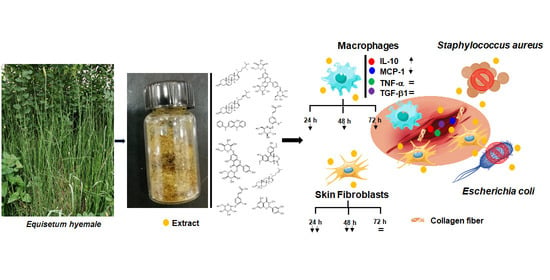
Horsetail (Equisetum hyemale) Extract Accelerates Wound Healing in Diabetic Rats by Modulating IL-10 and MCP-1 Release and Collagen Synthesis
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Elemental Analysis in the Raw Plant Material of E. hyemale
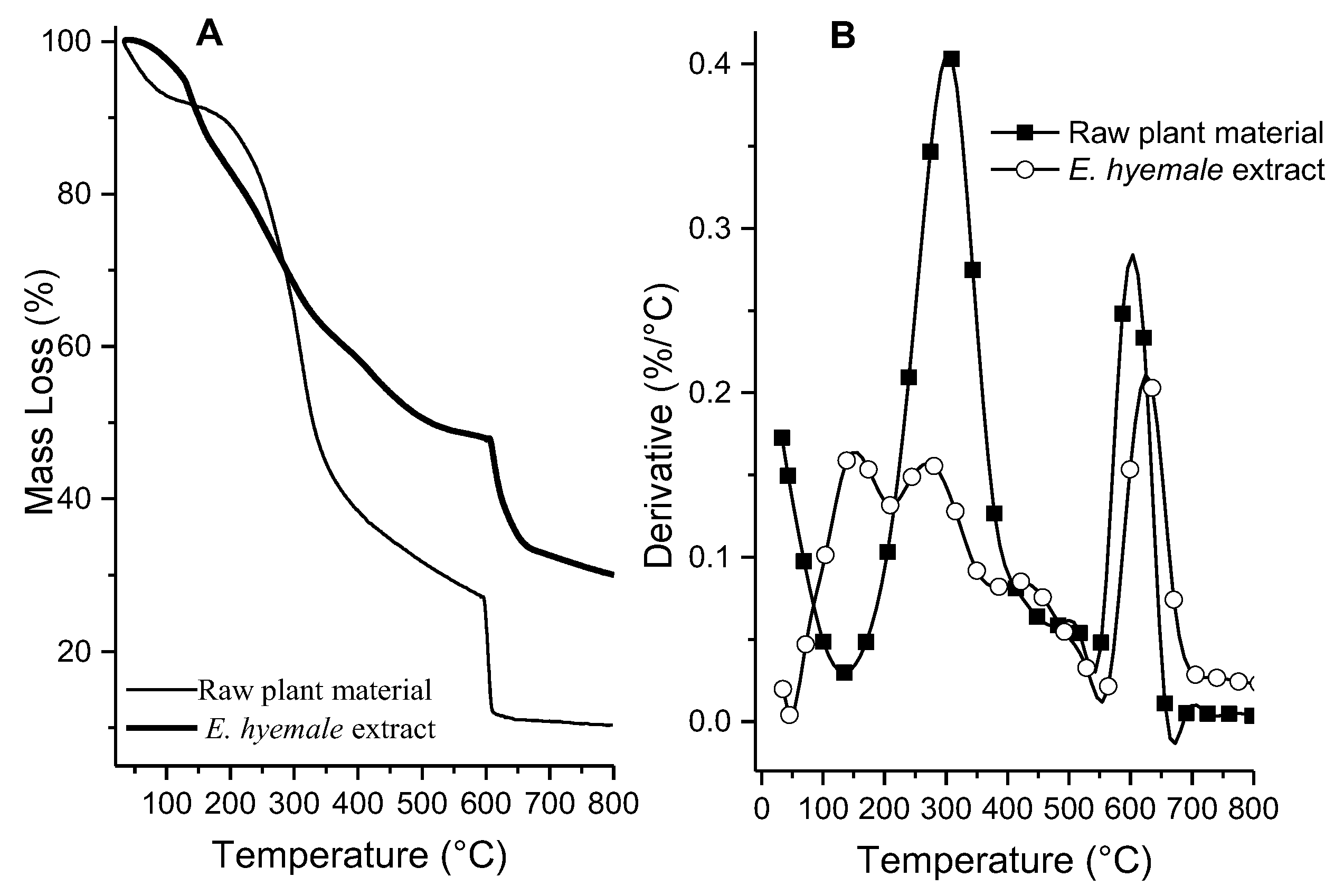
2.2. Thermal Analysis of Plant Extract
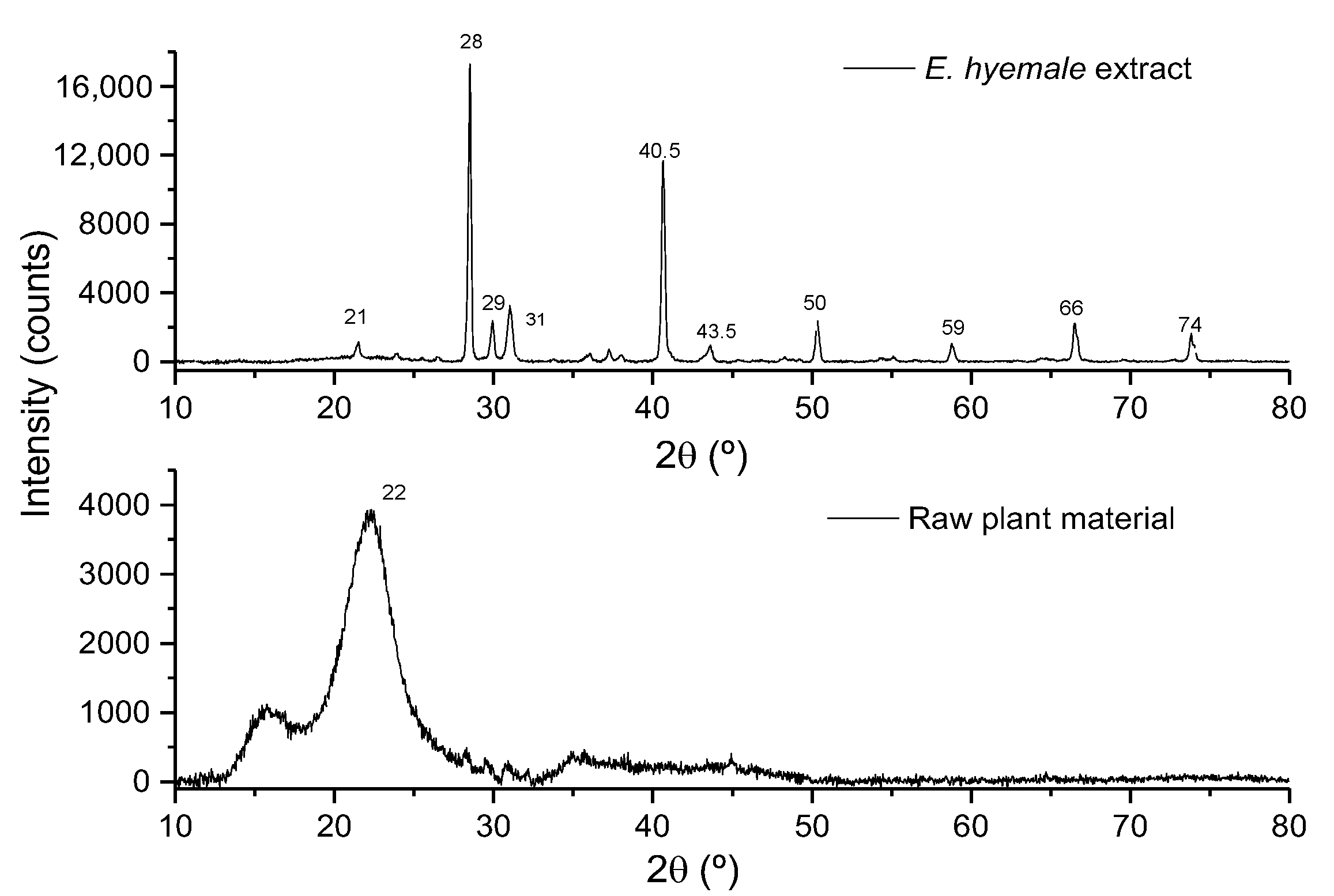
2.3. Evaluation of Surface Crystallinity by X-Ray Diffraction
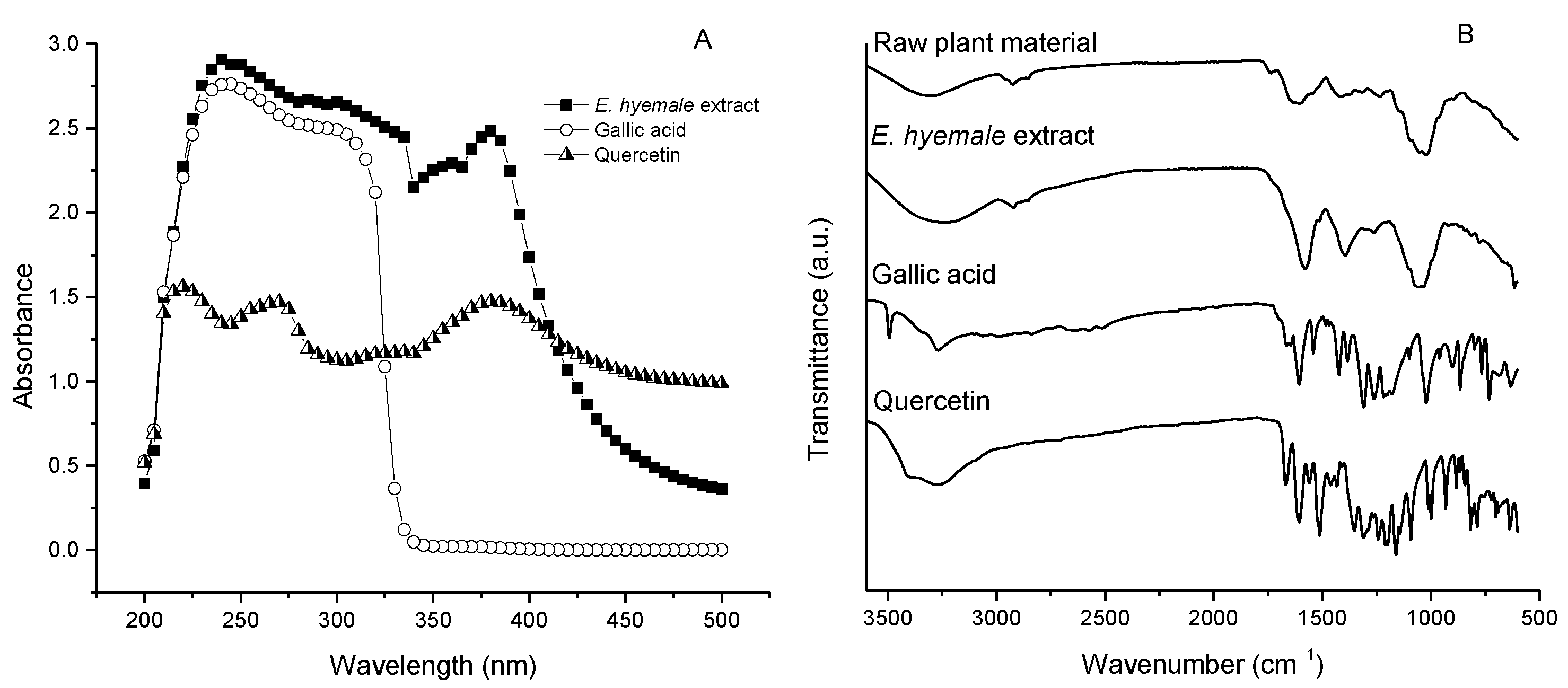
2.4. Chemical Structure of E. hyemale Extract Evaluated by UV/Vis Spectrophotometry and Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Total Polyphenol and Flavonoid Content in E. hyemale Extract
2.6. Identification of Compounds Contained in E. hyemale Extract by Chromatography
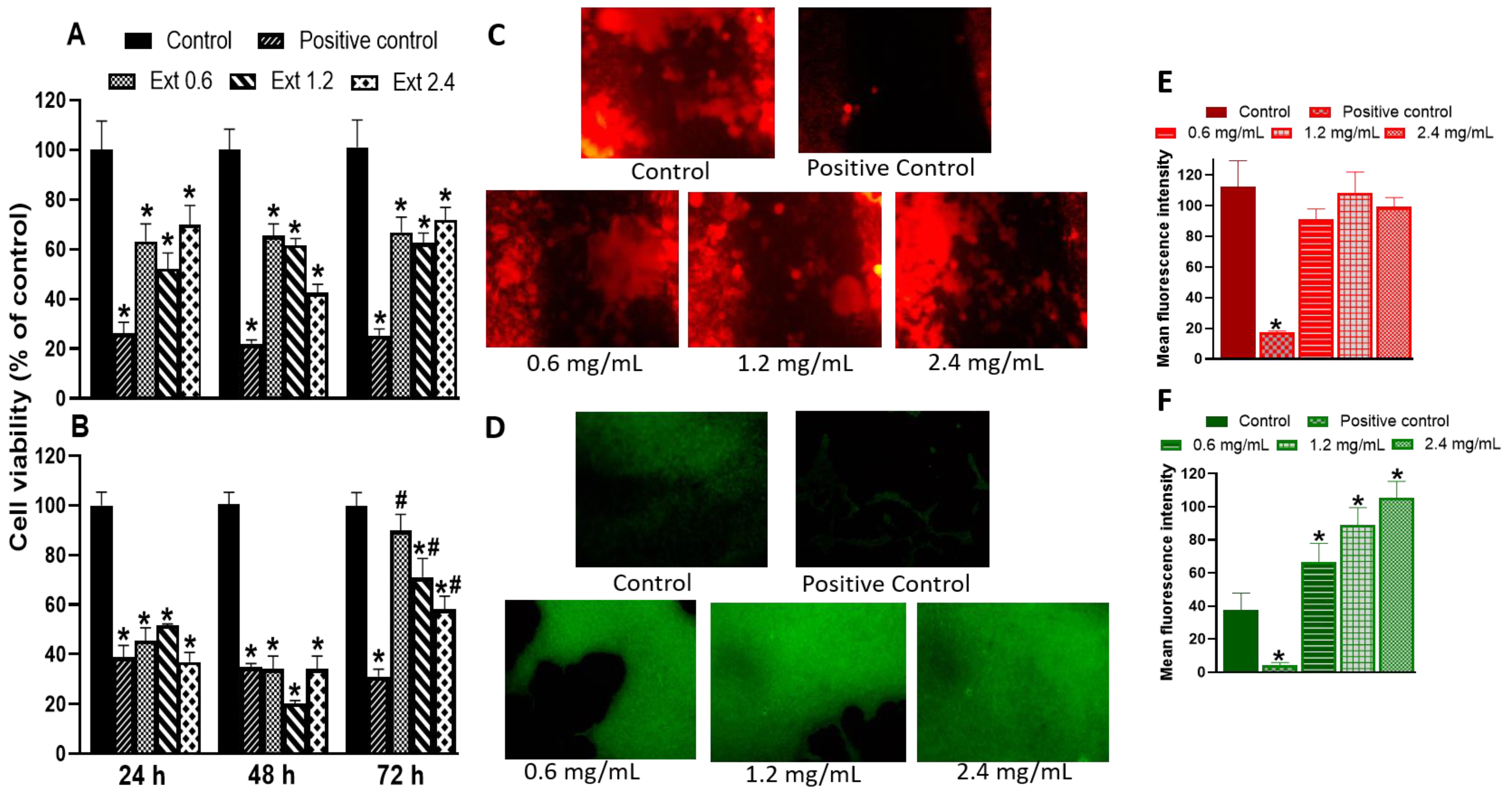
2.7. Effect of E. hyemale Extract on the Viability of RAW 264.7 Cells and Porcine Skin Fibroblasts
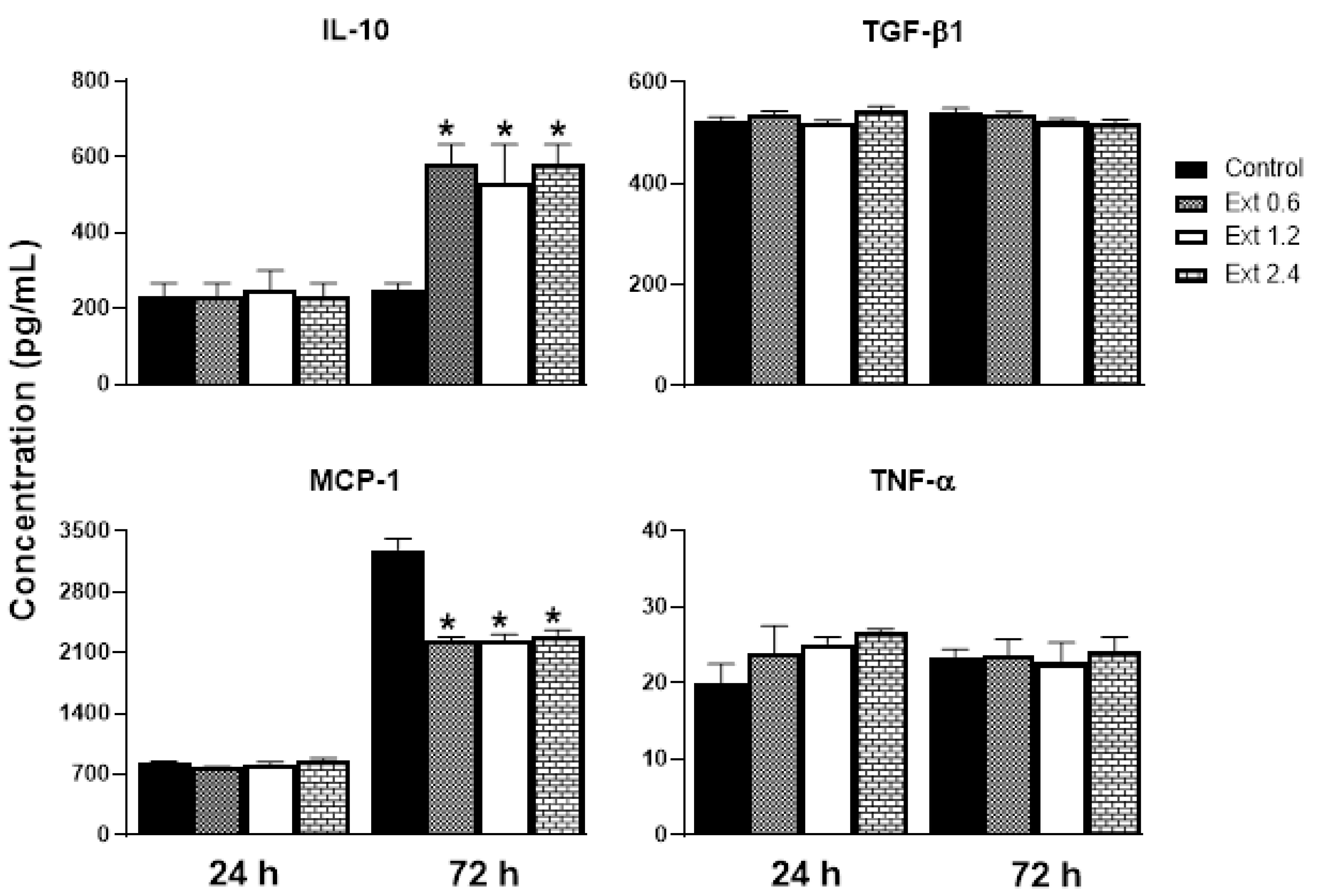
2.8. Effect of E. hyemale Extract on the Release of Inflammatory Mediators from RAW 264.7 Cells
2.9. Antimicrobial Activity of E. hyemale Extract
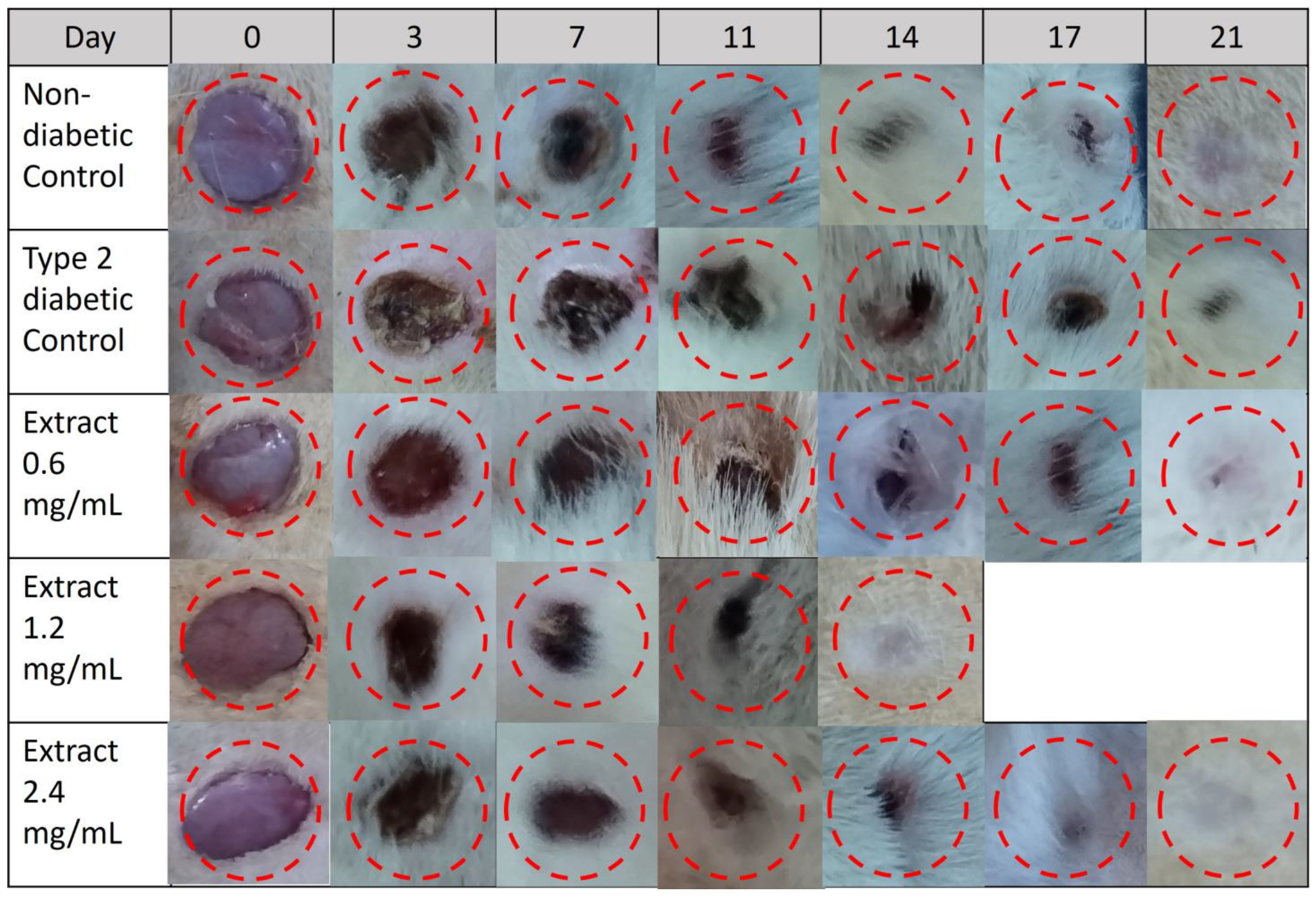
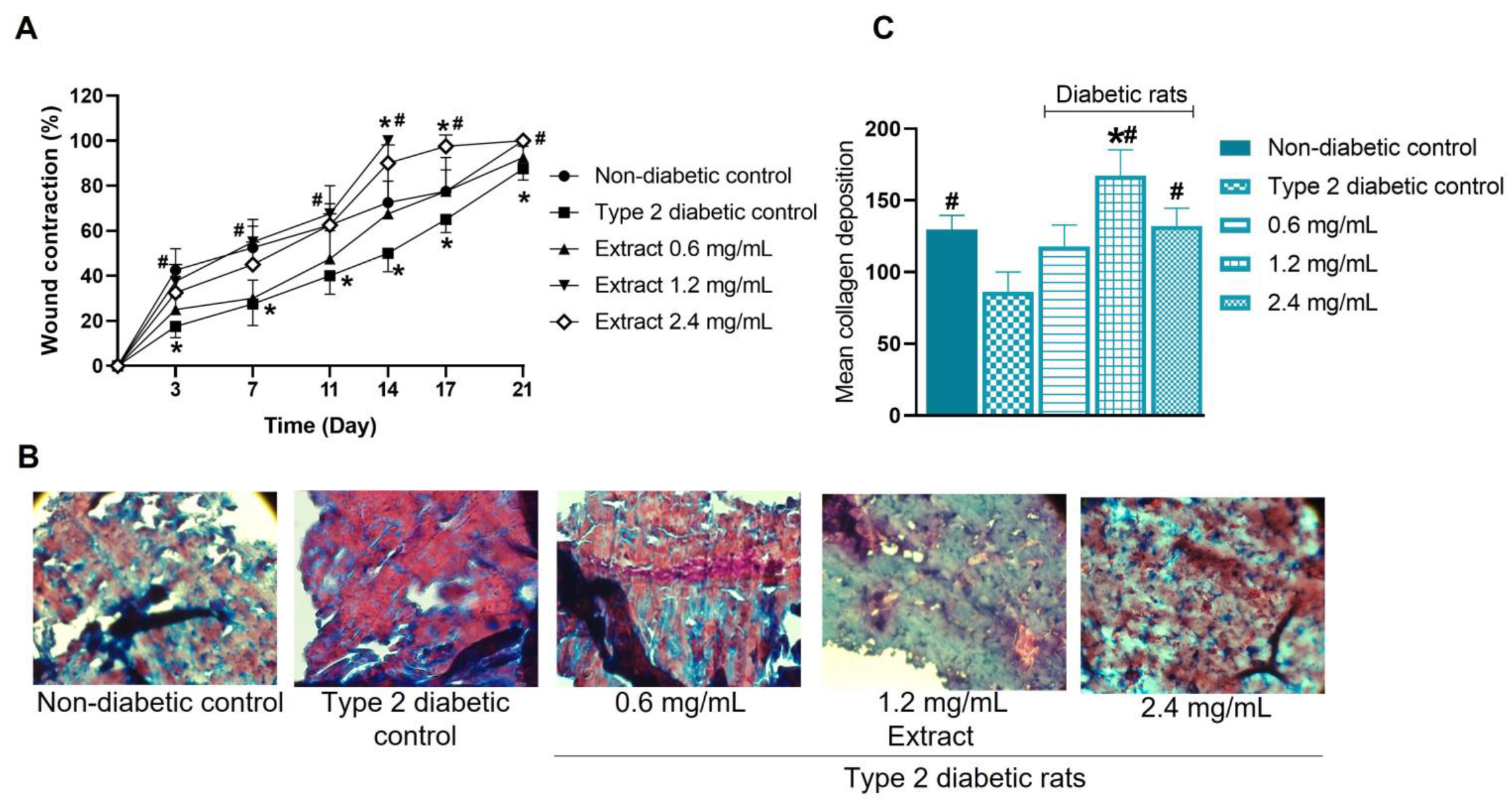
2.10. Effect of E. hyemale Extract on Wound Healing in Type 2 Diabetic Rats
3. Materials and Methods
3.1. Chemical Reagents and Materials
3.2. Plant Material
3.3. Elemental Analysis of Raw Plant Material by X-ray Fluorescence (XRF)
3.4. Extraction Procedure
3.5. Thermogravimetric Analysis (TGA) of Raw Material and Extract
3.6. X-ray Diffraction (XRD) Evaluation of the Raw Material and Extract
3.7. UV/Vis Spectrophotometry and Fourier Transform Infrared Spectroscopy (FTIR) Analysis of the Extract
3.8. Quantification of Total Polyphenols and Flavonoids in the Extract
3.9. Identification of Components of the Extract by Chromatography
3.9.1. Gas Chromatography (CG-MS)
3.9.2. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC-ESI-MS)
3.10. Cell Assays
3.10.1. Cells
3.10.2. Effect of Horsetail Extract on the Viability of RAW 264.7 Cells and Porcine Skin Fibroblasts
3.10.3. Effect of Horsetail Extract on the Proliferation of RAW 264.7 Cells and Porcine Skin Fibroblasts
3.11. Effect of Extract on Cytokine Release from RAW 264.7 Cells
3.12. Evaluation of Antimicrobial Activity of E. hyemale Extract
3.13. Wound-Healing Effect of E. hyemale Extract in Type 2 Diabetic Rats
3.14. Histological Analysis
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pryer, K.M.; Tomasi, C.; Wang, X.; Meineke, E.K.; Windham, M.D. Using computer vision on herbarium specimen images to discriminate among closely related horsetails (Equisetum). App. Plant Sci. 2020, 8, e11372. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Pérez, J.C.; Esparza-Aguilar, M.d.L.; Gómez-Campos, A. Importancia etnobotánica de una planta vascular sin semilla en México: Equisetum. Polibotánica 2006, 21, 61–74. [Google Scholar]
- Argueta, A. Atlas de las Plantas de la Medicina Tradicional Mexicana, 1st ed.; Istituto Nacional Indigenista: Ciudad de México, Mexico, 1994; pp. 1611–1786.
- Perez Gutierrez, R.M.; Laguna, G.Y.; Walkowski, A. Diuretic activity of Mexican equisetum. J. Ethnopharmacol. 1985, 14, 269–272. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, L.N.; Da Fonseca, A.C.C.; Wermelinger, G.F.; da Silva, D.P.D.; Pascoal, A.C.R.F.; Sawaya, A.C.H.F.; de Almeida, E.C.P.; do Amaral, B.S.; de Lima Moreira, D.; Robbs, B.K. New substances of Equisetum hyemale L. extracts and their in vivo antitumoral effect against oral squamous cell carcinoma. J. Ethnopharmacol. 2023, 303, 116043. [Google Scholar] [CrossRef] [PubMed]
- Carmignan, F.; Matias, R.; Carollo, C.A.; Dourado, D.M.; Fermiano, M.H.; Silva, B.A.K.; Bastos, P. Efficacy of application of Equisetum pyramidale Goldm. hydrogel for tissue restoration of induced skin lesions in Wistar rats. Braz. J. Biol. 2020, 80, 12–22. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, G.M.; Politi, F.A.; Rodrigues, E.R.; Souza-Moreira, T.M.; Moreira, R.R.; Cardoso, C.R.; Santos, L.C.; Pietro, R.C. Phytochemical Characterization, Antimicrobial Activity, and Antioxidant Potential of Equisetum hyemale L. (Equisetaceae) Extracts. J. Med. Food 2015, 18, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Alves, C.F.; Bonez, P.C.; de Souza, M.E.; Casagrande, C.; Freitas, L.; Dolwitsch, C.; Pires, F.; Rorato Sagrillo, M.; Fernandes de Brum, G.; Anraku de Campos, M.M.; et al. Antimicrobial, Cyto and Genotoxic Activities of Equisetum hyemale. Pharmacogn. J. 2019, 11, 1563–1571. [Google Scholar] [CrossRef]
- Dos Santos Alves, C.F.; Bonez, P.C.; de Souza, M.E.; da Cruz, R.C.; Boligon, A.A.; Piana, M.; Brum, T.F.; Rossi, G.G.; Jesus, R.D.; Grando, T.H.; et al. Antimicrobial, antitrypanosomal and antibiofilm activity of Equisetum hyemale. Microb. Pathog. 2016, 101, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, X.; Zheng, B.; Zhao, C. The optimization of ultrasonic-microwave assisted synergistic extraction of Lotus plumule extract rich in flavonoids and its hypoglycemic activity. Food Prod. Process. Nutr. 2021, 3, 23. [Google Scholar] [CrossRef]
- Carneiro, D.M.; Freire, R.C.; Honorio, T.C.; Zoghaib, I.; Cardoso, F.F.; Tresvenzol, L.M.; de Paula, J.R.; Sousa, A.L.; Jardim, P.C.; da Cunha, L.C. Randomized, Double-Blind Clinical Trial to Assess the Acute Diuretic Effect of Equisetum arvense (Field Horsetail) in Healthy Volunteers. Evid. Based Complement. Alternat. Med. 2014, 2014, 760683. [Google Scholar] [CrossRef] [PubMed]
- Grundemann, C.; Lengen, K.; Sauer, B.; Garcia-Kaufer, M.; Zehl, M.; Huber, R. Equisetum arvense (common horsetail) modulates the function of inflammatory immunocompetent cells. BMC Complement. Altern. Med. 2014, 14, 283. [Google Scholar] [CrossRef]
- Parrish, A.N.; Lange, I.; Samec, D.; Lange, B.M. Differential Accumulation of Metabolites and Transcripts Related to Flavonoid, Styrylpyrone, and Galactolipid Biosynthesis in Equisetum Species and Tissue Types. Metabolites 2022, 12, 403. [Google Scholar] [CrossRef]
- Ozay, Y.; Kasim Cayci, M.; Guzel-Ozay, S.; Cimbiz, A.; Gurlek-Olgun, E.; Sabri Ozyurt, M. Effects of Equisetum arvense Ointment on Diabetic Wound Healing in Rats. Wounds 2013, 25, 234–241. [Google Scholar]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Gianino, E.; Miller, C.; Gilmore, J. Smart Wound Dressings for Diabetic Chronic Wounds. Bioengineering 2018, 5, 51. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef]
- Laires, M.J.; Monteiro, C. Exercise, magnesium and immune function. Magnes. Res. 2008, 21, 92–96. [Google Scholar] [PubMed]
- Volpe, S.L. Magnesium in Disease Prevention and Overall Health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef]
- Ward, R.J.; Crichton, R.R.; Taylor, D.L.; Della Corte, L.; Srai, S.K.; Dexter, D.T. Iron and the immune system. J. Neural Transm. 2011, 118, 315–328. [Google Scholar] [CrossRef]
- Wang, N.; Ma, Y.; Shi, H.; Song, Y.; Guo, S.; Yang, S. Mg-, Zn-, and Fe-Based Alloys with Antibacterial Properties as Orthopedic Implant Materials. Front. Bioeng. Biotechnol. 2022, 10, 888084. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Siracusa, V. The Use of Thermal Techniques in the Characterization of Bio-Sourced Polymers. Materials 2021, 14, 1686. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solis, V.; Martinez-Guerra, E.; Balderrama, C.I.P.; Martinez, I.C.; Saavedra-Leos, M.Z. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries. Polymers 2019, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- De Assis, A.C.L.; Alves, L.P.; Malheiro, J.P.T.; Barros, A.R.A.; Pinheiro-Santos, E.E.; de Azevedo, E.P.; Silva Alves, H.D.; Oshiro-Junior, J.A.; Damasceno, B. Opuntia Ficus-Indica, L. Miller (Palma forrageira) as an Alternative Source of Cellulose for Production of Pharmaceutical Dosage Forms and Biomaterials: Extraction and Characterization. Polymers 2019, 11, 1124. [Google Scholar] [CrossRef] [PubMed]
- Masłowski, M.; Miedzianowska, J.; Czylkowska, A.; Strzelec, K. Horsetail (Equisetum arvense) as a Functional Filler for Natural Rubber Biocomposites. Materials 2020, 13, 2526. [Google Scholar] [CrossRef]
- Gierlinger, N.; Sapei, L.; Paris, O. Insights into the chemical composition of Equisetum hyemale by high resolution Raman imaging. Planta 2008, 227, 969–980. [Google Scholar] [CrossRef]
- Joshi, D.D. FTIR Spectroscopy: Herbal Drugs and Fingerprints. In Herbal Drugs and Fingerprints: Evidence Based Herbal Drugs, 1st ed.; Joshi, D.D., Ed.; Springer: Delhi, India, 2012; pp. 121–146. [Google Scholar]
- Durak, T.; Depciuch, J. Effect of plant sample preparation and measuring methods on ATR-FTIR spectra results. Environ. Exp. Bot. 2020, 169, 103915. [Google Scholar] [CrossRef]
- Milutinović, M.; Radovanović, N.; Rajilić-Stojanović, M.; Šiler-Marinković, S.; Dimitrijević, S.; Dimitrijević-Branković, S. Microwave-assisted extraction for the recovery of antioxidants from waste Equisetum arvense. Ind. Crops Prod. 2014, 61, 388–397. [Google Scholar] [CrossRef]
- Do Monte, F.H.; dos Santos, J.G., Jr.; Russi, M.; Lanziotti, V.M.; Leal, L.K.; Cunha, G.M. Antinociceptive and anti-inflammatory properties of the hydroalcoholic extract of stems from Equisetum arvense L. in mice. Pharmacol. Res. 2004, 49, 239–243. [Google Scholar] [CrossRef]
- Gomez, M.A.; Saenz, M.T.; Garcia, M.D.; Fernandez, M.A. Study of the topical anti-inflammatory activity of Achillea ageratum on chronic and acute inflammation models. Z. Naturforsch. C J. Biosci. 1999, 54, 937–941. [Google Scholar] [CrossRef]
- Navarro, A.; De las Heras, B.; Villar, A. Anti-inflammatory and immunomodulating properties of a sterol fraction from Sideritis foetens Clem. Biol. Pharm. Bull. 2001, 24, 470–473. [Google Scholar] [CrossRef]
- PubChem. Antiinflammatory Activity in Human Neutrophils Assessed as fMLP-Induced Superoxide Release after 5 min by Spectrometry. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/311335 (accessed on 20 June 2022).
- Chen, J.J.; Chen, P.H.; Liao, C.H.; Huang, S.Y.; Chen, I.S. New phenylpropenoids, bis(1-phenylethyl)phenols, bisquinolinone alkaloid, and anti-inflammatory constituents from Zanthoxylum integrifoliolum. J. Nat. Prod. 2007, 70, 1444–1448. [Google Scholar] [CrossRef]
- Niu, H.; Li, X.; Yang, A.; Jin, Z.; Wang, X.; Wang, Q.; Yu, C.; Wei, Z.; Dou, C. Cycloartenol exerts anti-proliferative effects on Glioma U87 cells via induction of cell cycle arrest and p38 MAPK-mediated apoptosis. J. BUON 2018, 23, 1840–1845. [Google Scholar]
- Zhang, Z.L.; Luo, Z.L.; Shi, H.W.; Zhang, L.X.; Ma, X.J. Research advance of functional plant pharmaceutical cycloartenol about pharmacological and physiological activity. Zhongguo Zhong Yao Za Zhi 2017, 42, 433–437. [Google Scholar] [CrossRef]
- Thuluva, S.C.; Igel, M.; Giesa, U.; Lutjohann, D.; Sudhop, T.; von Bergmann, K. Ratio of lathosterol to campesterol in serum predicts the cholesterol-lowering effect of sitostanol-supplemented margarine. Int. J. Clin. Pharmacol. Ther. 2005, 43, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Fons, F.; Froissard, D.; Bessière, J.-M.; Fruchier, A.; Buatois, B.; Rapior, S. Volatile Composition of Six Horsetails: Prospects and Perspectives. Nat. Prod. Commun. 2013, 8, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Giordani, C.; Waller, S.B.; Madrid, I.M.; Guterres, K.A.; de Matos, C.B.; Hoffmann, J.F.; de Castro, L.L.; Chaves, F.C.; de Faria, R.O.; Cleff, M.B. Chemical, antioxidant and cytotoxic profile of hydroalcoholic extracts of plants from Southern Brazil and their activity against pathogenic fungi isolated from dogs and cats with sensitivity and resistance to conventional antifungals. Nat. Prod. Res. 2022, 36, 3223–3228. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Liu, Q.; Cheng, X.; Zhou, Y.; Xiao, Y. Cell cycle arrest and cell apoptosis induced by Equisetum hyemale extract in murine leukemia L1210 cells. J. Ethnopharmacol. 2012, 144, 322–327. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; National Center for Advancing Translational Sciences (NCATS): Bethesda, MD, USA, 2004; pp. 353–377. [Google Scholar]
- Keith, C.T.; Borisy, A.A.; Stockwell, B.R. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discov. 2005, 4, 71–78. [Google Scholar] [CrossRef]
- Sundarraj, S.; Thangam, R.; Sreevani, V.; Kaveri, K.; Gunasekaran, P.; Achiraman, S.; Kannan, S. γ-Sitosterol from Acacia nilotica L. induces G2/M cell cycle arrest and apoptosis through c-Myc suppression in MCF-7 and A549 cells. J. Ethnopharmacol. 2012, 141, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Rajendra Prasad, N.; Karthikeyan, A.; Karthikeyan, S.; Reddy, B.V. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, L.; Zhang, Y.; Gao, Z. Kaempferol inhibits fibroblast collagen synthesis, proliferation and activation in hypertrophic scar via targeting TGF-β receptor type I. Biomed. Pharmacother. 2016, 83, 967–974. [Google Scholar] [CrossRef]
- Lampiasi, N.; Montana, G. The molecular events behind ferulic acid mediated modulation of IL-6 expression in LPS-activated Raw 264.7 cells. Immunobiology 2016, 221, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Zhong, L.; Lu, S.; Hu, P.; Pan, Y.; Ma, X.; Yan, B.; Wei, Z.; Yang, G. Quercetin promotes cutaneous wound healing in mice through Wnt/β-catenin signaling pathway. J. Ethnopharmacol. 2022, 290, 115066. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.N.S.; Nair, R.V.R.; Nair, A.P.R.; Nair, A.S.; Thyagarajan, S.; Johnson, A.J.; Baby, S. Antidiabetes constituents, cycloartenol and 24-methylenecycloartanol, from Ficus krishnae. PLoS ONE 2020, 15, e0235221. [Google Scholar] [CrossRef]
- Abate, G.; Zhang, L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia annua L. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, F.; Shen, M.; Jia, S.; Xie, J. Phytosterols Suppress Phagocytosis and Inhibit Inflammatory Mediators via ERK Pathway on LPS-Triggered Inflammatory Responses in RAW264.7 Macrophages and the Correlation with Their Structure. Foods 2019, 8, 582. [Google Scholar] [CrossRef]
- Singampalli, K.L.; Balaji, S.; Wang, X.; Parikh, U.M.; Kaul, A.; Gilley, J.; Birla, R.K.; Bollyky, P.L.; Keswani, S.G. The Role of an IL-10/Hyaluronan Axis in Dermal Wound Healing. Front. Cell. Dev. Biol. 2020, 8, 636. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef]
- Sabeva, N.S.; McPhaul, C.M.; Li, X.; Cory, T.J.; Feola, D.J.; Graf, G.A. Phytosterols differentially influence ABC transporter expression, cholesterol efflux and inflammatory cytokine secretion in macrophage foam cells. J. Nutr. Biochem. 2011, 22, 777–783. [Google Scholar] [CrossRef]
- Aherne, S.A.; O’Brien, N.M. Modulation of cytokine production by plant sterols in stimulated human Jurkat T cells. Mol. Nutr. Food Res. 2008, 52, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.J.; Yu, W.Q.; Zhang, Y.L.; Jiang, X.Q.; Zhang, F.Q. Effects of TiO2 nanotube layers on RAW 264.7 macrophage behaviour and bone morphogenetic protein-2 expression. Cell Prolifer. 2013, 46, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Liao, K.C.; Chen, C.H. 2-Phenylnaphthalene Derivatives Inhibit Lipopolysaccharide-Induced Pro-Inflammatory Mediators by Downregulating of MAPK/NF-kappaB Pathways in RAW 264.7 Macrophage Cells. PLoS ONE 2017, 12, e0168945. [Google Scholar] [CrossRef]
- Huang, C.; Li, W.; Zhang, Q.; Chen, L.; Chen, W.; Zhang, H.; Ni, Y. Anti-inflammatory activities of Guang-Pheretima extract in lipopolysaccharide-stimulated RAW 264.7 murine macrophages. BMC Complement. Altern. Med. 2018, 18, 46. [Google Scholar] [CrossRef]
- Cantuária, A.P.C.; Figueiredo, T.M.; Freire, M.S.; Lima, S.M.F.; Almeida, J.A.; Franco, O.L.; Rezende, T.M.B. The effects of glucose concentrations associated with lipopolysaccharide and interferon-gamma stimulus on mediators’ production of RAW 264.7 cells. Cytokine 2018, 107, 18–25. [Google Scholar] [CrossRef]
- Astashkina, A.; Mann, B.; Grainger, D.W. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol. Ther. 2012, 134, 82–106. [Google Scholar] [CrossRef]
- Brown, T.D. Techniques for mechanical stimulation of cells in vitro: A review. J. Biomech. 2000, 33, 3–14. [Google Scholar] [CrossRef]
- Búfalo, M.C.; Ferreira, I.; Costa, G.; Francisco, V.; Liberal, J.; Cruz, M.T.; Lopes, M.C.; Batista, M.T.; Sforcin, J.M. Propolis and its constituent caffeic acid suppress LPS-stimulated pro-inflammatory response by blocking NF-κB and MAPK activation in macrophages. J. Ethnopharmacol. 2013, 149, 84–92. [Google Scholar] [CrossRef]
- Endale, M.; Park, S.-C.; Kim, S.; Kim, S.-H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef]
- Bak, M.-J.; Hong, S.-G.; Lee, J.-W.; Jeong, W.-S. Red Ginseng Marc Oil Inhibits iNOS and COX-2 via NFκB and p38 Pathways in LPS-Stimulated RAW 264.7 Macrophages. Molecules 2012, 17, 13769–13786. [Google Scholar] [CrossRef]
- Palacz-Wrobel, M.; Borkowska, P.; Paul-Samojedny, M.; Kowalczyk, M.; Fila-Danilow, A.; Suchanek-Raif, R.; Kowalski, J. Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages. Biomed. Pharmacother. 2017, 93, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar] [CrossRef]
- Čanadanović-Brunet, J.M.; Ćetković, G.S.; Djilas, S.M.; Tumbas, V.T.; Savatović, S.S.; Mandić, A.I.; Markov, S.L.; Cvetković, D.D. Radical scavenging and antimicrobial activity of horsetail (Equisetum arvense L.) extracts. Int. J. Food Sci. Technol. 2009, 44, 269–278. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef] [PubMed]
- McRae, J.; Yang, Q.; Crawford, R.; Palombo, E. Review of the methods used for isolating pharmaceutical lead compounds from traditional medicinal plants. Environmentalist 2007, 27, 165–174. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Worley, S.D.; Broughton, R. The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. U-shaped dose-responses in biology, toxicology, and public health. Annu. Rev. Public Health 2001, 22, 15–33. [Google Scholar] [CrossRef]
- Karim, S.; Alkreathy, H.M.; Ahmad, A.; Khan, M.I. Effects of Methanolic Extract Based-Gel from Saudi Pomegranate Peels with Enhanced Healing Potential on Excision Wounds in Diabetic Rats. Front. Pharmacol. 2021, 12, 704503. [Google Scholar] [CrossRef] [PubMed]
- Nagar, H.K.; Srivastava, A.K.; Srivastava, R.; Kurmi, M.L.; Chandel, H.S.; Ranawat, M.S. Pharmacological Investigation of the Wound Healing Activity of Cestrum nocturnum (L.) Ointment in Wistar Albino Rats. J. Pharm. 2016, 2016, 9249040. [Google Scholar] [CrossRef]
- Lodhi, S.; Singhai, A.K. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua Linn. on streptozotocin induced diabetic rats. Asian Pac. J. Trop. Med. 2013, 6, 253–259. [Google Scholar] [CrossRef]
- Aly, S.H.; El-Hassab, M.A.; Elhady, S.S.; Gad, H.A. Comparative Metabolic Study of Tamarindus indica L.’s Various Organs Based on GC/MS Analysis, In Silico and In Vitro Anti-Inflammatory and Wound Healing Activities. Plants 2023, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, C.F. Comparative study of hydroalcoholic extracts of Bryophyllum pinnatum and Macrotyloma uniflorum for their antioxidant, antiurolithiatic, and wound healing potential. J. Appl. Biol. Biotechnol. 2022, 10, 196–205. [Google Scholar] [CrossRef]
- Mssillou, I.; Agour, A.; Slighoua, M.; Chebaibi, M.; Amrati, F.E.; Alshawwa, S.Z.; Kamaly, O.A.; El Moussaoui, A.; Lyoussi, B.; Derwich, E. Ointment-Based Combination of Dittrichia viscosa L. and Marrubium vulgare L. Accelerate Burn Wound Healing. Pharmaceuticals 2022, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.T.B.; Araújo-Filho, H.G.; Barreto, A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S.; Barreto, R.S.S. Wound healing properties of flavonoids: A systematic review highlighting the mechanisms of action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef]
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Choucry, M.A.; Kandil, Z.A. Antibacterial, antioxidant, and topical anti-inflammatory activities of Bergia ammannioides: A wound-healing plant. Pharm. Biol. 2016, 54, 215–224. [Google Scholar] [CrossRef]
- Solís-Salas, L.M.; Sierra-Rivera, C.A.; Cobos-Puc, L.E.; Ascacio-Valdés, J.A.; Silva-Belmares, S.Y. Antibacterial Potential by Rupture Membrane and Antioxidant Capacity of Purified Phenolic Fractions of Persea americana Leaf Extract. Antibiotics 2021, 10, 508. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Pearson, J. Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef]
- Ghasemi, A.; Khalifi, S.; Jedi, S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes (review). Acta Physiol. Hung. 2014, 101, 408–420. [Google Scholar] [CrossRef]
- Aceros, H.; Farah, G.; Cobos-Puc, L.; Stabile, A.M.; Noiseux, N.; Mukaddam-Daher, S. Moxonidine improves cardiac structure and performance in SHR through inhibition of cytokines, p38 MAPK and Akt. Br. J. Pharmacol. 2011, 164, 946–957. [Google Scholar] [CrossRef] [PubMed]

| Chemical Element | Concentration (%) | Concentration Based on Ash (%) |
|---|---|---|
| K | 40.00 | 5.72 |
| Ca | 23.97 | 3.42 |
| Si | 23.20 | 3.31 |
| Cl | 3.82 | 0.54 |
| S | 3.42 | 0.48 |
| P | 2.73 | 0.39 |
| Fe | 1.09 | 0.15 |
| Mg | 0.90 | 0.13 |
| Rt (min) | m/z [M-H]− | Identified Component | Family | Structure |
|---|---|---|---|---|
| 16.44 | 315.2 | Campesterol | Phytosterols | 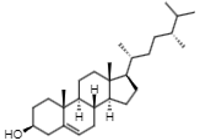 |
| 18.89 | 329.2 | γ-sitosterol | Phytosterols | 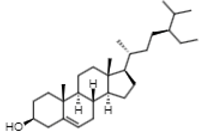 |
| 17.30 | 287.2 | Phenol, 2,4-bis(1-phenylethyl) | Phenylpropenoids |  |
| 20.80 | 41 | Cycloartenol | Phytosterols | 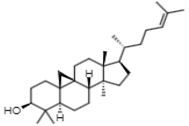 |
| Rt (min) | m/z [M-H]− | Identified Component | Family | Structure |
|---|---|---|---|---|
| 8.67 | 340.9 | Caffeic acid 4-O-glucoside | Hydroxycinnamic acids | 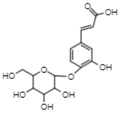 |
| 29.06 | 770.8 | Kaempferol 3,7,4’-O-triglucoside | Flavonols | 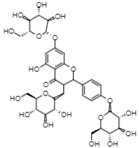 |
| 31.16 | 355 | Ferulic acid 4-O-glucoside | Methoxycinnamic acids |  |
| 35.52 | 355 | Conidendrin | Lignans | 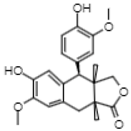 |
| 39.6 | 300.9 | Quercetin | Flavonols | 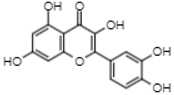 |
| 42.17 | 609 | Kaempferol 3,7-O-diglucoside | Flavonols | 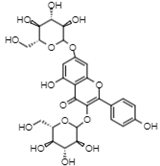 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguayo-Morales, H.; Sierra-Rivera, C.A.; Claudio-Rizo, J.A.; Cobos-Puc, L.E. Horsetail (Equisetum hyemale) Extract Accelerates Wound Healing in Diabetic Rats by Modulating IL-10 and MCP-1 Release and Collagen Synthesis. Pharmaceuticals 2023, 16, 514. https://doi.org/10.3390/ph16040514
Aguayo-Morales H, Sierra-Rivera CA, Claudio-Rizo JA, Cobos-Puc LE. Horsetail (Equisetum hyemale) Extract Accelerates Wound Healing in Diabetic Rats by Modulating IL-10 and MCP-1 Release and Collagen Synthesis. Pharmaceuticals. 2023; 16(4):514. https://doi.org/10.3390/ph16040514
Chicago/Turabian StyleAguayo-Morales, Hilda, Crystel A. Sierra-Rivera, Jesús A. Claudio-Rizo, and Luis E. Cobos-Puc. 2023. "Horsetail (Equisetum hyemale) Extract Accelerates Wound Healing in Diabetic Rats by Modulating IL-10 and MCP-1 Release and Collagen Synthesis" Pharmaceuticals 16, no. 4: 514. https://doi.org/10.3390/ph16040514
APA StyleAguayo-Morales, H., Sierra-Rivera, C. A., Claudio-Rizo, J. A., & Cobos-Puc, L. E. (2023). Horsetail (Equisetum hyemale) Extract Accelerates Wound Healing in Diabetic Rats by Modulating IL-10 and MCP-1 Release and Collagen Synthesis. Pharmaceuticals, 16(4), 514. https://doi.org/10.3390/ph16040514