The Relationship between Character Traits and In Vivo Cerebral Serotonin Transporter Availability in Healthy Subjects: A High-Resolution PET Study with C-11 DASB
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Assessment of Character Traits
4.3. Scan Protocol
4.4. Image Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cloninger, C.R.; Svrakic, D.M.; Przybeck, T.R. A Psychobiological Model of Temperament and Character. Arch. Gen. Psychiat. 1993, 50, 975–990. [Google Scholar] [CrossRef]
- Cloninger, C.R. The science of well-being: An integrated approach to mental health and its disorders. World Psychiatry 2006, 5, 71–76. [Google Scholar]
- Cloninger, C.R.; Zohar, A.H. Personality and the perception of health and happiness. J. Affect Disord. 2011, 128, 24–32. [Google Scholar] [CrossRef]
- Moreira, P.A.S.; Inman, R.A.; Cloninger, C.R. Disentangling the personality pathways to well-being. Sci. Rep. 2023, 13, 3353. [Google Scholar] [CrossRef]
- Komasi, S.; Rezaei, F.; Hemmati, A.; Rahmani, K.; Amianto, F.; Miettunen, J. Comprehensive meta-analysis of associations between temperament and character traits in Cloninger’s psychobiological theory and mental disorders. J. Int. Med. Res. 2022, 50, 03000605211070766. [Google Scholar] [CrossRef]
- Mitchell, R.L.C.; Phillips, L.H. The psychological, neurochemical and functional neuroanatomical mediators of the effects of positive and negative mood on executive functions. Neuropsychologia 2007, 45, 617–629. [Google Scholar] [CrossRef]
- Blum, K.; Chen, A.L.C.; Chen, T.J.H.; Bowirrat, A.; Downs, B.W.; Waite, R.L.; Reinking, J.; Kerner, M.; Braverman, D.; DiNubile, N.; et al. Genes and Happiness. Gene Ther. Mol. Biol. 2009, 13, 91–129. [Google Scholar]
- De Neve, J.E. Functional polymorphism (5-HTTLPR) in the serotonin transporter gene is associated with subjective well-being: Evidence from a US nationally representative sample. J. Hum. Genet. 2011, 56, 456–459. [Google Scholar] [CrossRef]
- De Neve, J.E.; Christakis, N.A.; Fowler, J.H.; Frey, B.S. Genes, Economics, and Happiness. J. Neurosci. Psychol. E 2012, 5, 193–211. [Google Scholar] [CrossRef]
- Rotenberg, V.S. “Genes of happiness and well being” in the context of search activity concept. Act. Nerv. Super. 2013, 55, 1–14. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Marazziti, D. Understanding the role of serotonin in psychiatric diseases. F1000Reserach 2017, 6, 180. [Google Scholar] [CrossRef]
- Comings, D.E.; Gade-Andavolu, R.; Gonzalez, N.; Wu, S.; Muhleman, D.; Blake, H.; Mann, M.B.; Dietz, G.; Saucier, G.; MacMurray, J.P. A multivariate analysis of 59 candidate genes in personality traits: The temperament and character inventory. Clin. Genet. 2000, 58, 375–385. [Google Scholar] [CrossRef]
- Gonda, X.; Fountoulakis, K.N.; Juhasz, G.; Rihmer, Z.; Lazary, J.; Laszik, A.; Akiskal, H.S.; Bagdy, G. Association of the s allele of the 5-HTTLPR with neuroticism-related traits and temperaments in a psychiatrically healthy population. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 106–113. [Google Scholar] [CrossRef]
- Alfimova, M.V.; Monakhov, M.V.; Golimbet, V.E.; Korovaitseva, G.I.; Lyashenko, G.L. Analysis of Associations between 5-HTT, 5-HTR2A, and GABRA6 Gene Polymorphisms and Health-Associated Personality Traits. Bull. Exp. Biol. Med. 2010, 149, 434–436. [Google Scholar] [CrossRef]
- Calati, R.; Signorelli, M.S.; Gressier, F.; Bianchini, O.; Porcelli, S.; Comings, D.E.; De Girolamo, G.; Aguglia, E.; MacMurray, J.; Serretti, A. Modulation of a number of genes on personality traits in a sample of healthy subjects. Neurosci. Lett. 2014, 566, 320–325. [Google Scholar] [CrossRef]
- Tuominen, L.; Salo, J.; Hirvonen, J.; Nagren, K.; Laine, P.; Melartin, T.; Isometsa, E.; Viikari, J.; Cloninger, C.R.; Raitakari, O.; et al. Temperament, character and serotonin activity in the human brain: A positron emission tomography study based on a general population cohort. Psychol. Med. 2013, 43, 881–894. [Google Scholar] [CrossRef]
- Kim, J.H.; Son, Y.D.; Kim, J.H.; Choi, E.J.; Lee, S.Y.; Joo, Y.H.; Kim, Y.B.; Cho, Z.H. Self-transcendence trait and its relationship with in vivo serotonin transporter availability in brainstem raphe nuclei: An ultra-high resolution PET-MRI study. Brain Res. 2015, 1629, 63–71. [Google Scholar] [CrossRef]
- Rubin, R.D.; Watson, P.D.; Duff, M.C.; Cohen, N.J. The role of the hippocampus in flexible cognition and social behavior. Front. Hum. Neurosci. 2014, 8, 742. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Beer, J.S.; Robertson, E.R.; Cooper, J.C.; Gabrieli, J.D.E.; Kihsltrom, J.F.; D’Esposito, M. The neural correlates of direct and reflected self-knowledge. Neuroimage 2005, 28, 797–814. [Google Scholar] [CrossRef]
- Van Schuerbeek, P.; Baeken, C.; De Raedt, R.; De Mey, J.; Luypaert, R. Individual differences in local gray and white matter volumes reflect differences in temperament and character: A voxel-based morphometry study in healthy young females. Brain Res. 2011, 1371, 32–42. [Google Scholar] [CrossRef]
- Samochowiec, J.; Fiszer-Piosik, E.; Kucharska-Mazur, J.; Horodnicki, J. The influence of genes on the development of personality. Psychiatr. Pol. 2000, 34, 99–109. [Google Scholar]
- Canli, T.; Lesch, K.P. Long story short: The serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 2007, 10, 1103–1109. [Google Scholar] [CrossRef]
- Mccourt, W.F.; Gurrera, R.J.; Cutter, H.S.G. Sensation Seeking and Novelty Seeking—Are They the Same. J. Nerv. Ment. Dis. 1993, 181, 309–312. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.; Lee, T. Relationship between sensory processing styles and personality. J. Korean Soc. Occup. Ther. 2010, 23, 35–45. [Google Scholar] [CrossRef]
- Choi, E.; Kim, Y.; Baek, D.; Hong, K.; Jung, H. The relationship between sensory processing feature and personality in early adolescents. J. Korean Soc. Occup. Ther. 2015, 18, 23–30. [Google Scholar] [CrossRef]
- Kim, S.K.; Kang, C.M.; Kwon, J.H.; Kim, M.K.; Kim, S.H.; Cho, Y.J.; Kim, E.Y. Effect of Sensory Processing Patterns on Temperament and Character Traits in Undergraduate Students. J. Korean Soc. Sens. Integr. Ther. 2022, 20, 38–47. [Google Scholar]
- Walker, E.P.; Tadi, P. Neuroanatomy, Nucleus Raphe. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2023. [Google Scholar]
- Peirson, A.R.; Heuchert, J.W.; Thomala, L.; Berk, M.; Plein, H.; Cloninger, C.R. Relationship between serotonin and the Temperament and Character Inventory. Psychiat. Res. 1999, 89, 29–37. [Google Scholar] [CrossRef]
- Nilsson, K.W.; Damberg, M.; Ohrvik, J.; Leppert, J.; Lindstrom, L.; Anckarsater, H.; Oreland, L. Genes encoding for AP-2 beta and the Serotonin Transporter are associated with the Personality Character Spiritual Acceptance. Neurosci. Lett. 2007, 411, 233–237. [Google Scholar] [CrossRef]
- Aoki, J.; Ikeda, K.; Murayama, O.; Yoshihara, E.; Ogai, Y.; Iwahashi, K. The association between personality, pain threshold and a single nucleotide polymorphism (rs3813034) in the 3’-untranslated region of the serotonin transporter gene (SLC6A4). J. Clin. Neurosci. 2010, 17, 574–578. [Google Scholar] [CrossRef]
- Saiz, P.A.; Garcia-Portilla, M.P.; Herrero, R.; Arango, C.; Corcoran, P.; Morales, B.; Bascaran, M.T.; Alvarez, V.; Coto, E.; Paredes, B.; et al. Interactions between functional serotonergic polymorphisms and demographic factors influence personality traits in healthy Spanish Caucasians. Psychiat. Genet. 2010, 20, 171–178. [Google Scholar] [CrossRef]
- Ham, B.J.; Kim, Y.H.; Choi, M.J.; Cha, J.H.; Choi, Y.K.; Lee, M.S. Serotonergic genes and personality traits in the Korean population. Neurosci. Lett. 2004, 354, 2–5. [Google Scholar] [CrossRef]
- Lorenzi, C.; Serretti, A.; Mandelli, L.; Tubazio, V.; Ploia, C.; Smeraldi, E. 5-HT1A polymorphism and self-transcendence in mood disorders. Am. J. Med. Genet. B 2005, 137, 33–35. [Google Scholar] [CrossRef]
- Aghajanian, G.K.; Marek, G.J. Serotonin and hallucinogens. Neuropsychopharmacology 1999, 21, S16–S23. [Google Scholar] [CrossRef]
- Geyer, M.A.; Vollenweider, F.X. Serotonin research: Contributions to understanding psychoses. Trends Pharmacol. Sci. 2008, 29, 445–453. [Google Scholar] [CrossRef]
- Hruby, R.; Nosalova, G.; Ondrejka, I.; Preiss, M. Personality Changes during Antidepressant Treatment. Psychiat. Danub. 2009, 21, 25–32. [Google Scholar]
- Borg, J.; Andree, B.; Soderstrom, H.; Farde, L. The serotonin system and spiritual experiences. Am. J. Psychiat. 2003, 160, 1965–1969. [Google Scholar] [CrossRef]
- McCrae, N.; Elliott, S. Spiritual Experiences in Temporal Lobe Epilepsy: A Literature Review. Br. J. Neurosci. Nurs. 2012, 8, 346–351. [Google Scholar] [CrossRef]
- Persinger, M.A.; Saroka, K.S.; Koren, S.A.; St-Pierre, L.S. The Electromagnetic Induction of Mystical and Altered States within the Laboratory. J. Conscious. Explor. Res. 2010, 1, 808–830. [Google Scholar]
- Mazzanti, C.M.; Lappalainen, J.; Long, J.C.; Bengel, D.; Naukkarinen, H.; Eggert, M.; Virkkunen, M.; Linnoila, M.; Goldman, D. Role of the serotonin transporter promoter polymorphism in anxiety-related traits. Arch. Gen. Psychiat. 1998, 55, 936–940. [Google Scholar] [CrossRef]
- Wiesbeck, G.A.; Weijers, H.G.; Wodarz, N.; Keller, H.K.; Michel, T.M.; Herrmann, M.J.; Boening, J. Serotonin transporter gene polymorphism and personality traits in primary alcohol dependence. World J. Biol. Psychiatry 2004, 5, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Matyka, D.; Jurczak, A.; Szkup, M.; Samochowiec, A.; Grzywacz, A.; Wieder-Huszla, S.; Grochans, E. The influence of the serotonergic system on the personality and quality of life of postmenopausal women. Clin. Interv. Aging 2017, 12, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Slifstein, M.; Laruelle, M. Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nucl. Med. Biol. 2001, 28, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Carson, R.E. Noise reduction in the simplified reference tissue model for neuroreceptor functional Imaging. J. Cereb. Blood Flow Metab. 2002, 22, 1440–1452. [Google Scholar] [CrossRef]
- Cunningham, V.J.; Hume, S.P.; Price, G.R.; Ahier, R.G.; Cremer, J.E.; Jones, A.K. Compartmental Analysis of Diprenorphine Binding to Opiate Receptors in the Rat in Vivo and Its Comparison with Equilibrium Data in Vitro. J. Cereb. Blood Flow Metab. 1991, 11, 1–9. [Google Scholar] [CrossRef]
- Lammertsma, A.A.; Hume, S.P. Simplified reference tissue model for PET receptor studies. Neuroimage 1996, 4, 153–158. [Google Scholar] [CrossRef]
- Kish, S.J.; Furukawa, Y.; Chang, L.-J.; Tong, J.; Ginovart, N.; Wilson, A.; Houle, S.; Meyer, J.H. Regional Distribution of Serotonin Transporter Protein in Postmortem Human Brain: Is the Cerebellum a SERT-Free Brain Region? Nucl. Med. Biol. 2005, 32, 123–128. [Google Scholar] [CrossRef]
- Ichise, M.; Liow, J.S.; Lu, J.Q.; Takano, T.; Model, K.; Toyama, H.; Suhara, T.; Suzuki, T.; Innis, R.B.; Carson, T.E. Linearized reference tissue parametric Imaging methods: Application to [C-11]DASB positron emission tomography studies of the serotonin transporter in human brain. J. Cereb. Blood Flow Metab. 2003, 23, 1096–1112. [Google Scholar] [CrossRef]
- Praschak-Rieder, N.; Wilson, A.A.; Hussey, D.; Carella, A.; Wei, C.; Ginovart, N.; Schwarz, M.J.; Zach, J.; Houle, S.; Meyer, J.H. Effects of tryptophan depletion on the serotonin transporter in healthy humans. Biol. Psychiat. 2005, 58, 825–830. [Google Scholar] [CrossRef]
- Tyrer, A.E.; Levitan, R.D.; Houle, S.; Wilson, A.A.; Nobrega, J.N.; Meyer, J.H. Increased Seasonal Variation in Serotonin Transporter Binding in Seasonal Affective Disorder. Neuropsychopharmacology 2016, 41, 2447–2454. [Google Scholar] [CrossRef]
- Smit, M.; Vallez Garcia, D.; de Jong, B.M.; Zoons, E.; Booij, J.; Dierckx, R.A.; Willemsen, A.T.; de Vries, E.F.; Bartels, A.L.; Tijssen, M.A. Relationships between Serotonin Transporter Binding in the Raphe Nuclei, Basal Ganglia, and Hippocampus with Clinical Symptoms in Cervical Dystonia: A [C-11]DASB Positron Emission Tomography Study. Front. Neurol. 2018, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Timmers, E.R.; Peretti, D.E.; Smit, M.; de Jong, B.M.; Dierckx, R.; Kuiper, A.; de Koning, T.J.; Vallez Garcia, D.; Tijssen, M.A.J. Serotonergic system in vivo with [(11)C]DASB PET scans in GTP-cyclohydrolase deficient dopa-responsive dystonia patients. Sci. Rep. 2022, 12, 6292. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994; Volume 4. [Google Scholar]
- First, M.B.; Spitzer, R.L.; Gibbon, M.; Williams, J.B.W. Structured Clinical Interview for DSM-IV Axis I Disorders Research Version (SCID-I); Biometrics Research; New York State Psychiatric Institute: New York, NY, USA, 1996. [Google Scholar]
- De Fruyt, F.; Van de Wiele, L.; Van Heeringen, C. Cloninger’s psychobiological model of temperament and character and the five-factor model of personality. Personal. Individ. Differ. 2000, 29, 441–452. [Google Scholar] [CrossRef]
- Cloninger, C.R.; Przybeck, T.R.; Svrakic, D.M.; Wetzel, R.D. The Temperament and Character Inventory (TCI): A Guide to Its Development and Use; Center for Psychobiology of Personality Washington University: St. Louis, MO, USA, 1994. [Google Scholar]
- Min, B.; Oh, H.; Lee, J. Temperament and Character Inventory-Revised-Short; Maumsarang: Seoul, Republic of Korea, 2007. [Google Scholar]
- Hong, I.K.; Chung, S.T.; Kim, H.K.; Kim, Y.B.; Son, Y.D.; Cho, Z.H. Ultra Fast Symmetry and SIMD-Based Projection-Backprojection (SSP) Algorithm for 3-D PET Image Reconstruction. IEEE Trans. Med. Imaging 2007, 26, 789–803. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Son, Y.D.; Joo, Y.H.; Lee, S.Y.; Kim, H.K.; Woo, M.K. Altered interregional correlations between serotonin transporter availability and cerebral glucose metabolism in schizophrenia: A high-resolution PET study using [C-11]DASB and [F-18]FDG. Schizophr. Res. 2017, 182, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Huang, C.C.; Lin, C.P.; Feng, J.; Joliot, M. Automated anatomical labelling atlas 3. Neuroimage 2020, 206, 116189. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Jajcay, L.; Tomecek, D.; Horacek, J.; Spaniel, F.; Hlinka, J. Brain Functional Connectivity Asymmetry: Left Hemisphere Is More Modular. Symmetry 2022, 14, 833. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Malison, R.T.; Seibyl, J.P.; Laruelle, M.; Klumpp, H.; Zoghbi, S.S.; Baldwin, R.M.; Innis, R.B. Age-related decline in central serotonin transporter availability with [I-123]beta-CIT SPECT. Neurobiol. Aging 2000, 21, 497–501. [Google Scholar] [CrossRef]
- Jovanovic, H.; Lundberg, J.; Karlsson, P.; Cerin, A.; Saijo, T.; Varrone, A.; Halldin, C.; Nordstrom, A.L. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage 2008, 39, 1408–1419. [Google Scholar] [CrossRef]
- Faria, D.D.; Duran, F.L.; Squarzoni, P.; Coutinho, A.M.; Garcez, A.T.; Santos, P.P.; Brucki, S.M.; de Oliveira, M.O.; Tres, E.S.; Forlenza, O.V.; et al. Topography of C-11-Pittsburgh compound B uptake in Alzheimer’s disease: A voxel-based investigation of cortical and white matter regions. Braz. J. Psychiat. 2019, 41, 101–111. [Google Scholar] [CrossRef] [PubMed]
| Variables | Mean ± SD/Number (%) |
|---|---|
| Demographic characteristics | |
| Age (years) | 29.9 ± 7.9 |
| Sex | |
| Male | 12 (50%) |
| Female | 12 (50%) |
| Education (years) | 15.0 ± 1.6 |
| Marital status | |
| Single | 16 (66.7%) |
| Married | 8 (33.3%) |
| Raw scores of TCI character scales | |
| Self-directedness | 54.7 ± 7.7 |
| Cooperativeness | 59.5 ± 7.9 |
| Self-transcendence | 22.7 ± 13.3 |
| [11C]DASB PET scan information | |
| Injected dose (MBq) | 684.0 ± 51.3 |
| Specific activity (GBq/μmol) | 47.3 ± 18.3 |
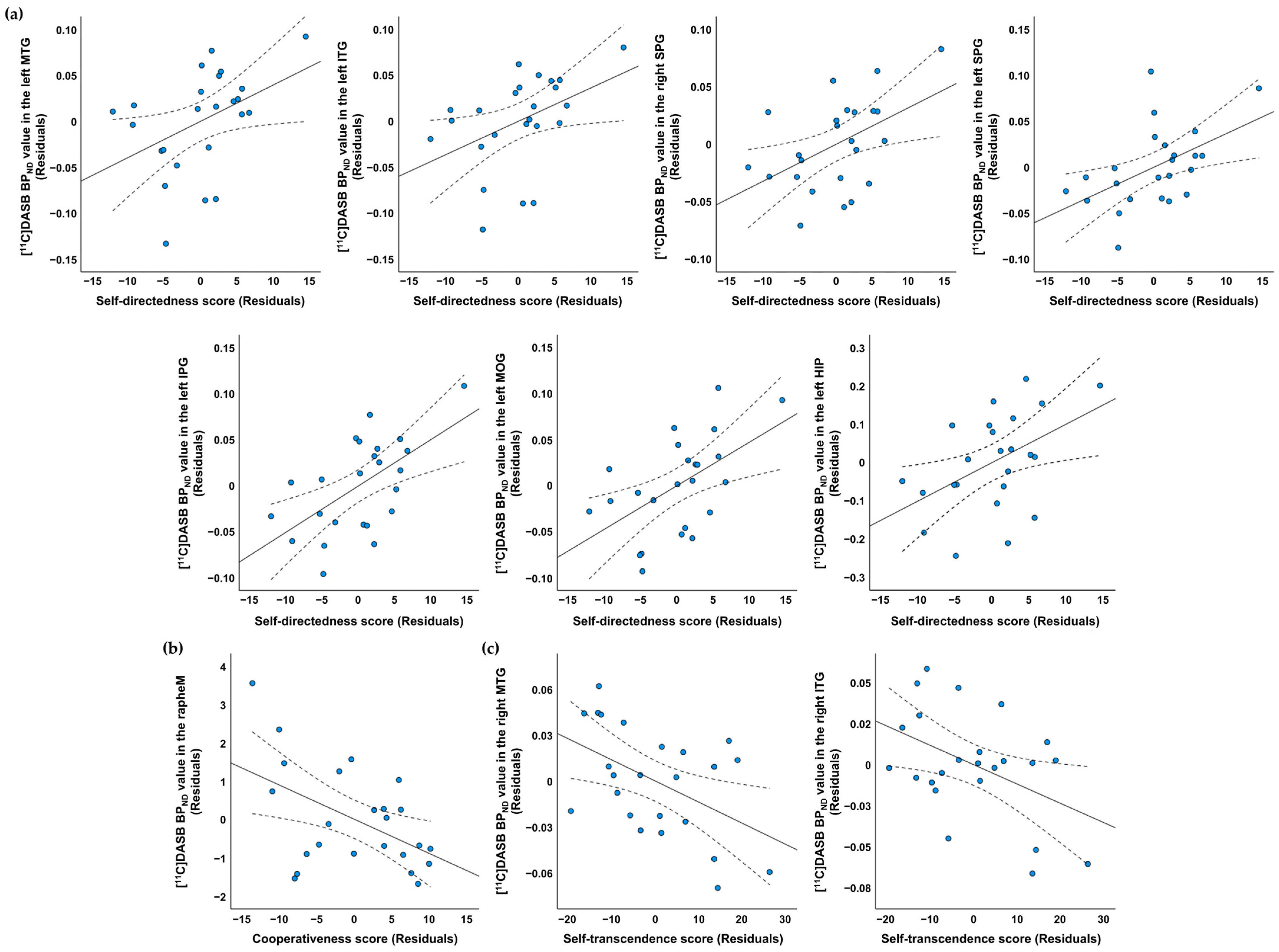
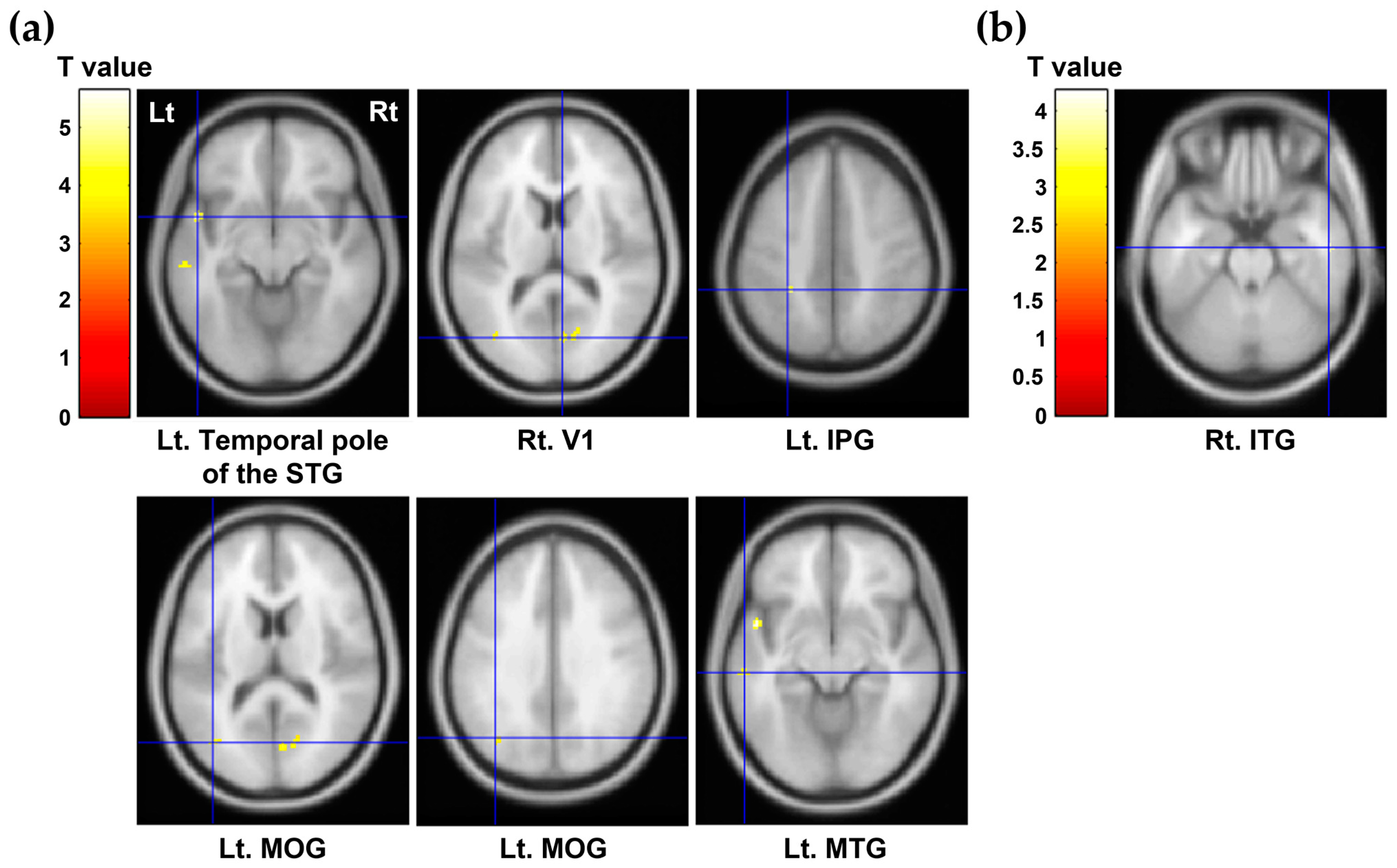
| Regions of Interest | Mean (SD) | Correlation Coefficients † (p Value) | ||
|---|---|---|---|---|
| Self-Directedness | Cooperativeness | Self-Transcendence | ||
| Rt. middle temporal gyrus | 0.29 (0.04) | −0.092 (0.683) | 0.095 (0.674) | −0.450 (0.035) * |
| Lt. middle temporal gyrus | 0.40 (0.06) | 0.428 (0.047) * | −0.004 (0.987) | −0.045 (0.842) |
| Rt. inferior temporal gyrus | 0.33 (0.06) | −0.042 (0.854) | −0.112 (0.619) | −0.446 (0.037) * |
| Lt. inferior temporal gyrus | 0.41 (0.05) | 0.433 (0.044) * | −0.045 (0.841) | −0.170 (0.449) |
| Rt. superior parietal gyrus | 0.23 (0.04) | 0.488 (0.021) * | 0.022 (0.924) | −0.122 (0.590) |
| Lt. superior parietal gyrus | 0.23 (0.04) | 0.508 (0.016) * | 0.066 (0.771) | 0.025 (0.913) |
| Lt. inferior parietal gyrus | 0.30 (0.05) | 0.593 (0.004) ** | 0.231 (0.301) | −0.026 (0.907) |
| Lt. middle occipital gyrus | 0.32 (0.05) | 0.542 (0.009) ** | −0.123 (0.584) | −0.053 (0.815) |
| Lt. hippocampus | 0.96 (0.14) | 0.477 (0.025) * | 0.232 (0.299) | −0.150 (0.505) |
| Median raphe nucleus | 2.06 (1.34) | −0.245 (0.272) | −0.497 (0.019) * | −0.155 (0.491) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Kim, H.-K.; Lee, S.-W.; Son, Y.-D.; Kim, J.-H. The Relationship between Character Traits and In Vivo Cerebral Serotonin Transporter Availability in Healthy Subjects: A High-Resolution PET Study with C-11 DASB. Pharmaceuticals 2023, 16, 759. https://doi.org/10.3390/ph16050759
Kim J-H, Kim H-K, Lee S-W, Son Y-D, Kim J-H. The Relationship between Character Traits and In Vivo Cerebral Serotonin Transporter Availability in Healthy Subjects: A High-Resolution PET Study with C-11 DASB. Pharmaceuticals. 2023; 16(5):759. https://doi.org/10.3390/ph16050759
Chicago/Turabian StyleKim, Jeong-Hee, Hang-Keun Kim, Sang-Wha Lee, Young-Don Son, and Jong-Hoon Kim. 2023. "The Relationship between Character Traits and In Vivo Cerebral Serotonin Transporter Availability in Healthy Subjects: A High-Resolution PET Study with C-11 DASB" Pharmaceuticals 16, no. 5: 759. https://doi.org/10.3390/ph16050759
APA StyleKim, J. -H., Kim, H. -K., Lee, S. -W., Son, Y. -D., & Kim, J. -H. (2023). The Relationship between Character Traits and In Vivo Cerebral Serotonin Transporter Availability in Healthy Subjects: A High-Resolution PET Study with C-11 DASB. Pharmaceuticals, 16(5), 759. https://doi.org/10.3390/ph16050759






