Continuous Manufacturing of Cocrystals Using 3D-Printed Microfluidic Chips Coupled with Spray Coating
Abstract
:1. Introduction
2. Results
2.1. Engineering of 3D-Printed Microfluidic Chips
2.2. Cocrystal Morphological Characterization
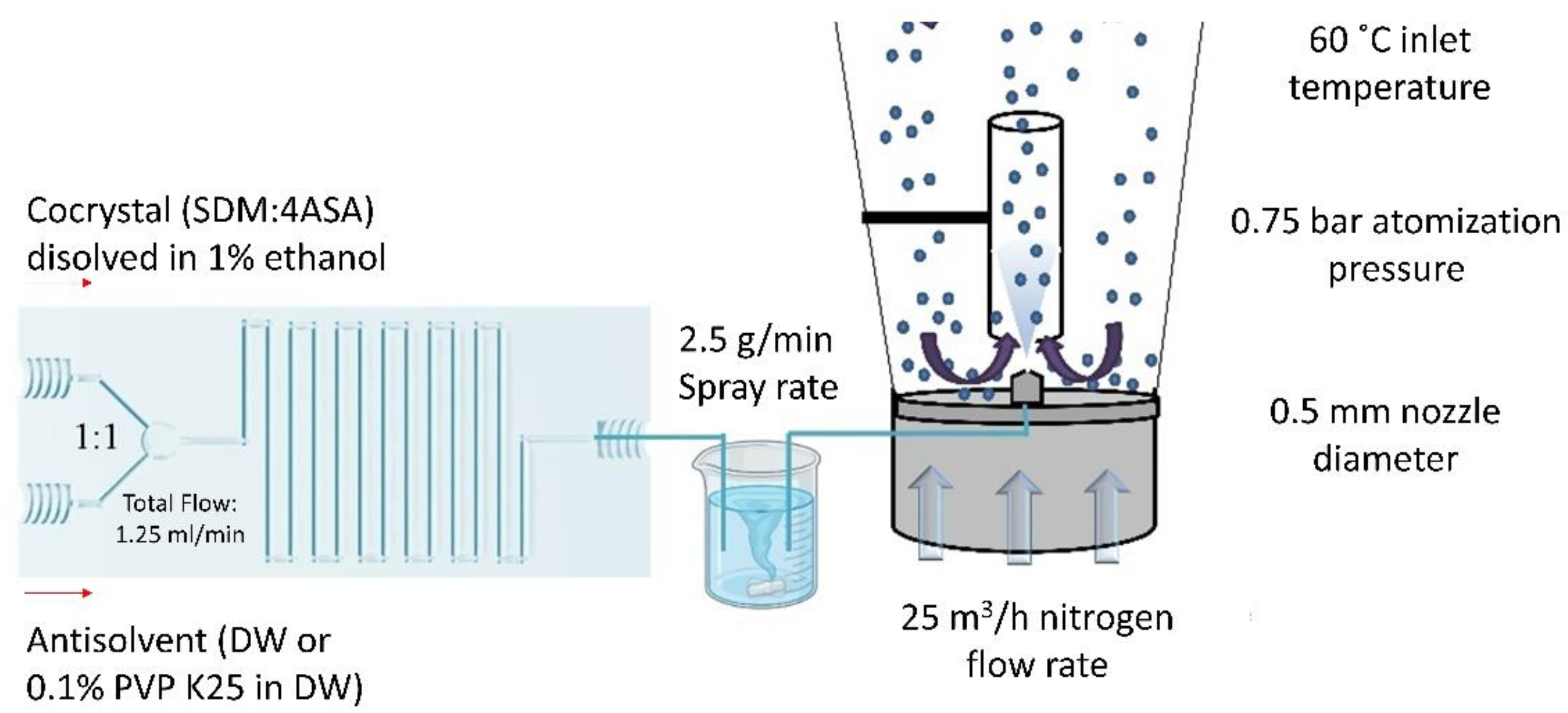
2.3. Continuous Manufacturing of Cocrystals Using a Solution-Based Microfluidic Approach Coupled with Spray Coating
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Design and 3D Printing of Microfluidic Chips
Geometrical Design
Stereolithography (SLA)
Imaging
3.2.2. Preparation of Cocrystal Formulations
Macroscale Solvent Evaporation
Microfluidic Mixing
Imaging
Powder X-ray Diffraction
3.2.3. Continuous Manufacturing of Cocrystals Using a Solution-Based Microfluidic Approach Coupled with Spray Coating
Method 1
Method 2
Method 3
Differential Scanning Calorimetry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stahly, G.P. Diversity in single-and multiple-component crystals. The search for and prevalence of polymorphs and cocrystals. Cryst. Growth Des. 2007, 7, 1007–1026. [Google Scholar] [CrossRef] [Green Version]
- Schultheiss, N.; Newman, A. Pharmaceutical cocrystals and their physicochemical properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [Green Version]
- Almarsson, Ö.; Zaworotko, M.J. Crystal engineering of the composition of pharmaceutical phases. Do pharmaceutical co-crystals represent a new path to improved medicines? Chem. Commun. 2004, 17, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Crystal engineering: A holistic view. Angew. Chem. Int. Ed. 2007, 46, 8342–8356. [Google Scholar]
- Trask, A.V. An overview of pharmaceutical cocrystals as intellectual property. Mol. Pharm. 2007, 4, 301–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, D.R.; Gallagher, K.H.; Healy, A.M. Emerging Nanonisation Technologies: Tailoring Crystalline Versus Amorphous Nanomaterials. Curr. Top. Med. Chem. 2015, 15, 2327–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, D.; Serrano, D.R.; Worku, Z.A.; Madi, A.M.; O’Connell, P.; Twamley, B.; Healy, A.M. Engineering of pharmaceutical cocrystals in an excipient matrix: Spray drying versus hot melt extrusion. Int. J. Pharm. 2018, 551, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Pekamwara, S.S.; Kulkarnia, D.A. Development and evaluation of bicomponent cocrystals of aceclofenac for efficient drug delivery with enhanced solubility and improved dissolution. Indian Drugs 2021, 58, 54–60. [Google Scholar] [CrossRef]
- Kulkarni, D. Accidental Formation of Eutectics during Crystal Engineering of Lamotrigine with Solubility Advantage and Drug Release Efficiency. Asian J. Pharm. 2021, 15. [Google Scholar] [CrossRef]
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical cocrystals: An overview. Int. J. Pharm. 2011, 419, 1–11. [Google Scholar] [CrossRef]
- Walsh, D.; Serrano, D.R.; Worku, Z.A.; Norris, B.A.; Healy, A.M. Production of cocrystals in an excipient matrix by spray drying. Int. J. Pharm. 2018, 536, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hornedo, N.; Nehm, S.J.; Seefeldt, K.F.; Pagan-Torres, Y.; Falkiewicz, C.J. Reaction crystallization of pharmaceutical molecular complexes. Mol. Pharm. 2006, 3, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Tripathi, D.; Rai, A.K. A Technical Approach of Solubility Enhancement of Poorly Soluble Drugs: Liquisolid Technique. Curr. Drug Del. 2020, 17, 638–650. [Google Scholar]
- Goyal, S.; Thorson, M.; Zhang, G.; Gong, Y.; Kenis, P. Microfluidic Approach to Cocrystal Screening of Pharmaceutical Parent Compounds. Cryst. Growth Des. 2012, 12, 6023–6034. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Douroumis, D.; Ross, S.A.; Nokhodchi, A. Advanced methodologies for cocrystal synthesis. Adv. Drug Del. Rev. 2017, 117, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.; Jensen, K.F. Microfluidic continuous seeded crystallization: Extraction of growth kinetics and impact of impurity on morphology. Cryst. Growth Des. 2012, 12, 6260–6266. [Google Scholar] [CrossRef]
- Chen, Z.; Shen, L.; Zhao, X.; Chen, H.; Xiao, Y.; Zhang, Y.; Yang, X.; Zhang, J.; Wei, J.; Hao, N. Acoustofluidic micromixers: From rational design to lab-on-a-chip applications. Appl. Mater. Today 2022, 26, 101356. [Google Scholar]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef]
- Kara, A.; Vassiliadou, A.; Ongoren, B.; Keeble, W.; Hing, R.; Lalatsa, A.; Serrano, D.R. Engineering 3D Printed Microfluidic Chips for the Fabrication of Nanomedicines. Pharmaceutics 2021, 13, 2134. [Google Scholar] [CrossRef]
- Grossjohann, C.; Serrano, D.R.; Paluch, K.J.; O’Connell, P.; Vella-Zarb, L.; Manesiotis, P.; McCabe, T.; Tajber, L.; Corrigan, O.I.; Healy, A.M. Polymorphism in sulfadimidine/4-aminosalicylic acid cocrystals: Solid-state characterization and physicochemical properties. J. Pharm. Sci. 2015, 104, 1385–1398. [Google Scholar] [CrossRef] [Green Version]
- Serrano, D.R.; Walsh, D.; O’Connell, P.; Mugheirbi, N.A.; Worku, Z.A.; Bolas-Fernandez, F.; Galiana, C.; Dea-Ayuela, M.A.; Healy, A.M. Optimising the in vitro and in vivo performance of oral cocrystal formulations via spray coating. Eur. J. Pharm. Biopharm. 2018, 124, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, D.; Zhao, S.; Huang, X.; Zhang, J.; Lv, Y.; Liu, X.; Lv, G.; Ma, X. Evaluate the ability of PVP to inhibit crystallization of amorphous solid dispersions by density functional theory and experimental verify. Eur. J. Pharm. Sci. 2017, 96, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; O’Connell, P.; Paluch, K.J.; Walsh, D.; Healy, A.M. Cocrystal habit engineering to improve drug dissolution and alter derived powder properties. J. Pharm. Pharmacol. 2016, 68, 665–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, D.R.; Persoons, T.; D’Arcy, D.M.; Galiana, C.; Dea-Ayuela, M.A.; Healy, A.M. Modelling and shadowgraph imaging of cocrystal dissolution and assessment of in vitro antimicrobial activity for sulfadimidine/4-aminosalicylic acid cocrystals. Eur. J. Pharm. Sci. 2016, 89, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Mugheirbi, N.A.; O’Connell, P.; Leddy, N.; Healy, A.M.; Tajber, L. Impact of Substrate Properties on the Formation of Spherulitic Films: A Case Study of Salbutamol Sulfate. Cryst. Growth Des. 2016, 16, 3853–3858. [Google Scholar] [CrossRef]
- Rolon, M.; Serrano, D.R.; Lalatsa, A.; de Pablo, E.; Torrado, J.J.; Ballesteros, M.P.; Healy, A.M.; Vega, C.; Coronel, C.; Bolas-Fernandez, F.; et al. Engineering Oral and Parenteral Amorphous Amphotericin B Formulations against Experimental Trypanosoma cruzi Infections. Mol. Pharm. 2017, 14, 1095–1106. [Google Scholar] [CrossRef] [Green Version]
- Kaerger, J.S.; Edge, S.; Price, R. Influence of particle size and shape on flowability and compactibility of binary mixtures of paracetamol and microcrystalline cellulose. Eur. J. Pharm. Sci. 2004, 22, 173–179. [Google Scholar] [CrossRef]
- Wilson, D.; Bunker, M.; Milne, D.; Jawor-Baczynska, A.; Powell, A.; Blyth, J.; Streather, D. Particle engineering of needle shaped crystals by wet milling and temperature cycling: Optimisation for roller compaction. Power Technol. 2018, 339, 641–650. [Google Scholar]
- Civati, F.; O’Malley, C.; Erxleben, A.; McArdle, P. Factors Controlling Persistent Needle Crystal Growth: The Importance of Dominant One-Dimensional Secondary Bonding, Stacked Structures, and van der Waals Contact. Cryst. Growth Des. 2021, 21, 3449–3460. [Google Scholar] [CrossRef]
- Gamble, J.F.; Tobyn, M.; Hamey, R. Application of image-based particle size and shape characterization systems in the development of small molecule pharmaceuticals. J. Pharm. Sci. 2015, 104, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Thipparaboina, R.; Modi, S.R.; Bansal, A.K.; Shastri, N.R. Effect of surfactant concentration on nifedipine crystal habit and its related pharmaceutical properties. J. Cryst. Growth 2015, 422, 44–51. [Google Scholar] [CrossRef]
- Pedu, S.N. Microfluidics and Modeling of Nucleation Rates in Cocrystal Systems. Ph.D. Thesis, Carnegie Mellon University, Pittsburgh, PA, USA, 2022. Available online: https://kilthub.cmu.edu/articles/thesis/Microfluidics_and_Modeling_of_Nucleation_Rates_in_Cocrystal_Systems/21508206 (accessed on 15 March 2023).
- Budiman, A.; Citraloka, Z.G.; Muchtaridi, M.; Sriwidodo, S.; Aulifa, D.L.; Rusdin, A. Inhibition of Crystal Nucleation and Growth in Aqueous Drug Solutions: Impact of Different Polymers on the Supersaturation Profiles of Amorphous Drugs-The Case of Alpha-Mangostin. Pharmaceutics 2022, 14, 2386. [Google Scholar] [CrossRef] [PubMed]
- Talwar, S.; Pawar, P.; Wu, H.; Sowrirajan, K.; Wu, S.; Igne, B.; Friedman, R.; Muzzio, F.J.; Drennen, J.K., 3rd. NIR Spectroscopy as an Online PAT Tool for a Narrow Therapeutic Index Drug: Toward a Platform Approach Across Lab and Pilot Scales for Development of a Powder Blending Monitoring Method and Endpoint Determination. AAPS J. 2022, 24, 103. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Scoutaris, N.; Maniruzzaman, M.; Moradiya, H.G.; Halsey, S.A.; Bradley, M.S.; Chowdhry, B.Z.; Snowden, M.J.; Douroumis, D. Implementation of transmission NIR as a PAT tool for monitoring drug transformation during HME processing. Eur. J. Pharm. Biopharm. 2015, 96, 106–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kara, A.; Kumar, D.; Healy, A.M.; Lalatsa, A.; Serrano, D.R. Continuous Manufacturing of Cocrystals Using 3D-Printed Microfluidic Chips Coupled with Spray Coating. Pharmaceuticals 2023, 16, 1064. https://doi.org/10.3390/ph16081064
Kara A, Kumar D, Healy AM, Lalatsa A, Serrano DR. Continuous Manufacturing of Cocrystals Using 3D-Printed Microfluidic Chips Coupled with Spray Coating. Pharmaceuticals. 2023; 16(8):1064. https://doi.org/10.3390/ph16081064
Chicago/Turabian StyleKara, Aytug, Dinesh Kumar, Anne Marie Healy, Aikaterini Lalatsa, and Dolores R. Serrano. 2023. "Continuous Manufacturing of Cocrystals Using 3D-Printed Microfluidic Chips Coupled with Spray Coating" Pharmaceuticals 16, no. 8: 1064. https://doi.org/10.3390/ph16081064
APA StyleKara, A., Kumar, D., Healy, A. M., Lalatsa, A., & Serrano, D. R. (2023). Continuous Manufacturing of Cocrystals Using 3D-Printed Microfluidic Chips Coupled with Spray Coating. Pharmaceuticals, 16(8), 1064. https://doi.org/10.3390/ph16081064









