Innate Vascular Failure by Application of Neuroleptics, Amphetamine, and Domperidone Rapidly Induced Severe Occlusion/Occlusion-like Syndromes in Rats and Stable Gastric Pentadecapeptide BPC 157 as Therapy
Abstract
:1. Introduction
2. Results
2.1. A Perilous Syndrome Occurred Peripherally and Centrally
2.1.1. Blood Pressure Disturbances
2.1.2. Thrombosis
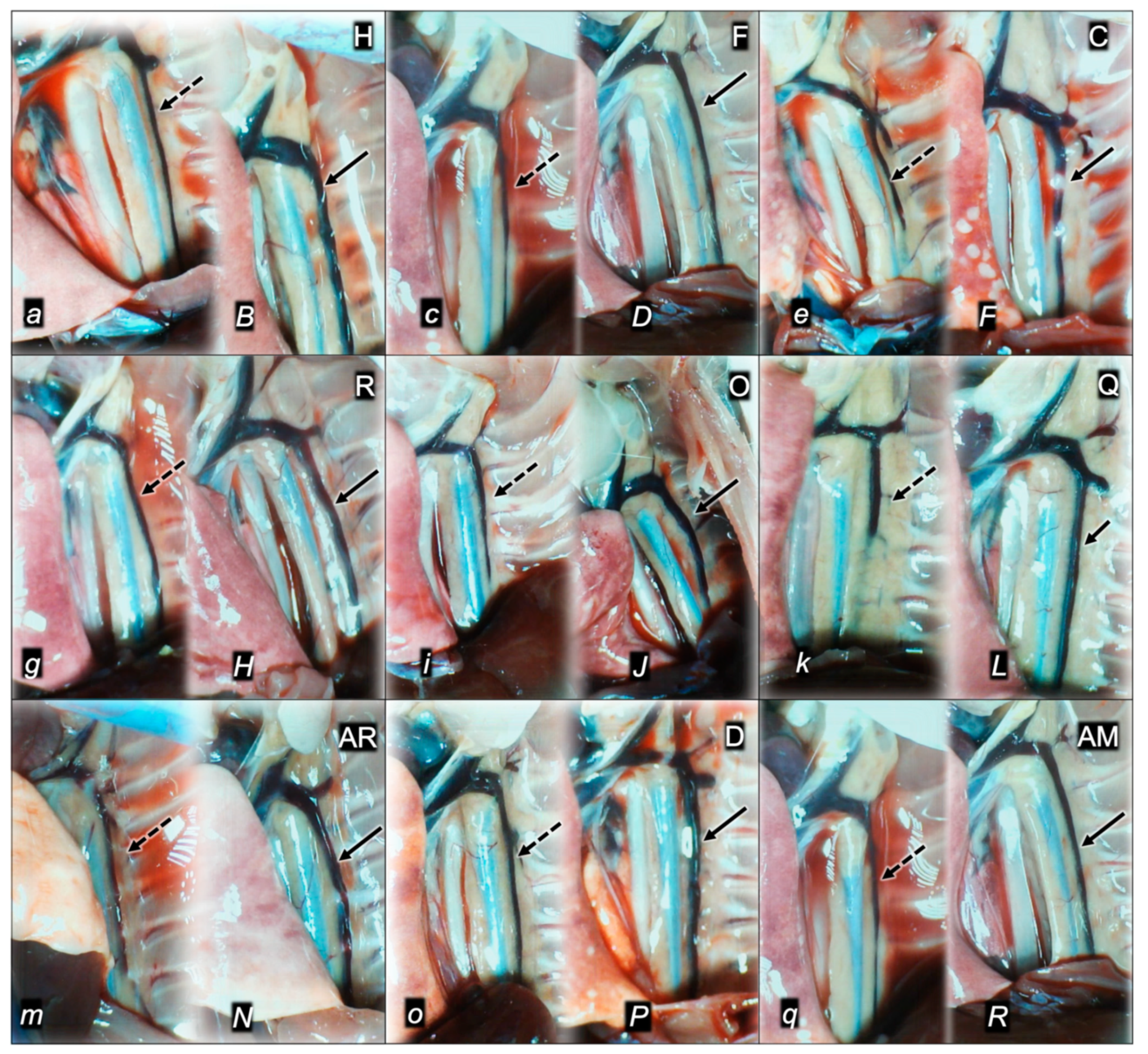
2.1.3. Collateral Pathways, Blood Vessels, and Gross Brain Presentation
2.1.4. Heart and ECG Disturbances
2.2. A Perilous Syndrome Occurred Peripherally
2.2.1. Gastrointestinal, Lung, Liver, Kidney, and Heart Lesions
2.2.2. Heart
2.2.3. Lung
2.2.4. Liver
2.2.5. Kidney
2.2.6. Gastrointestinal Lesions
2.3. A Perilous Syndrome Occurred Centrally
Brain Lesions, Cerebral and Cerebellar Cortex, Hypothalamus/Thalamus, and Hippocampus
2.4. Perilous Syndrome with Concomitant Application of the Dopamine Antagonist (Haloperidol) and Dopamine Agonist (Amphetamine)
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Experimental Protocol
4.4. Superior Sagittal Sinus, Portal, Caval Vein, and Abdominal Aorta Pressure Recording
4.5. ECG Recording
4.6. Thrombus Assessment
4.7. Brain Volume, Heart, and Vessel Volume Presentation
4.8. Gross Assessment of Gastrointestinal Lesions
4.9. Microscopy
4.9.1. Brain Histology
4.9.2. Lung Histology
4.9.3. Renal, Liver, and Heart Histology
4.9.4. Gastrointestinal Histology
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sikiric, P.; Gojkovic, S.; Krezic, I.; Smoday, I.M.; Kalogjera, L.; Zizek, H.; Oroz, K.; Vranes, H.; Vukovic, V.; Labidi, M.; et al. Stable gastric pentadecapeptide BPC 157 may recover brain–gut axis and gut–brain axis function. Pharmaceuticals 2023, 16, 676. [Google Scholar] [CrossRef]
- Sikiric, P.; Gojkovic, S.; Knezevic, M.; Tepes, M.; Strbe, S.; Vukojevic, J.; Duzel, A.; Kralj, T.; Krezic, I.; Zizek, H.; et al. Stable gastric pentadecapeptide BPC 157: Prompt particular activation of the collateral pathways. Curr. Med. Chem. 2023, 30, 1568–1573. [Google Scholar] [CrossRef]
- Sikiric, P.; Skrtic, A.; Gojkovic, S.; Krezic, I.; Zizek, H.; Lovric, E.; Sikiric, S.; Knezevic, M.; Strbe, S.; Milavic, M.; et al. Gastric pentadecapeptide BPC 157 in cytoprotection to resolve major vessel occlusion disturbances, ischemia-reperfusion injury following Pringle maneuver, and Budd-Chiari syndrome. World J. Gastroenterol. 2022, 28, 23–46. [Google Scholar] [CrossRef]
- Sikiric, P.; Udovicic, M.; Barisic, I.; Balenovic, D.; Zivanovic Posilovic, G.; Strinic, D.; Uzun, S.; Sikiric, S.; Krezic, I.; Zizek, H.; et al. Stable gastric pentadecapeptide BPC 157 as useful cytoprotective peptide therapy in the hearth disturbances, myocardial infarction, heart failure, pulmonary hypertension, arrhythmias, and thrombosis presentation. Biomedicines 2022, 10, 2696. [Google Scholar] [CrossRef]
- Staresinic, M.; Japjec, M.; Vranes, H.; Prtoric, A.; Zizek, H.; Krezic, I.; Gojkovic, S.; Smoday, I.M.; Oroz, K.; Staresinic, E.; et al. Stable gastric pentadecapeptide BPC 157 and striated, smooth, and heart muscle. Biomedicines 2022, 10, 3221. [Google Scholar] [CrossRef]
- Sikiric, P.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. Novel cytoprotective mediator, stable gastric pentadecapeptide BPC 157. Vascular recruitment and gastrointestinal tract healing. Curr. Pharm. Des. 2018, 24, 1990–2001. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Drmic, D.; Stupnisek, M.; Kokot, A.; Sever, M.; Zoricic, I.; Zoricic, Z.; Batelja, L.; et al. Stress in gastrointestinal tract and stable gastric pentadecapeptide BPC 157. Finally, do we have a solution? Curr. Pharm. Des. 2017, 23, 4012–4028. [Google Scholar] [CrossRef]
- Seiwerth, S.; Milavic, M.; Vukojevic, J.; Gojkovic, S.; Krezic, I.; Vuletic, L.B.; Pavlov, K.H.; Petrovic, A.; Sikiric, S.; Vranes, H.; et al. Stable gastric pentadecapeptide BPC 157 and wound healing. Front. Pharmacol. 2021, 12, 627533. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Kolenc, D.; Vuletic, L.B.; Drmic, D.; Grgic, T.; Strbe, S.; Zukanovic, G.; Crvenkovic, D.; et al. Brain-gut axis and pentadecapeptide BPC 157: Theoretical and practical implications. Curr. Neuropharmacol. 2016, 14, 857–865. [Google Scholar] [CrossRef]
- Vukojevic, J.; Milavic, M.; Perovic, D.; Ilic, S.; Cilic, A.Z.; Duran, N.; Strbe, S.; Zoricic, Z.; Filipcic, I.; Brecic, P.; et al. Pentadecapeptide BPC 157 and the central nervous system. Neural Regen. Res. 2022, 17, 482–487. [Google Scholar] [CrossRef]
- Vukojevic, J.; Siroglavic, M.; Kasnik, K.; Kralj, T.; Stancic, D.; Kokot, A.; Kolaric, D.; Drmic, D.; Sever, A.Z.; Barisic, I.; et al. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vascul. Pharmacol. 2018, 106, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Kolovrat, M.; Gojkovic, S.; Krezic, I.; Malekinusic, D.; Vrdoljak, B.; Kasnik Kovac, K.; Kralj, T.; Drmic, D.; Barisic, I.; Horvat Pavlov, K.; et al. Pentadecapeptide BPC 157 resolves Pringle maneuver in rats, both ischemia and reperfusion. World J. Hepatol. 2020, 12, 184–206. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, S.; Krezic, I.; Vrdoljak, B.; Malekinusic, D.; Barisic, I.; Petrovic, A.; Horvat Pavlov, K.; Kolovrat, M.; Duzel, A.; Knezevic, M.; et al. Pentadecapeptide BPC 157 resolves suprahepatic occlusion of the inferior caval vein, Budd-Chiari syndrome model in rats. World J. Gastrointest. Pathophysiol. 2020, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Pavlov, K.H.; Drmic, D.; et al. Complex syndrome of the complete occlusion of the end of the superior mesenteric vein, opposed with the stable gastric pentadecapeptide BPC 157 in rats. Biomedicines 2021, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Vranes, H.; Drmic, D.; Staroveski, M.; et al. Occluded superior mesenteric artery and vein. Therapy with the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 792. [Google Scholar] [CrossRef]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Vranes, H.; Knezevic, T.; Barisic, I.; Horvat Pavlov, K.; et al. Occlusion of the superior mesenteric artery in rats reversed by collateral pathways activation: Gastric pentadecapeptide BPC 157 therapy counteracts multiple organ dysfunction syndrome; intracranial, portal and caval hypertension; and aortal hypotension. Biomedicines 2021, 9, 609. [Google Scholar] [CrossRef]
- Kralj, T.; Kokot, A.; Zlatar, M.; Masnec, S.; Kasnik Kovac, K.; Milkovic Perisa, M.; Batelja Vuletic, L.; Giljanovic, A.; Strbe, S.; Sikiric, S.; et al. Stable gastric pentadecapeptide BPC 157 therapy of rat glaucoma. Biomedicines 2021, 10, 89. [Google Scholar] [CrossRef]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Pavlov, K.H.; Petrovic, A.; Batelja, L.; Milavic, M.; Sikiric, S.; et al. BPC 157 therapy and the permanent occlusion of the superior sagittal sinus in rat. Vascular recruitment. Biomedicines 2021, 9, 744. [Google Scholar] [CrossRef]
- Vukojevic, J.; Vrdoljak, B.; Malekinusic, D.; Siroglavic, M.; Milavic, M.; Kolenc, D.; Boban Blagaic, A.; Bateljam, L.; Drmic, D.; Seiwerth, S.; et al. The effect of pentadecapeptide BPC 157 on hippocampal ischemia/reperfusion injuries in rats. Brain Behav. 2020, 10, e01726. [Google Scholar] [CrossRef]
- Strbe, S.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Barisic, I.; Strinic, D.; Orct, T.; Vukojevic, J.; Ilic, S.; et al. Over-dose lithium toxicity as an occlusive-like syndrome in rats and gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 1506. [Google Scholar] [CrossRef]
- Tepes, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Madzar, Z.; Santak, G.; Batelja, L.; Milavic, M.; Sikiric, S.; Kocman, I.; et al. Stable gastric pentadecapeptide BPC 157 therapy for primary abdominal compartment syndrome in rats. Front. Pharmacol. 2021, 12, 718147. [Google Scholar] [CrossRef] [PubMed]
- Barisic, I.; Balenovic, D.; Udovicic, M.; Bardak, D.; Strinic, D.; Vlainic, J.; Vranes, H.; Smoday, I.M.; Krezic, I.; Milavic, M.; et al. Stable gastric pentadecapeptide BPC 157 may counteract myocardial infarction induced by isoprenaline in rats. Biomedicines 2022, 10, 265. [Google Scholar] [CrossRef]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Batelja Vuletic, L.; Milavic, M.; Sikiric, S.; Stilinovic, I.; Simeon, P.; et al. Robert’s intragastric alcohol-induced gastric lesion model as an escalated general peripheral and central syndrome, counteracted by the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Smoday, I.M.; Petrovic, I.; Kalogjera, L.; Vranes, H.; Zizek, H.; Krezic, I.; Gojkovic, S.; Skorak, I.; Hriberski, K.; Brizic, I.; et al. Therapy effect of the stable gastric pentadecapeptide BPC 157 on acute pancreatitis as vascular failure-induced severe peripheral and central syndrome in rats. Biomedicines 2022, 10, 1299. [Google Scholar] [CrossRef] [PubMed]
- Jelovac, N.; Sikiric, P.; Rucman, R.; Petek, M.; Perovic, D.; Konjevoda, P.; Marovic, A.; Seiwerth, S.; Grabarevic, Z.; Sumajstorcic, J.; et al. A novel pentadecapeptide, BPC 157, blocks the stereotypy produced acutely by amphetamine and the development of haloperidol-induced supersensitivity to amphetamine. Biol. Psychiatry 1998, 43, 511–519. [Google Scholar] [CrossRef]
- Jelovac, N.; Sikiric, P.; Rucman, R.; Petek, M.; Marovic, A.; Perovic, D.; Seiwerth, S.; Mise, S.; Turkovic, B.; Dodig, G.; et al. Pentadecapeptide BPC 157 attenuates disturbances induced by neuroleptics: The effect on catalepsy and gastric ulcers in mice and rats. Eur. J. Pharmacol. 1999, 379, 19–31. [Google Scholar] [CrossRef]
- Sikiric, P.; Jelovac, N.; Jelovac-Gjeldum, A.; Dodig, G.; Staresinic, M.; Anic, T.; Zoricic, I.; Rak, D.; Perovic, D.; Aralica, G.; et al. Pentadecapeptide BPC 157 attenuates chronic amphetamine-induced behavior disturbances. Acta Pharmacol. Sin. 2002, 23, 412–422. [Google Scholar]
- Zemba Cilic, A.; Zemba, M.; Cilic, M.; Balenovic, I.; Strbe, S.; Ilic, S.; Vukojevic, J.; Zoricic, Z.; Filipcic, I.; Kokot, A.; et al. Pentadecapeptide BPC 157 counteracts L-NAME-induced catalepsy. BPC 157, L-NAME, L-arginine, NO-relation, in the suited rat acute and chronic models resembling ’positive-like’ symptoms of schizophrenia. Behav. Brain Res. 2021, 396, 112919. [Google Scholar] [CrossRef]
- Zemba Cilic, A.; Zemba, M.; Cilic, M.; Strbe, S.; Ilic, S.; Vukojevic, J.; Zoricic, Z.; Filipcic, I.; Kokot, A.; Smoday, I.M.; et al. BPC 157, L-NAME, L-arginine, NO-relation, in the suited rat ketamine models resembling “negative-like” symptoms of schizophrenia. Biomedicines 2022, 10, 1462. [Google Scholar] [CrossRef]
- Robert, A. Cytoprotection by prostaglandins. Gastroenterology 1979, 77, 761–767. [Google Scholar] [CrossRef]
- Szabo, S.; Trier, J.S.; Brown, A.; Schnoor, J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology 1985, 88, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S. Experimental basis for a role for sulfhydryls and dopamine in ulcerogenesis: A primer for cytoprotection—organoprotection. Klin Wochenschr. 1986, 64 (Suppl. 7), 116–122. [Google Scholar] [PubMed]
- Hernandez, D.E.; Mason, G.A.; Walker, C.H.; Valenzuela, J.E. Dopamine receptors in human gastrointestinal mucosa. Life Sci. 1987, 41, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.E. Involvement of dopamine receptors in experimental ulceration. Int. J. Tissue React. 1987, 9, 407–411. [Google Scholar] [PubMed]
- Hernandez, D.E.; Walker, C.H.; Valenzuela, J.E.; Mason, G.A. Increased dopamine receptor binding in duodenal mucosa of duodenal ulcer patients. Dig. Dis. Sci. 1989, 34, 543–547. [Google Scholar] [CrossRef]
- Dietrich-Muszalska, A.; Rabe-Jablonska, J.; Nowak, P.; Kontek, B. The first- and second-generation antipsychotic drugs affect ADP-induced platelet aggregation. World J. Biol. Psychiatry 2010, 11 Pt 2, 268–275. [Google Scholar] [CrossRef]
- Allenet, B.; Schmidlin, S.; Genty, C.; Bosson, J.L. Antipsychotic drugs and risk of pulmonary embolism. Pharmacoepidemiol. Drug Saf. 2012, 21, 42–48. [Google Scholar] [CrossRef]
- Dietrich-Muszalska, A.; Rabe-Jablonska, J.; Olas, B. The effects of the second generation antipsychotics and a typical neuroleptic on collagen-induced platelet aggregation in vitro. World J. Biol. Psychiatry 2010, 11 Pt 2, 293–299. [Google Scholar] [CrossRef]
- Dai, L.; Zuo, Q.; Chen, F.; Chen, L.; Shen, Y. The association and influencing factors between antipsychotics exposure and the risk of VTE and PE: A systematic review and meta-analysis. Curr. Drug Targets. 2020, 21, 930–942. [Google Scholar] [CrossRef]
- Manoubi, S.A.; Boussaid, M.; Brahim, O.; Ouanes, S.; Mahjoub, Y.; Zarrouk, L.; Mesrati, M.A.; Aissaoui, A. Fatal pulmonary embolism in patients on antipsychotics: Case series, systematic review and meta-analysis. Asian J. Psychiatr. 2022, 73, 103105. [Google Scholar] [CrossRef]
- Jonsson, A.K.; Schill, J.; Olsson, H.; Spigset, O.; Hagg, S. Venous thromboembolism during treatment with antipsychotics: A review of current evidence. CNS Drugs 2018, 32, 47–64. [Google Scholar] [CrossRef]
- Nayak, S.; Parida, M.; Das, S.B.; Padhi, P.K.; Behera, M.; Patil, A.; Khurana, A.; Kumar Swain, S. Clinical characteristics and management of headache: A real-life prospective, observational study from a tertiary care center in Eastern India. Cureus 2020, 12, e12409. [Google Scholar] [CrossRef] [PubMed]
- Vichairuangthum, K. Acute myocardial infarction with left main thromboses in a young amphetamine addict. Arch. Med. Sci. Atheroscler Dis. 2020, 5, e45–e48. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, M.; Hermann, M.; Stähli, B.E.; Manka, R.; Maisano, F.; Alkadhi, H. Amphetamine-induced coronary artery dissection and massive aortic valve thrombus. Eur. Heart J. 2020, 41, 230. [Google Scholar] [CrossRef]
- den Uil, C.A.; Ligthart, J.M.R.; Mandigers, L.; den Dekker, W.K. Cocaine/amphetamine-induced accelerated atherosclerosis, coronary spasm and thrombosis, and refractory ventricular fibrillation. Eur. Heart J. Case Rep. 2019, 3, 1–2. [Google Scholar] [CrossRef]
- Bilic, I.; Zoricic, I.; Anic, T.; Separovic, J.; Stancic-Rokotov, D.; Mikus, D.; Buljat, G.; Ivankovic, D.; Aralica, G.; Prkacin, I.; et al. Haloperidol-stomach lesions attenuation by pentadecapeptide BPC 157, omeprazole, bromocriptine, but not atropine, lansoprazole, pantoprazole, ranitidine, cimetidine and misoprostol in mice. Life Sci. 2001, 68, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Separovic, J.; Buljat, G.; Anic, T.; Stancic-Rokotov, D.; Mikus, D.; Duplancic, B.; Marovic, A.; Zoricic, I.; Prkacin, I.; et al. Gastric mucosal lesions induced by complete dopamine system failure in rats. The effects of dopamine agents, ranitidine, atropine, omeprazole and pentadecapeptide BPC 157. J. Physiol. Paris. 2000, 94, 105–110. [Google Scholar] [CrossRef]
- Belosic Halle, Z.; Vlainic, J.; Drmic, D.; Strinic, D.; Luetic, K.; Sucic, M.; Medvidovic-Grubisic, M.; Pavelic Turudic, T.; Petrovic, I.; Belosic Halle, Z.; et al. Class side effects: Decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, L-NAME, pentadecapeptide BPC 157 and L-arginine. Inflammopharmacology 2017, 25, 511–522. [Google Scholar] [CrossRef]
- Strinic, D.; Belosic Halle, Z.; Luetic, K.; Nedic, A.; Petrovic, I.; Sucic, M.; Zivanovic Posilovic, G.; Balenovic, D.; Strbe, S.; Udovicic, M.; et al. BPC 157 counteracts QTc prolongation induced by haloperidol, fluphenazine, clozapine, olanzapine, quetiapine, sulpiride, and metoclopramide in rats. Life Sci. 2017, 186, 66–79. [Google Scholar] [CrossRef]
- Sikiric, P.; Drmic, D.; Boban Blagaic, A.; Tvrdeic, A.; Krezic, I.; Gojkovic, S.; Zizek, H.; Sikiric, S.; Strbe, S.; Smoday, I.M.; et al. Stable gastric pentadecapeptide BPC 157 and NO-system. In Nitric Oxide: From Research to Therapeutics; Ray, A., Gulati, K., Eds.; Advances in Biochemistry in Health and Disease 22; Springer Nature Switzerland AG: Cham, Switzerland, 2023; pp. 349–376. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr. Pharm. Des. 2014, 20, 1126–1135. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Rucman, R.; Petek, M.; Jagic, V.; Turkovic, B.; Rotkvic, I.; Mise, S.; Zoricic, I.; et al. The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure. Eur. J. Pharmacol. 1997, 332, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Turkovic, B.; Sikiric, P.; Seiwerth, S.; Mise, S.; Anic, T.; Petek, M. Stable gastric pentadecapeptide BPC 157 studied for inflammatory bowel disease (PLD-116, PL14736, Pliva) induces nitric oxide synthesis. Gastroenterology 2004, 126, 287. [Google Scholar]
- Stupnisek, M.; Kokot, A.; Drmic, D.; Hrelec Patrlj, M.; Zenko Sever, A.; Kolenc, D.; Radic, B.; Suran, J.; Bojic, D.; Vcev, A.; et al. Pentadecapeptide BPC 157 reduces bleeding and thrombocytopenia after amputation in rats treated with heparin, warfarin, L-NAME and L-arginine. PLoS ONE 2015, 10, e0123454. [Google Scholar] [CrossRef]
- Miller, R. Mechanisms of action of antipsychotic drugs of different classes, refractoriness to therapeutic effects of classical neuroleptics, and individual variation in sensitivity to their actions: PART I. Curr. Neuropharmacol. 2009, 7, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Lally, J.; MacCabe, J.H. Antipsychotic medication in schizophrenia: A review. Br. Med. Bull. 2015, 114, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Cheer, S.M.; Wagstaff, A.J. Quetiapine. A review of its use in the management of schizophrenia. CNS Drugs 2004, 18, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Reddymasu, S.C.; Soykan, I.; McCallum, R.W. Domperidone: Review of pharmacology and clinical applications in gastroenterology. Am. J. Gastroenterol. 2007, 102, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Strzelecki, A.; Weafer, J.; Stoops, W.W. Human behavioral pharmacology of stimulant drugs: An update and narrative review. Adv. Pharmacol. 2022, 93, 77–103. [Google Scholar]
- Docherty, J.R.; Alsufyani, H.A. Pharmacology of drugs used as stimulants. J. Clin. Pharmacol. 2021, 61 (Suppl. 2), S53–S69. [Google Scholar] [CrossRef]
- Ferrucci, M.; Limanaqi, F.; Ryskalin, L.; Biagioni, F.; Busceti, C.L.; Fornai, F. The effects of amphetamine and methamphetamine on the release of norepinephrine, dopamine and acetylcholine from the brainstem reticular formation. Front. Neuroanat. 2019, 13, 48. [Google Scholar] [CrossRef]
- Ozeki, Y.; Fujii, K.; Kurimoto, N.; Yamada, N.; Okawa, M.; Aoki, T.; Takahashi, J.; Ishida, N.; Horie, M.; Kunugi, H. QTc prolongation and antipsychotic medications in a sample of 1017 patients with schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.A.; Mythily, M.; Lum, A.; Goh, H.Y.; Chan, Y.H. Prolonged QTc intervals in medicated patients with schizophrenia. Hum. Psychopharmacol. 2003, 18, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.D.; Wang, X.Q.; Liu, X.P.; Zhao, K.X.; Fu, W.H.; Hao, X.R.; Zhao, X.L.; Huang, G.H.; Qu, S.C.; Bai, J.S.; et al. Sex difference in QTc prolongation in chronic institutionalized patients with schizophrenia on long-term treatment with typical and atypical antipsychotics. Psychopharmacology 2011, 216, 9–16. [Google Scholar] [CrossRef]
- Berling, I.; Isbister, G.K. Prolonged QT risk assessment in antipsychotic overdose using the QT nomogram. Ann. Emerg. Med. 2015, 66, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Stöllberger, C.; Huber, J.O.; Finsterer, J. Antipsychotic drugs and QT prolongation. Int. Clin. Psychopharmacol. 2005, 20, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Rmalho, D.; Freitas, J. Drug-induced life-threatening arrhythmias and sudden cardiac death: A clinical perspective of long QT, short QT and Brugada syndromes. Rev. Port. Cardiol. (Engl. Ed.) 2018, 37, 435–446. [Google Scholar] [CrossRef]
- Brugada, J.; Gussak, I.; Brugada, P. Short QT syndrome: A predictable story. Cardiology 2014, 128, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Gussak, I.; Brugada, P.; Brugada, J.; Wright, R.S.; Kopecky, S.L.; Chaitman, B.R.; Bjerregaard, P. Idiopathic short QT interval: A new clinical syndrome? Cardiology 2000, 94, 99–102. [Google Scholar] [CrossRef]
- Ramakrishna, H.; O’Hare, M.; Mookadam, F.; Gutsche, J.T.; Shah, R.; Augoustides, J.G. Sudden cardiac death and disorders of the QT interval: Anesthetic implications and focus on perioperative management. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1723–1733. [Google Scholar] [CrossRef]
- Shah, R.R. Drug-induced QT interval shortening: Potential harbinger of proarrhythmia and regulatory perspectives. Br. J. Pharmacol. 2010, 159, 58–69. [Google Scholar] [CrossRef]
- Schimpf, R.; Veltmann, C.; Papavassiliu, T.; Rudic, B.; Göksu, T.; Kuschyk, J.; Wolpert, C.; Antzelevitch, C.; Ebner, A.; Borggrefe, M.; et al. Drug-induced QT interval shortening following antiepileptic treatment with oral rufinamide. Heart Rhythm. 2012, 9, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Gamulin, O.; Oroz, K.; Coric, L.; Krajacic, M.; Skrabic, M.; Dretar, V.; Strbe, S.; Talapko, J.; Juzbasic, M.; Krezic, I.; et al. Fourier transform infrared spectroscopy reveals molecular changes in blood vessels of rats treated with pentadecapeptide BPC 157. Biomedicines 2022, 10, 3130. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Lee, C.H.; Chueh, H.Y.; Chang, G.J.; Huang, H.Y.; Lin, Y.; Pang, J.S. Modulatory effects of BPC 157 on vasomotor tone and the activation of Src-Caveolin-1-endothelial nitric oxide synthase pathway. Sci. Rep. 2020, 10, 17078. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Liu, H.T.; Wang, C.N.; Huang, H.Y.; Lin, Y.; Ko, Y.S.; Wang, J.S.; Chang, V.H.; Pang, J.S. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J. Mol. Med. 2017, 95, 323–333. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Toxicity by NSAIDs: Counteraction by stable gastric pentadecapeptide BPC 157. Curr. Pharm. Des. 2013, 19, 76–83. [Google Scholar]
- Park, J.M.; Lee, H.J.; Sikiric, P.; Hahm, K.B. BPC 157 rescued NSAID-cytotoxicity via stabilizing intestinal permeability and enhancing cytoprotection. Curr. Pharm. Des. 2020, 26, 2971–2981. [Google Scholar] [CrossRef]
- Stupnisek, M.; Franjic, S.; Drmic, D.; Hrelec, M.; Kolenc, D.; Radic, B.; Bojic, D.; Vcev, A.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 reduces bleeding time and thrombocytopenia after amputation in rats treated with heparin, warfarin or aspirin. Thromb. Res. 2012, 129, 652–659. [Google Scholar] [CrossRef]
- Konosic, S.; Petricevic, M.; Ivancan, V.; Konosic, L.; Goluza, E.; Krtalic, B.; Drmic, D.; Stupnisek, M.; Seiwerth, S.; Sikiric, P. Intragastric application of aspirin, clopidogrel, cilostazol, and BPC 157 in rats: Platelet aggregation and blood clot. Oxid. Med. Cell. Longev. 2019, 2019, 9084643. [Google Scholar] [CrossRef]
- Perovic, D.; Kolenc, D.; Bilic, V.; Somun, N.; Drmic, D.; Elabjer, E.; Buljat, G.; Seiwerth, S.; Sikiric, P. Stable gastric pentadecapeptide BPC 157 can improve the healing course of spinal cord injury and lead to functional recovery in rats. J. Orthop. Surg. Res. 2019, 14, 199. [Google Scholar] [CrossRef]
- Perovic, D.; Milavic, M.; Dokuzovic, S.; Krezic, I.; Gojkovic, S.; Vranes, H.; Bebek, I.; Bilic, V.; Somun, N.; Brizic, I.; et al. Novel therapeutic effects in rat spinal cord injuries: Recovery of the definitive and early spinal cord injury by the administration of pentadecapeptide BPC 157 therapy. Curr. Issues Mol. Biol. 2022, 44, 1901–1927. [Google Scholar] [CrossRef]
- Duzel, A.; Vlainic, J.; Antunovic, M.; Malekinusic, D.; Vrdoljak, B.; Samara, M.; Gojkovic, S.; Krezic, I.; Vidovic, T.; Bilic, Z.; et al. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: New insights. World J. Gastroenterol. 2017, 23, 8465–8488. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Tsai, W.C.; Lin, M.S.; Hsu, Y.H.; Pang, J.H.S. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J. Appl. Physiol. 2011, 110, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Tsai, W.C.; Hsu, Y.H.; Pang, J.H.S. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules 2014, 19, 19066–19077. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, K.; Sun, L.; Xue, X.; Zhang, C.; Shu, Z.; Mu, N.; Gu, J.; Zhang, W.; Wang, Y.; et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des. Devel. Ther. 2015, 9, 2485–2499. [Google Scholar] [CrossRef] [PubMed]
- Tkalcevic, V.I.; Cuzic, S.; Brajsa, K.; Mildner, B.; Bokulic, A.; Situm, K.; Perovic, D.; Glojnaric, I.; Parnham, M.J. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur. J. Pharmacol. 2007, 570, 212–221. [Google Scholar] [CrossRef]
- Wang, X.Y.; Qu, M.; Duan, R.; Shi, D.; Jin, L.; Gao, J.; Wood, J.D.; Li, J.; Wang, G.D. Cytoprotective mechanism of the novel gastric peptide BPC157 in gastrointestinal tract and cultured enteric neurons and glial cells. Neurosci. Bull. 2019, 35, 167–170. [Google Scholar] [CrossRef]
- Wu, H.; Wei, M.; Li, N.; Lu, Q.; Shrestha, S.M.; Tan, J.; Zhang, Z.; Wu, G.; Shi, R. Clopidogrel-induced gastric injury in rats is attenuated by stable gastric pentadecapeptide BPC 157. Drug Des. Devel. Ther. 2020, 14, 5599–5610. [Google Scholar] [CrossRef]
- Huang, B.S.; Huang, S.C.; Chen, F.H.; Chang, Y.; Mei, H.F.; Huang, H.Y.; Chen, W.Y.; Pang, J.S. Pentadecapeptide BPC 157 efficiently reduces radiation-induced liver injury and lipid accumulation through Kruppel-like factor 4 upregulation both in vivo and in vitro. Life Sci. 2022, 310, 121072. [Google Scholar] [CrossRef]
- Kang, E.A.; Han, Y.M.; An, J.M.; Park, Y.J.; Sikiric, P.; Kim, D.H.; Kwon, K.A.; Kim, Y.J.; Yang, D.; Tchah, H.; et al. BPC157 as potential agent rescuing from cancer cachexia. Curr. Pharm. Des. 2018, 24, 1947–1956. [Google Scholar] [CrossRef]
- Seiwerth, S.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. BPC 157 and standard angiogenic growth factors. Gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr. Pharm. Des. 2018, 24, 1972–1989. [Google Scholar] [CrossRef]
- Luetic, K.; Sucic, M.; Vlainic, J.; Halle, Z.B.; Strinic, D.; Vidovic, T.; Luetic, F.; Marusic, M.; Gulic, S.; Pavelic, T.T.; et al. Cyclophosphamide induced stomach and duodenal lesions as a NO-system disturbance in rats: L-NAME, L-arginine, stable gastric pentadecapeptide BPC 157. Inflammopharmacology 2017, 25, 255–264. [Google Scholar] [CrossRef]
- Sucic, M.; Luetic, K.; Jandric, I.; Drmic, D.; Sever, A.Z.; Vuletic, L.B.; Halle, Z.B.; Strinic, D.; Kokot, A.; Seiwerth, R.S.; et al. Therapy of the rat hemorrhagic cystitis induced by cyclophosphamide. Stable gastric pentadecapeptide BPC 157, L-arginine, L-NAME. Eur. J. Pharmacol. 2019, 861, 172593. [Google Scholar] [CrossRef]
- Sever, A.Z.; Sever, M.; Vidovic, T.; Lojo, N.; Kolenc, D.; Vuletic, L.B.; Drmic, D.; Kokot, A.; Zoricic, I.; Coric, M.; et al. Stable gastric pentadecapeptide BPC 157 in the therapy of the rats with bile duct ligation. Eur. J. Pharmacol. 2019, 847, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Amic, F.; Drmic, D.; Bilic, Z.; Krezic, I.; Zizek, H.; Peklic, M.; Klicek, R.; Pajtak, A.; Amic, E.; Vidovic, T.; et al. Bypassing major venous occlusion and duodenal lesions in rats, and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine. World J. Gastroenterol. 2018, 24, 5366–5378. [Google Scholar] [CrossRef] [PubMed]
- Gwyer, D.; Wragg, N.M.; Wilson, S.L. Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell Tissue Res. 2019, 377, 153–159. [Google Scholar] [CrossRef]
- Deek, S.A. BPC 157 as Potential Treatment for COVID-19. Med. Hypotheses 2021, 158, 110736. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D. The first Nobel prize for integrated systems physiology:Ivan Petrovich Pavlov, 1904. Physiology 2004, 19, 326–330. [Google Scholar] [CrossRef]
- Grabarevic, Z.; Tisljar, M.; Artukovic, B.; Bratulic, M.; Dzaja, P.; Seiwerth, S.; Sikiric, P.; Peric, J.; Geres, D.; Kos, J. The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicken. J. Physiol. Paris 1997, 91, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Tlak Gajger, I.; Ribaric, J.; Smodis Skerl, M.; Vlainic, J.; Sikiric, P. Stable gastric pentadecapeptide BPC 157 in honeybee (Apis mellifera) therapy, to control Nosema ceranae invasions in apiary conditions. J. Vet. Pharmacol. Ther. 2018, 41, 614–621. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Smodis Skerl, M.I.; Sostaric, P.; Suran, J.; Sikiric, P.; Vlainic, J. Physiological and immunological status of Adult honeybees (Apis mellifera) fed sugar syrup supplemented with pentadecapeptide BPC 157. Biology 2021, 10, 891. [Google Scholar] [CrossRef]
- Xu, C.; Sun, L.; Ren, F.; Huang, P.; Tian, Z.; Cui, J.; Zhang, W.; Wang, S.; Zhang, K.; He, L.; et al. Preclinical safety evaluation of body protective compound-157, a potential drug for treating various wounds. Regul. Toxicol. Pharmacol. 2020, 114, 104665. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Jing, X.; Tian, X.; Qin, M.C.; Xu, Z.H.; Wu, D.P.; Zhong, Z.G. Neuroprotective properties of Panax notoginseng saponins via preventing oxidative stress injury in SAMP8 mice. Evid. Based Complement Alternat. Med. 2017, 2017, 8713561. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Deigendesch, N.; Wittig, H.; Scheurer, E.; Lenz, C. Tissue sample analysis for post mortem determination of brain edema. Forensic Sci. Int. 2021, 323, 110808. [Google Scholar] [CrossRef] [PubMed]
- Chui, C.J.; McArdle, A.H.; Brown, R.; Scott, H.; Gurd, F. Intestinal mucosal lesion in low-flow states. Arch. Surg. 1970, 101, 478–483. [Google Scholar] [CrossRef]
- Lane, J.S.; Todd, K.E.; Lewis, M.P.; Gloor, B.; Ashley, S.W.; Reber, H.A.; McFadden, D.W.; Chandler, C.F. Interleukin-10 reduces the systemic inflammatory response in a murine model of intestinal ischemia/reperfusion. Surgery 1997, 122, 288–294. [Google Scholar] [CrossRef]

















| Medication (ip) at 5 min after Dopamine Drug Application | Blood Pressure and Thrombosis in Rats at 15 min Following Application of Haloperidol (H), Fluphenazine (F), Clozapine (C), Risperidone (R), Olanzapine (O), Quetiapine (Q), Aripiprazole (AR), Domperidone (D), Amphetamine (AM), and Amphetamine and Haloperidol (AM + H) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H | F | C | R | O | Q | AR | D | AM | AM + H | |
| Superior sagittal sinus pressure, mm Hg, Means ± SD | ||||||||||
| Control | 9 ± 1 | 8 ± 1 | 10 ± 2 | 12 ± 2 | 15 ± 2 | 13 ± 1 | 10 ± 1 | 5 ± 1 | 7 ± 1 | 8 ± 1 |
| BPC 157 10 μg/kg | −3 ± 1 * | −1 ± 1 * | −1 ± 1 * | −1 ± 1 * | −7 ± 1 * | −2 ± 1 * | −1 ± 1 * | −1 ± 1 * | −1 ± 1 * | −1 ± 1 * |
| BPC 157 10 ng/kg | −5 ± 1 * | −2 ± 1 * | −1 ± 1 * | −1 ± 1 * | −6 ± 1 * | −2 ± 1 * | −1 ± 1 * | −1 ± 1 * | −1 ± 1 * | −1 ± 1 * |
| Portal pressure, mm Hg, Means ± SD | ||||||||||
| Control | 17 ± 2 | 12 ± 1 | 15 ± 2 | 12 ± 1 | 18 ± 2 | 13 ± 2 | 12 ± 1 | 13 ± 1 | 13 ± 1 | 12 ± 1 |
| BPC 157 10 μg/kg | 5 ± 1 * | 4 ± 1 * | 4 ± 1 * | 5 ± 1 * | 4 ± 1 * | 3 ± 1 * | 4 ± 1 * | 3 ± 1 * | 4 ± 1 * | 5 ± 1 * |
| BPC 157 10 ng/kg | 5 ± 1 * | 3 ± 1 * | 2 ± 1 * | 5 ± 1 * | 5 ± 1 * | 4 ± 1 * | 5 ± 1 * | 3 ± 1 * | 4 ± 1 * | 4 ± 1 * |
| Caval pressure, mm Hg, Means ± SD | ||||||||||
| Control | 12 ± 2 | 11 ± 1 | 10 ± 1 | 11 ± 1 | 12 ± 2 | 10 ± 1 | 11 ± 1 | 11 ± 1 | 10 ± 2 | 10 ± 1 |
| BPC 157 10 μg/kg | 4 ± 1 * | 3 ± 1 * | 2 ± 1 * | 4 ± 1 * | 3 ± 1 * | 3 ± 1 * | 3 ± 1 * | 2 ± 1 * | 3 ± 1 * | 4 ± 1 * |
| BPC 157 10 ng/kg | 4 ± 1 * | 3 ± 1 * | 2 ± 1 * | 4 ± 1 * | 3 ± 1 * | 3 ± 1 * | 4 ± 1 * | 2 ± 1 * | 3 ± 1 * | 4 ± 1 * |
| Aortal pressure, mm Hg, Means ± SD | ||||||||||
| Control | 72 ± 5 | 55 ± 7 | 55 ± 8 | 53 ± 6 | 75 ± 5 | 52 ± 5 | 66 ± 5 | 52 ± 6 | 60 ± 5 | 55 ± 5 |
| BPC 157 10 μg/kg | 86 ± 4* | 80 ± 5 * | 73 ± 5 * | 82 ± 5 * | 90 ± 6 * | 73 ± 5 * | 78 ± 6 * | 76 ± 5 * | 75 ± 7 * | 78 ± 7 * |
| BPC 157 10 ng/kg | 90 ± 5 * | 83 ± 7 * | 75 ± 6 * | 85 ± 8 * | 92 ± 7 * | 76 ± 6 * | 79 ± 5 * | 79 ± 7 * | 77 ± 6 * | 80 ± 5 * |
| Superior sagittal sinus, thrombus mass, g, Means ± SD | ||||||||||
| Control | 0.0121 ± 0.0010 | 0.0062 ± 0.0009 | 0.081 ± 0.009 | 0.0047 ± 0.0006 | 0.049 ± 0.0008 | 0.086 ± 0.009 | 0.092 ± 0.01 | 0.0030 ± 0.0005 | 0.0028 ± 0.0005 | 0.0111 ± 0.005 |
| BPC 157 10 μg/kg | 0.0031 ± 0.0008 * | 0.0010 ± 0.0007 * | 0.012 ± 0.004 * | 0.0009 ± 0.0004 * | 0.006 ± 0.001 * | 0.015 ± 0.004 * | 0.032 ± 0.009 * | 0.0010 ± 0.0006 * | 0.0007 ± 0.0002 * | 0.0021 ± 0.0005 * |
| BPC 157 10 ng/kg | 0.0035 ± 0.0009 * | 0.0011 ± 0.0005 * | 0.010 ± 0.003 * | 0.0007 ± 0.0003 * | 0.008 ± 0.001 * | 0.012 ± 0.004 * | 0.030 ± 0.008 * | 0.0008 ± 0.0003 * | 0.0005 ± 0.0003 * | 0.0030 ± 0.0005 * |
| Portal vein, thrombus mass, g, Means ± SD | ||||||||||
| Control | 0.0265 ± 0.005 | 0.0106 ± 0.005 | 0.0127 ± 0.005 | 0.074 ± 0.008 | 0.079 ± 0.009 | 0.0107 ± 0.008 | 0.0107 ± 0.009 | 0.0046 ± 0.0009 | 0.0051 ± 0.0009 | 0.0165 ± 0.009 |
| BPC 157 10 μg/kg | 0.003 ± 0.0005 * | 0.0020 ± 0.0005 * | 0.0015 ± 0.0005 * | 0.032 ± 0.008* | 0.031 ± 0.008 * | 0.0025 ± 0.0009 * | 0.0035 ± 0.0007 * | 0.0017 ± 0.0005 * | 0.0021 ± 0.0007 * | 0.0010 ± 0.0009 * |
| BPC 157 10 ng/kg | 0.002 ± 0.0007 * | 0.0018 ± 0.0006 * | 0.0010 ± 0.0005 * | 0.028 ± 0.006 * | 0.033 ± 0.008 * | 0.0020 ± 0.0007 * | 0.0032 ± 0.0009 * | 0.0015 ± 0.0009 * | 0.0025 ± 0.0006 * | 0.0015 ± 0.0009 * |
| Inferior caval vein, thrombus mass, g, Means ± SD | ||||||||||
| Control | 0.0238 ± 0.007 | 0.0171 ± 0.008 | 0.0123 ± 0.006 | 0.0091 ± 0.0007 * | 0.0089 ± 0.0009 | 0.0095 ± 0.0008 | 0.0103 ± 0.005 | 0.0051 ± 0.0009 | 0.0040 ± 0.0007 * | 0.0138 ± 0.007 |
| BPC 157 10 μg/kg | 0.0072 ± 0.0007 * | 0.0071 ± 0.0008 * | 0.0050 ± 0.0009 * | 0.0041 ± 0.0008 * | 0.0035 ± 0.0007 * | 0.0047 ± 0.0008 * | 0.0040 ± 0.0007 * | 0.0020 ± 0.0007 * | 0.0018 ± 0.0007 * | 0.0052 ± 0.0009 * |
| BPC 157 10 ng/kg | 0.0068 ± 0.0008 * | 0.0065 ± 0.0008 * | 0.0055 ± 0.0007 * | 0.0035 ± 0.0006 * | 0.0038 ± 0.0007 * | 0.0045 ± 0.0007 * | 0.0045 ± 0.0009 * | 0.0016 ± 0.0006 * | 0.0020 ± 0.0008 * | 0.0048 ± 0.0007 * |
| Abdominal aorta, thrombus mass, g, Means ± SD | ||||||||||
| Control | 0.0204 ± 0.007 | 0.0113 ± 0.009 | 0.0101 ± 0.007 | 0.0081 ± 0.0009 | 0.0070 ± 0.001 | 0.0101 ± 0.007 | 0.0091 ± 0.001 | 0.0055 ± 0.0009 | 0.0047 ± 0.007 | 0.0104 ± 0.007 |
| BPC 157 10 μg/kg | 0.0036 ± 0.0005 * | 0.0060 ± 0.0007 * | 0.0042 ± 0.0005 * | 0.0041 ± 0.0008 * | 0.0029 ± 0.0005 * | 0.0045 ± 0.0006 * | 0.0032 ± 0.0007 * | 0.0025 ± 0.0006 * | 0.0021 ± 0.0007 * | 0.0026 ± 0.0008 * |
| BPC 157 10 ng/kg | 0.0040 ± 0.0007 * | 0.0055 ± 0.0009 * | 0.0035 ± 0.0007 * | 0.0043 ± 0.0007 * | 0.0025 ± 0.0005 * | 0.0048 ± 0.0007 * | 0.0038 ± 0.0008 * | 0.0022 ± 0.0007 * | 0.0018 ± 0.0008 * | 0.0030 ± 0.0009 * |
| Medication (ip) at 5 min after Dopamine Drug Application | Relative Volume (Control/Treated) (%) of the Brain, Heart, Azygos Vein, Inferior Caval Vein, Superior Mesenteric Vein, and Abdominal Aorta in Rats at 15 min Following Application of Haloperidol (H), Fluphenazine (F), Clozapine (C), Risperidone (R), Olanzapine (O), Quetiapine (Q), Aripiprazole (AR), Domperidone (D), Amphetamine (AM), and Amphetamine and Haloperidol (AM + H) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H | F | C | R | O | Q | AR | D | AM | AM + H | |
| Relative volume (control/treated) (%) of the brain, Means ± SD | ||||||||||
| BPC 157 10 μg/kg | 113 ± 3 * | 118 ± 2 * | 109 ± 3 * | 115 ± 3 * | 109 ± 3 * | 111 ± 3 * | 117 ± 2 * | 108 ± 2 * | 112 ± 3 * | 115 ± 3 * |
| BPC 157 10 ng/kg | 111 ± 3 * | 115 ± 3 * | 111 ± 2 * | 116 ± 2 * | 110 ± 2 * | 113 ± 2 * | 115 ± 3 * | 110 ± 2 * | 110 ± 3 * | 117 ± 3 * |
| Relative volume (control/treated) (%) of the heart, Means ± SD | ||||||||||
| BPC 157 10 μg/kg | 117 ± 4 * | 121 ± 5 * | 110 ± 3 * | 108 ± 3 * | 147 ± 6 * | 138 ± 6 * | 120 ± 4 * | 123 ± 3 * | 111 ± 3 * | 126 ± 5 * |
| BPC 157 10 ng/kg | 119 ± 3 * | 124 ± 4 * | 113 ± 4 * | 110 ± 3 * | 144 ± 7 * | 135 ± 7 * | 125 ± 5 * | 120 ± 5 * | 114 ± 3 * | 129 ± 6 * |
| Relative volume (control/treated) (%) of the azygos vein, Means ± SD | ||||||||||
| BPC 157 10 μg/kg | 69 ± 6 * | 40 ± 6 * | 31 ± 6 * | 43 ± 4 * | 36 ± 6 * | 50 ± 6 * | 43 ± 6 * | 25 ± 6 * | 45 ± 7 * | 39 ± 6 * |
| BPC 157 10 ng/kg | 66 ± 4 * | 44 ± 7 * | 29 ± 5 * | 40 ± 6 | 33 ± 5 * | 52 ± 7 * | 45 ± 5 * | 27 ± 7 * | 47 ± 6 * | 41 ± 7 * |
| Relative volume (control/treated) (%) of the inferior caval vein, Means ± SD | ||||||||||
| BPC 157 10 μg/kg | 151 ± 10 * | 139 ± 6 * | 151 ± 9 * | 112 ± 5 | 206 ± 12 | 127 ± 6 * | 123 ± 8 * | 125 ± 6 * | 117 ± 5 | 134 ± 9 * |
| BPC 157 10 ng/kg | 155 ± 12 * | 136 ± 7 * | 155 ± 12 * | 116 ± 6 * | 202 ± 14 | 125 ± 7 * | 120 ± 9 * | 128 ± 8 * | 119 ± 7 * | 136 ± 8 * |
| Relative volume (control/treated) (%) of the superior mesenteric vein, Means ± SD | ||||||||||
| BPC 157 10 μg/kg | 189 ± 12 * | 269 ± 22 * | 260 ± 15 * | 218 ± 14 * | 233 ± 19 * | 167 ± 17 * | 137 ± 12 * | 132 ± 15 * | 268 ± 18 * | 184 ± 16 * |
| BPC 157 10 ng/kg | 199 ± 19 * | 259 ± 24 * | 270 ± 14 * | 226 ± 19 * | 239 ± 16 * | 158 ± 15 * | 144 ± 16 * | 138 ± 17 * | 255 ± 22 * | 188 ± 18 * |
| Relative volume (control/treated) (%) of the abdominal aorta, Means ± SD | ||||||||||
| BPC 157 10 μg/kg | 69 ± 10 * | 66 ± 12 * | 58 ± 14 * | 58 ± 9 * | 67 ± 8 * | 77 ± 9 * | 55 ± 11 * | 60 ± 10 * | 70 ± 8 * | 78 ± 7 * |
| BPC 157 10 ng/kg | 66 ± 7 * | 68 ± 10 * | 62 ± 12 * | 62 ± 8 * | 64 ± 7 * | 73 ± 6 * | 50 ± 9 * | 58 ± 8 * | 66 ± 7 * | 74 ± 9 * |
| Medication (ip) at 5 min after Dopamine Drug Application | ECG Changes in Rats at 15 min Following Application of Haloperidol (H), Fluphenazine (F), Clozapine (C), Risperidone (R), Olanzapine (O), Quetiapine (Q), Aripiprazole (AR), Domperidone (D), Amphetamine (AM), and Amphetamine and Haloperidol (AM + H) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H | F | C | R | O | Q | AR | D | AM | AM + H | |
| PQ interval, msec Means ± SD | ||||||||||
| Control | 65 ± 5 | 66 ± 4 | 67 ± 4 | 65 ± 5 | 68 ± 5 | 65 ± 5 | 66 ± 5 | 65 ± 5 | 65 ± 5 | 50 ± 5 |
| BPC 157 10 μg/kg | 50 ± 5 * | 50 ± 5 * | 50 ± 5 * | 50 ± 5 * | 50 ± 5 * | 50 ± 5 * | 50 ± 5 * | 50 ± 5 * | 50 ± 5 * | 50 ± 5 |
| BPC 157 10 ng/kg | 50 ± 5 * | 50 ± 5 * | 50 ± 5 * | 50 ± 5 * | 50 ± 5 * | 50 ± 5 * | 50 ± 5 * | 50 ± 5 * | 50 ± 5 * | 50 ± 5 |
| QTc interval, msec, Means ± SD | ||||||||||
| Control | 241 ± 10 | 247 ± 10 | 251 ± 10 | 255 ± 10 | 248 ± 10 | 254 ± 10 | 250 ± 10 | 251 ± 10 | 175 ± 5 | 190 ± 10 |
| BPC 157 10 μg/kg | 187 ± 10 * | 185 ± 10 * | 190 ± 10 * | 185 ± 10 * | 185 ± 10 * | 188 ± 10 * | 185 ± 10 * | 195 ± 10 * | 195 ± 5 * | 185 ± 10 |
| BPC 157 10 ng/kg | 186 ± 10 * | 190 ± 10 * | 190 ± 10 * | 190 ± 10 * | 187 ± 10 * | 190 ±10 * | 190 ± 10 * | 190 ± 10 * | 190 ± 5 * | 190 ± 10 |
| Heart frequency, beats/min, Means ± SD | ||||||||||
| Control | 430 ± 10 | 450 ± 10 | 465 ± 10 | 485 ± 10 | 480 ± 10 | 445 ± 10 | 485 ± 10 | 465 ± 10 | 440 ± 10 | 440 ± 10 |
| BPC 157 10 μg/kg | 370 ± 10 * | 350 ± 10 * | 380 ± 10 * | 360 ± 10 * | 375 ± 10 * | 355 ± 10 * | 390 ± 10 * | 370 ± 10 * | 405 ± 10 * | 395 ± 10 * |
| BPC 157 10 ng/kg | 365 ± 10 * | 355 ± 10 * | 375 ± 10 * | 355 ± 10 * | 385 ± 10 * | 350 ± 10 * | 385 ± 10 * | 375 ± 10 * | 400 ± 10 * | 390 ± 10 * |
| Medication (ip) at 5 min after Dopamine Drug Application | Lesions Scored Microscopically (Heart, Lung, Liver, Kidney, and Stomach) or Macroscopically (Stomach) in Rats at 15 min Following Application of Haloperidol (H), Fluphenazine (F), Clozapine (C), Risperidone (R), Olanzapine (O), Quetiapine (Q), Aripiprazole (AR), Domperidone (D), Amphetamine (AM), and Amphetamine and Haloperidol (AM + H) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H | F | C | R | O | Q | AR | D | AM | AM + H | |
| Heart (scored 0–3, Min/Med/Max) | ||||||||||
| Control | 3/3/3 | 2/3/3 | 2/3/3 | 2/2/3 | 2/2/2 | 2/3/3 | 2/2/2 | 2/3/3 | 2/2/2 | 2/2/2 |
| BPC 157 10 μg/kg | 1/1/1 * | 0/0/0 * | 1/1/1 * | 0/1/1 * | 0/1/1 * | 1/2/2 * | 1/1/1 * | 1/1/1 * | 0/0/0 * | 0/0/0 * |
| BPC 157 10 ng/kg | 1/1/1 * | 0/0/0 * | 1/1/1 * | 0/1/1 * | 0/1/1 * | 1/2/2 * | 1/1/1 * | 1/1/1 * | 0/0/0 * | 0/0/0 * |
| Lung (scored 0–3, Min/Med/Max) | ||||||||||
| Control | 2/2/2 | 1/2/2 | 3/3/3 | 3/3/3 | 1/2/2 | 2/2/3 | 1/2/2 | 2/2/2 | 2/3/3 | 2/3/3 |
| BPC 157 10 μg/kg | 1/1/2 * | 0/0/0 * | 1/1/1 * | 0/1/1 * | 1/1/1 * | 1/1/2 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/0/0 * |
| BPC 157 10 ng/kg | 1/1/2 * | 0/0/0 * | 1/1/1 * | 0/1/1 * | 1/1/1 * | 1/1/2 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/0/0 * |
| Liver (scored 0–3, Min/Med/Max) | ||||||||||
| Control | 2/2/2 | 2/3/3 | 2/3/3 | 2/3/3 | 2/3/3 | 2/3/3 | 2/3/3 | 2/2/2 | 2/2/2 | 3/3/3 |
| BPC 157 10 μg/kg | 0/0/0 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 1/1/1 * | 0/1/1 * | 0/0/0 * | 0/1/1 * | 0/0/0 * | 0/0/0 * |
| BPC 157 10 ng/kg | 0/0/0 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 1/1/1 * | 0/1/1 * | 0/0/0 * | 0/1/1 * | 0/0/0 * | 0/0/0 * |
| Kidney (scored 0–3, Min/Med/Max) | ||||||||||
| Control | 2/2/2 | 2/2/2 | 2/2/2 | 1/2/2 | 2/2/2 | 2/3/3 | 2/3/3 | 2/3/3 | 3/3/3 | 2/2/2 |
| BPC 157 10 μg/kg | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 1/1/1 * | 1/1/1 * | 1/1/1 * | 1/1/1 * | 0/1/1 * |
| BPC 157 10 ng/kg | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 1/1/1 * | 0/1/1 * | 1/1/1 * | 0/1/1 * | 1/1/1 * | 0/1/1 * |
| Stomach (sum of longest diameters, mm, Means ± SD) | ||||||||||
| Control | 11 ± 2 | 5 ± 1 | 5 ± 2 | 5 ± 1 | 10 ± 2 | 3 ± 1 | 3 ± 1 | 12 ± 3 | 10 ± 2 | 10 ± 3 |
| BPC 157 10 μg/kg | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * |
| BPC 157 10 ng/kg | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * |
| Stomach (scored 0–15, Min/Med/Max) | ||||||||||
| Control | 2/2/2 | 1/1/1 | 1/1/1 | 2/2/2 | 2/2/2 | 2/2/2 | 1/2/2 | 1/2/2 | 1/1/1 | 2/3/3 |
| BPC 157 10 μg/kg | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * |
| BPC 157 10 ng/kg | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * |
| Medication (ip) at 5 min after Dopamine Drug Application | Lesions Scored Microscopically in the Cerebrum, Cerebellum, Hypothalamus, and Hippocampus in Rats at 15 min Following Application of Haloperidol (H), Fluphenazine (F), Clozapine (C), Risperidone (R), Olanzapine (O), Quetiapine (Q), Aripiprazole (AR), Domperidone (D), Amphetamine (AM), and Amphetamine and Haloperidol (AM + H) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H | F | C | R | O | Q | AR | D | AM | AM + H | |
| Cerebrum (scored 0–8, Min/Med/Ma) Percentage of karyopyknotic cells (%), Means ± SD | ||||||||||
| Control | 2/2/2 40 ± 5 | ½/2 35 ± 5 | 1/1/1 20 ± 5 | 1/1/2 25 ± 5 | 2/2/2 30 ± 5 | 2/2/2 30 ± 5 | 2/3/3 55 ± 5 | 2/3/3 60 ± 5 | 2/3/3 65 ± 5 | 3/3/3 60 ± 5 |
| BPC 157 10 μg/kg | 0/0/1 * 10 ± 10 * | 1/1/1 10 ± 5 * | 0/0/1 * 5 ± 5 * | 0/0/1 * 5 ± 5 * | 0/0/1 * 5 ± 5 * | 0/0/1 * 5 ± 5 * | 1/1/2 * 20 ± 10 * | 1/1/2 * 20 ± 10 * | 1/1/2 * 20 ± 10 * | 0/0/1 * 10 ± 10 * |
| BPC 157 10 ng/kg | 0/0/1 * 10 ± 10 * | 1/1/1 10 ± 5 * | 0/0/1 * 5 ± 5 * | 0/0/1 * 5 ± 5 * | 0/0/1 * 5 ± 5 * | 0/0/1 * 5 ± 5 * | 1/1/2 * 20 ± 10 * | 1/1/2 * 20 ± 10 * | 1/1/2 * 20 ± 10 * | 0/0/1 * 10 ± 10 * |
| Neuronal damage in the karyopyknotic areas, %, Means ± SD (10 HPF, 400×) | ||||||||||
| Control | 10 ± 2 | 10 ± 1 | 17 ± 3 | 15 ± 2 | 23 ± 3 | 10 ± 3 | 15 ± 3 | 32 ± 4 | 24 ± 3 | 33 ± 3 |
| BPC 157 10 μg/kg | 2 ± 2 * | 3 ± 2 * | 9 ± 2 * | 7 ± 2 * | 3 ± 3 * | 4 ± 2 * | 10 ± 1 | 13 ± 2 * | 7 ± 2 * | 19 ± 2 * |
| BPC 157 10 ng/kg | 2 ± 2 * | 2 ± 2 * | 10 ± 2 * | 5 ± 2 * | 3 ± 3 * | 4 ± 2 * | 9 ± 1 | 12 ± 2 * | 6 ± 2 * | 18 ± 2 * |
| Hemorrhage (% of total area), Means ± SD | ||||||||||
| Control | 40 ± 5 | 20 ± 2 | 0 ± 0 | 0 ± 0 | 40 ± 5 | 20 ± 2 | 20 ± 3 | 40 ± 5 | 40 ± 5 | 60 ± 5 |
| BPC 157 10 μg/kg | 10 ± 3 * | 10 ± 5 * | 0 ± 0 | 0 ± 0 | 10 ± 5 * | 10 ± 3 * | 10 ± 3 * | 20 ± 3 * | 10 ± 3 * | 20 ± 5 * |
| BPC 157 10 ng/kg | 9 ± 3 * | 8 ± 4 * | 0 ± 0 | 0 ± 0 | 9 ± 3 * | 9 ± 4 * | 11 ± 3 * | 20 ± 3 * | 10 ± 3 * | 20 ± 5 * |
| Edema (scored 0–3, Min/Med/Max) | ||||||||||
| Control | 3/3/3 | 3/3/3 | 2/3/3 | 3/3/3 | 3/3/3 | 2/3/3 | 2/3/3 | 2/3/3 | 2/3/3 | 3/3/3 |
| BPC 157 10 μg/kg | 1/1/1 * | 1/1/1 * | 0/1/1 * | 1/1/2 * | 1/1/1 * | 1/1/1 * | 1/1/2 * | 1/1/2 * | 2/2/2 * | 2/2/2 * |
| BPC 157 10 ng/kg | 1/1/1 * | 1/1/1 * | 0/1/1 * | 1/1/2 * | 1/1/1 * | 1/1/1 * | 1/1/2 * | 1/1/2 * | 2/2/2 * | 2/2/2 * |
| Cerebellum (scored 0–8, Min/Med/Ma) Percentage of karyopyknotic cells (%), Means ± SD | ||||||||||
| Control | ½/2 30 ± 10 | 1/1/1 20 ± 2 | 1/1/1 20 ± 2 | 1/1/1 18 ± 2 | 1/1/1 20 ± 2 | ½/2 30 ± 2 | 2/3/3 55 ± 5 | 1/1/1 20 ± 2 | 1/1/1 20 ± 2 | 2/3/3 60 ± 5 |
| BPC 157 10 μg/kg | 0/1/1 * 5 ± 5 * | 0/1/1 * 3 ± 2 * | 1/1/1 * 3 ± 2 * | 0/1/1 * 3 ± 2 * | 0/1/1 * 3 ± 2 * | 1/1/1 * 7 ± 2 * | 1/1/1 * 16 ± 4 * | 0/1/1 * 3 ± 2 * | 1/1/1 * 7 ± 2 * | 1/1/1 * 7 ± 2 * |
| BPC 157 10 ng/kg | 0/1/1 * 5 ± 5 * | 0/1/1 * 3 ± 2 * | 1/1/1 * 3 ± 2 * | 0/1/1 * 3 ± 2 * | 0/1/1 * 3 ± 2 * | 1/1/1 * 7 ± 2 * | 1/1/1 * 15 ± 5 * | 0/1/1 * 3 ± 2 * | 1/1/1 * 6 ± 2 * | 1/1/1 * 6 ± 2 * |
| Neuronal damage in the karyopyknotic areas, %, Means ± SD (10 HPF, 400×) | ||||||||||
| Control | 3 ± 1 | 7 ± 2 | 8 ± 3 | 10 ± 2 | 4 ± 1 | 8 ± 1 | 15 ± 2 | 5 ± 1 | 5 ± 2 | 18 ± 1 |
| BPC 157 10 μg/kg | 0 ± 0 * | 3 ± 1 * | 1 ± 1 * | 1 ± 1 * | 1 ± 1 * | 3 ± 1 * | 4 ± 1 * | 1 ± 1 * | 1 ± 1 * | 4 ± 2 |
| BPC 157 10 ng/kg | 0 ± 0 * | 3 ± 1 * | 1 ± 1 * | 1 ± 1 * | 1 ± 1 * | 2 ± 1 * | 3 ± 1 * | 1 ± 1 * | 1 ± 1 * | 3 ± 1 * |
| Hemorrhage (% of total area), Means ± SD | ||||||||||
| Control | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BPC 157 10 μg/kg | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BPC 157 10 ng/kg | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Edema (scored 0–3, Min/Med/Max) | ||||||||||
| Control | 3/3/3 | 2/2/2 | 2/3/3 | 2/2/2 | 2/3/3 | 2/2/2 | 2/3/3 | 2/3/3 | 2/2/2 | 2/3/3 |
| BPC 157 10 μg/kg | 1/1/1 * | 1/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 1/1/1 * | 0/1/1 * | 0/1/1 * | 1/1/1 * | 1/1/1 * |
| BPC 157 10 ng/kg | 1/1/1 * | 1/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 1/1/1 * | 0/1/1 * | 0/1/1 * | 1/1/1 * | 1/1/1 * |
| Hippocampus (scored 0–8, Min/Med/Max) Percentage of karyopyknotic cells (%), Means ± SD | ||||||||||
| Control | 1/1/1 13 ± 3 | 1/1/1 10 ± 1 | 1/1/1 10 ± 1 | 1/1/1 5 ± 0 | 1/1/1 10 ± 1 | 1/1/1 10 ± 1 | 1/1/1 10 ± 1 | 1/1/1 10 ± 1 | 1/1/1 10 ± 1 | 1/1/1 10 ± 1 |
| BPC 157 10 μg/kg | 0/1/1 * 3 ± 1 * | 0/1/1 * 3 ± 1 * | 0/1/1 * 3 ± 1 * | 0/1/1 * 3 ± 1 * | 0/0/0 * 0 ± 0 * | 0/1/1 * 3 ± 1 * | 0/1/1 * 2 ± 1 * | 0/1/1 * 3 ± 1 * | 0/1/1 * 3 ± 1 * | 0/1/1 * 3 ± 1 * |
| BPC 157 10 ng/kg | 0/1/1 * 3 ± 1 * | 0/1/1 * 3 ± 1 * | 0/1/1 * 3 ± 1 * | 0/1/1 * 3 ± 1 * | 0/0/0 * 0 ± 0 * | 0/1/1 * 3 ± 1 * | 0/1/1 * 2 ± 1 * | 0/1/1 * 3 ± 1 * | 0/1/1 * 3 ± 1 * | 0/1/1 * 3 ± 1 * |
| Neuronal damage in the karyopyknotic areas, %, Means ± SD (10 HPF, 400×) | ||||||||||
| Control | 3 ± 1 | 7 ± 1 | 2 ± 1 | 2 ± 1 | 9 ± 1 | 3 ± 1 | 6 ± 1 | 7 ± 1 | 2 ± 1 | 4 ± 1 |
| BPC 157 10 μg/kg | 1 ± 0 * | 1 ± 1 * | 1 ± 0 * | 1 ± 0 * | 0 ± 0 * | 1 ± 0 * | 1 ± 0 * | 1 ± 0 * | 1 ± 0 * | 2 ± 1 * |
| BPC 157 10 ng/kg | 1 ± 0 * | 1 ± 1 * | 1 ± 0 * | 1 ± 0 * | 0 ± 0 * | 1 ± 0 * | 1 ± 0 * | 1 ± 0 * | 1 ± 0 * | 2 ± 1 * |
| Hemorrhage (% of total area), Means ± SD | ||||||||||
| Control | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BPC 157 10 μg/kg | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BPC 157 10 ng/kg | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Edema (scored 0–3, Min/Med/Max) | ||||||||||
| Control | 2/2/2 | 2/3/3 | 2/3/3 | 2/3/3 | 2/3/3 | ½/3 | 2/2/2 | 2/2/2 | 2/2/2 | 2/2/2 |
| BPC 157 10 μg/kg | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/0/0 * | 0/1/1 * | 0/0/0 * | 0/1/1 * | 0/1/1 * | 1/1/1 * |
| BPC 157 10 ng/kg | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/0/0 * | 0/1/1 * | 0/0/0 * | 0/1/1 * | 0/1/1 * | 1/1/1 * |
| Hypothalamus (scored 0–8, Min/Med/Max) Percentage of karyopyknotic cells (%), Means ± SD | ||||||||||
| Control | 1/1/1 13 ± 2 | 1/1/1 13 ± 2 | 1/1/1 5 ± 1 | 1/1/1 5 ± 1 | 2/2/2 37 ± 3 | 1/1/1 20 ± 2 | 2/2/2 40 ± 2 | 1/1/1 10 ± 1 | 2/2/2 40 ± 1 | 2/2/2 30 ± 2 |
| BPC 157 10 μg/kg | 0/1/1 * 3 ± 2 * | 0/1/1 * 3 ± 2 * | 0/1/1 * 3 ± 2 * | 0/1/1 * 3 ± 2 * | 0/1/1 * 3 ± 2 * | 0/1/1 * 3 ± 2 * | 1/1/1 * 20 ± 2 * | 1/1/1 * 5 ± 2 * | 1/1/1 * 10 ± 1 * | 1/1/1 * 16 ± 4 |
| BPC 157 10 ng/kg | 0/1/1 * 3 ± 2 * | 0/1/1 * 3 ± 2 * | 0/1/1 * 3 ± 2 * | 0/1/1 * 3 ± 2 * | 0/1/1 * 3 ± 2 * | 0/1/1 * 3 ± 2 * | 1/1/1 * 20 ± 2 * | 1/1/1 * 5 ± 3 * | 1/1/1 * 10 ± 1 * | 1/1/1 * 15 ± 5 * |
| Neuronal damage in the karyopyknotic areas, %, Means ± SD (10 HPF, 400×) | ||||||||||
| Control | 4 ± 1 | 8 ± 2 | 6 ± 2 | 6 ± 2 | 22 ± 1 | 7 ± 1 | 22 ± 6 | 7 ± 1 | 22 ± 2 | 19 ± 3 |
| BPC 157 10 μg/kg | 1 ± 1 * | 1 ± 1 * | 1 ± 1 * | 2 ± 1 * | 3 ± 2 * | 1 ± 1 * | 9 ± 3 * | 3 ± 1 * | 5 ± 2 * | 9 ± 2 * |
| BPC 157 10 ng/kg | 1 ± 1 * | 1 ± 1 * | 1 ± 1 * | 2 ± 1 * | 3 ± 2 * | 1 ± 1 * | 8 ± 3 * | 3 ± 1 * | 5 ± 3 * | 10 ± 2 * |
| Hemorrhage (% of total area), Means ± SD | ||||||||||
| Control | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BPC 157 10 μg/kg | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BPC 157 10 ng/kg | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Edema (scored 0–3, Min/Med/Max) | ||||||||||
| Control | 3/3/3 | 2/2/2 | 2/2/2 | 2/2/2 | 2/3/3 | 2/2/2 | 2/3/3 | 2/2/2 | 2/3/3 | 2/3/3 |
| BPC 157 10 μg/kg | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 1/1/1 * | 2/2/2 * |
| BPC 157 10 ng/kg | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 0/1/1 * | 1/1/1 * | 2/2/2 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strbe, S.; Smoday, I.M.; Krezic, I.; Kalogjera, L.; Vukovic, V.; Zizek, H.; Gojkovic, S.; Vranes, H.; Barisic, I.; Sikiric, S.; et al. Innate Vascular Failure by Application of Neuroleptics, Amphetamine, and Domperidone Rapidly Induced Severe Occlusion/Occlusion-like Syndromes in Rats and Stable Gastric Pentadecapeptide BPC 157 as Therapy. Pharmaceuticals 2023, 16, 788. https://doi.org/10.3390/ph16060788
Strbe S, Smoday IM, Krezic I, Kalogjera L, Vukovic V, Zizek H, Gojkovic S, Vranes H, Barisic I, Sikiric S, et al. Innate Vascular Failure by Application of Neuroleptics, Amphetamine, and Domperidone Rapidly Induced Severe Occlusion/Occlusion-like Syndromes in Rats and Stable Gastric Pentadecapeptide BPC 157 as Therapy. Pharmaceuticals. 2023; 16(6):788. https://doi.org/10.3390/ph16060788
Chicago/Turabian StyleStrbe, Sanja, Ivan Maria Smoday, Ivan Krezic, Luka Kalogjera, Vlasta Vukovic, Helena Zizek, Slaven Gojkovic, Hrvoje Vranes, Ivan Barisic, Suncana Sikiric, and et al. 2023. "Innate Vascular Failure by Application of Neuroleptics, Amphetamine, and Domperidone Rapidly Induced Severe Occlusion/Occlusion-like Syndromes in Rats and Stable Gastric Pentadecapeptide BPC 157 as Therapy" Pharmaceuticals 16, no. 6: 788. https://doi.org/10.3390/ph16060788







