Study on the Intervention Mechanism of Cryptotanshinone on Human A2780 Ovarian Cancer Cell Line Using GC-MS-Based Cellular Metabolomics
Abstract
1. Introduction
2. Results
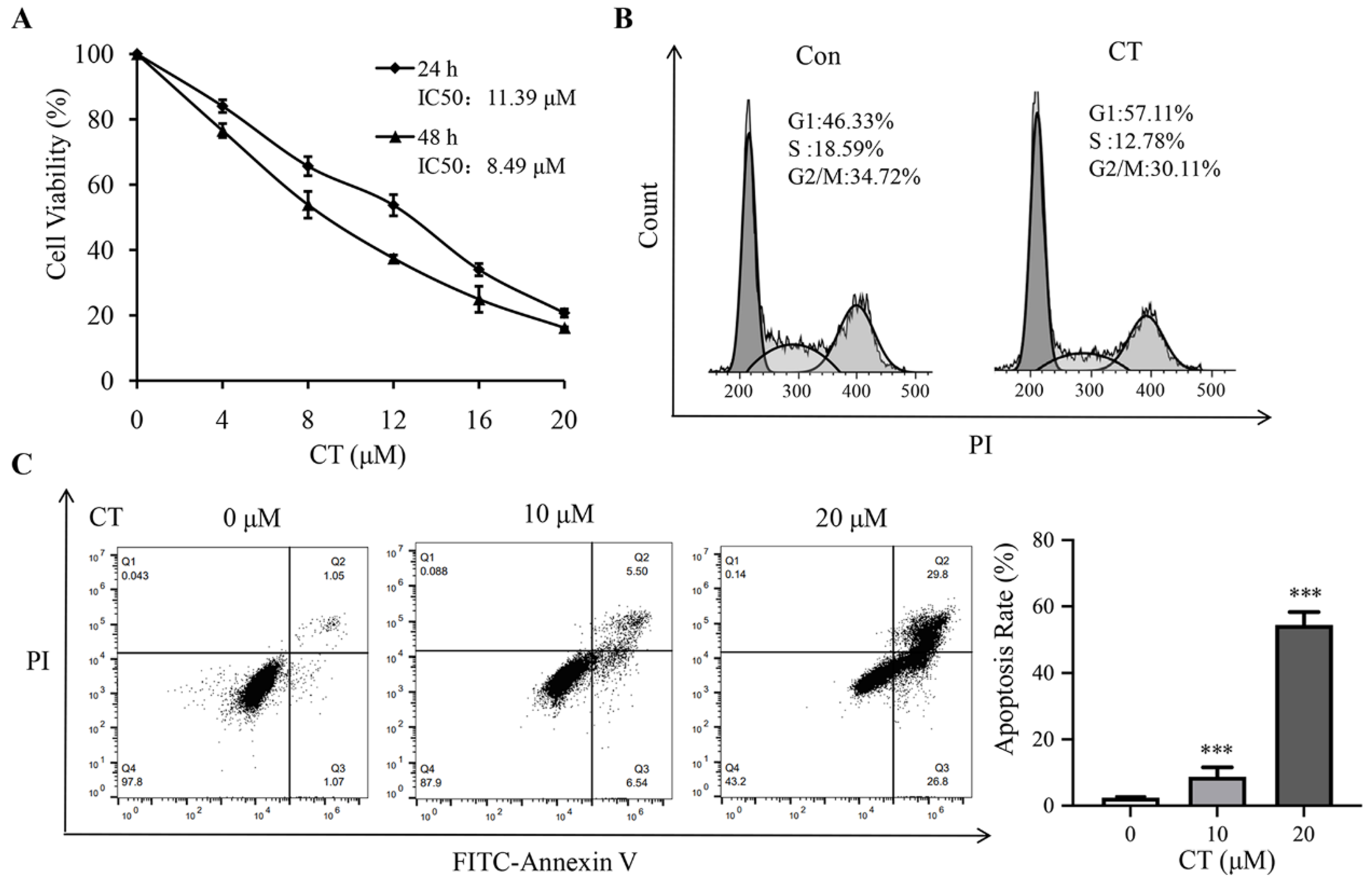
2.1. Effect of CT on Cell Viability Assay
2.2. Analysis of Cell Cycle Profiles and Apoptosis
2.3. GC-MS Cell Metabolomics Analysis
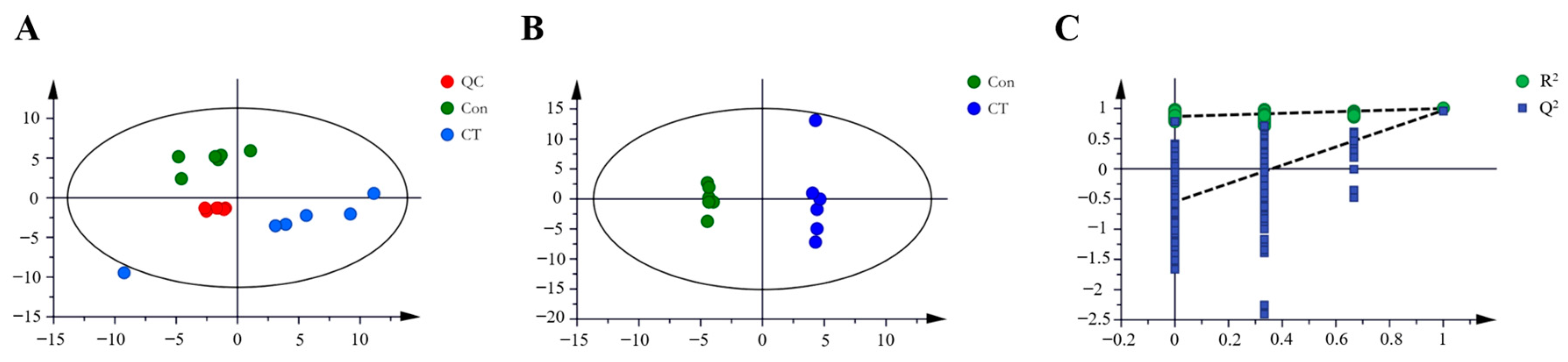
2.3.1. Discriminant Analysis of Model
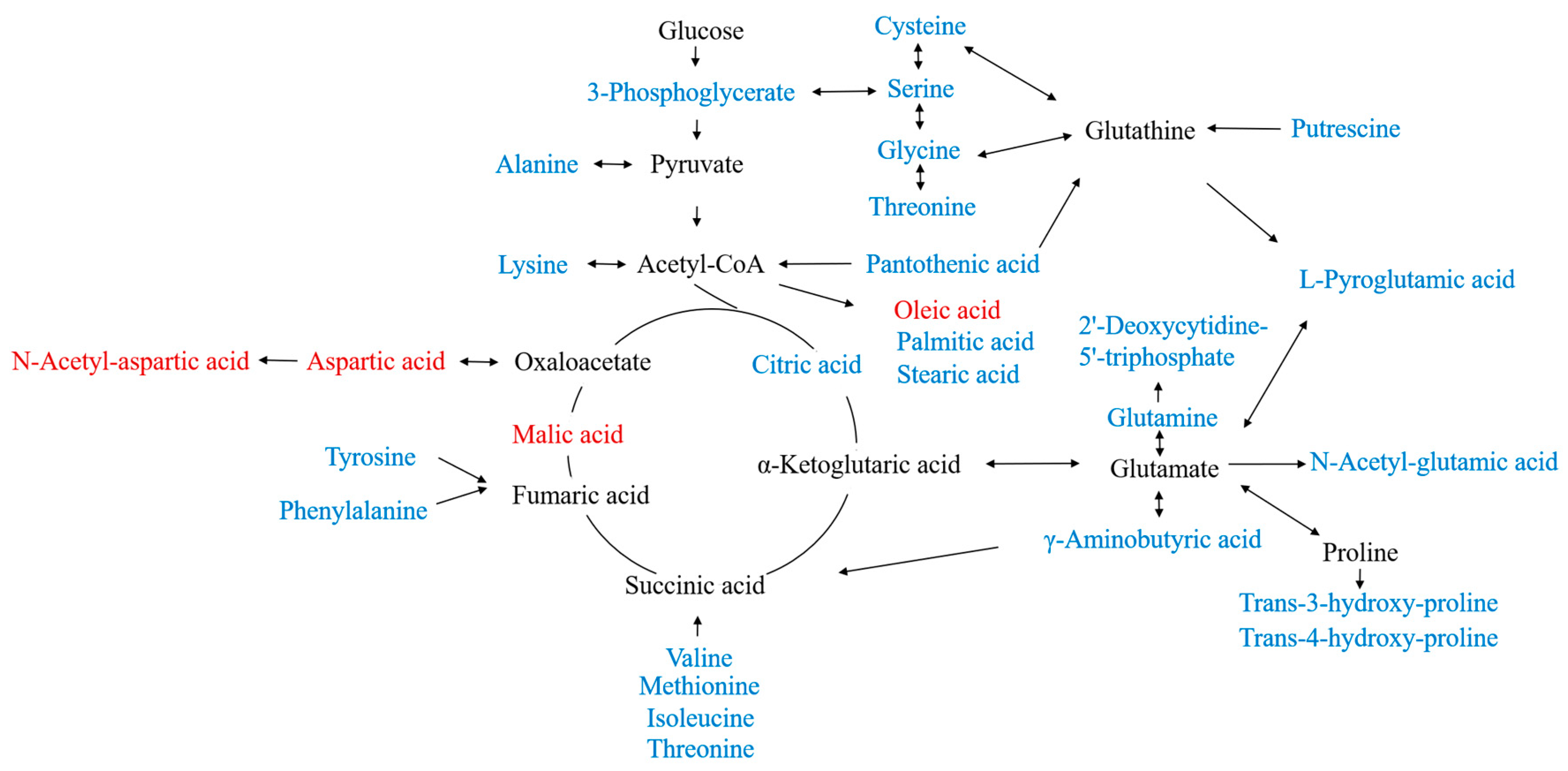
2.3.2. Identification of Differential Metabolites
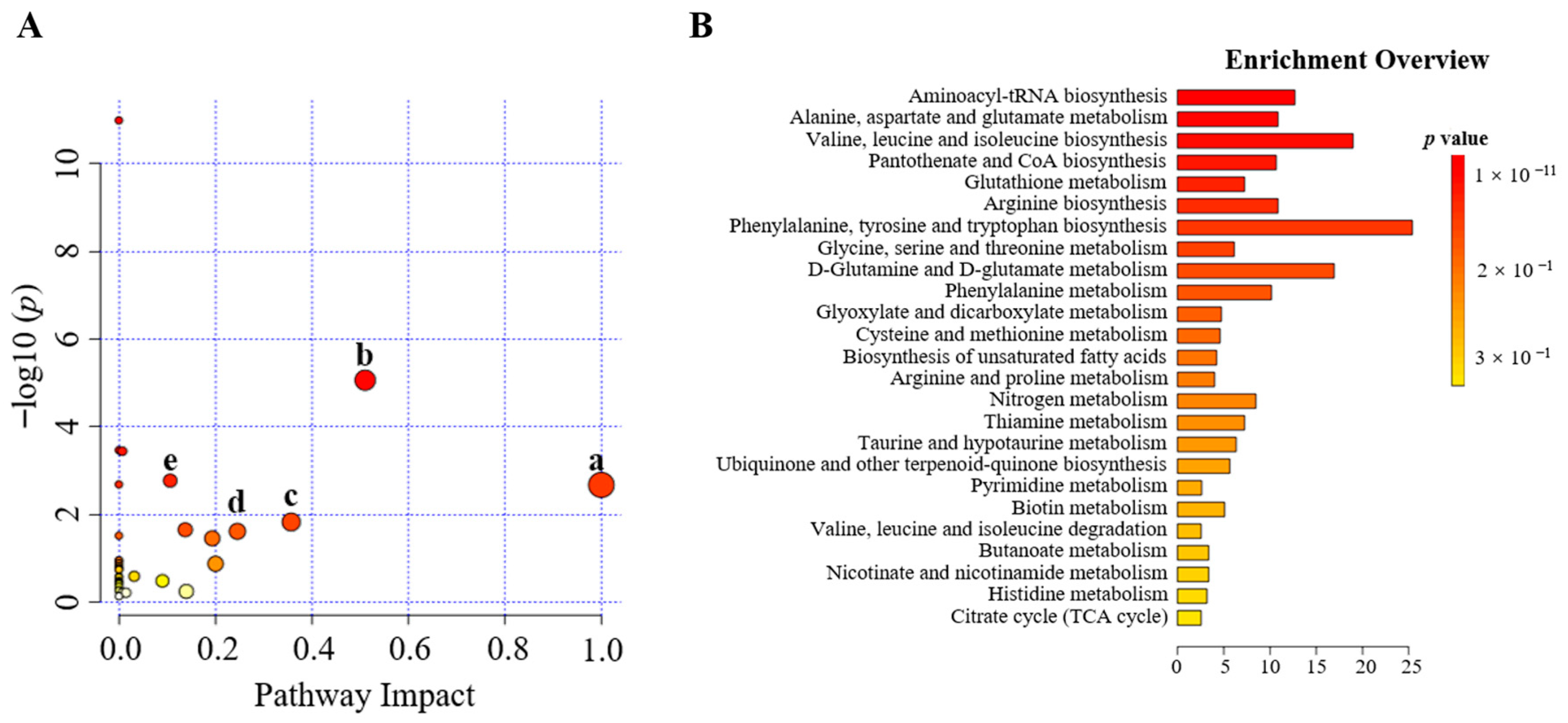
2.3.3. Metabolic Pathway Analysis
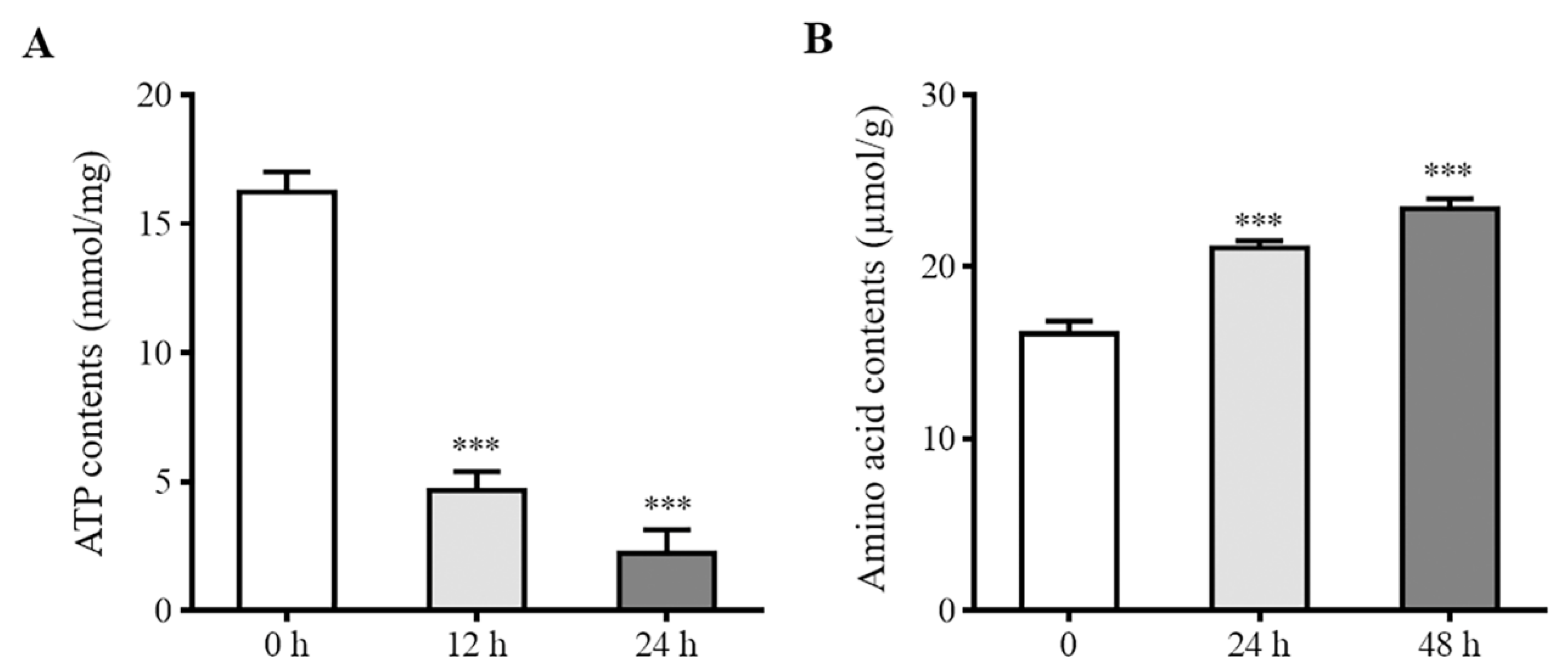
2.4. Determination of Intracellular ATP and Amino Acid Contents
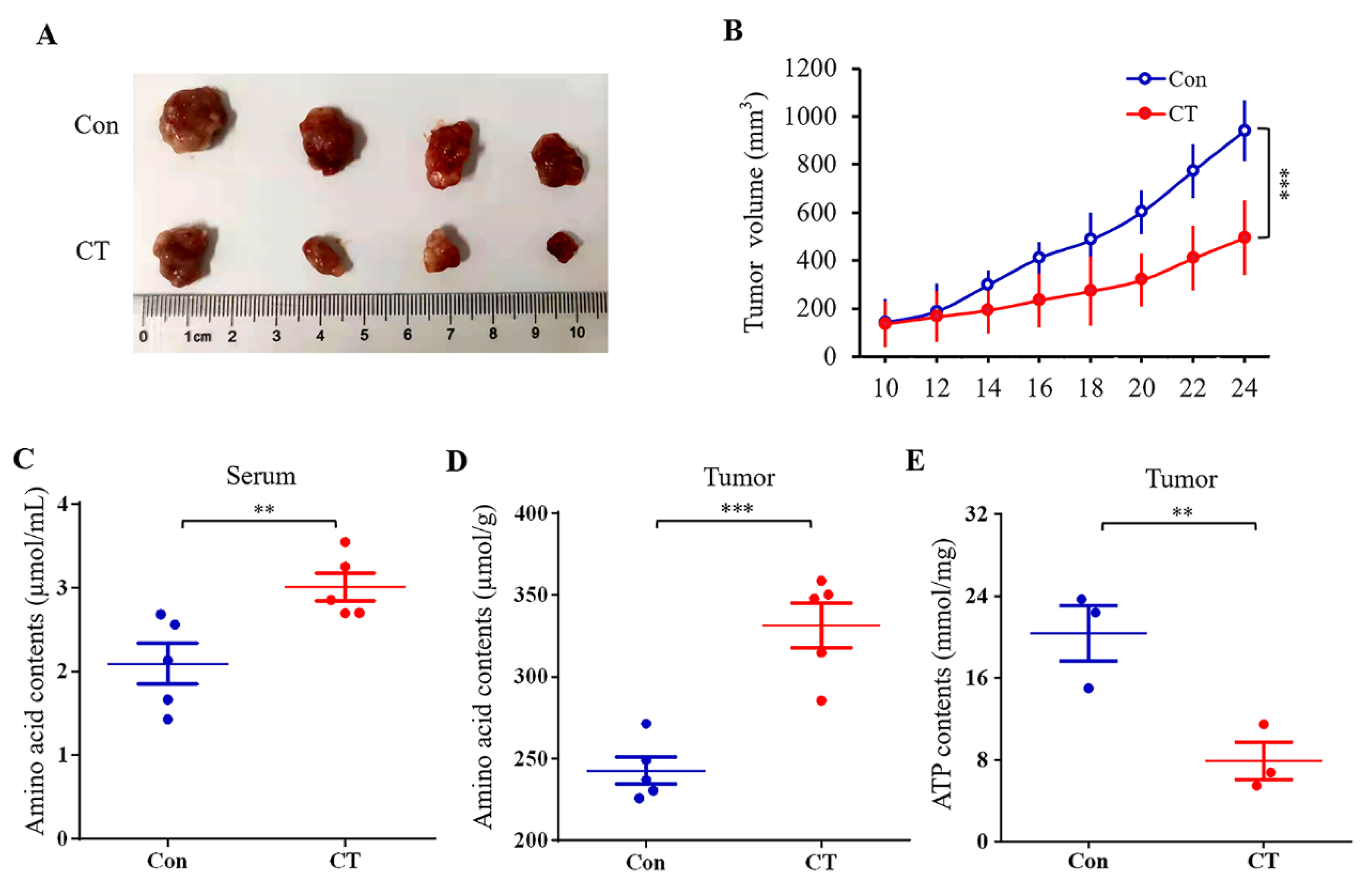
2.5. Effect of CT on A2780 Xenograft Tumor Growth
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Lines
4.3. Cell Proliferation Assay
4.4. Apoptosis and Cell Cycle Analysis
4.5. GC-MS Sample Preparation
4.6. GC-MS Analysis Conditions
4.7. Data Processing and Analysis
4.8. Determination of Intracellular ATP Contents
4.9. Determination of Intracellular Amino Acid Contents
4.10. Mouse Xenograft Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, J.; Gao, Y.; Lin, S.; Zou, X.; Zhu, Y.; Chen, X.; Wan, H.; Zhu, H. Effects of activating GABAB1 receptor on proliferation, migration, invasion and epithelial-mesenchymal transition of ovarian cancer cells. J. Ovarian Res. 2020, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Chen, H.D.; Yu, Y.W.; Li, N.; Chen, W.Q. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, V.D. Ovarian cancer biomarkers: Moving forward in early detection. Tumor Microenviron. 2020, 1219, 355–363. [Google Scholar]
- Khan, M.; Maryam, A.; Qazi, J.I.; Ma, T. Targeting Apoptosis and Multiple Signaling Pathways with Icariside II in Cancer Cells. Int. J. Biol. Sci. 2015, 11, 1100–1112. [Google Scholar] [CrossRef]
- Ashaq, A.; Maqbool, M.F.; Maryam, A.; Khan, M.; Shakir, H.A.; Irfan, M.; Qazi, J.I.; Li, Y.; Ma, T. Hispidulin: A novel natural compound with therapeutic potential against human cancers. Phytother Res. 2021, 35, 771–789. [Google Scholar] [CrossRef]
- Nagappan, A.; Kim, J.H.; Jung, D.Y.; Jung, M.H. Cryptotanshinone from the Salvia miltiorrhiza Bunge Attenuates Ethanol-Induced Liver Injury by Activation of AMPK/SIRT1 and Nrf2 Signaling Pathways. Int. J. Mol. Sci. 2019, 21, 265. [Google Scholar] [CrossRef]
- Li, H.; Gao, C.; Liu, C.; Liu, L.; Zhuang, J.; Yang, J.; Zhou, C.; Feng, F.; Sun, C.; Wu, J. A review of the biological activity and pharmacology of cryptotanshinone, an important active constituent in Danshen. Biomed. Pharmacother. 2021, 137, 111332. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Y.; Chen, G.; Huang, S. Molecular evidence of cryptotanshinone for treatment and prevention of human cancer. Anticancer Agents. Med. Chem. 2013, 13, 979–987. [Google Scholar] [CrossRef]
- Cai, P.; Sheng, G.; Jiang, S.; Wang, D.; Zhao, Z.; Huang, M.; Jin, J. Comparative Proteomics Analysis Reveals the Reversal Effect of Cryptotanshinone on Gefitinib-Resistant Cells in Epidermal Growth Factor Receptor-Mutant Lung Cancer. Front. Pharmacol. 2022, 13, 837055. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, J.; Ren, B.; Zhang, L.; Owusu, L.; Liu, L.; Zhang, J.; Tang, Y.; Li, W. Anti-tumor and chemosensitization effects of Cryptotanshinone extracted from Salvia miltiorrhiza Bge. on ovarian cancer cells in vitro. J. Ethnopharmacol. 2017, 205, 33–40. [Google Scholar] [CrossRef]
- Zhou, J.; Su, C.M.; Chen, H.A.; Du, S.; Li, C.W.; Wu, H.; Tsai, S.H.; Yeh, Y.T. Cryptanshinone inhibits the glycolysis and inhibits cell migration through PKM2/β-catenin axis in breast cancer. OncoTargets Ther. 2020, 13, 8629. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, Y.; Chen, L.; Liu, F.; Qi, Z.; Cheng, X.; Wang, Z. Cryptotanshinone suppresses cell proliferation and glucose metabolism via STAT3/SIRT3 signaling pathway in ovarian cancer cells. Cancer Med. 2018, 7, 4610–4618. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Pyun, W.Y.; Park, H.W. Cancer metabolism: Phenotype, signaling and therapeutic targets. Cells 2020, 9, 2308. [Google Scholar] [CrossRef] [PubMed]
- Lubes, G.; Goodarzi, M. GC–MS based metabolomics used for the identification of cancer volatile organic compounds as biomarkers. J. Pharm. Biomed. Anal. 2018, 147, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthie, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Park, I.J.; Yang, W.K.; Nam, S.H.; Hong, J.; Yang, K.R.; Kim, J.; Kim, S.S.; Choe, W.; Kang, I.; Ha, J. Cryptotanshinone induces G1 cell cycle arrest and autophagic cell death by activating the AMP-activated protein kinase signal pathway in HepG2 hepatoma. Apoptosis 2014, 19, 615–628. [Google Scholar] [CrossRef]
- Liang, Z.; Jin, C.; Bai, H.; Liang, G.; Su, X.; Wang, D.; Yao, J. Low rumen degradable starch promotes the growth performance of goats by increasing protein synthesis in skeletal muscle via the AMPK-mTOR pathway. Anim. Nutr. 2023, 13, 1–8. [Google Scholar] [CrossRef]
- Sitole, L.J.; Tugizimana, F.; Meyer, D. Multi-platform metabonomics unravel amino acids as markers of HIV/combination antiretroviral therapy-induced oxidative stress. J. Pharm. Biomed. Anal. 2019, 176, 112796. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, J.; Chen, Z.; Luo, Q.; Xu, J.; Song, G. Tumor-specific expansion of oxidative stress by glutathione depletion and use of a fenton nanoagent for enhanced chemodynamic therapy. ACS Appl. Mater. Interfaces 2019, 11, 30551–30565. [Google Scholar] [CrossRef]
- Svoboda, L.K.; The, S.S.; Sud, S.; Kerk, S.; Zebolsky, A.; Treichel, S.; Thomas, D.; Halbrook, C.J.; Lee, H.J.; Kremer, D.; et al. Menin regulates the serine biosynthetic pathway in Ewing sarcoma. J. Pathol. 2018, 245, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, T.; Cordes, T.; Handzlik, M.K.; You, L.; Lim, E.W.; Gengatharan, J.; Pinto, A.F.; Badur, M.G.; Kolar, M.J.; Wallace, M.; et al. Serine restriction alters sphingolipid diversity to constrain tumour growth. Nature 2020, 586, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, L.; Shi, Q.; Zhao, Y.; Lin, H.; Zhang, M.; Zhao, S.; Yang, Y.; Ling, Z.Q.; Guan, K.L.; et al. SIRT 3-dependent GOT 2 acetylation status affects the malate–aspartate NADH shuttle activity and pancreatic tumor growth. EMBO J. 2015, 34, 1110–1125. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Guo, Y.; Wang, C.; Liao, D.; Han, W.; Zhang, J.; Jiang, P. Systematic impacts of chronic unpredictable mild stress on metabolomics in rats. Sci. Rep. 2020, 10, 700. [Google Scholar] [CrossRef]
- Peterson, C.T.; Rodionov, D.A.; Osterman, A.L.; Peterson, S.N. B vitamins and their role in immune regulation and cancer. Nutrients 2020, 12, 3380. [Google Scholar] [CrossRef]
- Jia, N.; Ding, M.Z.; Du, J.; Pan, C.H.; Tian, G.; Lang, J.D.; Fang, J.H.; Gao, F.; Yuan, Y.J. Insights into mutualism mechanism and versatile metabolism of Ketogulonicigenium vulgare Hbe602 based on comparative genomics and metabolomics studies. Sci. Rep. 2016, 6, 23068. [Google Scholar] [CrossRef]
- Bertero, T.; Oldham, W.M.; Grasset, E.M.; Bourget, I.; Boulter, E.; Pisano, S.; Hofman, P.; Bellvert, F.; Meneguzzi, G.; Bulavin, D.V.; et al. Tumor-stroma mechanics coordinate amino acid availability to sustain tumor growth and malignancy. Cell Metab. 2019, 29, 124–140. [Google Scholar] [CrossRef]
- Sivanand, S.; Vander Heiden, M.G. Emerging Roles for Branched-Chain Amino Acid Metabolism in Cancer. Cancer Cell 2020, 37, 147–156. [Google Scholar] [CrossRef]
- Vettore, L.; Westbrook, R.L.; Tennant, D.A. New aspects of amino acid metabolism in cancer. Br. J. Cancer 2019, 122, 150–156. [Google Scholar] [CrossRef]
- Liu, N.; Shi, F.; Yang, L.; Liao, W.; Cao, Y. Oncogenic viral infection and amino acid metabolism in cancer progression: Molecular insights and clinical implications. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188724. [Google Scholar] [CrossRef]
- Plewa, S.; Horała, A.; Dereziński, P.; Klupczynska, A.; Nowak-Markwitz, E.; Matysiak, J.; Kokot, Z.J. Usefulness of Amino Acid Profiling in Ovarian Cancer Screening with Special Emphasis on Their Role in Cancerogenesis. Int. J. Mol. Sci. 2017, 18, 2727. [Google Scholar] [CrossRef] [PubMed]
- Bachmayr-Heyda, A.; Aust, S.; Auer, K.; Meier, S.M.; Schmetterer, K.G.; Dekan, S.; Gerner, C.; Pils, D. Integrative Systemic and Local Metabolomics with Impact on Survival in High-Grade Serous Ovarian Cancer. Clin. Cancer Res. 2017, 23, 2081–2092. [Google Scholar] [CrossRef] [PubMed]
- Horala, A.; Plewa, S.; Derezinski, P.; Klupczynska, A.; Matysiak, J.; Nowak-Markwitz, E.; Kokot, Z.J. Serum Free Amino Acid Profiling in Differential Diagnosis of Ovarian Tumors—A Comparative Study with Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 2167. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Byun, J.K.; Choi, Y.K.; Park, K.G. Targeting glutamine metabolism as a therapeutic strategy for cancer. Exp. Mol. Med. 2023, 55, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, Q.; Chen, S.; Zhou, Z.; Li, J.; Sun, H.; Liu, J.; Wang, G.; Zhou, F.; Sun, M. Metabolic intervention liposome for targeting glutamine-addiction of breast cancer. J. Control. Release 2022, 350, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mazi, T.A.; Sarode, G.V.; Czlonkowska, A.; Litwin, T.; Kim, K.; Shibata, N.M.; Medici, V. Dysregulated choline, methionine, and aromatic amino acid metabolism in patients with wilson disease: Exploratory metabolomic profiling and implications for hepatic and neurologic phenotypes. Int. J. Mol. Sci. 2019, 20, 5937. [Google Scholar] [CrossRef]
- Li, H.; Zhang, A.; Hao, Y.; Guan, H.; Lv, Z. Coexistence of Lambert–Eaton myasthenic syndrome and autoimmune encephalitis with anti-CRMP5/CV2 and anti-GABAB receptor antibodies in small cell lung cancer: A case report. Medicine 2018, 97, e0696. [Google Scholar] [CrossRef]
- Schuller, H.M. Regulatory role of G protein-coupled receptors in pancreatic cancer development and progression. Curr. Med. Chem. 2018, 25, 2566–2575. [Google Scholar] [CrossRef]
- Mansour, S.M.; Gomma, M.M.M.; Shafik, P.N. Proton MR spectroscopy and the detection of malignancy in ovarian masses. Br. J. Radiol. 2019, 92, 20190134. [Google Scholar] [CrossRef]
- Ma, F.H.; Li, Y.A.; Liu, J.; Li, H.M.; Zhang, G.F.; Qiang, J.W. Role of proton MR spectroscopy in the differentiation of borderline from malignant epithelial ovarian tumors: A preliminary study. J. Magn. Reson. 2019, 49, 1684–1693. [Google Scholar] [CrossRef]


| No. | Name | Average Rt (min) | p Value | VIP | Fold Change | Trend |
|---|---|---|---|---|---|---|
| 1 | Aspartic acid | 7.989 | 0.0000 | 1.68 | 0.40 | ↓ *** |
| 2 | Citric acid | 9.746 | 0.0000 | 1.60 | 2.80 | ↑ *** |
| 3 | 2′-Deoxycytidine -5’-triphosphate | 7.765 | 0.0001 | 1.58 | 3.03 | ↑ *** |
| 4 | Pantothenic acid | 10.642 | 0.0001 | 1.56 | 1.67 | ↑ *** |
| 5 | Malic acid | 7.781 | 0.0001 | 1.53 | 0.65 | ↓ *** |
| 6 | Cysteine | 8.224 | 0.0002 | 1.52 | 2.17 | ↑ *** |
| 7 | Serine | 6.946 | 0.0003 | 1.50 | 2.21 | ↑ *** |
| 8 | Phenylalanine | 8.676 | 0.0003 | 1.52 | 2.01 | ↑ *** |
| 9 | Valine | 5.914 | 0.0003 | 1.50 | 1.78 | ↑ *** |
| 10 | Tyrosine | 10.367 | 0.0004 | 1.50 | 2.35 | ↑ *** |
| 11 | Threonine | 6.495 | 0.0005 | 1.46 | 1.76 | ↑ *** |
| 12 | Isoleucine | 6.484 | 0.0006 | 1.47 | 1.87 | ↑ *** |
| 13 | L-Pyroglutamic acid | 8.034 | 0.0009 | 1.47 | 1.83 | ↑ *** |
| 14 | Glycine | 5.150 | 0.0009 | 1.45 | 1.58 | ↑ *** |
| 15 | Putrescine | 9.314 | 0.0011 | 1.40 | 2.01 | ↑ ** |
| 16 | Alanine | 5.024 | 0.0013 | 1.41 | 1.61 | ↑ ** |
| 17 | N-Acetyl-aspartic acid | 8.819 | 0.0015 | 1.40 | 0.74 | ↓ ** |
| 18 | Methionine | 8.000 | 0.0023 | 1.37 | 1.67 | ↑ ** |
| 19 | Lysine | 10.265 | 0.0036 | 1.37 | 2.08 | ↑ ** |
| 20 | Trans-3-hydroxy-proline | 8.018 | 0.0043 | 1.30 | 2.31 | ↑ ** |
| 21 | N-Acetyl-glutamic acid | 9.397 | 0.0050 | 1.30 | 1.86 | ↑ ** |
| 22 | Glutamine | 9.074 | 0.0065 | 1.28 | 2.81 | ↑ ** |
| 23 | Oleic acid | 11.619 | 0.0067 | 1.27 | 0.77 | ↓ ** |
| 24 | Trans-4-hydroxy-proline | 8.040 | 0.0107 | 1.22 | 1.42 | ↑ * |
| 25 | Stearic acid | 11.718 | 0.0174 | 1.14 | 1.10 | ↑ * |
| 26 | γ-Aminobutyric acid | 3.781 | 0.0216 | 1.15 | 1.06 | ↑ * |
| 27 | Palmitic acid | 10.813 | 0.0262 | 1.07 | 1.10 | ↑ * |
| 28 | 3-Phosphoglycerate | 9.688 | 0.0455 | 1.05 | 1.32 | ↑ * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Yin, S.; Gu, J.; Li, J.; Zhang, M.; Shan, J.; Wu, X.; Li, Y. Study on the Intervention Mechanism of Cryptotanshinone on Human A2780 Ovarian Cancer Cell Line Using GC-MS-Based Cellular Metabolomics. Pharmaceuticals 2023, 16, 861. https://doi.org/10.3390/ph16060861
Wang T, Yin S, Gu J, Li J, Zhang M, Shan J, Wu X, Li Y. Study on the Intervention Mechanism of Cryptotanshinone on Human A2780 Ovarian Cancer Cell Line Using GC-MS-Based Cellular Metabolomics. Pharmaceuticals. 2023; 16(6):861. https://doi.org/10.3390/ph16060861
Chicago/Turabian StyleWang, Tong, Shusheng Yin, Juan Gu, Jingjing Li, Mengmeng Zhang, Jinjun Shan, Xiao Wu, and Yongming Li. 2023. "Study on the Intervention Mechanism of Cryptotanshinone on Human A2780 Ovarian Cancer Cell Line Using GC-MS-Based Cellular Metabolomics" Pharmaceuticals 16, no. 6: 861. https://doi.org/10.3390/ph16060861
APA StyleWang, T., Yin, S., Gu, J., Li, J., Zhang, M., Shan, J., Wu, X., & Li, Y. (2023). Study on the Intervention Mechanism of Cryptotanshinone on Human A2780 Ovarian Cancer Cell Line Using GC-MS-Based Cellular Metabolomics. Pharmaceuticals, 16(6), 861. https://doi.org/10.3390/ph16060861








