Proof-of-Principle Study of Inflammasome Signaling Proteins as Diagnostic Biomarkers of the Inflammatory Response in Parkinson’s Disease
Abstract
:1. Introduction
2. Results
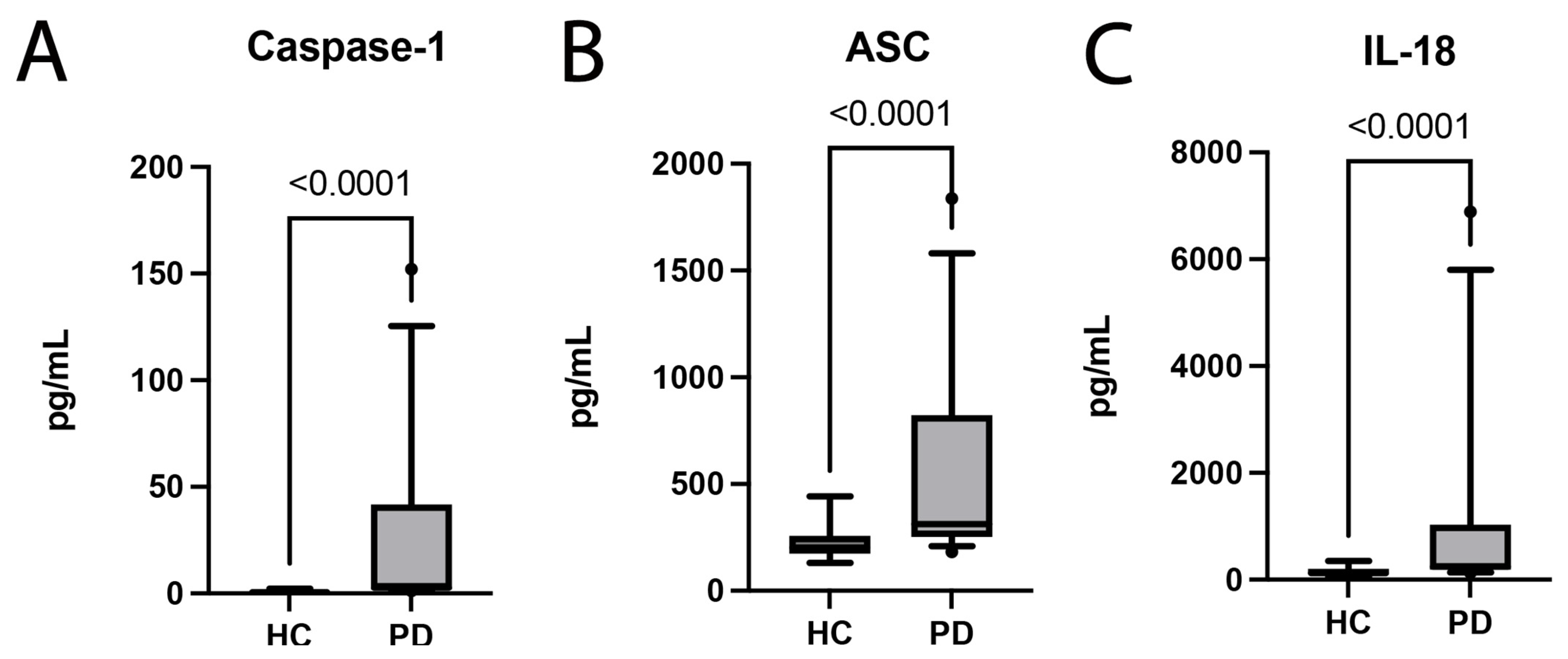
2.1. PD Patients Have Increased Plasma Levels of Inflammasome Proteins and Inflammatory Cytokines
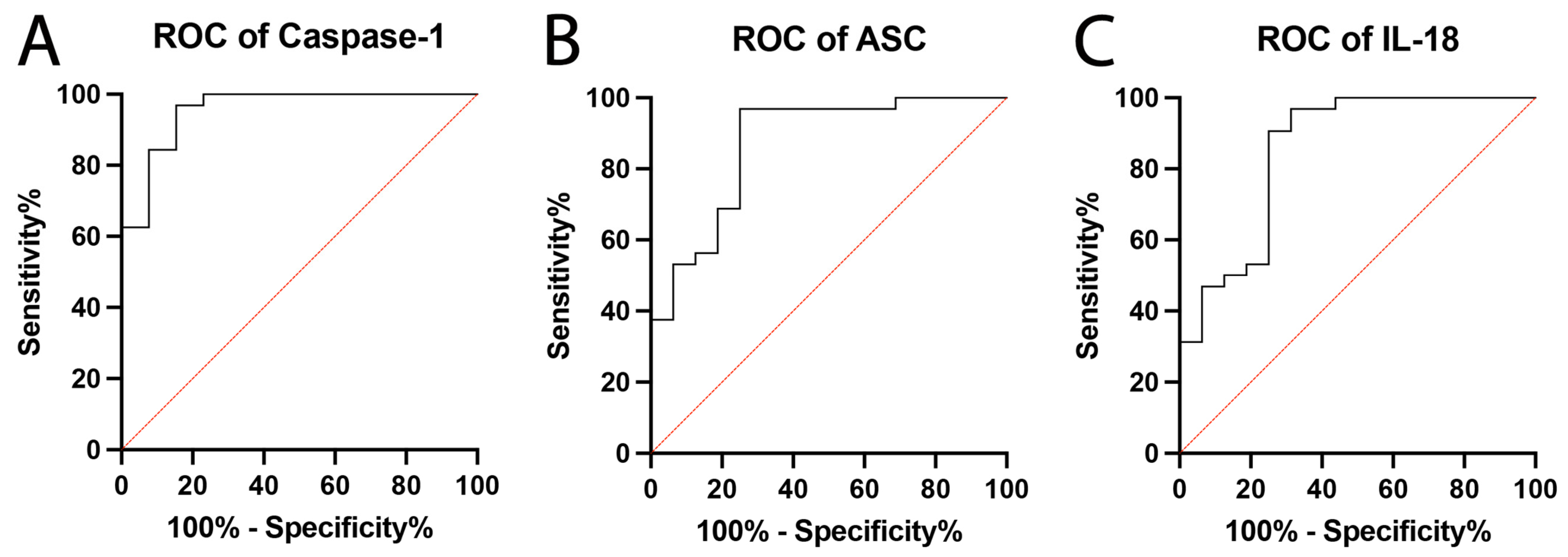
2.2. Inflammasome Proteins Are Reliable Biomarkers of PD
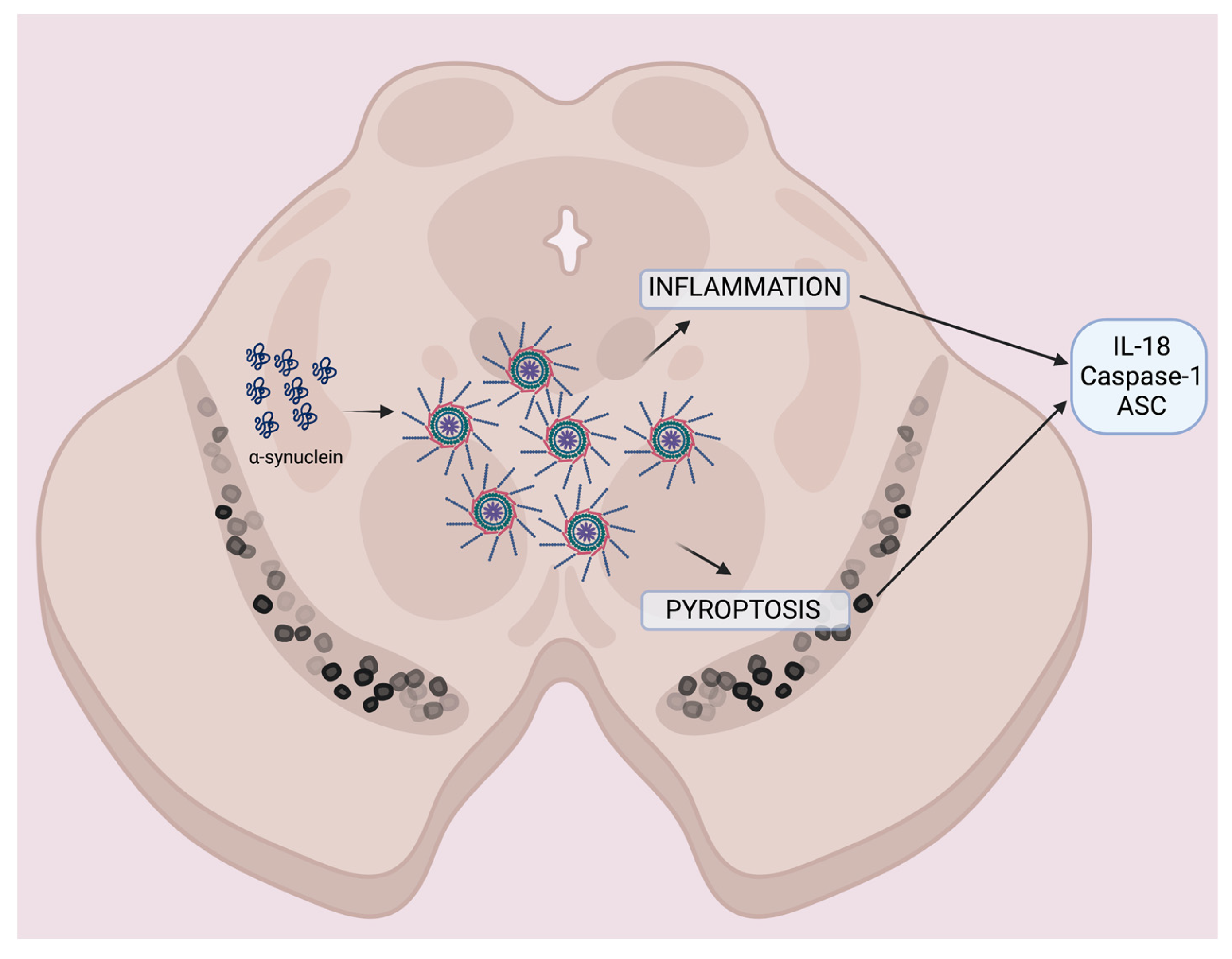
2.3. Caspase-1 and ASC Contribute to the Protein Levels of IL-18
3. Discussion
4. Materials and Methods
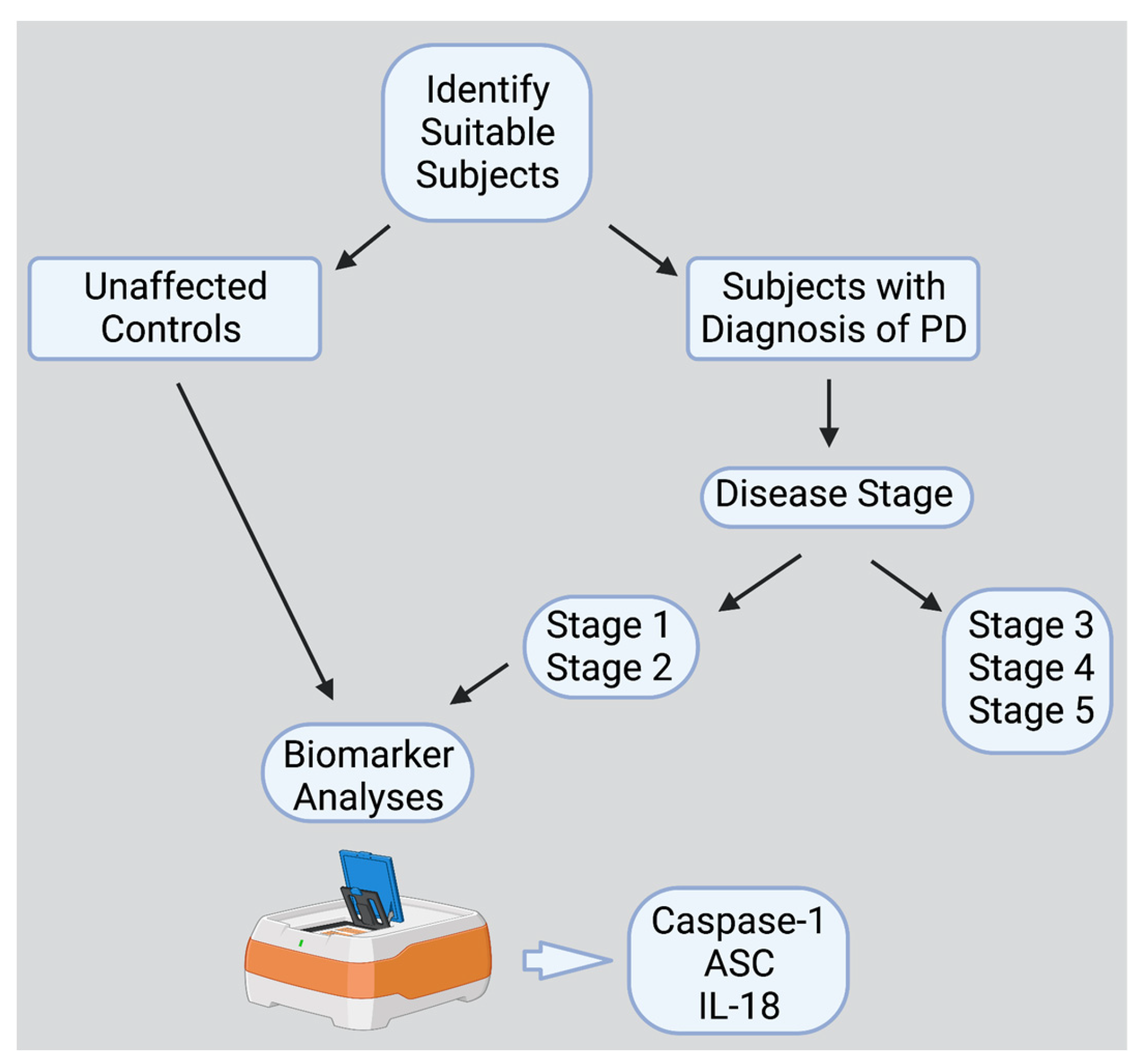
4.1. Participants
4.2. Simple Plex Assay
4.3. Statistical and Biomarker Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M.-Y. Pathological α-Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meade, R.M.; Fairlie, D.P.; Mason, J.M. Alpha-synuclein structure and Parkinson’s disease—Lessons and emerging principles. Mol. Neurodegener. 2019, 14, 29. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.E.; Akther, M.; Jakaria, M.; Kim, I.S.; Azam, S.; Choi, D.K. Targeting the microglial NLRP3 inflammasome and its role in Parkinson’s disease. Mov. Disord. 2020, 35, 20–33. [Google Scholar] [CrossRef]
- Yan, Y.Q.; Fang, Y.; Zheng, R.; Pu, J.L.; Zhang, B.R. NLRP3 Inflammasomes in Parkinson’s disease and their Regulation by Parkin. Neuroscience 2020, 446, 323–334. [Google Scholar] [CrossRef]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Kigerl, K.A.; de Rivero Vaccari, J.P.; Dietrich, W.D.; Popovich, P.G.; Keane, R.W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 2014, 258, 5–16. [Google Scholar] [CrossRef] [Green Version]
- de Rivero Vaccari, J.P.; Dietrich, W.D.; Keane, R.W. Therapeutics targeting the inflammasome after central nervous system injury. Transl. Res. 2016, 167, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.D. Inflammasome activation and assembly at a glance. J. Cell Sci. 2017, 130, 3955–3963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.S.; Bossaller, L.; De Nardo, D.; Ratter, J.M.; Stutz, A.; Engels, G.; Brenker, C.; Nordhoff, M.; Mirandola, S.R.; Al-Amoudi, A.; et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat. Immunol. 2014, 15, 727–737. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, R.; Albornoz, E.A.; Christie, D.C.; Langley, M.R.; Kumar, V.; Mantovani, S.; Robertson, A.A.B.; Butler, M.S.; Rowe, D.B.; O’Neill, L.A.; et al. Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 2018, 10, 4066. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xue, L.; Zheng, J.; Tian, X.; Zhang, Y.; Tong, Q. PPARss/delta agonist alleviates NLRP3 inflammasome-mediated neuroinflammation in the MPTP mouse model of Parkinson’s disease. Behav. Brain Res. 2019, 356, 483–489. [Google Scholar] [CrossRef]
- Mao, Z.; Liu, C.; Ji, S.; Yang, Q.; Ye, H.; Han, H.; Xue, Z. The NLRP3 Inflammasome is Involved in the Pathogenesis of Parkinson’s Disease in Rats. Neurochem. Res. 2017, 42, 1104–1115. [Google Scholar] [CrossRef]
- Qiao, C.; Zhang, L.X.; Sun, X.Y.; Ding, J.H.; Lu, M.; Hu, G. Caspase-1 Deficiency Alleviates Dopaminergic Neuronal Death via Inhibiting Caspase-7/AIF Pathway in MPTP/p Mouse Model of Parkinson’s Disease. Mol. Neurobiol. 2017, 54, 4292–4302. [Google Scholar] [CrossRef]
- Pike, A.F.; Varanita, T.; Herrebout, M.A.C.; Plug, B.C.; Kole, J.; Musters, R.J.P.; Teunissen, C.E.; Hoozemans, J.J.M.; Bubacco, L.; Veerhuis, R. alpha-Synuclein evokes NLRP3 inflammasome-mediated IL-1beta secretion from primary human microglia. Glia 2021, 69, 1413–1428. [Google Scholar] [CrossRef]
- Panicker, N.; Sarkar, S.; Harischandra, D.S.; Neal, M.; Kam, T.I.; Jin, H.; Saminathan, H.; Langley, M.; Charli, A.; Samidurai, M.; et al. Fyn kinase regulates misfolded alpha-synuclein uptake and NLRP3 inflammasome activation in microglia. J. Exp. Med. 2019, 216, 1411–1430. [Google Scholar] [CrossRef] [PubMed]
- Bliederhaeuser, C.; Grozdanov, V.; Speidel, A.; Zondler, L.; Ruf, W.P.; Bayer, H.; Kiechle, M.; Feiler, M.S.; Freischmidt, A.; Brenner, D.; et al. Age-dependent defects of alpha-synuclein oligomer uptake in microglia and monocytes. Acta Neuropathol. 2016, 131, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Codolo, G.; Plotegher, N.; Pozzobon, T.; Brucale, M.; Tessari, I.; Bubacco, L.; de Bernard, M. Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS ONE 2013, 8, e55375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniele, S.G.; Beraud, D.; Davenport, C.; Cheng, K.; Yin, H.; Maguire-Zeiss, K.A. Activation of MyD88-dependent TLR1/2 signaling by misfolded alpha-synuclein, a protein linked to neurodegenerative disorders. Sci. Signal. 2015, 8, ra45. [Google Scholar] [CrossRef] [Green Version]
- Klein, C.; Westenberger, A. Genetics of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a008888. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-Q.; Tan, L.; Yu, J.-T. The role of the LRRK2 gene in Parkinsonism. Mol. Neurodegener. 2014, 9, 47. [Google Scholar] [CrossRef] [Green Version]
- Dzamko, N.; Geczy, C.L.; Halliday, G.M. Inflammation is genetically implicated in Parkinson’s disease. Neuroscience 2015, 302, 89–102. [Google Scholar] [CrossRef]
- Mouton-Liger, F.; Rosazza, T.; Sepulveda-Diaz, J.; Ieang, A.; Hassoun, S.M.; Claire, E.; Mangone, G.; Brice, A.; Michel, P.P.; Corvol, J.C.; et al. Parkin deficiency modulates NLRP3 inflammasome activation by attenuating an A20-dependent negative feedback loop. Glia 2018, 66, 1736–1751. [Google Scholar] [CrossRef] [Green Version]
- Deleidi, M.; Gasser, T. The role of inflammation in sporadic and familial Parkinson’s disease. Cell Mol. Life Sci. 2013, 70, 4259–4273. [Google Scholar] [CrossRef]
- Cheng, H.C.; Ulane, C.M.; Burke, R.E. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010, 67, 715–725. [Google Scholar] [CrossRef]
- Willis, A.W.; Roberts, E.; Beck, J.C.; Fiske, B.; Ross, W.; Savica, R.; Van Den Eeden, S.K.; Tanner, C.M.; Marras, C.; Parkinson’s Foundation, P.G. Incidence of Parkinson disease in North America. NPJ Parkinson’s Dis. 2022, 8, 170. [Google Scholar] [CrossRef]
- Anderson, F.L.; von Herrmann, K.M.; Andrew, A.S.; Kuras, Y.I.; Young, A.L.; Scherzer, C.R.; Hickey, W.F.; Lee, S.L.; Havrda, M.C. Plasma-borne indicators of inflammasome activity in Parkinson’s disease patients. NPJ Parkinson’s Dis. 2021, 7, 2. [Google Scholar] [CrossRef]
- Ibrahim, N.; Kusmirek, J.; Struck, A.F.; Floberg, J.M.; Perlman, S.B.; Gallagher, C.; Hall, L.T. The sensitivity and specificity of F-DOPA PET in a movement disorder clinic. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 102–109. [Google Scholar] [PubMed]
- Fazio, P.; Svenningsson, P.; Forsberg, A.; Jönsson, E.G.; Amini, N.; Nakao, R.; Nag, S.; Halldin, C.; Farde, L.; Varrone, A. Quantitative Analysis of ¹⁸F-(E)-N-(3-Iodoprop-2-Enyl)-2β-Carbofluoroethoxy-3β-(4′-Methyl-Phenyl) Nortropane Binding to the Dopamine Transporter in Parkinson Disease. J. Nucl. Med. 2015, 56, 714–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Z.; Shi, M.; Chung, K.A.; Quinn, J.F.; Peskind, E.R.; Galasko, D.; Jankovic, J.; Zabetian, C.P.; Leverenz, J.B.; Baird, G.; et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain 2010, 133, 713–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollenhauer, B.; Locascio, J.J.; Schulz-Schaeffer, W.; Sixel-Döring, F.; Trenkwalder, C.; Schlossmacher, M.G. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: A cohort study. Lancet Neurol. 2011, 10, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Eusebi, P.; Giannandrea, D.; Biscetti, L.; Abraha, I.; Chiasserini, D.; Orso, M.; Calabresi, P.; Parnetti, L. Diagnostic utility of cerebrospinal fluid α-synuclein in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2017, 32, 1389–1400. [Google Scholar] [CrossRef]
- Reale, M.; Iarlori, C.; Thomas, A.; Gambi, D.; Perfetti, B.; Di Nicola, M.; Onofrj, M. Peripheral cytokines profile in Parkinson’s disease. Brain Behav. Immun. 2009, 23, 55–63. [Google Scholar] [CrossRef]
- Williams-Gray, C.H.; Wijeyekoon, R.; Yarnall, A.J.; Lawson, R.A.; Breen, D.P.; Evans, J.R.; Cummins, G.A.; Duncan, G.W.; Khoo, T.K.; Burn, D.J.; et al. Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov. Disord. 2016, 31, 995–1003. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Lu, M.; Du, R.-H.; Qiao, C.; Jiang, C.-Y.; Zhang, K.-Z.; Ding, J.-H.; Hu, G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol. Neurodegener. 2016, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Griffin, W.S.; Liu, L.; Li, Y.; Mrak, R.E.; Barger, S.W. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J. Neuroinflammation 2006, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, C.C.; Pott Godoy, M.C.; Tarelli, R.; Chertoff, M.; Depino, A.M.; Pitossi, F.J. Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1beta in the substantia nigra. Neurobiol. Dis. 2006, 24, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, T.; Pei, Z.; Miller, D.S.; Wu, X.; Block, M.L.; Wilson, B.; Zhang, W.; Zhou, Y.; Hong, J.S.; et al. Aggregated alpha-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J. 2005, 19, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Li, R.; Zhu, L.; Fu, B.; Yan, T. Salidroside ameliorates Parkinson’s disease by inhibiting NLRP3-dependent pyroptosis. Aging 2020, 12, 9405–9426. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Hwang, I.; Park, S.; Hong, S.; Hwang, B.; Cho, Y.; Son, J.; Yu, J.W. MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ. 2019, 26, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Liu, L.; Wang, X.; Ding, C.; Tian, Z.; Zhou, R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 2015, 160, 62–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, Z.; Zhou, Y.; Wang, L.; Xue, L.; Zheng, J.; Chen, L.; Tong, Q. NLRP3 Inflammasome Inhibition Prevents alpha-Synuclein Pathology by Relieving Autophagy Dysfunction in Chronic MPTP-Treated NLRP3 Knockout Mice. Mol. Neurobiol. 2021, 58, 1303–1311. [Google Scholar] [CrossRef]
- Chatterjee, K.; Roy, A.; Banerjee, R.; Choudhury, S.; Mondal, B.; Halder, S.; Basu, P.; Shubham, S.; Dey, S.; Kumar, H. Inflammasome and α-synuclein in Parkinson’s disease: A cross-sectional study. J. Neuroimmunol. 2020, 338, 577089. [Google Scholar] [CrossRef] [Green Version]
- von Herrmann, K.M.; Salas, L.A.; Martinez, E.M.; Young, A.L.; Howard, J.M.; Feldman, M.S.; Christensen, B.C.; Wilkins, O.M.; Lee, S.L.; Hickey, W.F.; et al. NLRP3 expression in mesencephalic neurons and characterization of a rare NLRP3 polymorphism associated with decreased risk of Parkinson’s disease. NPJ Parkinson’s Dis. 2018, 4, 24. [Google Scholar] [CrossRef]
- Martinez, E.M.; Young, A.L.; Patankar, Y.R.; Berwin, B.L.; Wang, L.; von Herrmann, K.M.; Weier, J.M.; Havrda, M.C. Editor’s Highlight: Nlrp3 Is Required for Inflammatory Changes and Nigral Cell Loss Resulting from Chronic Intragastric Rotenone Exposure in Mice. Toxicol. Sci. 2017, 159, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Le, W. Biomarkers for Parkinson’s Disease: How Good Are They? Neurosci. Bull. 2020, 36, 183–194. [Google Scholar] [CrossRef]
- Dinarello, C.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 Binding Protein. Front. Immunol. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Barcena, J.; Rodriguez Pilar, J.; Salazar, O.; Crespi, C.; Frontera, G.; Novo, M.A.; Guardiola, M.B.; Llompart-Pou, J.A.; Ibanez, J.; de Rivero Vaccari, J.P. Serum Caspase-1 as an Independent Prognostic Factor in Traumatic Brain Injured Patients. Neurocrit. Care 2022, 36, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Perez-Barcena, J.; Crespi, C.; Frontera, G.; Llompart-Pou, J.A.; Salazar, O.; Goliney, V.; Ibanez, J.; Bullock, M.R.; de Rivero Vaccari, J.P. Levels of caspase-1 in cerebrospinal fluid of patients with traumatic brain injury: Correlation with intracranial pressure and outcome. J. Neurosurg. 2020, 134, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Kerr, N.; Lee, S.W.; Perez-Barcena, J.; Crespi, C.; Ibanez, J.; Bullock, M.R.; Dietrich, W.D.; Keane, R.W.; de Rivero Vaccari, J.P. Inflammasome proteins as biomarkers of traumatic brain injury. PLoS ONE 2018, 13, e0210128. [Google Scholar] [CrossRef]
- Kattan, D.; Barsa, C.; Mekhijian, S.; Shakkour, Z.; Jammoul, M.; Doumit, M.; Zabala, M.C.P.; Darwiche, N.; Eid, A.H.; Mechref, Y.; et al. Inflammasomes as biomarkers and therapeutic targets in traumatic brain injury and related-neurodegenerative diseases: A comprehensive overview. Neurosci. Biobehav. Rev. 2023, 144, 104969. [Google Scholar] [CrossRef]
- Johnson, N.H.; Hadad, R.; Taylor, R.R.; Rodriguez Pilar, J.; Salazar, O.; Llompart-Pou, J.A.; Dietrich, W.D.; Keane, R.W.; Perez-Barcena, J.; de Rivero Vaccari, J.P. Inflammatory Biomarkers of Traumatic Brain Injury. Pharmaceuticals 2022, 15, 660. [Google Scholar] [CrossRef]
- Adamczak, S.; Dale, G.; de Rivero Vaccari, J.P.; Bullock, M.R.; Dietrich, W.D.; Keane, R.W. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: Clinical article. J. Neurosurg. 2012, 117, 1119–1125. [Google Scholar] [CrossRef] [Green Version]
- Kerr, N.; Garcia-Contreras, M.; Abbassi, S.; Mejias, N.H.; Desousa, B.R.; Ricordi, C.; Dietrich, W.D.; Keane, R.W.; de Rivero Vaccari, J.P. Inflammasome Proteins in Serum and Serum-Derived Extracellular Vesicles as Biomarkers of Stroke. Front. Mol. Neurosci. 2018, 11, 309. [Google Scholar] [CrossRef] [Green Version]
- Scott, X.O.; Stephens, M.E.; Desir, M.C.; Dietrich, W.D.; Keane, R.W.; de Rivero Vaccari, J.P. The Inflammasome Adaptor Protein ASC in Mild Cognitive Impairment and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 4674. [Google Scholar] [CrossRef]
- Keane, R.W.; Dietrich, W.D.; de Rivero Vaccari, J.P. Inflammasome Proteins as Biomarkers of Multiple Sclerosis. Front. Neurol. 2018, 9, 135. [Google Scholar] [CrossRef]
- Weaver, C.; Cyr, B.; de Rivero Vaccari, J.C.; de Rivero Vaccari, J.P. Inflammasome Proteins as Inflammatory Biomarkers of Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2020, 9, 27. [Google Scholar] [CrossRef]
- Tovar, A.; Gomez, A.; Serrano, A.; Blanco, M.P.; Galor, A.; Swaminathan, S.S.; de Rivero Vaccari, J.P.; Sabater, A.L. Role of Caspase-1 as a Biomarker of Ocular Surface Damage. Am. J. Ophthalmol. 2022, 239, 74–83. [Google Scholar] [CrossRef]
- de Rivero Vaccari, J.P.; Sawaya, M.E.; Brand, F., 3rd; Nusbaum, B.P.; Bauman, A.J.; Bramlett, H.M.; Dietrich, W.D.; Keane, R.W. Caspase-1 level is higher in the scalp in androgenetic alopecia. Dermatol. Surg. 2012, 38, 1033–1039. [Google Scholar] [CrossRef]
- Forouzandeh, M.; Besen, J.; Keane, R.W.; de Rivero Vaccari, J.P. The Inflammasome Signaling Proteins ASC and IL-18 as Biomarkers of Psoriasis. Front. Pharmacol. 2020, 11, 1238. [Google Scholar] [CrossRef]
- Syed, S.A.; Beurel, E.; Loewenstein, D.A.; Lowell, J.A.; Craighead, W.E.; Dunlop, B.W.; Mayberg, H.S.; Dhabhar, F.; Dietrich, W.D.; Keane, R.W.; et al. Defective Inflammatory Pathways in Never-Treated Depressed Patients Are Associated with Poor Treatment Response. Neuron 2018, 99, 914–924.e913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, N.H.; Keane, R.W.; de Rivero Vaccari, J.P. Renal and Inflammatory Proteins as Biomarkers of Diabetic Kidney Disease and Lupus Nephritis. Oxid. Med. Cell Longev. 2022, 2022, 5631099. [Google Scholar] [CrossRef] [PubMed]
- Cyr, B.; Keane, R.W.; de Rivero Vaccari, J.P. ASC, IL-18 and Galectin-3 as Biomarkers of Non-Alcoholic Steatohepatitis: A Proof of Concept Study. Int. J. Mol. Sci. 2020, 21, 8580. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; Agostini, S.; Hernis, A.; Zanzottera, M.; Bianchi, A.; Clerici, M. Circulatory miR-223-3p Discriminates Between Parkinson’s and Alzheimer’s Patients. Sci. Rep. 2019, 9, 9393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starhof, C.; Winge, K.; Heegaard, N.H.H.; Skogstrand, K.; Friis, S.; Hejl, A. Cerebrospinal fluid pro-inflammatory cytokines differentiate parkinsonian syndromes. J. Neuroinflammation 2018, 15, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Rivero Vaccari, J.P.; Dietrich, W.D.; Keane, R.W. Activation and regulation of cellular inflammasomes: Gaps in our knowledge for central nervous system injury. J. Cereb. Blood Flow Metab. 2014, 34, 369–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Biomarker | Area | Std. Error | 95% C.I. | p-Value |
|---|---|---|---|---|
| Caspase-1 | 0.9567 | 0.03207 | 0.8939 to 1.0 | <0.0001 |
| ASC | 0.8711 | 0.5714 | 0.7591 to 0.9831 | <0.0001 |
| IL-18 | 0.8535 | 0.0640 | 0.7281 to 0.9790 | <0.0001 |
| Biomarker | Cut-Off Point (pg/mL) | Sensitivity (%) | Specificity (%) | LR | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| Caspase-1 | >1.450 | 96.88 | 84.62 | 6.297 | 94 | 92 | 93 |
| ASC | >222.5 | 96.88 | 75 | 3.875 | 89 | 92 | 90 |
| IL-18 | >159.5 | 90.63 | 75 | 3.625 | 88 | 80 | 85 |
| IL-18 | Estimate | Std. Error | p-Value | Confidence Interval |
|---|---|---|---|---|
| Intercept | 907.1865 | 321.7597 | 0.00858 | 249.114 to 1565.259 |
| Caspase-1 | 60.6652 | 10.2042 | 1.85 × 10−6 | 39.795 to 81.535 |
| ASC | −2.7946 | 0.9669 | 0.00722 | −4.772 to −0.817 |
| Adjusted R2 | 0.7768 | |||
| p-value | 1.366 × 10−10 | |||
| BIC | 527.844 | |||
| RMSE | 781.5156 | |||
| Mean of residuals | 4.88 × 10−15 | |||
| DW Statistic | ||||
| rho ! = 0 | 0.896 | |||
| rho < 0 | 0.444 | |||
| rho > 0 | 0.582 |
| Sex | Males | 22 (69%) |
| Females | 10 (31%) | |
| Age (Range) | 41 to 82 | |
| Age (Median) | 66 years | |
| Disease Duration (Median) | 7 years | |
| Hoehn and Yahr Scale | Stage 1 | 8 (25%) |
| Stage 2 | 24 (75%) | |
| Deep Brain Stimulation | 8 (25%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera Ranaldi, E.d.l.R.M.; Nuytemans, K.; Martinez, A.; Luca, C.C.; Keane, R.W.; de Rivero Vaccari, J.P. Proof-of-Principle Study of Inflammasome Signaling Proteins as Diagnostic Biomarkers of the Inflammatory Response in Parkinson’s Disease. Pharmaceuticals 2023, 16, 883. https://doi.org/10.3390/ph16060883
Cabrera Ranaldi EdlRM, Nuytemans K, Martinez A, Luca CC, Keane RW, de Rivero Vaccari JP. Proof-of-Principle Study of Inflammasome Signaling Proteins as Diagnostic Biomarkers of the Inflammatory Response in Parkinson’s Disease. Pharmaceuticals. 2023; 16(6):883. https://doi.org/10.3390/ph16060883
Chicago/Turabian StyleCabrera Ranaldi, Erika d. l. R. M., Karen Nuytemans, Anisley Martinez, Corneliu C. Luca, Robert W. Keane, and Juan Pablo de Rivero Vaccari. 2023. "Proof-of-Principle Study of Inflammasome Signaling Proteins as Diagnostic Biomarkers of the Inflammatory Response in Parkinson’s Disease" Pharmaceuticals 16, no. 6: 883. https://doi.org/10.3390/ph16060883
APA StyleCabrera Ranaldi, E. d. l. R. M., Nuytemans, K., Martinez, A., Luca, C. C., Keane, R. W., & de Rivero Vaccari, J. P. (2023). Proof-of-Principle Study of Inflammasome Signaling Proteins as Diagnostic Biomarkers of the Inflammatory Response in Parkinson’s Disease. Pharmaceuticals, 16(6), 883. https://doi.org/10.3390/ph16060883









