From Bench to Bedside: Implications of Lipid Nanoparticle Carrier Reactogenicity for Advancing Nucleic Acid Therapeutics
Abstract
1. Introduction
2. Exploring LNPs as Xenobiotics in Reactogenic Responses
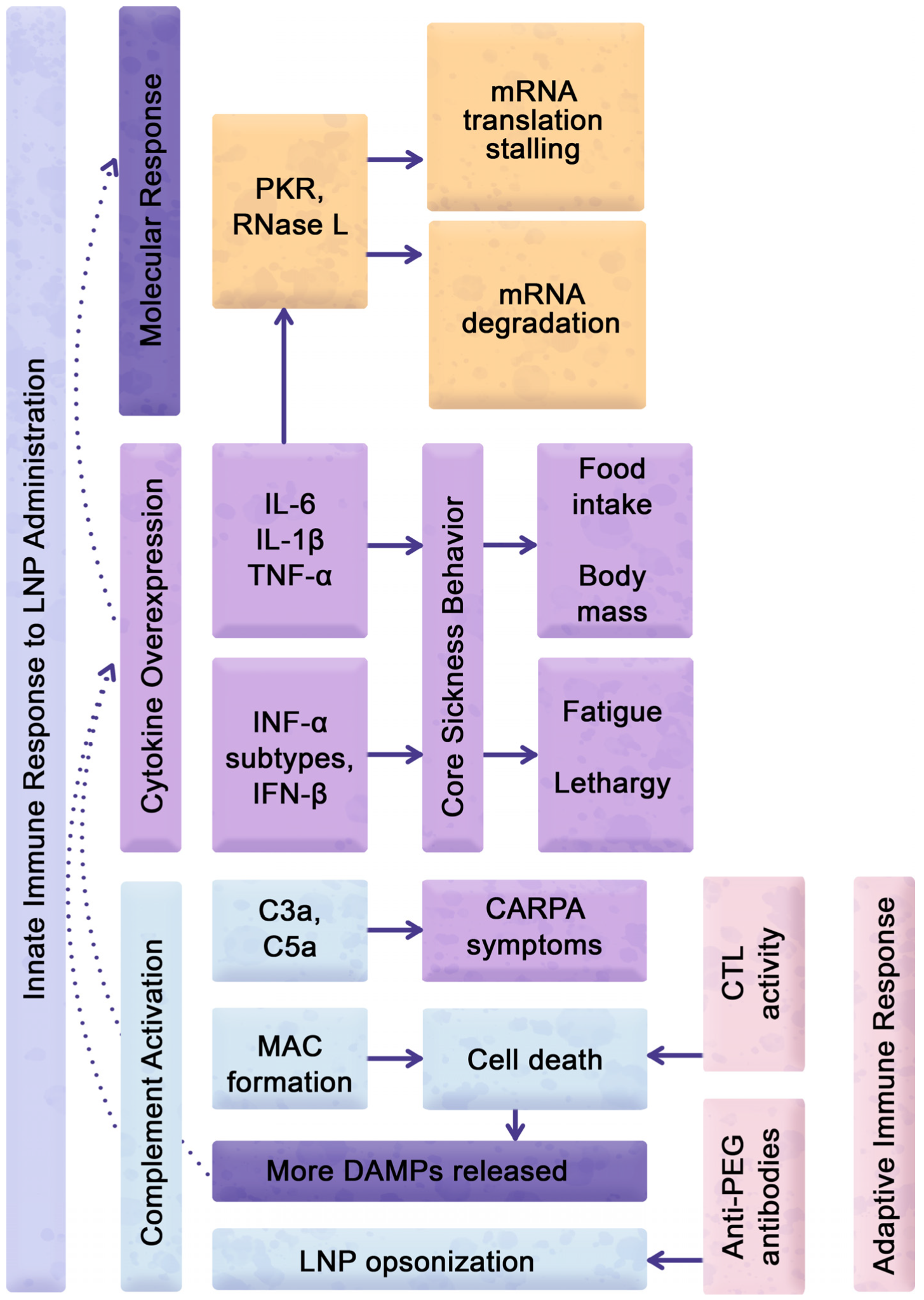
3. Assessment of Reactogenic Manifestations Following LNP Administration
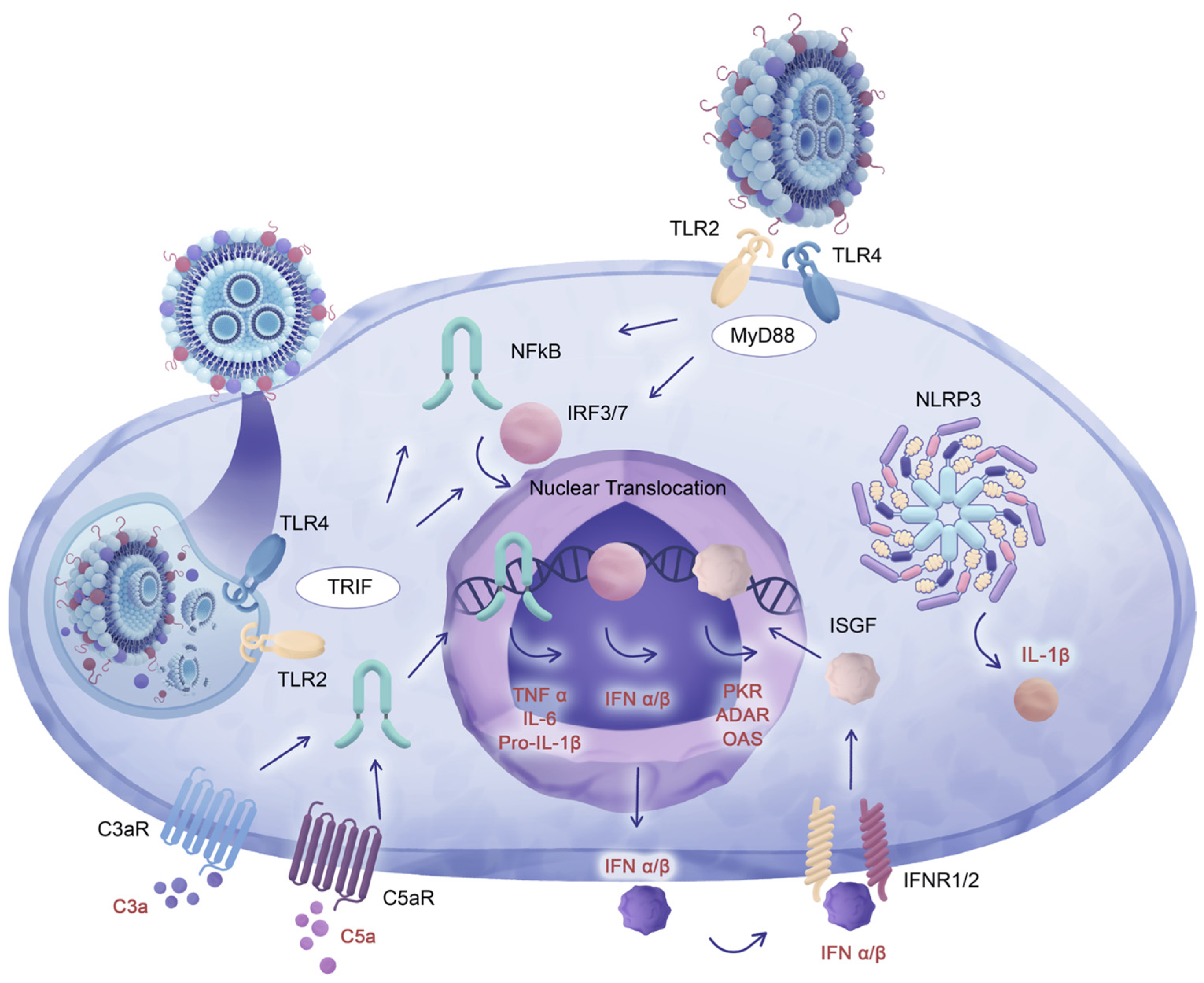
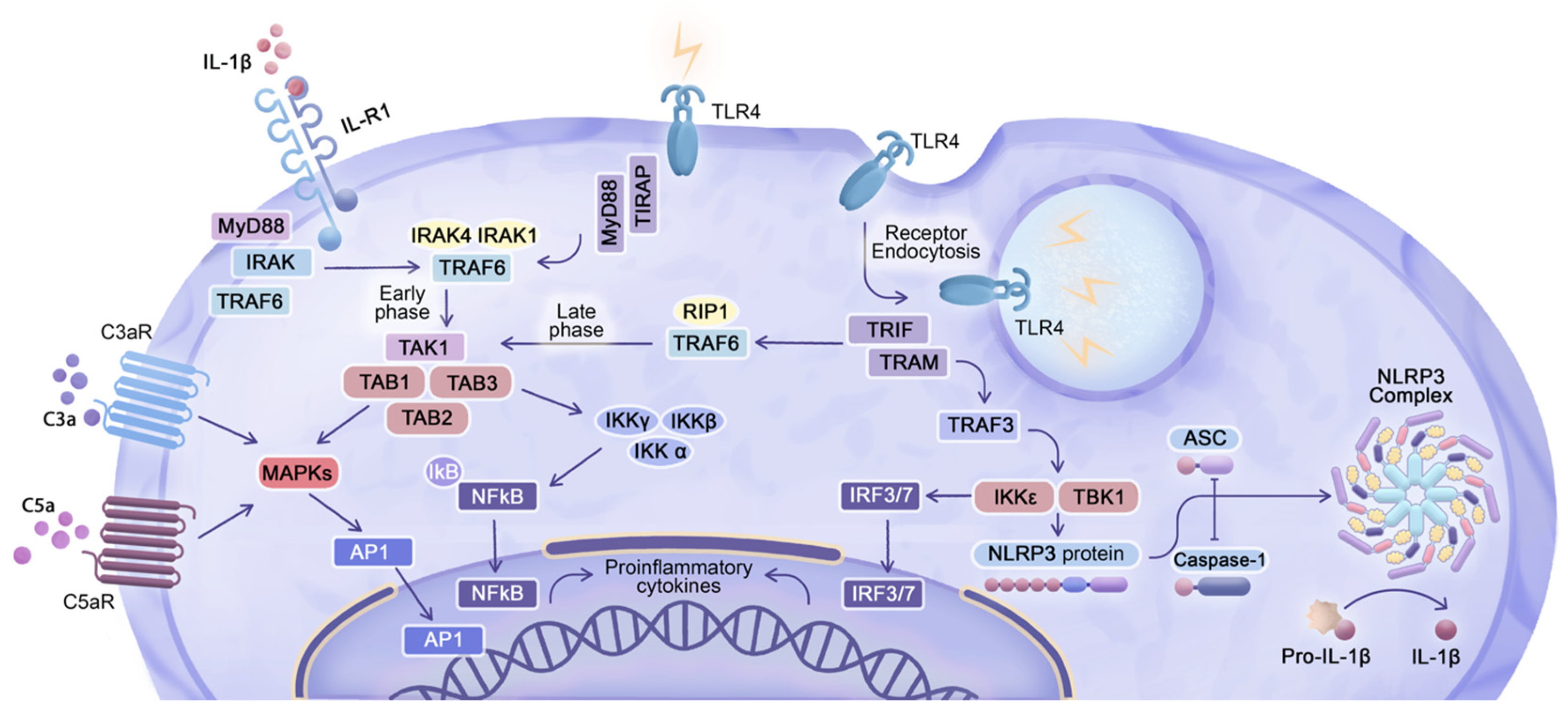
4. Cellular and Molecular Responses to LNP Carriers
5. Enhanced Cytokine Gene Expression in Response to eLNP Administration
6. LNP-Inducible Expression of Cytokines Modulating Sickness Behavior
7. Reactogenicity Interference with Translation of mRNA Delivered by LNP Carriers
8. Reactogenicity Interference with Multiple Injections of Lipid Nanoparticle Formulations
9. Conclusions and Next Steps in eLNP Reactogenicity Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP-1 | Activator Protein 1 |
| ASC | Apoptosis-Associated Speck-Like Protein Containing CARD |
| C3a | Complement Component 3a |
| C3aR | C3a Receptor |
| C5a | Complement Component 5a |
| C5aR | C5a Receptor |
| CARPA | Complement-Activation-Related Pseudoallergy |
| CTL | Cytotoxic T Lymphocytes |
| DAMPs | Danger-Associated Molecular Patterns |
| dsRNA | Double-stranded RNA |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| eLNP | Empty Lipid Nanoparticle |
| eIF2 | Eukaryotic Initiation Factor 2 |
| FDA | Food and Drug Administration |
| GFP | Green Fluorescent Protein |
| IFN | Interferon |
| IL | Interleukin |
| IL-1β | Interleukin 1 β |
| IL-1R | Interleukin-1 Receptor |
| IL-6 | Interleukin 6 |
| IRAKs | Interleukin-1-Receptor-Associated Kinases |
| IRF | Interferon Regulatory Factor |
| KO | Knock-Out |
| LNP | Lipid Nanoparticle |
| MAC | Membrane Attack Complex |
| MAPKs | Mitogen-Activated Protein Kinases |
| MC3 | Dlin-MC3-DMA |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| N/P ratio | Nitrogen-to-phosphate ratio |
| NLRP3 | NOD-, LRR-, and Pyrin Domain-Containing Protein 3 |
| NF-κB | Nuclear Factor-Kappa B |
| PAMP | Pathogen-Associated Molecular Pattern |
| PBMCs | Peripheral Blood Mononuclear Cells |
| PEG | Polyethylene Glycol |
| PKR | Protein Kinase R |
| RBC | Red Blood Cell |
| TAK1 | Transforming Growth Factor-β-Activated Kinase 1 |
| TBK1 | TANK-Binding Kinase 1 |
| TLR | Toll-Like Receptor |
| TLR4 | Toll-Like Receptor 4 |
| TRAF6 | TNF-Receptor-Associated Factor 6 |
| TRAM | Translocating Chain-Associating Membrane Protein |
| TRIF | TIR-Domain-Containing Adaptor-Inducing Interferon-β |
| WT | Wild-Type |
| WBC | White Blood Cell |
References
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the Bnt162b2 Mrna COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two Rna-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. Three Decades of Messenger Rna Vaccine Development. Nano Today 2019, 28, 100766. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the Mrna-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 Vaccine Bnt162b1 Elicits Human Antibody and T(H)1 T Cell Responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Sasso, J.M.; Ambrose, B.J.B.; Tenchov, R.; Datta, R.S.; Basel, M.T.; DeLong, R.K.; Zhou, Q.A. The Progress and Promise of RNA Medicine—An Arsenal of Targeted Treatments. J. Med. Chem. 2022, 65, 6975–7015. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An Mrna Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Matsumura, T.; Takano, T.; Takahashi, Y. Immune Responses Related to the Immunogenicity and Reactogenicity of COVID-19 Mrna Vaccines. Int. Immunol. 2022, 35, 213–220. [Google Scholar] [CrossRef]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following Mrna/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.; Shuai, Z.; Ye, D.; Pan, H. New-Onset Autoimmune Phenomena Post-COVID-19 Vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef]
- Ouldali, N.; Bagheri, H.; Salvo, F.; Antona, D.; Pariente, A.; Leblanc, C.; Tebacher, M.; Micallef, J.; Levy, C.; Cohen, R.; et al. Hyper Inflammatory Syndrome Following COVID-19 Mrna Vaccine in Children: A National Post-Authorization Pharmacovigilance Study. Lancet Reg. Health Eur. 2022, 17, 100393. [Google Scholar] [CrossRef]
- Hermann, E.A.; Lee, B.; Balte, P.P.; Xanthakis, V.; Kirkpatrick, B.D.; Cushman, M.; Oelsner, E. Association of Symptoms after COVID-19 Vaccination with Anti–SARS-CoV-2 Antibody Response in the Framingham Heart Study. JAMA Netw. Open 2022, 5, e2237908. [Google Scholar] [CrossRef]
- Sprent, J.; King, C. COVID-19 Vaccine Side Effects: The Positives About Feeling Bad. Sci. Immunol. 2021, 6, eabj9256. [Google Scholar] [CrossRef]
- Anderson, B.R.; Muramatsu, H.; Nallagatla, S.R.; Bevilacqua, P.C.; Sansing, L.H.; Weissman, D.; Karikó, K. Incorporation of Pseudouridine into Mrna Enhances Translation by Diminishing Pkr Activation. Nucleic Acids Res. 2010, 38, 5884–5892. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of Rna Recognition by Toll-Like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of Rna. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Karikó, K.; Ni, H.; Capodici, J.; Lamphier, M.; Weissman, D. mRNA Is an Endogenous Ligand for Toll-Like Receptor 3. J. Biol. Chem. 2004, 279, 12542–12550. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial Delivery Systems for Mrna Vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef]
- Ju, Y.; Carreño, J.M.; Simon, V.; Dawson, K.; Krammer, F.; Kent, S.J. Impact of Anti-Peg Antibodies Induced by SARS-CoV-2 Mrna Vaccines. Nat. Rev. Immunol. 2022, 23, 135–136. [Google Scholar] [CrossRef]
- Hause, A.M.; Baggs, J.; Marquez, P.; Abara, W.E.; Baumblatt, J.; Blanc, P.G.; Su, J.R.; Hugueley, B.; Parker, C.; Myers, T.R.; et al. Safety Monitoring of COVID-19 Mrna Vaccine Second Booster Doses among Adults Aged ≥50 Years—United States, March 29, 2022–July 10, 2022. MMWR 2022, 71, 971. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II Study of COVID-19 RNA Vaccine Bnt162b1 in Adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The Mrna-Lnp Platform’s Lipid Nanoparticle Component Used in Preclinical Vaccine Studies Is Highly Inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef] [PubMed]
- Cafri, G.; Gartner, J.J.; Zaks, T.; Hopson, K.; Levin, N.; Paria, B.C.; Parkhurst, M.R.; Yossef, R.; Lowery, F.J.; Jafferji, M.S.; et al. mRNA Vaccine-Induced Neoantigen-Specific T Cell Immunity in Patients with Gastrointestinal Cancer. J. Clin. Investig. 2020, 130, 5976–5988. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, B.N.; Alfagih, I.M.; Almurshedi, A.S. Lipid Nanoparticles as Delivery Systems for RNA-Based Vaccines. Pharmaceutics 2021, 13, 206. [Google Scholar] [CrossRef]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid Nanoparticles for Nucleic Acid Delivery: Current Perspectives. Adv. Drug Deliv. Rev. 2020, 154–155, 37–63. [Google Scholar] [CrossRef]
- Cheng, X.; Lee, R.J. The Role of Helper Lipids in Lipid Nanoparticles (Lnps) Designed for Oligonucleotide Delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 129–137. [Google Scholar] [CrossRef]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith, J.P.; Hou, S.; Esposito, A.A.; Ketova, T.; Welsher, K.; et al. Naturally-Occurring Cholesterol Analogues in Lipid Nanoparticles Induce Polymorphic Shape and Enhance Intracellular Delivery of Mrna. Nat. Commun. 2020, 11, 983. [Google Scholar] [CrossRef]
- Holland, J.W.; Hui, C.; Cullis, P.R.; Madden, T.D. Poly(Ethylene Glycol)–Lipid Conjugates Regulate the Calcium-Induced Fusion of Liposomes Composed of Phosphatidylethanolamine and Phosphatidylserine. Biochemistry 1996, 35, 2618–2624. [Google Scholar] [CrossRef]
- Kalyanram, P.; Puri, A.; Gupta, A. Thermotropic Effects of Pegylated Lipids on the Stability of Hpph-Encapsulated Lipid Nanoparticles (Lnp). J. Therm. Anal. Calorim. 2022, 147, 6337–6348. [Google Scholar] [CrossRef]
- Judge, A.; McClintock, K.; Phelps, J.R.; MacLachlan, I. Hypersensitivity and Loss of Disease Site Targeting Caused by Antibody Responses to Pegylated Liposomes. Mol. Ther. 2006, 13, 328–337. [Google Scholar] [CrossRef]
- Albertsen, C.H.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The Role of Lipid Components in Lipid Nanoparticles for Vaccines and Gene Therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro Story and the Clinical Translation of Nanomedicines Containing Nucleic Acid-Based Drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ishihara, H. Difference in the Lipid Nanoparticle Technology Employed in Three Approved Sirna (Patisiran) and Mrna (COVID-19 Vaccine) Drugs. Drug Metab. Pharmacokinet. 2021, 41, 100424. [Google Scholar] [CrossRef]
- Paloncýová, M.; Čechová, P.; Šrejber, M.; Kührová, P.; Otyepka, M. Role of Ionizable Lipids in SARS-CoV-2 Vaccines as Revealed by Molecular Dynamics Simulations: From Membrane Structure to Interaction with Mrna Fragments. J. Phys. Chem. Lett. 2021, 12, 11199–11205. [Google Scholar] [CrossRef]
- Suzuki, T.; Suzuki, Y.; Hihara, T.; Kubara, K.; Kondo, K.; Hyodo, K.; Yamazaki, K.; Ishida, T.; Ishihara, H. Peg Shedding-Rate-Dependent Blood Clearance of Pegylated Lipid Nanoparticles in Mice: Faster Peg Shedding Attenuates Anti-Peg Igm Production. Int. J. Pharm. 2020, 588, 119792. [Google Scholar] [CrossRef]
- McSweeney, M.D.; Shen, L.; DeWalle, A.C.; Joiner, J.B.; Ciociola, E.C.; Raghuwanshi, D.; Macauley, M.S.; Lai, S.K. Pre-Treatment with High Molecular Weight Free Peg Effectively Suppresses Anti-Peg Antibody Induction by Peg-Liposomes in Mice. J. Control. Release 2021, 329, 774–781. [Google Scholar] [CrossRef]
- Chen, B.-M.; Cheng, T.-L.; Roffler, S.R. Polyethylene Glycol Immunogenicity: Theoretical, Clinical, and Practical Aspects of Anti-Polyethylene Glycol Antibodies. ACS Nano 2021, 15, 14022–14048. [Google Scholar] [CrossRef]
- Lutz, J.; Lazzaro, S.; Habbeddine, M.; Schmidt, K.E.; Baumhof, P.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Heidenreich, R.; et al. Unmodified mRNA in Lnps Constitutes a Competitive Technology for Prophylactic Vaccines. NPJ Vaccines 2017, 2, 29. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified Mrna Vaccines Protect against Zika Virus Infection. Cell 2017, 168, 1114–1125.e10. [Google Scholar] [CrossRef]
- Freyn, A.W.; da Silva, J.R.; Rosado, V.C.; Bliss, C.M.; Pine, M.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Ferreira, L.C.d.S.; Weissman, D.; et al. A Multi-Targeting, Nucleoside-Modified Mrna Influenza Virus Vaccine Provides Broad Protection in Mice. Mol. Ther. 2020, 28, 1569–1584. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the Mrna-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Roth, N.; Schwendt, K.; Fotin-Mleczek, M.; Mueller, S.O.; Petsch, B. mRNA-Based SARS-CoV-2 Vaccine Candidate Cvncov Induces High Levels of Virus-Neutralising Antibodies and Mediates Protection in Rodents. NPJ Vaccines 2021, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Kalnin, K.V.; Plitnik, T.; Kishko, M.; Zhang, J.; Zhang, D.; Beauvais, A.; Anosova, N.G.; Tibbitts, T.; DiNapoli, J.; Ulinski, G.; et al. Immunogenicity and Efficacy of Mrna COVID-19 Vaccine Mrt5500 in Preclinical Animal Models. NPJ Vaccines 2021, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, Y.; Li, A.; Lin, J.; Hsieh, K.; Schneiderman, Z.; Zhang, P.; Zhu, Y.; Qiu, C.; Kokkoli, E.; et al. Payload Distribution and Capacity of Mrna Lipid Nanoparticles. Nat. Commun. 2022, 13, 5561. [Google Scholar] [CrossRef]
- Long, J.; Yu, C.; Zhang, H.; Cao, Y.; Sang, Y.; Lu, H.; Zhang, Z.; Wang, X.; Wang, H.; Song, G.; et al. Novel Ionizable Lipid Nanoparticles for SARS-CoV-2 Omicron Mrna Delivery. Adv. Healthc. Mater. 2023, 12, 2202590. [Google Scholar] [CrossRef]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression Kinetics of Nucleoside-Modified mRNA Delivered in Lipid Nanoparticles to Mice by Various Routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Röhss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient Targeting and Activation of Antigen-Presenting Cells In vivo after Modified Mrna Vaccine Administration in Rhesus Macaques. Mol. Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef]
- Sedic, M.; Senn, J.J.; Lynn, A.; Laska, M.; Smith, M.; Platz, S.J.; Bolen, J.; Hoge, S.; Bulychev, A.; Jacquinet, E.; et al. Safety Evaluation of Lipid Nanoparticle-Formulated Modified Mrna in the Sprague-Dawley Rat and Cynomolgus Monkey. Vet. Pathol. 2018, 55, 341–354. [Google Scholar] [CrossRef]
- Connors, J.; Joyner, D.; Mege, N.J.; Cusimano, G.M.; Bell, M.R.; Marcy, J.; Taramangalam, B.; Kim, K.M.; Lin, P.J.C.; Tam, Y.K.; et al. Lipid Nanoparticles (Lnp) Induce Activation and Maturation of Antigen Presenting Cells in Young and Aged Individuals. Commun. Biol. 2023, 6, 188. [Google Scholar] [CrossRef]
- Maier, M.A.; Jayaraman, M.; Matsuda, S.; Liu, J.; Barros, S.; Querbes, W.; Tam, Y.K.; Ansell, S.M.; Kumar, V.; Qin, J.; et al. Biodegradable Lipids Enabling Rapidly Eliminated Lipid Nanoparticles for Systemic Delivery of Rnai Therapeutics. Mol. Ther. 2013, 21, 1570–1578. [Google Scholar] [CrossRef]
- Alameh, M.-G.; Tombácz, I.; Bettini, E.; Lederer, K.; Ndeupen, S.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; et al. Lipid Nanoparticles Enhance the Efficacy of Mrna and Protein Subunit Vaccines by Inducing Robust T Follicular Helper Cell and Humoral Responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Tahtinen, S.; Tong, A.-J.; Himmels, P.; Oh, J.; Paler-Martinez, A.; Kim, L.; Wichner, S.; Oei, Y.; McCarron, M.J.; Freund, E.C.; et al. Il-1 and Il-1ra Are Key Regulators of the Inflammatory Response to Rna Vaccines. Nat. Immunol. 2022, 23, 532–542. [Google Scholar] [CrossRef]
- Qin, Z.; Bouteau, A.; Herbst, C.; Igyártó, B.Z. Pre-Exposure to Mrna-Lnp Inhibits Adaptive Immune Responses and Alters Innate Immune Fitness in an Inheritable Fashion. PLoS Pathog. 2022, 18, e1010830. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-Modified Mrna Vaccines Induce Potent T Follicular Helper and Germinal Center B Cell Responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef]
- Parhiz, H.; Brenner, J.S.; Patel, P.N.; Papp, T.E.; Shahnawaz, H.; Li, Q.; Shi, R.; Zamora, M.E.; Yadegari, A.; Marcos-Contreras, O.A.; et al. Added to Pre-Existing Inflammation, Mrna-Lipid Nanoparticles Induce Inflammation Exacerbation (Ie). J. Control. Release 2022, 344, 50–61. [Google Scholar] [CrossRef]
- Bradley, A.J.; Brooks, D.E.; Norris-Jones, R.; Devine, D.V. C1q Binding to Liposomes Is Surface Charge Dependent and Is Inhibited by Peptides Consisting of Residues 14-26 of the Human C1qa Chain in a Sequence Independent Manner. Biochim. Biophys. Acta 1999, 1418, 19–30. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hamad, I. Liposome-Mediated Triggering of Complement Cascade. J. Liposome Res. 2008, 18, 195–209. [Google Scholar] [CrossRef]
- Szebeni, J.; Baranyi, L.; Savay, S.; Milosevits, J.; Bodo, M.; Bunger, R.; Alving, C.R. The Interaction of Liposomes with the Complement System: In Vitro and in Vivo Assays. Methods Enzymol. 2003, 373, 136–154. [Google Scholar] [CrossRef]
- Szebeni, J.; Wassef, N.; Hartman, K.; Rudolph, A.; Alving, C. Complement Activation in Vitro by the Red Cell Substitute, Liposome-Encapsulated Hemoglobin: Mechanism of Activation and Inhibition by Soluble Complement Receptor Type 1. Transfusion 1997, 37, 150–159. [Google Scholar] [CrossRef]
- Neun, B.W.; Ilinskaya, A.N.; Dobrovolskaia, M.A. Analysis of Complement Activation by Nanoparticles. Methods Mol. Biol. 2018, 1682, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Pannuzzo, M.; Esposito, S.; Wu, L.-P.; Key, J.; Aryal, S.; Celia, C.; Di Marzio, L.; Moghimi, S.M.; Decuzzi, P. Overcoming Nanoparticle-Mediated Complement Activation by Surface Peg Pairing. Nano Lett. 2020, 20, 4312–4321. [Google Scholar] [CrossRef] [PubMed]
- Alving, C.R.; Swartz, G.M., Jr. Antibodies to Cholesterol, Cholesterol Conjugates and Liposomes: Implications for Atherosclerosis and Autoimmunity. Crit. Rev. Immunol. 1991, 10, 441–453. [Google Scholar] [PubMed]
- Alving, C.R.; Kinsky, S.C.; Haxby, J.A.; Kinsky, C.B. Antibody Binding and Complement Fixation by a Liposomal Model Membrane. Biochemistry 1969, 8, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- La-Beck, N.M.; Islam, R.; Markiewski, M.M. Nanoparticle-Induced Complement Activation: Implications for Cancer Nanomedicine. Front. Immunol. 2020, 11, 603039. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H.; El-Boubbou, K.; Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; et al. Clearance Properties of Nano-Sized Particles and Molecules as Imaging Agents: Considerations and Caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef]
- Sturgill, M.G.; Lambert, G.H. Xenobiotic-Induced Hepatotoxicity: Mechanisms of Liver Injury and Methods of Monitoring Hepatic Function. Clin. Chem. 1997, 43, 1512–1526. [Google Scholar] [CrossRef]
- Werner, M.; Costa, M.J.; Mitchell, L.G.; Nayar, R. Nephrotoxicity of Xenobiotics. Clin. Chim. Acta 1995, 237, 107–154. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte Recruitment during Infection and Inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Beyrau, M.; Bodkin, J.V.; Nourshargh, S. Neutrophil Heterogeneity in Health and Disease: A Revitalized Avenue in Inflammation and Immunity. Open Biol. 2012, 2, 120134. [Google Scholar] [CrossRef]
- Eash, K.J.; Greenbaum, A.M.; Gopalan, P.K.; Link, D.C. Cxcr2 and Cxcr4 Antagonistically Regulate Neutrophil Trafficking from Murine Bone Marrow. J. Clin. Investig. 2010, 120, 2423–2431. [Google Scholar] [CrossRef]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil Kinetics in Health and Disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef]
- Köhler, A.; De Filippo, K.; Hasenberg, M.; van den Brandt, C.; Nye, E.; Hosking, M.P.; Lane, T.E.; Männ, L.; Ransohoff, R.M.; Hauser, A.E.; et al. G-Csf-Mediated Thrombopoietin Release Triggers Neutrophil Motility and Mobilization from Bone Marrow via Induction of Cxcr2 Ligands. Blood 2011, 117, 4349–4357. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Hsu, C.-Y.; Aljuffali, I.A.; Chen, C.-H.; Chang, Y.-T.; Fang, J.-Y. Cationic Liposomes Evoke Proinflammatory Mediator Release and Neutrophil Extracellular Traps (Nets) toward Human Neutrophils. Colloids Surf. B Biointerfaces 2015, 128, 119–126. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Aljuffali, I.A.; Hung, C.-F.; Chen, C.-H.; Fang, J.-Y. The Impact of Cationic Solid Lipid Nanoparticles on Human Neutrophil Activation and Formation of Neutrophil Extracellular Traps (Nets). Chem. Biol. Interact. 2015, 235, 106–114. [Google Scholar] [CrossRef]
- Lin, M.-H.; Lin, C.-F.; Yang, S.-C.; Hung, C.-F.; Fang, J.-Y. The Interplay between Nanoparticles and Neutrophils. J. Biomed. Nanotechnol. 2018, 14, 66–85. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Z.-T.; Xu, C.-F.; Lu, Z.-D.; Luo, Y.-L.; Wang, J. Optimization of Lipid-Assisted Nanoparticle for Disturbing Neutrophils-Related Inflammation. Biomaterials 2018, 172, 92–104. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage Biology in Development, Homeostasis and Disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Serbina, N.V.; Jia, T.; Hohl, T.M.; Pamer, E.G. Monocyte-Mediated Defense against Microbial Pathogens. Annu. Rev. Immunol. 2008, 26, 421–452. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A Guide to Chemokines and Their Receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.E., III; Peppas, N.A. Opsonization, Biodistribution, and Pharmacokinetics of Polymeric Nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Akashi, T.; Tsujii, Y.; Yamamoto, M.; Tabata, Y. Blood Clearance and Biodistribution of Polymer Brush-Afforded Silica Particles Prepared by Surface-Initiated Living Radical Polymerization. Biomacromolecules 2012, 13, 927–936. [Google Scholar] [CrossRef]
- Cai, D.; Gao, W.; Li, Z.; Zhang, Y.; Xiao, L.; Xiao, Y. Current Development of Nano-Drug Delivery to Target Macrophages. Biomedicines 2022, 10, 1203. [Google Scholar] [CrossRef]
- Lonez, C.; Bessodes, M.; Scherman, D.; Vandenbranden, M.; Escriou, V.; Ruysschaert, J.-M. Cationic Lipid Nanocarriers Activate Toll-Like Receptor 2 and Nlrp3 Inflammasome Pathways. Nanomedicine 2014, 10, 775–782. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Bowie, A.G. The Family of Five: Tir-Domain-Containing Adaptors in Toll-Like Receptor Signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef]
- Murphy, J.E.; Padilla, B.E.; Hasdemir, B.; Cottrell, G.S.; Bunnett, N.W. Endosomes: A Legitimate Platform for the Signaling Train. Proc. Natl. Acad. Sci. USA 2009, 106, 17615–17622. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of Adaptor Trif in the Myd88-Independent Toll-Like Receptor Signaling Pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome Maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef]
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief Update on Endocytosis of Nanomedicines. Adv. Drug Deliv. Rev. 2019, 144, 90–111. [Google Scholar] [CrossRef]
- Herrera, M.; Kim, J.; Eygeris, Y.; Jozic, A.; Sahay, G. Illuminating Endosomal Escape of Polymorphic Lipid Nanoparticles That Boost Mrna Delivery. Biomater. Sci. 2021, 9, 4289–4300. [Google Scholar] [CrossRef]
- Hui, S.; Langner, M.; Zhao, Y.; Ross, P.; Hurley, E.; Chan, K. The Role of Helper Lipids in Cationic Liposome-Mediated Gene Transfer. Biophys. J. 1996, 71, 590–599. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Stirling, D.C.; Wang, Z.; Flight, K.E.; Brown, J.C.; Blakney, A.K.; McKay, P.F.; Cunliffe, R.F.; Murugaiah, V.; Fox, C.B.; et al. Formulation, Inflammation, and Rna Sensing Impact the Immunogenicity of Self-Amplifying Rna Vaccines. Mol. Ther. Nucleic Acids 2023, 31, 29–42. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Sen, G.C. dsRNA-activation of TLR3 and RLR signaling: Gene induction-dependent and independent effects. J. Interferon Cytokine Res. 2014, 34, 427–436. [Google Scholar] [CrossRef]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef]
- Noris, M.; Remuzzi, G. Overview of Complement Activation and Regulation. Semin. Nephrol. 2013, 33, 479–492. [Google Scholar] [CrossRef]
- Szebeni, J.; Muggia, F.; Gabizon, A.; Barenholz, Y. Activation of Complement by Therapeutic Liposomes and Other Lipid Excipient-Based Therapeutic Products: Prediction and Prevention. Adv. Drug Deliv. Rev. 2011, 63, 1020–1030. [Google Scholar] [CrossRef]
- Aksamit, R.R.; Falk, W.; Leonard, E.J. Chemotaxis by Mouse Macrophage Cell Lines. J. Immunol. 1981, 126, 2194–2199. [Google Scholar] [CrossRef]
- Lett-Brown, M.A.; Leonard, E.J. Histamine-Induced Inhibition of Normal Human Basophil Chemotaxis to C5a. J. Immunol. 1977, 118, 815–818. [Google Scholar] [CrossRef]
- Peng, Q.; Li, K.; Patel, H.; Sacks, S.H.; Zhou, W. Dendritic Cell Synthesis of C3 Is Required for Full T Cell Activation and Development of a Th1 Phenotype. J. Immunol. 2006, 176, 3330–3341. [Google Scholar] [CrossRef] [PubMed]
- Ehrengruber, M.U.; Geiser, T.; Deranleau, D.A. Activation of Human Neutrophils by C3a and C5a. Comparison of the Effects on Shape Changes, Chemotaxis, Secretion, and Respiratory Burst. FEBS Lett. 1994, 346, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Lalli, P.N.; Strainic, M.G.; Lin, F.; Medof, M.E.; Heeger, P.S. Decay Accelerating Factor Can Control T Cell Differentiation into Ifn-Gamma-Producing Effector Cells via Regulating Local C5a-Induced Il-12 Production. J. Immunol. 2007, 179, 5793–5802. [Google Scholar] [CrossRef] [PubMed]
- Markiewski, M.M.; DeAngelis, R.A.; Benencia, F.; Ricklin-Lichtsteiner, S.K.; Koutoulaki, A.; Gerard, C.; Coukos, G.; Lambris, J.D. Modulation of the Antitumor Immune Response by Complement. Nat. Immunol. 2008, 9, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Henz, B.M.; Krüger-Krasagakes, S.; Köhl, J.; Burger, R.; Guhl, S.; Haase, I.; Lippert, U.; Zuberbier, T. C3a and C5a Stimulate Chemotaxis of Human Mast Cells. Blood 1997, 89, 2863–2870. [Google Scholar] [CrossRef]
- Asgari, E.; Le Friec, G.; Yamamoto, H.; Perucha, E.; Sacks, S.S.; Köhl, J.; Cook, H.T.; Kemper, C. C3a Modulates Il-1β Secretion in Human Monocytes by Regulating Atp Efflux and Subsequent Nlrp3 Inflammasome Activation. Blood 2013, 122, 3473–3481. [Google Scholar] [CrossRef]
- Takabayashi, T.; Vannier, E.; Clark, B.D.; Margolis, N.H.; Dinarello, C.A.; Burke, J.F.; Gelfand, J.A. A New Biologic Role for C3a and C3a Desarg: Regulation of Tnf-Alpha and Il-1 Beta Synthesis. J. Immunol. 1996, 156, 3455–3460. [Google Scholar] [CrossRef]
- Kumar, S.; Basu, M.; Ghosh, P.; Ansari, A.; Ghosh, M.K. COVID-19: Clinical Status of Vaccine Development to Date. Br. J. Clin. Pharmacol. 2023, 89, 114–149. [Google Scholar] [CrossRef]
- Hervé, C.; Laupeze, B.; Del Giudice, G.; Didierlaurent, A.M.; Da Silva, F.T. The How’s and What’s of Vaccine Reactogenicity. NPJ Vaccines 2019, 4, 39. [Google Scholar] [CrossRef]
- Hayden, M.S.; West, A.P.; Ghosh, S. Nf-Kappab and the Immune Response. Oncogene 2006, 25, 6758–6780. [Google Scholar] [CrossRef]
- Negishi, H.; Taniguchi, T.; Yanai, H. The Interferon (Ifn) Class of Cytokines and the Ifn Regulatory Factor (Irf) Transcription Factor Family. Cold Spring Harb. Perspect. Biol. 2018, 10, a028423. [Google Scholar] [CrossRef]
- Tanaka, T.; Legat, A.; Adam, E.; Steuve, J.; Gatot, J.-S.; Vandenbranden, M.; Ulianov, L.; Lonez, C.; Ruysschaert, J.-M.; Muraille, E.; et al. Dic14-Amidine Cationic Liposomes Stimulate Myeloid Dendritic Cells through Toll-Like Receptor 4. Eur. J. Immunol. 2008, 38, 1351–1357. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Loré, K.; Pardi, N. Innate Immune Mechanisms of Mrna Vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to Nf-Kappab by Toll-Like Receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Medzhitov, R.; Preston-Hurlburt, P.; Kopp, E.; Stadlen, A.; Chen, C.; Ghosh, S.; Janeway, C.A., Jr. Myd88 Is an Adaptor Protein in the Htoll/Il-1 Receptor Family Signaling Pathways. Mol. Cell 1998, 2, 253–258. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The Nlrp3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Frenois, F.; Moreau, M.; O’connor, J.; Lawson, M.; Micon, C.; Lestage, J.; Kelley, K.W.; Dantzer, R.; Castanon, N. Lipopolysaccharide Induces Delayed Fosb/Deltafosb Immunostaining within the Mouse Extended Amygdala, Hippocampus and Hypothalamus, That Parallel the Expression of Depressive-Like Behavior. Psychoneuroendocrinology 2007, 32, 516–531. [Google Scholar] [CrossRef]
- Burfeind, K.G.; Michaelis, K.A.; Marks, D.L. The Central Role of Hypothalamic Inflammation in the Acute Illness Response and Cachexia. Semin. Cell Dev. Biol. 2016, 54, 42–52. [Google Scholar] [CrossRef]
- Engström, L.; Ruud, J.; Eskilsson, A.; Larsson, A.; Mackerlova, L.; Kugelberg, U.; Qian, H.; Vasilache, A.M.; Larsson, P.; Engblom, D.; et al. Lipopolysaccharide-Induced Fever Depends on Prostaglandin E2 Production Specifically in Brain Endothelial Cells. Endocrinology 2012, 153, 4849–4861. [Google Scholar] [CrossRef]
- Timper, K.; Denson, J.L.; Steculorum, S.M.; Heilinger, C.; Engström-Ruud, L.; Wunderlich, C.M.; Rose-John, S.; Wunderlich, F.T.; Brüning, J.C. Il-6 Improves Energy and Glucose Homeostasis in Obesity via Enhanced Central Il-6 Trans-Signaling. Cell Rep. 2017, 19, 267–280. [Google Scholar] [CrossRef]
- Wallenius, K.; Wallenius, V.; Sunter, D.; Dickson, S.L.; Jansson, J.-O. Intracerebroventricular Interleukin-6 Treatment Decreases Body Fat in Rats. Biochem. Biophys. Res. Commun. 2002, 293, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.; Swarbrick, M.; Rose-John, S.; Rincon, M.; Robertson, G.; et al. A Switch from White to Brown Fat Increases Energy Expenditure in Cancer-Associated Cachexia. Cell Metab. 2014, 20, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Wallenius, V.; Wallenius, K.; Ahrén, B.; Rudling, M.; Carlsten, H.; Dickson, S.L.; Ohlsson, C.; Jansson, J.-O. Interleukin-6-Deficient Mice Develop Mature-Onset Obesity. Nat. Med. 2002, 8, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Nov, O.; Shapiro, H.; Ovadia, H.; Tarnovscki, T.; Dvir, I.; Shemesh, E.; Kovsan, J.; Shelef, I.; Carmi, Y.; Voronov, E.; et al. Interleukin-1β Regulates Fat-Liver Crosstalk in Obesity by Auto-Paracrine Modulation of Adipose Tissue Inflammation and Expandability. PLoS ONE 2013, 8, e53626. [Google Scholar] [CrossRef]
- Konsman, J.P.; Parnet, P.; Dantzer, R. Cytokine-Induced Sickness Behaviour: Mechanisms and Implications. Trends Neurosci. 2002, 25, 154–159. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Han, M.S.; White, A.; Perry, R.J.; Camporez, J.-P.; Hidalgo, J.; Shulman, G.I.; Davis, R.J. Regulation of Adipose Tissue Inflammation by Interleukin 6. Proc. Natl. Acad. Sci. USA 2020, 117, 2751–2760. [Google Scholar] [CrossRef]
- Kistner, T.M.; Pedersen, B.K.; Lieberman, D.E. Interleukin 6 as an Energy Allocator in Muscle Tissue. Nat. Metab. 2022, 4, 170–179. [Google Scholar] [CrossRef]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous Adipose Tissue Releases Interleukin-6, but Not Tumor Necrosis Factor-A, in Vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar] [CrossRef]
- López-Ferreras, L.; Longo, F.; Richard, J.E.; Eerola, K.; Shevchouk, O.T.; Tuzinovic, M.; Skibicka, K.P. Key Role for Hypothalamic Interleukin-6 in Food-Motivated Behavior and Body Weight Regulation. Psychoneuroendocrinology 2021, 131, 105284. [Google Scholar] [CrossRef]
- A Dinarello, C. Biologic Basis for Interleukin-1 in Disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef]
- Ren, K.; Torres, R. Role of Interleukin-1beta during Pain and Inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef]
- Ahima, R.S.; Antwi, D.A. Brain Regulation of Appetite and Satiety. Endocrinol. Metab. Clin. N. Am. 2008, 37, 811–823. [Google Scholar] [CrossRef]
- Katahira, M.; Iwasaki, Y.; Aoki, Y.; Oiso, Y.; Saito, H. Cytokine Regulation of the Rat Proopiomelanocortin Gene Expression in Att-20 Cells. Endocrinology 1998, 139, 2414–2422. [Google Scholar] [CrossRef]
- Pereda, M.P.; Lohrer, P.; Kovalovsky, D.; Castro, C.P.; Goldberg, V.; Losa, M.; Chervín, A.; Berner, S.; Molina, H.; Stalla, G.K.; et al. Interleukin-6 Is Inhibited by Glucocorticoids and Stimulates Acth Secretion and Pomc Expression in Human Corticotroph Pituitary Adenomas. Exp. Clin. Endocrinol. Diabetes 2000, 108, 202–207. [Google Scholar] [CrossRef]
- Laddha, N.C.; Dwivedi, M.; Mansuri, M.S.; Singh, M.; Patel, H.H.; Agarwal, N.; Shah, A.M.; Begum, R. Association of Neuropeptide Y (Npy), Interleukin-1b (Il1b) Genetic Variants and Correlation of Il1b Transcript Levels with Vitiligo Susceptibility. PLoS ONE 2014, 9, e107020. [Google Scholar] [CrossRef]
- Braun, T.P.; Zhu, X.; Szumowski, M.; Scott, G.D.; Grossberg, A.J.; Levasseur, P.R.; Graham, K.; Khan, S.; Damaraju, S.; Colmers, W.F.; et al. Central Nervous System Inflammation Induces Muscle Atrophy via Activation of the Hypothalamic–Pituitary–Adrenal Axis. J. Exp. Med. 2011, 208, 2449–2463. [Google Scholar] [CrossRef]
- Kumar, V. Toll-Like Receptors in Sepsis-Associated Cytokine Storm and Their Endogenous Negative Regulators as Future Immunomodulatory Targets. Int. Immunopharmacol. 2020, 89 Pt B, 107087. [Google Scholar] [CrossRef]
- Yang, T.; Yang, Y.; Wang, D.; Li, C.; Qu, Y.; Guo, J.; Shi, T.; Bo, W.; Sun, Z.; Asakawa, T. The Clinical Value of Cytokines in Chronic Fatigue Syndrome. J. Transl. Med. 2019, 17, 213. [Google Scholar] [CrossRef]
- Trask, P.C.; Paterson, A.G.; Esper, P.; Pau, J.; Redman, B. Longitudinal Course of Depression, Fatigue, and Quality of Life in Patients with High Risk Melanoma Receiving Adjuvant Interferon. Psycho-Oncology 2004, 13, 526–536. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Bender, C.; Agarwala, S.; Tarhini, A.; Shipe-Spotloe, J.; Smelko, B.; Donnelly, S.; Stover, L.; Goh, B.-C.; Lee, S.-C.; et al. Mechanisms and Management of Toxicities Associated with High-Dose Interferon Alfa-2b Therapy. Clin. Oncol. 2002, 20, 3703–3718. [Google Scholar] [CrossRef] [PubMed]
- Malik, U.R.; Makower, D.F.; Wadler, S. Interferon-Mediated Fatigue. CA Cancer J. Clin. 2001, 92, 1664–1668. [Google Scholar] [CrossRef]
- Russell, A.; Hepgul, N.; Nikkheslat, N.; Borsini, A.; Zajkowska, Z.; Moll, N.; Forton, D.; Agarwal, K.; Chalder, T.; Mondelli, V.; et al. Persistent Fatigue Induced by Interferon-Alpha: A Novel, Inflammation-Based, Proxy Model of Chronic Fatigue Syndrome. Psychoneuroendocrinology 2019, 100, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Rönnblom, L.; Leonard, D. Interferon Pathway in Sle: One Key to Unlocking the Mystery of the Disease. Lupus Sci. Med. 2019, 6, e000270. [Google Scholar] [CrossRef]
- Dighriri, I.M.; Alhusayni, K.M.; Mobarki, A.Y.; Aljerary, I.S.; Alqurashi, K.A.; Aljuaid, F.A.; Alamri, K.A.; Mutwalli, A.A.; Maashi, N.A.; Aljohani, A.M.; et al. Pfizer-Biontech COVID-19 Vaccine (Bnt162b2) Side Effects: A Systematic Review. Cureus 2022, 14, e23526. [Google Scholar] [CrossRef]
- Rabail, R.; Ahmed, W.; Ilyas, M.; Rajoka, M.S.R.; Hassoun, A.; Khalid, A.R.; Khan, M.R.; Aadil, R.M. The Side Effects and Adverse Clinical Cases Reported after COVID-19 Immunization. Vaccines 2022, 10, 488. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 Vaccines: Modes of Immune Activation and Future Challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]
- Dalpke, A.H.; Helm, M. RNA Mediated Toll-Like Receptor Stimulation in Health and Disease. RNA Biol. 2012, 9, 828–842. [Google Scholar] [CrossRef]
- Ning, S.; Pagano, J.S.; Barber, G.N. Irf7: Activation, Regulation, Modification and Function. Genes Immun. 2011, 12, 399–414. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. Ifnγ: Signalling, Epigenetics and Roles in Immunity, Metabolism, Disease and Cancer Immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef]
- Delehedde, C.; Even, L.; Midoux, P.; Pichon, C.; Perche, F. Intracellular Routing and Recognition of Lipid-Based Mrna Nanoparticles. Pharmaceutics 2021, 13, 945. [Google Scholar] [CrossRef]
- Lokugamage, M.P.; Gan, Z.; Zurla, C.; Levin, J.; Islam, F.Z.; Kalathoor, S.; Sato, M.; Sago, C.D.; Santangelo, P.J.; Dahlman, J.E. Mild Innate Immune Activation Overrides Efficient Nanoparticle-Mediated Rna Delivery. Adv. Mater. 2020, 32, e1904905. [Google Scholar] [CrossRef]
- Duan, L.-J.; Wang, Q.; Zhang, C.; Yang, D.-X.; Zhang, X.-Y. Potentialities and Challenges of Mrna Vaccine in Cancer Immunotherapy. Front. Immunol. 2022, 13, 923647. [Google Scholar] [CrossRef]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The Clinical Progress of Mrna Vaccines and Immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- García, M.A.; Gil, J.; Ventoso, I.; Guerra, S.; Domingo, E.; Rivas, C.; Esteban, M. Impact of Protein Kinase Pkr in Cell Biology: From Antiviral to Antiproliferative Action. Microbiol. Mol. Biol. Rev. 2006, 70, 1032–1060. [Google Scholar] [CrossRef]
- Goh, K.C.; Deveer, M.J.; Williams, B.R. The Protein Kinase Pkr Is Required for P38 Mapk Activation and the Innate Immune Response to Bacterial Endotoxin. EMBO J. 2000, 19, 4292–4297. [Google Scholar] [CrossRef]
- Ito, T.; Yang, M.; May, W.S. Rax, a Cellular Activator for Double-Stranded RNA-Dependent Protein Kinase during Stress Signaling. J. Biol. Chem. 1999, 274, 15427–15432. [Google Scholar] [CrossRef]
- Nakamura, T.; Furuhashi, M.; Li, P.; Cao, H.; Tuncman, G.; Sonenberg, N.; Gorgun, C.Z.; Hotamisligil, G.S. Double-Stranded RNA-Dependent Protein Kinase Links Pathogen Sensing with Stress and Metabolic Homeostasis. Cell 2010, 140, 338–348. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Liu, M.; Zhu, D.; Chen, S.; Zhang, S.; et al. The Role of Host Eif2α in Viral Infection. Virol. J. 2020, 17, 112. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Wek, S.A.; McGrath, B.C.; Scheuner, D.; Kaufman, R.J.; Cavener, D.R.; Wek, R.C.; Rademakers, S.; Volker, M.; Hoogstraten, D.; et al. Phosphorylation of the Alpha Subunit of Eukaryotic Initiation Factor 2 Is Required for Activation of Nf-Kappab in Response to Diverse Cellular Stresses. Mol. Cell. Biol. 2003, 23, 5651–5663. [Google Scholar] [CrossRef]
- Burke, J.M.; Moon, S.L.; Matheny, T.; Parker, R. Rnase L Reprograms Translation by Widespread Mrna Turnover Escaped by Antiviral Mrnas. Mol. Cell 2019, 75, 1203–1217.e5. [Google Scholar] [CrossRef] [PubMed]
- Gusho, E.; Baskar, D.; Banerjee, S. New Advances in Our Understanding of the “Unique” Rnase L in Host Pathogen Interaction and Immune Signaling. Cytokine 2020, 133, 153847. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.M.; Ripin, N.; Ferretti, M.B.; Clair, L.A.S.; Worden-Sapper, E.R.; Salgado, F.; Sawyer, S.L.; Perera, R.; Lynch, K.W.; Parker, R. Rnase L Activation in the Cytoplasm Induces Aberrant Processing of Mrnas in the Nucleus. PLoS Pathog. 2022, 18, e1010930. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Ferguson, T.; Zitvogel, L.; Kroemer, G. Immunogenic and Tolerogenic Cell Death. Nat. Rev. Immunol. 2009, 9, 353–363. [Google Scholar] [CrossRef]
- Dunkelberger, J.R.; Song, W.-C. Complement and Its Role in Innate and Adaptive Immune Responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Szebeni, J. Complement Activation-Related Pseudoallergy: A New Class of Drug-Induced Acute Immune Toxicity. Toxicology 2005, 216, 106–121. [Google Scholar] [CrossRef]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-Peg Antibodies: Properties, Formation, Testing and Role in Adverse Immune Reactions to Pegylated Nano-Biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef]
- Szebeni, J.; Simberg, D.; González-Fernández, A.; Barenholz, Y.; Dobrovolskaia, M.A. Roadmap and Strategy for Overcoming Infusion Reactions to Nanomedicines. Nat. Nanotechnol. 2018, 13, 1100–1108. [Google Scholar] [CrossRef]
- Ju, Y.; Lee, W.S.; Pilkington, E.H.; Kelly, H.G.; Li, S.; Selva, K.J.; Wragg, K.M.; Subbarao, K.; Nguyen, T.H.O.; Rowntree, L.C.; et al. Anti-Peg Antibodies Boosted in Humans by SARS-CoV-2 Lipid Nanoparticle Mrna Vaccine. ACS Nano 2022, 16, 11769–11780. [Google Scholar] [CrossRef]
- Oberli, M.A.; Reichmuth, A.M.; Dorkin, J.R.; Mitchell, M.J.; Fenton, O.S.; Jaklenec, A.; Anderson, D.G.; Langer, R.; Blankschtein, D. Lipid Nanoparticle Assisted Mrna Delivery for Potent Cancer Immunotherapy. Nano Lett. 2017, 17, 1326–1335. [Google Scholar] [CrossRef]
- Bevers, S.; Kooijmans, S.A.; Van de Velde, E.; Evers, M.J.; Seghers, S.; Gitz-Francois, J.J.; van Kronenburg, N.C.; Fens, M.H.; Mastrobattista, E.; Hassler, L.; et al. mRNA-Lnp Vaccines Tuned for Systemic Immunization Induce Strong Antitumor Immunity by Engaging Splenic Immune Cells. Mol. Ther. 2022, 30, 3078–3094. [Google Scholar] [CrossRef]
- Dilliard, S.A.; Cheng, Q.; Siegwart, D.J. On the Mechanism of Tissue-Specific Mrna Delivery by Selective Organ Targeting Nanoparticles. Proc. Natl. Acad. Sci. USA 2021, 118, e2109256118. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kato, Y.; Edahiro, R.; Søndergaard, J.N.; Murakami, T.; Amiya, S.; Nameki, S.; Yoshimine, Y.; Morita, T.; Takeshima, Y.; et al. Consecutive Bnt162b2 Mrna Vaccination Induces Short-Term Epigenetic Memory in Innate Immune Cells. J. Clin. Investig. 2022, 7, e163347. [Google Scholar] [CrossRef]


| Category | Common Name | IUPAC Name | Chemical Structure |
|---|---|---|---|
| Ionizable Lipid | Dlin-MC3-DMA a | (6Z,9Z,28Z,31Z)-6,9,28,31-Heptatriacontatetraen-19-yl 4-(dimethylamino)butanoate |  |
| Ionizable Lipid | SM-102 b | 9-Heptadecanyl 8-{(2-hydroxyethyl)[6-oxo-6-(undecyloxy)hexyl]amino}octanoate |  |
| Ionizable Lipid | ALC-0315 c | [(4-Hydroxybutyl)imino]di-6,1-hexanediyl bis(2-hexyldecanoate) |  |
| Helper Lipid | DSPC | (2R)-2,3-Bis(stearoyloxy)propyl 2-(trimethylammonio)ethyl phosphate | 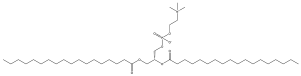 |
| Stabilizing component | Cholesterol | (3β)-Cholest-5-en-3-ol |  |
| Shielding component | PEG | poly(oxyethylene) | 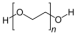 |
| Reference | eLNP Composition | Ionizable Lipid | eLNP Dose |
|---|---|---|---|
| [46] | IL: DSPC: cholesterol: PEG-lipid at a molar ratio of 50:10:38.5:1 | MC3 and YK009 | Not provided; mRNA–LNP dose was equivalent to 10 µg mRNA administered via the IM, SQ, or ID routes |
| [23] | IL: phosphatidylcholine: cholesterol: PEG-lipid at a molar ratio of 50:10:38.5:1.5 as described in [47] | IL under US10221127B2 patent (Acuitas Therapeutics) | 10 μg administered in 4 spots, 2.5 μg/spot, ID, and IV; 10 μg administered IN |
| [48] | Valera LLC, a Moderna Therapeutics Venture, supplied all vaccines. In-house formulation: IL: DSPC: cholesterol: PEG-lipid: GLA at a molar ratio of 50:9.83:38.5:1.5:0.17 | Not discussed | 50 μg administered per site, injected ID |
| [49] | IL: DSPC: cholesterol: PEG-lipid at a molar ratio of 50:10:38.5:1.5 | MC3 ionizable lipid | Equivalent to 0.3 mg/kg mRNA–LNP dose, injected IV |
| [50] | IL: DSPC: cholesterol: PEG-lipid at a molar ratio of 55:10:32.5:2.5 as described in [51] | IL under US10221127B2 patent (Acuitas Therapeutics) | Equivalent to 5 μg/mL total lipids or ~7.5 μg/mL ionizable lipids; eLNP used in in vitro study |
| [52] | The LNP formulation used in this study is proprietary to Acuitas Therapeutics | IL under US10221127 patent (Acuitas Therapeutics) | Total lipid content: 900 μg; equivalent to the lipid content of 30 μg mRNA-LNP ID and IV |
| [53] | IL: DSPC: cholesterol: PEG-lipid at a molar ratio of 50:20:28:2 for the MC3 or 50:10:38.5:1.5 for the SM-102 formulations | MC3 and SM-102 | eLNP doses are not provided; eLNP injected IV |
| [54] | IL: phosphatidylcholine: cholesterol: PEG-lipid at a molar ratio of 50:10:38.5:1.5 mol/mol) as described in [47,55] | IL under US10221127 patent (Acuitas Therapeutics) | Equivalent to the lipid content of 2.5 μg mRNA-LNP injected ID |
| [56] | IL: DSPC: cholesterol: PEG-lipid at a molar ratio of 50:10:38.5:1.5 | MC3 and C12-200 | 2 mg/kg lipids or dose equivalent to 0.32 mg mRNA/kg, injected IV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzun, T.; Moses, A.S.; Diba, P.; Sattler, A.L.; Taratula, O.R.; Sahay, G.; Taratula, O.; Marks, D.L. From Bench to Bedside: Implications of Lipid Nanoparticle Carrier Reactogenicity for Advancing Nucleic Acid Therapeutics. Pharmaceuticals 2023, 16, 1088. https://doi.org/10.3390/ph16081088
Korzun T, Moses AS, Diba P, Sattler AL, Taratula OR, Sahay G, Taratula O, Marks DL. From Bench to Bedside: Implications of Lipid Nanoparticle Carrier Reactogenicity for Advancing Nucleic Acid Therapeutics. Pharmaceuticals. 2023; 16(8):1088. https://doi.org/10.3390/ph16081088
Chicago/Turabian StyleKorzun, Tetiana, Abraham S. Moses, Parham Diba, Ariana L. Sattler, Olena R. Taratula, Gaurav Sahay, Oleh Taratula, and Daniel L. Marks. 2023. "From Bench to Bedside: Implications of Lipid Nanoparticle Carrier Reactogenicity for Advancing Nucleic Acid Therapeutics" Pharmaceuticals 16, no. 8: 1088. https://doi.org/10.3390/ph16081088
APA StyleKorzun, T., Moses, A. S., Diba, P., Sattler, A. L., Taratula, O. R., Sahay, G., Taratula, O., & Marks, D. L. (2023). From Bench to Bedside: Implications of Lipid Nanoparticle Carrier Reactogenicity for Advancing Nucleic Acid Therapeutics. Pharmaceuticals, 16(8), 1088. https://doi.org/10.3390/ph16081088








