Therapeutic Investigation of Palm Oil Mill Effluent-Derived Beta-Carotene in Streptozotocin-Induced Diabetic Retinopathy via the Regulation of Blood–Retina Barrier Functions
Abstract
:1. Introduction
2. Results
2.1. Effect of PBC in the OMR Test as Blood–Retina Barrier Function-Associated Visual Acuity Actions
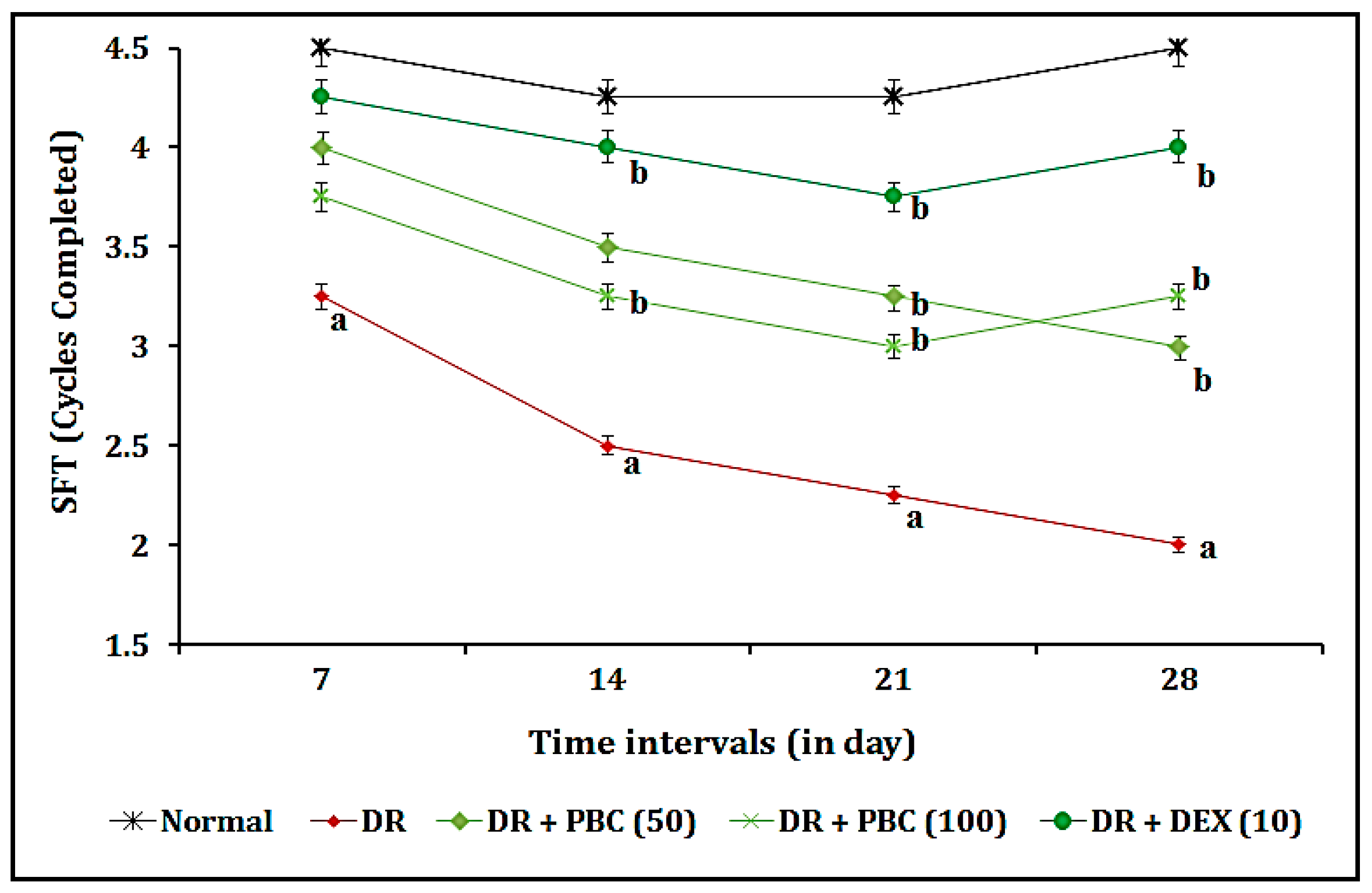
2.2. Effect of PBC in VCFT on Blood–Retina Barrier Functions
2.3. Effect of PBC on Biomarker Estimations
2.4. Effect of PBC on Blood Glucose Level Changes
2.5. Effect of PBC on Retinal Tissue Biomarker Changes
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals Used
4.3. Estimation of Blood Glucose Level
4.4. Experimental Design
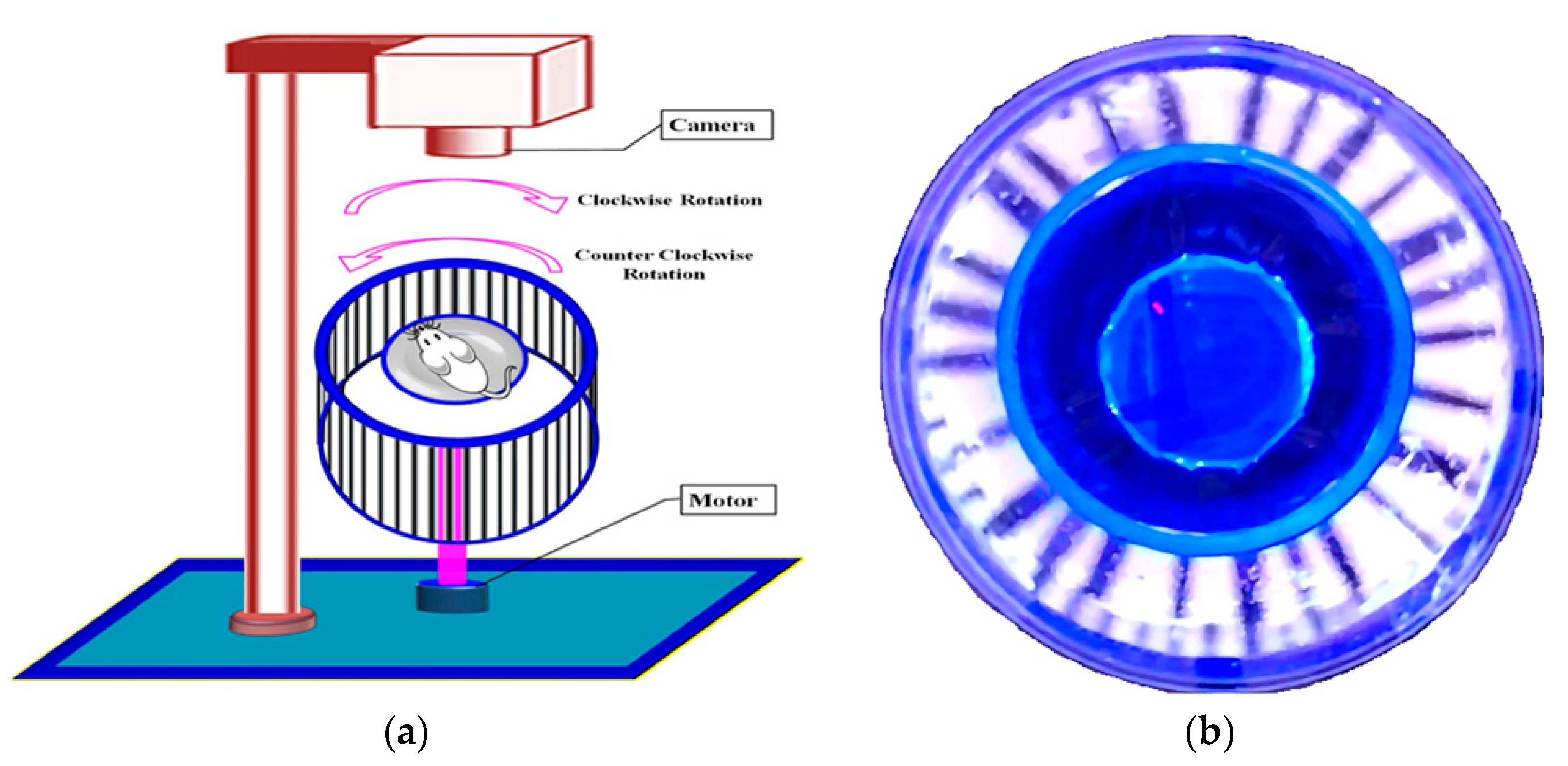
4.5. Assessment of OMR Using an Optokinetic Device
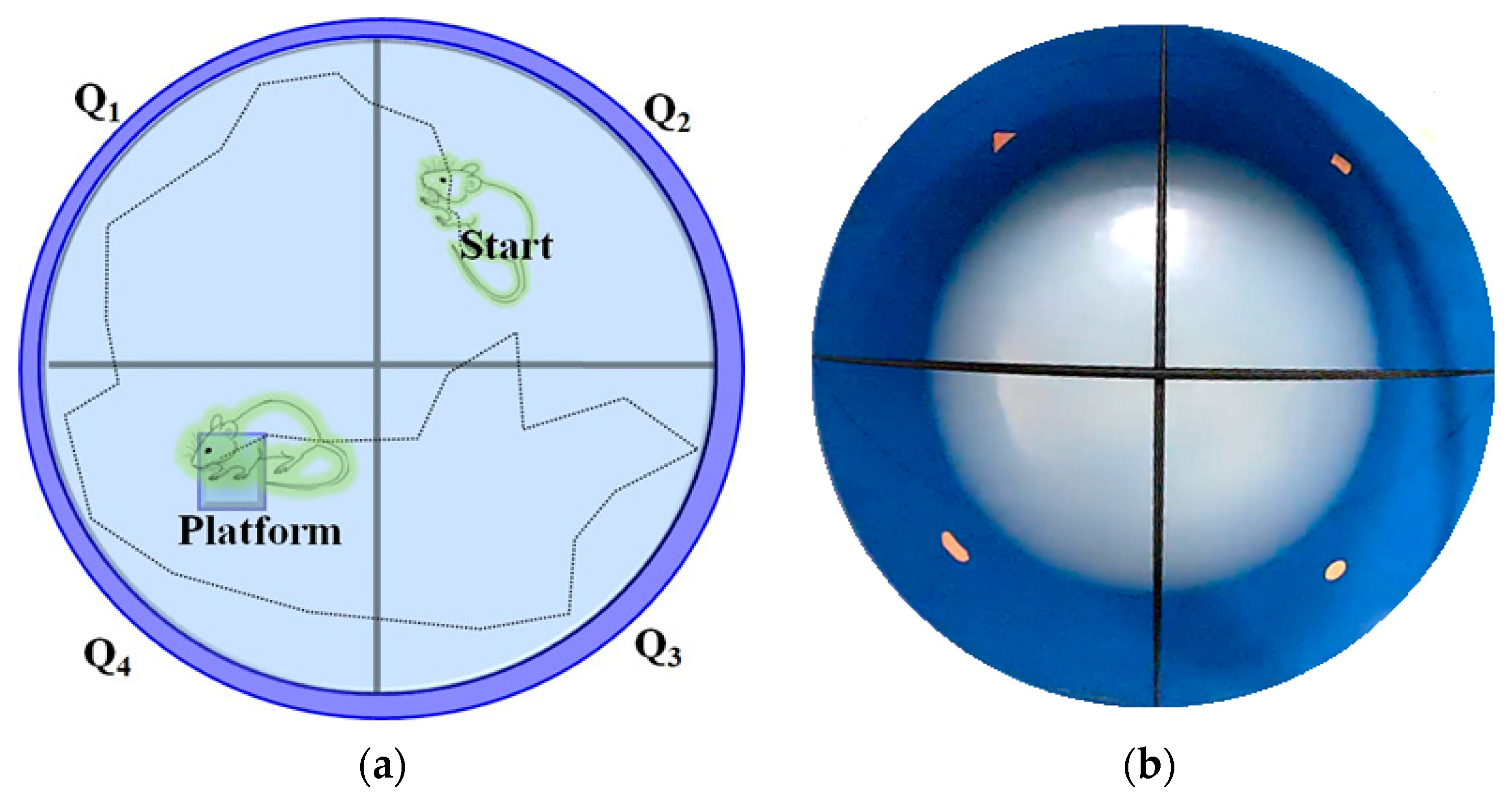
4.6. Assessment of Visual-Cue Function Test (VCFT) by MORRIS Water-Maze Device
4.7. Estimations of Diabetic Retinal Tissue (Blood–Retina Barrier) Marker Changes
4.7.1. GSH Estimation as an Indicator of Non-Enzymatic Oxidative Stress
4.7.2. TBARS Estimation as an Indicator of Lipid Peroxidation
4.7.3. Catalase Estimation as an Indicator of Enzymatic Oxidative Stress
4.7.4. Estimation of Retinal Tissue Proteins
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sripriya, S.; Raman, R.; Soumittra, N.; Pandian, A.J. Current Research Perspectives in Understanding Diabetic Retinopathy. Adv. Vis. Res. Vol. I 2017, 483, 259–274. [Google Scholar]
- Zhou, M.; Wu, L.; Hu, Q.; Wang, C.; Ye, J.; Chen, T.; Wan, P. Visual Impairments Are Associated With Retinal Microvascular Density in Patients With Parkinson’s Disease. Front. Neurosci. 2021, 15, 718820. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Tay, W.T.; Ikram, M.K.; Ong, Y.T.; De Silva, D.A.; Chow, K.Y.; Wong, T.Y. Retinal Microvascular Changes and Risk of Stroke: The Singapore Malay Eye Study. Stroke 2013, 44, 2402–2408. [Google Scholar] [CrossRef]
- Liberski, S.; Kaluzny, B.J.; Kocięcki, J. Methanol-Induced Optic Neuropathy: A Still-Present Problem. Arch. Toxicol. 2022, 96, 431–451. [Google Scholar] [CrossRef] [PubMed]
- Surek, C.C.; Said, S.A.; Perry, J.D.; Zins, J.E. Retrobulbar Injection for Hyaluronic Acid Gel Filler-Induced Blindness: A Review of Efficacy and Technique. Aesthetic Plast. Surg. 2019, 43, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Geamănu Pancă, A.; Popa-Cherecheanu, A.; Marinescu, B.; Geamănu, C.D.; Voinea, L.M. Retinal Toxicity Associated with Chronic Exposure to Hydroxychloroquine and Its Ocular Screening. Review. J. Med. Life 2014, 7, 322–326. [Google Scholar] [PubMed]
- Sharma, P.; Sharma, R. Toxic Optic Neuropathy. Indian J. Ophthalmol. 2011, 59, 137–141. [Google Scholar] [CrossRef]
- Lloyd, M.J.; Fraunfelder, F.W. Drug-Induced Optic Neuropathies. Drugs Today 2007, 43, 827–836. [Google Scholar] [CrossRef]
- Olivares, A.M.; Althoff, K.; Chen, G.F.; Wu, S.; Morrisson, M.A.; DeAngelis, M.M.; Haider, N. Animal Models of Diabetic Retinopathy. Curr. Diabetes Rep. 2017, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, R.; Hoyer, S. Local Action of the Diabetogenic Drug, Streptozotocin, on Glucose and Energy Metabolism in Rat Brain Cortex. Neurosci. Lett. 1991, 128, 199–202. [Google Scholar] [CrossRef]
- Grieb, P. Intracerebroventricular Streptozotocin Injections as a Model of Alzheimer’s Disease: In Search of a Relevant Mechanism. Mol. Neurobiol. 2016, 53, 1741–1752. [Google Scholar] [CrossRef]
- Al-Awar, A.; Kupai, K.; Veszelka, M.; Szűcs, G.; Attieh, Z.; Murlasits, Z.; Török, S.; Pósa, A.; Varga, C. Experimental Diabetes Mellitus in Different Animal Models. J. Diabetes Res. 2016, 2016, 9051426. [Google Scholar] [CrossRef] [PubMed]
- Hafez, S.; Coucha, M.; Bruno, A.; Fagan, S.C.; Ergul, A. Hyperglycemia, Acute Ischemic Stroke, and Thrombolytic Therapy. Transl. Stroke Res. 2014, 5, 442–453. [Google Scholar] [CrossRef]
- Adki, K.M.; Kulkarni, Y.A. Potential Biomarkers in Diabetic Retinopathy. Curr. Diabetes Rev. 2020, 16, 971–983. [Google Scholar] [PubMed]
- Lelyte, I.; Ahmed, Z.; Kaja, S.; Kalesnykas, G. Structure-Function Relationships in the Rodent Streptozotocin-Induced Model for Diabetic Retinopathy: A Systematic Review. J. Ocul. Pharmacol. Ther. 2022, 38, 271–286. [Google Scholar] [CrossRef]
- Cameron, N.E.; Cotter, M.A. Neurovascular Dysfunction in Diabetic Rats. Potential Contribution of Autoxidation and Free Radicals Examined Using Transition Metal Chelating Agents. J. Clin. Investig. 1995, 96, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Gui, F.; You, Z.; Fu, S.; Wu, H.; Zhang, Y. Endothelial Dysfunction in Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.G.; Varatharajan, R.; Muthuraman, A. The Attenuating Effect of Beta-Carotene on Streptozotocin Induced Diabetic Vascular Dementia Symptoms in Rats. Molecules 2022, 27, 4293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wu, X.; Pinos, I.; Abraham, B.M.; Barrett, T.J.; von Lintig, J.; Fisher, E.A.; Amengual, J. β-Carotene Conversion to Vitamin A Delays Atherosclerosis Progression by Decreasing Hepatic Lipid Secretion in Mice. J. Lipid Res. 2020, 61, 1491–1503. [Google Scholar] [CrossRef]
- Miller, A.P.; Coronel, J.; Amengual, J. The Role of β-Carotene and Vitamin A in Atherogenesis: Evidences from Preclinical and Clinical Studies. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158635. [Google Scholar] [CrossRef]
- Teikari, J.M.; Laatikainen, L.; Rapola, J.M.; Virtamo, J.; Haukka, J.; Liesto, K.; Taylor, P.; Heinonen, O.P. Retinal Vascular Changes Following Supplementation with Alpha-Tocopherol or Beta-Carotene. Acta Ophthalmol. Scand. 1998, 76, 68–73. [Google Scholar] [CrossRef]
- Coronel, J.; Pinos, I.; Amengual, J. β-Carotene in Obesity Research: Technical Considerations and Current Status of the Field. Nutrients 2019, 11, 842. [Google Scholar] [CrossRef]
- de Vries, J.J.; Chang, A.B.; Bonifant, C.M.; Shevill, E.; Marchant, J.M. Vitamin A and Beta (β)-Carotene Supplementation for Cystic Fibrosis. Cochrane Database Syst. Rev. 2018, 8, CD006751. [Google Scholar] [CrossRef] [PubMed]
- Hira, S.; Saleem, U.; Anwar, F.; Sohail, M.F.; Raza, Z.; Ahmad, B. β-Carotene: A Natural Compound Improves Cognitive Impairment and Oxidative Stress in a Mouse Model of Streptozotocin-Induced Alzheimer’s Disease. Biomolecules 2019, 9, 441. [Google Scholar] [CrossRef]
- Paramakrishnan, N.; Chavan, L.; Lim, K.G.; Paramaswaran, Y.; Muthuraman, A. Reversal of Neuralgia Effect of Beta Carotene in Streptozotocin-Associated Diabetic Neuropathic Pain in Female Zebrafish via Matrix Metalloprotease-13 Inhibition. Pharmaceuticals 2023, 16, 157. [Google Scholar] [CrossRef]
- Levy, Y.; Zaltzberg, H.; Ben-Amotz, A.; Kanter, Y.; Aviram, M. β-Carotene Affects Antioxidant Status in Non-Insulin-Dependent Diabetes Mellitus. Pathophysiology 1999, 6, 157–161. [Google Scholar] [CrossRef]
- Mushtaq, M.; Anwar, F. A Centum of Valuable Plant Bioactives; Academic Press: Cambridge, MA, USA, 2021; ISBN 0128229241. [Google Scholar]
- Sangkharak, K.; Pichid, N.; Tewan, Y.; Kingman, P. Separation of Carotenes and Vitamin E from Palm Oil Mill Effluent Using Silica from Agricultural Waste as an Adsorbent. Walailak J. Sci. Technol. (WJST) 2016, 13, 939–947. [Google Scholar]
- Kim, I.-J.; Ko, K.-C.; Kim, C.-S.; Chung, W.-I. Isolation and Characterization of cDNAs Encoding β-Carotene Hydroxylase in Citrus. Plant Sci. 2001, 161, 1005–1010. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Chan, C.Y.; Abd Shukor, S.R.; Mashitah, M.D.; Sunarti, A.R. Isolation of Carotenes from Palm Oil Mill Effluent and Its Use as a Source of Carotenes. Desalination Water Treat. 2009, 7, 251–256. [Google Scholar] [CrossRef]
- Tsuchihashi, H.; Kigoshi, M.; Iwatsuki, M.; Niki, E. Action of β-Carotene as an Antioxidant against Lipid Peroxidation. Arch. Biochem. Biophys. 1995, 323, 137–147. [Google Scholar] [CrossRef]
- Silveira, E.R.; Moreno, F.S. Natural Retinoids and β-Carotene: From Food to Their Actions on Gene Expression. J. Nutr. Biochem. 1998, 9, 446–456. [Google Scholar] [CrossRef]
- McAllister, I.L.; Vijayasekaran, S.; Zhang, D.; McLenachan, S.; Chen, F.K.; Yu, D.-Y. Neuronal Degeneration and Associated Alterations in Cytokine and Protein in an Experimental Branch Retinal Venous Occlusion Model. Exp. Eye Res. 2018, 174, 133–146. [Google Scholar] [CrossRef]
- Kovacova, A.; Shotliff, K. Eye Problems in People with Diabetes: More than Just Diabetic Retinopathy. Pract. Diabetes 2022, 39, 34–39a. [Google Scholar] [CrossRef]
- Li, J.; Yan, M.; Zhang, Y.; Xie, M.; Yan, L.; Chen, J. Serum Neuron-Specific Enolase Is Elevated as a Novel Indicator of Diabetic Retinopathy Including Macular Oedema. Diabet. Med. A J. Br. Diabet. Assoc. 2015, 32, 102–107. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Drel, V.R.; Kumagai, A.K.; Szábo, C.; Pacher, P.; Stevens, M.J. Early Diabetes-Induced Biochemical Changes in the Retina: Comparison of Rat and Mouse Models. Diabetologia 2006, 49, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- Kermorvant-Duchemin, E.; Pinel, A.C.; Lavalette, S.; Lenne, D.; Raoul, W.; Calippe, B.; Behar-Cohen, F.; Sahel, J.-A.; Guillonneau, X.; Sennlaub, F. Neonatal Hyperglycemia Inhibits Angiogenesis and Induces Inflammation and Neuronal Degeneration in the Retina. PLoS ONE 2013, 8, e79545. [Google Scholar] [CrossRef] [PubMed]
- Lutchmansingh, F.K.; Hsu, J.W.; Bennett, F.I.; Badaloo, A.V.; McFarlane-Anderson, N.; Gordon-Strachan, G.M.; Wright-Pascoe, R.A.; Jahoor, F.; Boyne, M.S. Glutathione Metabolism in Type 2 Diabetes and Its Relationship with Microvascular Complications and Glycemia. PLoS ONE 2018, 13, e0198626. [Google Scholar] [CrossRef]
- Hakki Kalkan, I.; Suher, M. The Relationship between the Level of Glutathione, Impairment of Glucose Metabolism and Complications of Diabetes Mellitus. Pak. J. Med. Sci. 2013, 29, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Alışık, M.; Işik, M.U. The Relationship between Choroidal Thickness and Intracellular Oxidised-Reduced Glutathione and Extracellular Thiol-Disulfide Homeostasis at Different Stages of Diabetic Retinopathy. Curr. Eye Res. 2021, 46, 367–372. [Google Scholar] [CrossRef]
- Al-Shabrawey, M.; Smith, S. Prediction of Diabetic Retinopathy: Role of Oxidative Stress and Relevance of Apoptotic Biomarkers. EPMA J. 2010, 1, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Desmarchelier, C.; El, S.N.; Keijer, J.; van Schothorst, E.; Rühl, R.; Borel, P. β-Carotene in the Human Body: Metabolic Bioactivation Pathways–from Digestion to Tissue Distribution and Excretion. Proc. Nutr. Soc. 2019, 78, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Palace, V.P.; Khaper, N.; Qin, Q.; Singal, P.K. Antioxidant Potentials of Vitamin A and Carotenoids and Their Relevance to Heart Disease. Free. Radic. Biol. Med. 1999, 26, 746–761. [Google Scholar] [CrossRef]
- Tinoush, B.; Shirdel, I.; Wink, M. Phytochemicals: Potential Lead Molecules for MDR Reversal. Front. Pharmacol. 2020, 11, 832. [Google Scholar] [CrossRef]
- Umigai, N.; Tanaka, J.; Tsuruma, K.; Shimazawa, M.; Hara, H. Crocetin, a Carotenoid Derivative, Inhibits VEGF-Induced Angiogenesis via Suppression of p38 Phosphorylation. Curr. Neurovasc. Res. 2012, 9, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Njie-Mbye, Y.F.; Kulkarni-Chitnis, M.; Opere, C.A.; Barrett, A.; Ohia, S.E. Lipid Peroxidation: Pathophysiological and Pharmacological Implications in the Eye. Front. Physiol. 2013, 4, 366. [Google Scholar] [CrossRef] [PubMed]
- Augustine, J.; Troendle, E.P.; Barabas, P.; McAleese, C.A.; Friedel, T.; Stitt, A.W.; Curtis, T.M. The Role of Lipoxidation in the Pathogenesis of Diabetic Retinopathy. Front. Endocrinol. 2021, 11, 621938. [Google Scholar] [CrossRef]
- Kwong-Han, K.; Zunaina, E.; Hanizasurana, H.; Che-Badariah, A.A.; Che-Maraina, C.H. Comparison of Catalase, Glutathione Peroxidase and Malondialdehyde Levels in Tears among Diabetic Patients with and without Diabetic Retinopathy. J. Diabetes Metab. Disord. 2022, 21, 681–688. [Google Scholar] [CrossRef]
- Álvarez-Barrios, A.; Álvarez, L.; García, M.; Artime, E.; Pereiro, R.; González-Iglesias, H. Antioxidant Defenses in the Human Eye: A Focus on Metallothioneins. Antioxidants 2021, 10, 89. [Google Scholar] [CrossRef]
- Giordano, C.R.; Roberts, R.; Krentz, K.A.; Bissig, D.; Talreja, D.; Kumar, A.; Terlecky, S.R.; Berkowitz, B.A. Catalase Therapy Corrects Oxidative Stress-Induced Pathophysiology in Incipient Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3095–3102. [Google Scholar] [CrossRef]
- Lutton, E.M.; Razmpour, R.; Andrews, A.M.; Cannella, L.A.; Son, Y.-J.; Shuvaev, V.V.; Muzykantov, V.R.; Ramirez, S.H. Acute Administration of Catalase Targeted to ICAM-1 Attenuates Neuropathology in Experimental Traumatic Brain Injury. Sci. Rep. 2017, 7, 3846. [Google Scholar] [CrossRef]
- Joseph, A.; Nyambura, C.W.; Bondurant, D.; Corry, K.; Beebout, D.; Wood, T.R.; Pfaendtner, J.; Nance, E. Formulation and Efficacy of Catalase-Loaded Nanoparticles for the Treatment of Neonatal Hypoxic-Ischemic Encephalopathy. Pharmaceutics 2021, 13, 1131. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, P.G.; Ferrington, D.A.; Kannan, R. Glutathione Metabolism and the Novel Role of Mitochondrial GSH in Retinal Degeneration. Antioxidants 2021, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, M.; Ustundag, Y.; Akdağ, A.; Neselioglu, S.; Erel, O. The Thiol-Disulfide Homeostasis and Coenzyme Q10 in Conjunction with Vitamin E Effect on Retinopathy Prematurity. Open Ophthalmol. J. 2019, 13, 23–28. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, Q.; Diao, L. Geraniin Ameliorates Streptozotocin-Induced Diabetic Retinopathy in Rats via Modulating Retinal Inflammation and Oxidative Stress. Arab. J. Chem. 2023, 16, 104396. [Google Scholar] [CrossRef]
- Ola, M.S.; Alanazi, A.Z.; Malik, A.; Malik, A.; Ahmed, M.; Al-Rejaie, S.S.; Alhomida, A.S. Loranthus Regularis Ameliorates Neurodegenerative Factors in the Diabetic Rat Retina. Appl. Sci. 2022, 12, 2875. [Google Scholar] [CrossRef]
- Sadikan, M.Z.; Nasir, N.A.A.; Iezhitsa, I.; Agarwal, R. Antioxidant and Anti-Apoptotic Effects of Tocotrienol-Rich Fraction against Streptozotocin-Induced Diabetic Retinopathy in Rats. Biomed. Pharmacother. 2022, 153, 113533. [Google Scholar] [CrossRef] [PubMed]
- Kini, D.; Rekha, D.; Arunkumar, N.; Shetty, D.; Bhoja, S.; Anupama, N.; Bhagyalakshmi, K.; Gokul, M.M. Potential Protective Role of Beta Carotene on Cadmium Induced Brain and Kidney Damage. Indian J. Public Health Res. Dev. 2019, 10, 509–512. [Google Scholar] [CrossRef]
- Akkara, P.J.; Sabina, E.P. A Biochemical Approach to the Anti-Inflammatory, Antioxidant and Antiapoptotic Potential of Beta-Carotene as a Protective Agent against Bromobenzene-Induced Hepatotoxicity in Female Wistar Albino Rats. J. Appl. Biomed. 2020, 18, 87–95. [Google Scholar] [CrossRef]
- Rocha, F.; Yumi Sugahara, L.; Leimann, F.V.; de Oliveira, S.M.; da Silva Brum, E.; Calhelha, R.C.; Barreiro, M.F.; Ferreira, I.C.F.R.; Porto Ineu, R.; Gonçalves, O.H. Nanodispersions of Beta-Carotene: Effects on Antioxidant Enzymes and Cytotoxic Properties. Food Funct. 2018, 9, 3698–3706. [Google Scholar] [CrossRef]
- Liu, S.; Ju, Y.; Gu, P. Experiment-Based Interventions to Diabetic Retinopathy: Present and Advances. Int. J. Mol. Sci. 2022, 23, 7005. [Google Scholar] [CrossRef]
- Ramsey, L.B.; Pounds, S.; Cheng, C.; Cao, X.; Yang, W.; Smith, C.; Karol, S.E.; Liu, C.; Panetta, J.C.; Inaba, H.; et al. Genetics of Pleiotropic Effects of Dexamethasone. Pharm. Genom. 2017, 27, 294–302. [Google Scholar] [CrossRef]
- Whitcup, S.M.; Cidlowski, J.A.; Csaky, K.G.; Ambati, J. Pharmacology of Corticosteroids for Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Y.; Gao, L.; Li, X.; Li, M.; Guo, J. Dexamethasone Inhibits Leukocyte Accumulation and Vascular Permeability in Retina of Streptozotocin-Induced Diabetic Rats via Reducing Vascular Endothelial Growth Factor and Intercellular Adhesion Molecule-1 Expression. Biol. Pharm. Bull. 2008, 31, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hu, R.; Mao, S.; Xu, M.; Shi, W.; Chen, L. Dexamethasone Attenuates Airway Inflammation in Mouse Models with Allergic Asthma. Basic Clin. Med. 2023, 43, 402. [Google Scholar]
- Alhaddad, A.; Radwan, A.; Mohamed, N.A.; Mehanna, E.T.; Mostafa, Y.M.; El-Sayed, N.M.; Fattah, S.A. Rosiglitazone Mitigates Dexamethasone-Induced Depression in Mice via Modulating Brain Glucose Metabolism and AMPK/mTOR Signaling Pathway. Biomedicines 2023, 11, 860. [Google Scholar] [CrossRef]
- Alawadhi, M.; Kilarkaje, N.; Mouihate, A.; Al-Bader, M.D. Role of Progesterone on Dexamethasone-Induced Alterations in Placental Vascularization and Progesterone Receptors in Rats. Biol. Reprod. 2023, 108, 133–149. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.S.; Jeong, J.H.; Yoon, J.S.; Hwang, S.H.; Kim, S.-Y.; Yeon, S.H.; Ryu, J.-H. Extract of Alnus Japonica Prevents Dexamethasone-Induced Muscle Atrophy in Mice. J. Funct. Foods 2023, 101, 105419. [Google Scholar] [CrossRef]
- Kanda, A.; Hirose, I.; Yoshida, S.; Murata, M.; Ishida, S. Dexamethasone Attenuates Hypoxia-and Diabetes-Induced Retinal Galectin-1 Expression via Reducing Hypoxia-Inducible Factor-1α Protein in Vitro and in Vivo. Investig. Ophthalmol. Vis. Sci. 2020, 61, 747. [Google Scholar]
- Bhandari, S.; Gabrielle, P.-H.; Nguyen, V.; Daien, V.; Viola, F.; Bougamha, W.; Young, S.; Romero-Nuñez, B.; Figueras-Roca, M.; Zarranz-Ventura, J. Dexamethasone Implant for Diabetic Macular Oedema: 1-Year Treatment Outcomes from the Fight Retinal Blindness! Registry. Ophthalmol. Ther. 2022, 11, 797–810. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, T.; Deng, T.; Wang, Z. Protective Effects of Astaxanthin against Diabetic Retinal Vessels and pro-Inflammatory Cytokine Synthesis. Int. J. Clin. Exp. Med. 2019, 12, 4725–4734. [Google Scholar]
- Huang, J.; Weinstein, S.J.; Yu, K.; Männistö, S.; Albanes, D. Serum Beta Carotene and Overall and Cause-Specific Mortality: A Prospective Cohort Study. Circ. Res. 2018, 123, 1339–1349. [Google Scholar] [CrossRef]
- Takitani, K.; Matsumura, H.; Nakakura, H.; Ashida, A.; Tamai, H. Expression of β-Carotene 15, 15′-monooxygenase Gene and Retinol Status in Rats with Puromycin Aminonucleoside-Induced Nephrosis. Biofactors 2008, 33, 293–300. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Sithamparam, K.; Zulkali, M.M.D.; Ismail, S. Extraction of Residue Oil from Palm Oil Mill Effluent (POME) Using Organic Solvent. ASEAN J. Sci. Technol. Dev. 2003, 20, 385–394. [Google Scholar] [CrossRef]
- Siew, W.L.; Tang, T.S.; Tan, Y.A. PORIM Test Methods; Palm Oil Research Institute of Malaysia: Selangor, Malaysia, 1995. [Google Scholar]
- Yuan, F.; Liu, Y.; Liu, F.; Peng, Y.; Tian, J. Intraperitoneal Administration of the Globular Adiponectin Gene Ameliorates Diabetic Nephropathy in Wistar Rats. Mol. Med. Rep. 2014, 9, 2293–2300. [Google Scholar] [CrossRef]
- Villena Gonzales, W.; Mobashsher, A.T.; Abbosh, A. The Progress of Glucose monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef]
- Prusky, G.T.; Douglas, R.M.; Nelson, L.; Shabanpoor, A.; Sutherland, R.J. Visual Memory Task for Rats Reveals an Essential Role for Hippocampus and Perirhinal Cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 5064–5068. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, F.; Sajgo, S.; Kretschmer, V.; Badea, T.C. A System to Measure the Optokinetic and Optomotor Response in Mice. J. Neurosci. Methods 2015, 256, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.G.M. Spatial Localization Does Not Require the Presence of Local Cues. Learn. Motiv. 1981, 12, 239–260. [Google Scholar] [CrossRef]
- Prusky, G.T.; West, P.W.R.; Douglas, R.M. Behavioral Assessment of Visual Acuity in Mice and Rats. Vis. Res. 2000, 40, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, C.M.; Glen, E.F. Cage Colour Preferences and Effects of Home Cage Colour on Anxiety in Laboratory Mice. Anim. Behav. 2003, 66, 1085–1092. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Hadwan, M.H. Simple Spectrophotometric Assay for Measuring Catalase Activity in Biological Tissues. BMC Biochem. 2018, 19, 7. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]


| Groups | Day 0 (mmol/L) | Day 3 (mmol/L) | Day 8 (mmol/L) | Day 28 (mmol/L) |
|---|---|---|---|---|
| Normal | 6.1 ± 0.5 | 6.4 ± 0.6 | 6.3 ± 0.6 | 6.5 ± 0.5 |
| DR | 6.2 ± 0.7 | 24.7 ± 1.4 a | 25.3 ± 1.3 a | 29.7 ± 1.1 a |
| DR + PBC (50) | 6.3 ± 0.5 | 25.8 ± 1.1 a | 26.3 ± 1.0 a | 11.2 ± 1.4 b |
| DR + PBC (100) | 6.4 ± 0.4 | 24.2 ± 1.2 a | 24.2 ± 1.5 a | 9.4 ± 1.2 b |
| DR + DEX (10) | 5.9 ± 0.3 | 23.1 ± 1.8 a | 25.4 ± 1.4 a | 8.2 ± 1.3 b |
| Groups | GSH (µmol/mg of Protein) | TBARS (nmol/mg of Protein) | Catalase (U/mg of Protein) |
|---|---|---|---|
| Normal | 24.8 ± 1.5 | 1.3 ± 0.05 | 15.2 ± 1.4 |
| DR | 7.94 ± 1.4 a | 3.4 ± 0.08 a | 2.9 ± 1.3 a |
| DR + PBC (50) | 13.3 ± 1.2 b | 2.3 ± 0.03 b | 5.2 ± 0.8 b |
| DR + PBC (100) | 10.5 ± 1.4 b | 1.9 ± 0.04 b | 4.8 ± 0.3 b |
| DR + DEX (10) | 8.6 ± 1.7 b | 1.6 ± 0.07 b | 4.4 ± 1.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paramaswaran, Y.; Subramanian, A.; Paramakrishnan, N.; Ramesh, M.; Muthuraman, A. Therapeutic Investigation of Palm Oil Mill Effluent-Derived Beta-Carotene in Streptozotocin-Induced Diabetic Retinopathy via the Regulation of Blood–Retina Barrier Functions. Pharmaceuticals 2023, 16, 647. https://doi.org/10.3390/ph16050647
Paramaswaran Y, Subramanian A, Paramakrishnan N, Ramesh M, Muthuraman A. Therapeutic Investigation of Palm Oil Mill Effluent-Derived Beta-Carotene in Streptozotocin-Induced Diabetic Retinopathy via the Regulation of Blood–Retina Barrier Functions. Pharmaceuticals. 2023; 16(5):647. https://doi.org/10.3390/ph16050647
Chicago/Turabian StyleParamaswaran, Yamunna, Aswinprakash Subramanian, Nallupillai Paramakrishnan, Muthusamy Ramesh, and Arunachalam Muthuraman. 2023. "Therapeutic Investigation of Palm Oil Mill Effluent-Derived Beta-Carotene in Streptozotocin-Induced Diabetic Retinopathy via the Regulation of Blood–Retina Barrier Functions" Pharmaceuticals 16, no. 5: 647. https://doi.org/10.3390/ph16050647
APA StyleParamaswaran, Y., Subramanian, A., Paramakrishnan, N., Ramesh, M., & Muthuraman, A. (2023). Therapeutic Investigation of Palm Oil Mill Effluent-Derived Beta-Carotene in Streptozotocin-Induced Diabetic Retinopathy via the Regulation of Blood–Retina Barrier Functions. Pharmaceuticals, 16(5), 647. https://doi.org/10.3390/ph16050647







