Tocotrienol-Rich Fraction Ameliorates the Aluminium Chloride-Induced Neurovascular Dysfunction-Associated Vascular Dementia in Rats
Abstract
:1. Introduction
2. Results
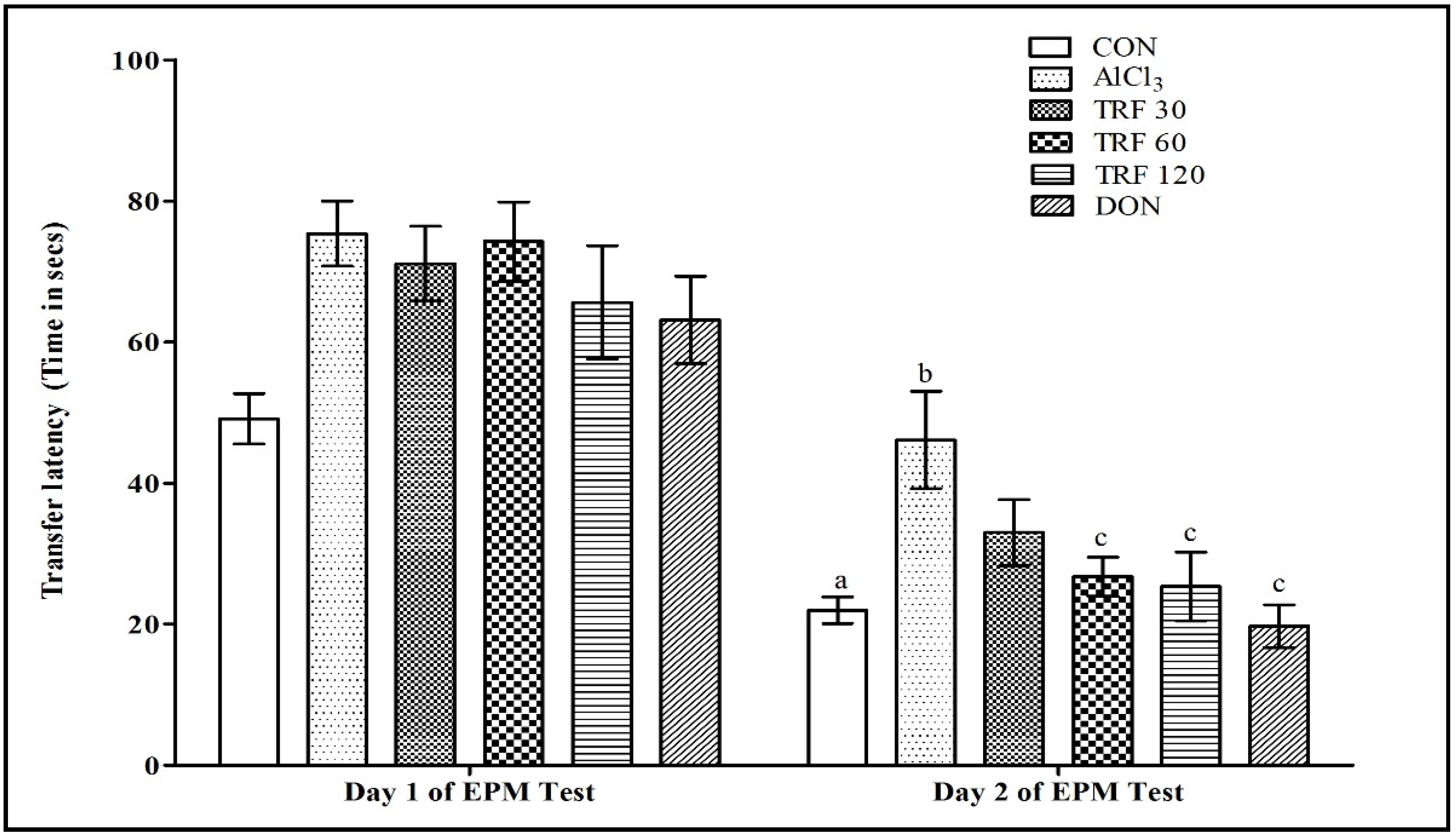
2.1. Effect of TRF on Learning and Memory Function during the Elevated Plus Maze (EPM) Test
2.2. Effect of TRF on Brain-Thiobarbituric Acid Reactive Substances (TBARS) Levels
2.3. Effect of TRF on Serum Nitrite Levels
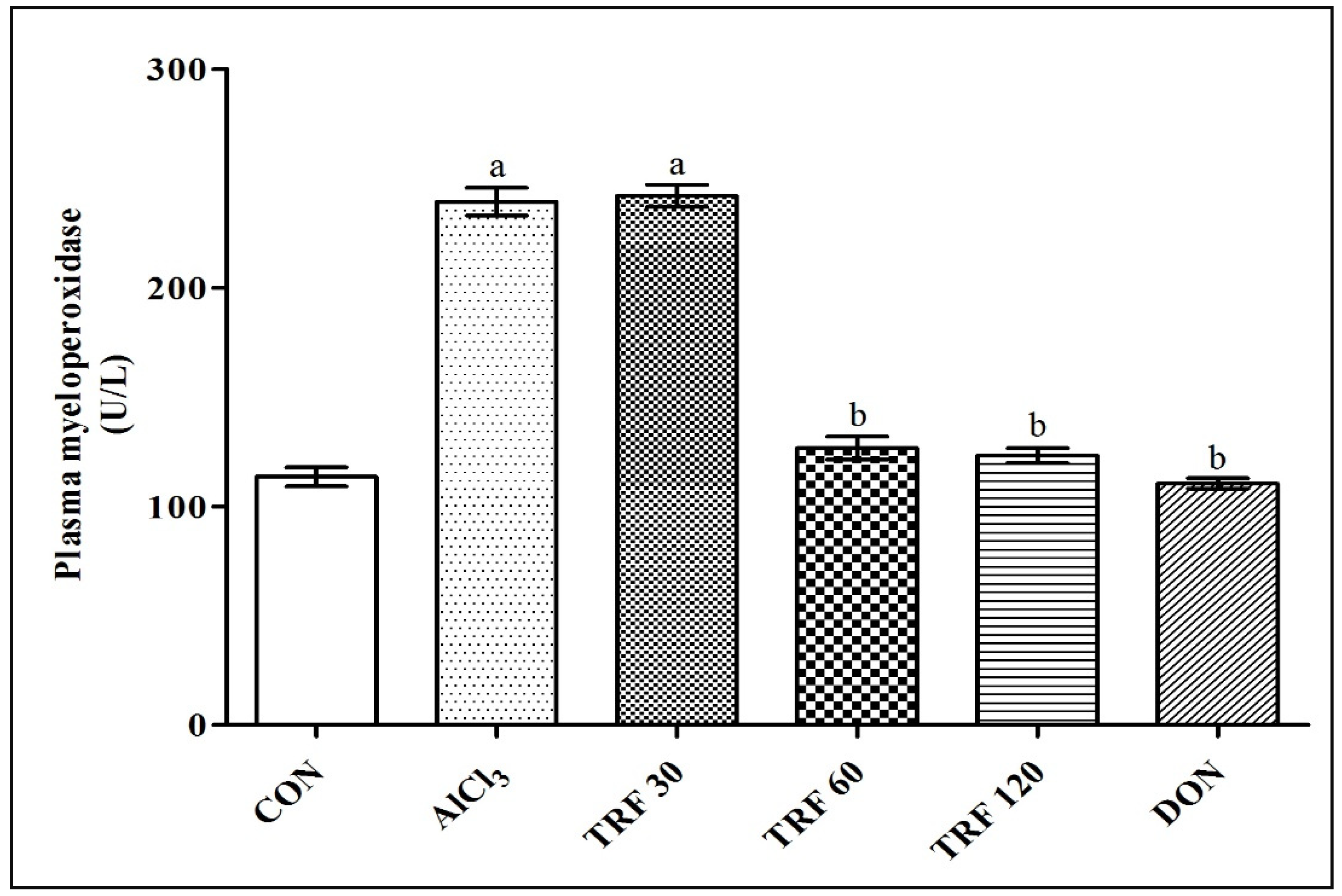
2.4. Effect of TRF on Plasma Myeloperoxidase (MPO) Levels
2.5. Effect of TRF on Hippocampus PDGF-C Expression
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals and Assay Kits
4.3. Experimental Design
- CON: Normal control rats received an equal volume of vehicle.
- AlCl3: AlCl3-induced rats received an equal volume of vehicle.
- TRF 30: AlCl3-induced rats received TRF 30 mg/kg/bw; p.o.
- TRF 60: AlCl3-induced rats received TRF 60 mg/kg/bw; p.o.
- TRF 120: AlCl3-induced rats received TRF 120 mg/kg/bw; p.o.
- DON: AlCl3-induced rats received DON 1 mg/kg/bw; p.o. [63].
4.4. Elevated Plus Maze (EPM) Test
4.5. Blood Sample Collection and Brain Homogenate Preparation
4.6. Estimation of Brain-Thiobarbituric Acid Reactive Substances
4.7. Estimation of Serum Nitrite
4.8. Estimation of Plasma Myeloperoxidase
4.9. Immunohistochemistry Study for Identification of PDGF-C Expression in Hippocampus
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tariq, S.; Barber, P.A. Dementia Risk and Prevention by Targeting Modifiable Vascular Risk Factors. J. Neurochem. 2018, 144, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Shabir, O.; Berwick, J.; Francis, S.E. Neurovascular Dysfunction in Vascular Dementia, Alzheimer’s and Atherosclerosis. BMC Neurosci. 2018, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Wolters, F.J.; Ikram, M.A. Epidemiology of Vascular Dementia. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Zhou, Z.-F.; Zhu, Y.-G.; Wan, Z.-K.; Yang, M.-W.; Hong, F.-F.; Yang, S.-L. Pharmacological Treatment of Vascular Dementia: A Molecular Mechanism Perspective. Aging Dis. 2021, 12, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Sashindranath, M.; Nandurkar, H.H. Endothelial Dysfunction in the Brain. Stroke 2021, 52, 1895–1904. [Google Scholar] [CrossRef]
- Martins-Filho, R.K.; Zotin, M.C.; Rodrigues, G.; Pontes-Neto, O. Biomarkers Related to Endothelial Dysfunction and Vascular Cognitive Impairment: A Systematic Review. Dement. Geriatr. Cogn. Disord. 2020, 49, 365–374. [Google Scholar] [CrossRef]
- Quick, S.; Moss, J.; Rajani, R.M.; Williams, A. A Vessel for Change: Endothelial Dysfunction in Cerebral Small Vessel Disease. Trends Neurosci. 2021, 44, 289–305. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, X.; Zhang, L.; Shen, J. Neurovascular Unit: A Critical Role in Ischemic Stroke. CNS Neurosci. Ther. 2021, 27, 7–16. [Google Scholar] [CrossRef]
- Patwa, J.; Flora, S.J.S. Heavy Metal-Induced Cerebral Small Vessel Disease: Insights into Molecular Mechanisms and Possible Reversal Strategies. Int. J. Mol. Sci. 2020, 21, 3862. [Google Scholar] [CrossRef]
- Bai, T.; Yu, S.; Feng, J. Advances in the Role of Endothelial Cells in Cerebral Small Vessel Disease. Front. Neurol. 2022, 13, 861714. [Google Scholar] [CrossRef]
- Frisardi, V.; Solfrizzi, V.; Kehoe, P.G.; Imbimbo, B.P.; Vendemiale, G.; Capurso, A.; Panza, F. Aluminium in the Diet, Cognitive Decline and Dementia. In Handbook of Behavior, Food and Nutrition; Springer: New York, NY, USA, 2011; pp. 2829–2850. [Google Scholar]
- Maya, S.; Prakash, T.; Madhu, K.D.; Goli, D. Multifaceted Effects of Aluminium in Neurodegenerative Diseases: A Review. Biomed. Pharmacother. 2016, 83, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, A.; Srivastava, S.; Siow, R.C.M.; Modo, M.; Fraser, P.A.; Mann, G.E. Targeting the Nrf2–Keap1 Antioxidant Defence Pathway for Neurovascular Protection in Stroke. J. Physiol. 2011, 589, 4125–4136. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.J.C.; Nelson, L.; Slade, J.Y.; Oakley, A.E.; Khundakar, A.A.; Kalaria, R.N. Morphometry of the Hippocampal Microvasculature in Post-Stroke and Age-Related Dementias. Neuropathol. Appl. Neurobiol. 2014, 40, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Alsalahi, A.; Imam, M.U.; Ooi, D.J.; Khaza’ai, H.; Aljaberi, M.A.; Shamsudin, M.N.; Idrus, Z. Safety and Neuroprotective Efficacy of Palm Oil and Tocotrienol-Rich Fraction from Palm Oil: A Systematic Review. Nutrients 2020, 12, 521. [Google Scholar] [CrossRef]
- Atia, A.; Alrawaiq, N.S.; Abdullah, A. Tocotrienols Activate Nrf2 Nuclear Translocation and Increase the Antioxidant- Related Hepatoprotective Mechanism in Mice Liver. Curr. Pharm. Biotechnol. 2021, 22, 1085–1098. [Google Scholar] [CrossRef]
- Sadikan, M.Z.; Nasir, N.A.A.; Iezhitsa, I.; Agarwal, R. Antioxidant and Anti-Apoptotic Effects of Tocotrienol-Rich Fraction against Streptozotocin-Induced Diabetic Retinopathy in Rats. Biomed. Pharmacother. 2022, 153, 113533. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Mathai, M.; Zulli, A. Revisiting the Therapeutic Potential of Tocotrienol. BioFactors Oxf. Engl. 2022, 48, 813–856. [Google Scholar] [CrossRef]
- Zainal, Z.; Khaza’ai, H.; Kutty Radhakrishnan, A.; Chang, S.K. Therapeutic Potential of Palm Oil Vitamin E-Derived Tocotrienols in Inflammation and Chronic Diseases: Evidence from Preclinical and Clinical Studies. Food Res. Int. 2022, 156, 111175. [Google Scholar] [CrossRef]
- Shaikh, S.A.; Varatharajan, R.; Muthuraman, A. Palm Oil Derived Tocotrienol-Rich Fraction Attenuates Vascular Dementia in Type 2 Diabetic Rats. Int. J. Mol. Sci. 2022, 23, 13531. [Google Scholar] [CrossRef]
- Koladiya, R.U.; Jaggi, A.S.; Singh, N.; Sharma, B.K. Beneficial Effects of Donepezil on Vascular Endothelial Dysfunction-Associated Dementia Induced by L-Methionine in Rats. J. Health Sci. 2009, 55, 215–225. [Google Scholar] [CrossRef]
- Yokel, R.A. Aluminum in Food–The Nature and Contribution of Food Additives; IntechOpen: London, UK, 2012; ISBN 978-953-51-0067-6. [Google Scholar]
- Nayak, P. Conjecturable Role of Aluminum in Pathophysiology of Stroke. In Metal Ion in Stroke; Li, Y.V., Zhang, J.H., Eds.; Springer Series in Translational Stroke Research; Springer: New York, NY, USA, 2012; pp. 649–680. ISBN 978-1-4419-9663-3. [Google Scholar]
- Zhao, Y.; Dang, M.; Zhang, W.; Lei, Y.; Ramesh, T.; Priya Veeraraghavan, V.; Hou, X. Neuroprotective Effects of Syringic Acid against Aluminium Chloride Induced Oxidative Stress Mediated Neuroinflammation in Rat Model of Alzheimer’s Disease. J. Funct. Foods 2020, 71, 104009. [Google Scholar] [CrossRef]
- Ojha, P.S.; Biradar, P.R.; Tubachi, S.; Patil, V.S. Evaluation of Neuroprotective Effects of Canna Indica L against Aluminium Chloride Induced Memory Impairment in Rats. Adv. Tradit. Med. 2022, 23, 539–556. [Google Scholar] [CrossRef]
- Durani, L.W.; Hamezah, H.S.; Ibrahim, N.F.; Yanagisawa, D.; Nasaruddin, M.L.; Mori, M.; Azizan, K.A.; Damanhuri, H.A.; Makpol, S.; Wan Ngah, W.Z. Tocotrienol-Rich Fraction of Palm Oil Improves Behavioral Impairments and Regulates Metabolic Pathways in AβPP/PS1 Mice. J. Alzheimers Dis. 2018, 64, 249–267. [Google Scholar] [CrossRef]
- Nagapan, G.; Meng Goh, Y.; Shameha Abdul Razak, I.; Nesaretnam, K.; Ebrahimi, M. The Effects of Prenatal and Early Postnatal Tocotrienol-Rich Fraction Supplementation on Cognitive Function Development in Male Offspring Rats. BMC Neurosci. 2013, 14, 77. [Google Scholar] [CrossRef]
- Taridi, N.M.; Yahaya, M.F.; Teoh, S.L.; Latiff, A.A.; Ngah, W.Z.W.; Das, S.; Mazlan, M. Tocotrienol Rich Fraction (TRF) Supplementation Protects against Oxidative DNA Damage and Improves Cognitive Functions in Wistar Rats. Clin. Ter. 2011, 162, 93–98. [Google Scholar] [PubMed]
- Bai, T.; Li, M.; Liu, Y.; Qiao, Z.; Wang, Z. Inhibition of Ferroptosis Alleviates Atherosclerosis through Attenuating Lipid Peroxidation and Endothelial Dysfunction in Mouse Aortic Endothelial Cell. Free Radic. Biol. Med. 2020, 160, 92–102. [Google Scholar] [CrossRef]
- Yongxia, Z.; Jian, X.; Suyuan, H.; Aixin, N.; Lihong, Z. Isolation and Characterization of Ergosterol from Monascus Anka for Anti-Lipid Peroxidation Properties. J. Mycol. Med. 2020, 30, 101038. [Google Scholar] [CrossRef]
- Exley, C. The Pro-Oxidant Activity of Aluminum. Free Radic. Biol. Med. 2004, 36, 380–387. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.M.; Shehata, M.G.; Alsulami, T.; Badr, A.N.; Elbakatoshy, M.R.; Ali, H.S.; El-Sohaimy, S.A. Characterization of Orange Peel Extract and Its Potential Protective Effect against Aluminum Chloride-Induced Alzheimer’s Disease. Pharmaceuticals 2023, 16, 12. [Google Scholar] [CrossRef]
- Kantar, D.; Acun, A.D.; Danışman, B. Effects of Thymoquinone on Scopolamine-Induced Spatial and Echoic Memory Changes through Regulation of Lipid Peroxidation and Cholinergic Impairment. Behav. Brain Res. 2022, 431, 113972. [Google Scholar] [CrossRef]
- Elmorsy, E.; Elsharkawy, E.; Alhumaydhi, F.A.; Salama, M. The Protective Effect of Indian Catechu Methanolic Extract against Aluminum Chloride-Induced Neurotoxicity, A Rodent Model of Alzheimer’s Disease. Heliyon 2021, 7, e06269. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Zhao, J.; Xing, X.; Zhang, C.; Meng, H. Neuroprotective Effects of D-(-)-Quinic Acid on Aluminum Chloride-Induced Dementia in Rats. Evid. Based Complement. Alternat. Med. 2020, 2020, 5602597. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological Potential of Tocotrienols: A Review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, J.; Chakraborty, R.; Raychaudhuri, U. The 21st Century Form of Vitamin E--Tocotrienol. Curr. Pharm. Des. 2011, 17, 2196–2205. [Google Scholar] [CrossRef]
- Budin, S.B.; Othman, F.; Louis, S.R.; Bakar, M.A.; Das, S.; Mohamed, J. The Effects of Palm Oil Tocotrienol-Rich Fraction Supplementation on Biochemical Parameters, Oxidative Stress and the Vascular Wall of Streptozotocin-Induced Diabetic Rats. Clin. Sao Paulo Braz. 2009, 64, 235–244. [Google Scholar] [CrossRef]
- Kuhad, A.; Chopra, K. Attenuation of Diabetic Nephropathy by Tocotrienol: Involvement of NFkB Signaling Pathway. Life Sci. 2009, 84, 296–301. [Google Scholar] [CrossRef]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Abdul-Rahman, M.; Al-Wahaibi, N.; Mohammed, J. Tocotrienol-Rich Fraction from Palm Oil Prevents Oxidative Damage in Diabetic Rats. Sultan Qaboos Univ. Med. J. 2014, 14, e95–e103. [Google Scholar] [CrossRef]
- Wong, R.S.Y.; Radhakrishnan, A.K. Tocotrienol Research: Past into Present. Nutr. Rev. 2012, 70, 483–490. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J. Antioxidant and Antiproliferative Properties of a Tocotrienol-Rich Fraction from Grape Seeds. Food Chem. 2009, 114, 1386–1390. [Google Scholar] [CrossRef]
- Virk, D.; Kumar, A.; Jaggi, A.S.; Singh, N. Ameliorative Role of Rolipram, PDE-4 Inhibitor, against Sodium Arsenite–Induced Vascular Dementia in Rats. Environ. Sci. Pollut. Res. 2021, 28, 63250–63262. [Google Scholar] [CrossRef]
- Corzo, L.; Zas, R.; Rodríguez, S.; Fernández-Novoa, L.; Cacabelos, R. Decreased Levels of Serum Nitric Oxide in Different Forms of Dementia. Neurosci. Lett. 2007, 420, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-Y.; Hong, F.-F.; Yang, S.-L. The Roles of Nitric Oxide Synthase/Nitric Oxide Pathway in the Pathology of Vascular Dementia and Related Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 4540. [Google Scholar] [CrossRef] [PubMed]
- Norsidah, K.-Z.; Asmadi, A.Y.; Azizi, A.; Faizah, O.; Kamisah, Y. Palm Tocotrienol-Rich Fraction Improves Vascular Proatherosclerotic Changes in Hyperhomocysteinemic Rats. Evid. Based Complement. Alternat. Med. 2013, 2013, e976967. [Google Scholar] [CrossRef]
- Bosche, B.; Molcanyi, M.; Noll, T.; Rej, S.; Zatschler, B.; Doeppner, T.R.; Hescheler, J.; Müller, D.J.; Macdonald, R.L.; Härtel, F.V. A Differential Impact of Lithium on Endothelium-Dependent but Not on Endothelium-Independent Vessel Relaxation. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 67, 98–106. [Google Scholar] [CrossRef]
- Haupt, M.; Zechmeister, B.; Bosche, B.; Lieschke, S.; Zheng, X.; Zhang, L.; Venkataramani, V.; Jin, F.; Hein, K.; Weber, M.S.; et al. Lithium Enhances Post-Stroke Blood-Brain Barrier Integrity, Activates the MAPK/ERK1/2 Pathway and Alters Immune Cell Migration in Mice. Neuropharmacology 2020, 181, 108357. [Google Scholar] [CrossRef] [PubMed]
- Karel, M.F.A.; Roosen, M.G.C.H.; Tullemans, B.M.E.; Zhang, C.E.; Staals, J.; Cosemans, J.M.E.M.; Koenen, R.R. Characterization of Cerebral Small Vessel Disease by Neutrophil and Platelet Activation Markers Using Artificial Intelligence. J. Neuroimmunol. 2022, 367, 577863. [Google Scholar] [CrossRef]
- Mohd Nor, N.A.; Budin, S.B.; Zainalabidin, S.; Jalil, J.; Sapian, S.; Jubaidi, F.F.; Mohamad Anuar, N.N. The Role of Polyphenol in Modulating Associated Genes in Diabetes-Induced Vascular Disorders. Int. J. Mol. Sci. 2022, 23, 6396. [Google Scholar] [CrossRef]
- Chianca, M.; Panichella, G.; Fabiani, I.; Giannoni, A.; Aimo, A.; Franco, A.D.; Vergaro, G.; Grigoratos, C.; Castiglione, V.; Cipolla, C.M. Bidirectional Relationship between Cancer and Heart Failure: Insights on Circulating Biomarkers. Front. Cardiovasc. Med. 2022, 9, 1739. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Zhang, Y.; Fang, F.; Liu, J.; Xia, Y.; Liu, Y. Cardiac Biomarkers for the Detection and Management of Cancer Therapy-Related Cardiovascular Toxicity. J. Cardiovasc. Dev. Dis. 2022, 9, 372. [Google Scholar] [CrossRef]
- Cheng, H.S.; Ton, S.H.; Tan, J.B.L.; Abdul Kadir, K. The Ameliorative Effects of a Tocotrienol-Rich Fraction on the AGE-RAGE Axis and Hypertension in High-Fat-Diet-Fed Rats with Metabolic Syndrome. Nutrients 2017, 9, 984. [Google Scholar] [CrossRef]
- Saw, T.Y.; Malik, N.A.; Lim, K.P.; Teo, C.W.L.; Wong, E.S.M.; Kong, S.C.; Fong, C.W.; Petkov, J.; Yap, W.N. Oral Supplementation of Tocotrienol-Rich Fraction Alleviates Severity of Ulcerative Colitis in Mice. J. Nutr. Sci. Vitaminol. 2019, 65, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhan, Y.; Jiang, Q.; Lu, W.; Li, X. Expression and Function of PDGF-C in Development and Stem Cells. Open Biol. 2021, 11, 210268. [Google Scholar] [CrossRef] [PubMed]
- Moriya, J.; Wu, X.; Zavala-Solorio, J.; Ross, J.; Liang, X.H.; Ferrara, N. Platelet-Derived Growth Factor C Promotes Revascularization in Ischemic Limbs of Diabetic Mice. J. Vasc. Surg. 2014, 59, 1402–1409.e4. [Google Scholar] [CrossRef] [PubMed]
- Grismaldo, A.; Sobrevia, L.; Morales, L. Role of Platelet-Derived Growth Factor c on Endothelial Dysfunction in Cardiovascular Diseases. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130188. [Google Scholar] [CrossRef]
- Grismaldo Rodríguez, A.; Zamudio Rodríguez, J.A.; Mendieta, C.V.; Quijano Gómez, S.; Sanabria Barrera, S.; Morales Álvarez, L. Effect of Platelet-Derived Growth Factor C on Mitochondrial Oxidative Stress Induced by High d-Glucose in Human Aortic Endothelial Cells. Pharmaceuticals 2022, 15, 639. [Google Scholar] [CrossRef]
- Ting, T.M.; King, J.H.; Ho, K.L.; Lau, H.L.N. Wound Healing Potential of Palm Oil Tocotrienols Rich Fraction. Food Res. 2021, 5, 394–403. [Google Scholar] [CrossRef]
- Xu, C.; Bentinger, M.; Savu, O.; Moshfegh, A.; Sunkari, V.; Dallner, G.; Swiezewska, E.; Catrina, S.-B.; Brismar, K.; Tekle, M. Mono-Epoxy-Tocotrienol-α Enhances Wound Healing in Diabetic Mice and Stimulates in Vitro Angiogenesis and Cell Migration. J. Diabetes Complicat. 2017, 31, 4–12. [Google Scholar] [CrossRef]
- Liaquat, L.; Sadir, S.; Batool, Z.; Tabassum, S.; Shahzad, S.; Afzal, A.; Haider, S. Acute Aluminum Chloride Toxicity Revisited: Study on DNA Damage and Histopathological, Biochemical and Neurochemical Alterations in Rat Brain. Life Sci. 2019, 217, 202–211. [Google Scholar] [CrossRef]
- Lee, H.; Lim, Y. Tocotrienol-Rich Fraction Supplementation Reduces Hyperglycemia-Induced Skeletal Muscle Damage through Regulation of Insulin Signaling and Oxidative Stress in Type 2 Diabetic Mice. J. Nutr. Biochem. 2018, 57, 77–85. [Google Scholar] [CrossRef]
- Chiroma, S.M.; Hidayat Baharuldin, M.T.; Mat Taib, C.N.; Amom, Z.; Jagadeesan, S.; Adenan, M.I.; Mohd Moklas, M.A. Protective Effect of Centella Asiatica against D-Galactose and Aluminium Chloride Induced Rats: Behavioral and Ultrastructural Approaches. Biomed. Pharmacother. 2019, 109, 853–864. [Google Scholar] [CrossRef]
- Dhingra, D.; Parle, M.; Kulkarni, S. Memory Enhancing Activity of Glycyrrhiza Glabra in Mice. J. Ethnopharmacol. 2004, 91, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, V.; Riju, T.; Venkatesh, S.; Babu, G. Memory Enhancing Activity of Lawsonia inermis Linn. Leaves against Scopolamine Induced Memory Impairment in Swiss Albino Mice. Orient. Pharm. Exp. Med. 2017, 17, 127–142. [Google Scholar] [CrossRef]
- Rishitha, N.; Muthuraman, A. Preventative Effects of Alpha-Naphtho Flavone in Vascular Dementia. Front. Biosci. Elite 2020, 12, 79–94. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]


| Treatment | Brain TBARS (nM/mg of Protein) |
|---|---|
| CON | 59.40 ± 2.84 |
| AlCl3 | 83.58 ± 4.80 a |
| TRF 30 | 68.71 ± 1.48 |
| TRF 60 | 53.14 ± 5.21 b |
| TRF 120 | 53.84 ± 3.83 b |
| DON | 51.15 ± 1.38 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, S.A.; Muthuraman, A. Tocotrienol-Rich Fraction Ameliorates the Aluminium Chloride-Induced Neurovascular Dysfunction-Associated Vascular Dementia in Rats. Pharmaceuticals 2023, 16, 828. https://doi.org/10.3390/ph16060828
Shaikh SA, Muthuraman A. Tocotrienol-Rich Fraction Ameliorates the Aluminium Chloride-Induced Neurovascular Dysfunction-Associated Vascular Dementia in Rats. Pharmaceuticals. 2023; 16(6):828. https://doi.org/10.3390/ph16060828
Chicago/Turabian StyleShaikh, Sohrab A., and Arunachalam Muthuraman. 2023. "Tocotrienol-Rich Fraction Ameliorates the Aluminium Chloride-Induced Neurovascular Dysfunction-Associated Vascular Dementia in Rats" Pharmaceuticals 16, no. 6: 828. https://doi.org/10.3390/ph16060828
APA StyleShaikh, S. A., & Muthuraman, A. (2023). Tocotrienol-Rich Fraction Ameliorates the Aluminium Chloride-Induced Neurovascular Dysfunction-Associated Vascular Dementia in Rats. Pharmaceuticals, 16(6), 828. https://doi.org/10.3390/ph16060828






