Abstract
Hyperglycemia, which is a chronic metabolic condition caused by either a defect in insulin secretion or insulin resistance, is a hallmark of diabetes mellitus (DM). Sustained hyperglycemia leads to the onset and development of many health complications. Despite the number of available antidiabetic medications on the market, there is still a need for novel treatment agents with increased efficacy and fewer adverse effects. Many medicinal plants offer a rich supply of bioactive compounds that have remarkable pharmacological effects with less toxicity and side effects. According to published evidence, natural antidiabetic substances influence pancreatic β-cell development and proliferation, inhibit pancreatic β-cell death, and directly increase insulin output. Pancreatic ATP-sensitive potassium channels play an essential role in coupling glucose metabolism to the secretion of insulin. Although much of the literature is available on the antidiabetic effects of medicinal plants, very limited studies discuss their direct action on pancreatic KATP. The aim of this review is to focus on the modulatory effects of antidiabetic medicinal plants and their active constituents on pancreatic KATP. The KATP channel should be regarded as a key therapeutic milestone in the treatment of diabetes. Therefore, continuous research into the interaction of medicinal plants with the KATP channel is crucial.
1. Introduction
Hyperglycemia, which is a chronic condition caused by either insulin secretion problems or insulin resistance, is a hallmark of diabetes mellitus (DM). Numerous complications, such as the production of reactive oxygen species (ROS), which cause the oxidation of lipids and membrane damage, are brought on and developed by prolonged hyperglycemia. Additionally, the excessive non-enzymatic glycation of proteins and the production of advanced glycation end products (AGE) are formed [1]. As a result of glycation changes, the pathophysiology of diabetes can become worse. Diabetes management continues to be a major issue on a global scale, and a cure has not yet been found. According to the World Health Organization (WHO), currently, about 350 million individuals in the world have diabetes [2].
Despite the fact that there are several well-known synthetic antidiabetic medications, their ineffectiveness and undesirable side effects have increased interest in finding novel and alternative antidiabetic treatments. Because of their capacity to boost insulin production, reduce glucose absorption via the intestine, and alter insulin sensitivity, a number of medicinal plants have recently been found to be beneficial for antidiabetic treatment [3,4]. Flavonoids, terpenoids, alkaloids, carotenoids, and other secondary metabolites from medicinal herbs have been documented for their safe action in diabetes [3,5]. Over 800 plants have been claimed to possess antidiabetic properties with no known adverse effects and low toxicity in comparison to synthesized drugs [6]. However, the search for new antihyperglycemic drugs from natural plants which have safe effects is still desirable.
2. Therapeutic Options and Their Limitations
Currently, there are five classes of oral hypoglycemic drugs: each work differently to treat the underlying causes of diabetes. The mechanisms by which a hypoglycemic effect is achieved include (1) the stimulation of insulin production (sulfonylureas and meglitinides), (2) stimulation of peripheral glucose absorption (thiazolidinediones and biguanides), (3) the delay of carbohydrate absorption from the intestine (alpha-glucosidase), and (4) a reduction in hepatic gluconeogenesis (biguanides). Lifestyle modifications combined with the use of hypoglycemic agents are important to accomplish long-term metabolic control in diabetes [7]. Despite this, up to date, complete recovery from diabetes has not been reported, and drug treatment does not always prevent late-stage diabetic problems [8].
Although the early effects of oral hypoglycemic medications may be helpful, they may also gradually lose their potency in a significant majority of diabetic patients. Currently, available medications have a number of negative effects. For instance, sulfonylurea can lead to weight gain and hyperinsulinemia. Meglitinides have a similar mechanism of action to sulfonylureas [9], but they have a lower risk of weight gain and hypoglycemia episodes, making them a better alternative for patients who experience these adverse effects [10]. Other side effects include fatigue and lactic acidosis (biguanides), flatulence and diarrhea (alpha-glucosidase inhibitor), and an elevated LDL-cholesterol level (thiazolidinediones) [11].
3. Role of KATP Channels in Diabetes
Adenosine triphosphate-sensitive potassium (KATP) channels of pancreatic β-cells are crucial for glucose metabolism because they control insulin production in response to an increase in ATP concentration [12]. KATP channels have the general purpose of coupling membrane potential and potassium (K+) conductance to the metabolic state of the cell via detecting the intracellular ATP content. It is generally known that glucose stimulates the release of insulin from β-cells through glycolysis and mitochondrial oxidation, which raises the intracellular ATP/ADP ratio. As a result, KATP channels close, resulting in membrane depolarization. This then causes the plasma membrane’s voltage-gated L-type Ca2+ channels to open, Ca2+ to enter, and the exocytosis of insulin-secreting granules to be activated [13].
The β-cell KATP channel is a multimeric complex made up of two different types of subunits: a pore-forming component, Kir6.2, and a regulatory subunit, the sulfonylurea receptor SUR1 [12,14]. For KATP channels to operate, both subunits must be present. The Kir6.2 location is where ATP mediates channel blockage, while the SUR1 nucleotide-binding domain is where MgADP activates the channel. KATP channels are thought to be interesting targets for the development of medications for the treatment of diabetes because they have a rich and diversified pharmacology with many substances acting as particular inhibitors or activators. Effective insulin secretagogues, such as glibenclamide and tolbutamide, bypass cell metabolism and directly block KATP channels to cause insulin release [15,16]. In this review, we focus on the interaction of medicinal plants and their active constituents with the KATP channel. The KATP channel should be regarded as a key therapeutic milestone in the treatment of diabetes. Therefore, understanding the mechanism behind the interaction of medicinal plants with the KATP channel can facilitate drug development for the treatment of diabetes.
4. Effect of Medicinal Plants and Their Active Constituents on KATP Channel
4.1. Lupinus mutabilis
Lupinus mutabilis is a native legume of South America that belongs to the Fabaceae family. The plant is often referred to as Tarwi or Andean lupin. Previous in vitro and in vivo studies provided evidence that Lupinus species have a hypoglycemic activity which was attributed to the presence of the protein γ-conglutin as well as quinolizidine alkaloids [17,18]. To date, more than 20 distinct quinolizidine alkaloids have been identified in the L. mutabilis alkaloid fraction [19].
Earlier studies examined the effect of raw L. mutabilis on blood sugar and insulin levels in normal and dysglycemic human subjects. The consumption of L. mutabilis by hyperglycemic patients resulted in a considerable decrease in blood glucose levels, while consumption of the same doses by healthy adults did not cause a significant alteration in blood glucose and insulin levels. Interestingly, the effects of L. mutabilis were stronger in people with greater basal glucose levels. In addition, treatment with L. mutabilis reduced insulin resistance in patients with diabetes [20]. Cooked L. mutabilis seeds have also been shown to lower glycemia in people with type 2 diabetes, according to clinical research. This action is likely due to the alkaloid content of L. mutabilis [21]. Wiedemann et al. looked at the effect of lupanine (Figure 1), one of the main quinolizidine alkaloids found in Lupinus mutabilis seeds, on a clonal rat-derived β-cell line (INS-1E) and on rats pre-treated with streptozotocin (STZ). The potentiating effect of lupanine began to manifest in L-arginine-treated islets at a glucose concentration of 8 mmol/L. Lupanine also increased the expression of the Ins-1 gene. Electrophysiological studies have shown that lupanine directly and dose-dependently blocked KATP channels. An analysis of the stimulus-secretion coupling revealed that membrane depolarization and the increase in the frequency of Ca2+ action potentials (APs) were linked to the potentiating impact on secretion. In diabetic rats pretreated with STZ, lupanine reduced plasma glucose levels and boosted glycemic control. Lupanine dramatically increased insulin secretion in the presence of 15 mmol/L glucose. Although orally administered lupanine did not cause hypoglycemia, it improved glycemic control in STZ-diabetic rats [22]. The finding that the effect of lupanine depends on the glycemic status of the subject is in agreement with the human studies mentioned above [20]. The lupin alkaloid sparteine has also been demonstrated to block KATP channels in mouse β cells and in the insulin-secreting cell line (HIT-T15). When compared to lupanine, sparteine’s inhibitory impact was far more obvious and strong enough to cause insulin secretion in unstimulated islets at micromolar doses [23,24].
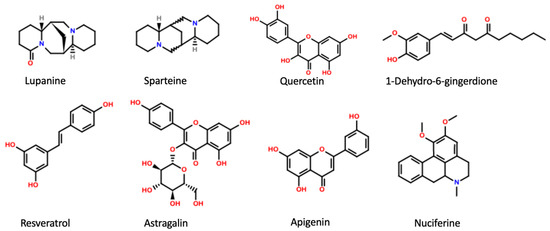
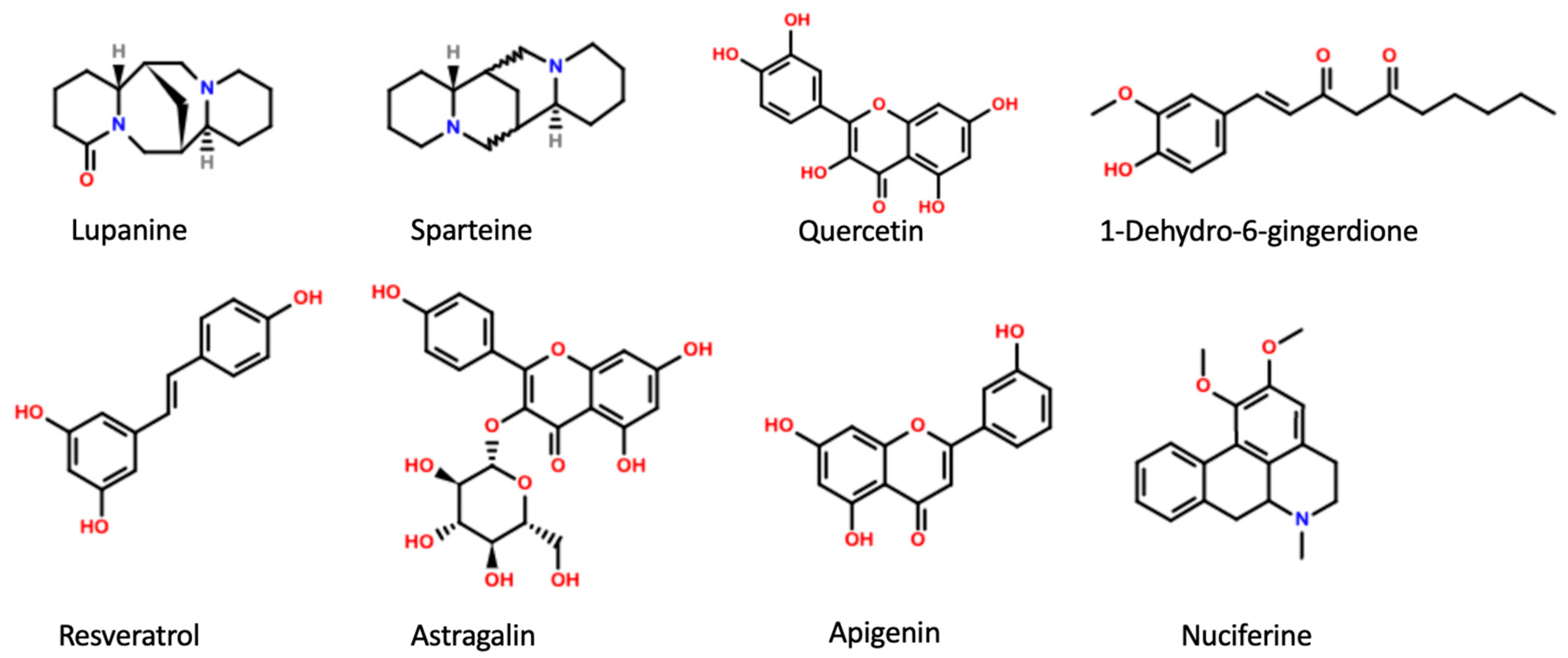
Figure 1.
Main active plant constituents with inhibitory effect on KATP channels.
4.2. Belamcanda chinensis
Belamcanda chinensis is a member of the Iridaceae family. The dried rhizome of B. chinensis was traditionally utilized to treat throat disease and inflammation in Chinese medicine [25]. Several flavonoids were identified in the leaves of the plant [26]. Wu et al. studied the effect of the aqueous extract of B. chinensis leaves on normal and STZ-induced diabetic rats. The B. chinensis extract (400, 800, 1600 mg/kg, p.o.) decreased oral glucose tolerance and fasting blood glucose levels in both healthy and diabetic rats. Additionally, it raised serum insulin levels in healthy rats. This effect was reversed in the presence of the KATP channel opener nicorandil and Ca+2 channel blocker nifedipine. The results indicate that the B. chinensis extract induces insulin release via the KATP channel-dependent pathway [27]. The results of a recent study conducted by the same group provided evidence that the extracts of B. chinensis leaves significantly decreased hyperglycemia, ameliorated insulin sensitivity, and prevented hepatic gluconeogenesis in KK-Ay mice [28].
4.3. Hyphaene thebaica
Hyphaene thebaica (Areceaeae), also known as doum, is a type of palm tree commonly found in Egypt, Sudan, and other countries in Africa. The fruit of H. thebaica is edible and rich in proteins, carbohydrates, lipids, iron, calcium, and other minerals. The fruit has a variety of phytochemical substances, such as flavonoids, hydroxy cinnamates, essential oils, and saponins. A phytochemical analysis of H. thebaica revealed the presence of more than ten different flavonoids, in addition to tannins, saponins, glycosides, and essential oils [29,30]. The antidiabetic potential of H. thebaica has been reported in earlier studies. In alloxan-induced diabetic rats, the water-soluble fraction of the fruit enhanced glucose and insulin tolerance and significantly decreased glycosylated hemoglobin levels [29]. In STZ-treated diabetic rats, the aqueous extracts of H. thebaica caused a significant reduction in blood glucose levels, improved the relative expression of insulin, and decreased the relative expression of inflammatory mediators TNF-α and TGF-β. According to histopathological studies, treatment with the aqueous extract successfully reversed the β-cell necrosis brought on by STZ and returned it to its normal form. The pancreatic KATP channel was identified as a target for flavonoids: the principal bioactive phytochemicals present in the aqueous extract of H. thebaica [31]. Although many flavonoids extracted from H. thebaica have been shown to increase glucose and insulin tolerance and reduce blood levels of glycosylated hemoglobin [29], their antidiabetic effects have not been fully elucidated. In addition, the data on the effects of flavonoids on pancreatic KATP are very scarce.
4.4. Eucalyptus citriodora
Eucalyptus citriodora (lemon-scented gum) belongs to the Myrtaceae family. This plant is known for its analgesic, anti-inflammatory, antioxidant, antimicrobial, and antipyretic activities [32,33,34]. According to earlier research, E. citriodora has characteristics that can be used in the treatment of diabetes. The phytochemicals in E. citriodora have a range of antidiabetic effects that are mediated by several mechanisms. It has been demonstrated that E. citriodora reduces hyperglycemia in alloxan-treated diabetic rats. The plant leaves contain betulinic acid and corosolic acid, which have been shown to have a hypoglycemic effect by promoting the translocation of GLUT-4 [35]. In BRIN-BD11 cells and isolated mouse pancreatic islets, E. citriodora leaves stimulated concentration-dependent insulin release from β-cells. Interestingly, this effect was inhibited by diazoxide, verapamil, and extracellular Ca2+ depletion, suggesting that KATP channels, L-type Ca2+ channels, and the Ca2+ influx were involved in the insulin secretory activities. Supporting this finding, the extract depolarized the plasma membrane of β-cells and caused an increase in intracellular Ca2+. In high-fat-fed diabetic rats, the oral application of E. citriodora for nine days enhanced glycemic control and β-cell activity. These effects were attributed to the presence of phytochemicals such as quercitrin, isoquercitrin, and rhodomyrtosone E [32]. In fact, the effect of quercetin (which exists mostly in its glycoside form quercitrin) on insulin release was reported earlier. Electrophysiological studies have shown that quercetin depolarized the β-cells membrane, stimulated an increase in intracellular Ca2+ concentration, and caused a 50% decrease in the current through KATP channels [36].
4.5. Leonurus sibiricus
Leonurus sibiricus is an herbaceous plant that belongs to the family Lamiaceae which was found in various locations in Asia and America. Many Asian nations have utilized L. sibiricus in traditional medicine to treat type 2 DM. The literature has several reports on L. sibiricus’s pharmacological properties. For instance, the plant has been demonstrated to display significant antioxidant as well as anti-inflammatory action in rats and humans [37].
Schmidt et al. studied the impact of the L. sibiricus plant extract on INS-1E insulinoma cell proliferation, electrophysiological characteristics, intracellular Ca2+ concentration, and insulin production. Both the aqueous and methanolic extracts from L. sibiricuss dramatically boosted the rat INS-1E cells’ ability to secrete insulin. The aqueous extract blocked KATP, depolarized membranes, and raised intracellular Ca2+ concentrations when applied acutely. The electrophysiological effects were intriguingly similar to those of tolbutamide: the sulfonylurea antidiabetic agent. Additionally, the administration of the aqueous extract induced INS 1-E cell growth. The results of this investigation lend support to the use of L. sibiricus in conventional medicine for the treatment of DM [38]
The β-cells responses to flavonoid quercetin and its glycoside rutin, which are present in the L. sibiricus extract, were examined by Kittl et al. [36]. Rutin did not affect insulin secretion or the electrophysiological activity of rat INS-1 cells, despite the fact that these phytochemicals are known to possess insulinotropic characteristics in vivo and in vitro studies. The transitory KATP channel blockage and intracellular Ca2+ stimulation of quercetin, on the other hand, likely contributed to its ability to abruptly promote insulin secretion.
4.6. Portulaca oleracea
Portulaca oleracea is a member of the Portulacaceae family that is extensively found throughout temperate and tropical regions of the world. The plant has been reported to have many biological activities, such as hypoglycemic, antioxidant, and anti-inflammatory effects. Alkaloids, terpenoids, coumarins, and flavonoids have all been found in this plant [39].
The effect of the P. oleracea extract on insulin secretion in INS-1 pancreatic β-cells and its mechanism of action were studied by Park and Han. The results of this investigation demonstrated a dose-dependent rise in insulin production by INS-1 pancreatic β-cells in response to P. oleracea extract application. The co-administration of diazoxide or verapamil, along with the P. oleracea extract, blocked membrane depolarization and Ca2+ influx and, consequently, inhibited insulin secretion. The findings imply that the membrane depolarization and closing of KATP channels in INS-1 pancreatic cells may be related to the insulinotropic action of the P. oleracea extract. Supporting these results, treatment with P. oleracea extract, along with the depolarizing concentration of KCl, greatly enhanced insulin secretion in comparison to treatment with KCl alone [40]. It is important to mention that P. oleracea is rich in many phytochemicals, such as polyphenols, terpenoids, and alkaloids [39]. For example, the triterpenoid 3β-hidroxihop-22(29)ene (3-BHO) was found to regulate the Ca2+ influx through the KATP channel-dependent route in rat pancreatic islets [41]. Collectively, the insulin secretory effects of P. oleracea extract could be attributed to the presence of active phytochemicals such as flavonoids and triterpenoids.
4.7. Cichorium intybus
Cichorium intybus, commonly known as chicory, is a member of the Compositae family. It is widely used in India as a traditional treatment for diabetes mellitus. Pushparaj et al. tested the antidiabetic effect of this plant on STZ-induced diabetic rats [42]. The results of this study showed that the daily administration of the ethanolic extract of C. intybus (125 mg/kg) for 14 days attenuated serum glucose by 20%. A similar finding was observed in a more recent study where the administration of the ethanolic extract to STZ-induced diabetic rats lowered plasma glucose and improved their lipid profile. In addition, the extract had antioxidant properties and modulated an oxidative stress system in diabetic rats [43].
Resveratrol is one of the polyphenols that are present in large amounts in C. intybus [44]. Resveratrol is well-reported for its potent antidiabetic actions by reducing glucose levels, ameliorating the parameters of oxidative damage, and preserving β-cells [45]. A whole-cell voltage-clamp study on a mouse β-cell line revealed that resveratrol significantly inhibited the KATP current at a concentration of 3 μmol/l and stimulated insulin secretion by causing membrane depolarization [46]. Supporting this finding, Hambrock et al. demonstrated that resveratrol has the ability to bind to SUR, the regulatory subunit of the KATP channel, and displace glibenclamide [47].
4.8. Cassia alata
Cassia alata (Caesalpiniaceae) is a shrub that is mostly utilized in traditional medicine in tropical nations such as Malaysia, Brazil, and Indonesia. The principal pharmacological actions listed in the literature for Cassia alata are the anti-inflammatory, antidiabetic, antifungal, and antibacterial effects [48,49,50].
Astragalin is one of the main flavonoids found in C. alata leaves. Rey et al. measured the glucose level and secretion of insulin in fasting Wistar rats after the oral administration of astragalin. The results of their study showed that astragalin at a concentration of 10 mg/kg significantly enhanced insulin secretion and lowered glycemia within 15–180 min of application. In isolated pancreatic cells, glibenclamide and diazoxide were applied to test the possibility that astragalin caused an increase in the Ca2+ influx that was mediated by KATP channels. The results showed that the Ca2+ influx increased 2.3 times more than the control when astragalin was applied in the presence of glibenclamide. On the other hand, diazoxide alone considerably reduced the influx of Ca2+. Furthermore, the stimulatory action of astragalin was reduced by almost 50% when diazoxide was present. These findings suggest that astragalin may act as an insulin secretagogue and support glucose homeostasis through a mechanism combining KATP, L-type Ca2+ channels, and the sarcoendoplasmic reticulum Ca2+ transport ATPase (SERCA) that allow astragalin to induce Ca2+ influx in pancreatic cells [51].
4.9. Momordica charantia
Momordica charantia, also known as bitter melon, is a member of the Cucurbitaceae family and is mainly grown in Asia, Africa, and South America [52]. The plant’s medicinal effects and nutritive values have been well-known for decades. The plant is used in folk medicine around the world to treat a variety of pathologies, particularly diabetes, but also inflammation and cancer, due to the presence of several biologically active compounds [53]. It has been well established that M. charantia extract helps type 2 diabetic patients by reducing blood sugar levels.
Using different diabetic animal models, the bitter melon’s hypoglycaemic impact has been demonstrated [54,55]. In alloxan-induced diabetic rats, blood glucose tolerance was considerably improved with the chronic oral administration of M. charantia fruit juice [56]. In male ddY diabetic mice, the main pure cucurbutanoid compounds in M. charantia were shown to exhibit hypoglycaemic effects. The effects on blood glucose were nonetheless considerable, even if they were less pronounced than those of glibenclamide at the same dose [57].
Recently, the effect of bitter melon fruit on insulin secretion from pancreatic β-cells and their underlying mechanism was studied by Shimada et al. The results of this study showed that the fruit extract of bitter melon acutely lowered blood glucose levels in healthy and in STZ-induced diabetic animals. Furthermore, the extract enhanced insulin release from β-cells independent of the dose of glucose. The enhancement of insulin secretion by the fruit extract appeared to be caused by an observed increase in ATP generation in β-cells. Since increased ATP concentration in the β-cell results in the closure of KATP channels, it can be concluded that the effect of the bitter melon extract is directly linked to the inhibition of KATP channels. It is suggested that the hydrophobic components of bitter melon fruit may stimulate glucose metabolism in β-cells by upregulating the enzymes responsible for glucose metabolism and ATP synthesis in the mitochondria [58]. Supporting this finding, earlier studies have also reported that M. charantia extracts impact the enzymes involved in the glycolytic cascade, in addition to increasing insulin secretion and decreasing insulin resistance [59].
4.10. Zingiber officinale
Zingiber officinale (ginger) is an herbaceous plant belonging to the Zingiberaceae family. The plant is frequently used as a flavoring food. Ginger is also one of the most well-known therapeutic plants in traditional medicine for its antioxidant, anti-inflammatory, antinausea, anti-emetic, and anticancer effects [60]. Accumulated evidence has shown that ginger and its constituents gingerol, shogaol, and paradol have hypoglycemic effects by increasing insulin sensitivity and lowering the chance of developing DM. Zhu et al. showed that 6-shogaol and 6-gingerol suppressed the synthesis of AGEs by capturing methylglyoxal (MGO), the precursor of AGEs, in an in vitro experiment [61].
In high-fat diet-fed mice, 6-gingerol [62] and 6-paradol [63] decreased plasma glucose and insulin levels. In Leprdb/db type 2 diabetic mice, the oral administration of 6-gingerol (200 mg/kg) for 4 weeks enhanced the cell membrane expression of glucose transporter type 4 (GLUT4) and activated glycogen synthase 1, which promoted glucose uptake in skeletal muscles [64]. Mahluji et al. showed that consumption of ginger by individuals with type 2 diabetes resulted in lower levels of insulin, low-density lipoprotein cholesterol, and triglycerides, a lower homeostasis model assessment index, and a higher quantitative insulin sensitivity check index. Therefore, it was suggested that ginger could be a helpful treatment to lessen DM-associated complications [65].
In comparison to either fresh or dried ginger, the steaming process has been proven to improve the efficacy of the ginger. According to the findings by Cheng et al., steamed ginger (120 °C, 4 h) had an antiproliferative effect that was approximately two times stronger than that of dry and fresh ginger [66]. A recent study revealed that steamed ginger contains more than 1-Dehydro-6-gingerdione and may increase the secretion of insulin through the inhibition of KATP channels in pancreatic cells. Using zebrafish as an animal model, the steamed ginger extract showed a higher efficacy in the recovery of alloxan-induced damage in pancreatic islets. Interestingly, the co-treatment of pancreatic islets with diazoxide in the presence of a steamed ginger extract and 1-Dehydro-6-gingerdione, resulted in considerably smaller pancreatic islets compared to pancreatic islets treated with a steamed ginger extract or 1-Dehydro-6-gingerdione alone. According to these findings, it was suggested that steamed ginger extract and 1-Dehydro-6-gingerdione increase insulin secretion by blocking KATP channels in pancreatic cells [67].
4.11. Kalanchoe pinnata
Kalanchoe pinnata (Crassulaceae) is a traditional medicinal plant that has been reported for its immunomodulatory [68], hepatoprotective [69], hypoglycemic [70], and anticancer [71] effects. Patil et al. assessed the antihyperglycemic and insulin secretagogue capabilities of K. pinnata in STZ-treated rats. Similar to glibenclamide, the dichloromethane component of the plant displayed a glucose-independent insulin secretagogue effect. Fasting blood glucose levels were dramatically lowered in STZ-induced diabetic rats after treatment with 10 mg/kg body weight of dichloromethane fraction. In addition, lipid profile and insulin levels were also normal. Moreover, glycated hemoglobin decreased to 8.4% from 12.9% in the diabetic controls. In vitro experiments showed that the insulin secretagogue activity of the dichloromethane fraction was dose-dependent and abolished by diazoxide. Therefore, the insulin secretagogue effect could be attributed to the closure of KATP channels [70]. Similarly, Kalanchoe crenata, another species of Kalanchoe, was also demonstrated to have antihyperglycemic effects in prior research. The modification of insulin sensitivity was hypothesized to be the cause of its antihyperglycemic action [4].
4.12. Heritiera fomes
Heritiera fomes is an evergreen tree belonging to the Sterculiaceae family which grows abundantly in Sundarbans. H. fomes has been extensively used in the treatment of various ailments, such as diabetes and cardiac and hepatic disorders [72]. Although H. fomes has remarkable therapeutic potential, there is little information on its mechanism of action or the composition of the active constituents that could be in charge of such favorable effects. Nonetheless, it is known that H. fomes contains a variety of potentially significant phytochemicals such as polyphenols, tannins, and carotenoids [72,73].
A recent study conducted by Ansari et al. tested the effect of hot water extract of H. fomes on insulin release from BRIN BD11 cells and isolated mouse islets, as well as on glucose homeostasis in high-fat-fed rats [73]. The oral administration of hot water extract to high-fat-fed rats significantly improved glucose tolerance and plasma insulin responses. Basal and glucose-induced insulin production was concentration-dependently enhanced by the hot water extract of H. fomes in BRIN BD11 cells and isolated mouse islets. The presence of the KATP channel blocker tolbutamide or depolarization with KCl did not interfere with the insulin secretory effect of the hot water extract H. fomes. Nevertheless, the effect was reduced by the KATP channel opener diazoxide, suggesting that the cellular actions of the hot water extract of H. fomes may entail the closing of KATP channels in addition to KATP channel-independent actions. In agreement with this finding, the L-type voltage-dependent Ca2+ channel blocker verapamil reduced the activity of the hot water extract of H. fomes, indicating that Ca2+ channels play a role in insulin secretion. This finding was further supported by the membrane depolarizing effect of the hot water extract and the resulting increase in intracellular Ca2+ in BRIN-BD11 cells. Phytochemical analysis revealed the presence of quercetin as the main active constituent of the water extract of H. fomes [73].
4.13. Enicostemma littorale
Enicostemma littorale is a member of the Gentianaceae family. According to previous research, the plant possesses anti-inflammatory [74], anti-cancer [75], and hypoglycemic effects. The long-term treatment (30 days) of diabetic rats with the aqueous extract of E. littorale has shown a significant reduction in blood glucose and glycosylated hemoglobin levels [76]. In alloxan-induced diabetic rats, a single dosage of the aqueous extract of E. littorale has demonstrated a substantial rise in blood insulin levels [77]. Using rat pancreatic islets, the insulinotropic effect of the aqueous extract of E. littorale was also confirmed. The extract potentiated glucose-induced insulin release from isolated rat pancreatic islets and was partially able to reverse the effect of diazoxide. Furthermore, the glucose-induced insulin release was unaffected by the presence of the Ca2+ channel blocker nimodipine. The results of this study indicated that the aqueous extract of E. littorale may possess a glucose-lowering effect by potentiating the release of insulin when blood sugar levels are elevated. This effect was dependent on KATP channels and did not require Ca2+ influx [77].
4.14. Tabernanthe iboga
Tabernanthe iboga belongs to the Apocynaceae family and is widely grown in the tropical forest of Central Africa. Root bark preparations have long been used in traditional medicine in Central and West African regions for the management of type 2 diabetes [78]. Despite this, scientific evidence on the antidiabetic effects of T. iboga is very limited.
Souza et al. showed that the aqueous extract of T. iboga had sulfonylureas-like action on insulin release [79]. In isolated pancreatic rat islets, the aqueous extract significantly increased the secretion of insulin in response to mM glucose stimulatory concentrations by closing KATP channels and increasing the influx of Ca2+ [79]. Similarly, Bading-Taïka et al. also showed that T. iboga aqueous extract (50 to 200 mg/kg p.o.) had a hypoglycemic impact on healthy rats [78]. This effect was delayed and only manifested after 3 h, which may be indicative of the intracellular action of the compound as previously demonstrated in isolated pancreatic β-cells by Souza et al. [79]. Additionally, T. iboga aqueous extract showed antihyperglycemic effects on non-fasted and fasted blood glucose in fructose-fed STZ diabetic rats [78].
4.15. Teucrium polium
Teucrium polium belongs to the family Lamiaceae and is mostly found in the Mediterranean and western Irano-Turanian regions [80]. This medicinal plant, also known as calpoureh, is traditionally used as a spice, a hypoglycemic agent, or a herbal tea by herbalists. Many therapeutic properties of this plant have been documented, including antioxidant, anti-inflammatory, hypolipidemic, and hypoglycemic effects [80,81,82]. Mirghazanfari et al. investigated the impact of T. polium extracts on insulin production from an isolated rat pancreas [83]. Interestingly, only the methanolic extract, but not the aqueous extract of T. polium, caused a significant increase in insulin release. According to the study findings, both diazoxide and verapamil decreased the insulinotropic activity of the T. polium extract, suggesting that the mechanism of insulin secretion was mediated by Ca2+ channel activation, KATP channel inhibition, or both. Moreover, it was determined that only the methanolic extract of T. polium contained the chemical apigenin, which is responsible for the insulinotropic activity of T. polium [83].
4.16. Nelumbo nucifera
Nelumbo nucifera (Lotus) is a perennial aquatic plant that belongs to the family Nelumbonaceae [84]. All plant parts have been widely used in traditional medicine for many medicinal purposes. For example, the herbal tea from dry leaves of N. nucifera is used to lose weight and decrease body fat index, while herbal tea from stamens is commonly used to improve the circulatory system and to decrease blood glucose and lipid levels [85]. Nguyen et al. found that the alkaloid nuciferine extracted from N. nucifera stimulated insulin secretion in both isolated islets and INS-1E cells. Nuciferine acted primarily by closing KATP channels. The effect of nuciferine was eliminated by diazoxide and nimodipine and decreased by protein kinase A and protein kinase C inhibition, indicating that the alkaloid also acted through KATP channel-independent pathways. Interestingly, nuciferine had a weaker affinity for binding to the sulfonylurea receptor, a stronger effect on insulin secretion, and less cytotoxicity compared to glibenclamide [86].
4.17. Gynostemma pentaphyllum
Gynostemma pentaphyllum, also known as jiaogulan, is a perennial climbing vine that belongs to the family Cucurbitaceae and is found across many Asian countries. The key phytochemicals include saponins and sterols [87]. Many studies have established the pharmacological benefits of G. pentaphyllum. The plant’s great potential for scavenging free radicals, in addition to its anti-inflammatory, hypoglycemic, and anticancer properties, have all been previously noted [88,89,90,91]. In both randomly selected type 2 diabetic individuals and diabetic animal models, the hypoglycemic effects of G. pentaphyllum were demonstrated. The administration of G. pentaphyllum tea to diabetic patients enhanced their ability to tolerate glucose by increasing their insulin sensitivity [91]. Similarly, in the Goto-Kakizaki rat, G. pentaphyllum extract lowered hepatic glucose production [92]. Moreover, G. pentaphyllum saponins were found to cause hypoglycemia and hypolipidemia in the diabetic rat model [93]. A more recent study examined the mechanism of antidiabetic action on the aqueous extract of G. pentaphyllum in Goto-Kakizaki diabetic rats [94]. According to the study findings, the plant’s extract enhanced glucose tolerance, raised plasma insulin levels and boosted the release of insulin from isolated rat islets. Moreover, extract-induced insulin release was partially mediated via KATP channels, L-type Ca2+ channels, and protein kinase: a system and pertussis toxin-sensitive Ge-protein at high glucose levels [94]. The insulinotropic characteristics of G. pentaphyllum were attributed to the presence of phanoside, an active gypenoside molecule, which has been reported to possess a strong insulin-releasing action [95].
4.18. Swietenia humilis
Swietenia humilis, commonly known as zopilote, belongs to the family Meliaceae. The plant is among the species widely used to alleviate diabetes and dyslipidemia [96,97,98] Tetranortriterpenoids of the mexicanolide class were recently isolated from the seeds of S. humilis. Ovalle-Magallanes et al. tested the effects of three mexicanoloides in vivo (using STZ-treated mice) and in vitro (using INSE1, H4IIE, and C2C12 cells) to determine the mechanisms of antihyperglycemic activity on S. humilis [97]. The results of this study demonstrated that the mexicanolide 2-hydroxy-destigloyl-6-deoxyswietenine acetates modulate glucose homeostasis by interacting with a variety of pharmacological targets including the pancreas (KATP channels), liver (glucose-6-phosphatase) and skeletal muscle (mitochondria and possibly glucose transporters). However, the fact that the compound retained a minimal antihyperglycemic effect in the presence of diazoxide indicated that KATP channels were only partially involved in the antihyperglycemic action of this compound.
Table 1 summarizes the effects of different antidiabetic medicinal plants and their active constituents on KATP channels.

Table 1.
Effect of antidiabetic medicinal plants and their active constituents on insulin secretion through the inhibition of pancreatic KATP channels.
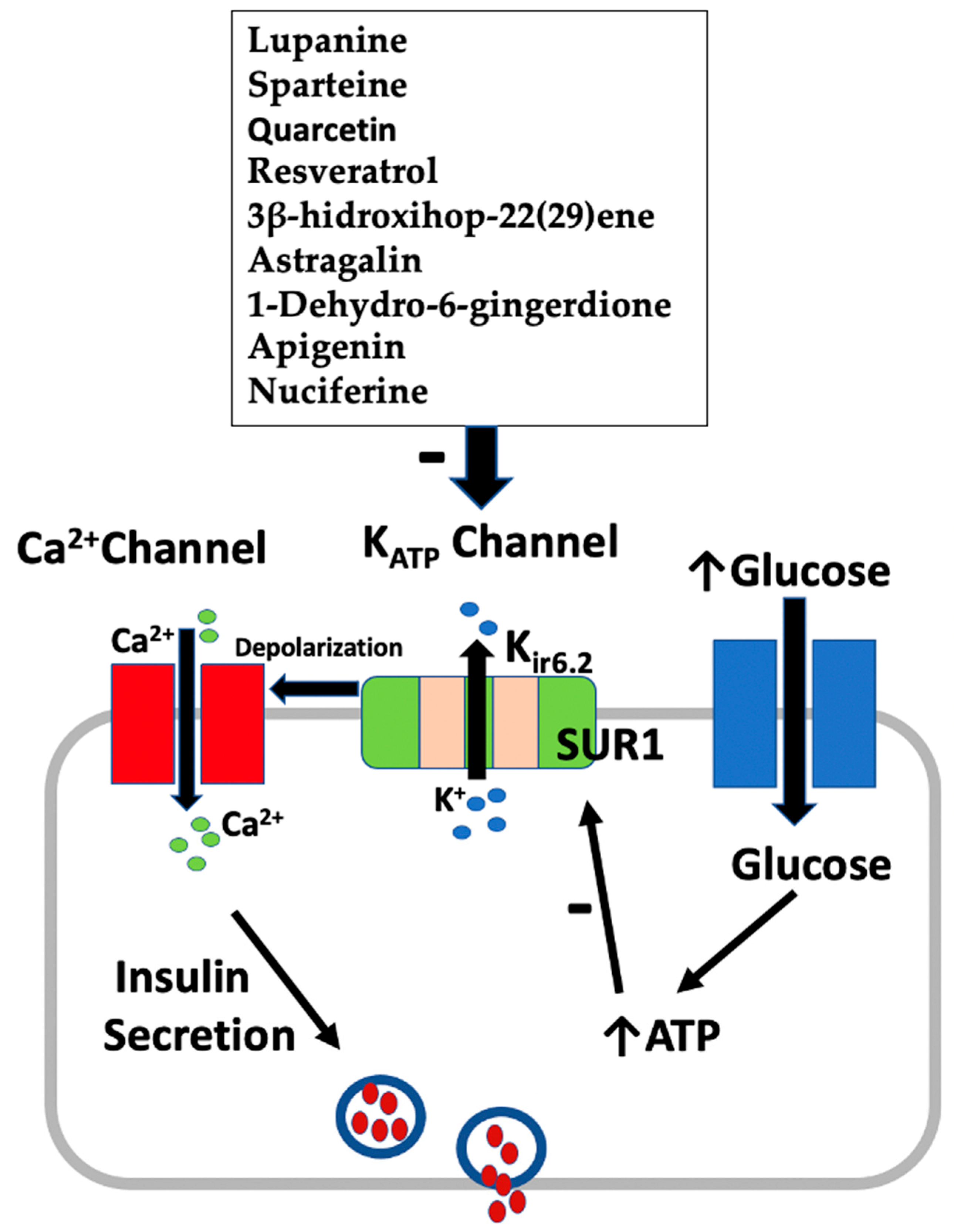
Figure 2 summarizes the mechanism of insulin secretion from pancreatic β-cells by active plant constituents.
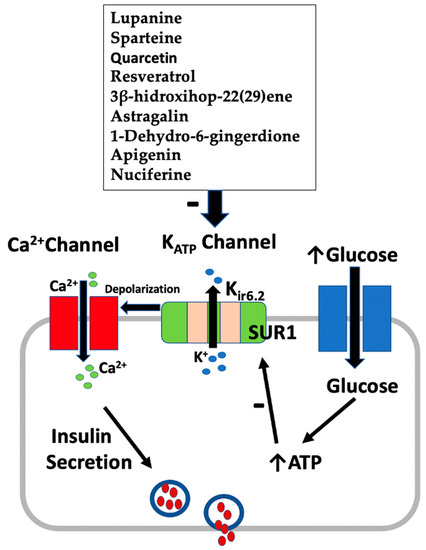
Figure 2.
Effect of active plant constituents on insulin secretion from β-cells.
5. Other Plants with Potential Modulatory Effect on KATP Channels
5.1. Berberis aristata
Berberis aristata belongs to the family Berberidaceae. The plant is native to the northern Himalayan region and is commonly referred to as Citra or Daruhaldi. The extract of the roots of B. aristata has potent antioxidant and anti-hyperglycemic effects [99,100]. Berberine is the main antidiabetic substance extracted from this plant. Berberine is known to act mainly by mimicking the action of insulin, reducing insulin resistance, and upregulating the expression of insulin receptors [99]. In a 6-month randomized controlled study in type 1 diabetic subjects, the Berberis aristata/Silybum marianum combination reduced the body’s use of insulin during insulin treatment. Hb1Ac levels were decreased in comparison to the baseline. Furthermore, fasting and postprandial levels of plasma glucose were also decreased in comparison to the baseline and placebo [101]. Similarly, a year-long placebo-controlled trial found that this combination, which intended to increase the poor bioavailability of berberine when taken orally, had significant antidiabetic benefits in type 2 diabetic subjects [102].
The effect of berberine on the KATP channel was demonstrated in an earlier study. In a whole-cell voltage clamp experiment, berberine inhibited the cromakalim-induced outward K+ currents in a concentration-dependent manner and decreased open channel probability. Such inhibition was completely blocked by glibenclamide, indicating that the observed effect of berberine is due to the inhibition of the KATP channel. However, the effect of this compound was tested on cardiac KATP only, and currently, no studies have shown the link between berbeine and pancreatic KATP [103].
5.2. Psacalium decompositum
Psacalium decompositum (Asteraceae), sometimes referred to as “matarique,” is a prominent remedy in Mexican folk medicine for the treatment of neuralgia, hepatic and renal colic, rheumatic illnesses, discomfort, and ulcers [104]. Previous research looked at the mechanism underlying the hypoglycemic effects of aqueous root and rhizome decoctions of P. decompositum. The hypoglycemic effects of the three sesquiterpenoids, cacalol, cacalol acetate, and cacalone epimer mixture, were investigated. Similar to glibenclamide, all the tested compounds inhibited KATP channels in aortic smooth muscle rings. The sesquiterpenoids may have a stronger affinity for the SUR2 subunit of KATP channels in aortic smooth muscle than the SUR1 subunit in pancreatic β-cells since they were less successful than glibenclamide in decreasing plasma glucose levels [105].
5.3. Annona cherimola
Annona cherimola is a perennial plant that belongs to the Annonaceae family. The plant’s fruit is known as “annona” or “cherimoya” [96]. The leaves were used in traditional medicine as a remedy to treat gastrointestinal disorders [106]. Studies have also demonstrated that the leaves possess antidepressants [107] and pro-apoptotic properties [108]. Interestingly, the administration of the tea infusion extract of leaves from A. cherimola improved blood glucose and glycated hemoglobin levels in mice [109]. Recently, Valdes et al. demonstrated that the ethanolic extract of A. cherimola and the flavonoid rutin administered alone and in combination with oral antidiabetic drugs caused a significant reduction in hyperglycemia, glycated hemoglobin, and hyperlipidemia in STZ-treated mice [110]. Although many phytochemicals with reported hypoglycemic and insulin-secreting effects (such as flavonoids, alkaloids, and sesquiterpenes) have been isolated from A. cherimola, there are currently no studies on the effect of this plant extract on KATP channels.
5.4. Bougainvillea spectabilis
Bougainvillea spectabilis, which is commonly known as Bougainvillae, belongs to the Nyctaginaceae family. It is a woody and thorny vine cultivated in the tropical and subtropical regions of India. The plant leaves are rich in flavonoids, quinones, phenols, sterols, triterpenoids, glycosides, and tannins [111]. The extract of its leaves contains pinitol, which has been shown to possess insulin-like activity [112]. The daily administration of the aqueous extract of B. spectabilis leaves at a dose of 100 mg/kg body weight for 28 days resulted in a significant reduction in hyperglycemia in STZ-pretreated rats [111]. A recent study has shown that the methanol extract of B. spectabilis leaves has an antinociceptive effect, possibly through the modulation of KATP channels. Therefore, further investigation is needed to test the effect of B. spectabilis extracts on KATP channels in the pancreas.
5.5. Lycium barbarum
Lycium barbarum belongs to the family Solanaceae. The plant is well known as “Ausaj” and “Box Thorn” and has been used in traditional medicine to treat several ailments such as cancer and hepatitis and to reduce blood sugar [113].
Recently, Hager et al. identified many distinct plant extracts and some of their bioactive constituents as modulators of insulin production in β-cells. In this study, L. barbarum extracts were identified among the highest plant extracts with stimulatory activity on insulin secretion [114]. The effect of the polysaccharide galactomannan from L. barbarum fruit was studied in alloxan-induced diabetic rats. The maximal effect of the polysaccharide extract (500 mg/kg) administered with glibenclamide occurred at 6 h and faded after 24 h. A chronic application, where glibenclamide and polysaccharide galactomannan was repeatedly administered once daily for 21 days, further resulted in a substantial decrease in blood glucose levels compared to the diabetic control group. Considering the similar hypoglycemic effects determined by the polysaccharide galactomannan with respect to glibenclamide, it was proposed that their mechanism of action was the same (via the inhibition of KATP channels) [113]. However, further data are needed to confirm the KATP channels-dependent hypoglycemic effect of L. barbarum.
6. Conclusions
Medicinal plants have been extensively used in folk medicine to treat DM. KATP channels are metabolic detectors that are required for regular insulin production. Although a lot of information is available on the antidiabetic effects of medicinal plants and their active constituents, very limited studies discuss their direct action on pancreatic KATP. The available data affirms the need for further research into the interaction of medicinal plants with the KATP channel. Future studies are anticipated to use natural substances as lead structures for the manufacture of therapeutic drugs that target KATP channels.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Dariya, B.; Nagaraju, G.P. Advanced glycation end products in diabetes, cancer and phytochemical therapy. Drug Discov. Today 2020, 25, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Malviya, N.; Jain, S.; Malviya, S. Antidiabetic potential of medicinal plants. Acta Pol. Pharm. 2010, 67, 113–118. [Google Scholar] [PubMed]
- Kamgang, R.; Mboumi, R.Y.; Fondjo, A.F.; Tagne, M.A.; N’Dillé, G.P.; Yonkeu, J.N. Antihyperglycaemic potential of the water-ethanol extract of Kalanchoe crenata (Crassulaceae). J. Nat. Med. 2008, 62, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Al Kury, L.T.; Abdoh, A.; Ikbariah, K.; Sadek, B.; Mahgoub, M. In vitro and in vivo antidiabetic potential of monoterpenoids: An update. Molecules 2022, 27, 182. [Google Scholar] [CrossRef]
- Kirtikar, K.; Basu, B. Indian medicinal plants. Indian Med. Plants. 1935, 2, 1347–1348. [Google Scholar]
- Tan, S.Y.; Mei Wong, J.L.; Sim, Y.J.; Wong, S.S.; Mohamed Elhassan, S.A.; Tan, S.H.; Ling Lim, G.P.; Rong Tay, N.W.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, P.; Pandey, R.; Srivatava, R.; Goswami, S. Alternative therapies useful in the management of diabetes: A systematic review. J. Pharm. Bioallied Sci. 2011, 3, 504–512. [Google Scholar]
- Becker, M.; Galler, A.; Raile, K. Meglitinide analogues in adolescent patients with HNF1A-MODY (MODY 3). Pediatrics 2014, 133, e775–e779. [Google Scholar] [CrossRef]
- Tran, L.; Zielinski, A.; Roach, A.H.; Jende, J.A.; Householder, A.M.; Cole, E.E.; Atway, S.A.; Amornyard, M.; Accursi, M.L.; Shieh, S.W. Pharmacologic treatment of type 2 diabetes: Oral medications. Ann. Pharmacother. 2015, 49, 540–556. [Google Scholar] [CrossRef]
- Ganesan, K.; Rana, M.B.M.; Sultan, S. Oral hypoglycemic medications. In StatPearls; StatPerals Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Inagaki, N.; Gonoi, T.; Clement IV, J.P.; Namba, N.; Inazawa, J.; Gonzalez, G.; Aguilar-Bryan, L.; Seino, S.; Bryan, J. Reconstitution of I KATP: An inward rectifier subunit plus the sulfonylurea receptor. Science 1995, 270, 1166–1170. [Google Scholar] [CrossRef]
- Rutter, G.A. Nutrient–secretion coupling in the pancreatic islet β-cell: Recent advances. Mol. Asp. Med. 2001, 22, 247–284. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Rorsman, P. Electrophysiology of the pancreatic beta-cell. Prog. Biophys. Mol. Biol. 1989, 54, 87–143. [Google Scholar] [CrossRef]
- Trapp, S.; Tucker, S.J.; Ashcroft, F.M. Activation and inhibition of K-ATP currents by guanine nucleotides is mediated by different channel subunits. Proc. Natl. Acad. Sci. USA 1997, 94, 8872–8877. [Google Scholar] [CrossRef]
- Ashcroft, F.M. ATP-sensitive potassium channelopathies: Focus on insulin secretion. J. Clin. Investig. 2005, 115, 2047–2058. [Google Scholar] [CrossRef]
- Magni, C.; Sessa, F.; Accardo, E.; Vanoni, M.; Morazzoni, P.; Scarafoni, A.; Duranti, M. Conglutin gamma, a lupin seed protein, binds insulin in vitro and reduces plasma glucose levels of hyperglycemic rats. J. Nutr. Biochem. 2004, 15, 646–650. [Google Scholar] [CrossRef]
- García López, P.M.; de la Mora, P.G.; Wysocka, W.; Maiztegui, B.; Alzugaray, M.E.; Del Zotto, H.; Borelli, M.I. Quinolizidine alkaloids isolated from Lupinus species enhance insulin secretion. Eur. J. Pharm. 2004, 504, 139–142. [Google Scholar] [CrossRef]
- Witte, L. Quinolizidine alkaloids in seeds of Lupinus mutabilis. J. Agric. Food Chem 1983, 31, 934938. [Google Scholar]
- Fornasini, M.; Castro, J.; Villacrés, E.; Narváez, L.; Villamar, M.P.; Baldeón, M.E. Hypoglycemic effect of Lupinus mutabilis in healthy volunteers and subjects with dysglycemia. Nutr. Hosp. 2012, 27, 425–433. [Google Scholar]
- Baldeón, M.E.; Castro, J.; Villacrés, E.; Narváez, L.; Fornasini, M. Hypoglycemic effect of cooked Lupinus mutabilis and its purified alkaloids in subjects with type-2 diabetes. Nutr. Hosp. 2012, 27, 1261–1266. [Google Scholar]
- Wiedemann, M.; Gurrola-Díaz, C.M.; Vargas-Guerrero, B.; Wink, M.; García-López, P.M.; Düfer, M. Lupanine Improves Glucose Homeostasis by Influencing KATP Channels and Insulin Gene Expression. Molecules 2015, 20, 19085–19100. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, G.; Nenquin, M.; Schmeer, W.; Mathot, F.; Meissner, H.; Henquin, J.-C. Sparteine increases insulin release by decreasing the K+ permeability of the B-cell membrane. Biochem. Pharmacol. 1985, 34, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, F.M.; Kerr, A.J.; Gibson, J.S.; Williams, B.A. Amantadine and sparteine inhibit ATP-regulated K-currents in the insulin-secreting β-cell line, HIT-T15. Br. J. Pharmacol. 1991, 104, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, K.; Xu, J.; Yang, D.; Zhang, C.; Wang, Z.; Li, M. Belamcanda chinensis (L.) DC-An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2016, 186, 1–13. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, C.-M.; Dai, R.-J.; Li, L.; Yu, Y.-H.; Li, Y.; Meng, W.-W.; Zhang, L.; Zhang, Y.; Deng, Y.-L. Combination of HPLC chromatogram and hypoglycemic effect identifies isoflavones as the principal active fraction of Belamcanda chinensis leaf extract in diabetes treatment. J. Chromatogr. B 2011, 879, 371–378. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Chen, Y.; Lao, X.; Sheng, L.; Dai, R.; Meng, W.; Deng, Y. Hypoglycemic effect of Belamcanda chinensis leaf extract in normal and STZ-induced diabetic rats and its potential active faction. Phytomedicine Int. J. Phytother. Phytopharm. 2011, 18, 292–297. [Google Scholar] [CrossRef]
- Guo, Y.; Dai, R.; Deng, Y.; Sun, L.; Meng, S.; Xin, N. Hypoglycemic activity of the extracts of Belamcanda chinensis leaves (BCLE) on KK-Ay mice. Biomed. Pharmacother. 2019, 110, 449–455. [Google Scholar] [CrossRef]
- Salib, J.Y.; Michael, H.N.; Eskande, E.F. Anti-diabetic properties of flavonoid compounds isolated from Hyphaene thebaica epicarp on alloxan induced diabetic rats. Pharmacogn. Res. 2013, 5, 22–29. [Google Scholar] [CrossRef]
- Saber, F.R.; Aly, S.H.; Khallaf, M.A.; El-Nashar, H.A.; Fahmy, N.M.; El-Shazly, M.; Radha, R.; Prakash, S.; Kumar, M.; Taha, D. Hyphaene thebaica (Areceaeae) as a Promising Functional Food: Extraction, Analytical Techniques, Bioactivity, Food, and Industrial Applications. Food Anal. Methods 2022, 15, 1–21. [Google Scholar] [CrossRef]
- Shady, N.H.; Hassan, H.A.; Elrehany, M.A.; Kamel, M.S.; Saber, E.A.; Maher, S.A.; Abo-Elsoud, F.A.; Sayed, A.M.; Abdelmohsen, U.R.; Gaber, S.S. Hyphaene thebaica (doum)-derived extract alleviates hyperglycemia in diabetic rats: A comprehensive in silico, in vitro and in vivo study. Food Funct. 2021, 12, 11303–11318. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Insulinotropic and antidiabetic properties of Eucalyptus citriodora leaves and isolation of bioactive phytomolecules. J. Pharm. Pharmacol. 2021, 73, 1049–1061. [Google Scholar] [CrossRef]
- Juergens, U.; Stöber, M.; Schmidt-Schilling, L.; Kleuver, T.; Vetter, H. Antiinflammatory effects of euclyptol (1.8-cineole) in bronchial asthma: Inhibition of arachidonic acid metabolism in human blood monocytes ex vivo. Eur. J. Med. Res. 1998, 3, 407–412. [Google Scholar]
- Gomes-Carneiro, M.R.; Felzenszwalb, I.; Paumgartten, F.J. Mutagenicity testing of (±)-camphor, 1, 8-cineole, citral, citronellal, (−)-menthol and terpineol with the Salmonella/microsome assay. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 1998, 416, 129–136. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Zhao, P.; Zhou, Q.; Mei, Z.; Yang, G.; Yang, X.; Feng, Y. Chemical constituents from Eucalyptus citriodora Hook leaves and their glucose transporter 4 translocation activities. Bioorganic Med. Chem. Lett. 2014, 24, 3096–3099. [Google Scholar] [CrossRef]
- Kittl, M.; Beyreis, M.; Tumurkhuu, M.; Furst, J.; Helm, K.; Pitschmann, A.; Gaisberger, M.; Glasl, S.; Ritter, M.; Jakab, M. Quercetin Stimulates Insulin Secretion and Reduces the Viability of Rat INS-1 Beta-Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2016, 39, 278–293. [Google Scholar] [CrossRef]
- Sayed, M.A.; Alam, M.A.; Islam, M.S.; Ali, M.T.; Ullah, M.E.; Shibly, A.Z.; Ali, M.A.; Hasan-Olive, M.M. Leonurus sibiricus L. (honeyweed): A review of its phytochemistry and pharmacology. Asian Pac. J. Trop. Biomed. 2016, 6, 1076–1080. [Google Scholar] [CrossRef]
- Schmidt, S.; Jakab, M.; Jav, S.; Streif, D.; Pitschmann, A.; Zehl, M.; Purevsuren, S.; Glasl, S.; Ritter, M. Extracts from Leonurus sibiricus L. increase insulin secretion and proliferation of rat INS-1E insulinoma cells. J. Ethnopharmacol. 2013, 150, 85–94. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Xin, H.-L.; Rahman, K.; Wang, S.-J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef]
- Park, J.E.; Han, J.S. A Portulaca oleracea L. extract promotes insulin secretion via a K+ ATP channel dependent pathway in INS-1 pancreatic β-cells. Nutr. Res. Pract. 2018, 12, 183. [Google Scholar] [CrossRef]
- Castro, A.J.G.; Cazarolli, L.H.; Carvalho, F.K.d.; Luz, G.d.; Altenhofen, D.; Santos, A.R.S.d.; Pizzolatti, M.G.; Silva, F.R.M.B. Acute effect of 3β-hidroxihop-22(29)ene on insulin secretion is mediated by GLP-1, potassium and calcium channels for the glucose homeostasis. J. Steroid Biochem. Mol. Biol. 2015, 150, 112–122. [Google Scholar] [CrossRef]
- Pushparaj, P.N.; Low, H.K.; Manikandan, J.; Tan, B.K.H.; Tan, C.H. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2007, 111, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Borji, A.; Tabasi, S.H. Effects of Cichorium intybus linn on blood glucose, lipid constituents and selected oxidative stress parameters in streptozotocin-induced diabetic rats. Cardiovasc. Hematol. Disord. Drug Targets 2013, 13, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.R.; Manaa, A.; Sheta, E.; Ghareeb, D.A.; Abd-Elmonem, N.M. The Synergetic Effect of Egyptian Portulaca oleracea L. (Purslane) and Cichorium intybus L. (Chicory) Extracts against Glucocorticoid-Induced Testicular Toxicity in Rats through Attenuation of Oxidative Reactions and Autophagy. Antioxidants 2022, 11, 1272. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Anti-diabetic effects of resveratrol. Ann. New York Acad. Sci. 2011, 1215, 34–39. [Google Scholar] [CrossRef]
- Chen, W.-P.; Chi, T.-C.; Chuang, L.-M.; Su, M.-J. Resveratrol enhances insulin secretion by blocking KATP and KV channels of beta cells. Eur. J. Pharmacol. 2007, 568, 269–277. [Google Scholar] [CrossRef]
- Hambrock, A.; de Oliveira Franz, C.B.; Hiller, S.; Grenz, A.; Ackermann, S.; Schulze, D.U.; Drews, G.; Osswald, H. Resveratrol binds to the sulfonylurea receptor (SUR) and induces apoptosis in a SUR subtype-specific manner. J. Biol. Chem. 2007, 282, 3347–3356. [Google Scholar] [CrossRef]
- Hennebelle, T.; Weniger, B.; Joseph, H.; Sahpaz, S.; Bailleul, F. Senna alata. Fitoterapia 2009, 80, 385–393. [Google Scholar] [CrossRef]
- Hazni, H.; Ahmad, N.; Hitotsuyanagi, Y.; Takeya, K.; Choo, C.-Y. Phytochemical constituents from Cassia alata with inhibition against methicillin-resistant Staphylococcus aureus (MRSA). Planta Med. 2008, 74, 1802–1805. [Google Scholar] [CrossRef]
- Varghese, G.K.; Bose, L.V.; Habtemariam, S. Antidiabetic components of Cassia alata leaves: Identification through α-glucosidase inhibition studies. Pharm. Biol. 2013, 51, 345–349. [Google Scholar] [CrossRef]
- Rey, D.; Miranda Sulis, P.; Alves Fernandes, T.; Gonçalves, R.; Silva Frederico, M.J.; Costa, G.M.; Aragon, M.; Ospina, L.F.; Mena Barreto Silva, F.R. Astragalin augments basal calcium influx and insulin secretion in rat pancreatic islets. Cell Calcium 2019, 80, 56–62. [Google Scholar] [CrossRef]
- Bortolotti, M.; Mercatelli, D.; Polito, L. Momordica charantia, a nutraceutical approach for inflammatory related diseases. Front. Pharmacol. 2019, 10, 486. [Google Scholar] [CrossRef]
- Tan, S.P.; Kha, T.C.; Parks, S.E.; Roach, P.D. Bitter melon (Momordica charantia L.) bioactive composition and health benefits: A review. Food Rev. Int. 2016, 32, 181–202. [Google Scholar] [CrossRef]
- Liu, Z.; Gong, J.; Huang, W.; Lu, F.; Dong, H. The effect of Momordica charantia in the treatment of diabetes mellitus: A review. Evid. Based Complement. Altern. Med. 2021, 2021, 3796265. [Google Scholar] [CrossRef]
- Han, J.H.; Tuan, N.Q.; Park, M.H.; Quan, K.T.; Oh, J.; Heo, K.S.; Na, M.; Myung, C.S. Cucurbitane triterpenoids from the fruits of Momordica charantia improve insulin sensitivity and glucose homeostasis in streptozotocin-induced diabetic mice. Mol. Nutr. Food Res. 2018, 62, 1700769. [Google Scholar] [CrossRef]
- Chaturvedi, P.; George, S.; Milinganyo, M.; Tripathi, Y. Effect of Momordica charantia on lipid profile and oral glucose tolerance in diabetic rats. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 954–956. [Google Scholar]
- Harinantenaina, L.; Tanaka, M.; Takaoka, S.; Oda, M.; Mogami, O.; Uchida, M.; Asakawa, Y. Momordica charantia constituents and antidiabetic screening of the isolated major compounds. Chem. Pharm. Bull. 2006, 54, 1017–1021. [Google Scholar] [CrossRef]
- Shimada, T.; Kato, F.; Dwijayanti, D.R.; Nagata, T.; Kinoshita, A.; Okuyama, T.; Nishizawa, M.; Mukai, E. Bitter melon fruit extract enhances intracellular ATP production and insulin secretion from rat pancreatic β-cells. Br. J. Nutr. 2022, 127, 377–383. [Google Scholar] [CrossRef]
- Pahlavani, N.; Roudi, F.; Zakerian, M.; Ferns, G.A.; Navashenaq, J.G.; Mashkouri, A.; Ghayour-Mobarhan, M.; Rahimi, H. Possible molecular mechanisms of glucose-lowering activities of Momordica charantia (karela) in diabetes. J. Cell. Biochem. 2019, 120, 10921–10929. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Y.; Wang, P.; Ahmedna, M.; Sang, S. Bioactive ginger constituents alleviate protein glycation by trapping methylglyoxal. Chem. Res. Toxicol. 2015, 28, 1842–1849. [Google Scholar] [CrossRef]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Specific bioactive compounds in ginger and apple alleviate hyperglycemia in mice with high fat diet-induced obesity via Nrf2 mediated pathway. Food Chem. 2017, 226, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-K.; Tsai, Y.-H.; Korinek, M.; Hung, P.-H.; El-Shazly, M.; Cheng, Y.-B.; Wu, Y.-C.; Hsieh, T.-J.; Chang, F.-R. 6-Paradol and 6-shogaol, the pungent compounds of ginger, promote glucose utilization in adipocytes and myotubes, and 6-paradol reduces blood glucose in high-fat diet-fed mice. Int J Mol Sci 2017, 18, 168. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.B.; Mohsin, M.; Razu, B.A.; Hossain, M.T.; Mahzabeen, S.; Unnoor, N.; Muna, I.A.; Akhter, F.; Kabir, A.U.; Hannan, J.M.A. [6]-Gingerol, from Zingiber officinale, potentiates GLP-1 mediated glucose-stimulated insulin secretion pathway in pancreatic β-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Lepr(db/db) type 2 diabetic mice. BMC Complement Altern Med. 2017, 17, 395. [Google Scholar]
- Mahluji, S.; Attari, V.E.; Mobasseri, M.; Payahoo, L.; Ostadrahimi, A.; Golzari, S.E. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2013, 64, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-L.; Liu, Q.; Peng, Y.-B.; Qi, L.-W.; Li, P. Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem. 2011, 129, 1785–1792. [Google Scholar] [CrossRef]
- Nam, Y.H.; Hong, B.N.; Rodriguez, I.; Park, M.S.; Jeong, S.Y.; Lee, Y.-G.; Shim, J.H.; Yasmin, T.; Kim, N.W.; Koo, Y.T. Steamed ginger may enhance insulin secretion through KATP channel closure in pancreatic β-cells potentially by increasing 1-dehydro-6-gingerdione content. Nutrients 2020, 12, 324. [Google Scholar] [CrossRef]
- Cruz, E.A.; Da-Silva, S.A.; Muzitano, M.F.; Silva, P.M.; Costa, S.S.; Rossi-Bergmann, B. Immunomodulatory pretreatment with Kalanchoe pinnata extract and its quercitrin flavonoid effectively protects mice against fatal anaphylactic shock. Int. Immunopharmacol. 2008, 8, 1616–1621. [Google Scholar] [CrossRef]
- Yadav, N.P.; Dixit, V.K. Hepatoprotective activity of leaves of Kalanchoe pinnata Pers. J. Ethnopharmacol. 2003, 86, 197–202. [Google Scholar] [CrossRef]
- Patil, S.B.; Dongare, V.R.; Kulkarni, C.R.; Joglekar, M.M.; Arvindekar, A.U. Antidiabetic activity of Kalanchoe pinnata in streptozotocin-induced diabetic rats by glucose independent insulin secretagogue action. Pharm. Biol. 2013, 51, 1411–1418. [Google Scholar] [CrossRef]
- Supratman, U.; Fujita, T.; Akiyama, K.; Hayashi, H.; Murakami, A.; Sakai, H.; Koshimizu, K.; Ohigashi, H. Anti-tumor promoting activity of bufadienolides from Kalanchoe pinnata and K. daigremontiana x tubiflora. Biosci. Biotechnol. Biochem. 2001, 65, 947–949. [Google Scholar] [CrossRef]
- Mahmud, I.; Islam, M.K.; Saha, S.; Barman, A.K.; Rahman, M.M.; Anisuzzman, M.; Rahman, T.; Al-Nahain, A.; Jahan, R.; Rahmatullah, M. Pharmacological and Ethnomedicinal Overview of Heritiera fomes: Future Prospects. Int. Sch. Res. Not. 2014, 2014, 938543. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H. Insulin secretory and antidiabetic actions of Heritiera fomes bark together with isolation of active phytomolecules. PLoS ONE 2022, 17, e0264632. [Google Scholar] [CrossRef]
- Sadique, J.; Chandra, T.; Thenmozhi, V.; Elango, V. The anti-inflammatory activity of Enicostemma littorale and Mollugo cerviana. Biochem. Med. Metab. Biol. 1987, 37, 167–176. [Google Scholar] [CrossRef]
- Kavimani, S.; Manisenthlkumar, K.T. Effect of methanolic extract of Enicostemma littorale on Dalton’s ascitic lymphoma. J. Ethnopharmacol. 2000, 71, 349–352. [Google Scholar] [CrossRef]
- Maroo, J.; Vasu, V.T.; Gupta, S. Dose dependent hypoglycemic effect of aqueous extract of Enicostemma littorale Blume in alloxan induced diabetic rats. Phytomedicine Int. J. Phytother. Phytopharm. 2003, 10, 196–199. [Google Scholar] [CrossRef]
- Maroo, J.; Vasu, V.T.; Aalinkeel, R.; Gupta, S. Glucose lowering effect of aqueous extract of Enicostemma littorale Blume in diabetes: A possible mechanism of action. J. Ethnopharmacol. 2002, 81, 317–320. [Google Scholar] [CrossRef]
- Bading-Taïka, B.; Souza, A.; Bourobou Bourobou, H.-P.; Lione, L.A. Hypoglycaemic and anti-hyperglycaemic activity of Tabernanthe iboga aqueous extract in fructose-fed streptozotocin type 2 diabetic rats. Adv. Tradit. Med. 2021, 21, 281–295. [Google Scholar] [CrossRef]
- Souza, A.; Mbatchi, B.; Herchuelz, A. Induction of insulin secretion by an aqueous extract of Tabernanhte iboga Baill. (Apocynaceae) in rat pancreatic islets of Langerhans. J. Ethnopharmacol. 2011, 133, 1015–1020. [Google Scholar] [CrossRef]
- Afifi, F.; Al-Khalidi, B.; Khalil, E. Studies on the in vivo hypoglycemic activities of two medicinal plants used in the treatment of diabetes in Jordanian traditional medicine following intranasal administration. J. Ethnopharmacol. 2005, 100, 314–318. [Google Scholar] [CrossRef]
- Rasekh, H.; Khoshnood-Mansourkhani, M.; Kamalinejad, M. Hypolipidemic effects of Teucrium polium in rats. Fitoterapia 2001, 72, 937–939. [Google Scholar] [CrossRef]
- Tariq, M.; Ageel, A.; Al-Yahya, M.; Mossa, J.; Al-Said, M. Anti-inflammatory activity of Teucrium polium. Int. J. Tissue React. 1989, 11, 185–188. [Google Scholar] [PubMed]
- Mirghazanfari, S.M.; Keshavarz, M.; Nabavizadeh, F.; Soltani, N.; Kamalinejad, M. The effect of “Teucrium polium L.” extracts on insulin release from in situ isolated perfused rat pancreas in a newly modified isolation method: The role of Ca2+ and K+ channels. Iran. Biomed. J. 2010, 14, 178–185. [Google Scholar] [PubMed]
- Lin, Z.; Zhang, C.; Cao, D.; Damaris, R.N.; Yang, P. The Latest Studies on Lotus (Nelumbo nucifera)—An Emerging Horticultural Model Plant. Int J Mol Sci 2019, 20, 3680. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Pinthong, D.; Hano, C. Flavonoids from Nelumbo nucifera Gaertn. a Medicinal Plant: Uses in Traditional Medicine, Phytochemistry and Pharmacological Activities. Medicines 2018, 5, 127. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Ta, T.N.; Pham, T.H.; Nguyen, Q.T.; Pham, H.D.; Mishra, S.; Nyomba, B.L. Nuciferine stimulates insulin secretion from beta cells-an in vitro comparison with glibenclamide. J. Ethnopharmacol. 2012, 142, 488–495. [Google Scholar] [CrossRef]
- Ahmed, A.; Saleem, M.A.; Saeed, F.; Afzaal, M.; Imran, A.; Nadeem, M.; Ambreen, S.; Imran, M.; Hussain, M.; Al Jbawi, E. Gynostemma pentaphyllum an immortal herb with promising therapeutic potential: A comprehensive review on its phytochemistry and pharmacological perspective. Int. J. Food Prop. 2023, 26, 808–832. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, W.; Huang, H.; Slavin, M.; Zhao, Y.; Whent, M.; Blackford, J.; Lutterodt, H.; Zhou, H.; Chen, P. Chemical composition of five commercial Gynostemma pentaphyllum samples and their radical scavenging, antiproliferative, and anti-inflammatory properties. J. Agric. Food Chem. 2010, 58, 11243–11249. [Google Scholar] [CrossRef]
- Lin, J.-M.; Lin, C.-C.; Chiu, H.-F.; Yang, J.-J.; Lee, S.-G. Evaluation of the anti-inflammatory and liver-protective effects of Anoectochilus formosanus, Ganoderma lucidum and Gynostemma pentaphyllum in rats. Am. J. Chin. Med. 1993, 21, 59–69. [Google Scholar] [CrossRef]
- Lu, K.-W.; Tsai, M.-L.; Chen, J.-C.; Hsu, S.-C.; Hsia, T.-C.; Lin, M.-W.; Huang, A.-C.; Chang, Y.-H.; Ip, S.-W.; Lu, H.-F. Gypenosides inhibited invasion and migration of human tongue cancer SCC4 cells through down-regulation of NFκB and matrix metalloproteinase-9. Anticancer Res. 2008, 28, 1093–1099. [Google Scholar]
- Huyen, V.; Phan, D.; Thang, P.; Hoa, N.; Östenson, C. Antidiabetic effect of Gynostemma pentaphyllum tea in randomly assigned type 2 diabetic patients. Horm. Metab. Res. 2010, 42, 353–357. [Google Scholar] [CrossRef]
- Yassin, K.; Huyen, V.; Hoa, K.; Östenson, C. Herbal extract of gynostemma pentaphyllum decreases hepatic glucose output in type 2 diabetic goto-kakizaki rats. Int. J. Biomed. Sci. IJBS 2011, 7, 131. [Google Scholar]
- Megalli, S.; Davies, N.M.; Roufogalis, B.D. Anti-hyperlipidemic and hypoglycemic effects of Gynostemma pentaphyllum in the Zucker fatty rat. J. Pharm. Pharm. Sci 2006, 9, 281–291. [Google Scholar]
- Lokman, E.F.; Gu, H.F.; Wan Mohamud, W.N.; Ostenson, C.G. Evaluation of Antidiabetic Effects of the Traditional Medicinal Plant Gynostemma pentaphyllum and the Possible Mechanisms of Insulin Release. Evid. Based Complement. Altern. Med. Ecam 2015, 2015, 120572. [Google Scholar]
- Norberg, A.; Hoa, N.K.; Liepinsh, E.; Van Phan, D.; Thuan, N.D.; Jörnvall, H.; Sillard, R.; Ostenson, C.-G. A novel insulin-releasing substance, phanoside, from the plant Gynostemma pentaphyllum. J. Biol. Chem. 2004, 279, 41361–41367. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef]
- Ovalle-Magallanes, B.; Navarrete, A.; Haddad, P.S.; Tovar, A.R.; Noriega, L.G.; Tovar-Palacio, C.; Mata, R. Multi-target antidiabetic mechanisms of mexicanolides from Swietenia humilis. Phytomedicine Int. J. Phytother. Phytopharm. 2019, 58, 152891. [Google Scholar] [CrossRef]
- Ovalle-Magallanes, B.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Mata, R. Hypoglycemic and antihyperglycemic effects of phytopreparations and limonoids from Swietenia humilis. Phytochemistry 2015, 110, 111–119. [Google Scholar] [CrossRef]
- Potdar, D.; Hirwani, R.; Dhulap, S. Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia 2012, 83, 817–830. [Google Scholar] [CrossRef]
- Rathi, B.; Sahu, J.; Koul, S.; Kosha, R. Detailed pharmacognostical studies on Berberis aristata DC plant. Anc. Sci. Life 2013, 32, 234. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Maffioli, P. The role of a fixed Berberis aristata/Silybum marianum combination in the treatment of type 1 diabetes mellitus. Clin. Nutr. 2016, 35, 1091–1095. [Google Scholar] [CrossRef]
- Guarino, G.; Strollo, F.; Carbone, L.; Della Corte, T.; Letizia, M.; Marino, G.; Gentile, S. Bioimpedance analysis, metabolic effects and safety of the association Berberis aristata/Bilybum marianum: A 52-week double-blind, placebo-controlled study in obese patients with type 2 diabetes. J. Biol. Regul. Homeost. Agents 2017, 31, 495–502. [Google Scholar] [PubMed]
- Wang, Y.-X.; Zheng, Y.-M.; Zhou, X.-B. Inhibitory effects of berberine on ATP-sensitive K+ channels in cardiac myocytes. Eur. J. Pharmacol. 1996, 316, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Estrada, M.; Chilpa, R.R.; Apan, T.R.; Lledias, F.; Hansberg, W.; Arrieta, D.; Aguilar, F.J. Anti-inflammatory activity of cacalol and cacalone sesquiterpenes isolated from Psacalium decompositum. J. Ethnopharmacol. 2006, 105, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.G.; Oropeza, M.; Torres-Sosa, C.; Jimenez-Estrada, M.; Reyes-Chilpa, R. Sesquiterpenoids from antidiabetic Psacalium decompositum block ATP sensitive potassium channels. J. Ethnopharmacol. 2009, 123, 489–493. [Google Scholar] [CrossRef]
- Calzada, F.; Correa-Basurto, J.; Barbosa, E.; Mendez-Luna, D.; Yepez-Mulia, L. Antiprotozoal constituents from Annona cherimola Miller, a plant used in Mexican traditional medicine for the treatment of diarrhea and dysentery. Pharmacogn. Mag. 2017, 13, 148. [Google Scholar]
- Martínez-Vázquez, M.; Estrada-Reyes, R.; Araujo Escalona, A.G.; Ledesma Velázquez, I.; Martínez-Mota, L.; Moreno, J.; Heinze, G. Antidepressant-like effects of an alkaloid extract of the aerial parts of Annona cherimolia in mice. J. Ethnopharmacol. 2012, 139, 164–170. [Google Scholar] [CrossRef]
- Ammoury, C.; Younes, M.; El Khoury, M.; Hodroj, M.H.; Haykal, T.; Nasr, P.; Sily, M.; Taleb, R.I.; Sarkis, R.; Khalife, R.; et al. The pro-apoptotic effect of a Terpene-rich Annona cherimola leaf extract on leukemic cell lines. BMC Complement Altern Med 2019, 19, 365. [Google Scholar] [CrossRef]
- Martínez-Solís, J.; Calzada, F.; Barbosa, E.; Valdés, M. Antihyperglycemic and Antilipidemic Properties of a Tea Infusion of the Leaves from Annona cherimola Miller on Streptozocin-Induced Type 2 Diabetic Mice. Molecules 2021, 26, 2408. [Google Scholar] [CrossRef]
- Valdes, M.; Calzada, F.; Martínez-Solís, J.; Martínez-Rodríguez, J. Antihyperglycemic Effects of Annona cherimola Miller and the Flavonoid Rutin in Combination with Oral Antidiabetic Drugs on Streptozocin-Induced Diabetic Mice. Pharmaceuticals 2023, 16, 112. [Google Scholar] [CrossRef]
- Chauhan, P.; Mahajan, S.; Kulshrestha, A.; Shrivastava, S.; Sharma, B.; Goswamy, H.M.; Prasad, G.B.K.S. Bougainvillea spectabilis Exhibits Antihyperglycemic and Antioxidant Activities in Experimental Diabetes. J. Evid. Based Complement. Altern. Med. 2015, 21, 177–185. [Google Scholar] [CrossRef]
- Adebayo, J.O.; Adesokan, A.A.; Olatunji, L.A.; Buoro, D.O.; Soladoye, A.O. Effect of ethanolic extract of Bougainvillea spectabilis leaves on haematological and serum lipid variables in rats. Biokemistri 2005, 17, 45–50. [Google Scholar] [CrossRef]
- Al-Fartosy, A.J. Protective effect of galactomannan extracted from Iraqi Lycium Barbarum L. fruits against alloxan-induced diabetes in rats. Am. J. Biochem. Biotechnol. 2015, 11, 73. [Google Scholar] [CrossRef]
- Hager, R.; Pitsch, J.; Kerbl-Knapp, J.; Neuhauser, C.; Ollinger, N.; Iken, M.; Ranner, J.; Mittermeier-Kleßinger, V.; Dawid, C.; Lanzerstorfer, P.; et al. A High-Content Screen for the Identification of Plant Extracts with Insulin Secretion-Modulating Activity. Pharmaceuticals 2021, 14, 809. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).