Examination of Risk Factors and Expression Patterns of Atypical Femoral Fractures Using the Japanese Adverse Drug Event Report Database: A Retrospective Pharmacovigilance Study
Abstract
:1. Introduction
2. Results
2.1. Creation of Data Analysis Tables
2.2. Risk Factors for AFF
2.2.1. Relationship between Patient Background and AFF
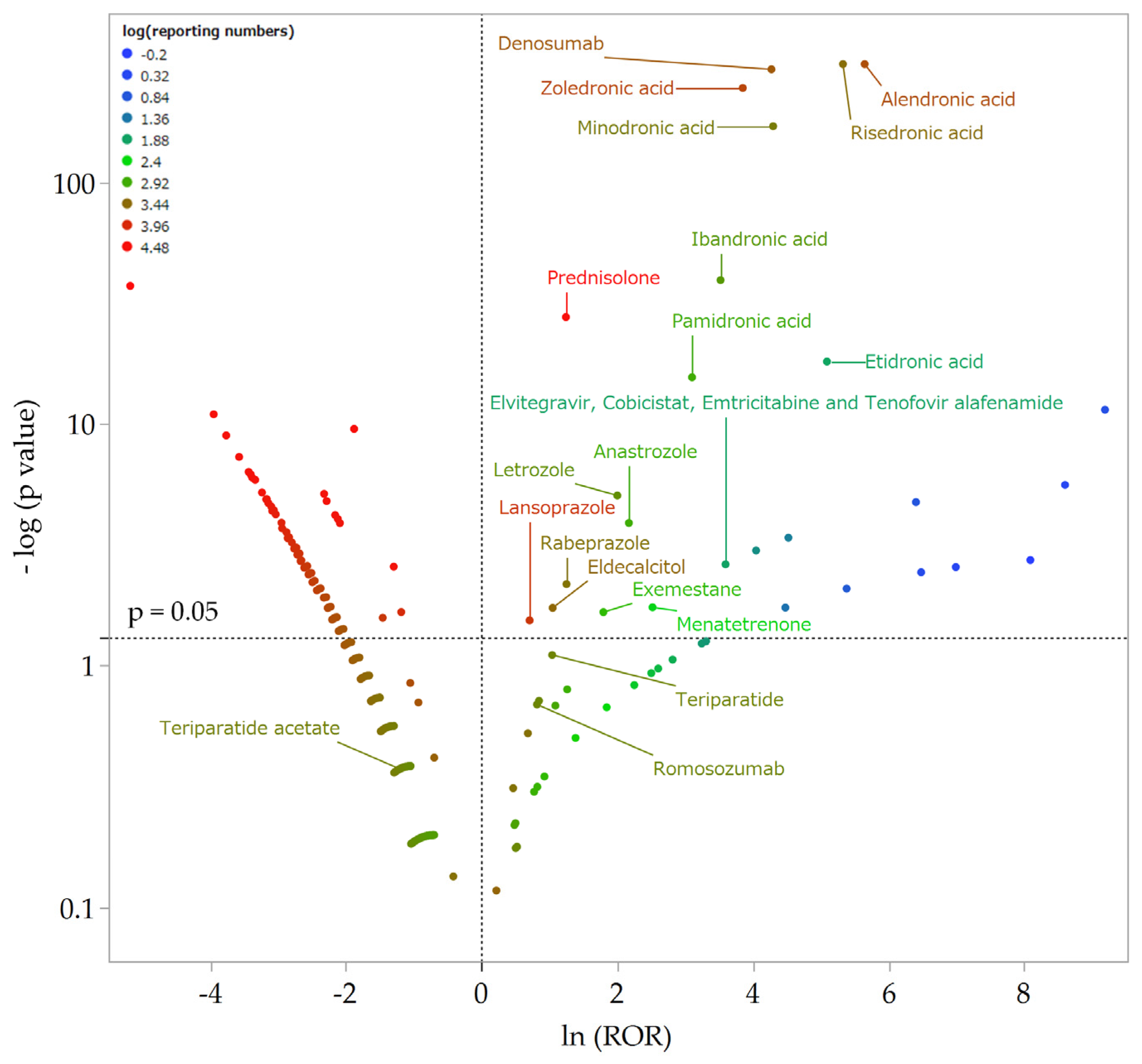
2.2.2. Relationship between Suspected Drugs and AFF
2.2.3. Multiple Logistic Regression Analysis
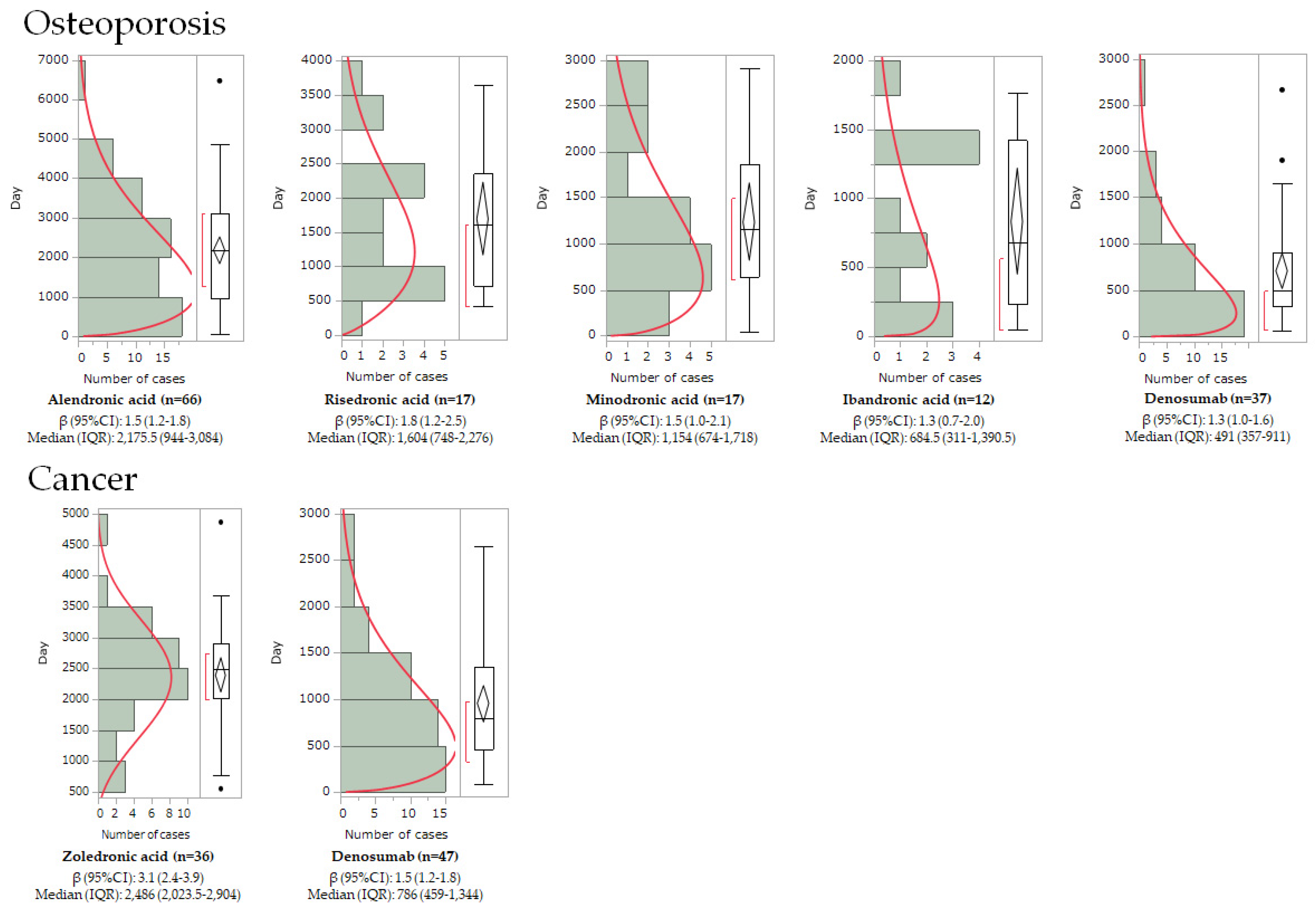
2.3. Onset Pattern Analysis Using Weibull Distribution
3. Discussion
3.1. Risk Factors for AFF
3.2. Patterns for AFF Onset Timelines
3.3. Limitations
4. Materials and Methods
4.1. Preparation of JADER and Data Tables for Analysis
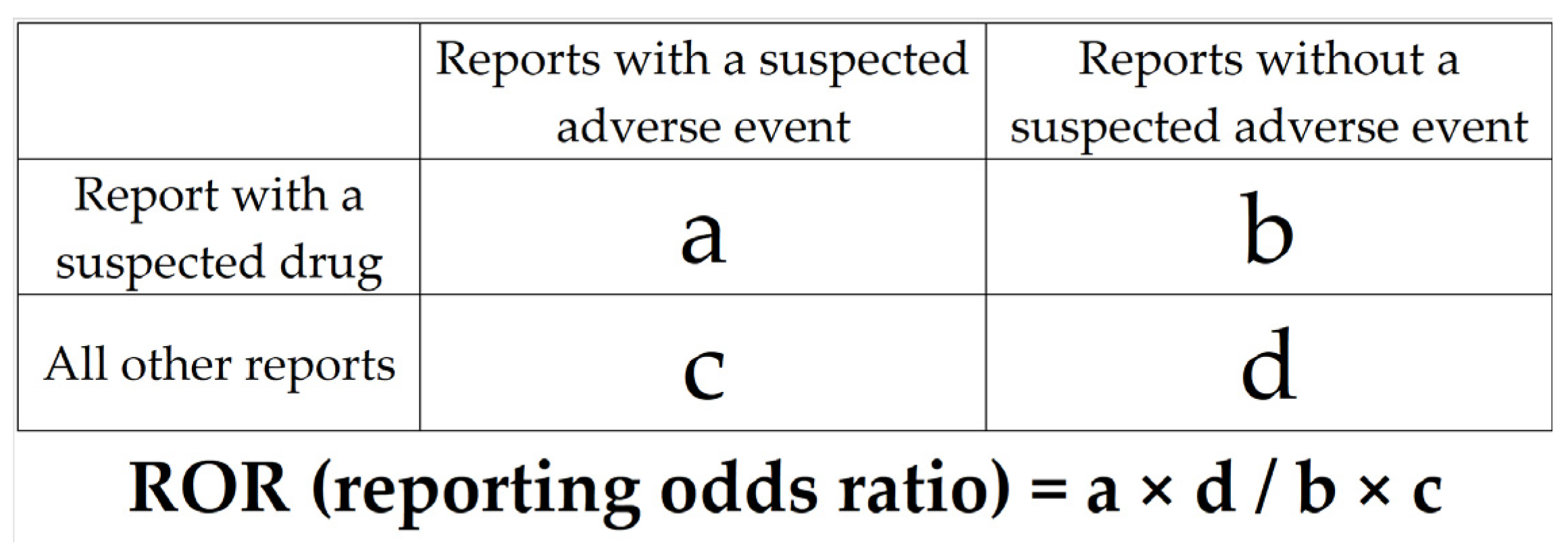
4.2. Risk Factors for AFFs
4.3. Onset Pattern Analysis of AFF by Weibull Distribution
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Odvina, C.V.; Zerwekh, J.E.; Rao, D.S.; Maalouf, N.; Gottschalk, F.A.; Pak, C.Y. Severely suppressed bone turnover: A potential complication of alendronate therapy. J. Clin. Endocrinol. Metab. 2005, 90, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Shane, E.; Burr, D.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; Dempster, D.W.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014, 29, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Toriumi, S.; Kobayashi, A.; Sueki, H.; Yamamoto, M.; Uesawa, Y. Exploring the Mechanisms Underlying Drug-Induced Fractures Using the Japanese Adverse Drug Event Reporting Database. Pharmaceuticals 2021, 13, 1299. [Google Scholar] [CrossRef] [PubMed]
- Van de Laarschot, D.M.; McKenna, M.J.; Abrahamsen, B.; Langdahl, B.; Cohen-Solal, M.; Guañabens, N.; Eastell, R.; Ralston, S.H.; Zillikens, M.C. Medical Management of Patients After Atypical Femur Fractures: A Systematic Review and Recommendations from the European Calcified Tissue Society. J. Clin. Endocrinol. Metab. 2020, 105, 1682–1699. [Google Scholar] [CrossRef]
- Meling, T.; Nawab, A.; Harboe, K.; Fosse, L. AFF in elderly women: A fracture registry-based cohort study. Bone Jt. J. 2014, 96, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef]
- Peddi, P.; Lopez-Olivo, M.A.; Pratt, G.F.; Suarez-Almazor, M.E. Denosumab in patients with cancer and skeletal metastases: A systematic review and meta-analysis. Cancer Treat. Rev. 2013, 39, 97–104. [Google Scholar] [CrossRef]
- El Osta, L.; El Osta, N.; El Osta, H. Benefits and potential risks of bisphosphonate therapy A narrative review. J. Med. Liban. 2016, 64, 228–237. [Google Scholar] [CrossRef]
- Adler, R.A.; Fuleihan, G.E.-H.; Bauer, D.C.; Camacho, P.M.; Clarke, B.L.; Clines, G.A.; Compston, J.E.; Drake, M.T.; Edwards, B.J.; Favus, M.J.; et al. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2016, 1, 16–35. [Google Scholar] [CrossRef]
- McClung, M.; Harris, S.T.; Miller, P.D.; Bauer, D.C.; Davison, K.S.; Dian, L.; Hanley, D.A.; Kendler, D.L.; Yuen, C.K.; Lewiecki, E.M. Bisphosphonate therapy for osteoporosis: Benefits, risks, and drug holiday. Am. J. Med. 2013, 126, 13–20. [Google Scholar] [CrossRef]
- Pharmaceutical and Medical Devices Agency. Available online: https://www.pmda.go.jp/safety/info-services/drugs/adr-info/suspected-adr/0005.html (accessed on 16 September 2022).
- Kamimura, H.; Setsu, T.; Kimura, N.; Miyazawa, M.; Kaneko, S.; Kamimura, K.; Tsuchiya, A.; Uesawa, Y.; Terai, S. Analysis of drug-induced liver-related adverse event trend reporting between 1997 and 2019. Hepatol. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, V.R.; Sauzet, O.; Evans, S.J. A signal detection method to detect adverse drug reactions using a parametric time-to-event model in simulated cohort data. Drug Saf. 2012, 35, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Zenke, Y.; Ikeda, S.; Fukuda, F.; Tanaka, M.; Tanaka, H.; Hirano, F.; Sakai, A. Study of Atypical Femoral Fracture Cases Coupled in a Multicenter Study. J. UOEH 2016, 38, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Taormina, D.P.; Marcano, A.I.; Karia, R.; Egol, K.A.; Tejwani, N.C. Symptomatic AFF are related to underlying hip geometry. Bone 2014, 63, 1–6. [Google Scholar] [CrossRef]
- Velasco, S.; Kim, S.; Bleakney, R.; Jamal, S.A. The clinical characteristics of patients with hip fractures in typical locations and AFF. Arch. Osteoporos. 2014, 9, 171. [Google Scholar] [CrossRef]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO Study Group. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef]
- Xia, J.; Luo, R.; Guo, S.; Yang, Y.; Ge, S.; Xu, G.; Zeng, R. Prevalence and Risk Factors of Reduced Bone Mineral Density in Systemic Lupus Erythematosus Patients: A Meta-Analysis. Biomed. Res. Int. 2019, 2019, 3731648. [Google Scholar] [CrossRef]
- Jin, S.; Hsieh, E.; Peng, L.; Yu, C.; Wang, Y.; Wu, C.; Wang, Q.; Li, M.; Zeng, X. Incidence of fractures among patients with rheumatoid arthritis: A systematic review and meta-analysis. Osteoporos. Int. 2018, 6, 1263–1275. [Google Scholar] [CrossRef]
- al-Janadi, M.; al-Balla, S.; Al-Dalaan, A.; Raziuddin, S. Cytokine profile in systemic lupus erythematosus, rheumatoid arthritis, and other rheumatic diseases. J. Clin. Immunol. 1993, 13, 58–67. [Google Scholar] [CrossRef]
- Linker-Israeli, M.; Deans, R.J.; Wallace, D.J.; Prehn, J.; Ozeri-Chen, T.; Klinenberg, J.R. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J. Immunol. 1991, 147, 117–123. [Google Scholar] [CrossRef]
- Tanaka, Y.; Watanabe, K.; Suzuki, M.; Saito, K.; Oda, S.; Suzuki, H.; Eto, S.; Yamashita, U. Spontaneous production of bone-resorbing lymphokines by B cells in patients with systemic lupus erythematosus. J. Clin. Immunol. 1989, 5, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Lu, C.; Zhang, L.; Zhou, X.; Zou, H. Osteoporosis in patients with rheumatoid arthritis is associated with serum immune regulatory cellular factors. Clin. Rheumatol. 2022, 41, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Yu, J.; Rho, J. Bone Loss Triggered by the Cytokine Network in Inflammatory Autoimmune Diseases. J. Immunol. Res. 2015, 2015, 832127. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, A.C.; Black, D.; Perrin, N.; Rosales, A.G.; Friess, D.; Boardman, D.; Dell, R.; Santora, A.; Chandler, J.M.; Rix, M.M.; et al. Incidence and demography of femur fractures with and without atypical features. J. Bone Miner. Res. 2012, 27, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; San Martin, J.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, doubleblind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Baron, R.; Ferrari, S.; Russell, R.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef]
- Thompson, R.N.; Armstrong, C.L.; Heyburn, G. Bilateral AFF in a patient prescribed denosumab—A case report. Bone 2014, 61, 44–47. [Google Scholar] [CrossRef]
- Takahashi, M.; Ozaki, Y.; Kizawa, R.; Masuda, J.; Sakamaki, K.; Kinowaki, K.; Umezu, T.; Kondoh, C.; Tanabe, Y.; Tamura, N.; et al. Atypical femoral fracture in patients with bone metastasis receiving denosumab therapy: A retrospective study and systematic review. BMC Cancer 2019, 19, 980. [Google Scholar] [CrossRef]
- Starr, J.; Tay, Y.K.D.; Shane, E. Current Understanding of Epidemiology, Pathophysiology, and Management of Atypical Femur Fractures. Curr. Osteoporos. Rep. 2018, 16, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.T.; McComsey, G.A.; King, M.S.; Qaqish, R.B.; Bernstein, B.M.; da Silva, B.A. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J. Acquir. Immune Defic. Syndr. 2009, 51, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; Weinstein, R.S. New developments in the pathogenesis and treatment of steroid-induced osteoporosis. J. Bone Miner. Res. 1999, 14, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S.; Jilka, R.L.; Parfitt, A.M.; Manolagas, S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J. Clin. Investig. 1998, 102, 274–282. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Chen, J.R.; Powers, C.C.; Stewart, S.A.; Landes, R.D.; Bellido, T.; Jilka, R.L.; Parfitt, A.M.; Manolagas, S.C. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J. Clin. Investig. 2002, 109, 1041–1048. [Google Scholar] [CrossRef]
- Mazziotti, G.; Angeli, A.; Bilezikian, J.P.; Canalis, E.; Giustina, A. Glucocorticoid-induced osteoporosis: An update. Trends Endocrinol. Metab. 2006, 17, 144–149. [Google Scholar] [CrossRef]
- Yang, Y.X.; Lewis, J.D.; Epstein, S.; Metz, D.C. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006, 296, 2947–2953. [Google Scholar] [CrossRef]
- Yu, E.W.; Bauer, S.R.; Bain, P.A.; Bauer, D.C. Proton pump inhibitors and risk of fractures: A meta-analysis of 11 international studies. Am. J. Med. 2011, 124, 519–526. [Google Scholar] [CrossRef]
- Ngamruengphong, S.; Leontiadis, G.I.; Radhi, S.; Dentino, A.; Nugent, K. Proton pump inhibitors and risk of fracture: A systematic review and meta-analysis of observational studies. Am. J. Gastroenterol. 2011, 106, 1209–1218, quiz 1219. [Google Scholar] [CrossRef]
- Matuszewska, A.; Nowak, B.; Rzeszutko, M.; Zduniak, K.; Szandruk, M.; Jędrzejuk, D.; Landwójtowicz, M.; Bolanowski, M.; Pieśniewska, M.; Kwiatkowska, J.; et al. Effects of long-term administration of pantoprazole on bone mineral density in young male rats. Pharmacol. Rep. 2016, 68, 1060–1064. [Google Scholar] [CrossRef]
- Sheraly, A.R.; Lickorish, D.; Sarraf, F.; Davies, J.E. Use of gastrointestinal proton pump inhibitors to regulate osteoclast-mediated resorption of calcium phosphate cements in vivo. Curr. Drug. Deliv. 2009, 6, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Prause, M.; Seeliger, C.; Unger, M.; Rosado Balmayor, E.; van Griensven, M.; Haug, A.T. Pantoprazole decreases cell viability and function of human osteoclasts in vitro. Mediators Inflamm. 2015, 2015, 413097. [Google Scholar] [CrossRef] [PubMed]
- Van Geel, T.A.; Geusens, P.P.; Winkens, B.; Sels, J.P.; Dinant, G.J. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle mass, muscle strength and bone mineral density in postmenopausal women: A cross-sectional study. Eur. J. Endocrinol. 2009, 160, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Adams, J.E.; Coleman, R.E.; Howell, A.; Hannon, R.A.; Cuzick, J.; Mackey, J.R.; Beckmann, M.W.; Clack, G. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J. Clin. Oncol. 2008, 26, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Geiger, E.J.; Eastell, R.; Vittinghoff, E.; Li, B.H.; Ryan, D.S.; Dell, R.M.; Adams, A.L. Atypical Femur Fracture Risk versus Fragility Fracture Prevention with Bisphosphonates. N. Engl. J. Med. 2020, 20, 743–753. [Google Scholar] [CrossRef]
- Pariente, A.; Avillach, P.; Salvo, F.; Thiessard, F.; Miremont-Salamé, G.; Fourrier-Reglat, A.; Haramburu, F.; Bégaud, B.; Moore, N. Effect of competition bias in safety signal generation: Analysis of a research database of spontaneous reports in France. Drug Saf. 2012, 35, 855–864. [Google Scholar] [CrossRef]
- Avillach, P.; Salvo, F.; Thiessard, F.; Miremont-Salamé, G.; Fourrier-Reglat, A.; Haramburu, F.; Bégaud, B.; Moore, N.; Pariente, A. Pilot evaluation of an automated method to decrease false-positive signals induced by co-prescriptions in spontaneous reporting databases. Pharmacoepidemiol. Drug Saf. 2014, 23, 186–194. [Google Scholar] [CrossRef]
- MedDRA Japanese Maintenance Organization. Available online: https://www.pmrj.jp/jmo/php/indexj.php (accessed on 16 September 2022).
- Hirooka, T.; Yamada, M. Evaluation of AEs Risk Using the “Japanese Adverse Drug Event Report Database” of PMDA. In Proceedings of the SAS User General Assembly, Tokyo, Japan, 1–3 December 2012; pp. 263–270. [Google Scholar]
- Hosoya, R.; Ishii-Nozawa, R.; Kurosaki, K.; Uesawa, Y. Analysis of Factors Associated with Hiccups Using the FAERS Database. Pharmaceuticals 2021, 15, 27. [Google Scholar] [CrossRef]
- Toriumi, S.; Kobayashi, A.; Uesawa, Y. Comprehensive Study of the Risk Factors for Medication-Related Osteonecrosis of the Jaw Based on the Japanese Adverse Drug Event Report Database. Pharmaceuticals 2020, 13, 467. [Google Scholar] [CrossRef]
- Watanabe, H.; Matsushita, Y.; Watanabe, A.; Maeda, T.; Nukui, K.; Ogawa, Y.; Sawa, J.; Maeda, H. Early detection of important safety information. Jpn. J. Biomet. 2004, 25, 37–60. [Google Scholar] [CrossRef]
- Okunaka, M.; Kano, D.; Uesawa, Y. Nuclear Receptor and Stress Response Pathways Associated with Antineoplastic Agent-Induced Diarrhea. Int. J. Mol. Sci. 2022, 23, 12407. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Wang, S.J.; Tsai, C.A.; Lin, C.J. Selection of differentially expressed genes in microarray data analysis. Pharm. J. 2007, 7, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, K.; Uesawa, Y. Molecular Initiating Events Associated with Drug-Induced Liver Malignant Tumors: An Integrated Study of the FDA Adverse Event Reporting System and Toxicity Predictions. Biomolecules 2021, 11, 944. [Google Scholar] [CrossRef]
- Hosoya, R.; Uesawa, Y.; Ishii-Nozawa, R.; Kagaya, H. Analysis of factors associated with hiccups based on the Japanese Adverse Drug Event Report database. PLoS ONE 2017, 12, e0172057. [Google Scholar] [CrossRef]
- Kan, Y.; Nagai, J.; Uesawa, Y. Evaluation of antibiotic-induced taste and smell disorders using the FDA adverse event reporting system database. Sci. Rep. 2021, 11, 9625. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Ikesue, H.; Satake, R.; Inoue, M.; Yoshida, Y.; Tanaka, M.; Matsumoto, K.; Wakabayashi, W.; Oura, K.; Muroi, N.; et al. Osteonecrosis of the Jaw Caused by Denosumab in Treatment-Naïve and Pre-Treatment with Zoledronic Acid Groups: A Time-to-Onset Study Using the Japanese Adverse Drug Event Report (JADER) Database. Drugs Real World Outcomes 2022, 9, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Asada, M.; Uesawa, Y. Trends in reporting embolic and thrombotic events after COVID-19 vaccination: A retrospective, pharmacovigilance study. PLoS ONE 2022, 17, e0269268. [Google Scholar] [CrossRef]
| Patient Backgrounds | AFF (n = 1879) | Non-AFF (n = 2,032,839) | p-Value |
|---|---|---|---|
| Sex (male/female) # | 102/1729 (1831) | 1,003,206/966,165 (1,977,662) | <0.001 ### |
| Age * | 69.8 ± 13.7 (1638) | 59. 5± 21.5 (1,895,047) | <0.001 *** |
| Height (cm) * | 55.4 ± 14.5 (395) | 54.6 ± 16.4 (919,036) | 0.07 |
| Weight (kg) * | 151.6 ± 8.2 (378) | 157.3 ± 18.4 (786,612) | <0.001 *** |
| BMI * | 24.0 ± 5.6 (378) | 21.9 ± 4.5 (763,182) | <0.001 *** |
| Cancer † | 368 (1414) | 592,264 (1,520,573) | 1.0 |
| Osteoporosis † | 757 (1414) | 67,308 (1,520,573) | <0.001 ††† |
| Arthritis † | 225 (1414) | 120,158 (1,520,573) | <0.001 ††† |
| SLE † | 136 (1414) | 91,005 (1,520,573) | <0.001 ††† |
| Renal disorder † | 43 (1414) | 157,231 (1,520,573) | 1.0 |
| Drug | Drug Class | AEF (1879) | Non-AFF (2,037,648) | Reporting Ratio | ROR | 95% Confidence Interval | p-Value |
|---|---|---|---|---|---|---|---|
| Alendronic acid | BP | 665 | 3937 | 14.50% | 283.02 | 256.20–312.65 | <0.001 ** |
| Risedronic acid | BP | 344 | 2222 | 13.40% | 205.47 | 181.50–232.60 | <0.001 ** |
| Zoledronic acid | BP | 201 | 5197 | 3.70% | 46.94 | 40.46–54.47 | <0.001 ** |
| Minodronic acid | BP | 120 | 1898 | 5.90% | 73.44 | 60.73–88.80 | <0.001 ** |
| Ibandronic acid | BP | 35 | 1158 | 2.90% | 33.83 | 24.15–47.39 | <0.001 ** |
| Pamidronic acid | BP | 16 | 811 | 1.90% | 22.22 | 13.62–36.26 | <0.001 ** |
| Etidronic acid | BP | 10 | 70 | 12.50% | 162.33 | 84.75–310.92 | <0.001 ** |
| Denosumab | Anti-RANKL antibody | 210 | 3580 | 5.50% | 71.63 | 61.83–82.98 | <0.001 ** |
| Prednisolone | Corticosteroid | 119 | 39,152 | 0.30% | 3.46 | 2.88–4.17 | <0.001 ** |
| Lansoprazole | PPI | 13 | 7240 | 0.20% | 2.03 | 1.19–3.47 | 0.029 * |
| Rabeprazole | PPI | 7 | 2333 | 0.30% | 3.49 | 1.70–7.16 | 0.007 * |
| Letrozole | Aromatase inhibitor | 9 | 1399 | 0.60% | 7.39 | 3.90–14.01 | <0.001 ** |
| Anastrozole | Aromatase inhibitor | 6 | 806 | 0.70% | 8.76 | 4.04–18.98 | <0.001 ** |
| Exemestane | Aromatase inhibitor | 3 | 631 | 0.50% | 6.02 | 2.10–17.22 | 0.022 * |
| Eldecalcitol | Vitamin D | 7 | 2860 | 0.20% | 2.85 | 1.39–5.84 | 0.019 * |
| Menatetrenone | Vitamin K | 2 | 218 | 0.90% | 12.42 | 3.57–43.23 | 0.018 * |
| Teriparatide | Parathyroid hormone | 4 | 1727 | 0.23% | 2.83 | 1.11–7.14 | 0.078 |
| Teriparatide acetate | Parathyroid hormone | 0 | 1810 | 0.00% | 0.30 | 0.02–4.79 | 0.423 |
| Romosozumab | Sclerostin inhibition | 3 | 1678 | 0.18% | 2.26 | 0.79–6.46 | 0.204 |
| Elvitegravir, Cobicistat, emtricitabine, and tenofovir alafenamide fumarate | HIV drugs | 2 | 74 | 2.60% | 36.42 | 10.32–128.52 | 0.002 * |
| Risk Factor | Drug Class | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|
| Etidronic acid | BP | 1150.66 | 318.65–4155.08 | <0.001 ** |
| Alendronic acid | BP | 502.28 | 324.05–778.54 | <0.001 ** |
| Minodronic acid | BP | 390.66 | 225.92–675.53 | <0.001 ** |
| Risedronic acid | BP | 343.26 | 202.95–580.56 | <0.001 ** |
| Zoledronic acid | BP | 300.67 | 184.95–488.80 | <0.001 ** |
| Ibandronic acid | BP | 132.99 | 53.51–330.5 | <0.001 ** |
| Denosumab | Anti-RANKL antibody | 705.40 | 464.27–1071.76 | <0.001 ** |
| Prednisolone | Corticosteroid | 26.35 | 15.28–45.45 | <0.001 ** |
| Rabeprazole | PPI | 39.12 | 9.38–163.09 | 0.001 * |
| Lansoprazole | PPI | 18.28 | 5.61–59.53 | 0.001 * |
| Exemestane | Aromatase inhibitor | 58.39 | 7.93–429.77 | 0.013 * |
| Letrozole | Aromatase inhibitor | 24.86 | 3.38–182.84 | 0.034 * |
| Eldecalcitol | Vitamin D | 16.25 | 3.83–69.03 | 0.007 * |
| Menatetrenone | Vitamin K | 289.92 | 67.35–1247.98 | <0.001 ** |
| Female | N/A | 4.02 | 2.81–5.74 | <0.001 ** |
| Osteoporosis | N/A | 2.53 | 1.91–3.37 | <0.001 ** |
| SLE | N/A | 1.91 | 1.27–2.89 | 0.004 * |
| Arthritis | N/A | 1.36 | 1.01–1.82 | 0.037 * |
| Age (unit) | N/A | 0.99 | 0.99–1 | 0.137 |
| Age (range) | N/A | 0.52 | 0.24–1.15 | 0.137 |
| BMI (unit) | N/A | 1.11 | 1.09–1.13 | <0.001 ** |
| BMI (range) | N/A | 3047.24 | 775.66–11,971.25 | <0.001 ** |
| Drug | Drug Class | n | Median | Interquartile Range | Scale Parameter | Shape Parameter | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25% | 75% | α | 95% CI | β | 95% CI | Pattern | ||||
| Osteoporosis | ||||||||||
| Alendronic acid | BP | 66 | 2176 | 944 | 3084 | 2398.7 | 2017–2835.3 | 1.5 | 1.2–1.8 | wear-out failure |
| Risedronic acid | BP | 17 | 1604 | 748 | 2276 | 1919.1 | 1408.3–2564 | 1.8 | 1.2–2.5 | wear-out failure |
| Minodronic acid | BP | 17 | 1122 | 604 | 1718 | 1360.8 | 937.6–1936.5 | 1.5 | 1.0–2.1 | wear-out failure |
| Ibandronic acid | BP | 12 | 685 | 311 | 1390 | 889.8 | 519.4–1473.8 | 1.3 | 0.7–2.0 | wear-out failure |
| Denosumab | Anti-RANKL antibody | 37 | 491 | 357 | 911 | 769.5 | 585–1000 | 1.3 | 1.0–1.6 | wear-out failure |
| Cancer | ||||||||||
| Zoledronic acid | BP | 36 | 2486 | 2023 | 2904 | 2668.3 | 2377.3–2982.1 | 3.1 | 2.4–3.9 | wear-out failure |
| Denosumab | Anti-RANKL antibody | 47 | 786 | 459 | 1344 | 1053.1 | 851.3–1291.2 | 1.5 | 1.2–1.8 | wear-out failure |
| Osteoporosis | Cancer | ||||
|---|---|---|---|---|---|
| Code | SMQ | Code | SMQ | Code | SMQ |
| 20000178 | Osteoporosis/osteopenia | 20000092 | Malignant-disorder-related state | 20000203 | Prostate tumor unidentified in detail |
| Arthritis | 20000094 | Tumor marker | 20000204 | Malignant skin tumor | |
| Code | SMQ | 20000110 | Neoplasm of the oropharynx | 20000205 | Skin tumor unidentified in detail |
| 20000216 | Arthritis | 20000194 | Malignant tumor | 20000206 | Malignant uterus/salpingioma |
| Systemic lupus erythematosus | 20000195 | Tumor unidentified in detail | 20000207 | Uterus/salpingioma unidentified in detail | |
| Code | SMQ | 20000196 | Malignant biliary tract neoplasm | 20000208 | Malignant hepatophyma |
| 20000045 | Systemic lupus erythematosus | 20000197 | Biliary tract neoplasm unknown in detail | 20000209 | Hepatophyma unidentified in detail |
| Renal disorder | 20000198 | Malignant breast tumor | 20000215 | Malignant lymphoma | |
| Code | SMQ | 20000199 | Breast tumor unknown in detail | ||
| 20000003 | Acute renal failure | 20000200 | Malignant ovarian tumor | ||
| 20000181 | Renal vessel disorder | 20000201 | Ovarian tumor unidentified in detail | ||
| 20000213 | Chronic kidney disease | 20000202 | Malignant prostate tumor | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toriumi, S.; Mimori, R.; Sakamoto, H.; Sueki, H.; Yamamoto, M.; Uesawa, Y. Examination of Risk Factors and Expression Patterns of Atypical Femoral Fractures Using the Japanese Adverse Drug Event Report Database: A Retrospective Pharmacovigilance Study. Pharmaceuticals 2023, 16, 626. https://doi.org/10.3390/ph16040626
Toriumi S, Mimori R, Sakamoto H, Sueki H, Yamamoto M, Uesawa Y. Examination of Risk Factors and Expression Patterns of Atypical Femoral Fractures Using the Japanese Adverse Drug Event Report Database: A Retrospective Pharmacovigilance Study. Pharmaceuticals. 2023; 16(4):626. https://doi.org/10.3390/ph16040626
Chicago/Turabian StyleToriumi, Shinya, Ryuji Mimori, Haruhiko Sakamoto, Hitoshi Sueki, Munehiro Yamamoto, and Yoshihiro Uesawa. 2023. "Examination of Risk Factors and Expression Patterns of Atypical Femoral Fractures Using the Japanese Adverse Drug Event Report Database: A Retrospective Pharmacovigilance Study" Pharmaceuticals 16, no. 4: 626. https://doi.org/10.3390/ph16040626
APA StyleToriumi, S., Mimori, R., Sakamoto, H., Sueki, H., Yamamoto, M., & Uesawa, Y. (2023). Examination of Risk Factors and Expression Patterns of Atypical Femoral Fractures Using the Japanese Adverse Drug Event Report Database: A Retrospective Pharmacovigilance Study. Pharmaceuticals, 16(4), 626. https://doi.org/10.3390/ph16040626







