Effects of Natural Product-Derived Compounds on Inflammatory Pain via Regulation of Microglial Activation
Abstract
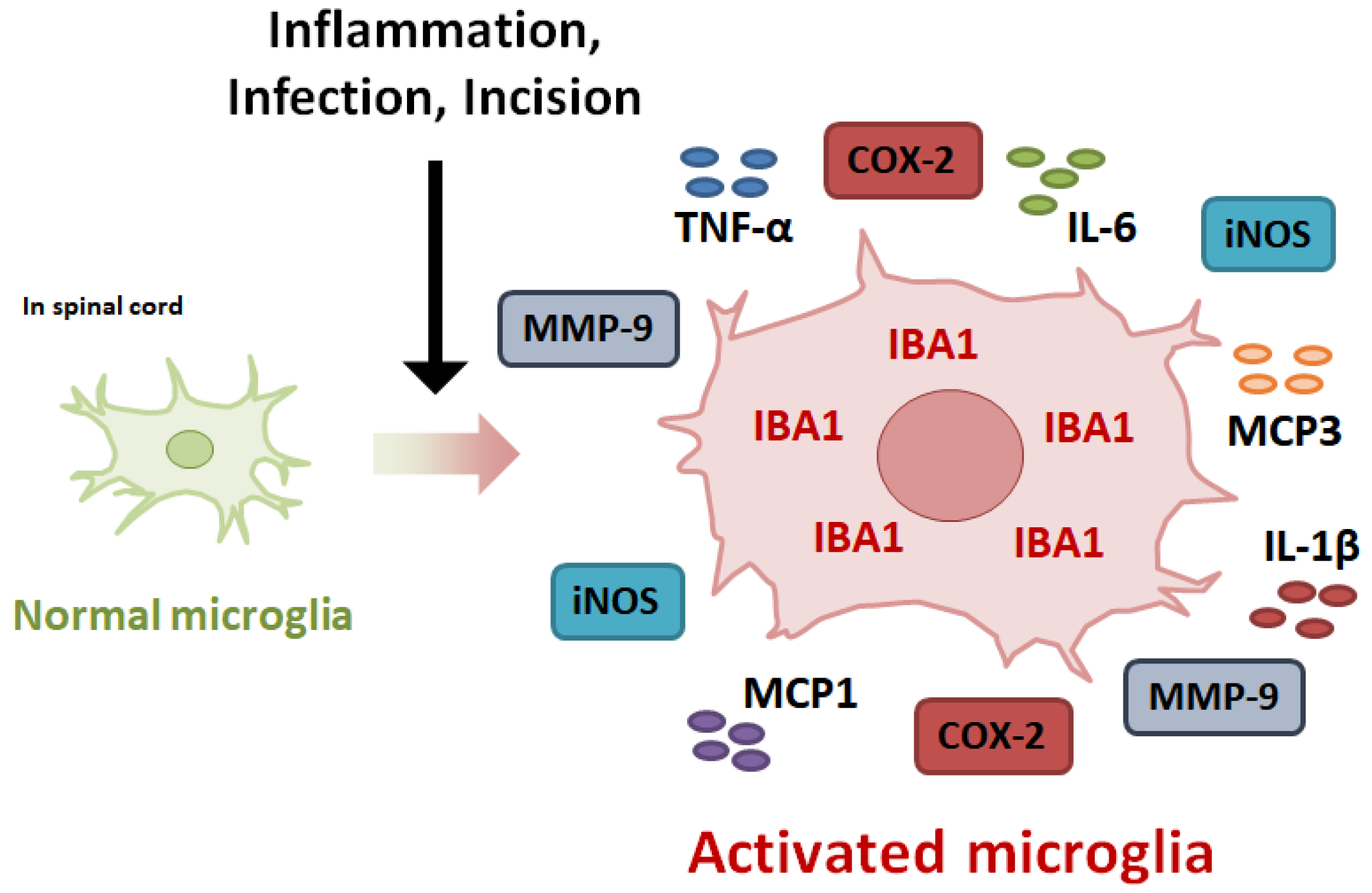
:1. Introduction
2. Mechanism Underlying the Development of Inflammatory Pain
3. Expression of Inflammatory Mediators in Activated Microglia
3.1. Inducible Nitric Oxide Synthase
3.2. Cyclooxygenase-2
3.3. Matrix Metalloproteinases-9
3.4. Pro-Inflammatory Cytokines
3.4.1. TNF-α
3.4.2. Interleukin-1β
3.4.3. Interelukin-6
3.4.4. Monocyte Chemoattractant Protein-1
3.4.5. Monocyte Chemoattractant Protein-3
4. Intracellular Signaling in Activated Microglia
4.1. Nuclear Factor-κB
4.2. Mitogen-Activated Protein Kinase
4.3. Janus Kinase 2 (JAK2)/Signal Transducer and Activator of Transcription 3
4.4. Nuclear Factor-Erythroid 2-Related Factor 2
4.5. Autophagy
5. Natural Product-Derived Compounds against Microglial Activation-Mediated Inflammatory Pain
5.1. 3,5-Dicaffeoylquinic Acid
5.2. Chlorogenic Acid
5.3. Ferulic Acid
5.4. 6-Gingerol
5.5. Curcumin
5.6. Kaempferol
5.7. Quercetin
5.8. Formononetin
5.9. Naringenin
5.10. Resveratrol
5.11. Honokiol
5.12. Ligustilide
5.13. Glycyrrhizin
5.14. Docosahexaenoic Acid
5.15. Paeoniflorin
5.16. Sinomenine
5.17. Muscone
5.18. Urolithins
| Class of Phytochemicals | Subclass | Major Compound | Source | Targeting Inflammatory Mediators | Targeting Intracellular Signaling | Inducer in Animal Model | Safety Dosage in Clinical Study | Effects in Clinical Study | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Phenolics | Phenolic acid | 3,5-Dicaffeoylquinic acid | Arctium lappa, aster yomena | TNF-α, IL-1β, IL-6, MCP1, MCP3, iNOS, COX2 | JAK2/STAT3, Autophagy | CFA | [24] | ||
| Phenolics | Phenolic acid | Chlorogenic acid | NO, iNOS, TNF-α | NF-κB | Carrageenan, Formalin | 480 mg/day for 8 weeks | Improvements in neuronal function | [102,103,104,105] | |
| Phenolics | Phenolic acid | Ferulic acid | ferula asafetida | TNF-α, iNOS | NF-κB, JNK | Formalin | 1000 mg/day for 6 weeks | Anti-oxidant, anti-inflammation | [106,107,108,109]. |
| Phenolics | Phenolic acid | 6-gingerol | zingiber officinale | NO, iNOS, IL-1β, IL-6 | STAT3 | Acetic acid, Formalin, Carrageenan | 20 mg/day for 12 weeks | [110,111,113] | |
| Phenolics | flavonoids | Curcumin | Curcuma longa | NO, PGE2, iNOS, COX2, TNF-α, IL-1β, IL-6 | NF-κB, MAPK, Nrf2 | CFA | 1200 mg/day for 6 days | Analgesic effects, anti-inflammation | [114,115,116,117] |
| Phenolics | flavonoids | Kaempferol | Tea, broccoli | NO, PGE2, iNOS, COX2, MMP-9, TNF-α, IL-1β, IL-6 | NF-κB, JNK, ERK, p38 | Formalin | 50 mg/day for 4 weeks | Anti-inflammation | [118,119,120,121,122] |
| Phenolics | flavonoids | Quercetin | NO, iNOS, TNF-α | NF-κB, Nrf2, ERK | CFA | 500 mg/day for 8 weeks | Analgesic effects, anti-inflammation | [123,124,125] | |
| Phenolics | flavonoids | Formononetin | Trifolium pretense L. | TNF-α, IL-1β, IL-6, iNOS, COX2 | NF-κB | CFA | [126,127] | ||
| Phenolics | flavonoids | Naringenin | TNF-α, IL-1β, iNOS | MAPK | Carrageenan, Capsaicin, CFA, PGE2 | 900 mg for a day | [129,130,131] | ||
| Phenolics | stilbenes | Resveratrol | grape | TNF-α, IL-1β, iNOS | NF-κB, Autophagy | CFA | 500 mg/day for 90 days | Analgesic effects, anti-inflammation | [133,134,135] |
| Phenolics | lignan | Honokiol | Magnolia officinlis | NO, iNOS, TNF-α, IL-1β, IL-6 | Autophagy | Carrageenan, CFA | 50 mg/kg for a week | [136,137,138,139] | |
| Non-phenolics | phthalide | Ligustilide | the roof of Angelica sinensis | NO, iNOS, COX-2, TNF-α, IL-1β, IL-6, MCP1 | NF-κB | CFA, Acetic acid, Formalin | [140,141,142] | ||
| Non-phenolics | saponin | Glycyrrhizin | Glycyrrhiza glabra | NO, TNF-α, IL-1β, IL-6 | NF-κB | CFA | 450 mg/day for 4 weeks | Anti-inflammation | [144,145] |
| Non-phenolics | omega-3 fatty acid | Docosahexaenoic acid | Omega-3 polyunsaturated fatty acid | TNF-α, IL-1β, IL-6, MCP1, CCL3, CXCL10 | p38 | Carrageenan | [146] | ||
| Non-phenolics | monoterpene | Paeoniflorin | Paeonia lactiflora | TNF-α, IL-1β, IL-6 | NF-κB | CFA | 35.8 mg/day for 7 days | [148,149] | |
| Non-phenolics | alkaloid | Sinomenine | Sinomenium acutum | NO, TNF-α, IL-1β, IL-6, MCP1 | NF-κB, p38 | CFA | 40 mg/day for 3 months | Anti-inflammation | [150,151,152] |
| Muscone | Musk | NO, TNF-α, IL-1β, IL-6 | JAK2/STAT3 | CFA | [89] | ||||
| Urolithins | Secondary metabolite | TNF-α, IL-1β, IL-6, iNOS, and COX-2 | ERK, p38, and NF-κB | Surgery | 1000 mg/day for 4 months | Anti-inflammation | [154,155,156] | ||
| Muscone | Musk | NO, TNF-α, IL-1β, IL-6 | JAK2/STAT3 | CFA | [89] | ||||
| Urolithins | Secondary metabolite | TNF-α, IL-1β, IL-6, iNOS, and COX-2 | ERK, p38, and NF-κB | Surgery | 1000 mg/day for 4 months | Anti-inflammation | [154,155,156] |
6. Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zelaya, C.E.; Dahlhamer, J.M.; Lucas, J.W.; Connor, E.M. Chronic Pain and High-Impact Chronic Pain among US Adults, 2019. 2020. Available online: https://stacks.cdc.gov/view/cdc/97308 (accessed on 7 June 2023).
- Lee, J.; Jotwani, R.; Robert, S.W. The economic cost of racial disparities in chronic pain. J. Comp. Eff. Res. 2020, 9, 903–906. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, L.A.; Cox, B.J.; Enns, M.W. Mood and anxiety disorders associated with chronic pain: An examination in a nationally representative sample. Pain 2003, 106, 127–133. [Google Scholar] [CrossRef]
- Reddi, D.; Curran, N.; Stephens, R. An introduction to pain pathways and mechanisms. Br. J. Hosp. Med. 2013, 74 (Suppl. S12), C188–C191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs); StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, M.; Huh, Y.; Ji, R.R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef]
- Zhang, L.; Berta, T.; Xu, Z.Z.; Liu, T.; Park, J.Y.; Ji, R.R. TNF-α contributes to spinal cord synaptic plasticity and inflammatory pain: Distinct role of TNF receptor subtypes 1 and 2. Pain 2011, 152, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Xie, R.G.; Gao, Y.J.; Park, C.K.; Lu, N.; Luo, C.; Wang, W.T.; Wu, S.X.; Ji, R.R. Spinal CCL2 Promotes Central Sensitization, Long-Term Potentiation, and Inflammatory Pain via CCR2: Further Insights into Molecular, Synaptic, and Cellular Mechanisms. Neurosci. Bull. 2018, 34, 13–21. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Zhang, L.; Cheng, J.K.; Ji, R.R. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008, 28, 5189–5194. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [Green Version]
- Vergne-Salle, P.; Bertin, P. Chronic pain and neuroinflammation. Jt. Bone Spine 2021, 88, 105222. [Google Scholar] [CrossRef]
- Lim, E.Y.; Kim, Y.T. Food-Derived Natural Compounds for Pain Relief in Neuropathic Pain. BioMed Res. Int. 2016, 2016, 7917528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Mauborgne, A.; Masson, J.; Bourgoin, S.; Kayser, V.; Hamon, M.; Pohl, M. Differential implication of proinflammatory cytokine interleukin-6 in the development of cephalic versus extracephalic neuropathic pain in rats. J. Neurosci. 2008, 28, 8489–8501. [Google Scholar] [CrossRef] [Green Version]
- Imai, S.; Ikegami, D.; Yamashita, A.; Shimizu, T.; Narita, M.; Niikura, K.; Furuya, M.; Kobayashi, Y.; Miyashita, K.; Okutsu, D.; et al. Epigenetic transcriptional activation of monocyte chemotactic protein 3 contributes to long-lasting neuropathic pain. Brain 2013, 136, 828–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, N.; Yi, M.H.; Murugan, M.; Xie, M.; Parusel, S.; Peng, J.; Eyo, U.B.; Hunt, C.L.; Dong, H.; Wu, L.J. Spinal microglia contribute to sustained inflammatory pain via amplifying neuronal activity. Mol. Brain 2022, 15, 86. [Google Scholar] [CrossRef]
- Yi, M.H.; Liu, Y.U.; Liu, K.; Chen, T.; Bosco, D.B.; Zheng, J.; Xie, M.; Zhou, L.; Qu, W.; Wu, L.J. Chemogenetic manipulation of microglia inhibits neuroinflammation and neuropathic pain in mice. Brain Behav. Immun. 2021, 92, 78–89. [Google Scholar] [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef] [Green Version]
- Rocha, P.A.; Ferreira, A.F.B.; Da Silva, J.T.; Alves, A.S.; Martins, D.O.; Britto, L.R.G.; Chacur, M. Effects of selective inhibition of nNOS and iNOS on neuropathic pain in rats. Mol. Cell Neurosci. 2020, 105, 103497. [Google Scholar] [CrossRef]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Kim, Y.; Lee, C.; Kim, Y.T. 3,5-Dicaffeoylquinic acid attenuates microglial activation-mediated inflammatory pain by enhancing autophagy through the suppression of MCP3/JAK2/STAT3 signaling. Biomed. Pharmacother. 2022, 153, 113549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Gao, S.J.; Sun, J.; Li, D.Y.; Wu, J.Y.; Song, F.H.; Liu, D.Q.; Zhou, Y.Q.; Mei, W. DKK3 ameliorates neuropathic pain via inhibiting ASK-1/JNK/p-38-mediated microglia polarization and neuroinflammation. J. Neuroinflamm. 2022, 19, 129. [Google Scholar] [CrossRef] [PubMed]
- Kuboyama, K.; Tsuda, M.; Tsutsui, M.; Toyohara, Y.; Tozaki-Saitoh, H.; Shimokawa, H.; Yanagihara, N.; Inoue, K. Reduced spinal microglial activation and neuropathic pain after nerve injury in mice lacking all three nitric oxide synthases. Mol. Pain 2011, 7, 50. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhou, Y.; Li, X.C.; Ma, X.; Mi, W.L.; Chu, Y.X.; Wang, Y.Q.; Mao-Ying, Q.L. Neuronal GRK2 regulates microglial activation and contributes to electroacupuncture analgesia on inflammatory pain in mice. Biol. Res. 2022, 55, 5. [Google Scholar] [CrossRef]
- Osborne, M.G.; Coderre, T.J. Effects of intrathecal administration of nitric oxide synthase inhibitors on carrageenan-induced thermal hyperalgesia. Br. J. Pharmacol. 1999, 126, 1840–1846. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Deng, H.; Chen, X.; Lin, Y.; Xie, X.; Bo, Z. The efficacy and safety of selective COX-2 inhibitors for postoperative pain management in patients after total knee/hip arthroplasty: A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 39. [Google Scholar] [CrossRef] [Green Version]
- Hoozemans, J.J.; Rozemuller, A.J.; Janssen, I.; De Groot, C.J.; Veerhuis, R.; Eikelenboom, P. Cyclooxygenase expression in microglia and neurons in Alzheimer’s disease and control brain. Acta Neuropathol. 2001, 101, 2–8. [Google Scholar] [CrossRef]
- Sinatra, R. Role of COX-2 inhibitors in the evolution of acute pain management. J. Pain Symptom Manag. 2002, 24, S18–S27. [Google Scholar] [CrossRef]
- Cho, N.; Moon, E.H.; Kim, H.W.; Hong, J.; Beutler, J.A.; Sung, S.H. Inhibition of Nitric Oxide Production in BV2 Microglial Cells by Triterpenes from Tetrapanax papyriferus. Molecules 2016, 21, 459. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Li, G.; Tong, T.; Chen, J. Micheliolide suppresses LPS-induced neuroinflammatory responses. PLoS ONE 2017, 12, e0186592. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Jin, C.Y.; Kim, C.H.; Yoo, Y.H.; Choi, S.H.; Kim, G.Y.; Yoon, H.M.; Park, H.T.; Choi, Y.H. Isorhamnetin alleviates lipopolysaccharide-induced inflammatory responses in BV2 microglia by inactivating NF-κB, blocking the TLR4 pathway and reducing ROS generation. Int. J. Mol. Med. 2019, 43, 682–692. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Lim, E.Y.; Kim, Y.T. The inhibitory effects of Aster yomena extract on microglial activation-mediated inflammatory response and pain by modulation of the NF-κB and MAPK signaling pathways. J. Funct. Foods 2021, 85, 104659. [Google Scholar] [CrossRef]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.T. Erythronium japonicum Alleviates Inflammatory Pain by Inhibiting MAPK Activation and by Suppressing NF-κB Activation via ERK/Nrf2/HO-1 Signaling Pathway. Antioxidants 2020, 9, 626. [Google Scholar] [CrossRef] [PubMed]
- Konnecke, H.; Bechmann, I. The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clin. Dev. Immunol. 2013, 2013, 914104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.R.; Xu, Z.Z.; Wang, X.; Lo, E.H. Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol. Sci. 2009, 30, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, Y.; Xu, Z.Z.; Wang, X.; Park, J.Y.; Zhuang, Z.Y.; Tan, P.H.; Gao, Y.J.; Roy, K.; Corfas, G.; Lo, E.H.; et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 2008, 14, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; Kim, H.S. Inhibitory mechanism of MMP-9 gene expression by ethyl pyruvate in lipopolysaccharide-stimulated BV2 microglial cells. Neurosci. Lett. 2011, 493, 38–43. [Google Scholar] [CrossRef]
- Kular, L.; Rivat, C.; Lelongt, B.; Calmel, C.; Laurent, M.; Pohl, M.; Kitabgi, P.; Melik-Parsadaniantz, S.; Martinerie, C. NOV/CCN3 attenuates inflammatory pain through regulation of matrix metalloproteinases-2 and -9. J. Neuroinflamm. 2012, 9, 36. [Google Scholar] [CrossRef]
- Leung, L.; Cahill, C.M. TNF-α and neuropathic pain—A review. J. Neuroinflamm. 2010, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.W.; Chen, S.X.; Li, Q.Y.; Zang, Y. Neuroimmune Mechanisms Underlying Neuropathic Pain: The Potential Role of TNF-α-Necroptosis Pathway. Int. J. Mol. Sci. 2022, 23, 7191. [Google Scholar] [CrossRef] [PubMed]
- Kuno, R.; Wang, J.; Kawanokuchi, J.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Autocrine activation of microglia by tumor necrosis factor-α. J. Neuroimmunol. 2005, 162, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hankittichai, P.; Lou, H.J.; Wikan, N.; Smith, D.R.; Potikanond, S.; Nimlamool, W. Oxyresveratrol Inhibits IL-1β-Induced Inflammation via Suppressing AKT and ERK1/2 Activation in Human Microglia, HMC3. Int. J. Mol. Sci. 2020, 21, 6054. [Google Scholar] [CrossRef]
- Davis, R.L.; Buck, D.J.; McCracken, K.; Cox, G.W.; Das, S. Interleukin-1β-induced inflammatory signaling in C20 human microglial cells. Neuroimmunol. Neuroinflamm. 2018, 5, 50. [Google Scholar] [CrossRef]
- Samad, T.A.; Moore, K.A.; Sapirstein, A.; Billet, S.; Allchorne, A.; Poole, S.; Bonventre, J.V.; Woolf, C.J. Interleukin-1β-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 2001, 410, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Gui, W.S.; Wei, X.; Mai, C.L.; Murugan, M.; Wu, L.J.; Xin, W.J.; Zhou, L.J.; Liu, X.G. Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol. Pain 2016, 12, 1744806916646784. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.; Kelley, K.W.; Johnson, R.W. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc. Natl. Acad. Sci. USA 2008, 105, 7534–7539. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.; Li, K.; Zhang, F.Y.; Zhang, Z.K.; Light, A.R.; Fu, K.Y. Dissociation of spinal microglia morphological activation and peripheral inflammation in inflammatory pain models. J. Neuroimmunol. 2007, 192, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhou, W.; Xu, X.; Ge, X.; Wang, F.; Zhang, G.Q.; Miao, L.; Deng, X. Aprepitant Inhibits JNK and p38/MAPK to Attenuate Inflammation and Suppresses Inflammatory Pain. Front. Pharmacol. 2021, 12, 811584. [Google Scholar] [CrossRef]
- Zanjani, T.M.; Sabetkasaei, M.; Mosaffa, N.; Manaheji, H.; Labibi, F.; Farokhi, B. Suppression of interleukin-6 by minocycline in a rat model of neuropathic pain. Eur. J. Pharmacol. 2006, 538, 66–72. [Google Scholar] [CrossRef]
- Bose, S.; Cho, J. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch. Pharm. Res. 2013, 36, 1039–1050. [Google Scholar] [CrossRef]
- Thacker, M.A.; Clark, A.K.; Bishop, T.; Grist, J.; Yip, P.K.; Moon, L.D.; Thompson, S.W.; Marchand, F.; McMahon, S.B. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur. J. Pain 2009, 13, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Dansereau, M.A.; Midavaine, E.; Begin-Lavallee, V.; Belkouch, M.; Beaudet, N.; Longpre, J.M.; Melik-Parsadaniantz, S.; Sarret, P. Mechanistic insights into the role of the chemokine CCL2/CCR2 axis in dorsal root ganglia to peripheral inflammation and pain hypersensitivity. J. Neuroinflamm. 2021, 18, 79. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, J.; Jiang, X.; Qian, W.; Yang, T.; Sun, X.; Chen, Z.; Zhu, Q. Neuron-derived CCL2 contributes to microglia activation and neurological decline in hepatic encephalopathy. Biol. Res. 2017, 50, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Q.; Li, Y.; Pei, G. Polysaccharides from Ganoderma lucidum attenuate microglia-mediated neuroinflammation and modulate microglial phagocytosis and behavioural response. J. Neuroinflamm. 2017, 14, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiatkowski, K.; Popiolek-Barczyk, K.; Piotrowska, A.; Rojewska, E.; Ciapala, K.; Makuch, W.; Mika, J. Chemokines CCL2 and CCL7, but not CCL12, play a significant role in the development of pain-related behavior and opioid-induced analgesia. Cytokine 2019, 119, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C.; Liu, Z.G. A special issue on NF-κB signaling and function. Cell Res. 2011, 21, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Sun, S.C. NF-κB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [Green Version]
- Brás, J.P.; Bravo, J.; Freitas, J.; Barbosa, M.A.; Santos, S.G.; Summavielle, T.; Almeida, M.I. TNF-α-induced microglia activation requires miR-342: Impact on NF-kB signaling and neurotoxicity. Cell Death Dis. 2020, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xiao, L.; Zhong, Z.; Wang, L.; Li, Z.; Pan, X.; Liu, Z. Astaxanthin acts via LRP-1 to inhibit inflammation and reverse lipopolysaccharide-induced M1/M2 polarization of microglial cells. Oncotarget 2017, 8, 69370–69385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, C.J.; Hossain, M.M.; Richardson, J.R.; Aleksunes, L.M. Inflammatory regulation of ATP binding cassette efflux transporter expression and function in microglia. J. Pharmacol. Exp. Ther. 2012, 343, 650–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, I.H.; Hong, J.; Suh, E.C.; Kim, J.H.; Lee, H.; Lee, J.E.; Lee, S.; Kim, C.H.; Kim, D.W.; Jo, E.K.; et al. Role of microglial IKKbeta in kainic acid-induced hippocampal neuronal cell death. Brain 2008, 131, 3019–3033. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, X. Bupivacaine effectively relieves inflammation-induced pain by suppressing activation of the NF-κB signalling pathway and inhibiting the activation of spinal microglia and astrocytes. Exp. Ther. Med. 2017, 13, 1074–1080. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, G.; Li, M.; Zhang, Z. Oleanolic acid administration alleviates neuropathic pain after a peripheral nerve injury by regulating microglia polarization-mediated neuroinflammation. RSC Adv. 2020, 10, 12920–12928. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Yarza, R.; Vela, S.; Solas, M.; Ramirez, M.J. c-Jun N-terminal Kinase (JNK) Signaling as a Therapeutic Target for Alzheimer’s Disease. Front. Pharmacol. 2015, 6, 321. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Andersen, J.K. The role of c-Jun N-terminal kinase (JNK) in Parkinson’s disease. IUBMB Life 2003, 55, 267–271. [Google Scholar] [CrossRef]
- Waetzig, V.; Czeloth, K.; Hidding, U.; Mielke, K.; Kanzow, M.; Brecht, S.; Goetz, M.; Lucius, R.; Herdegen, T.; Hanisch, U.K. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 2005, 50, 235–246. [Google Scholar] [CrossRef]
- Wang, Y.R.; Xu, H.; Tao, M.; Xu, L.H.; Fu, X.C. Ligustilide Relieves Complete Freund’s Adjuvant-induced Mechanical Hyperalgesia through Inhibiting the Activation of Spinal c-Jun N-terminal Kinase/c-Jun Pathway in Rats. Pharmacogn. Mag. 2017, 13, 634–638. [Google Scholar] [CrossRef]
- Gasco, H.A.; Ros-Bernal, F.; Castillo-Gómez, E.; Olucha-Bordonau, F. MAPK/ERK Dysfunction in Neurodegenerative Diseases. Available online: https://pdfs.semanticscholar.org/25e1/3caa0ba99099e466e319cf166cfe22d4a2a9.pdf (accessed on 7 June 2023).
- Zhuang, Z.Y.; Gerner, P.; Woolf, C.J.; Ji, R.R. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005, 114, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Jayasooriya, R.G.P.T.; Dilshara, M.G.; Choi, Y.H.; Jeong, Y.-K.; Kim, N.D.; Kim, G.-Y. Caffeine suppresses lipopolysaccharide-stimulated BV2 microglial cells by suppressing Akt-mediated NF-κB activation and ERK phosphorylation. Food Chem. Toxicol. 2012, 50, 4270–4276. [Google Scholar] [CrossRef]
- Ryu, K.-Y.; Lee, H.-J.; Woo, H.; Kang, R.-J.; Han, K.-M.; Park, H.; Lee, S.M.; Lee, J.-Y.; Jeong, Y.J.; Nam, H.-W. Dasatinib regulates LPS-induced microglial and astrocytic neuroinflammatory responses by inhibiting AKT/STAT3 signaling. J. Neuroinflamm. 2019, 16, 190. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, S.B.; Gao, Y.J.; Xing, J.L.; Xian, H.; Li, Z.Z.; Shen, S.N.; Wu, S.X.; Luo, C.; Xie, R.G. Spinal CCL2 Promotes Pain Sensitization by Rapid Enhancement of NMDA-Induced Currents Through the ERK-GluN2B Pathway in Mouse Lamina II Neurons. Neurosci. Bull. 2020, 36, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.L.; Zhang, Z.J.; Xie, R.G.; Jiang, B.C.; Ji, R.R.; Gao, Y.J. Chemokine CXCL1 enhances inflammatory pain and increases NMDA receptor activity and COX-2 expression in spinal cord neurons via activation of CXCR2. Exp. Neurol. 2014, 261, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Boehm, J.; Lee, J.C. p38 MAP kinases: Key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2003, 2, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Suter, M.R. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.R.; Samad, T.A.; Jin, S.X.; Schmoll, R.; Woolf, C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002, 36, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Chen, C.; Meng, Q.X.; Wu, Y.; Wu, H.; Zhao, T.B. Crosstalk between Activated Microglia and Neurons in the Spinal Dorsal Horn Contributes to Stress-induced Hyperalgesia. Sci. Rep. 2016, 6, 39442. [Google Scholar] [CrossRef] [Green Version]
- Taves, S.; Berta, T.; Liu, D.L.; Gan, S.; Chen, G.; Kim, Y.H.; Van de Ven, T.; Laufer, S.; Ji, R.R. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav. Immun. 2016, 55, 70–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Zhang, W.; Cao, Q.; Zou, L.; Fan, X.; Qi, C.; Yan, Y.; Song, B.; Wu, B. JAK2/STAT3 pathway regulates microglia polarization involved in hippocampal inflammatory damage due to acute paraquat exposure. Ecotoxicol. Environ. Saf. 2022, 234, 113372. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, C.; Chen, H.; Hu, Y.; Tian, L.; Pan, J.; Geng, M. Aβ-induced microglial cell activation is inhibited by baicalin through the JAK2/STAT3 signaling pathway. Int. J. Neurosci. 2014, 124, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Porro, C.; Cianciulli, A.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Curcumin Regulates Anti-Inflammatory Responses by JAK/STAT/SOCS Signaling Pathway in BV-2 Microglial Cells. Biology 2019, 8, 51. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Xie, X.; Qin, K.; Xu, L.; Peng, J.; Li, X.; Li, X.; Liu, Z. Dexamethasone and potassium canrenoate alleviate hyperalgesia by competitively regulating IL-6/JAK2/STAT3 signaling pathway during inflammatory pain in vivo and in vitro. Immun. Inflamm. Dis. 2022, 10, e721. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, G.; Han, F.; Liang, W.; Jiao, Y.; Li, Z.; Li, L. Muscone relieves inflammatory pain by inhibiting microglial activation-mediated inflammatory response via abrogation of the NOX4/JAK2-STAT3 pathway and NLRP3 inflammasome. Int. Immunopharmacol. 2020, 82, 106355. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Ren, P.; Chen, J.; Li, B.; Zhang, M.; Yang, B.; Guo, X.; Chen, Z.; Cheng, H.; Wang, P.; Wang, S.; et al. Nrf2 Ablation Promotes Alzheimer’s Disease-Like Pathology in APP/PS1 Transgenic Mice: The Role of Neuroinflammation and Oxidative Stress. Oxid. Med. Cell Longev. 2020, 2020, 3050971. [Google Scholar] [CrossRef]
- Rojo, A.I.; Pajares, M.; Garcia-Yague, A.J.; Buendia, I.; Van Leuven, F.; Yamamoto, M.; Lopez, M.G.; Cuadrado, A. Deficiency in the transcription factor NRF2 worsens inflammatory parameters in a mouse model with combined tauopathy and amyloidopathy. Redox Biol. 2018, 18, 173–180. [Google Scholar] [CrossRef]
- Velagapudi, R.; El-Bakoush, A.; Olajide, O.A. Activation of Nrf2 Pathway Contributes to Neuroprotection by the Dietary Flavonoid Tiliroside. Mol. Neurobiol. 2018, 55, 8103–8123. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Lv, O.; Zhou, F.; Li, Q.; Wu, Z.; Zheng, Y. Linalool Inhibits LPS-Induced Inflammation in BV2 Microglia Cells by Activating Nrf2. Neurochem. Res. 2015, 40, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.O.; Egea, J.; Lorrio, S.; Rojo, A.I.; Cuadrado, A.; Lopez, M.G. Nrf2-mediated haeme oxygenase-1 up-regulation induced by cobalt protoporphyrin has antinociceptive effects against inflammatory pain in the formalin test in mice. Pain 2008, 137, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhu, M.; Che, X.; Wang, H.; Liang, X.J.; Wu, C.; Xue, X.; Yang, J. Lipopolysaccharide induces neuroinflammation in microglia by activating the MTOR pathway and downregulating Vps34 to inhibit autophagosome formation. J. Neuroinflamm. 2020, 17, 18. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zheng, X.; Liu, L.; Hu, Y.; Zhu, Q.; Zhang, J.; Wang, H.; Gu, E.W.; Yang, Z.; Xu, G. Caloric Restriction Alleviates CFA-Induced Inflammatory Pain via Elevating β-Hydroxybutyric Acid Expression and Restoring Autophagic Flux in the Spinal Cord. Front. Neurosci. 2022, 16, 828278. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Tao, B.; Fan, L.; Yaster, M.; Zhang, Y.; Tao, Y.X. mTOR and its downstream pathway are activated in the dorsal root ganglion and spinal cord after peripheral inflammation, but not after nerve injury. Brain Res. 2013, 1513, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Qi, R.; Zhang, J.; Wang, Z.; Wang, H.; Hu, C.; Zhao, Y.; Bie, M.; Wang, Y.; Fu, Y.; et al. Chlorogenic acid inhibits LPS-induced microglial activation and improves survival of dopaminergic neurons. Brain Res. Bull. 2012, 88, 487–494. [Google Scholar] [CrossRef]
- dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; de Souza, G.E. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef] [Green Version]
- Agudelo-Ochoa, G.M.; Pulgarin-Zapata, I.C.; Velasquez-Rodriguez, C.M.; Duque-Ramirez, M.; Naranjo-Cano, M.; Quintero-Ortiz, M.M.; Lara-Guzman, O.J.; Munoz-Durango, K. Coffee Consumption Increases the Antioxidant Capacity of Plasma and Has No Effect on the Lipid Profile or Vascular Function in Healthy Adults in a Randomized Controlled Trial. J. Nutr. 2016, 146, 524–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Ali, T.; Alam, S.I.; Ullah, R.; Zeb, A.; Lee, K.W.; Rutten, B.P.F.; Kim, M.O. Ferulic Acid Rescues LPS-Induced Neurotoxicity via Modulation of the TLR4 Receptor in the Mouse Hippocampus. Mol. Neurobiol. 2019, 56, 2774–2790. [Google Scholar] [CrossRef]
- Priebe, A.; Hunke, M.; Tonello, R.; Sonawane, Y.; Berta, T.; Natarajan, A.; Bhuvanesh, N.; Pattabiraman, M.; Chandra, S. Ferulic acid dimer as a non-opioid therapeutic for acute pain. J. Pain Res. 2018, 11, 1075. [Google Scholar] [CrossRef] [Green Version]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef] [Green Version]
- Di Giacomo, S.; Percaccio, E.; Gulli, M.; Romano, A.; Vitalone, A.; Mazzanti, G.; Gaetani, S.; Di Sotto, A. Recent Advances in the Neuroprotective Properties of Ferulic Acid in Alzheimer’s Disease: A Narrative Review. Nutrients 2022, 14, 3709. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, S.; Zhang, Z.; Gu, Y.; Xia, S.; Bao, X.; Cao, X.; Xu, Y. 6-Gingerol attenuates microglia-mediated neuroinflammation and ischemic brain injuries through Akt-mTOR-STAT3 signaling pathway. Eur. J. Pharmacol. 2020, 883, 173294. [Google Scholar] [CrossRef]
- Young, H.Y.; Luo, Y.L.; Cheng, H.Y.; Hsieh, W.C.; Liao, J.C.; Peng, W.H. Analgesic and anti-inflammatory activities of [6]-gingerol. J. Ethnopharmacol. 2005, 96, 207–210. [Google Scholar] [CrossRef]
- Sharma, S.; Shukla, M.K.; Sharma, K.C.; Tirath; Kumar, L.; Anal, J.M.H.; Upadhyay, S.K.; Bhattacharyya, S.; Kumar, D. Revisiting the therapeutic potential of gingerols against different pharmacological activities. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 633–647. [Google Scholar] [CrossRef]
- Konmun, J.; Danwilai, K.; Ngamphaiboon, N.; Sripanidkulchai, B.; Sookprasert, A.; Subongkot, S. A phase II randomized double-blind placebo-controlled study of 6-gingerol as an anti-emetic in solid tumor patients receiving moderately to highly emetogenic chemotherapy. Med. Oncol. 2017, 34, 69. [Google Scholar] [CrossRef]
- Gao, F.; Lei, J.; Zhang, Z.; Yang, Y.; You, H. Curcumin alleviates LPS-induced inflammation and oxidative stress in mouse microglial BV2 cells by targeting miR-137-3p/NeuroD1. RSC Adv. 2019, 9, 38397–38406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory Effects of Curcumin in Microglial Cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Vinayak, M. Curcumin attenuates CFA induced thermal hyperalgesia by modulation of antioxidant enzymes and down regulation of TNF-α, IL-1β and IL-6. Neurochem. Res. 2015, 40, 463–472. [Google Scholar] [CrossRef]
- Satoskar, R.R.; Shah, S.J.; Shenoy, S.G. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986, 24, 651–654. [Google Scholar] [PubMed]
- Park, S.E.; Sapkota, K.; Kim, S.; Kim, H.; Kim, S.J. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br. J. Pharmacol. 2011, 164, 1008–1025. [Google Scholar] [CrossRef] [Green Version]
- Jabbari, S.; Bananej, M.; Zarei, M.; Komaki, A.; Hajikhani, R. Effects of intrathecal and intracerebroventricular microinjection of kaempferol on pain: Possible mechanisms of action. Res. Pharm. Sci. 2021, 16, 203–216. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, M.; Mizokami, T.; Ito, H.; Ikeda, Y. A randomized, placebo-controlled trial evaluating the safety of excessive administration of kaempferol aglycone. Food Sci. Nutr. 2023. [Google Scholar] [CrossRef]
- Kang, C.H.; Choi, Y.H.; Moon, S.K.; Kim, W.J.; Kim, G.Y. Quercetin inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-κB pathway and activating the Nrf2-dependent HO-1 pathway. Int. Immunopharmacol. 2013, 17, 808–813. [Google Scholar] [CrossRef]
- Kumar, S.; Vinayak, M. Quercetin Ameliorates CFA-Induced Chronic Inflammatory Hyperalgesia via Modulation of ROS-Mediated ERK1/2 Signaling and Inhibition of Spinal Glial Activation In Vivo. Neuromol. Med. 2020, 22, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Javadi, F.; Ahmadzadeh, A.; Eghtesadi, S.; Aryaeian, N.; Zabihiyeganeh, M.; Rahimi Foroushani, A.; Jazayeri, S. The Effect of Quercetin on Inflammatory Factors and Clinical Symptoms in Women with Rheumatoid Arthritis: A Double-Blind, Randomized Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 9–15. [Google Scholar] [CrossRef] [PubMed]
- El-Bakoush, A.; Olajide, O.A. Formononetin inhibits neuroinflammation and increases estrogen receptor beta (ERβ) protein expression in BV2 microglia. Int. Immunopharmacol. 2018, 61, 325–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.S.; Guan, S.Y.; Liu, A.; Yue, J.; Hu, L.N.; Zhang, K.; Yang, L.K.; Lu, L.; Tian, Z.; Zhao, M.G.; et al. Anxiolytic effects of Formononetin in an inflammatory pain mouse model. Mol. Brain 2019, 12, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, S.K.L.; Shanmugam, M.K.; Fan, L.; Fraser, S.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Bishayee, A. Focus on Formononetin: Anticancer Potential and Molecular Targets. Cancers 2019, 11, 611. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Wei, Y.Z.; Wang, G.Q.; Li, D.D.; Shi, J.S.; Zhang, F. Targeting MAPK Pathways by Naringenin Modulates Microglia M1/M2 Polarization in Lipopolysaccharide-Stimulated Cultures. Front. Cell Neurosci. 2018, 12, 531. [Google Scholar] [CrossRef] [Green Version]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Fattori, V.; Manchope, M.F.; Mizokami, S.S.; Casagrande, R.; Verri, W.A., Jr. Naringenin reduces inflammatory pain in mice. Neuropharmacology 2016, 105, 508–519. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef]
- Cara, K.C.; Beauchesne, A.R.; Wallace, T.C.; Chung, M. Effects of 100% Orange Juice on Markers of Inflammation and Oxidation in Healthy and At-Risk Adult Populations: A Scoping Review, Systematic Review, and Meta-analysis. Adv. Nutr. 2022, 13, 116–137. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, L.; Liu, X.; Lu, T.; Xie, C.; Jia, J. Resveratrol Attenuates Microglial Activation via SIRT1-SOCS1 Pathway. Evid. Based Complement. Alternat. Med. 2017, 2017, 8791832. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Liu, S.; Shu, H.; Crawford, J.; Xing, Y.; Tao, F. Resveratrol alleviates temporomandibular joint inflammatory pain by recovering disturbed gut microbiota. Brain Behav. Immun. 2020, 87, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Marouf, B.H.; Hussain, S.A.; Ali, Z.S.; Ahmmad, R.S. Resveratrol Supplementation Reduces Pain and Inflammation in Knee Osteoarthritis Patients Treated with Meloxicam: A Randomized Placebo-Controlled Study. J. Med. Food 2018, 21, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Fu, Y.S.; Tsai, M.J.; Cheng, H.; Weng, C.F. Natural Compounds from Herbs that can Potentially Execute as Autophagy Inducers for Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rickert, U.; Cossais, F.; Heimke, M.; Arnold, P.; Preusse-Prange, A.; Wilms, H.; Lucius, R. Anti-inflammatory properties of Honokiol in activated primary microglia and astrocytes. J. Neuroimmunol. 2018, 323, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Ullah, M.Z.; Khan, A.U.; Afridi, R.; Rasheed, H.; Khan, A.; Ali, H.; Kim, Y.S.; Khan, S. Antihyperalgesic Properties of Honokiol in Inflammatory Pain Models by Targeting of NF-κB and Nrf2 Signaling. Front. Pharmacol. 2018, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Eliaz, I.; Weil, E. Intravenous Honokiol in Drug-Resistant Cancer: Two Case Reports. Integr. Cancer Ther. 2020, 19, 1534735420922615. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.D.; Zhao, L.X.; Wang, X.T.; Gao, Y.J.; Zhang, Z.J. Ligustilide inhibits microglia-mediated proinflammatory cytokines production and inflammatory pain. Brain Res. Bull. 2014, 109, 54–60. [Google Scholar] [CrossRef]
- Wang, J.; Du, J.R.; Wang, Y.; Kuang, X.; Wang, C.Y. Z-ligustilide attenuates lipopolysaccharide-induced proinflammatory response via inhibiting NF-κB pathway in primary rat microglia. Acta Pharmacol. Sin. 2010, 31, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Yu, Y.; Ke, Y.; Wang, C.; Zhu, L.; Qian, Z.M. Ligustilide attenuates pain behavior induced by acetic acid or formalin. J. Ethnopharmacol. 2007, 112, 211–214. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Han, Y.; Tian, Y.; Wu, P.; Xin, A.; Wei, X.; Shi, Y.; Zhang, Z.; Su, G.; et al. Pharmacokinetics, tissue distribution, and safety evaluation of a ligustilide derivative (LIGc). J. Pharm. Biomed. Anal. 2020, 182, 113140. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zeng, H.; Wang, Q.; Yu, Q.; Wu, J.; Feng, Y.; Deng, P.; Zhang, H. Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-kB pathway. Exp. Cell Res. 2018, 369, 112–119. [Google Scholar] [CrossRef]
- Cao, Z.Y.; Liu, Y.Z.; Li, J.M.; Ruan, Y.M.; Yan, W.J.; Zhong, S.Y.; Zhang, T.; Liu, L.L.; Wu, R.; Wang, B.; et al. Glycyrrhizic acid as an adjunctive treatment for depression through anti-inflammation: A randomized placebo-controlled clinical trial. J. Affect. Disord. 2020, 265, 247–254. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, L.X.; Cao, D.L.; Gao, Y.J. Spinal injection of docosahexaenoic acid attenuates carrageenan-induced inflammatory pain through inhibition of microglia-mediated neuroinflammation in the spinal cord. Neuroscience 2013, 241, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L.; March, L.M.; Aitken, D.; Lester, S.E.; Battersby, R.; Hynes, K.; Fedorova, T.; Proudman, S.M.; James, M.; Cleland, L.G.; et al. Fish oil in knee osteoarthritis: A randomised clinical trial of low dose versus high dose. Ann. Rheum. Dis. 2016, 75, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Xu, G.; Zhang, X.; Xu, L.; Zhou, H.; Ma, Z.; Shen, X.; Zhu, J.; Shen, R. Paeoniflorin Attenuates Inflammatory Pain by Inhibiting Microglial Activation and Akt-NF-κB Signaling in the Central Nervous System. Cell Physiol. Biochem. 2018, 47, 842–850. [Google Scholar] [CrossRef]
- Li, X.; Shi, F.; Zhang, R.; Sun, C.; Gong, C.; Jian, L.; Ding, L. Pharmacokinetics, Safety, and Tolerability of Amygdalin and Paeoniflorin after Single and Multiple Intravenous Infusions of Huoxue-Tongluo Lyophilized Powder for Injection in Healthy Chinese Volunteers. Clin. Ther. 2016, 38, 327–337. [Google Scholar] [CrossRef]
- Shukla, S.M.; Sharma, S.K. Sinomenine inhibits microglial activation by Aβ and confers neuroprotection. J. Neuroinflamm. 2011, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhang, Y.; He, X.; Fan, S. Protective effects of sinomenine on CFA-induced inflammatory pain in rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 2018–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Zhang, Y.; Zhu, W.; Ma, C.; Ruan, J.; Long, H.; Wang, Y. Sinomenine Inhibits the Progression of Rheumatoid Arthritis by Regulating the Secretion of Inflammatory Cytokines and Monocyte/Macrophage Subsets. Front. Immunol. 2018, 9, 2228. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cheng, Y.; Rao, M.; Tang, M.; Dong, Z. Muscone Induces CYP1A2 and CYP3A4 Enzyme Expression in L02 Human Liver Cells and CYP1A2 and CYP3A11 Enzyme Expression in Kunming Mice. Pharmacology 2017, 99, 205–215. [Google Scholar] [CrossRef]
- Xu, J.; Yuan, C.; Wang, G.; Luo, J.; Ma, H.; Xu, L.; Mu, Y.; Li, Y.; Seeram, N.P.; Huang, X.; et al. Urolithins Attenuate LPS-Induced Neuroinflammation in BV2Microglia via MAPK, Akt, and NF-κB Signaling Pathways. J. Agric. Food Chem. 2018, 66, 571–580. [Google Scholar] [CrossRef]
- D’Amico, D.; Olmer, M.; Fouassier, A.M.; Valdes, P.; Andreux, P.A.; Rinsch, C.; Lotz, M. Urolithin A improves mitochondrial health, reduces cartilage degeneration, and alleviates pain in osteoarthritis. Aging Cell 2022, 21, e13662. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; D’Amico, D.; Shankland, E.; Bhayana, S.; Garcia, J.M.; Aebischer, P.; Rinsch, C.; Singh, A.; Marcinek, D.J. Effect of Urolithin A Supplementation on Muscle Endurance and Mitochondrial Health in Older Adults: A Randomized Clinical Trial. JAMA Netw. Open. 2022, 5, e2144279. [Google Scholar] [CrossRef]
- Niu, J.; Straubinger, R.M.; Mager, D.E. Pharmacodynamic Drug-Drug Interactions. Clin. Pharmacol. Ther. 2019, 105, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, Z.; Li, Z.; Li, J.; Li, Y. Muscone reduced the hypnotic and analgesic effect of ketamine in mice. J. Chin. Med. Assoc. 2020, 83, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, C.; Kim, Y.T. Effects of Natural Product-Derived Compounds on Inflammatory Pain via Regulation of Microglial Activation. Pharmaceuticals 2023, 16, 941. https://doi.org/10.3390/ph16070941
Park J, Lee C, Kim YT. Effects of Natural Product-Derived Compounds on Inflammatory Pain via Regulation of Microglial Activation. Pharmaceuticals. 2023; 16(7):941. https://doi.org/10.3390/ph16070941
Chicago/Turabian StylePark, Joon, Changho Lee, and Yun Tai Kim. 2023. "Effects of Natural Product-Derived Compounds on Inflammatory Pain via Regulation of Microglial Activation" Pharmaceuticals 16, no. 7: 941. https://doi.org/10.3390/ph16070941
APA StylePark, J., Lee, C., & Kim, Y. T. (2023). Effects of Natural Product-Derived Compounds on Inflammatory Pain via Regulation of Microglial Activation. Pharmaceuticals, 16(7), 941. https://doi.org/10.3390/ph16070941





