Therapeutic Potential of Hibiscus sabdariffa Linn. in Attenuating Cardiovascular Risk Factors
Abstract
:1. Introduction
2. Origin and Beneficial Use of Roselle
Bioactive Compounds of Roselle
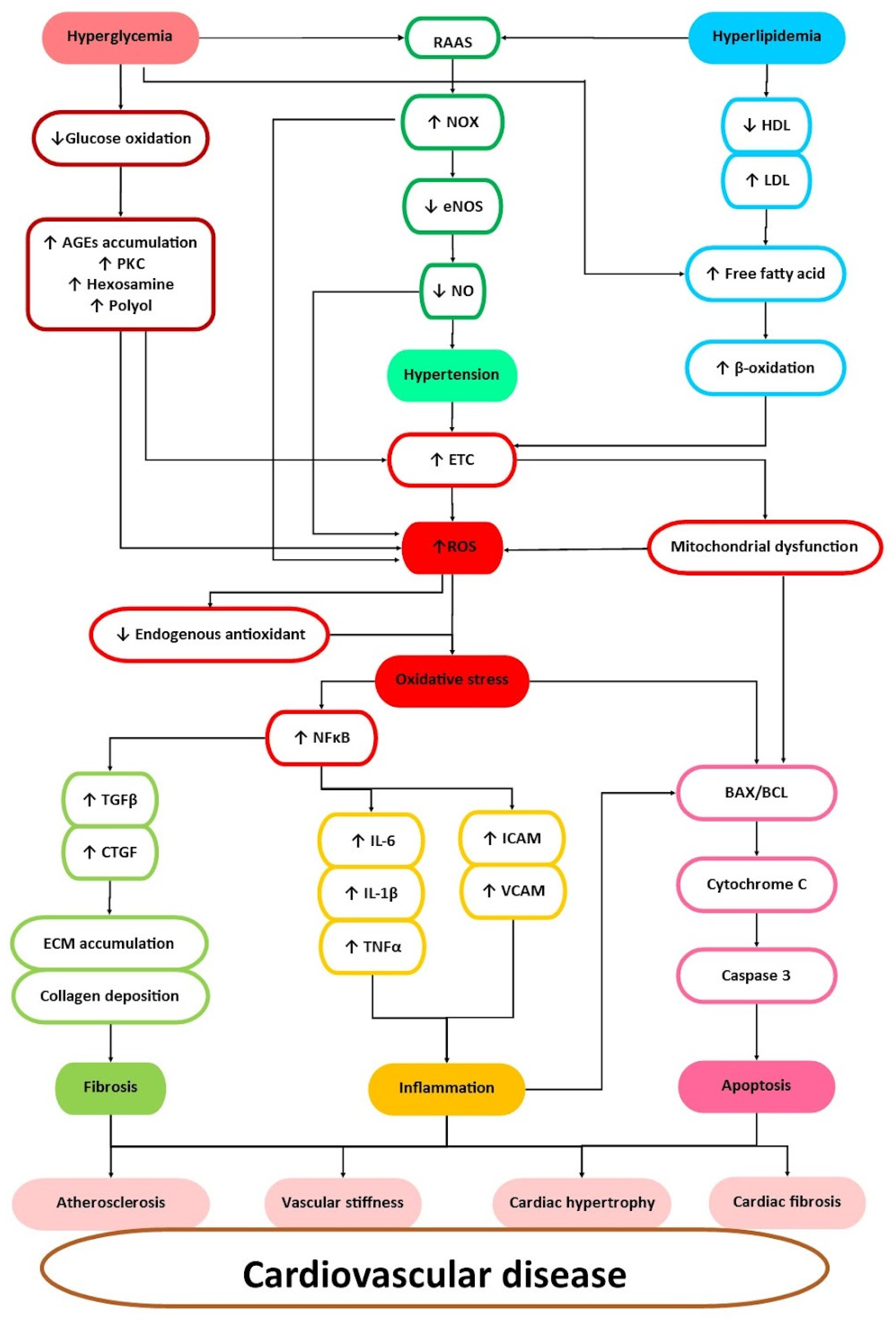
3. Cardiovascular Risk Factors and Their Management
4. Roselle in the Attenuation of Cardiovascular Diseases Risk Factors
4.1. Anti-Hypertensive
4.2. Anti-Hyperlipidemic
4.3. Anti-Hyperglycemic
4.4. Antioxidant
4.5. Anti-Inflammatory
4.6. Anti-Fibrosis
5. Clinical Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| ACE | Angiotensin-Converting Enzyme |
| AGE | Advanced Glycation End-Product |
| AMPK | 5′ Adenosine Monophosphate-Activated Protein Kinase |
| AOPP | Advanced Oxidation Protein Product |
| BNP | B-Type Natriuretic Peptide |
| CAT | Catalase |
| CRP | C-Reactive Protein |
| CTGF | Connective Tissue Growth Factor |
| CVD | Cardiovascular Disease |
| DM | Diabetes Mellitus |
| ECM | Extracellular Matrix |
| eNOS | Endothelial Nitric Oxide Synthase |
| GSH | Glutathione |
| HDL | High-Density Lipoprotein |
| HMG-CoA | 3-Hydroxy-3-Methylglutaryl Coenzyme A |
| ICAM | Intracellular Adhesion Molecule |
| IL | Interleukin |
| LDL | Low-Density Lipoprotein |
| MDA | Malondialdehyde |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NO | Nitric Oxide |
| NOX | Reduced Nicotinamide Adenine Dinucleotide Phosphate Oxidase |
| PKC | Protein Kinase C |
| RAAS | Renin–Angiotensin–Aldosterone System |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| SERCA | Sarcoendoplasmic Reticulum Calcium Transport ATPase |
| SREBP-1 | Sterol Regulatory Element-Binding Protein-1 |
| SOD | Superoxide Dismutase |
| TG | Triglyceride |
| TGF-β | Transforming Growth Factor Beta |
| TNFα | Tumor Necrosis Factor Alpha |
| VCAM | Vascular Adhesion Molecule |
| VLDL | Very Low-Density Lipoprotein |
References
- EHN. Heart Failure and Cardiovascular Diseases. Available online: https://ehnheart.org/publications-and-papers/publications/1202:heart-failure-and-cardiovascular-diseases.html (accessed on 22 April 2019).
- Stewart, J.; Manmathan, G.; Wilkinson, P. Primary prevention of cardiovascular disease: A review of contemporary guidance and literature. JRSM Cardiovasc. Dis. 2017, 6, 2048004016687211. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 June 2021).
- Capewell, S.; Ford, E.S.; Croft, J.B.; Critchley, J.A.; Greenlund, K.J.; Labarthe, D.R. Cardiovascular risk factor trends and potential for reducing coronary heart disease mortality in the United States of America. Bull. World Health Organ. 2010, 88, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Thuan, D.T.B.; Phu, H.T.; Nguyen, T.H.D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G.K.; Eid, A.H.; Pintus, G. Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Front. Pharmacol. 2020, 11, 422. [Google Scholar] [CrossRef]
- Hamjane, N.; Benyahya, F.; Nourouti, N.G.; Mechita, M.B.; Barakat, A. Cardiovascular diseases and metabolic abnormalities associated with obesity: What is the role of inflammatory responses? A systematic review. Microvasc. Res. 2020, 131, 104023. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Diteepeng, T.; del Monte, F.; Luciani, M. The long and winding road to target protein misfolding in cardiovascular diseases. Eur. J. Clin. Investig. 2021, 51, e13504. [Google Scholar] [CrossRef]
- Sapian, S.; Taib, I.S.; Latip, J.; Katas, H.; Chin, K.-Y.; Nor, N.A.M.; Jubaidi, F.F.; Budin, S.B. Therapeutic Approach of Flavonoid in Ameliorating Diabetic Cardiomyopathy by Targeting Mitochondrial-Induced Oxidative Stress. Int. J. Mol. Sci. 2021, 22, 11616. [Google Scholar] [CrossRef]
- Kar, S.; Kambis, T.N.; Mishra, P.K. Hydrogen sulfide-mediated regulation of cell death signaling ameliorates adverse cardiac remodeling and diabetic cardiomyopathy. Am. J. Physiol. Circ. Physiol. 2019, 316, H1237–H1252. [Google Scholar] [CrossRef]
- Rippe, J.M. Lifestyle Strategies for Risk Factor Reduction, Prevention, and Treatment of Cardiovascular Disease. Am. J. Lifestyle Med. 2018, 13, 204–212. [Google Scholar] [CrossRef]
- Ajani, E.O.; Bamisaye, F.A.; Amusa, T.O.; Atolani, O.; Kola-Mustapha, A.T.; Njinga, N.S.; Quadri, L.A.; Bakare-Odunola, M.T.; Oladiji, A.T.; Kambizi, L. Roselle hibiscus sabdarrifa calyces extracts modulates cardiovascular disease risk and kidney dysfunctions in diabetic rats. Plant Arch. 2021, 21, 1350–1359. [Google Scholar] [CrossRef]
- Si, L.Y.-N.; Ali, S.A.M.; Latip, J.; Fauzi, N.M.; Budin, S.B.; Zainalabidin, S. Roselle is cardioprotective in diet-induced obesity rat model with myocardial infarction. Life Sci. 2017, 191, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, V.H.; Ramachandra, C.T. Roselle (Hibiscus sabdariffa L.) Calyces: A Potential Source of Natural Color and Its Health Benefits. In Food bioactives; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 169–190. [Google Scholar] [CrossRef]
- Patel, S. Hibiscus sabdariffa: An ideal yet under-exploited candidate for nutraceutical applications. Biomed. Prev. Nutr. 2014, 4, 23–27. [Google Scholar] [CrossRef]
- Islam, M. Food and medicinal values of Roselle (Hibiscus sabdariffa L. Linne Malvaceae) plant parts: A review. Open J. Nutr. Food Sci. 2019, 1, 1003. [Google Scholar]
- Salem, M.A.; Zayed, A.; Beshay, M.E.; Abdel Mesih, M.M.; Ben Khayal, R.F.; George, F.A.; Ezzat, S.M. Hibiscus sabdariffa L.: Phytoconstituents, nutritive, and pharmacological applications. Adv. Tradit. Med. 2021, 22, 497–507. [Google Scholar] [CrossRef]
- Singh, P.; Khan, M.; Hailemariam, H. Nutritional and health importance of Hibiscus sabdariffa: A review and indication for research needs. J. Nutr. Health Food Eng. 2017, 6, 00212. [Google Scholar]
- El-Nagerabi, S.A.; Al-Bahry, S.N.; Elshafie, A.E.; AlHilali, S. Effect of Hibiscus sabdariffa extract and Nigella sativa oil on the growth and aflatoxin B1 production of Aspergillus flavus and Aspergillus parasiticus strains. Food Control 2012, 25, 59–63. [Google Scholar] [CrossRef]
- Hapsari, B.W.; Setyaningsih, W. Methodologies in the analysis of phenolic compounds in roselle (Hibiscus sabdariffa L.): Composition, biological activity, and beneficial effects on human health. Horticulturae 2021, 7, 35. [Google Scholar] [CrossRef]
- Ariyabukalakorn, V.; Panthong, S.; Itharat, A. Effects and chemical contents of hydrolysis modification of aqueous roselle extract to reflect the antioxidant and anti-inflammatory effects. Sci. Technol. Asia 2019, 24, 115–125. [Google Scholar]
- Sapian, S.; Taib, I.S.; Katas, H.; Latip, J.; Zainalabidin, S.; Hamid, Z.A.; Anuar, N.N.M.; Budin, S.B. The Role of Anthocyanin in Modulating Diabetic Cardiovascular Disease and Its Potential to Be Developed as a Nutraceutical. Pharmaceuticals 2022, 15, 1344. [Google Scholar] [CrossRef]
- Hinojosa-Gómez, J.; San Martín-Hernández, C.; Heredia, J.B.; León-Félix, J.; Osuna-Enciso, T.; Muy-Rangel, M.D. Anthocyanin induction by drought stress in the calyx of roselle cultivars. Molecules 2020, 25, 1555. [Google Scholar] [CrossRef] [PubMed]
- Si, L.Y.-N.; Kamisah, Y.; Ramalingam, A.; Lim, Y.C.; Budin, S.B.; Zainalabidin, S. Roselle supplementation prevents nicotine-induced vascular endothelial dysfunction and remodelling in rats. Appl. Physiol. Nutr. Metab. 2017, 42, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Supercritical CO2 extraction of bioactive compounds from Hibiscus sabdariffa. J. Supercrit. Fluids 2018, 147, 213–221. [Google Scholar] [CrossRef]
- Bedi, P.S.; Bekele, M.; Gure, G. Phyto-chemistry and Pharmacological Activities of Hibiscus sabdariffa Linn—A Review. Int. Res. J. Pure Appl. Chem. 2020, 21, 41–54. [Google Scholar] [CrossRef]
- Rambe, P.S.; Putra, I.B.; Yosi, A. The effect of roselle leaf (Hibiscus sabdariffa L.) extract gel on wound healing. J. Med. Life 2022, 15, 1246–1251. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef]
- Phewphong, S.; Roschat, W.; Namwongsa, K.; Wonam, A.; Kaisri, T.; Duangpakdee, P.; Leelatam, T.; Moonsin, P.; Promarak, V. Evaluation of the Nutritional, Minerals, and Antioxidant Potential of Roselle (Hibiscus sabdariffa Linn.) Seeds from Roi Et Province in the Northeastern Region of Thailand. Trends Sci. 2023, 20, 6664. [Google Scholar] [CrossRef]
- Morales, R.B.; Carrillo, R.E.M.; Ruiz, E.J.; Martínez, M.T.S.; Sánchez, M.L.M.; Herrera, L.M.S. Potencial de la jamaica (Hibiscus sabdariffa L.) en la elaboración de alimentos funcionales con actividad antioxidante. Rev. Mex. De Agronegocios 2014, 35, 1082–1088. [Google Scholar]
- Izquierdo-Vega, J.A.; Arteaga-Badillo, D.A.; Sánchez-Gutiérrez, M.; Morales-González, J.A.; Vargas-Mendoza, N.; Gómez-Aldapa, C.A.; Castro-Rosas, J.; Delgado-Olivares, L.; Madrigal-Bujaidar, E.; Madrigal-Santillán, E. Organic acids from Roselle (Hibiscus sabdariffa L.)—A brief review of its pharmacological effects. Biomedicines 2020, 8, 100. [Google Scholar] [CrossRef]
- Lim, Y.-C.; Budin, S.B.; Othman, F.; Latip, J.; Zainalabidin, S. Roselle Polyphenols Exert Potent Negative Inotropic Effects via Modulation of Intracellular Calcium Regulatory Channels in Isolated Rat Heart. Cardiovasc. Toxicol. 2016, 17, 251–259. [Google Scholar] [CrossRef]
- Jung, E.; Kim, Y.; Joo, N. Physicochemical properties and antimicrobial activity of Roselle (Hibiscus sabdariffa L.). J. Sci. Food Agric. 2013, 93, 3769–3776. [Google Scholar] [CrossRef]
- Abou-Arab, A.A.; Abu-Salem, F.M.; Abou-Arab, E.A. Physico-chemical properties of natural pigments (anthocyanin) extracted from Roselle calyces (Hibiscus subdariffa). J. Am. Sci. 2011, 7, 445–456. [Google Scholar]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef]
- Dai, H.; Abu Much, A.; Maor, E.; Asher, E.; Younis, A.; Xu, Y.; Lu, Y.; Liu, X.; Shu, J.; Bragazzi, N.L. Global, regional, and national burden of ischaemic heart disease and its attributable risk factors, 1990–2017: Results from the Global Burden of Disease Study 2017. Eur. Hear. J.-Qual. Care Clin. Outcomes 2022, 8, 50–60. [Google Scholar] [CrossRef]
- Sarrafzadegan, N.; Mohammmadifard, N. Cardiovascular disease in Iran in the last 40 years: Prevalence, mortality, morbidity. Challenges and strategies for cardiovascular prevention. Arch. Iran. Med. 2019, 22, 204–210. [Google Scholar]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Haybar, H.; Shahrabi, S.; Rezaeeyan, H.; Shirzad, R.; Saki, N. Endothelial Cells: From Dysfunction Mechanism to Pharmacological Effect in Cardiovascular Disease. Cardiovasc. Toxicol. 2018, 19, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.H.; Abel, E.D. Basic Mechanisms of Diabetic Heart Disease. Circ. Res. 2020, 126, 1501–1525. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Blesso, C.N. Antioxidant properties of anthocyanins and their mechanism of action in atherosclerosis. Free. Radic. Biol. Med. 2021, 172, 152–166. [Google Scholar] [CrossRef]
- Park, J.J. Epidemiology, Pathophysiology, Diagnosis and Treatment of Heart Failure in Diabetes. Diabetes Metab. J. 2021, 45, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Molehin, O.R.; Adefegha, S.A.; Adeyanju, A.A. Role of Oxidative Stress in the Pathophysiology of Type 2 Diabetes and Cardiovascular Diseases. In Role Oxidative Stress Pathophysiology of Diseases; Springer: Berlin/Heidelberg, Germany, 2020; pp. 277–297. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Soulaidopoulos, S.; Sfikakis, P.P.; Dimitroulas, T.; Kitas, G.D. Effect of Biologics on Cardiovascular Inflammation: Mechanistic Insights and Risk Reduction. J. Inflamm. Res. 2021, 14, 1915–1931. [Google Scholar] [CrossRef] [PubMed]
- Nor, N.A.M.; Budin, S.B.; Zainalabidin, S.; Jalil, J.; Sapian, S.; Jubaidi, F.F.; Anuar, N.N.M. The Role of Polyphenol in Modulating Associated Genes in Diabetes-Induced Vascular Disorders. Int. J. Mol. Sci. 2022, 23, 6396. [Google Scholar] [CrossRef]
- De Geest, B.; Mishra, M. Role of Oxidative Stress in Diabetic Cardiomyopathy. Antioxidants 2022, 11, 784. [Google Scholar] [CrossRef]
- Abdelazeem, A.H.; Abuelsaad, A.S.A.; Abdel-Moniem, A.; Abdel-Gabbar, M. Association of metabolic syndrome components with alterations in oxidative stress and cytokines expression. J. Taibah Univ. Sci. 2021, 15, 928–940. [Google Scholar] [CrossRef]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef]
- Abdulmajeed, N.A. Therapeutic ability of some plant extracts on aflatoxin B1 induced renal and cardiac damage. Arab. J. Chem. 2011, 4, 1–10. [Google Scholar] [CrossRef]
- Rasouli, H.; Nayeri, F.D.; Khodarahmi, R. May phytophenolics alleviate aflatoxins-induced health challenges? A holistic insight on current landscape and future prospects. Front. Nutr. 2022, 9, 981984. [Google Scholar] [CrossRef] [PubMed]
- Byrne, N.J.; Rajasekaran, N.S.; Abel, E.D.; Bugger, H. Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy. Free. Radic. Biol. Med. 2021, 169, 317–342. [Google Scholar] [CrossRef]
- Ghani, M.A.A.; Ugusman, A.; Latip, J.; Zainalabidin, S. Role of Terpenophenolics in Modulating Inflammation and Apoptosis in Cardiovascular Diseases: A Review. Int. J. Mol. Sci. 2023, 24, 5339. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef]
- Kucukler, S.; Darendelioğlu, E.; Caglayan, C.; Ayna, A.; Yıldırım, S.; Kandemir, F.M. Zingerone attenuates vancomycin-induced hepatotoxicity in rats through regulation of oxidative stress, inflammation and apoptosis. Life Sci. 2020, 259, 118382. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.D.; Lopez, V.A.; Roberts, C.S.; Solomon, H.A.; Burke, G.L.; Kuller, L.; Tracy, R.; Yanez, D.; Psaty, B.M. Combined Association of Lipids and Blood Pressure in Relation to Incident Cardiovascular Disease in the Elderly: The Cardiovascular Health Study. Am. J. Hypertens. 2010, 23, 161–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Vittinghoff, E.; Pletcher, M.J.; Allen, N.B.; Al Hazzouri, A.Z.; Yaffe, K.; Balte, P.P.; Alonso, A.; Newman, A.B.; Ives, D.G.; et al. Associations of Blood Pressure and Cholesterol Levels During Young Adulthood with Later Cardiovascular Events. J. Am. Coll. Cardiol. 2019, 74, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Fogacci, F.; Agnoletti, D.; Cicero, A.F.G. Hypertension and Dyslipidemia Combined Therapeutic Approaches. High Blood Press. Cardiovasc. Prev. 2022, 29, 221–230. [Google Scholar] [CrossRef]
- Emanuelsson, F.; Marott, S.; Tybjærg-Hansen, A.; Nordestgaard, B.G.; Benn, M. Impact of Glucose Level on Micro- and Macrovascular Disease in the General Population: A Mendelian Randomization Study. Diabetes Care 2020, 43, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, T. Intensive Glucose Lowering and Cardiovascular Disease Prevention in Diabetes. Circulation 2010, 122, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Song, Y.-M.; Ebrahim, S.; Lawlor, D.A. Fasting Blood Glucose and the Risk of Stroke and Myocardial Infarction. Circulation 2009, 119, 812–819. [Google Scholar] [CrossRef]
- Hansen, E.; Grimm, D.; Wehland, M. Current Knowledge about the New Drug Firibastat in Arterial Hypertension. Int. J. Mol. Sci. 2022, 23, 1459. [Google Scholar] [CrossRef]
- Komala, M.G.; Ong, S.G.; Qadri, M.U.; Elshafie, L.M.; Pollock, C.A.; Saad, S. Investigating the Regulatory Process, Safety, Efficacy and Product Transparency for Nutraceuticals in the USA, Europe and Australia. Foods 2023, 12, 427. [Google Scholar] [CrossRef]
- Balogun, M.E.; Besong, E.E.; Obimma, J.N.; Iyare, E.E.; Nwachukwu, D.C. Ameliorative effect of aqueous extract of Hibiscus sabdariffa (Roselle) on salt-induced hypertension in wistar rats. Pharmacologyonline 2019, 2, 247–258. [Google Scholar]
- Salam, M.A.; Ibrahim, B.; El-Batran, S.; El-Gengaihi, S.E.; Baker, D. Study of the possible antihypertensive and hypolipidemic effects of an herbal mixture on l-name-induced hypertensive rats. Asian J. Pharm. Clin. Res. 2016, 9, 85–90. [Google Scholar]
- Zainalabidin, S.; Shahidin, S.; Budin, S.B. Hibiscus sabdariffa Linn. (Roselle) protects against nicotine-induced heart damage in rats. Sains Malays. 2016, 45, 207–214. [Google Scholar]
- Si, L.-Y.; Ramalingam, A.; Ali, S.S.; Aminuddin, A.; Ng, P.-Y.; Latip, J.; Kamisah, Y.; Budin, S.B.; Zainalabidin, S. Roselle attenuates cardiac hypertrophy after myocardial infarction in vivo and in vitro. EXCLI J. 2019, 18, 876–892. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.L.M.; Zainalabidin, S.; Fauzi, N.M.; Budin, S.B. Hibiscus sabdariffa (roselle) polyphenol-rich extract averts cardiac functional and structural abnormalities in type 1 diabetic rats. Appl. Physiol. Nutr. Metab. 2018, 43, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.L.M.; Budin, S.B.; Nasir, S.N.M.; Yusoff, N.A.; Fauzi, N.M.; Zainalabidin, S. Hibiscus sabdariffa (roselle) polyphenol-rich extract prevents the aortic oxidative damage in type 1 diabetic rats. J. Teknol. 2018, 80, 11165. [Google Scholar] [CrossRef]
- Ajay, M.; Chai, H.; Mustafa, A.; Gilani, A.; Mustafa, M. Mechanisms of the anti-hypertensive effect of Hibiscus sabdariffa L. calyces. J. Ethnopharmacol. 2007, 109, 388–393. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.F.; Hessin, A.F.; Abdelbaset, M.; Ogaly, H.A.; Abd-Elsalam, R.M.; Hassan, S.M. Antihypertensive effects of roselle-olive combination in L-NAME-induced hypertensive rats. Oxidative Med. Cell. Longev. 2017, 2017, 9460653. [Google Scholar] [CrossRef]
- Burkhardt, R. Hyperlipidemia and cardiovascular disease: Reinforcement for ‘lower is better’. Curr. Opin. Lipidol. 2015, 26, 468–469. [Google Scholar] [CrossRef]
- Adefolalu, F.; Salawa, J.; Gara, T.; Abubakar, A. Hypoglycemic and Hypolipidemic Effect of Methanol Extract of Hibiscus Sabdariffa Seed in Alloxan Induced Diabetic Albino Rats. Niger. J. Basic Appl. Sci. 2019, 27, 151–156. [Google Scholar] [CrossRef]
- Abidin, Z.; Budin, S.B. Hibiscus sabdariffa Linn. (Roselle) polyphenols-rich extract prevents hyperglycemia-induced cardiac oxidative stress and mitochondrial damage in diabetic rats. Sains Malays. 2020, 49, 2499–2506. [Google Scholar]
- Farombi, E.; Ige, O. Hypolipidemic and antioxidant effects of ethanolic extract from dried calyx of Hibiscus sabdariffa in alloxan-induced diabetic rats. Fundam. Clin. Pharmacol. 2007, 21, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Ochani, P.C.; D’Mello, P. Antioxidant and antihyperlipidemic activity of Hibiscus sabdariffa Linn. leaves and calyces extracts in rats. Indian J. Exp. Biol. 2009, 47, 276–282. [Google Scholar]
- Yang, M.-Y.; Peng, C.-H.; Chan, K.-C.; Yang, Y.-S.; Huang, C.-N.; Wang, C.-J. The Hypolipidemic Effect of Hibiscus sabdariffa Polyphenols via Inhibiting Lipogenesis and Promoting Hepatic Lipid Clearance. J. Agric. Food Chem. 2010, 58, 850–859. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G. Aqueous extracts of Roselle (Hibiscus sabdariffa Linn.) varieties inhibit α-amylase and α-glucosidase activities in vitro. J. Med. Food 2013, 16, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Mardiah, M.; Zakaria, F.; Prangdimurti, E.; Damanik, R. The effect of roselle extract (Hibiscus sabdariffa Linn.) on blood glucose level and total antioxidant level on diabetic rat induced by streptozotocin. IOSR J. Pharm. 2014, 4, 8–16. [Google Scholar]
- Rosemary, R.; Haro, G. Antidiabetic effect of roselle calyces extract (Hibiscus sabdariffa L.) in streptozotocin induced mice. Int. J. PharmTech Res. 2014, 6, 1703–1711. [Google Scholar]
- Wisetmuen, E.; Pannangpetch, P.; Kongyingyoes, B.; Kukongviriyapan, U.; Yutanawiboonchai, W.; Itharat, A.; Wisetmuen, E. Insulin secretion enhancing activity of roselle calyx extract in normal and streptozotocin-induced diabetic rats. Pharmacogn. Res. 2013, 5, 65–70. [Google Scholar] [CrossRef]
- Mashi, J.A.M.; Shehu, D.; Idris, R.; Sa`id, A.M.; Dangambo, M.; Babagana, K.; Ya’u, M.; Babandi, A. Comparative Study of Different Solvents Extract of Persea Americana Leaf on Alloxan Induced Hyperglycemic Rats. Asian J. Biol. Sci. 2019, 12, 67–72. [Google Scholar] [CrossRef]
- El Barky, A.H.S.; Alm-Eldeen, A.-E.; Hafez, A.; Mohamed, T. Saponins and Their Potential 958 Role in Diabetes Mellitus. Diabetes Manag. 2017, 7, 148–158. [Google Scholar]
- Morales-Luna, E.; Pérez-Ramírez, I.F.; Salgado, L.M.; Castaño-Tostado, E.; Gómez-Aldapa, C.A.; Reynoso-Camacho, R. The main beneficial effect of roselle (Hibiscus sabdariffa) on obesity is not only related to its anthocyanin content. J. Sci. Food Agric. 2018, 99, 596–605. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Ramalingam, A.; Budin, S.B.; Lim, Y.-C.; Si, Y.-N.L.; Zainalabidin, S. Dietary UKMR-1 roselle supplementation prevents nicotine-induced cardiac injury by inhibiting myocardial oxidative stress. Sains Malays. 2016, 45, 1131–1137. [Google Scholar]
- Khongrum, J.; Yingthongchai, P.; Boonyapranai, K.; Wongtanasarasin, W.; Donrung, N.; Sukketsiri, W.; Prachansuwan, A.; Chonpathompikunlert, P. Antidyslipidemic, Antioxidant, and Anti-inflammatory Effects of Jelly Drink Containing Polyphenol-Rich Roselle Calyces Extract and Passion Fruit Juice with Pulp in Adults with Dyslipidemia: A Randomized, Double-Blind, Placebo-Controlled Trial. Oxidative Med. Cell Longev. 2022, 2022, 4631983. [Google Scholar] [CrossRef]
- Sun, B.; Li, F.; Zhang, X.; Wang, W.; Shao, J.; Zheng, Y. Delphinidin-3-O-glucoside, an active compound of Hibiscus sabdariffa calyces, inhibits oxidative stress and inflammation in rabbits with atherosclerosis. Pharm. Biol. 2022, 60, 247–254. [Google Scholar] [CrossRef]
- Ali, S.S.; Mohamed, S.F.A.; Rozalei, N.H.; Boon, Y.W.; Zainalabidin, S. Anti-fibrotic Actions of Roselle Extract in Rat Model of Myocardial Infarction. Cardiovasc. Toxicol. 2018, 19, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Meraiyebu, A.; Olaniyan, O.; Eneze, C.; Anjorin, Y.; Dare, J. Anti-inflammatory activity of methanolic extract of Hibiscus sabdariffa on carrageenan induced inflammation in wistar rat. Int. J. Pharm. Sci. Invent. 2013, 2, 22–24. [Google Scholar]
- Nyam, K.; Sin, L.; Kamariah, L. Phytochemical Analysis and Anti-Inflammatory Effect of Kenaf and Roselle Seeds. Malays. J. Nutr. 2015, 21, 245–254. [Google Scholar]
- Ali, S.; Mohamed, A.; Mohammed, G. Fatty acid composition, anti-inflammatory and analgesic activities of Hibiscus sabdariffa Linn. seeds. J. Adv. Veter-Anim. Res. 2014, 1, 50. [Google Scholar] [CrossRef]
- Lubis, M.; Siregar, G.A.; Bangun, H.; Ilyas, S. The effect of roselle flower petals extract (Hibiscus sabdariffa Linn.) on reducing inflammation in dextran sodium sulfateinduced colitis. Med. Glas 2020, 17, 395–401. [Google Scholar] [CrossRef]
- Mahadevan, N.; Shivali; Pradeep, K. Hibiscus sabdariffa Linn.—An overview. Nat. Prod. Radiance 2009, 8, 77–83. [Google Scholar]
- Gondokesumo, M.E.; Kusuma, H.S.; Widowati, W. α-/β-Glucosidase and α-Amylase Inhibitory Activities of Roselle (Hibiscus sabdariffa L.) Ethanol Extract. Mol. Cell. Biomed. Sci. 2017, 1, 34–40. [Google Scholar] [CrossRef]
- Jubaidi, F.F.Z.S.; Ying, L.; Mohammed Yusof, N.L.; Budin, S.B. Potential of Hibiscus Sabdariffa Linn. Polyphenol-Rich Extract in Improving Diabetes-Induced Vascular Functional and Structural Abnormalities in Rats. Sains Malays. 2021, 50, 1959–1970. [Google Scholar] [CrossRef]
- Harmili, H.; Fadlilah, S.; Sucipto, A. Effectiveness of hibiscus sabdariffa on blood pressure of hypertension patients. J. Keperawatan Respati Yogyak. 2021, 8, 99–102. [Google Scholar]
- Aroonsiriwatana, S.; Sangwatanaroj, S. The effects of roselle (Hibiscus sabdariffa) and stevia (Stevia rebaudiana) on hypertension in patients with type 2 diabetes at king chulalongkorn memorial hospital. Asean Heart J. 2012, 20, 26–34. [Google Scholar]
- Anggraini, S.S.; Nur, S.A.; Morika, H.D.; Dewi, R.I.S. Rosella flower tea on blood pressure reduction in hypertension patients. Proceeding Int. Conf. Syedza St. 2020, 7, 4777–4780. [Google Scholar] [CrossRef]
- Ritonga, N.J.; Setiani, O.; Umaroh, U.; Budhi R, K.; Amri, F. Roselle flower (Hibiscus sabdariffa) in the treatment of hypertension in postpartum mothers. Belitung Nurs. J. 2017, 3, 229–237. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Jalali-Khanabadi, B.-A.; Afkhami-Ardekani, M.; Fatehi, F.; Noori-Shadkam, M. The effects of sour tea (Hibiscus sabdariffa) on hypertension in patients with type II diabetes. J. Hum. Hypertens. 2008, 23, 48–54. [Google Scholar] [CrossRef]
- Elkafrawy, N.; Younes, K.; Naguib, A.; Badr, H.; Kamal Zewain, S.; Kamel, M.; Raoof, G.F.A.; El-Desoky, A.M.; Mohamed, S. Antihypertensive efficacy and safety of a standardized herbal medicinal product of Hibiscus sabdariffa and Olea europaea extracts (NW Roselle): A phase-II, randomized, double-blind, captopril-controlled clinical trial. Phytother. Res. 2020, 34, 3379–3387. [Google Scholar] [CrossRef]
- Al-Shafei, A.I.; El-Gendy, O.A. Effects of Roselle on arterial pulse pressure and left ventricular hypertrophy in hyper-tensive patients. Saudi Med. J. 2013, 34, 1248–1254. [Google Scholar]
- AL-Jawad, F.H.; Hashim, H.; Al-Attar, Z.; Al-Ani, A.H. Changing the lipid profile and renal functions by Allium sativum, Nigella sativa and Hibiscus sabdariffa in essential hypertensive patients. World J. Pharm. Phamaceutical. Sci. 2018, 4, 125–134. [Google Scholar]
- Asgary, S.; Soltani, R.; Zolghadr, M.; Keshvari, M.; Sarrafzadegan, N. Evaluation of the effects of roselle (Hibiscus sabdariffa L.) on oxidative stress and serum levels of lipids, insulin and hs-CRP in adult patients with metabolic syndrome: A double-blind placebo-controlled clinical trial. J. Complement. Integr. Med. 2016, 13, 175–180. [Google Scholar] [CrossRef]
- Abubakar, S.M.; Ukeyima, M.T.; Spencer, J.P.; Lovegrove, J.A. Acute effects of Hibiscus sabdariffa calyces on post-prandial blood pressure, vascular function, blood lipids, biomarkers of insulin resistance and inflammation in humans. Nutrients 2019, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Najafpour Boushehri, S.; Karimbeiki, R.; Ghasempour, S.; Ghalishourani, S.S.; Pourmasoumi, M.; Hadi, A.; Mbabazi, M.; Pour, Z.K.; Assarroudi, M.; Mahmoodi, M. The efficacy of sour tea (Hibiscus sabdariffa L.) on selected cardiovascular disease risk factors: A systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 2020, 34, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.R.; Zulfiqar, S.; Holmes, M.; Marshall, L.; Dye, L.; Boesch, C. A systematic review and meta-analysis of the effects of Hibiscus sabdariffa on blood pressure and cardiometabolic markers. Nutr. Rev. 2022, 80, 1723–1737. [Google Scholar] [CrossRef] [PubMed]



| Activity | Roselle Part | Extract Type | Dose | Duration of the Study | Study Model | Mechanism of Action | References |
|---|---|---|---|---|---|---|---|
| Anti-hypertensive | Leaves | Aqueous extract | 100, 200, 400 mg/kg | 6 weeks | In vivo | Reduced systolic and diastolic blood pressure, mean arterial blood pressure, and heart rate. | [64] |
| Calyx | Aqueous extract | 13.347, 26.694 mg/kg | 4 weeks | In vivo | Prevented an increase in blood pressure. | [65] | |
| Calyces | Aqueous extract | 100 mg/kg | 21 days | In vivo | Decreased blood pressure and heart rate in nicotine-exposed rats. | [66] | |
| Calyx | Aqueous extract | 100 mg/kg | 28 days | In vivo | Exerted vasodilatory effects on the aortic ring, activated potassium channel, and reduced blood pressure. | [25] | |
| Calyx | Hydro-alcoholic powdered extract | 125, 250, 500 mg/kg (4 weeks) | 4 weeks | In vivo | Decreased systolic and diastolic blood pressure, reversed the suppression of nitric oxide, lowered ACE activity, and enhanced eNOS gene and protein expression. | [71] | |
| Calyx | Methanol extract | 10 ng/mL to 1 mg/mL | - | Ex vivo | Induced vasodilator effect in isolated aortas via nitric oxide/cGMP-relaxant pathway and inhibited Ca2+ influx. | [70] | |
| Calyx | Aqueous extract | 100 mg/kg | 28 days | In vivo | Ameliorated cardiac systolic and diastolic dysfunction; improved coronary flow and cardiac output. | [13] | |
| Calyx | Roselle polyphenol extract | 125 to 2000 µg/mL | - | Ex vivo | Lowered systolic function, reduced heart rate, increased relaxation, and improved coronary blood flow. | [33] | |
| Calyx | Aqueous extract | 100 mg/kg | 28 days | In vivo and in vitro | Ameliorated myocardial infarction-induced cardiac systolic and diastolic dysfunction, improved left ventricular developed pressure, attenuated angiotensin II-induced cardiomyocyte hypertrophy. | [67] | |
| Calyx | Polyphenol-rich Extract | 100 mg/kg | 8 weeks | In vivo | Exerted hypotensive effects by reducing systolic blood pressure. | [69] | |
| Calyx | Polyphenol-rich extract | 100 mg/kg | 8 weeks | In vivo | Improved cardiac contractility and relaxation rate by improving left ventricular developed pressure and coronary blood flow. | [68] | |
| Anti-hyperlipidemic | Calyces | Aqueous | 100 mg/kg | 28 days | In vivo | Reduced the level of LDL-C in the blood through LDL hydrolysis and lipolysis. | [13] |
| Calyces and leaves | Ethanolic extract | 500 mg/kg | 30 days | In vivo | Inhibited the intestinal absorption of cholesterol, interfered with lipoprotein production, and induced hepatic LDL receptor expression. | [76] | |
| Flower | Ethanolic | 100 and 200 mg/kg | 28 days | In vivo | Reduced cholesterol by 29%, VLDL-C by 36%, LDL-C by 40%, and atherogenic index by 32%. | [75] | |
| Calyces | Polyphenol-rich extract | 100 mg/kg | 8 weeks | In vivo | Reduced serum levels of LDL/HDL and TG/HDL | [68] | |
| Seed | Methanol extract | 400, 200 mg/kg | 14 days | In vivo | Lowered the concentration of TG, LDL-C, and cholesterol while increasing the HDL concentration. | [73] | |
| Calyces | Polyphenol-rich extract | 100 mg/kg | 28 days | In vivo | Reduced serum level of LDL-C and increased the level of HDL-C. | [74] | |
| Calyces | Polyphenol-rich extract (HPE) | 100 mg/kg | 8 weeks | In vivo | Reduced plasma TG, cholesterol, and LDL-C, and increased level of HDL-C. | [69] | |
| Leaves | Lyophilized extract and polyphenols | 1%, 2%, 0.1%, 0.2% (10 weeks) | 10 weeks | In vivo and in vitro | Suppressed the expression of fatty acid synthesis in hepatocytes; inhibited hepatic intracellular lipid accumulation and lipid synthesis. | [77] | |
| Anti-hyperglycemic | Calyces (white and red) | Aqueous | 0–200 µL | - | In vitro | Reduced hyperglycemia through inhibition of α-amylase and α-glucoside; inhibited the breakdown of starch into oligosaccharides and monosaccharides. | [78] |
| Calyces | Ethanol | 0–37.5 µL | - | In vitro | Reduced the concentration of α-amylase and α and β-glucosidase in a concentration-dependent manner, inhibited glucosidase, and disrupted hydrolysis and the release of glucose. | [95] | |
| Calyces | N-hexane, ethyl acetate, and ethanol extract | 200, 400, and 600 mg/kg | - | In vivo | Bioflavonoids in roselle mimic insulin by inhibiting blood glucose levels and stimulating insulin secretion and formation of glycogen in muscle cells. | [80] | |
| Calyces | Polyphenol-rich extract | 100 mg/kg | 28 days | In vivo | Reduced plasma glucose and dyslipidemia. | [74] | |
| Seed | Methanol extract | 400, 200 mg/kg | 14 days | In vivo | Decreased plasma glucose through regeneration of β-pancreatic cells. Saponins in the roselle lower blood glucose through the restoration of insulin response or induction of insulin release from the pancreas. | [73] | |
| Antioxidative | Calyces | Aqueous and methanolic | 750 and 500 mg/100 mL potable water ad libitum | 16 weeks | In vivo | Organic acids (hibiscus acid), phenolic acids, anthocyanin, and flavonoids inhibit the damaging effect of oxidative stress. | [84] |
| Calyces | Aqueous | 100 mg/kg | 28 days | In vivo | Reduced NOX2 gene expression and 8-isoprostane level; increased the level of antioxidants SOD and GSH. | [13] | |
| Calyces | Polyphenol-rich extract | 100 mg/kg | 8 weeks | In vivo | Inhibited MDA and AOPP in the aorta with notably increased levels of GSH antioxidants. | [96] | |
| Calyces | Polyphenol-rich extract | 100 mg/kg | 8 weeks | In vivo | Reduced MDA and AOPP levels. Enhanced the production of GSH. | [69] | |
| Calyces | Polyphenol-rich extract | 100 mg/kg | 4 weeks | In vivo | Attenuated oxidative stress and cellular damage; decreased the mitochondrial complex I activity. | [74] | |
| Calyces | Polyphenol-rich extract | 100 mg/kg (28 days) | 8 weeks | In vivo | Decreased the mitochondrial structural damage and increased the overexpression of MnSOD or SOD2 in mitochondria. | [68] | |
| White and red calyces | Aqueous | 0–200 µL | - | In vivo | Roselle chelates Fe2+ and scavenges OH− radical. Decreased lipid peroxidation and oxidative damage in the pancreas. | [78] | |
| Calyces | Polyphenol-rich extract | 100 mg/kg | 28 days | In vivo | Enhanced the production of SOD, SOD2, GSH, and CAT; attenuated the production of heart lipid peroxidation (TBARS) and (AOPP). | [74] | |
| Calyces | Aqueous | 100 mg/kg | 28 days | In vivo and in vitro | Inhibited the gene expression of oxidative stress (NOX2 and 8-isoprostane level) and increased the level of antioxidant SOD and GSH in post-myocardial infarction rats. | [67] | |
| Isolated delphinidin-3-O-sambubioside | - | 10 and 20 mg/kg | 12 weeks | In vivo | Upregulated the mRNA expression of aortic GSH-PX and SOD1. | [88] | |
| Calyces | Aqueous | 100 mg/kg | 28 days | In vivo | Enhanced the production of Cu/Zn-SOD and GSH as well as the GSH/GSSC ratio. | [86] | |
| Anti-inflammatory | Isolated delphinidin-3-O-sambubioside | - | 10 and 20 mg/kg | 12 weeks | In vivo | Inhibited the production of aortic ICAM-1, MCP-1, VCAM-1, CRP, IL-6, and TNF-α. | [88] |
| Calyces | Aqueous | 100 mg/kg | 28 days | In vivo | Enhanced the intracellular LDH activity in the heart after nicotine administration. | [86] | |
| Calyces | Aqueous | 100 mg/kg | 10 days | In vivo | Reduced the plasma level of troponin-T, IL-6, and IL-10 and attenuated the expression of IL-10 in MI rats. | [89] | |
| Leaves | Methanolic | 250 and 500 mg/kg | In vivo | Decreased paw diameter. | [90] | ||
| Seed | Oil and extract | 500 mg/kg | 5 h | In vivo | Inhibited inflammation by reducing the synthesis, release, or action of the inflammatory mediators or antagonizing the activity of the mediators after release. | [91] | |
| Seed | Petroleum ether | 2–8 mL/kg | In vivo | Reduced chronic and acute inflammation in a dose-dependent manner through suppression of cyclooxygenase and prostaglandin synthesis. | [92] | ||
| Flower petals | Ethanol | 300 mg/kg | 28 days | In vivo | Promoted the increase in IL10 production and inhibited the synthesis of IL6 and TNF-α production. | [93] | |
| Anti-fibrosis | Calyx | Aqueous extract | 100 mg/kg | 28 days | In vivo and in vitro | Suppressed the production of collagen I and collagen III in myocardial infarction. | [67] |
| Calyx | Aqueous extract | 100 mg/kg | 28 days | In vivo | Decreased interstitial collagen deposition and downregulated BNP. | [13] | |
| Calyx | Aqueous extract | 100 mg/kg | 10 days | In vivo | Reduced collagen deposition; downregulated collagen III expression. | [67] | |
| Calyx | Hydro-alcoholic powdered extract | 125, 250, 500 mg/kg | 4 weeks | In vivo | Attenuated extensive collagen fiber deposition and myocardial fibrosis. | [71] |
| Roselle Part | Study Design | Subjects | Dose | Treatment Duration | Effects | References |
|---|---|---|---|---|---|---|
| Calyx powder | Double-blind, placebo-controlled | 40 adult patients with metabolic syndrome | 500 mg/day | 4 weeks | Reduced serum TG and systolic blood pressure | [105] |
| Polyphenol-rich roselle calyx extract in the jelly drink | Randomized, double-blind, placebo-controlled trial | 42 adults with dyslipidemia | 300 mL/day | 8 weeks | Decreased levels of LDL-C, TNFα, and MDA and increased GSH level | [87] |
| Standardized herbal medicinal product containing roselle | Phase II, randomized, double-blind, captopril-controlled clinical trial | 134 patients with grade 1 essential hypertension | 1200 mg | 8 weeks | Reduced blood pressure, lowered TG, reduced mean serum renin and ACE | [102] |
| Roselle sour tea | Double-blind, randomized controlled trial | 60 diabetic patients with mild hypertension | 2 g of roselle tea in 240 mL boiling water two times/day | 1 month | Decreased systolic blood pressure and mean pulse pressure | [101] |
| Roselle infusion | Non-randomized, quasi-experimental study | 50 subjects with moderate essential hypertension | 2 g in 250 mL of boiling water/day | 4 weeks | Lowered pulse pressure and heart rate, reduced ventricular hypertrophy | [103] |
| Roselle infusion | Prospective randomized clinical-case study | 24 hypertensive patients | 15 g/day | 4 weeks | Lowered arterial systolic and diastolic blood pressure, suppressed LDL level, reduced cholesterol level, enhanced HDL level, diminished TG level | [104] |
| Roselle calyx infusion | Quasi-experimental with a pre-test and post-test nonequivalent control group | 17 people with hypertension | 2 g roselle-infused drink/day | 7 days | Reduced systolic and diastolic blood pressure | [97] |
| Roselle calyx infusion | Randomized clinical case study | 40 diabetic patients with mild hypertension | 240 mL of roselle two times/day | 1 month | Reduced systolic and diastolic blood pressure | [98] |
| Roselle flower tea | A quasi-experimental design with a two-group pre-test and post-test design | 16 patients with hypertension | 2 weeks | [99] | ||
| Roselle dried flower infusion | A quasi-experimental study with a nonequivalent control group design | 30 postpartum mothers with hypertension | 10 g brewed with 200 mL water/day | 6 h | Reduced systolic and diastolic blood pressure | [100] |
| Roselle calyx tea | Randomized, controlled, single-blinded, 2-meal cross-over study | 25 participants with 1 to 10% CVD risk factors | 7.5 g calyx in 250 mL Buxton water | 4 h | Decreased postprandial systolic and diastolic blood pressure, reduced serum glucose, plasma insulin, serum TG, CRP level, improved antioxidant response curve | [106] |
| Roselle sour tea | Meta-analysis, randomized clinical trials | 362 participants with hypertension | - | - | Reduced fasting plasma glucose, lowered systolic and diastolic blood pressure | [107] |
| Roselle tea | Meta-analysis, meta-regression | 415 participants with hypertension and cardiometabolic markers | - | - | Reduced systolic and diastolic blood pressure, lowered LDL level | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapian, S.; Ibrahim Mze, A.A.; Jubaidi, F.F.; Mohd Nor, N.A.; Taib, I.S.; Abd Hamid, Z.; Zainalabidin, S.; Mohamad Anuar, N.N.; Katas, H.; Latip, J.; et al. Therapeutic Potential of Hibiscus sabdariffa Linn. in Attenuating Cardiovascular Risk Factors. Pharmaceuticals 2023, 16, 807. https://doi.org/10.3390/ph16060807
Sapian S, Ibrahim Mze AA, Jubaidi FF, Mohd Nor NA, Taib IS, Abd Hamid Z, Zainalabidin S, Mohamad Anuar NN, Katas H, Latip J, et al. Therapeutic Potential of Hibiscus sabdariffa Linn. in Attenuating Cardiovascular Risk Factors. Pharmaceuticals. 2023; 16(6):807. https://doi.org/10.3390/ph16060807
Chicago/Turabian StyleSapian, Syaifuzah, Asma Ali Ibrahim Mze, Fatin Farhana Jubaidi, Nor Anizah Mohd Nor, Izatus Shima Taib, Zariyantey Abd Hamid, Satirah Zainalabidin, Nur Najmi Mohamad Anuar, Haliza Katas, Jalifah Latip, and et al. 2023. "Therapeutic Potential of Hibiscus sabdariffa Linn. in Attenuating Cardiovascular Risk Factors" Pharmaceuticals 16, no. 6: 807. https://doi.org/10.3390/ph16060807
APA StyleSapian, S., Ibrahim Mze, A. A., Jubaidi, F. F., Mohd Nor, N. A., Taib, I. S., Abd Hamid, Z., Zainalabidin, S., Mohamad Anuar, N. N., Katas, H., Latip, J., Jalil, J., Abu Bakar, N. F., & Budin, S. B. (2023). Therapeutic Potential of Hibiscus sabdariffa Linn. in Attenuating Cardiovascular Risk Factors. Pharmaceuticals, 16(6), 807. https://doi.org/10.3390/ph16060807








