Insulin Sensitization by PPARγ and GLUT-4 Overexpression/Translocation Mediates the Antidiabetic Effect of Plantago australis †
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Characterization
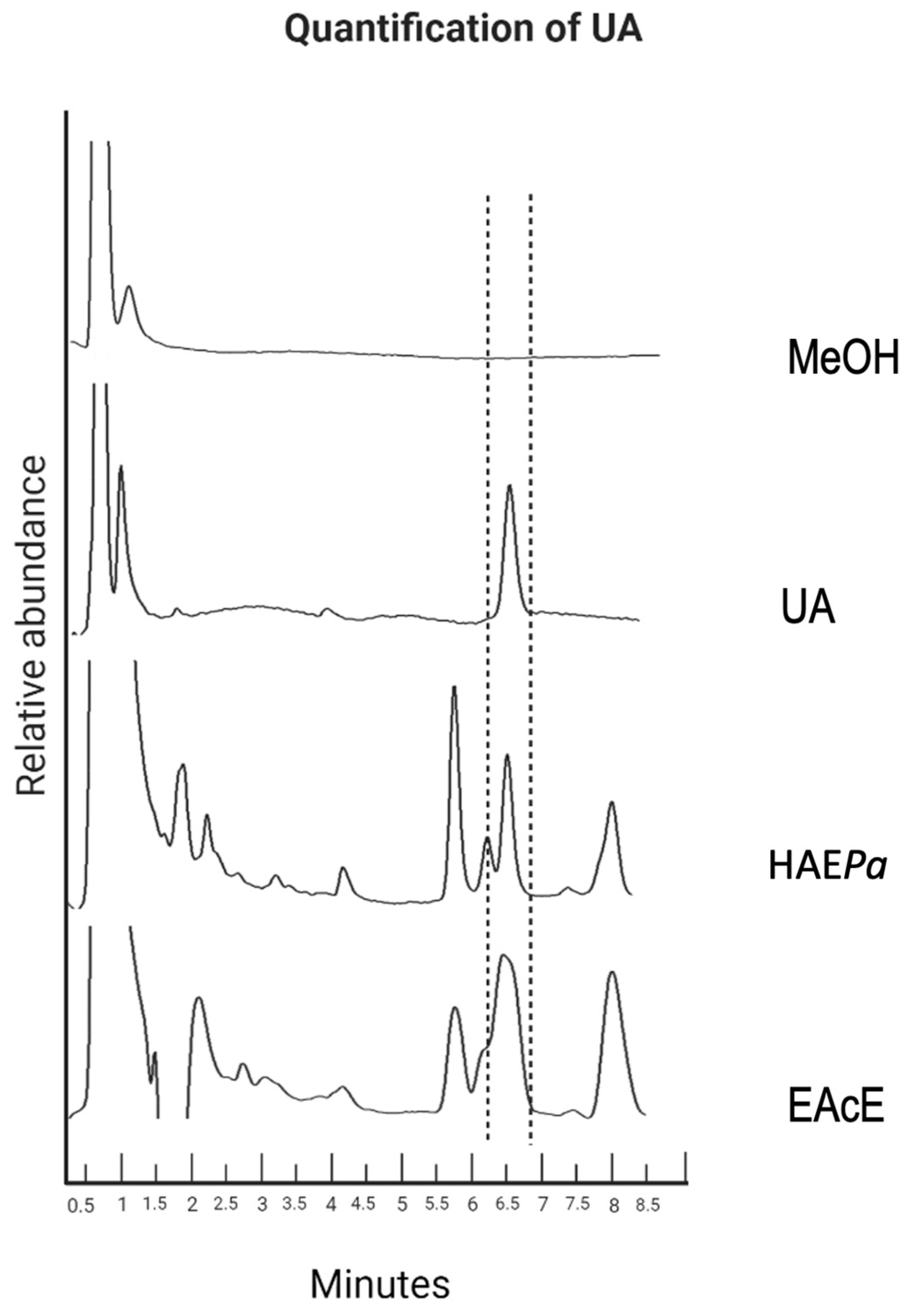
2.1.1. Identification and Quantification of UA by HPLC
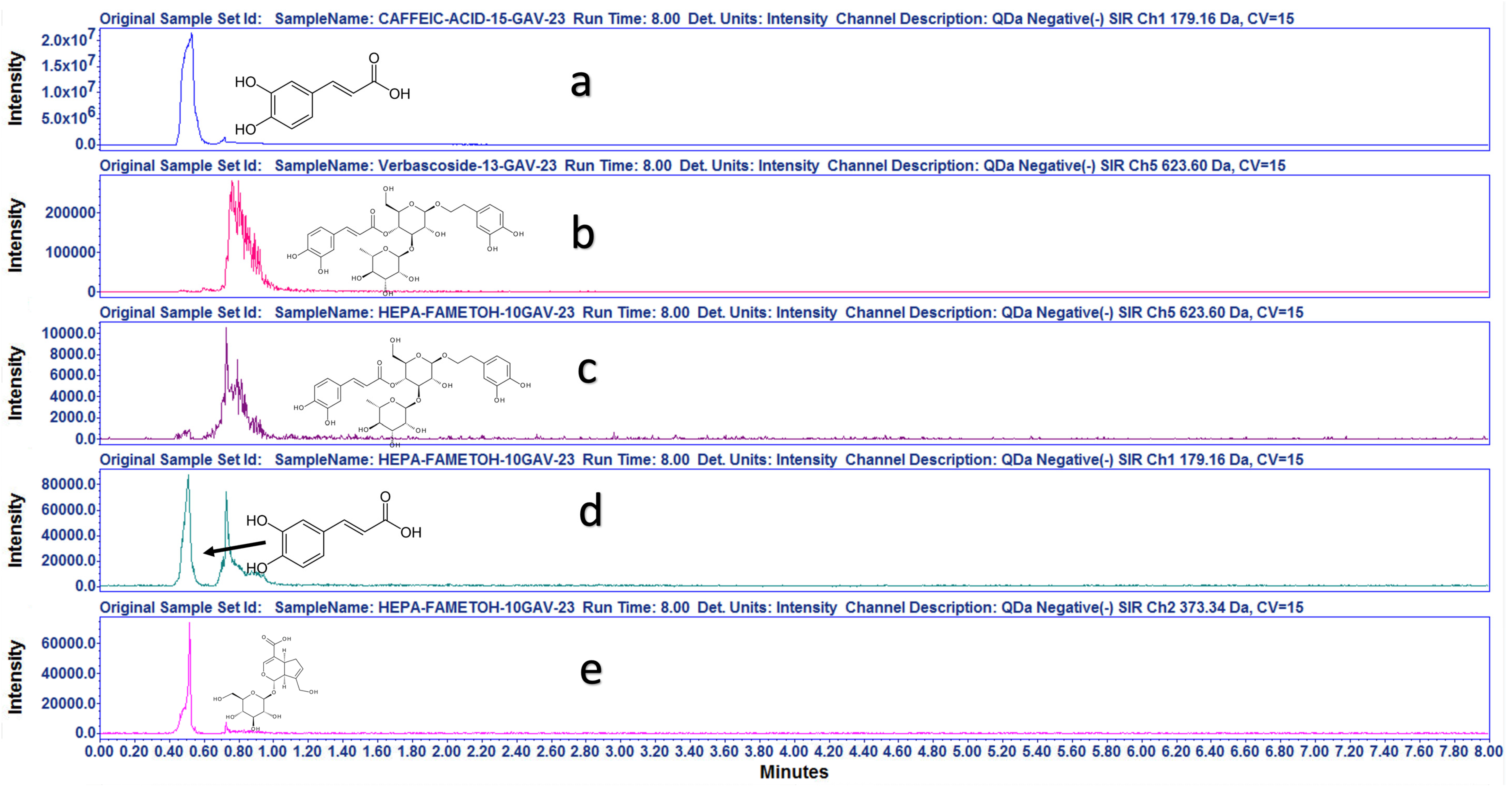
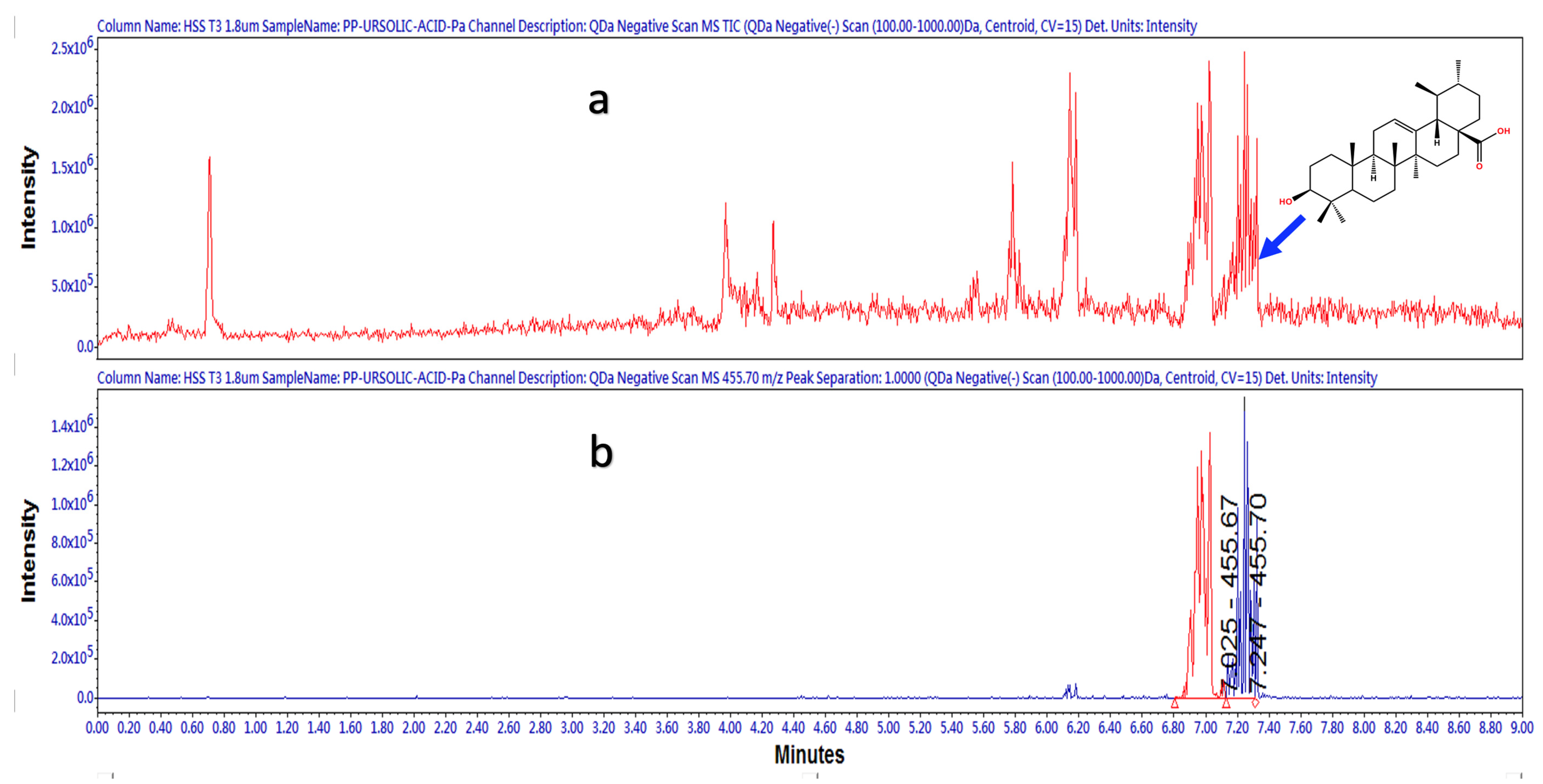
2.1.2. UPLC-ESI-MS Strategy for Compounds Identification
2.2. In Vivo Pharmacological Studies
2.2.1. Oral Glucose Tolerance Tests
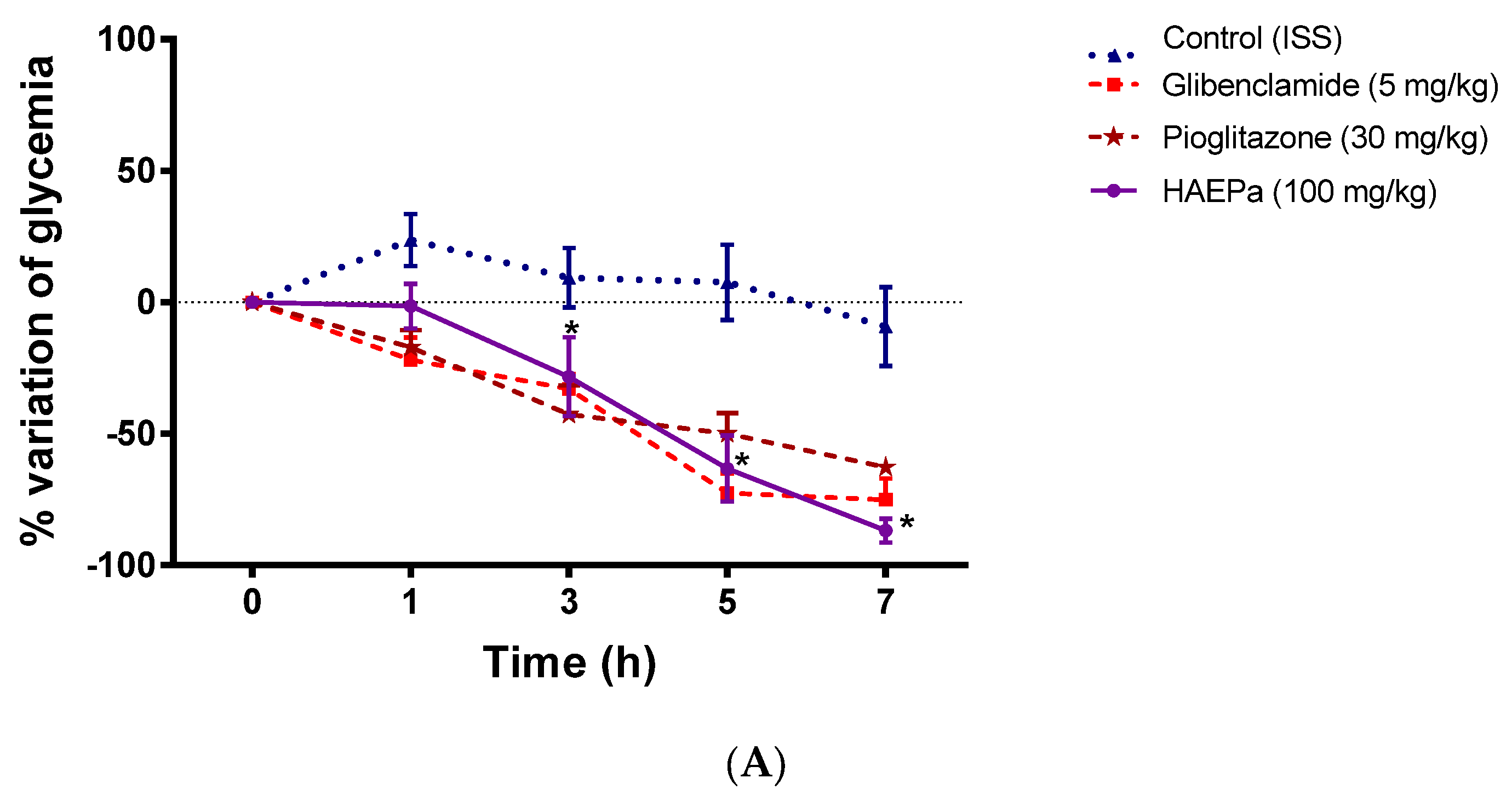
2.2.2. Acute Antidiabetic Assay
2.3. In Vitro Pharmacologic Assays: RNAm Expression of PPARγ and GLUT4
2.4. In Vivo Toxicological Studies
2.4.1. Acute Toxic Class Method (LD50 Estimation)
2.4.2. Sub-Chronic Toxicity Study
3. Materials and Methods
3.1. Chemicals and Drugs
3.2. Plant Material Collection
3.3. Preparation of the Extracts
3.4. Phytochemical Study: Identification and Quantification of Ursolic Acid (UA) by HPLC Method
3.5. LC-MS Characterization
3.6. In Vivo Pharmacologic Studies
3.6.1. Animals
3.6.2. Oral Glucose or Sucrose Tolerance Tests
3.6.3. Induction of Diabetes
3.6.4. Acute Antidiabetic Assay
3.7. In Vitro Pharmacologic Assays
3.7.1. mRNA Expression Analysis of PPARγ and GLUT
3.7.2. Glut GLUT4 Translocation
3.7.3. α-Glucosidases Inhibition
3.8. In Vivo Toxicological Studies
3.8.1. Acute Toxic Class Method (LD50 Estimation)
3.8.2. Sub-Chronic Toxicity Study
3.9. Results Presentation and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guías ALAD de Diagnóstico, Control y Tratamiento de la Diabetes Mellitus Tipo 2. 2009. Available online: https://www.paho.org/hq/dmdocuments/2010/Guias_ALAD_2009.pdf (accessed on 26 March 2022). (In Spanish).
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://fmdiabetes.org/wp-content/uploads/2022/01/IDF_Atlas_10th_Edition_2021-comprimido.pdf (accessed on 26 March 2022).
- Luher, N.S.T.; Geymonat, A.E.B.; Acuña, J.N.Z. Adherencia al tratamiento en pacientes con Diagnóstico de Diabetes Mellitus Tipo II. Biomedicina 2015, 10, 20–33. (In Spanish) [Google Scholar]
- Mata, R.; Figueroa, M.; Navarrete, A.; Rivero-Cruz, I. Chemistry and biology of selected Mexican medicinal plants. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A., Falk, H., Gibbons, S., Kobayashi, J., Asakawa, Y., Liu, J.K., Eds.; Springer: Cham, Switzerland, 2019; Volume 108, pp. 1–142. [Google Scholar]
- Hernandez-Galicia, E.; Aguilar-Contreras, A.; Aguilar-Santamaria, L.; Roman-Ramos, R.; Chavez-Miranda, A.A.; Garcia-Vega, L.M.; Flores-Saenz, J.L.; Alarcon-Aguilar, F.J. Studies on hypoglycemic activity of mexican medicinal plants. Proc. West. Pharmacol. Soc. 2002, 45, 118–124. [Google Scholar]
- Biblioteca Digital de la Medicina Tradicional Mexicana. 2009. Available online: http://www.medicinatradicionalmexicana.unam.mx/index.php (accessed on 26 March 2021). (In Spanish).
- Monroy-Ortiz, C.; Castillo-España, P. Plantas Medicinales Utilizadas en el Estado de Morelos, 2nd ed.; Universidad Autónoma de Morelos, CONABIO: Cuernavaca, Mexico, 2007; 405p. (In Spanish) [Google Scholar]
- Dorigoni, P.A.; Ghedini, P.C.; Fróes, L.F.; Baptista, K.C.; Ethur, A.B.M.; Baldisserotto, B.; Bürger, M.E.; Almeida, C.E.; Lopes, A.M.V.; Záchia, R.A. Levantamento de dados sobre plantas medicinais de uso popular no municıpio de São João do Polêsine, RS, Brasil. I—Relação entre enfermidades e espécies utilizadas. Rev. Bras. Pl. Med. 2001, 4, 69–79. (In Portuguese) [Google Scholar]
- Palmeiro, N.S.; Esteves-Almeida, C.; Ghedini, P.C.; Goulart, L.S.; Baldisserotto, B. Analgesic and anti-inflammatory properties of Plantago australis hydroalcoholic extract. Acta Farm. Bonaer. 2002, 21, 89–92. [Google Scholar]
- Somavilla, N.; Canto-Dorow, T.S. Levantamento das plantas medicinais utilizadas em bairros de Santa Maria—RS. Cienc. Nat. 1996, 18, 131–148. (In Portuguese) [Google Scholar] [CrossRef]
- Sperotto, N.D.D.M.; Steffens, L.; Veríssimo, R.M.; Henn, J.G.; Péres, V.F.; Vianna, P.; Chies, J.A.B.; Roehe, A.; Saffi, J.; Moura, D.J. Wound healing and anti-inflammatory activities induced by a Plantago australis hydroethanolic extract standardized in verbascoside. J. Ethnopharmacol. 2018, 225, 178–188. [Google Scholar] [CrossRef]
- Rønsted, N.; Göbel, E.; Franzyk, H.; Jensen, S.R.; Olsen, C.E. Chemotaxonomy of Plantago. Iridoid glucosides and caffeoyl phenylethanoid glycosides. Phytochemistry 2000, 55, 337–348. [Google Scholar] [CrossRef]
- Henn, J.G.; Steffens, L.; Sperotto, N.D.D.M.; Ponce, B.D.S.; Veríssimo, R.M.; Boaretto, F.B.M.; Hassemer, G.; Péres, V.F.; Schirmer, H.; Picada, J.N.; et al. Toxicological evaluation of a standardized hydroethanolic extract from leaves of Plantago australis and its major compound, verbascoside. J. Ethnopharmacol. 2019, 229, 145–156. [Google Scholar] [CrossRef]
- Nemitz, M.C.; Banderó, F.; Zanetti, G.D.; Zanotto, C.Z.; Manfron, M.P. Phenolic compounds and antioxidant activity of the leaves of Plantago australis L. (Plantaginaceae). Lat. Am. J. Pharm. 2010, 29, 1082–1087. [Google Scholar]
- Colin-Lozano, B.; Torres-Gomez, H.; Hidalgo-Figueroa, S.; Chávez-Silva, F.; Estrada-Soto, S.; Almanza-Pérez, J.C.; Navarrete-Vazquez, G. Synthesis, In Vitro, In Vivo and In Silico Antidiabetic Bioassays of 4-Nitro(thio)phenoxyisobutyric Acids Acting as Unexpected PPARγ Modulators: An In Combo Study. Pharmaceuticals 2022, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.M.V.; Estrada-Soto, S.E.; de Jesús Arellano-García, J.; Rivera-Leyva, J.C.; Castillo-España, P.; Flores, A.F.; Perea-Arango, I. Methyl Jasmonate and Salicylic Acid Enhanced the Production of Ursolic and Oleanolic Acid in Callus Cultures of Lepechinia caulescens. Pharmacogn. Mag. 2017, 13, S886. [Google Scholar] [CrossRef]
- Ramírez-Espinosa, J.J.; Rios, M.Y.; López-Martínez, S.; López-Vallejo, F.; Medina-Franco, J.L.; Paoli, P.; Camici, G.; Navarrete-Vázquez, G.; Ortiz-Andrade, R.; Estrada-Soto, S. Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP-1B: In vitro, in silico, and in vivo approaches. Eur. J. Med. Chem. 2011, 46, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WPR/RC52/7: A Draft Regional Strategy for Traditional Medicine in Western Pacific. In Proceedings of the WHO Regional Committee, 52nd Session, Brunei Darussalam, 10–14 September 2001. [Google Scholar]
- Gao, H.; Liu, Z.; Song, F.; Xing, J.; Zheng, Z.; Liu, S. A Strategy for Identification and Structural Characterization of Compounds from Plantago asiatica L. by Liquid Chromatography-Mass Spectrometry Combined with Ion Mobility Spectrometry. Molecules 2022, 27, 4302. [Google Scholar] [CrossRef]
- Wei, W.; Wang, X.; Hou, J.; Yao, C.; Feng, Z.; Zhang, J.; Han, S.; Deng, Y.; Huang, Y.; Wu, W.; et al. Implementation of a Single Quadrupole Mass Spectrometer for Fingerprint Analysis: Venenum bufonis as a Case Study. Molecules 2018, 23, 3020. [Google Scholar] [CrossRef] [PubMed]
- Franzyk, H.; Jaroszewski, J.W.; Jensen, S. Chemotaxonomy and evolution of Plantago L. Plant Syst. Evol. 2003, 242, 63–82. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Matsabisa, M.G.; Ibrahim, M.A.; Erukainure, O.L.; Chabalala, M.H.; Islam, M.S. Medicinal plants with concomitant anti-diabetic and anti-hypertensive effects as potential sources of dual acting therapies against diabetes and hypertension: A review. J. Ethnopharmacol. 2019, 235, 329–360. [Google Scholar] [CrossRef]
- Oršolić, N.; Sirovina, D.; Odeh, D.; Gajski, G.; Balta, V.; Šver, L.; Jembrek, M.J. Efficacy of Caffeic Acid on Diabetes and Its Complications in the Mouse. Molecules 2021, 26, 3262. [Google Scholar] [CrossRef]
- Xu, X.H.; Su, Q.; Zang, Z.H. Simultaneous determination of oleanolic acid and ursolic acid by RP-HPLC in the leaves of Eriobotrya japonica Lindl. J. Pharm. Anal. 2012, 2, 238–240. [Google Scholar] [CrossRef]
- Zacchigna, M.; Cateni, F.; Faudale, M.; Sosa, S.; Della Loggia, R. Rapid HPLC Analysis for Quantitative Determination of the Two Isomeric Triterpenic Acids, Oleanolic acid and Ursolic acid, in Plantago Major. Sci. Pharm. 2009, 77, 79–86. [Google Scholar] [CrossRef]
- Kartini, K.; Wati, N.; Gustav, R.; Wahyuni, R.; Anggada, Y.F.; Hidayani, R.; Raharjo, A.; Islamie, R.; Putra, S.E.D. Wound healing effects of Plantago major extract and its chemical compounds in hyperglycemic rats. Food Biosci. 2021, 41, 100937. [Google Scholar] [CrossRef]
- Wu, L.; Georgiev, M.I.; Cao, H.; Nahar, L.; El-Seedi, H.R.; Sarker, S.D.; Xiao, J.; Lu, B. Therapeutic potential of phenylethanoid glycosides: A systematic review. Med. Res. Rev. 2020, 40, 2605–2649. [Google Scholar] [CrossRef] [PubMed]
- Abud, M.A.; Nardello, A.L.; Torti, J.F. Hypoglycemic Effect due to Insulin Stimulation with Plantago major in Wistar Rats. Med. Aromat. Plants 2017, 6, 292. [Google Scholar] [CrossRef]
- Parsa, H.; Moradi-Khaligh, Z.; Rajabi, S.; Ranjbar, K.; Komaki, A. Swimming training and Plantago psyllium ameliorate cognitive impairment and glucose tolerance in streptozotocin–nicotinamide-induced type 2 diabetic rats. J. Physiol. Sci. 2021, 71, 37. [Google Scholar] [CrossRef]
- Yoshida, T.; Rikimaru, K.; Sakai, M.; Nishibe, S.; Fujikawa, T.; Tamura, Y. Plantago lanceolata L. leaves prevent obesity in C57BL/6 J mice fed a high-fat diet. Nat. Prod. Res. 2013, 27, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, S.; Romano, A. The medicinal potential of plants from the genus Plantago (Plantaginaceae). Ind. Crops Prod. 2016, 83, 213–226. [Google Scholar] [CrossRef]
- Rodríguez-Morán, M.; Guerrero-Romero, F.; Lazcano-Burciaga, G. Lipid- and glucose-lowering efficacy of Plantago psyllium in type II diabetes. J. Diabetes Complicat. 1998, 12, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Ruas, N.R.; Pereira, A.C.; Silva Pereira, L.L.; Germano, C.M.; da Cunha, E.F.F.; de Carvalho, A.A.; Lameira, O.A.; Pereira Pinto, J.E.B.; Vilela Bertolucci, S.K. Inhibition of α-Glycosidase by Lippia dulcis Trevir. (Verbenaceae) Preparations, Quantification of Verbascoside, and Study of Its Molecular Docking. Chem. Biodivers. 2023, 20, e202200760. [Google Scholar] [CrossRef]
- Bermúdez, V.; Bermúdez, F.; Arraiz, N.; Leal, E.; Linares, S.; Mengual, E.; Valdelamar, S.; Rodríguez, M.; Seyfi, H.; Amell, A.; et al. Biología molecular de los transportadores de glucosa: Clasificación, estructura y distribución. Arch. Venez. Farmacol. Ter. 2007, 26, 76–86. (In Spanish) [Google Scholar]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiong, L.; Wang, W.J. Reviews on α-glucosidase inhibitor from plant secondary metabolites. Zhongguo Zhong Yao Za Zhi 2017, 42, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- De Moura Sperotto, N.D. Avaliação das atividades antiinflamatória e cicatrizante de um extrato hidroetanólico de Plantago australis e do seu composto verbascosídeo, em modelos in vitro. In Tesis de Maestría; Universidade Federal de Ciências da Saúde de Porto Alegre: Porto Alegre, Brazil, 2016. (In Portuguese) [Google Scholar]
- Clark, R.B. The role of PPARs in inflammation and immunity. J. Leukoc. Biol. 2002, 71, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in vitro studies on Austria’s folk medicine- an unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef]
- Fernyhough, M.E.; Okine, E.; Hausmanc, G.; Vierck, J.L.; Dodsona, M.V. PPARγ and GLUT4 expression as developmental regulators/markers for preadipocyte differentiation into an adipocyte. Domest. Anim. Endocrinol. 2007, 33, 367–378. [Google Scholar] [CrossRef]
- Anandharajan, R.; Jaiganesh, S.; Shankernarayanan, N.P.; Viswakarmac, R.A.; Balakrishnand, A. In vitro glucose uptake activity of Aegles marmelos and Syzygium cumini by activation of Glut4, PI3 kinase and PPARgamma in L6 myotubes. Phytomedicine 2005, 13, 434–441. [Google Scholar] [CrossRef]
- Larsen, T.M.; Toubro, S.; Astrup, A. PPARgamma agonists in the treatment of type II diabetes: Is increased fatness commensurate with long-term efficacy? Int. J. Obes. and Relat. Metab. Disord. 2003, 27, 147–161. [Google Scholar] [CrossRef]
- Wang, Y.X. PPARs: Diverse regulators in energy metabolism and metabolic diseases. Cell Res. 2010, 20, 124–137. [Google Scholar] [CrossRef]
- Yuet Ping, K.; Darah, I.; Chen, Y.; Sreeramanan, S.; Sasidharan, S. Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. BioMed Res. Int. 2013, 2013, 182064. [Google Scholar] [CrossRef]
- Cortés, L.; Montoro, M.A. Capítulo 48: Datos de laboratorio: Pruebas hepáticas alteradas. In Gastroenterología y Hepatología. Problemas Comunes en la Práctica Clínica, 2nd ed.; Montoro, M.A., García Pagán, J.C., Jarpyo, Eds.; Jarpyo Editores: Madrid, Spain, 2012. (In Spanish) [Google Scholar]
- Limdi, J.K.; Hyde, G.M. Evaluation of abnormal liver function tests. Postgrad. Med. J. 2003, 79, 307–312. [Google Scholar] [CrossRef]
- Kaneko, J.J. Clinical Biochemistry of Domestic Animals, 4th ed.; Academic Press: San Diego, CA, USA, 1989; Volume 932. [Google Scholar]
- Palmeiro, N.; Almeida, C.; Ghedini, P.; Goulart, L.; Pereira, M.; Huber, S.; da Silva, J.; Lopes, S. Oral subchronic toxicity of aqueous crude extract of Plantago australis leaves. J. Ethnopharmacol. 2003, 88, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Guadarrama, A.B.; Yáñez-Ibarra, G.; Cancino-Marentes, M.E.; González-Ibarra, P.; Ortiz-Andrade, R.; Sánchez-Recillas, A.; Rodríguez-Carpena, J.-G.; Aguirre-Vidal, Y.; Medina-Diaz, I.-M.; Ávila-Villarreal, G. Chromatographic Techniques and Pharmacological Analysis as a Quality Control Strategy for Serjania triquetra a Traditional Medicinal Plant. Pharmaceuticals 2022, 15, 1289. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Kojima, R.; Ito, M. Strain differences in the diabetogenic activity of streptozotocin in mice. Biol. Pharm. Bull. 2006, 29, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Giles-Rivas, D.; Estrada-Soto, S.; Aguilar-Guadarrama, A.B.; Almanza-Pérez, J.; García-Jiménez, S.; Colín-Lozano, B.; Navarrete-Vázquez, G.; Villalobos-Molina, R. Antidiabetic effect of Cordia morelosana, chemical and pharmacological studies. J. Ethnopharmacol. 2020, 251, 112543. [Google Scholar] [CrossRef]
- Chávez-Silva, F.; Cerón-Romero, L.; Arias-Durán, L.; Navarrete-Vázquez, G.; Almanza-Pérez, J.; Román-Ramos, R.; Ramírez-Ávila, G.; Perea-Arango, I.; Villalobos-Molina, R.; Estrada-Soto, S. Antidiabetic effect of Achillea millefollium through multitarget interactions: α-glucosidases inhibition, insulin sensitization and insulin secretagogue activities. J. Ethnopharmacol. 2018, 212, 1–7. [Google Scholar] [CrossRef]
- Giacoman-Martínez, A.; Alarcón-Aguilar, F.J.; Zamilpa, A.; Huang, F.; Romero-Nava, R.; Román-Ramos, R.; Almanza-Pérez, J.C. α-Amyrin induces GLUT4 translocation mediated by AMPK and PPARδ/γ in C2C12 myoblasts. Can. J. Physiol. Pharmacol. 2021, 99, 935–942. [Google Scholar] [CrossRef]
- Loza-Rodríguez, H.; Estrada-Soto, S.; Alarcón-Aguilar, F.J.; Huang, F.; Aquino-Jarquín, G.; Fortis-Barrera, Á.; Giacoman-Martínez, A.; Almanza-Pérez, J.C. Oleanolic acid induces a dual agonist action on PPARγ/α and GLUT4 translocation: A pentacyclic triterpene for dyslipidemia and type 2 diabetes. Eur. J. Pharmacol. 2020, 883, 173252. [Google Scholar] [CrossRef]
- Youn, I.; Chen, S.N.; Simmler, C.; Bisson, J.; Pauli, G. “UrsolicAcid_400MHz_DMSOd6_jdf.zip”, Ursolic Acid 400MHz DMSOd6 NMR Data. Harvard Dataverse, V1. NMR. 2018. Available online: https://doi.org/10.7910/DVN/RXF88X/HUJVBE (accessed on 16 March 2023). [CrossRef]








| Doses of HAEPa (mg/kg) | Deaths | DL50 | Classification (GHS) |
|---|---|---|---|
| 5 | 0 | >2000 mg/kg | Category 4 |
| 50 | 0 | ||
| 300 | 0 | ||
| 2000 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada-Soto, S.; Ornelas-Mendoza, K.; Navarrete-Vázquez, G.; Chávez-Silva, F.; Almanza-Pérez, J.C.; Villalobos-Molina, R.; Ortiz-Barragán, E.; Loza-Rodríguez, H.; Rivera-Leyva, J.C.; Flores-Flores, A.; et al. Insulin Sensitization by PPARγ and GLUT-4 Overexpression/Translocation Mediates the Antidiabetic Effect of Plantago australis. Pharmaceuticals 2023, 16, 535. https://doi.org/10.3390/ph16040535
Estrada-Soto S, Ornelas-Mendoza K, Navarrete-Vázquez G, Chávez-Silva F, Almanza-Pérez JC, Villalobos-Molina R, Ortiz-Barragán E, Loza-Rodríguez H, Rivera-Leyva JC, Flores-Flores A, et al. Insulin Sensitization by PPARγ and GLUT-4 Overexpression/Translocation Mediates the Antidiabetic Effect of Plantago australis. Pharmaceuticals. 2023; 16(4):535. https://doi.org/10.3390/ph16040535
Chicago/Turabian StyleEstrada-Soto, Samuel, Kathia Ornelas-Mendoza, Gabriel Navarrete-Vázquez, Fabiola Chávez-Silva, Julio Cesar Almanza-Pérez, Rafael Villalobos-Molina, Erandi Ortiz-Barragán, Hilda Loza-Rodríguez, Julio César Rivera-Leyva, Angélica Flores-Flores, and et al. 2023. "Insulin Sensitization by PPARγ and GLUT-4 Overexpression/Translocation Mediates the Antidiabetic Effect of Plantago australis" Pharmaceuticals 16, no. 4: 535. https://doi.org/10.3390/ph16040535
APA StyleEstrada-Soto, S., Ornelas-Mendoza, K., Navarrete-Vázquez, G., Chávez-Silva, F., Almanza-Pérez, J. C., Villalobos-Molina, R., Ortiz-Barragán, E., Loza-Rodríguez, H., Rivera-Leyva, J. C., Flores-Flores, A., Perea-Arango, I., Rodríguez-Carpena, J.-G., & Ávila-Villarreal, G. (2023). Insulin Sensitization by PPARγ and GLUT-4 Overexpression/Translocation Mediates the Antidiabetic Effect of Plantago australis. Pharmaceuticals, 16(4), 535. https://doi.org/10.3390/ph16040535










