, K. Artepillin C in Brazilian propolis induces G0/G1 arrest via stimulation of Cip1/p21 expression in human colon cancer cells. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Anderson Cancer Cent. 2005, 44, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Buahorm, S.; Puthong, S.; Palaga, T.; Lirdprapamongkol, K.; Phuwapraisirisan, P.; Svasti, J.; Chanchao, C. Cardanol isolated from Thai Apis mellifera propolis induces cell cycle arrest and apoptosis of BT-474 breast cancer cells via p21 upregulation. DARU J. Pharm. Sci. 2015, 23, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, M.; Chen, D.; Yang, C.S. Dietary polyphenols may affect DNA methylation. J. Nutr. 2007, 137, 223S–228S. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.J.; Park, H.J.; Chung, H.-J.; Min, H.-Y.; Park, E.-J.; Hong, J.-Y.; Lee, S.K. Inhibitory effects of caffeic acid phenethyl ester on cancer cell metastasis mediated by the down-regulation of matrix metalloproteinase expression in human HT1080 fibrosarcoma cells. J. Nutr. Biochem. 2006, 17, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Emerging adjuvant therapy for cancer: Propolis and its constituents. J. Diet. Suppl. 2016, 13, 245–268. [Google Scholar] [CrossRef]
- Ahn, M.-R.; Kunimasa, K.; Ohta, T.; Kumazawa, S.; Kamihira, M.; Kaji, K.; Uto, Y.; Hori, H.; Nagasawa, H.; Nakayama, T. Suppression of tumor-induced angiogenesis by Brazilian propolis: Major component artepillin C inhibits in vitro tube formation and endothelial cell proliferation. Cancer Lett. 2007, 252, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Daleprane, J.B.; Schmid, T.; Dehne, N.; Rudnicki, M.; Menrad, H.; Geis, T.; Ikegaki, M.; Ong, T.P.; Brüne, B.; Abdalla, D.S. Suppression of hypoxia-inducible factor-1α contributes to the antiangiogenic activity of red propolis polyphenols in human endothelial cells. J. Nutr. 2012, 142, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meneghelli, C.; Joaquim, L.S.D.; Félix, G.L.Q.; Somensi, A.; Tomazzoli, M.; da Silva, D.A.; Berti, F.V.; Veleirinho, M.B.R.; Recouvreux, D.d.O.S.; de Mattos Zeri, A.C. Southern Brazilian autumnal propolis shows anti-angiogenic activity: An in vitro and in vivo study. Microvasc. Res. 2013, 88, 1–11. [Google Scholar] [CrossRef]
- Ozturk, G.; Ginis, Z.; Akyol, S.; Erden, G.; Gurel, A.; Akyol, O. The anticancer mechanism of caffeic acid phenethyl ester (CAPE): Review of melanomas, lung and prostate cancers. Eur. Rev. Med. Pharm. Sci. 2012, 16, 2064–2068. [Google Scholar]
- Silva, L.M.d.; Souza, P.d.; Jaouni, S.K.A.; Harakeh, S.; Golbabapour, S.; de Andrade, S.F. Propolis and its potential to treat gastrointestinal disorders. Evid.-Based Complement. Altern. Med. 2018, 2018, 2035820. [Google Scholar] [CrossRef] [Green Version]
- Oršolić, N.; Bašić, I. Water-soluble derivative of propolis and its polyphenolic compounds enhance tumoricidal activity of macrophages. J. Ethnopharmacol. 2005, 102, 37–45. [Google Scholar] [CrossRef]
- Wang, L.-C.; Lin, Y.-L.; Liang, Y.-C.; Yang, Y.-H.; Lee, J.-H.; Yu, H.-H.; Wu, W.-M.; Chiang, B.-L. The effect of caffeic acid phenethyl ester on the functions of human monocyte-derived dendritic cells. BMC Immunol. 2009, 10, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misir, S.; Aliyazicioglu, Y.; Demir, S.; Turan, I.; Hepokur, C. Effect of Turkish propolis on miRNA expression, cell cycle, and apoptosis in human breast cancer (MCF-7) cells. Nutr. Cancer 2020, 72, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Omene, C.; Kalac, M.; Wu, J.; Marchi, E.; Frenkel, K.; O’Connor, O.A. Propolis and its active component, caffeic acid phenethyl ester (CAPE), modulate breast cancer therapeutic targets via an epigenetically mediated mechanism of action. J. Cancer Sci. Ther. 2013, 5, 334–342. [Google Scholar] [PubMed]
- Yilmaz, U.C.; Bagca, B.G.; Karaca, E.; Durmaz, A.; Durmaz, B.; Aykut, A.; Kayalar, H.; Avci, C.B.; Susluer, S.Y.; Pariltay, E.; et al. Propolis Extract Regulates microRNA Expression in Glioblastoma and Brain Cancer Stem Cells. Anti-Cancer Agents Med. Chem. 2022, 22, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Cogulu, O.; Biray, C.; Gunduz, C.; Karaca, E.; Aksoylar, S.; Sorkun, K.; Salih, B.; Ozkinay, F. Effects of Manisa propolis on telomerase activity in leukemia cells obtained from the bone marrow of leukemia patients. Int. J. Food Sci. Nutr. 2009, 60, 601–605. [Google Scholar] [CrossRef]
- Jung, B.i.; Kim, M.s.; Kim, H.A.; Kim, D.; Yang, J.; Her, S.; Song, Y.S. Caffeic acid phenethyl ester, a component of beehive propolis, is a novel selective estrogen receptor modulator. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Kapare, H.; Lohidasan, S.; Sinnathambi, A.; Mahadik, K. Standardization, anti-carcinogenic potential and biosafety of Indian propolis. J. Ayurveda Integr. Med. 2019, 10, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Hotta, S.; Tazawa, S.; Arai, Y.; Kato, K.; Ichihara, K. Chemical constituents of Brazilian Propolis from the state of Bahia and their growth inhibitory activities against cancer cells. Biosci. Biotechnol. Biochem. 2018, 82, 417–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, S.P.; Hemsley, F.; Djamgoz, M.B. Caffeic acid phenethyl ester: Inhibition of metastatic cell behaviours via voltage-gated sodium channel in human breast cancer in vitro. Int. J. Biochem. Cell Biol. 2016, 71, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, N.L.; Obradovic, A.D.; Vukic, M.D.; Jovanovic, D.; Djurdjevic, P.M. Cytotoxic, proapoptotic and antioxidative potential of flavonoids isolated from propolis against colon (HCT-116) and breast (MDA-MB-231) cancer cell lines. Food Res. Int. 2018, 106, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Frión-Herrera, Y.; Gabbia, D.; Scaffidi, M.; Zagni, L.; Cuesta-Rubio, O.; De Martin, S.; Carrara, M. The Cuban Propolis Component Nemorosone Inhibits Proliferation and Metastatic Properties of Human Colorectal Cancer Cells. Int. J. Mol. Sci. 2020, 21, 1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumazaki, M.; Shinohara, H.; Taniguchi, K.; Yamada, N.; Ohta, S.; Ichihara, K.; Akao, Y. Propolis cinnamic acid derivatives induce apoptosis through both extrinsic and intrinsic apoptosis signaling pathways and modulate of miRNA expression. Phytomedicine 2014, 21, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Lei, Y.-h.; Su, M.; Li, D.-j.; Zhang, N.; Shen, Y.-q. Kaempferol inhibits proliferation of human prostate cancer PC-3 cells via down-regulation of PCNA and VCAM-1. Chin. Pharmacol. Bull. 2011, 4, 553–557. [Google Scholar]
- Tolba, M.F.; Esmat, A.; Al-Abd, A.M.; Azab, S.S.; Khalifa, A.E.; Mosli, H.A.; Abdel-Rahman, S.Z.; Abdel-Naim, A.B. Caffeic acid phenethyl ester synergistically enhances docetaxel and paclitaxel cytotoxicity in prostate cancer cells. IUBMB Life 2013, 65, 716–729. [Google Scholar] [CrossRef]
- Kuo, Y.-Y.; Lin, H.-P.; Huo, C.; Su, L.-C.; Yang, J.; Hsiao, P.-H.; Chiang, H.-C.; Chung, C.-J.; Wang, H.-D.; Chang, J.-Y. Caffeic acid phenethyl ester suppresses proliferation and survival of TW2. 6 human oral cancer cells via inhibition of Akt signaling. Int. J. Mol. Sci. 2013, 14, 8801–8817. [Google Scholar] [CrossRef] [Green Version]
- Bernardino, P.N.; Bersano, P.R.O.; Neto, J.F.L.; Sforcin, J.M. Positive effects of antitumor drugs in combination with propolis on canine osteosarcoma cells (spOS-2) and mesenchymal stem cells. Biomed. Pharmacother. 2018, 104, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nani, B.D.; Franchin, M.; Lazarini, J.G.; Freires, I.A.; da Cunha, M.G.; Bueno-Silva, B.; de Alencar, S.M.; Murata, R.M.; Rosalen, P.L. Isoflavonoids from Brazilian red propolis down-regulate the expression of cancer-related target proteins: A pharmacogenomic analysis. Phytother. Res. 2018, 32, 750–754. [Google Scholar] [CrossRef]
- Skiba, M.; Szliszka, E.; Kunicka, M.; Krol, W. Effect of ethanol extract of propolis (EEP) on interleukin 8 release by human gastric adenocarcinoma cells (AGS) infected with Helicobacter pylori. Cent.-Eur. J. Immunol. 2011, 36, 65–69. [Google Scholar]
- Rouibah, H.; Kebsa, W.; Lahouel, M.; Zihlif, M.; Ahram, M.; Aburmeleih, B.; Mustafa, E.; Al-Ameer, H.J. Algerian propolis potentiates doxorubicin mediated anticancer effect against human pancreatic PANC-1 cancer cell line through cell cycle arrest, apoptosis induction and P-glycoprotein inhibition. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2018, 18, 375–387. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Nguyen, M.T.; Nguyen, N.T.; Awale, S. Chemical constituents of propolis from vietnamese trigona minor and their antiausterity activity against the panc-1 human pancreatic cancer cell line. J. Nat. Prod. 2017, 80, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Zhang, W.; Wu, G.; Ren, J.; Lu, H.; Li, Z.; Han, X. Synergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells. Biomed. Pharmacother. 2016, 84, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Ebeed, N.; Abdelmegeed, S. Modulator impacts of propolis extract against doxorubicin mediated carcinogenesis on hepatocellular carcinoma and drosophila somatic cells. Egypt. J. Genet. Cytol. 2017, 46, 389–407. [Google Scholar] [CrossRef]
- Yilmaz, U.C.; Bagca, B.G.; Karaca, E.; Durmaz, A.; Durmaz, B.; Aykut, A.; Kayalar, H.; Avci, C.B.; Susluer, S.Y.; Gunduz, C. Evaluation of the miRNA profiling and effectiveness of the propolis on B-cell acute lymphoblastic leukemia cell line. Biomed. Pharmacother. 2016, 84, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Żukowska, R.; Borawska, M.H.; Fiedorowicz, A.; Naliwajko, S.K.; Sawicka, D.; Car, H. Propolis changes the anticancer activity of temozolomide in U87MG human glioblastoma cell line. BMC Complement. Altern. Med. 2013, 13, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milošević-Đorđević, O.; Grujičić, D.; Radović, M.; Vuković, N.; Žižić, J.; Marković, S. In vitro chemoprotective and anticancer activities of propolis in human lymphocytes and breast cancer cells. Arch. Biol. Sci. 2015, 67, 571–581. [Google Scholar] [CrossRef]
- Padmavathi, R.; Senthilnathan, P.; Sakthisekaran, D. Therapeutic effect of propolis and paclitaxel on hepatic phase I and II enzymes and marker enzymes in dimethylbenz (a) anthracene-induced breast cancer in female rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 349–354. [Google Scholar] [CrossRef]
- Salim, E.I.; Abd El-Magid, A.D.; Farara, K.M.; Maria, D.S. Antitumoral and antioxidant potential of Egyptian propolis against the PC3 prostate cancer cell line. Asian Pac. J. Cancer Prev. 2015, 16, 7641–7651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, A.E.Z.; Artika, I.; Suryani; Minarni; Wiendarlina, I.Y.; Utami, N.; Sriwahyuni, D. The combination of propolis and curcuma zanthorrhiza as anti-breast cancer materials. Int. Res. J. Pharm. 2019, 10, 41–47. [Google Scholar] [CrossRef]



| Chemical Groups Found in Propolis | Examples | Reference |
|---|---|---|
| Polyphenols | Flavonoids (e.g., quecentin, islapinin, chrysin, galangin, alnusin, pinocembrin, naringenin, pinostribon, ermanin, formononetin, and biochanin A) | [32,33,37,38,39,40,41,42] |
| Phenolic esters (e.g., 4-phenyl coumarin) | ||
| Phenolic aldehydes (e.g., 4-hydroxybenzaldehyde) | ||
| Ketone derivatives (e.g., 3-oxo-oleanolic and 3-oxo-ursolic acids) | ||
| Flavanonols (e.g., dihydrokaempfero) | ||
| Benzoic acid Derivatives | Gentisic acid, protocatechuic acid, salicylic acid phenylmethyl ester, and gallic acid | [34,35] |
| Benzaldehyde compounds | Vanillin, protocatechualdehyde, and caproic aldehydes | [34,35]. |
| Cinnamic acid derivatives | Caffeic acid, isoferulic acid, cinnamic acid methyl ester, artepillin C, and capillartemisin A | [36,39,43] |
| Aliphatic hydrocarbons | Eicosine, tricosane, and heneicosane | [36,44] |
| Sugars | Fructose, glucose, glucitol, talose, ribose, and glycoside | [36,45] |
| Vitamins | B1, B6, C, and E | [36,45,46] |
| Amino acids | Alanine, cysteine, butyric acid, isoleucine, leucine, and valine | [36,46] |
| Fatty acids | Oleic, linoleic, stearic, eicosenoic, palmitoleic, and palmitic acid | [36,47] |
| Esters | Methyl palmitate, phenylethyl caffeate, benzyl benzoate, ethyl palmitate, tetradecyl caffeate, and stearic acid methyl ester | [36] |
| Alcohols | Benzyl alcohol, coumarine, and xanthorrhoeol | [36] |
| Minerals | Sodium, zinc, magnesium, potassium calcium, aluminium, and lead | [36,48] |
| Others | Enzymes, waxy acids, fatty acids, and aliphatic acids | [36] |
| Cancer Type | Cell Lines | Propolis Type/ Active Compound | Anticancer Mechanism(s) | Reference |
|---|---|---|---|---|
| Breast Cancer | MCF-7 | CAPE | Modulation of the estrogen receptor | [169] |
| New Zealand | Growth arrest of cells by downregulation of mortalin and activation of p53 tumor suppressor protein | [112] | ||
| Indian propolis | Cytotoxicity “less dense and rounded cells” | [170] | ||
| Brazilian propolis | Anti-proliferation | [171] | ||
| Turkish propolis | Cell cycle arrest at G1 Apoptosis through increasing pro-apoptotic protein levels (p21, Bax, p53, p53-Ser46, p53-Ser15) and decreasing MMP Alters the miRNA expression of tumor suppression gene (miR 34, 15a, and 16-5p), miR 21 and breast cancer gene (BRCA ½) | [165] | ||
| MDA-MB-231 | Cuban red propolis | Apoptosis by modulation of PI3K/Akt, p38 MAPK, and ERK1/2 signaling pathways and enhancing ROS generation and loss of mitochondrial potential. | [130] | |
| CAPE | Inhibit invasion/metastasis and cell motility through blockage of voltage-gated sodium channels | [172] | ||
| New Zealand propolis | Cell cycle arrest by downregulation of mortalin and activation of p53 | [112] | ||
| Serbian propolis | Cytotoxicity, proapoptotic, and antioxidative | [173] | ||
| BT-474 | Thai Apis mellifera propolis | Cell cycle arrest and apoptosis via p21 upregulation | [154] | |
| Colon Cancer | HCT-116 | Chinese propolis | Apoptosis | [137] |
| Genistein | G2/M Cell cycle arrest and apoptosis | [152] | ||
| Brazilian Propolis | Cell cycle arrest via Cip1/p21 expression | [153] | ||
| Polish propolis | Apoptosis by DNA condensation | [124] | ||
| Serbian propolis | Cytotoxicity, proapoptotic and antioxidative | [173] | ||
| SW-480 | Genistein | G2/M cell cycle arrest and apoptosis | [152] | |
| SW620 | CAPE | Anti-angiogenesis and anti-metastasis via VEGF and MMPs inhibition | [24] | |
| Trigona incisa propolis | Apoptosis via increasing ROS | [125] | ||
| HT-29 | Cuban Propolis | Cell cycle arrest and apoptosis | [174] | |
| Indian propolis | Cytotoxicity “less dense and rounded cells” | [170] | ||
| DLD-1 | Propolis cinnamic acid | TRAIL-dependent apoptosis | [175] | |
| Brazilian propolis | Anti-proliferative | [171] | ||
| Prostate Cancer | PC-3 | Kaempferol | Anti- proliferation by downregulation of PCNA and VCAM-1 | [176] |
| CAPE | Apoptosis by decreasing the cIAP-1/2 | [129] | ||
| Anti-inflammatory through suppression of COX-2/LOX and NF-κB signaling, 5-a reductase enzyme inhibition | [161] | |||
| DU145, PC-3 | Brazilian propolis | Downregulation of cyclin A, B, and D1, and Cdk and upregulation of p21 | [97] | |
| Chrysin | Mitochondrial-mediated apoptosis and ER stress and down-regulating ERK1/2 | [131] | ||
| CAPE | PI3K/Akt downregulation | [132] | ||
| Activation of the non-canonical Wnt-signaling pathway and synergistically act with docetaxel and paclitaxel | [177] | |||
| LNCaP, DU145 | Poland propolis | TRAIL-mediated apoptosis | [133] | |
| Oral Cancers | SCC15/25, CAL27 | CAPE | Cell cycle arrest p53 and Rb modulation Downregulation of oncogenes MIFT and K-Ras | [151] |
| KB | Brazilian Brown propolis | COX-2 inhibition | [25] | |
| YD15, HSC-4 HN22 | CAPE | Apoptosis via up-regulation of Bax and Puma proteins | [17] | |
| TW2.6 | CAPE | Alteration of c-Jun N-terminal kinase, ERK1/2, NF-κB, and Akt signaling | [178] | |
| CAL27 | Chrysin, Caffeic Acid, p-Coumaric Acid, and Ferulic Acid | PRODH/POX (proline degradation/proline oxidase) dependent apoptosis | [123] | |
| Melanoma | A375 | Chinese Propolis | Apoptosis, cell cycle arrest, and autophagy via NLRP1 Inflammatory Pathway | [145] |
| Chrysin | Apoptosis (Bax activation) by upregulating p38 MAPK Downregulating the ERK1/2 signaling pathway | [128] | ||
| A2058 | CAPE | Suppression of the production of pro-inflammatory cytokines Production of anti-inflammatory cytokines (IL-10) | [161] | |
| B16F10 | Galangin | Apoptosis via mitochondrial pathway and sustained activation of p38 MAPK | [127] | |
| Chrysin | Apoptosis and mitochondrial membrane potential loss through upregulating p38 mitogen-activated protein kinase (MAPK) and p62 Downregulation of tyrosinase activity (anti-melanogenesis) by modulating microphthalmia-associated transcription factor | [128] | ||
| Osteosarcoma | U2OS | New Zealand propolis | Cell cycle arrest by downregulation of mortalin and activation of p53 | [112] |
| Brazilian green propolis | Activation of p53 and growth arrest | [113] | ||
| Apoptosis | [179] | |||
| Lung Carcinoma | A549 | New Zealand propolis | Cell cycle arrest by downregulation of mortalin and activation of p53 | [112] |
| Brazilian green propolis | Activation of p53 and growth arrest | [113] | ||
| Brazilian propolis | Anti-proliferative | [171] | ||
| Cervical Cancer | HeLa | Brazilian red propolis | Downregulation the expression alpha tubulin, tubulin, histone H3 and prostaglandin E synthase. | [180] |
| New Zealand propolis | Cell cycle arrest mediated by downregulation of mortalin and activation of p53 | [112] | ||
| Gastric Cancer | AGS | Iran propolis | Downregulation the mRNA expression of PLD1 gene | [120] |
| Poland propolis | Interleukin (IL)-8 suppression | [181] | ||
| Pancreatic Cancer | PANC-1 | Algerian propolis | Cell cycle arrest, Apoptosis, P-Glycoprotein Inhibition | [182] |
| Vietnamese Trigona minor | Antiausterity | [183] | ||
| Oesophageal carcinoma | Eca9706, TE-1, and EC109 | Galangin | Apoptosis and cell cycle arrest | [184] |
| Hepatocellular Carcinoma | HEp-2 | Chinese and Egyptian propolis | Protection against doxorubicin-induced genotoxicity | [185] |
| Neuroblastoma | IMR32 | New Zealand propolis | Cell cycle arrest by downregulation of mortalin and activation of p53 | [112] |
| Fibrosarcoma | HT1080 | Brazilian green propolis | Cell cycle arrest via activation of p53 | [113] |
| Leukemia | CCRF-SB | Turkish propolis | Apoptosis | [186] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altabbal, S.; Athamnah, K.; Rahma, A.; Wali, A.F.; Eid, A.H.; Iratni, R.; Al Dhaheri, Y. Propolis: A Detailed Insight of Its Anticancer Molecular Mechanisms. Pharmaceuticals 2023, 16, 450. https://doi.org/10.3390/ph16030450
Altabbal S, Athamnah K, Rahma A, Wali AF, Eid AH, Iratni R, Al Dhaheri Y. Propolis: A Detailed Insight of Its Anticancer Molecular Mechanisms. Pharmaceuticals. 2023; 16(3):450. https://doi.org/10.3390/ph16030450
Chicago/Turabian StyleAltabbal, Suhib, Khawla Athamnah, Aaesha Rahma, Adil Farooq Wali, Ali H. Eid, Rabah Iratni, and Yusra Al Dhaheri. 2023. "Propolis: A Detailed Insight of Its Anticancer Molecular Mechanisms" Pharmaceuticals 16, no. 3: 450. https://doi.org/10.3390/ph16030450





