Impact of Sirolimus versus Mycophenolate Mofetil on Kidney Function after Calcineurin Inhibitor Dose Reduction in Liver Transplant Recipients
Abstract
1. Introduction
2. Results
2.1. Pre-Operation Baseline Patient Characteristics
2.2. Intra- and Post-Operative Factors
2.3. Patterns of Immunosuppressant Treatment
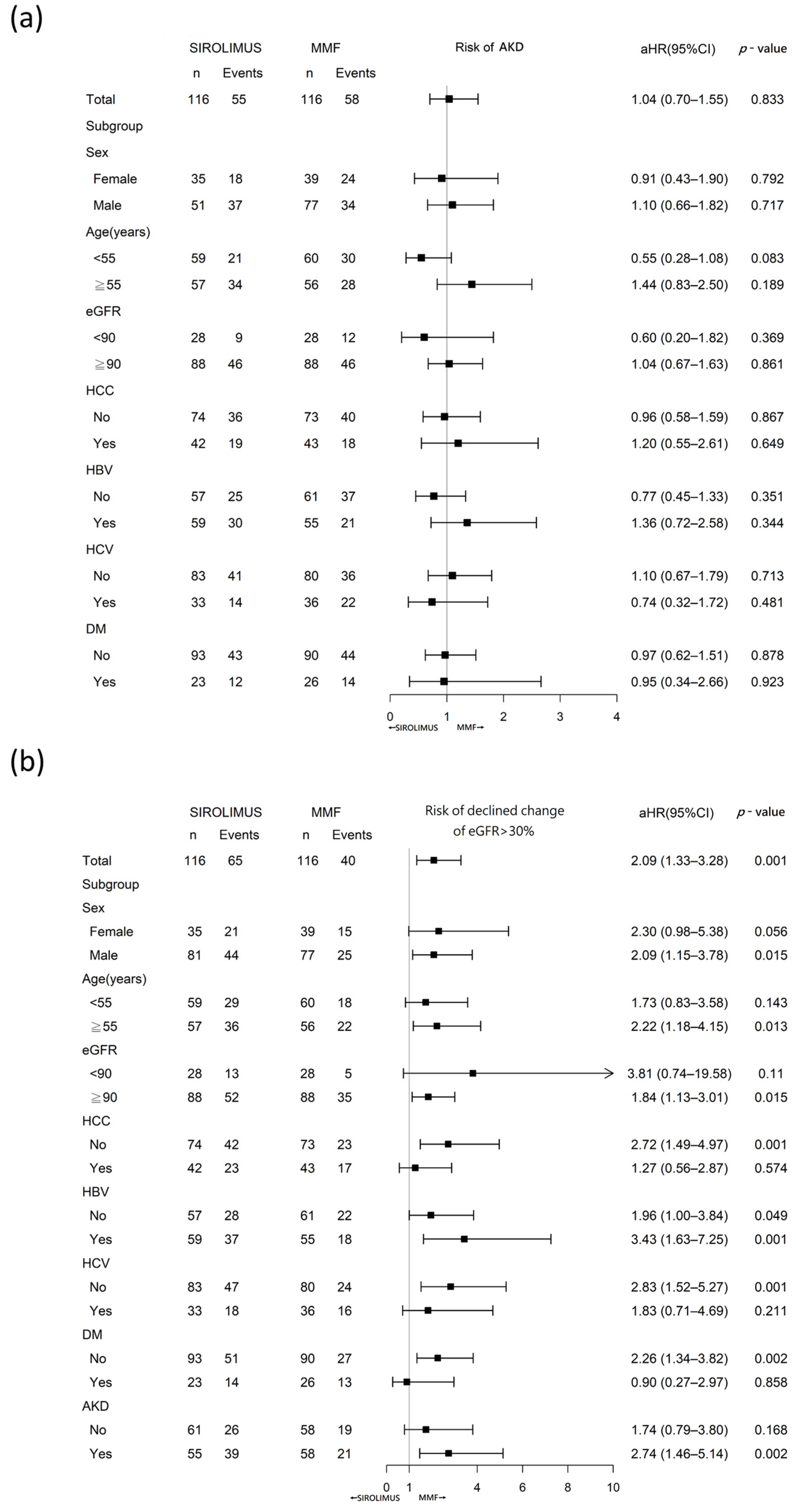
2.4. Adverse Kidney Outcomes
2.5. Acute Kidney Disease
2.6. eGFR Decline from Baseline
2.7. Changes in eGFR over Time
3. Discussion
4. Materials and Methods
4.1. Patients and Study Design
4.2. Outcomes
4.3. Covariates
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, C.B.; Humar, A. Liver transplantation: Current and future. Abdom. Radiol. N. Y. 2021, 46, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Di Maira, T.; Little, E.C.; Berenguer, M. Immunosuppression in liver transplant. Best Pract. Res. Clin. Gastroenterol. 2020, 46–47, 101681. [Google Scholar] [CrossRef] [PubMed]
- Bahirwani, R.; Reddy, K.R. Outcomes after liver transplantation: Chronic kidney disease. Liver Transpl. 2009, 15 (Suppl. S2), S70–S74. [Google Scholar] [CrossRef]
- Allen, A.M.; Kim, W.R.; Therneau, T.M.; Larson, J.J.; Heimbach, J.K.; Rule, A.D. Chronic kidney disease and associated mortality after liver transplantation--a time-dependent analysis using measured glomerular filtration rate. J. Hepatol. 2014, 61, 286–292. [Google Scholar] [CrossRef]
- Ojo, A.O.; Held, P.J.; Port, F.K.; Wolfe, R.A.; Leichtman, A.B.; Young, E.W.; Arndorfer, J.; Christensen, L.; Merion, R.M. Chronic renal failure after transplantation of a nonrenal organ. N. Engl. J. Med. 2003, 349, 931–940. [Google Scholar] [CrossRef]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.S.; Bilous, R.W.; Coresh, J. Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef]
- Trinh, E.; Alam, A.; Tchervenkov, J.; Cantarovich, M. Impact of acute kidney injury following liver transplantation on long-term outcomes. Clin. Transplant. 2017, 31, e12863. [Google Scholar] [CrossRef]
- Guo, M.; Gao, Y.; Wang, L.; Zhang, H.; Liu, X.; Zhang, H. Early Acute Kidney Injury Associated with Liver Transplantation: A Retrospective Case-Control Study. Med. Sci. Monit. 2020, 26, e923864. [Google Scholar] [CrossRef]
- Hilmi, I.A.; Damian, D.; Al-Khafaji, A.; Planinsic, R.; Boucek, C.; Sakai, T.; Chang, C.C.; Kellum, J.A. Acute kidney injury following orthotopic liver transplantation: Incidence, risk factors, and effects on patient and graft outcomes. Br. J. Anaesth. 2015, 114, 919–926. [Google Scholar] [CrossRef]
- Klaus, F.; Keitel da Silva, C.; Meinerz, G.; Carvalho, L.M.; Goldani, J.C.; Cantisani, G.; Zanotelli, M.L.; Duro Garcia, V.; Keitel, E. Acute kidney injury after liver transplantation: Incidence and mortality. Transplant. Proc. 2014, 46, 1819–1821. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, Y.; Xia, Q.; Wang, S.; Qiu, Y.; Che, M.; Dai, H.; Qian, J.; Ni, Z.; Axelsson, J.; et al. Strong impact of acute kidney injury on survival after liver transplantation. Transplant. Proc. 2010, 42, 3634–3638. [Google Scholar] [CrossRef]
- O’Riordan, A.; Wong, V.; McCormick, P.A.; Hegarty, J.E.; Watson, A.J. Chronic kidney disease post-liver transplantation. Nephrol. Dial. Transplant. 2006, 21, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Francoz, C.; Asrani, S.K.; Khemichian, S.; Pham, T.A.; Sung, R.S.; Genyk, Y.S.; Nadim, M.K. Acute Kidney Injury After Liver Transplantation. Transplantation 2018, 102, 1636–1649. [Google Scholar] [CrossRef] [PubMed]
- Fussner, L.A.; Heimbach, J.K.; Fan, C.; Dierkhising, R.; Coss, E.; Leise, M.D.; Watt, K.D. Cardiovascular disease after liver transplantation: When, What, and Who Is at Risk. Liver Transpl. 2015, 21, 889–896. [Google Scholar] [CrossRef]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef]
- Dong, V.; Nadim, M.K.; Karvellas, C.J. Post-Liver Transplant Acute Kidney Injury. Liver Transpl. 2021, 27, 1653–1664. [Google Scholar] [CrossRef]
- Colliou, E.; Del Bello, A.; Milongo, D.; Muscari, F.; Vallet, M.; Tack, I.; Kamar, N. Kidney Failure after Liver Transplantation. Transplantology 2021, 2, 315–335. [Google Scholar] [CrossRef]
- Fairbanks, K.D.; Eustace, J.A.; Fine, D.; Thuluvath, P.J. Renal function improves in liver transplant recipients when switched from a calcineurin inhibitor to sirolimus. Liver Transpl. 2003, 9, 1079–1085. [Google Scholar] [CrossRef]
- Watson, C.J.; Gimson, A.E.; Alexander, G.J.; Allison, M.E.; Gibbs, P.; Smith, J.C.; Palmer, C.R.; Bradley, J.A. A randomized controlled trial of late conversion from calcineurin inhibitor (CNI)-based to sirolimus-based immunosuppression in liver transplant recipients with impaired renal function. Liver Transpl. 2007, 13, 1694–1702. [Google Scholar] [CrossRef]
- Asrani, S.K.; Wiesner, R.H.; Trotter, J.F.; Klintmalm, G.; Katz, E.; Maller, E.; Roberts, J.; Kneteman, N.; Teperman, L.; Fung, J.J.; et al. De novo sirolimus and reduced-dose tacrolimus versus standard-dose tacrolimus after liver transplantation: The 2000–2003 phase II prospective randomized trial. Am. J. Transplant. 2014, 14, 356–366. [Google Scholar] [CrossRef]
- Kaltenborn, A.; Schrem, H. Mycophenolate mofetil in liver transplantation: A review. Ann. Transplant. 2013, 18, 685–696. [Google Scholar] [CrossRef]
- Levitsky, J.; O’Leary, J.G.; Asrani, S.; Sharma, P.; Fung, J.; Wiseman, A.; Niemann, C.U. Protecting the Kidney in Liver Transplant Recipients: Practice-Based Recommendations From the American Society of Transplantation Liver and Intestine Community of Practice. Am. J. Transplant. 2016, 16, 2532–2544. [Google Scholar] [CrossRef] [PubMed]
- Boudjema, K.; Camus, C.; Saliba, F.; Calmus, Y.; Salame, E.; Pageaux, G.; Ducerf, C.; Duvoux, C.; Mouchel, C.; Renault, A.; et al. Reduced-dose tacrolimus with mycophenolate mofetil vs. standard-dose tacrolimus in liver transplantation: A randomized study. Am. J. Transplant. 2011, 11, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Pageaux, G.P.; Rostaing, L.; Calmus, Y.; Duvoux, C.; Vanlemmens, C.; Hardgwissen, J.; Bernard, P.H.; Barbotte, E.; Vercambre, L.; Bismuth, M.; et al. Mycophenolate mofetil in combination with reduction of calcineurin inhibitors for chronic renal dysfunction after liver transplantation. Liver Transpl. 2006, 12, 1755–1760. [Google Scholar] [CrossRef]
- Barri, Y.M.; Sanchez, E.Q.; Jennings, L.W.; Melton, L.B.; Hays, S.; Levy, M.F.; Klintmalm, G.B. Acute kidney injury following liver transplantation: Definition and outcome. Liver Transpl. 2009, 15, 475–483. [Google Scholar] [CrossRef]
- Coresh, J.; Turin, T.C.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Appel, L.J.; Arima, H.; Chadban, S.J.; Cirillo, M.; Djurdjev, O.; et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014, 311, 2518–2531. [Google Scholar] [CrossRef] [PubMed]
- Persson, F.; Rossing, P.; Vart, P.; Chertow, G.M.; Hou, F.F.; Jongs, N.; McMurray, J.J.V.; Correa-Rotter, R.; Bajaj, H.S.; Stefansson, B.V.; et al. Efficacy and Safety of Dapagliflozin by Baseline Glycemic Status: A Prespecified Analysis From the DAPA-CKD Trial. Diabetes Care 2021, 44, 1894–1897. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Xu, E.; Al-Aly, Z. Kidney Outcomes in Long COVID. J. Am. Soc. Nephrol. 2021, 32, 2851–2862. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Kaewput, W.; Thamcharoen, N.; Bathini, T.; Watthanasuntorn, K.; Lertjitbanjong, P.; Sharma, K.; Salim, S.A.; Ungprasert, P.; Wijarnpreecha, K.; et al. Incidence and Impact of Acute Kidney Injury after Liver Transplantation: A Meta-Analysis. J. Clin. Med. 2019, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.Q.; Gonwa, T.A.; Levy, M.F.; Goldstein, R.M.; Mai, M.L.; Hays, S.R.; Melton, L.B.; Saracino, G.; Klintmalm, G.B. Preoperative and perioperative predictors of the need for renal replacement therapy after orthotopic liver transplantation. Transplantation 2004, 78, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Pawarode, A.; Fine, D.M.; Thuluvath, P.J. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver Transpl. 2003, 9, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Inker, L.A.; Matsushita, K.; Greene, T.; Willis, K.; Lewis, E.; de Zeeuw, D.; Cheung, A.K.; Coresh, J. GFR decline as an end point for clinical trials in CKD: A scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am. J. Kidney Dis. 2014, 64, 821–835. [Google Scholar] [CrossRef]
- Cantarovich, M.; Tchervenkov, J.; Paraskevas, S.; Ghali, P.; Wong, P.; Deschenes, M.; Chaudhury, P.; Hassanain, M.; Vrochides, D.; Metrakos, P.; et al. Early changes in kidney function predict long-term chronic kidney disease and mortality in patients after liver transplantation. Transplantation 2011, 92, 1358–1363. [Google Scholar] [CrossRef]
- Nashan, B.; Saliba, F.; Durand, F.; Barcena, R.; Herrero, J.I.; Mentha, G.; Neuhaus, P.; Bowles, M.; Patch, D.; Bernardos, A.; et al. Pharmacokinetics, efficacy, and safety of mycophenolate mofetil in combination with standard-dose or reduced-dose tacrolimus in liver transplant recipients. Liver Transpl. 2009, 15, 136–147. [Google Scholar] [CrossRef]
- Neuberger, J.M.; Mamelok, R.D.; Neuhaus, P.; Pirenne, J.; Samuel, D.; Isoniemi, H.; Rostaing, L.; Rimola, A.; Marshall, S.; Mayer, A.D.; et al. Delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: The ‘ReSpECT’ study. Am. J. Transplant. 2009, 9, 327–336. [Google Scholar] [CrossRef]
- Baldan, N.; Rigotti, P.; Furian, L.; Margani, G.; Ekser, B.; Frison, L.; De Martin, S.; Palatini, P. Co-administration of sirolimus alters tacrolimus pharmacokinetics in a dose-dependent manner in adult renal transplant recipients. Pharmacol. Res. 2006, 54, 181–185. [Google Scholar] [CrossRef]
- Park, S.I.; Felipe, C.R.; Pinheiro-Machado, P.G.; Garcia, R.; Fernandes, F.B.; Casarini, D.E.; Tedesco-Silva, H., Jr.; Medina-Pestana, J.O. Tacrolimus pharmacokinetic drug interactions: Effect of prednisone, mycophenolic acid or sirolimus. Fundam. Clin. Pharmacol. 2009, 23, 137–145. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, C.C.; Wang, C.C.; Wang, S.H.; Liu, Y.W.; Yong, C.C.; Lin, T.L.; Li, W.F.; Concejero, A.M.; Chen, C.L. The 4-week serum creatinine level predicts long-term renal dysfunction after adult living donor liver transplantation. Transplant. Proc. 2012, 44, 772–775. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, Y.; Tang, H.; Yao, J.; Wang, G.; Yang, Y.; Chen, G. Prediction of chronic kidney disease progression used by calcineurin inhibitor concentration and estimated glomerular filtration rate early after liver transplantation. Niger. J. Clin. Pract. 2020, 23, 1387–1394. [Google Scholar] [CrossRef]
- DuBay, D.; Smith, R.J.; Qiu, K.G.; Levy, G.A.; Lilly, L.; Therapondos, G. Sirolimus in liver transplant recipients with renal dysfunction offers no advantage over low-dose calcineurin inhibitor regimens. Liver Transpl. 2008, 14, 651–659. [Google Scholar] [CrossRef]
- Li, L.C.; Hsu, C.N.; Lin, C.C.; Cheng, Y.F.; Hu, T.H.; Chen, D.W.; Lee, C.H.; Nakano, T.; Chen, C.L. Proteinuria and baseline renal function predict mortality and renal outcomes after sirolimus therapy in liver transplantation recipients. BMC Gastroenterol. 2017, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kriesche, H.U.; Schold, J.D.; Srinivas, T.R.; Howard, R.J.; Fujita, S.; Kaplan, B. Sirolimus in combination with tacrolimus is associated with worse renal allograft survival compared to mycophenolate mofetil combined with tacrolimus. Am. J. Transplant. 2005, 5, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Tasdogan, B.E.; Ma, M.; Simsek, C.; Saberi, B.; Gurakar, A. Update on Immunosuppression in Liver Transplantation. Euroasian J. Hepatogastroenterol. 2019, 9, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Salvador, N.G.A.; Wee, S.Y.; Lin, C.C.; Wu, C.C.; Lu, H.I.; Lin, T.L.; Lee, W.F.; Chan, Y.C.; Lin, L.M.; Chen, C.L. Clinical Outcomes of Tuberculosis in Recipients After Living Donor Liver Transplantation. Ann. Transplant. 2018, 23, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Geissler, E.K.; Schnitzbauer, A.A.; Zulke, C.; Lamby, P.E.; Proneth, A.; Duvoux, C.; Burra, P.; Jauch, K.W.; Rentsch, M.; Ganten, T.M.; et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation 2016, 100, 116–125. [Google Scholar] [CrossRef]
- Tsai, K.F.; Li, L.C.; Hsu, C.N.; Lin, C.C.; Lin, Y.H.; Cheng, Y.F.; Wang, C.C.; Chen, C.L. Effects of Conversion From Calcineurin Inhibitors to Sirolimus or Everolimus on Renal Function and Possible Mechanisms in Liver Transplant Recipients. J. Clin. Pharmacol. 2019, 59, 326–334. [Google Scholar] [CrossRef]
- Chen, L.I.; Guh, J.Y.; Wu, K.D.; Chen, Y.M.; Kuo, M.C.; Hwang, S.J.; Chen, T.H.; Chen, H.C. Modification of diet in renal disease (MDRD) study and CKD epidemiology collaboration (CKD-EPI) equations for Taiwanese adults. PLoS ONE 2014, 9, e99645. [Google Scholar] [CrossRef]
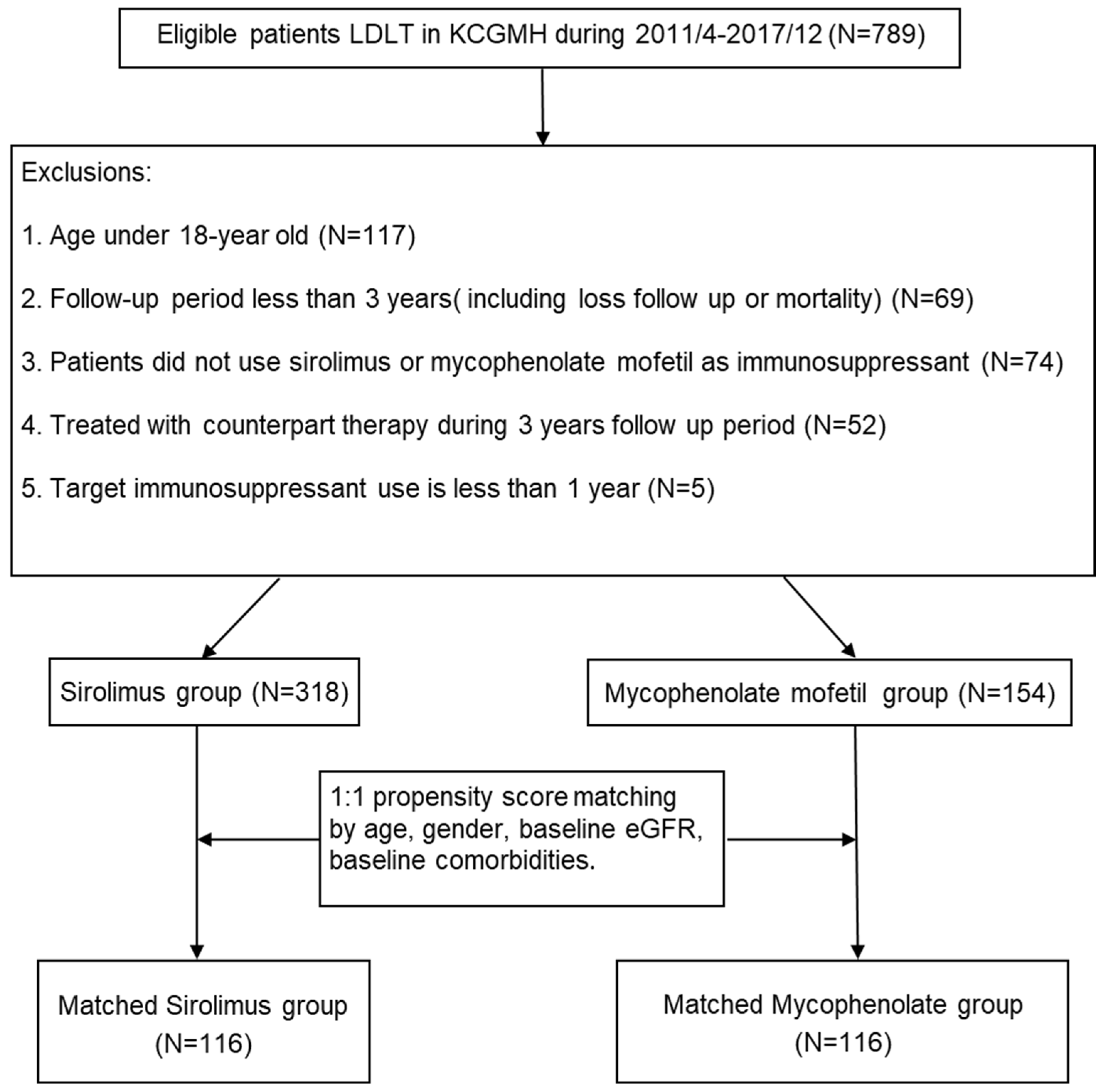

| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Sirolimus | MMF | SMD | Sirolimus | MMF | SMD | |
| N = 318 | N = 154 | N = 116 | N = 116 | |||
| Age (mean (SD)) | 54.3 (8.6) | 51.7 (10.5) | 0.27 | 52.3 (10.2) | 52.3 (9.5) | 0.004 |
| eGFR, mL/min/1.73 m2 (mean (SD)) | 86.9 (25.4) | 100.4 (15.2) | 0.57 | 97.1 (18.3) | 100.2 (14.9) | <0.001 |
| eGFR < 60 | 53.0 (16.7) | 3.0 (1.9) | 2.0 (1.7) | 2.0 (1.7) | ||
| eGFR 60–89.9 | 85.0 (26.7) | 33.0 (21.4) | 26.0 (22.4) | 26.0 (22.4) | ||
| eGFR ≧ 90 | 180.0 (56.6) | 118.0 (76.6) | 88.0 (75.9) | 88.0 (75.9) | ||
| Hypertension (%) | 67.0 (21.1) | 24.0 (15.6) | 0.14 | 13.0 (11.2) | 16.0 (13.8) | 0.078 |
| Diabetes mellitus (%) | 81.0 (25.5) | 35.0 (22.7) | 0.06 | 23.0 (19.8) | 26.0 (22.4) | 0.063 |
| Male sex (%) | 248.0 (78.0) | 104.0 (67.5) | 0.24 | 81.0 (69.8) | 77.0 (66.4) | 0.074 |
| HBV (%) | 140.0 (44.0) | 69.0 (44.8) | 0.02 | 59.0 (50.9) | 55.0 (47.4) | 0.069 |
| HCV (%) | 78.0 (24.5) | 59.0 (38.3) | 0.30 | 33.0 (28.4) | 36.0 (31.0) | 0.057 |
| HCC (%) | 154.0 (48.4) | 44.0 (28.6) | 0.42 | 42.0 (36.2) | 43.0 (37.1) | 0.018 |
| baseline proteinuria > 1+ (%) | 39.0 (12.3) | 9.0 (5.8) | 0.23 | 8.0 (6.90) | 9.0 (7.8) | 0.033 |
| MELD score (mean (SD)) | 13.8 (7.6) | 13.5 (6.9) | 0.05 | 14.1 (7.6) | 13.6 (7.3) | 0.071 |
| BMI (mean (SD)) | 25.55 (3.9) | 24.5 (4.3) | 0.25 | 25.0 (4.0) | 24.9 (4.5) | 0.025 |
| baseline albumin (mean (SD)) | 3.2 (0.60) | 3.06 (0.61) | 0.28 | 3.1 (0.5) | 3.2 (0.6) | 0.037 |
| baseline Hb (mean (SD)) | 10.5 (2.3) | 10.3 (2.2) | 0.10 | 10.6 (2.2) | 10.4 (2.3) | 0.099 |
| Outcomes | Event N | Sirolimus (N = 116) | MMF (N = 116) | p Value |
|---|---|---|---|---|
| N(%) | N(%) | |||
| AKD | 114 | 55 (47.4) | 58 (50) | 0.693 |
| advanced AKD | 25 | 13 (11.2) | 12 (10.3) | 0.832 |
| EGFR decline > 30% from baseline | 105 | 65 (56.0) | 40 (34.5) | 0.001 |
| EGFR decline > 50% from baseline | 27 | 23 (19.8) | 4 (3.5) | 0.0001 |
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Variables | B | 95% CI | p Value | B | 95% CI | p Value |
| Treatment | ||||||
| Sirolimus | −7.03 | (−10.39, −3.68) | <0.0001 | −4.37 | (−6.89, −1.85) | 0.0007 |
| Mycophenolate mofetil | Ref | Ref | ||||
| TIME | −0.37 | (−0.48, −0.25) | <0.0001 | −0.46 | (−0.54, −0.37) | <0.0001 |
| Sirolimus * TIME | −0.12 | (−0.28, 0.03) | 0.1179 | −0.18 | (−0.30, −0.06) | 0.0040 |
| Mycophenolate mofetil * TIME | Ref | Ref | ||||
| Baseline proteinuria | −4.99 | (−8.05, −1.92) | 0.0016 | |||
| HBV | −0.20 | (−2.08, 1.68) | 0.8306 | |||
| HCV | 1.97 | (−0.12, 4.06) | 0.0648 | |||
| HCC | 1.03 | (−0.88, 2.94) | 0.2884 | |||
| AGE | −0.38 | (−0.46, −0.29) | <0.0001 | |||
| MELD | −0.12 | (−0.24, 0.00) | 0.0453 | |||
| baseline eGFR group | 18.67 | (16.73, 20.61) | <0.0001 | |||
| Tacrolimus 6 m trough | 0.22 | (−0.13, 0.57) | 0.2077 | |||
| Tacrolimus index date trough | 0.90 | (0.44, 1.36) | 0.0001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, H.-Y.; Li, L.-C.; Hsu, C.-N.; Lin, C.-C.; Chan, Y.-C.; Wang, C.-C.; Chen, C.-L. Impact of Sirolimus versus Mycophenolate Mofetil on Kidney Function after Calcineurin Inhibitor Dose Reduction in Liver Transplant Recipients. Pharmaceuticals 2023, 16, 1087. https://doi.org/10.3390/ph16081087
Chiang H-Y, Li L-C, Hsu C-N, Lin C-C, Chan Y-C, Wang C-C, Chen C-L. Impact of Sirolimus versus Mycophenolate Mofetil on Kidney Function after Calcineurin Inhibitor Dose Reduction in Liver Transplant Recipients. Pharmaceuticals. 2023; 16(8):1087. https://doi.org/10.3390/ph16081087
Chicago/Turabian StyleChiang, Heng-Yi, Lung-Chih Li, Chien-Ning Hsu, Chih-Che Lin, Yi-Chia Chan, Chih-Chi Wang, and Chao-Long Chen. 2023. "Impact of Sirolimus versus Mycophenolate Mofetil on Kidney Function after Calcineurin Inhibitor Dose Reduction in Liver Transplant Recipients" Pharmaceuticals 16, no. 8: 1087. https://doi.org/10.3390/ph16081087
APA StyleChiang, H.-Y., Li, L.-C., Hsu, C.-N., Lin, C.-C., Chan, Y.-C., Wang, C.-C., & Chen, C.-L. (2023). Impact of Sirolimus versus Mycophenolate Mofetil on Kidney Function after Calcineurin Inhibitor Dose Reduction in Liver Transplant Recipients. Pharmaceuticals, 16(8), 1087. https://doi.org/10.3390/ph16081087







