Abstract
Due to the increasing limitations and negative impacts of the current options for preventing and managing diseases, including chemotherapeutic drugs and radiation, alternative therapies are needed, especially ones utilizing and maximizing natural products (NPs). NPs abound with diverse bioactive primary and secondary metabolites and compounds with therapeutic properties. Marine probiotics are beneficial microorganisms that inhabit marine environments and can benefit their hosts by improving health, growth, and disease resistance. Several studies have shown they possess potential bioactive and therapeutic actions against diverse disease conditions, thus opening the way for possible exploitation of their benefits through their application. Pseudoalteromonas spp. are a widely distributed heterotrophic, flagellated, non-spore-forming, rod-shaped, and gram-negative marine probiotic bacteria species with reported therapeutic capabilities, including anti-cancer and -bacterial effects. This review discusses the basic concepts of marine probiotics and their therapeutic effects. Additionally, a survey of the anticancer and antibacterial effects of Pseudoalteromonas spp. is presented. Finally, marine probiotic production, advances, prospects, and future perspectives is presented.
1. Introduction
Natural products (NPs) are highly structurally diversified, ubiquitous life forms and derivatives of living organisms and minerals. They have been used extensively, especially in traditional medicine, to manage diverse diseases [1]. They have also contributed immensely to drug discovery in making current conventional drugs, serving as a direct source of medicinal substances, raw materials in drug production, lead compound design prototypes, and taxonomic biomarkers for new drug search and discovery [2]. NPs are associated with prominent, apparent beneficial properties [3] (as shown in Table 1) against conventional drugs or radiotherapy (as implicated in cancer management), including minimal side effects, toxicity, allergenicity, and low-cost isolation, identification, characterization, and production.
Synthetic drugs, including antibiotics used in aquaculture, primarily aim to prevent infection, leading to fatalities of “aquatic products” with correspondingly low productivity. However, their irrational use undermines the purpose. Although used optimally, it does not guarantee a clean bill of zero health risks to humans [4]. These drugs, including chloramphenicol, sulfamethazine, oxytetracycline, and furazolidone, remain residues in aquatic animals, with their bioaccumulation and biomagnification linked to human carcinogenicity [5,6,7]. Cancer is a leading cause of death globally, with yearly increasing cases [8] and an estimated 19 and 10 million new occurrences and deaths, respectively, in 2020 [9]. The risk/predisposing factors impact the DNA repair system following mutation in a single cell [10]. The pioneer single precancer cell produces many neoplastic cells and tissues via specific mechanisms, leading to undesirable physiological states of the cell, the surrounding cells, tissues, and the entire system. Clinically managing or controlling cancer cells often involves using single and multiple chemotherapeutic drugs to kill or destroy benign or malignant tumor cells and applying radiation to target sites [11,12]. Chemotherapy usually consists of administering high doses of synthetic chemicals for extended durations. Thus, the chemotherapeutic management of cancer has obvious negative implications. Most notable are the drop in the quality/standard of living of most recipient patients due to the extensive side effects of chemotherapeutic drugs on the surrounding cells and systems, the cost of acquiring and maintaining the therapy, and the contribution of chemicals to increasing drug resistance [10]. Radiation therapy has negative implications, including bioaccumulation of radiation, inadequate replacement of damaged stem cells, and injury [13].
NPs are also being exploited to produce anti-infective agents, especially with emerging infectious diseases, antibiotic resistance, and the dearth of new chemotherapeutic agents. Furthermore, the importance of NPs with immunomodulatory and antioxidant properties in combating bioterrorism cannot be overemphasized. The inadequacies in current therapeutic options highlight the need for safer, cheaper, and cost-effective alternative therapies, mainly from various natural products (NPs) that are abundant in the environment. One awakening mindset involves using marine probiotics for their inherent enormous potential [3]. Marine probiotics are a group of natural microbial dwellers of the marine ecosystem that, through their biological activities, apparently contribute to improving aquatic life forms through disease resistance, growth, stress tolerance, reproduction, and water quality [14,15]. They are being exploited for possible utilization in disease prevention and management and have many genera and species belonging to different phyla, including Pseudoalteromonas spp. The cold-adapted Pseudoalteromonas spp. are diverse understudied marine probiotics usually resident in the extreme marine environment and has shown great potential for therapeutic and biotechnological applications [16]. Despite limited literature, they are reported to elicit bioactive metabolites/compounds with potential medicinal applications, including anti-cancer and -bacterial therapies [17,18]. Also, there is no existing review on the aggregation of studies on the species. This review aims to discuss the potential of marine probiotics with a specific focus on Pseudoalteromonas spp. for cancer prevention, management, and antibacterial effects. The first is the background, basic concepts, sources, and classifications of marine probiotics; second, some general therapeutic effects of marine probiotics; third, an extensive survey of the anticancer and antibacterial effects of Pseudoalteromonas spp; and finally, a general review of marine probiotic production, prospects, advances, and future perspectives.

Table 1.
Comparison of side effects of chemotherapeutic drugs, radiation (as applied in cancer management), and natural products.
Table 1.
Comparison of side effects of chemotherapeutic drugs, radiation (as applied in cancer management), and natural products.
| S/N | Therapy | Side Effects | Therapy Cost | Production Cost | Drug Resistance | Bioaccumulation | Mutation | Injury | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Chemotherapeutic drugs | Extensive side effects | High cost of acquiring and maintaining therapy | High cost of development and production of candidate drugs | Contribution of chemicals to increasing drug resistance | Bioaccumulation of chemicals | Not applicable | Not applicable | [19] |
| 2 | Radiation | Extensive side effects | High cost of acquiring and maintaining therapy | Not applicable | Not Applicable | Bioaccumulation of radiation | Induction of mutation | Inadequate replacement of damaged stem cells | [13] |
| 3 | Natural products | Minimal side effects | Relatively low cost of acquiring and maintaining therapy | Relatively low cost of isolation, identification, characterization, and production | Little or no contribution to resistance | Little or no accumulation | No induction of mutation | No injuries | [20] |
2. Methodology
This paper is organized into three parts. The first part is a general review of the background of marine probiotics, sources, and some therapeutic effects. The second part is an extensive survey presenting relevant literature on the involvement of Pseudoalteromonas spp. in the prevention and management of cancer and bacterial diseases. The third section reviews marine probiotic production, prospects, advances, and future perspectives.
For the second part, the classical literature search of titles, abstracts, and keywords across four databases, including PubMed, ScienceDirect, Scopus, and google scholar (as a secondary database), was done using the ‘Pseudoalteromonas spp.’, ‘cancer’, ‘anticancer’, and antibacterial’, as keywords. The study used most original articles published in the last ten (10) years (2013–2023). The literature search was performed using the Boolean connectors: “And” or “Or” where necessary. Original articles reporting the anticancer and antibacterial effects of Pseudoalteromonas spp. in the stated years were included. All articles outside the study year range, not written in English, not reporting on Pseudoalteromonas spp., review articles, and not reporting anticancer and antibacterial effects were excluded. Some of the limitations of the review include the exclusion of subscription-based articles requiring payment for access. Also, most articles obtained using the keywords were review articles.
3. Probiotics, Sources, and Classifications
Probiotics resulting from the Greek words “Pro bios,” which translates to “for life” [21], are a class of microorganisms, including bacteria, viruses (such as bacteriophages), and fungi (yeast and mold) that can be ingested or topically applied for dietary and numerous medicinal (physiological and immunological) purposes [8,22,23]. Examples include Bifidobacterium, Streptococcus, Bacillus, Escherichia coli, Lactobacillus, Saccharomyces, Coccobacilli, and Propionibacterium and are in varying classifications, mechanisms of action, and corresponding functions. They preserve specific qualities, including, but not limited to, the ability to inhibit pathogens in the gut, navigate and survive via intestinal transit and gastric/bile secretions, adhere to the mucosa of the intestine, have immunomodulating and other biological effects [10,24]. Probiotic strains should be thoroughly characterized, safe for the intended application, backed by at least one successful human clinical trial per generally accepted scientific criteria, and alive in adequate numbers in the product at the time of usage [25]. Other considerations in choosing for medicinal purposes include non-toxicity, non-pathogenicity, beneficial effects, and appreciable shelf life. Probiotics have been applied in managing diverse medical conditions [10]. Also, several preclinical and clinical trials have suggested potent therapeutic applications.
Probiotics are traditionally used and present in fermented food products (e.g., milk, yogurt, cheese, buttermilk, kombucha, sauerkraut, and tempeh) and supplements [10]. The major classifications of probiotics include lactic acid (e.g., Streptococcus, Lactobacillus, Enterococcus, and Bifidobacterium) and non-lactic acid strains (e.g., Bacillus, Clostridium, and Propionibacterium), yeasts (e.g., Saccharomyces, Candida, and Debaryomyces) and viruses, with each group exhibiting different mode of operation [26]. Based on ecosystems, terrestrial and aquatic or marine-based probiotics are the classifications [27]. Probiotics are involved in the production of inhibitory substances which prevent the adhesion of pathogens to the intestinal epithelium, direct inhibition of gram-negative bacteria, regulation of short-chain fatty acids, downregulation of proinflammatory cytokines, colonization of intestinal permeability, regulation of electrolyte adsorption, maintenance of the immune response of the intestine, and maintenance of lipid metabolism [26].
4. Marine Probiotics and General Therapeutic Potentials
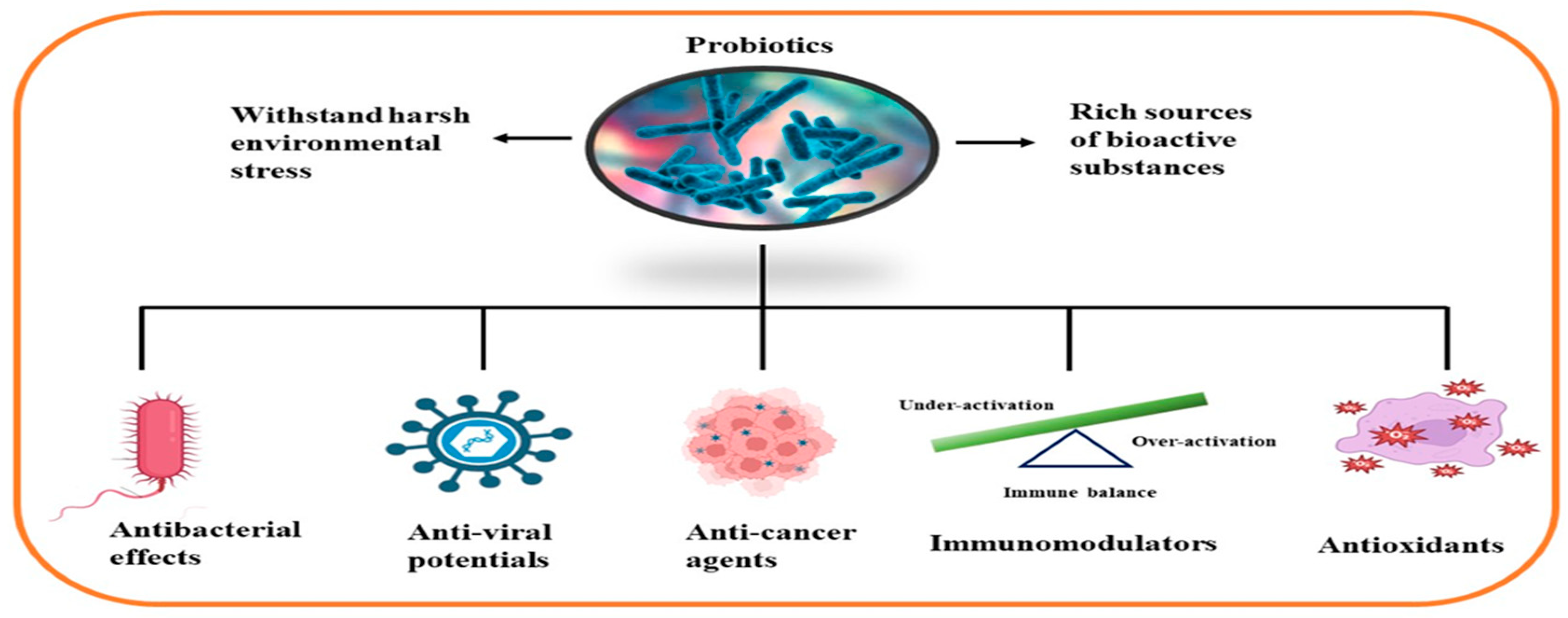
Marine probiotics abound in the aquatic ecosystems. Indigenous and exogenous microbiota from aquatic animals is the primary source for isolating probiotic strains, with the Lactobacilli, Pseudomonas, Shewanella, Fluorescens, Phaeobacter, and Bifidobacteria being the most common genera [28,29]. They have many beneficial roles, including the protection of aquatic life forms. They naturally act as disease control agents in aquatic plants and fishes, promote growth, improve digestion and immune systems, provide sources of nutrients, improve water quality, encourage reproduction, form beneficial relationships with the host and the environment, enhance gut health and immune response in higher animals, and improve human health by preventing and treating various diseases such as cancer, gastrointestinal disorders, respiratory infections, and skin diseases [21,30,31]. They also engage in the blockage of the pathogen’s ability to utilize certain nutrients, prevention of their attachment to the host environment, distortion of the enzymatic activities of the pathogens, enhancement of the quality of water, stimulation of the immune system, and improvement of host nutrition. Marine probiotics are a promising source of novel bioactive compounds with anticancer, antibacterial, immunomodulatory, antioxidant, anti-inflammatory, and antiviral properties, as have been implicated in several studies [27,32,33,34,35,36,37,38,39].
Studies have detailed the potential of yeast as a probiotic for cancer management [40], especially the isolates from marine ecosystems [41,42,43,44,45] and floras of the gut system [42]. Various marine yeast microbiota genera with potential anticancer effects have been identified, including Saccharomyces, the most studied genera, particularly in colorectal cancer, as discussed by Sambrani et al. [40]. Others are Candida, Debaryomyces, Kluyveromyces, Pichia, Saccharomyces, Cryptococcus, Rhodosporidium, Rhodotorula, Sporobolomyces, Mrakiafrigida, Guehomyces pullulans, Rhodotorula, Rhodosporidium, Yarrowialipolytica, Aureobasidium, Metschnikowia spp., Torulopsis spp., Pichia, Kluyveromyces, Williopsis, Pseudozyma spp., Hansenula, Trichosporon, Filobasidium, Leucosporidium, Mrakiafrigida, Guehomyces pullulans, Metschnikowia, Rhodotorula, Cystobasidium, and Yamadazyma [45,46]. The lactic acid bacteria (LAB) genera and species, including Lactobacillus (L. casei, L. acidophilus, L. fermentum, L. delbrueckii, L. helveticus, L. paracasei, L. pentosus, L. plantarum, L. salivarius, L. rhamnosus GG, L. johnsonii), Bifidobacterium (B. bifidum, B. longum, B. lactis, B. infantis, B. breve, B. adolescentis), Leuconostoc spp. (Ln. lactis, Ln. mesenteroidessubsp. Cremoris, Ln. mesenteroides subsp. dextranicum), and Streptococcus spp. (S. salivarius subsp. thermophilus) are highly diversified and studied probiotic bacteria implicated in the inhibition and apoptosis of various human cancer cells [32,47,48,49]. Other reported bacterial probionts include Bacillus (B. fermenticus, B. subtilis), Clostridium butyricum, Enterococcus faecium, Pediococcus pentosaceus, Lactococcus lactis, Propionibacterium, and Streptococcus thermophilus [49]. Actinomycetes such as Streptomyces and Micromonosporaceae are also promising candidates. Trioxacarcins A–C, anthraquinone, Macrodiolide tartrolon D, and Streptokordin obtained from Streptomyces spp. exhibited significant antitumor/cytotoxic activities against various cancer cell lines [50,51,52].
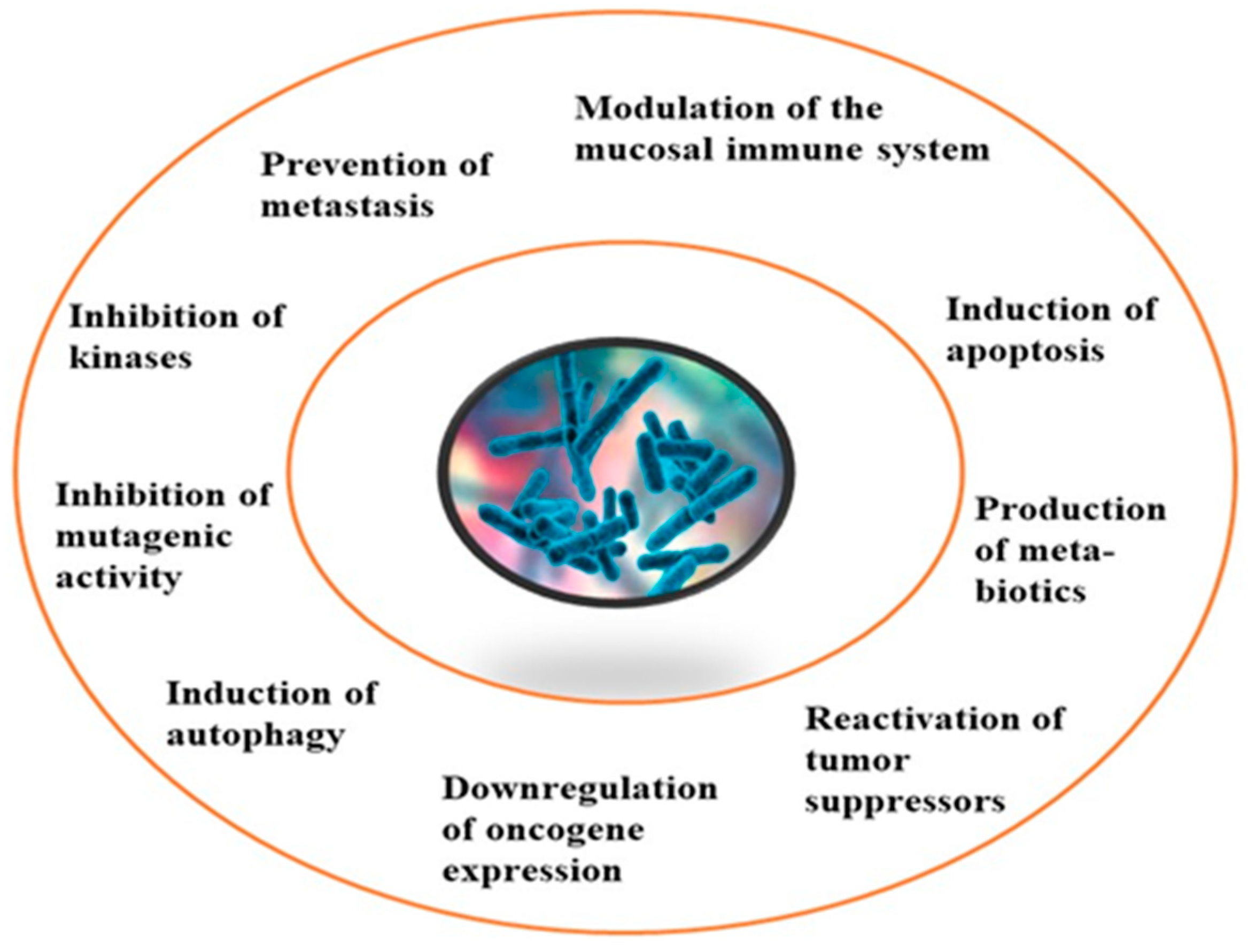
Although limited information is available on cancer management among marine probiotics, few probiotics are known to possess anticancer properties through several mechanisms (Figure 1), primarily due to meta-biotics, which consist of structural components, metabolites, and signaling molecules with specific chemical structures as shown in Enterococcus lactis IW5 [53,54,55]. These components optimize various physiological functions of the host, including regulatory, metabolic, and behavioral reactions. Marine probiotics, such as Lactobacilli and Bifidobacteria, modify the mucosa by increasing the production of chemokines and host defense peptides, inducing dendritic cell maturation, and increasing cell proliferation and apoptosis [51]. Marine probiotics can modulate cancer by inducing apoptosis, inhibiting mutagenic activity, downregulating oncogene expression, inducing autophagy, inhibiting kinases, reactivating tumor suppressors, preventing metastasis, and producing meta-biotics, as already shown in B. animalis, B. infantis, B. bifidum, L. paracasei, L. acidophilus, and L. plantarum I-UL4 against MFC7 cancer cells [53,54]. Although probiotics alone may not suffice in treating cancer, they can mitigate colorectal cancer (CRC) by enhancing the efficacy of treatments and acting on the immune system [56]. Studies have shown that probiotic strains, specifically lactic acid bacteria mixtures, can differentially induce and modulate macrophage pro- and anti-inflammatory cytokines and phagocytosis [53]. It also can mitigate the effects of DMH-induced colon shortening and positively affect leukocyte count and colon tumor growth [56]. The combinations also induce the excretion of proinflammatory IL-18 by tumor cells and are crucial in mitigating CRC [56]. Probiotics generally exhibit antitumor activities by enhancing the intestinal microbiota, degrading possible carcinogens, and modulating gut-associated and systemic immune responses [57]. Worthy of mention is that several anticancer drugs of marine origin are in clinical use with sufficient approvals, including cytarabine, vidarabine, nelarabine, fludarabine phosphate, trabectedin, eribulin mesylate, brentuximab vedotin, polatuzumabvedotin, enfortumabvedotin, belantamabmafodotin, plitidepsin, lurbinectedin, bryostatins, discodermolide, eleutherobin, and sarcodictyin [41,58].
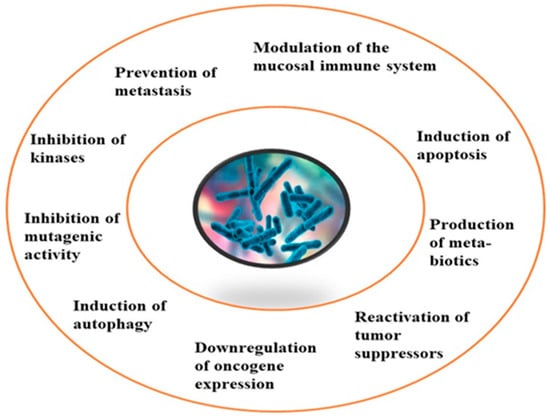
Figure 1.
General mechanism of action of cancer prevention and management.
With the increasing popularity of antibiotic stewardship, the misuse and abuse of antimicrobials in developed and developing countries have remained high, with an attendant increase in the development of bacterial resistance. Especially with its forecasted implications [59], possible avenues for developing newer antimicrobials must be exploited. Marine probiotics are a potential source of antimicrobial substances. Pereira et al. [59] showed that marine-isolated L. lactis and E. faecium produce effective bacteriocin antibiotics [59]. The bacteriocin-producing potential of Lactococcus spp. also agrees with Sarika et al.’s study [60]. Other marine probiotics that can produce antibacterial substances have continued to emerge. Pseudoalteromonas spp. and Vibrio spp. produced antibacterial substances with activity comparable to established antibiotics [61]. Marine lactic acid bacteria of the genera Lactococcus spp., Enterococcus spp., Lactobacillus spp., and Leuconostoc spp. have been reported to produce antimicrobial substances against Vibrio spp. and Photobacterium spp. [62]. Kaktchan et al. [63] revealed that Lactococcus spp., cultured in an earthen pond, could produce a bacteriocin-like substance that inhibits the growth of Vibrio spp. and Pseudomonas aeruginosa.
Immune system arsenals generally recognize viral antigens, preventing their multiplication within the host [64]. However, research should remain proactive, as infective agents constantly change and could sprout newer pathogenic strains. Although the exact mechanisms of action are still unclear, some probiotics have shown a solid ability to prevent viral multiplication in fish and can be used as antiviral agents. Lactobacillus spp. and B. subtilis boosted viral resistance by preventing viral infections in Paralychthus olivaceus and grouper fish, respectively [65,66]. The production of antiviral compounds by Pseudoalteromonas spp. has protected Prawns and Sea breams against viral pathogens [67]. Other studies have also shown the possibility and potential of deploying probiotics in the marine environment as agents to prevent viral infections [68,69].
A robust immune system correlates well with defense against infections and diseases. Studies on the immune-enhancing potential of marine probiotics have shown promising results [33,37]. Wasana et al. [47] reported the expression of the immunomodulatory genes in zebrafish larvae exposed to a novel Pseudoalteromonas xiamenensis, a marine probiotic that induced the down-regulation of proinflammatory cytokine genes. In another study, a significant reduction in the levels of proinflammatory cytokines was observed in CRC patients who received six viable probiotics of Lactobacillus and Bifidobacterium strains [70].
The propensity of reactive oxygen and reactive nitrogen species as free radicals to alter the body’s proteins, lipids, and DNA is a significant cause of some human diseases [71]. Due to synthetic antioxidants’ toxic effects [72], marine probiotics have been studied as a natural source of antioxidants. In a study, Alsharmmari et al. [73] espoused the novel marine probiotic, Enterococcus durans, which possesses an efficient antioxidant potential. Other studies that corroborated and supported the possible use of marine probiotics as potential sources of antioxidants are those by Husain et al. [73] and Angulo et al. [74].
Representative marine probiotic-derived drugs and their microbial sources are presented in Table 2. Therapeutic potentials of marine probiotics are illustrated in Figure 2.
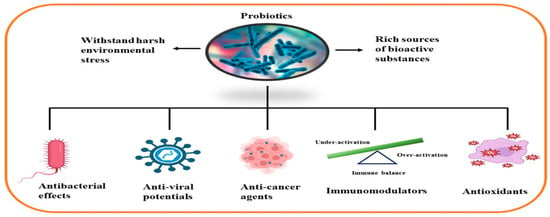
Figure 2.
Therapeutic potentials of marine probiotics.

Table 2.
Representative marine probiotic-derived drugs and their microbial sources.
Table 2.
Representative marine probiotic-derived drugs and their microbial sources.
| Bioactivity | Drugs | Microbial Source | Reference |
|---|---|---|---|
| Anticancer | Actinomycin, Salinosporamide A (Marizomib®) (NPI-0052), Plinabulin, Enzastaurin, Lestaurtinib, Becatecarin, GSK2857916, Ladiratuzumab vedotin, Tisotumab vedotin, Glembatumumab vedotin, Denintuzumab mafodotin, Midostaurin (Rydapt®), Pinatuzumab vedotin, ASG-15ME, Lifastuzumab vedotin, Vandortuzumab vedotin, UCN-01 | Streptomyces sp., Salinospora tropica, Aspergillus sp., Streptomyces staurosporeus, Saccharothrix aerocolonigenes, Caldora penicillata | [75,76] |
| Antimicrobial | Gageotetrins A–C, Gageopeptides A–D, Ieodoglucomide 1, 2, Marinopyrrole A, Merochlorin A, Anthracimycin | Bacillus subtillis 109GGC020, Bacillus licheniformis 09IDYM23, Streptomyces sp. | [77,78] |
| Immunomodulatory | Brentuximab vedotin, Polatuzumab vedotin (DCDS-4501A), Belantamab madofotin-blmf | Symploca sp. VP642, Cyanobacteria | [79] |
| Antioxidant | Hexaricins F, Asperchalasine I | Streptosporangium sp. CGMCC 4.7309, Mycosphaerella sp. SYSU-DZG0 | [80] |
| Antiinflammatory | Cyclic peptide cyclomarin, Violaceomide A, Dehydrocurvularin | Streptomyces sp., Aspergillus terreus H010, Penicillium sumatrense | [78,80] |
5. Pseudoalteromonas spp.; Therapeutic Potentials and Systematic Survey
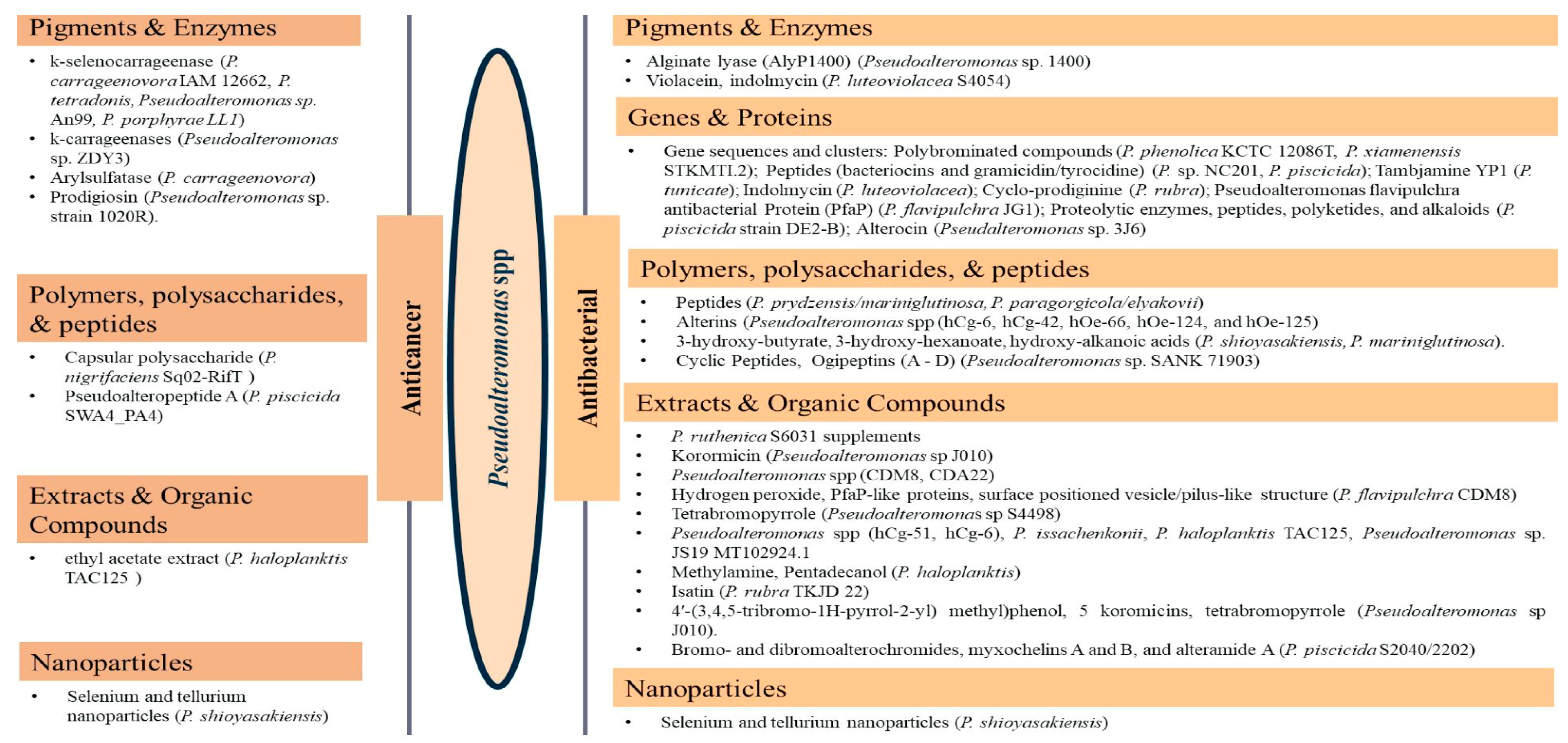
Of the class Gammaproteobacteria; the cold-adapted Pseudoalteromonas spp. are widely distributed heterotrophic, flagellated, non-spore-forming, rod-shaped gram-negative marine probiotic bacteria with up to 37 to 48 specified species [70,71,72] proposed and separated from Alteromonas as a genus in the last 28 years [81]. The relatively low number of studies on the genus could be attributed to the recency of the genus proposal. In addition, it is difficult to reach and manage these isolates [82]. They are abundant, comprise 20 to 60% of the marine microbial community, and are often associated with particles [83]. They are well known for possessing and releasing a broad range of extracellular bioactive substances, including enzymes, pigments, protease, and polysaccharides, with many biotechnological and pharmaceutical applications [84,85,86]. They are implicated in the cycling of nutrients, including carbon and nitrogen, and in the intestinal microbiota of marine life forms, assisting in maintaining physiological functions [87]. Recent studies have shown the Pseudoalteromonas spp. to possess diverse bioactive/therapeutic potentials involving their metabolites, including having anticancer (Table 3) and antibacterial effects (Table 4) via different mechanisms. They are presented here in four categories, including (1) genes and proteins/enzymes, (2) polymers, polysaccharides, and peptides, (3) extracts and organic compounds, and (4) nanoparticles, as studies have been reported in these lines.

Table 3.
Anticancer activities of Pseudoalteromonas spp.
Table 3.
Anticancer activities of Pseudoalteromonas spp.
| Category | Pseudoalteromonas sp. | Study | Method | Active Agent | Structure | Mechanism of Action | References |
|---|---|---|---|---|---|---|---|
| Proteins/enzymes | Pseudoalteromonas sp. Xi13, (genomically associated with P. carrageenovora IAM 12662 and Pseudoalteromonas sp. An99) | Comparison of immobilized and lyophilized k-Selenocarrageenase produced from Pseudoalteromonas sp. Xi13 and The complete genome sequencing of Pseudoalteromonas sp. Xi13 and anticancer effects of the selenium oligosaccharides. | Response Surface Methodology, 16S rRNA gene sequencing, and Cell mass detection of cancer cells | k-Selenocarrageenase | 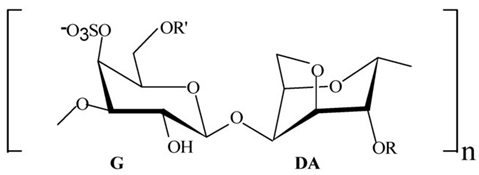 | k-Selenocarrageenase acts by degrading k-Selenocarrageenan to selenium oligosaccharides, possibly involving complex glycoside hydrolase | [88,89] |
| P. tetradonis, P. porphyrae LL1, Pseudoalteromonas sp. ZDY3 | Catalytic activity and thermostability enhancement of two similar mutants k-Carrageenases. The expression of the same enzyme from P. porphyrae LL1 in B. choshinensis | PoPMuSiC algorithm. Cloning, transformation, and enzyme activity assays. Enzyme isolation, purification, LC-HRMS | k-Carrageenases. | 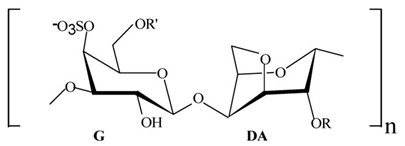 | Produces even numbered k-Carrageenan oligosaccharides by catalyzing k-Carrageenans | [90,91,92] | |
| P. carrageenovora | Characterization (pH and temperature) of arylsulfatase from P. carrageenovora mutants | Library construction, sequencing, expression, purification, and determination of arylsulfatase activity following pH and temperature variations. | Arylsulfatase |  | The use in the detection of cancer cells was not stated. However, arylsulfatase enables the arylsulfatase hydrolysis into inorganic sulfate and aryl compounds. | [93] | |
| Pseudoalteromonas sp. strain 1020R | Determined the effects of prodigiosins produced by Pseudoalteromonas sp. strain 1020R on the protein kinases and phosphate as targets in the cell death of cancer cells through apoptosis using leukemia cell lines. | Cytotoxicity, protein phosphatase, and kinase assay | Prodigiosins (2-methyl-3-pentylprodiginine, 2-methyl-3-heptylprodiginine, 2-methyl-3-butylprodiginine, and 2-methyl-3-hexylprodiginine) | 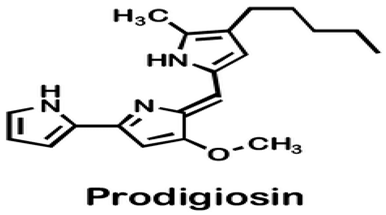 | Protein-phosphatase inhibited cytotoxicity of the leukemia cancer cells. Also, that mechanism activation is independent of the p53 protein. | [94] | |
| Polymer, polysaccharides, and peptides | P. nigrifaciens Sq02-RifT | Assay of the antitumor effect on colon cancer cells by capsular polysaccharide isolated from P. nigrifaciens Sq02-RifT | Chemical and spectroscopic methods, Cell viability assay | Capsular polysaccharide | 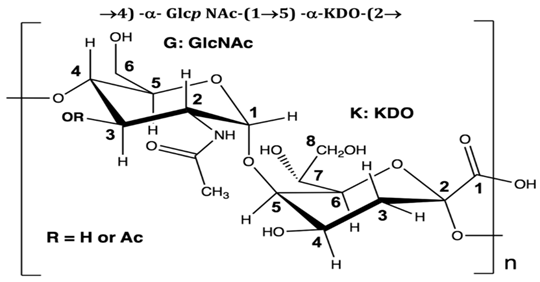 | Activate Caspases -3 and -9 on the CaCo-2 and HCT-116 of the cancer cells inducing apoptosis. | [95] |
| P. piscicida SWA4_PA4. | Iron chelating activity and cytotoxicity effects (against human T lymphocyte cells) of novel Pseudoalteropeptide A (lipopeptide) isolated from P. piscicida SWA4_PA4. | Peptide isolation, spectroscopy, and viability assay | Pseudoalteropeptide A | 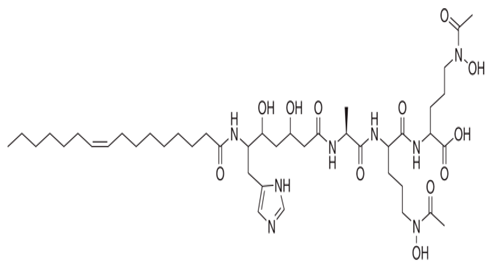 | Iron chelating activity and cytotoxicity effects | [96] | |
| Extracts and organic compounds | P. haloplanktis TAC125 | The antiproliferative effects of ethyl acetate crude extract and compounds of marine P. haloplanktis TAC125 against A549 lung epithelial cancer cells | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) viability assay, bioassay-guided purification, and mass spectrometry | 4-hydroxybenzoic acid | 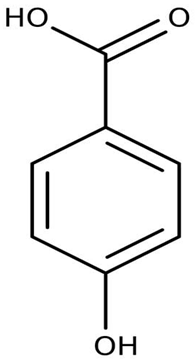 | Activates specific well-regulated molecular pathways, Pyroptotic cell death signaling, which triggers the transcription of Caspase -1 and subsequent proinflammatory cytokines | [97] |
| Nanoparticles | P. shioyasakiensis | Reduction and cytotoxicity effects of P. shioyasakiensis-based nanoparticles. | Estimation of cell viabilities. | Selenium and tellurium nanoparticles of P. shioyasakiensis | - | Different pathways and enzymes, including reductases, siderophores, glutaredoxin, and glutathione, in converting selenium and tellurium to their nanoparticles; however, the specific mechanisms used by the isolates were not covered. | [83] |
5.1. Marine Probiotic Pseudoalteromonas spp. in the Prevention/Management of Cancer
5.1.1. Anticancer Pseudoalteromonas spp. Proteins/Enzymes
Denge et al. [88] compared immobilized and lyophilized k-selenocarrageenase, a potential adjuvant for cancer drugs obtained from Pseudoalteromonas sp. Xi13. Although the lyophilized enzyme had a better recovery rate (70%), the immobilized enzyme exhibited better stability after several uses. Mechanistically, k-selenocarrageenase acts by degrading k-selenocarrageenan to selenium oligosaccharides, addressing the issues of water solubility and the high molecular weight of the former [88]. In a study involving the complete genome sequencing of the same isolate, Wang et al. [89] suggested the mechanism of the k-Selenocarrageenase action to involve complex glycoside hydrolase. They also confirmed the anticancer effects of selenium oligosaccharides (specifically, disaccharides and tetrasaccharides) in a concentration-dependent manner in HeLa cervical cancer cells. Hong et al. [90] reported the catalytic activity and thermostability enhancement of two mutant k-carrageenases produced from P. tetradonis, which produces even numbered k-carrageenan oligosaccharides by catalyzing k-carrageenans via the PoPMuSiC algorithm. Compared to wild-type k-carrageenase, the enzyme mutants displayed better enzyme activity, attributed to better enzyme flexibility and fewer structural deviations. Zhao et al. [91] demonstrated the expression of the same enzyme from P. porphyrae LL1 in Brevibacillus choshinensis, which also presented considerable stability across a broad pH range. They showed that Mg2+ drastically enhanced the enzyme’s activity and that the enzymatic hydrolysates were made of An-G4S-type neocarrabiose units, the end products of which were neo-carratetraose. Also, Zhao et al. [92] isolated stable, high hydrolyzing k-carrageenase from Pseudoalteromonas sp. ZDY3 with Km and Kcal/Km of 3.67 mg/mL and 53 mL/mg/s, respectively. The enzyme end-products were k-neocarratetraose and k-neocarrabiose. Arylsulfatase, which plays a potential role in cancer cell detection, was isolated by Zhu et al. [93] from a library of P. carrageenovora arylsulfatase mutants, which showed improved thermal and pH stability compared with the wild-type enzyme. Soliev et al. [94] determined the effects of prodigiosin (2-methyl-3-pentylprodiginine, 2-methyl-3-heptylprodiginine, 2-methyl-3-butylprodiginine, and 2-methyl-3-hexylprodiginine) produced by Pseudoalteromonas sp. strain 1020R on protein kinases and phosphate as targets of cancer cell death through apoptosis in leukemia cell lines (HL60, K562, and U937). They reported the dose-dependent inhibition of two phosphates, including protein phosphatase 2A and tyrosine phosphatase 1B. However, the prodigiosins were largely inactive against the protein kinases. Thus, they suggested protein–phosphatase inhibition-based cytotoxicity in leukemia cancer cells. Also, the mechanism activation is independent of the p53 protein owing to the low concentration of the prodigiosin in the leukemia cell amidst the apoptosis.
5.1.2. Anticancer Pseudoalteromonas spp. Polymer, Polysaccharides, and Peptides
Di Guida et al. [95] reported antitumor effects against colon cancer cells (compared to the untreated cell, reducing 60% of colon cancer cell viabilities at 600 μg/mL after 72 h and with a nontoxic lower cell viability reduction (20%), when compared to the untreated control cells, at 200 μg/mL, after 72 h) by a capsular polysaccharide made up of N-acetylated aminosugars, isolated from P. nigrifaciens Sq02-RifT and obtained within a fish gut, which activates Caspases-3 and -9 on the CaCo-2 and HCT-116 of the cancer cells and induces apoptosis. Ueoka et al. [96] showed the iron chelating activity and cytotoxic effects (against human T lymphocyte cells) of a novel Pseudoalteropeptide A (lipopeptide) isolated from P. piscicida SWA4_PA4.
5.1.3. Anticancer Pseudoalteromonas spp. Extracts and Organic Compounds
The antiproliferative activity of ethyl acetate extract and the bioactive compounds of P. haloplanktis TAC125 against A549 lung epithelial cancer cells were investigated by Sannino et al. [97]. 4-hydroxybenzoic acid from the extract was identified as an anticancer cell proliferation (with IC50 value ≤ 1 μg/mL) compound in the bacterial extract; it exhibited a specific gene and protein level well-regulated pathway termed pyroptotic cell death signaling, which triggers the transcription of Caspase-1 (an inflammasome) and subsequently the release of IL18β and IL18 encoding genes (proinflammatory cytokines).

Table 4.
Antibacterial activities of Pseudoalteromonas spp.
Table 4.
Antibacterial activities of Pseudoalteromonas spp.
| Category | Pseudoalteromonas sp. | Study | Method | Active Agent | Structure | Mechanism of Action | Reference |
|---|---|---|---|---|---|---|---|
| Genes and proteins/enzymes | Pseudoalteromonas sp. 1400 | P. aeruginosa-implicated biofilm inhibition by alginolytic enzymes produced by thirty-six bacterial isolates, including Pseudoalteromonas sp. 1400 | Enzyme isolation, activity, antibiofilm assays, immunofluorescent staining, and chromatography | Alginate lyase (AlyP1400) | 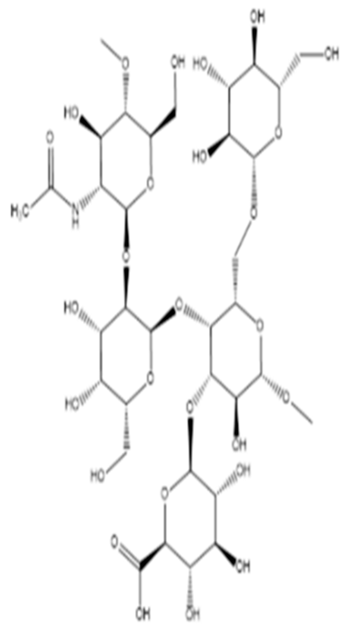 | Dual lyase activity of degrading poly-glucuronic and -mannuronic acids. Hydrolytic and disruptive effects of the enzyme against the extracellular alginate produced by P. aeruginosa CF27 | [98,99] |
| P. luteoviolacea S4054 | Assay of the bioactive violacein and indolmycin produced by a mutant P. luteoviolacea S4054 | Antibacterial/diffusion, UHPLC-UV/Vis studies | Violacein, indolmycin | 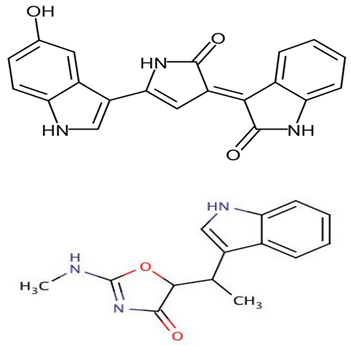 | Not available | [100] | |
| P. phenolica KCTC 12086T | The complete genome elucidation of P. phenolica KCTC 12086T, which could produce antibiotic compounds | Genome sequencing techniques | Polybrominated compounds: polybrominated-diphenyl ethers, -bipyrroles, and -biphenyls | 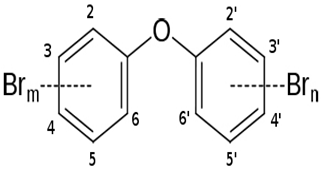 | Not available | [101] | |
| P. xiamenensis STKMTI.2 | The complete genome elucidation of P. xiamenensis STKMTI.2 | Genome sequencing techniques | Brominated marine phenols/pyrroles and secondary metabolites (peptides, butyrolactone, prodigiosin, RiPP-like, and Lant I) | 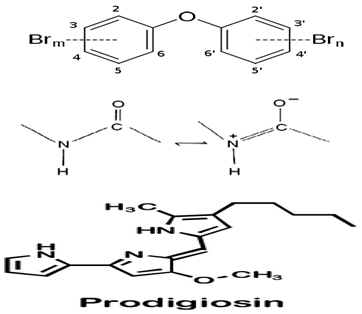 | Not available | [102] | |
| Pseudoalteromonas sp. NC201 | The complete genome elucidation of Pseudoalteromonas sp. NC201 | Genome sequencing techniques | Peptides (bacteriocins and gramicidin/tyrocidine) | 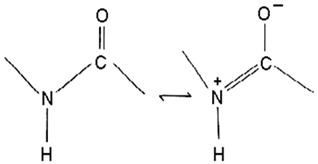 | Not available | [103,104] | |
| P. tunicate | Studied and sequenced the tam operon, which initiates the synthesis of P. tunicate-produced tambjamine YP1, a natural bipyrrole antibiotic | Genome sequencing techniques, mass spectrometry analysis | Tambjamine YP1 | 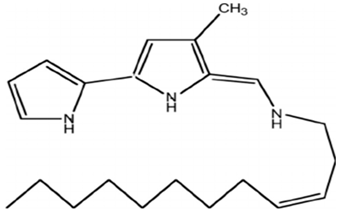 | Not available | [105] | |
| P. luteoviolacea | Analyzed different geographic sort isolates of P. luteoviolacea for biosynthetic richness and diversity | Metabolomic, Genome sequencing techniques, mass spectrometry analysis | Indolmycin | 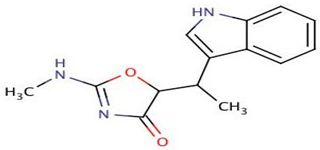 | Not available | [106] | |
| P. rubra | Genomic study of P. rubra gene responsible for cycloprodiginine production. | Genome sequencing techniques | Cyclo-prodiginine | 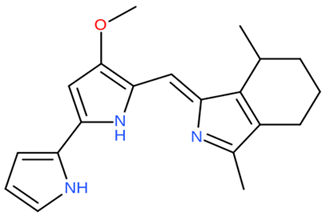 | Not available | [107] | |
| P. flavipulchra JG1 | Genome sequence of P. flavipulchra JG1 responsible for producing P. flavipulchra antibacterial Protein (PfaP) for specific genes or clusters responsible for the expression of the antibacterial protein, proven to have inhibitory effects against certain bacteria. | Genome sequencing techniques | P. flavipulchra antibacterial Protein (PfaP) | - | P. flavipulchra antibacterial Protein (PfaP) oxidatively deaminates certain amino acids to α-keto acids, hydrogen peroxide, and ammonium. Hydrogen peroxide decomposes into other metabolites, which exhibit antimicrobial effects. | [108] | |
| P. piscicida | Genome sequencing of P. piscicida 36Y_RITHPW produces bioactive compounds with inhibition effects against multi-resistant V. parahaemolyticus implicated in Shrimp hepatopancreatic necrosis disease. | Genome sequencing techniques | Bacteriocins, peptides |  | Not available | [109] | |
| P. piscicida strain DE2-B | Genome sequence of P. piscicida strain DE2-B | Genome sequencing techniques | Proteolytic enzymes, peptides, polyketides, and alkaloids |  | Not availables | [110] | |
| Pseudalteromonas sp. 3J6 | Sequence elucidation and antibiofilm effect of Pseudalteromonas sp. 3J6 | Genome sequencing techniques, Antibiofilm assay | Alterocin |  | Not available | [111] | |
| Polymer, Polysaccharides, and peptides | P. prydzensis/mariniglutinosa, P. paragorgicola/elyakovii | Isolation, identification of peptides and bacteria of Oysters hemolymph and their antibacterial effects. | Antimicrobial activities | Peptides |  | Not available | [112] |
| Pseudoalteromonas spp (Designgated hCg-6, hCg-42, hOe-66, hOe-124, and hOe-125) | Alterins isolated using the bacterial cell-free culture were subjected to antibacterial assays against some gram-negative bacteria. Mechanism of alterin activity determined via lipopolysaccharide binding assays | Minimal Inhibitory Concentration (MIC), UPLC-HRMS | Alterins | 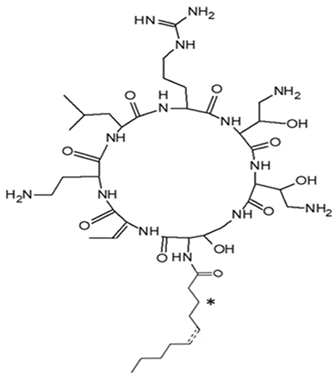 | Provide the depolarization of the bacterial membrane and the subsequent cell permeabilization and lysis. | [113,114] | |
| P. shioyasakiensis and P. mariniglutinosa. | Poly-hydroxybutyrate-co hydroxy-hexanoate (PHBH) degrading abilities of certain gram-negative bacteria, including P. shioyasakiensis and P. mariniglutinosa. | Bacterial isolation, degradation, and isolation of monomers, antibacterial assay | 3-hydroxy-butyrate, 3-hydroxy-hexanoate, hydroxy-alkanoic acids | 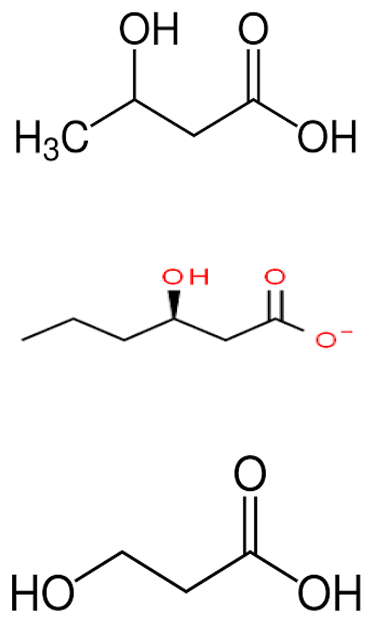 | Degradation of Poly-hydroxybutyrate-co hydroxy-hexanoate (PHBH) to antibacterial monomers | [115] | |
| Pseudoalteromonas sp. SANK 71903 | Tested an array of secondary metabolites of Pseudoalteromonas sp. SANK 71903 for potential inhibition effects against lipopolysaccharide (LPS) | LPS, inhibition assay, MIC | Cyclic Peptides, Ogipeptins (A–D) | 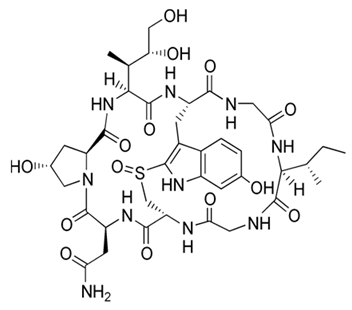 | LPS inhibition by CD14 binding and cytokine secretion blockage from LPS-stimulated cells. | [116] | |
| Extracts and organic compounds | P. ruthenica S6031. | In-vitro and vivo studies by Wasana et al. [117] demonstrated the probiotic potentials of P. ruthenica S6031, especially against E. piscicida. | Agar spot, In vivo studies | P. ruthenica S6031 supplements | - | Not available | [117] |
| Pseudoalteromonas sp. J010 | Determination of the antibacterial effect of korormicin against Vibrio sp. | MIC, sequence data studies | Korormicin | 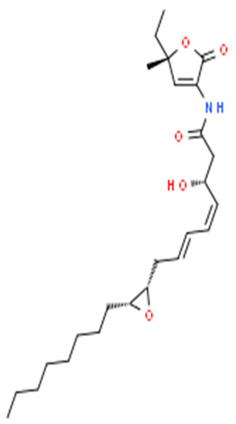 | Korormicin releases reactive O2 species via the electron transfer initiation in the enzyme, which promotes O2 and some redox cofactors reactions. | [118] | |
| Pseudoalteromonas spp. coded CDM8 and CDA22. | In vitro antibacterial activity and in vivo probiotic potential against V. parahaemolyticus, and PCR evaluations of two Pseudoaltermonas spp. | In vitro, in vivo, PCR evaluations | Pseudoalteromonas spp. coded CDM8 and CDA22 | - | Reduction of the copy number of V. parahaemolyticus toxin production gene, PirAvp | [119] | |
| P. flavipulchra CDM8 | Described the antibacterial mechanisms of P. flavipulchra CDM8 with potent inhibition activities against Bacillus spp. and Vibrio spp. | Antimicrobial biofilm assays and microscopy | Hydrogen peroxide, PfaP-like antibacterial proteins, and other molecules, surface positioned vesicle/pilus-like structure | 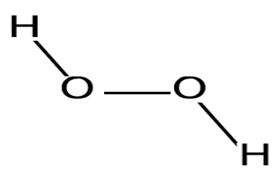 | The transferable outer surface positioned vesicle/pilus-like structure likely contributes to the inhibition activities observed and is described as a novel mechanism of antibacterial activity by the isolate. | [120] | |
| Pseudoalteromonas sp. type strain S4498 | Identification and determination of the mechanisms of secondary metabolites of Pseudoalteromonas sp. type strain S4498 | Use of antiSMASH and bioassays | Tetrabromopyrrole | 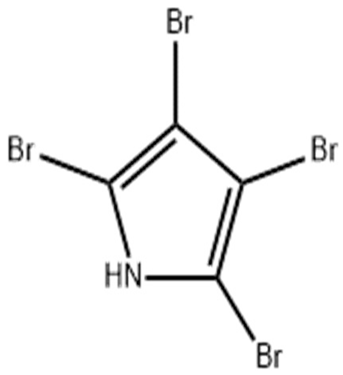 | Tetrabromopyrrole induces its antimicrobial effects through its signaling properties, induction of cellular stress, and the activities of dibromo-maleimide, its oxidized by-product. | [121] | |
| Pseudoalteromonas sp. (designated as hCg-51) | Assay of the different cell-free cultures of 843 species/strains obtained within the haemolymphs microbiota of bivalve species from the sea | Well diffusion and hemolymph-associated strain assays | Pseudoalteromonas sp. (designated as hCg-51) | - | Not available | [122] | |
| Pseudolateromonas spp., Pseudolateromonas sp. (hCg-6) | Assay of a collection of 11 Pseudolateromonas spp. obtained from the hemolymph of mussels and oysters for their antibacterial activities against V. harveyi. | Well diffusion, PCR, and hemolymph-associated strain assays | Pseudolateromonas spp., Pseudolateromonas sp. (hCg-6) | - | Not available | [123] | |
| P. issachenkonii, P. haloplanktis TAC125 | Antibacterial activities against E. coli, S. epidermis, and K. rhizophila by solvents extracts two Pseudoalteromonas spp., among other marine bacteria isolated from the surface of various marine macroalgae. | Well diffusion assay and phylogenetics | P. issachenkonii, P. haloplanktis TAC125 | - | Not available | [124] | |
| P. haloplanktis TAC125 | Antibiofilm activity of the culture supernatant of P. haloplanktis TAC125 against S. epidermidis | Antibiofilm assay | P. haloplanktis TAC125 | - | Disruption of the S. epidermidis biofilm multicellular structure. | [125] | |
| P. haloplanktis TAC125 | Culture, fermentation, and antibacterial activity of methylamine from P. haloplanktis TAC125 | Fermentation, minimum volatile inhibitory concentrations | Methylamine |  | Sannino et al., via a defined culture and fermentation system, produced and accumulated methylamine, a volatile organic compound (VOC), from P. haloplanktis TAC125, which by the minimum volatile inhibitory concentrations, presented a dose-dependent inhibition of different strains of Burkholderia spp. and E. coli. | [126] | |
| P. haloplanktis | Assay of the antibacterial properties of pentadecanol, a metabolite of P. haloplanktis, and the derivatives against Listeria monocytogenes | Minimum inhibitory concentrations. | Pentadecanol |  | Not available | [127] | |
| P. haloplanktis TAC125 | Antibiofilm activity against S. epidermis O-47, S. epidermidis RP62A | Antibiofilm assay | Pentadecanol | 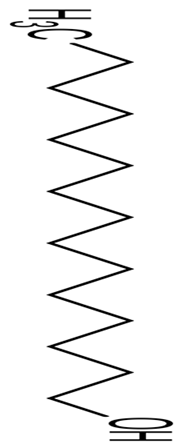 | The interference of the quorum sensing system of the S. epidermis by the AI-2 signaling process. | [128] | |
| P. rubra TKJD 22 | Isolation of organic compounds from Tunicate-associated bacteria and antibacterial activities of compounds. | Sequencing, NMR, Minimum inhibitory concentration | Isatin | 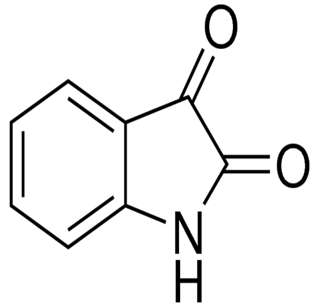 | Not available | [129] | |
| Pseudoalteromonas strain J010 | Isolation of bioactive compounds from the ethanol extract of Pseudoalteromonas strain J010 and determination of their antimicrobial activities. | Disc-diffusion assay, MS, and NMR techniques. | 4′-(3,4,5-tribromo-1 H-pyrrol-2-yl) methyl) phenol (a bromopyrrole), 5 koromicins, and tetrabromopyrrole | 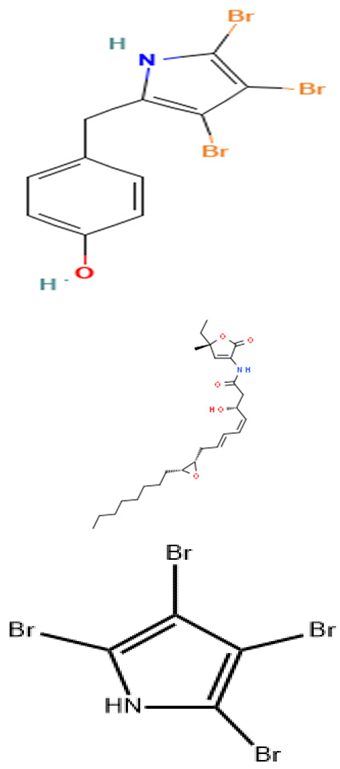 | Not available | [130] | |
| P. piscicida S2040 | Isolation of bioactive compounds from the extracts of P. piscicida S2040 and determination of their antimicrobial activities. | Disc-diffusion assay, MS, and NMR techniques. | Bromo- and dibromoalterochromides, myxochelins A and B, and alteramide A | 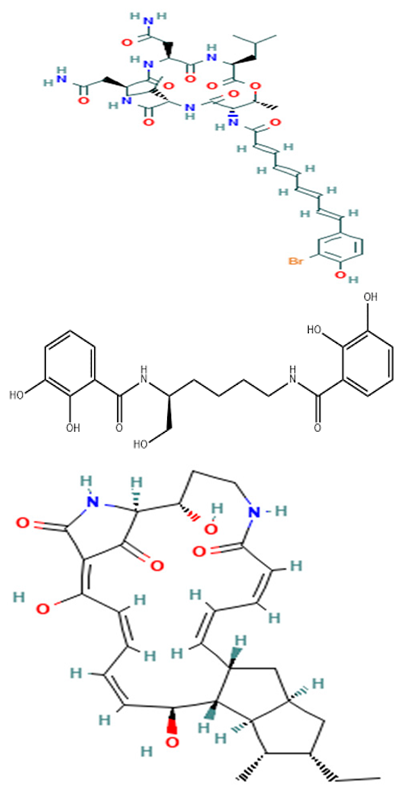 | Not available | [131] | |
| P. piscicida 2202 | Antibacterial activity of P. piscicida 2202 isolated from Modiolus kurillenis | Antimicrobial activity | P. piscicida 2202 | - | Not available | [132] | |
| Pseudoalteromonas sp. JS19 MT102924.1 | Extraction, phytochemical assay, and antibacterial effect of Pseudoalteromonas sp. JS19 MT102924.1 | Antimicrobial activity, FTIR | Pseudoalteromonas sp. JS19 MT102924.1 | - | Not available | [133] | |
| Nanoparticles | P. shioyasakiensis | Antimicrobial effects of P. shioyasakiensis-based nanoparticles | Inhibitory zones and minimum inhibitory concentrations | Selenium and tellurium nanoparticles of P. shioyasakiensis | - | The study suggests the contribution of different pathways and different enzymes, including reductases, siderophores, glutaredoxin, and glutathione, in the conversion of selenium and tellurium to their nanoparticles; however, the particular mechanisms used by the isolates were not covered. | [83] |
NB: ‘*’ in the Alterocin/Alterin structure is hydroxylation at C3 position.
5.1.4. Anticancer Pseudoalteromonas Nanoparticles
Beleneva et al. [83] used a green technology to synthesize the nanoparticles of selenium and tellurium using three different isolates of P. shioyasakiensis from a marine test facility in Vietnam, which presented high reduction process effects and human breast cancer and dermal fibroblasts cytotoxicity effects, with the selenium-based nanoparticles of the isolates outperforming the tellurium-based nanoparticles.
5.2. Antibacterial Activities of Pseudoalteromonas spp.
5.2.1. Antibacterial Pseudoalteromonas spp. Genes and Proteins/Enzymes
Daboor et al. [98] demonstrated the P. aeruginosa-implicated biofilm inhibition by alginolytic enzymes produced by thirty-six bacterial isolates, including Pseudoalteromonas sp. 1400, which yielded alginate lyase and had the highest alginolytic activity. The purified enzyme had dual lyase activity of degrading poly-glucuronic and -mannuronic acids and had significant combined antibiofilm activities with carbenicillin and ciprofloxacin, reducing the P. aeruginosa biofilms’ surface area, biovolume, and thickness. In a follow-up investigation, using immunofluorescent staining and chromatography, Daboor et al. [99] confirmed the antibiofilm-enhancing effects of the alginate lyase enzyme, AlyP1400, against P. aeruginosa CF2F-implicated biofilm. Also, the hydrolytic and disruptive impact of the enzyme against the extracellular alginate produced by P. aeruginosa CF27 is reported to be instrumental to the anti-biofilm’s activities. Thogersen et al. [100] assayed the bioactive violacein and indolmycin produced by a mutant P. luteoviolacea S4054, which had inhibition activities against Staphylococcus aureus and Vibrio anguillarum. The UHPLC-UV/Vis studies utilized in comparing the expression of the bioactive compounds with those produced by a wild strain revealed a lower and higher production of violacein and indolmycin, respectively, compared to the wild strain. The study concluded that although the significant antibacterial compounds of Pseudoalteromonas sp. are yet to be identified, the two bioactive compounds also contribute to the observed antibacterial properties.
The complete genome elucidation of P. phenolica KCTC 12086T, which could produce antibiotic compounds including polybrominated-diphenyl ethers, -bipyrroles, and -biphenyls, with proven antibacterial effects against certain bacteria, including methicillin-resistant S. aureus, E. faecium, Enterococcus seriolicida, and Enterococcus faecalis has been reported by Choe et al. [101]. The result showed the presence of two 4,868,993 bp chromosomes with 4,264,659 bp coding regions, which encode a total of 4168 proteins. Also, 28 rRNA (9 operons), six ncRNAs, 113 tRNAs, eight pseudo-genes, and one tmRNA were detected. Furthermore, the bmp cluster (1–10), responsible for producing polybrominated compounds, was observed at different nucleotide positions on the chromosome. Other hydrolysis enzyme coding regions reported, including collagenases, phytase, chitinases, and proteases detected, further support the potential applicability of the isolate. Similarly, the genome of P. xiamenensis STKMTI.2 isolated from a mangrove soil sediment in Indonesia was elucidated by Handayani et al. [102], which consisted of 4,563,326 bp with a GC content of 43.2%, two circular and linear plasmids, one chromosome, 25 rRNAs, four ncRNAs, 4824 coding sequences, and a CRISPR. The CRISPR gene detected was attributed to the production of brominated marine phenols/pyrroles and secondary metabolites, including bmp 8 and 9 and peptides, butyrolactone, prodigiosin, RiPP-like, and Lant I, and justified the suspicion of P. xiamenensis STKMTI.2 for generating broad-spectrum antimicrobial compounds. The genome of Pseudoalteromonas sp. NC201 (from the coastal area of New Caledonia) with antibacterial potentials and has already been assessed for its probiotic effects through enhancement of survival rates in Litopenaeus stylirostris infected with Vibrio nigripulchritudo [103] was also sequenced by Sorieul et al. [104]. The analysis revealed 115 contigs (>100 bp). Of the contigs, 65 presented six scaffolds with an approximate GC content of 43.25%; two were the largest and represented 4.13 Mbp chromosome and 1.24 Mbp chromid, respectively. The remaining sequences comprised insertion sequences and ribosomal RNA operons in repetitive regions and clusters. These results indicated the ability of the isolate to synthesize antibacterial peptides, including bacteriocins and gramicidin/tyrocidine. In addition, the lodA and lodB genes, which are related to amino acid oxidases, suggested the production of oxygen peroxide, which can have bacterial inhibitory effects. Marchetti et al. [105] studied and sequenced the tam operon, which initiates the synthesis of P. tunicate-produced tambjamine YP1, a natural bipyrrole antibiotic, and reported to possess other bioactive potentials. They described the tam operon as possessing 19 genes, comprising a fused C-terminal acyl carrier protein and N-terminal adenylation domain, bound C 11 and 12 acyl-adenylate intermediates, transfers chain length of fatty acids from C6–C13 to an isolated acyl carrier protein domain, and thus shows the initiations of the production of tambjamine YP1 via the linkage of the pyrrole and fatty acid pathways. Maansson et al. [106] analyzed different geographic sort isolates of P. luteoviolacea for biosynthetic richness and diversity using metabolomic and sequencing techniques. The study reported enormous diversity in the 13 analyzed isolates, with only 2 and 7% of the chemical features and biosynthetic genes, respectively, common in the isolates. Genome sequencing of the isolates revealed the presence of biosynthetic clusters that were attributed to the generation of indolmycin, an antibacterial compound. Rond et al. [107] studied P. rubra, a cyclized prodiginine-producing bacterium. The genome sequence revealed an unclustered gene responsible for the enzyme that catalyzes regiospecific C-H and prodigiosin cyclization to cyclo-prodigiosin. Prodiginines are natural products with potent bioactive properties, including antibacterial and anticancer effects. Yu et al. [108] sequenced P. flavipulchra JG1, responsible for producing Pseudoalteromonas flavipulchra antibacterial protein (PfaP) for specific genes or clusters responsible for the expression of the antibacterial protein, which has been proven to have inhibitory effects against certain bacteria, including Bacillus spp., Aeromonas spp., and Vibrio spp. The isolate also produced p-hydroxybenzoic acid, trans-cinnamic acid, N-hydroxybenzoisoxazolone, 6-bromoindolyl-3-acetic acid, and 2′-deoxyadenosine, with inhibitory effects against some microorganisms, including the previously mentioned bacterial species. The results showed that P. flavipulchra JG1 had a total of 5,565,361 bp with a GC content and open reading frames of 43.23% and 4913. The tandem repeats, transposons, and insert sequences were 180, 143, and 5, respectively. It possesses a complex system of genes belonging to ABC-type antimicrobial peptide transport and siderophore export systems, penicillin-binding and beta-lactamase class C proteins, and efflux pumps, all contributing to the generation of bioactive metabolites. Specifically, PfaP, the reported most potent bioactive metabolite of the isolate, oxidizes certain amino acids to α-keto acids, hydrogen peroxide, and ammonium. Hydrogen peroxide decomposes into other metabolites, which exhibit antimicrobial effects. Diaz et al. [109] also sequenced P. piscicida 36Y_RITHPW, which produces bioactive compounds with inhibition effects against multi-resistant Vibrio parahaemolyticus implicated in shrimp hepatopancreatic necrosis disease. Summarily, 4548, 4217, and 71 genes, protein-coding sequences, and RNA sequences were reported. In addition to other findings, the authors elucidated the expression of bacteriocins and peptides (ribosomally produced and responsible for antibacterial properties) by 12 genes in a cluster, including polyketide synthase/non-ribosomal peptide synthase (PKS/NRPS), lantipeptide gene, type 1 PKS, 7 NRPS, and aryl-polyene/NRPS hybrid clusters. In agreement, the genome sequence of P. piscicida strain DE2-B by Richard et al. [110] revealed relatively the same genes responsible for the production of antimicrobial proteolytic enzymes and compounding, including peptides, polyketides, and alkaloids; thus, the P. piscicida strain DE2-B’s ability to inhibit some bacteria, including, V. parahaemolyticus. Jouault et al. [111] sequenced Pseudalteromonas sp. 3J6 and identified gene sequences, including the alt gene, which encodes 139 residue proteins and is responsible for the expression of alterocin, a protein implicated in inhibiting P. aeruginosa biofilms. Generally, the genome, GC content, and coding region of the Pseudalteromonas sp. 3J6 were approximately 4.6 Mb, 39.93%, and 3789, respectively.
5.2.2. Antibacterial Pseudoalteromonas spp. Polymer, Polysaccharides, and Peptides
On the isolation, identification, and bacterial origin suspicion of low molecular weight antimicrobial peptides of oyster hemolymph, Defer et al. [112] assayed the culture supernatant of different bacteria resident in the hemolymph for their antibacterial effects. Three strains of Pseudoalteromonas spp., designated hCg-6, hCg-10, and hCg-42, with sequence results suspecting the first two to be P. prydzensis/mariniglutinosa and the last to be P. paragorgicola/elyakovii, displayed ranging antibacterial activities against Aeromonas hydrophila CIP 7614, Listonella anguillarum NCBIM 829, Yersinia ruckeri ATCC 29473, Bacillus megaterium ATCC 10778, Lactococcus garviae ATCC 43921, and Salmonella enterica CIP 829. Two of the strains (hCg-6 and hCg-42) also showed BLIS-production abilities. Desriac et al. [113] showed the antibacterial activity of seven alterins with ranging molecular masses (924–982 Da) extracted from two Pseudoalteromonas spp. (hCg-6 and hCg-42) of healthy hemolymph microbiota of Crassostrea gigas (oyster) against several gram-negative bacteria, including Vibrio harveyi ORM4 and V. parahaemolyticus 13-028A/3. The produced alterins belong to the family of cationic cyclo-lipopeptides, which bind to bacterial lipopolysaccharides, provoking membrane depolarization and subsequent cell permeability and lysis [113]. Similarly, Offret et al. [114] obtained alterins from a group of 5 Pseudoalteromonas spp. isolated from the oyster hemolymph and also demonstrated ranging antibacterial activities against E. coli ATCC 25922, V. harveyi OMM4, V. pectenicida CIP 105190, V. tasmaniensis LGP32, and V. tapetis CECT 4600. These studies suggest that alterins can be potent lipopolysaccharide-neutralizing and synergistic antibiotic peptides. The polymer poly-hydroxybutyrate-co-hydroxy-hexanoate (PHBH), through its degradation products, including 3-hydroxy-butyrate and -hexanoate and hydroxy-alkanoic acids, is reported to have antibacterial activities. A review of a specific study found that it inhibits Vibrio penaeicida and increases the survival rate of shrimp exposed to V. penaeicida following consumption of PHBH-supplemented feed [115]. However, the study [108] reported the high PHBH degrading abilities of certain gram-negative bacteria, including P. shioyasakiensis and P. mariniglutinosa. This degradation action showed inhibitory/suppression effects against V. penaeicida through the generation of antibacterial monomer products, thus suggesting the potential application in aquaculture for the protection of shrimps against infection by V. penaeicida through diet supplementation with PHBH and the corresponding implicated PHBH-degrading P. shioyasakiensis and P. mariniglutinosa. No inhibitory effects were observed when V. penaeicida was challenged with Pseudoalteromonas spp., without PHBH. Kozuma et al. [116] tested an array of secondary metabolites of Pseudoalteromonas sp. SANK 71903 has potential inhibitory effects against lipopolysaccharides (LPS) and typical LPS-bearing bacteria. Cyclic Peptides, Ogipeptins (A–D), closely related to Polymyxin B, were identified and showed the ability to inhibit LPS through CD14 binding with 1C50 values of Ogipeptin-A = 4.8 nm, -B = 6.0 nm, -C = 4.1 nm, and -D = 5.6 nm and blockage of cytokine secretion from LPS-stimulated cells. They showed antibacterial effects against E. coli, with minimum inhibitory concentrations ranging from 0.25–1.0 μg/mL.
5.2.3. Antibacterial Pseudoalteromonas spp. Extracts and Organic Compounds
In vitro and in vivo studies by Wasana et al. [117] demonstrated the probiotic potential of P. ruthenica S6031. Broth culture spots of the isolate on microbial lawns of P. aeruginosa, Edwardsiella piscicida, A. hydrophila, and Candida albicans revealed significant positive inhibition against E. piscicida and A. hydrophila. In E. piscicida-challenged Zebra fish, there was higher cumulative per cent survival of the animals exposed to P. ruthenica-supplemented feed, outperforming the control group without supplements. It also revealed the induction of several immune-stress response gene transcripts and the downregulation of the proinflammatory genes. Thus, the study showed that the treatment group animals had better disease tolerance than the control group, making P. ruthenica S6031 a good potential probiotic isolate. The organic compound, korormicin, produced by many Pseudoalteromonas spp., including Pseudoalteromonas strain J010, is acknowledged by Maynard et al. [118] to have antibacterial activities, including against Vibrio spp., Aliivibrio sp, and Pseudomonas sp. They, however, through MICs and DNA sequence data, disclosed that, though korormicin is a potent inhibitor of NA+-pumping NADH (quinone oxidoreductase), the antibacterial effects are not due to the inhibition of the stated enzyme, but to the release of reactive O2 species via the electron transfer initiation in the enzyme, which promotes O2 and some redox cofactor’s reaction. In vitro, in vivo, and PCR evaluations by Wang et al. [119] showed antibacterial activities against V. parahaemolyticus, respective enhancement and decrease of the survival rates of V. parahaemolyticus-infected shrimps following exposure to Pseudoaltermonas spp-supplemented feed and shrimp hindgut presumptive Vibrio sp. counts, and reduced the copy number of V. parahaemolyticus toxin production gene, PirAvp, respectively, by two Pseudoalteromonas spp. coded CDM8 and CDA22. Following proper identification, Wang et al. [120] described the antibacterial mechanisms of one of the isolates, P. flavipulchra CDM8, which also showed potent inhibition activities against Bacillus spp. and Vibrio spp. Using antimicrobial biofilm assays and microscopy, they showed its broad-spectrum antimicrobial activity against both gram-positive and -negative bacteria using filter-impregnated culture from the isolate. They described the mechanism to involve P. flavipulchra CDM8 metabolites, including hydrogen peroxide, PfaP-like antibacterial proteins, and other molecules. They also identified a transferable outer surface-positioned vesicle/pilus-like structure, which likely contributes to the observed inhibition activities and is described as a novel mechanism of antibacterial activity by the isolate. Using several genome analysis tools, including the antiSMASH (a secondary metabolite prediction tool) and bioassays, Paulsen et al. [121] identified, among others, a highly halogenated tetrabromopyrrole as the main antibacterial metabolite of Pseudoalteromonas sp. type strain S4498. Genome studies revealed that the strain had a genome size of 5.4 Mb and a GC content of 43%. However, they suggested that tetrabromopyrrole induced its antimicrobial effects through its signaling properties, cellular stress induction, and dibromo maleimide activity, the oxidized by-product. Desriac et al. [122], in an assay of the different cell-free cultures of 843 species/strains obtained within the haemolymphs microbiota of bivalve species from the sea, showed the Pseudoalteromonas strains to possess the most potent antimicrobial abilities (against 12 marine gram-positive and -negative pathogens), with one Pseudoalteromonas sp. (designated as hCg-51) beating the other isolates with inhibition at an extreme dilution of 1:1024. They also presented a dose-dependent beneficial effect of the strain on the hemocyte survival rates. Thus, suggesting their strong probiotic potential. Similarly, Offret et al. [123] assayed a collection of 11 Pseudolateromonas spp. obtained from the hemolymph of mussels and oysters for their antibacterial activities against V. harveyi. The results showed that more than half of the isolates (54%) had varying inhibition activities, with one coded hCg-6 outperforming the others in the collection, with an impressive inhibition zone of 19 mm. The Pseudoalteromons sp. (hCg-6) also revealed significant probiotic activities following the increment in the survival rate of Abalone exposed to hCg-6 and subsequently infected with V. harveyi. Also, Tangestani et al. [124] reported antibacterial activities against E. coli, Staphylococcus epidermis, and Kocuria rhizophila by solvent extracts of two Pseudoalteromonas spp., among other marine bacteria isolated from the surface of various marine macroalgae. The two relevant isolates were phylogenetically confirmed to be P. issachenkonii and P. haloplanktis TAC125, associated with the seaweeds Splachnidium rugosum, and Carpophyllum maschalocarpum, respectively. Papa et al. [125] reported the antibiofilm activity of the culture supernatant of P. haloplanktis TAC125 against S. epidermidis by disrupting the S. epidermidis biofilm multicellular structure. Sannino et al. [126], via a defined culture and fermentation system, produced and accumulated methylamine, a volatile organic compound (VOC), from P. haloplanktis TAC125, which by the minimum volatile inhibitory concentrations, presented a dose-dependent inhibition of different strains of Burkholderia spp. and E. coli. Venuti et al. [127] assayed the antibacterial properties of pentadecanol, a metabolite of P. haloplanktis, and the derivatives against Listeria monocytogenes, using the minimum inhibitory concentrations. The results showed that the derivatives, including the ester, acetal, and carboxylic acid, had no inhibitory effects against the test isolates; however, pentadecanol showed potent antibacterial properties and exhibited a MIC of 0.6 mg/mL. Similarly, Casillo et al. [128] isolated pentadacanol from the same P. haloplanktis, and they demonstrated its antibiofilm activity against S. epidermis. They suggested the mechanism of action of inhibiting the biofilm from involving the interference of the quorum sensing system of the S. epidermis through the AI-2 signaling process. Using 16S RNA sequencing and NMR elucidation, an organic compound was obtained and determined to be isatin, respectively, from P. rubra TKJD 22 associated with marine tunicates by Ayuningrum et al. [129]. The isatin demonstrated inhibition effects against both the laboratory-isolated and MDR-ESBL E. coli. Tebben et al. [130] isolated bioactive compounds from the ethanol extract of Pseudoalteromonas strain J010, which were elucidated using MS and NMR techniques. Among others, novel 4′-(3,4,5-tribromo-1H-pyrrol-2-yl) methyl)phenol (a bromopyrrole), 5 koromicins, and tetrabromopyrrole were identified and presented ranging antibacterial activities against S. aureus and other gram-negative bacteria, including Vibrio spp., P. aeruginosa, Pseudoalteromonas spp., and Shewanella aquimarina. In another study, following the inhibition activities of the crude extract fractions of Pseudoalteromonas piscicida S2040 against P. aeruginosa, Sonnenschein et al. [131] isolated bromo- and dibromoalterochromides, myxochelins A and B, and alteramide A, chosen for their antibacterial effects. Eliseikina et al. [132] isolated Pseudoalteromonas piscicida 2202, a natural flora of Modiolus kurillenis, which had selective antimicrobial activity against S. aureus, C. albicans, and B. subtilis and no significant activity against E. coli and P. aeruginosa. They, however, suggested caution in the application as potent probiotic agent. Among other heterotrophic bacteria, Setiaji et al. [133] employed Pseudoalteromonas sp. JS19 MT102924.1 in an ethyl acetate extraction system to yield secondary metabolites, through which the phytochemical analysis showed the presence of alkanes, carbonyls, alcohols, and alkenes. The extract had antibacterial activities with inhibition zones of 9.8, 10.8, and 9.8 mm against A. hydrophilia, Vibrio alginolyticus, and P. aeruginosa, respectively.
5.2.4. Antibacterial Pseudoalteromonas spp. Nanoparticles
Beleneva et al. [83] reported high concentration (500 and 1000 μg/mL) antimicrobial effects of the selenium and tellurium-based nanoparticles of the isolates of P. shioyasakiensis against typed cultures of E. coli, P. aeruginosa, S. aureus, B. subtilis, and additionally a fungus (C. albicans), with the tellurium-based nanoparticles having overall more significant effects.
6. Discussion
The review revealed that none of the literature evaluated the genetic basis for the anticancer properties of Pseudoalteromonas spp. However, involved k-selenocarrageenases/k-carrageenans, arylsulfatase, and prodigiosin as the pigments and enzymes, capsular polysaccharides, and polysaccharides and Pseudoalteropeptide A in the polymer, polysaccharide, and peptide category, a crude extract of P. haloplanktis, selenium, and tellurium nanoparticles in the reported anticancer activities. The reported antibacterial properties involved the production of bioactive pigments and enzymes, including alginate lyase (AlyP1400), violacein, and indolmycin, specific gene sequences and clusters responsible for the expression and production of polybrominated compounds, peptides, polyketides, alkaloids, tambjamine YP1, cylco-prodigine, PFaP, alterocins/alterins, and other compounds including 3-hydroxy-butyrate, 3-hydroxy-hexanoate, hydroxy-alkanoic acids, korormicin, hydrogen peroxide, tetrabromopyrrole, methylamine, isatin, brominated compounds, and nanoparticles by representative tests Psesudoaltromonas spp. Much of the literature summarized in Figure 3, however, is currently in the preliminary stages; without an in-depth analysis of the mechanistic and physiological basis of the observed bioactive properties as such, the current review provided the basis and a standpoint for the advancement of the studies including the isolation and extensive characterization of the bioactive compounds with a view to possible clinical application and commercialization.
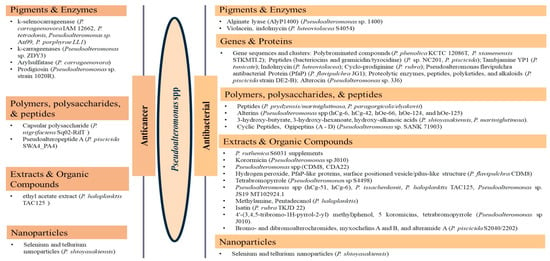
Figure 3.
A pictorial presentation of the anticancer and antibacterial constituents of Pseudoalteromonas spp.
7. Production and Development of Marine Probiotics
Marine probiotics have proven therapeutic applications; however, adequate exploitation and utilization of these properties hinge on optimal industrial-scale production and development. These involve several steps, including isolation, identification, and characterization of potential probiotic strains and testing their efficacy and safety in various applications [134,135]. The first step in marine probiotics production is isolating and identifying likely probiotic strains from marine environments and involves collecting samples from marine ecosystems, such as seawater, sediments, and aquatic organisms and isolating potential probiotic strains using standard microbiological and molecular techniques. The identified strains are then characterized for their morphology, physiology, and biochemistry to profile their potential as probiotics [135]. They are screened and selected for their probiotic properties, including their ability to survive in the host gut system, produce anticancer and antimicrobial compounds, or stimulate the host’s immune system [136]. The selected strains are tested for their safety and efficacy in various applications [137], after which they are mass-produced. Marine probiotics can be produced using different techniques such as batch, fed-batch, and continuous cultures [138,139]. The production process involves growing the selected strains in a suitable growth medium and harvesting and processing the cells to obtain the final product [137,140]. The produced marine probiotics are formulated into an appropriate delivery system, such as capsules, powders, or liquid formulations, to maintain viability, stability, and efficacy during storage and transportation [141,142], and the final application of the produced probiotics in the intended fields, such as aquaculture, animal feed, and human health.
However, the current industrial production of marine probiotics is hindered by several factors, most importantly, the high cost of culture media required for optimal growth [28]. Optimal media for propagating marine probiotics should contain sufficient organic nitrogen, peptones, and protein hydrolysates from various sources. These media constituents are, however, expensive [28]. However, alternative sources of these essential elements/compounds from nature, such as organic marine wastes from fish and other life forms, are imperative. An example is the sourcing of fish peptones from different fish waste materials and by-products by Vázquez et al. [28] which resulted in 120 times better growth of the test probiotics Pseudomonas fluorescens and Phaeobacter sp. The development of these media nutrient requirements is ongoing through various studies and, with time, will ensure the scale-up of the production of proven probiotics for commercial use in the treatment of different medical conditions, including cancer and bacterial infections [143].
8. Limitations, Prospects, Future Perspectives
Studies in aquaculture have identified diverse marine bacterial species as potent probiotics [111,112,113]. Some identified bacteria can be considered safe (GRAS) for humans, while others are pathogenic. Since these marine probiotics have shown the potential to produce substances that could be of health benefit to humans, there is a need for innovative research to harness these benefits. In the development of marine probiotics, one major challenge is to isolate and identify potential strains [32,144]. In addressing low specificity and side effects associated with chemotherapeutic drugs and radiotherapy, there is a need to search for novel and non-toxic compounds from natural sources [79]. While the potential of terrestrial probiotics in cancer management has been explored, the function of marine probiotics is not yet fully understood. Several studies, including animal models and cell lines, have shown the promising therapeutic effects of marine probiotics. However, clinical trials are necessary to understand the therapeutic mechanisms [51] fully. Randomized, double-anonymized, placebo-controlled clinical trials are crucial to gaining broader acceptance in the medical community [49,57]. Although drug discovery and development from marine sources have inherent limitations, advances in analytical instrumentation, screening platforms, scalable synthetic approaches, and antibody-drug conjugates (ADCs) have expanded the clinical arsenal for therapeutic application [145]. In addition, omics approaches, including probiotic genome, metagenome, transcriptome, and metatranscriptome sequencing, are yet to be fully explored in cancer studies. These methods can enhance the detection and understanding of several bioactive mechanisms.
Biotechnological concepts and approaches are still evolving. In this review, we strongly recommend two possible biotechnological approaches that could yield beneficial products from these organisms; first, the selective identification and deletion of the virulence-causing gene(s) in the pathogenic marine probiotics while optimizing the expression of the genes responsible for producing the beneficial substances. The process affords the removal of the virulence factor and ensures the safety of use. This approach has yielded substances of pharmaceutical importance [146] and hindered the expression of patulin, a mycotoxin by P. expansum [147]. Second, the elucidation, manipulation, and optimization of the genes responsible for producing the bioactive agents using recombinant DNA (rDNA) technologies [148].
9. Conclusions
The need for alternative medicinal agents to synthetic/chemotherapeutic drugs and radiation (as it applies to cancer) in managing prevalent diseases is evident. Marine probiotics are, however, proven to be viable bioactive synthetic microorganisms. Specifically, Pseudoalteromonas spp., a group of marine probiotics, has shown potential therapeutic application against diseases, including cancer and bacterial-implicated diseases, through their activities and byproducts or metabolites. The production of therapeutic/bioactive agents from marine probiotics looks promising; however, the need for intensified research cannot be over-emphasized to enable optimal utilization of the potential therein.
Author Contributions
Conceptualization, O.C.E., D.P.B. and S.C.E.; writing—original draft preparation, O.C.E., D.P.B. and S.C.E.; writing—review and editing, O.C.E., D.P.B., S.C.E., S.A.E., C.N.O., V.M.B. and M.M.D.C.V. All authors have read and agreed to the published version of the manuscript.
Funding
Financial Support: Project funding by FAPESP (São Paulo, Brazil) (Refs. No. 2022/10775-9 (Project PsgPhageKill) and 2018/05522-9 (Project PsaPhageKill)), is hereby gratefully acknowledged. This work also received support from CNPq, in the form of a Research Productivity (PQ) fellowship granted to Victor M. Balcão (Ref. No. 301978/2022-0).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug. Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Ciênc. 2019, 91 (Suppl. S3), e20190105. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Code of Practice for Fish and Fishery Products. FAO and WHO; 2020. Available online: http://www.fao.org/documents/card/en/c/cb0658en (accessed on 20 March 2023).
- Chen, Z.; Guo, J.; Jiang, Y.; Shao, Y. High concentration and high dose of disinfectants and antibiotics used during the COVID-19 pandemic threaten human health. Environ. Sci. Eur. 2021, 33, 11. [Google Scholar] [CrossRef]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef]
- The Rules Governing Medicinal Products in the European Union. In GMP/ISO Quality Audit Manual for Healthcare Manufacturers and Their Suppliers, (Volume 2—Regulations, Standards, and Guidelines), 6th ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 257–316. Available online: https://www.taylorfrancis.com/books/9780203026656/chapters/10.3109/9780203026656-14 (accessed on 20 March 2023).
- Tripathy, A.; Dash, J.; Kancharla, S.; Kolli, P.; Mahajan, D.; Senapati, S.; Jena, M.K. Probiotics: A promising candidate for management of colorectal cancer. Cancers 2021, 13, 3178. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bedada, T.L.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Kesler, S.R.; Ahles, T.A.; Morrow, G.R. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry 2014, 26, 102–113. [Google Scholar] [CrossRef]
- Sultana, A.; Smith, C.T.; Cunningham, D.; Starling, N.; Neoptolemos, J.P.; Ghaneh, P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J. Clin. Oncol. 2007, 25, 2607–2615. [Google Scholar] [CrossRef]
- Majeed, H.; Gupta, V. Adverse Effects of Radiation Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK563259/ (accessed on 4 March 2023).
- Tarnecki, A.M.; Wafapoor, M.; Phillips, R.N.; Rhody, N.R. Benefits of a Bacillus probiotic to larval fish survival and transport stress resistance. Sci. Rep. 2019, 9, 4892. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.P.; Ibáñez, A.L.; Monroy, H.O.A.; Ramírez, S.H.C. Use of probiotics in aquaculture. ISRN Microbiol. 2012, 2012, 916845. [Google Scholar] [CrossRef] [PubMed]
- Borchert, E.; Knobloch, S.; Dwyer, E.; Flynn, S.; Jackson, S.A.; Jóhannsson, R.; Marteinsson, V.T.; O’Gara, F.; Dobson, A.D.W. Biotechnological Potential of Cold Adapted Pseudoalteromonas spp. Isolated from ‘Deep Sea’ Sponges. Mar. Drugs 2017, 15. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5484134/ (accessed on 13 May 2023).
- Paulsen, S.S.; Strube, M.L.; Bech, P.K.; Gram, L.; Sonnenschein, E.C. Marine chitinolytic Pseudoalteromonas represents an untapped reservoir of bioactive potential. mSystem 2019, 4, e00060-19. [Google Scholar] [CrossRef] [PubMed]
- Offret, C.; Desriac, F.; Le Chevalier, P.; Mounier, J.; Jégou, C.; Fleury, Y. Spotlight on antimicrobial metabolites from the marine bacteria Pseudoalteromonas: Chemodiversity and ecological significance. Mar. Drugs 2016, 14, 129. [Google Scholar] [CrossRef]
- Leighl, N.B.; Nirmalakumar, S.; Ezeife, D.A.; Gyawali, B. An arm and a leg: The rising cost of cancer drugs and impact on access. Am. Soc. Clin. Oncol. Educ. Book. 2021, 41, e1–e12. [Google Scholar] [CrossRef]
- Dzobo, K. The role of natural products as sources of therapeutic agents for innovative drug discovery. Compr. Pharmacol. 2022, 408–422. [Google Scholar]
- Sayes, C.; Leyton, Y.; Riquelme, C. Probiotic bacteria as an healthy alternative for fish aquaculture. Antibiot. Use Anim. Savic. Ed. Rij. Croat. InTech. Publ. 2018, 115–132. [Google Scholar]
- Blackall, L.L.; Dungan, A.M.; Hartman, L.M.; van Oppen, M.J. Probiotics for corals. Microbiol. Aust. 2020, 41, 100–104. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Swelum, A.A.; Abo Ghanima, M.M.; Shukry, M.; Omar, A.A.; Taha, A.E.; Salem, H.M.; El-Tahan, A.M.; El-Tarabily, K.A.; El-Hack, E.A. Shrimp production, the most important diseases that threaten it, and the role of probiotics in confronting these diseases: A review. Res. Vet. Sci. 2022, 144, 126–140. [Google Scholar] [CrossRef]
- Saraf, K.; Shashikanth, M.C.; Priy, T.; Chaitanya, N.C. Probiotics-do they have a role in medicine and dentistry. J. Assoc. Physicia. Ind. 2010, 58, 488–490. [Google Scholar]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to qualify mas “Probiotic” in foods and dietary supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Sen, M. Role of probiotics in health and disease—A review. Int. J. Adv. Life. Sci. Res. 2019, 30, 1–11. [Google Scholar]
- Mujeeb, I.; Ali, S.H.; Qambarani, M.; Ali, S.A. Marine bacteria as potential probiotics in aquaculture. J. Microbiol. Biotechnol. Food Sci. 2022, e5631. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Durán, A.; Nogueira, M.; Menduíña, A.; Antunes, J.; Freitas, A.C.; Gomes, A.M. Production of marine probiotic bacteria in a cost-effective marine media based on peptones obtained from discarded fish by-products. Microorganism 2020, 8, 1121. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Bhatnagar, I.; Kang, K.H. Development of marine probiotics: Prospects and approach. Adv. Food. Nutr. Res. 2012, 65, 353–362. [Google Scholar]
- Madison, D.; Schubiger, C.; Lunda, S.; Mueller, R.S.; Langdon, C. A marine probiotic treatment against the bacterial pathogen Vibrio coralliilyticus to improve the performance of Pacific (Crassostrea gigas) and Kumamoto (C. sikamea) oyster larvae. Aquaculture 2022, 560, 738611. [Google Scholar] [CrossRef]
- Sumithra, T.G.; Reshma, K.J.; Christo, J.P.; Anusree, V.N.; Drisya, D.; Kishor, T.G.; Revathi, D.N.; Sanil, N.K. A glimpse towards cultivable hemolymph microbiota of marine crabs: Untapped resource for aquatic probiotics/antibacterial agents. Aquaculture 2019, 501, 119–127. [Google Scholar] [CrossRef]
- Anas, K.K.; Mathew, S. Marine Nutraceuticals; ICAR-Central Institute of Fisheries Technology: Willingdon Island, Kochi, Kerala, India, 2018. [Google Scholar]
- Makled, S.O.; Hamdan, A.M.; El-Sayed, A.F.M. Growth promotion and immune stimulation in Nile tilapia, Oreochromis niloticus, fingerlings following dietary administration of a novel marine probiotic, Psychrobacter maritimus S. Probiot. Antimicrob. Proteins 2020, 12, 365–374. [Google Scholar] [CrossRef]
- Koga, A.; Goto, M.; Hayashi, S.; Yamamoto, S.; Miyasaka, H. Probiotic effects of a marine purple non-sulfur bacterium, Rhodovulum sulfidophilum KKMI01, on Kuruma Shrimp (Marsupenaeus japonicus). Microorganisms 2022, 10, 244. [Google Scholar] [CrossRef]
- Norouzi, H.; Danesh, A.; Mohseni, M.; Rabbani, K.M. Marine actinomycetes with probiotic potential and bioactivity against multidrug-resistant bacteria. Int. J. Mol. Cell. Med. 2018, 7, 44–52. [Google Scholar]
- Reyes-Becerril, M.; Alamillo, E.; Angulo, C. Probiotic and immunomodulatory activity of marine yeast Yarrowia lipolytica strains and response against Vibrio parahaemolyticus in Fish. Probiot. Antimicrob. Proteins 2021, 13, 1292–1305. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.M.; El-Sayed, A.F.M.; Mahmoud, M.M. Effects of a novel marine probiotic, Lactobacillus plantarum AH 78, on growth performance and immune response of Nile tilapia (Oreochromis niloticus). J. Appl. Microbiol. 2016, 120, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, F.A.; Padilla, D.; Real, F.; Ramos, S.M.J.; Acosta-Hernández, B.; Sánchez, H.A.; García-Álvarez, N.; Rosario Medina, I.; Silva Sergent, F.; Déniz, S.; et al. Screening of new potential probiotics strains against Photobacterium damselae Subsp. piscicida for marine aquaculture. Animals 2021, 11, 2029. [Google Scholar] [CrossRef] [PubMed]
- Victor, O.E. A Review on the Application and Benefits of Probiotics Supplements in Fish Culture. Oceanogr. Fish Open Access J. 2020, 11. Available online: https://juniperpublishers.com/ofoaj/OFOAJ.MS.ID.555817.php (accessed on 20 March 2023). [CrossRef]
- Sambrani, R.; Abdolalizadeh, J.; Kohan, L.; Jafari, B. Recent advances in the application of probiotic yeasts, particularly Saccharomyces, as an adjuvant therapy in the management of cancer with focus on colorectal cancer. Mol. Biol. Rep. 2021, 48, 951–960. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Montalbano, A.; Cueto, M.; Díaz Marrero, A.R.; Deniz, I.; Erdoğan, A.; Lukić Bilela, L.; Moulin, C.; Taffin-de-Givenchy, E.; et al. Marine anticancer agents: An overview with a particular focus on their chemical classes. Mar. Drugs 2020, 18, 619. [Google Scholar] [CrossRef]
- El-Baz, A.F.; El-Enshasy, H.A.; Shetaia, Y.M.; Mahrous, H.; Othman, N.Z.; Yousef, A.E. Semi-industrial scale production of a new yeast with probiotic traits, Cryptococcus sp. YMHS, isolated from the red sea. Probiot. Antimicrob. Prot. 2018, 10, 77–88. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Ângulo, C.; Ângulo, M.; Esteban, M.Á. Probiotic properties of Debaryomyces hansenii BCS004 and their immunostimulatory effect in supplemented diets for gilthead seabream (Sparus aurata). Aquac. Res. 2021, 52, 2715–2726. [Google Scholar] [CrossRef]
- Tseng, C.C.; Lin, Y.J.; Liu, W.; Lin, H.Y.; Chou, H.Y.; Thia, C.; Wu, J.H.; Chang, J.S.; Wen, Z.H.; Chang, J.J.; et al. Metabolic engineering probiotic yeast produces 3S, 3′S-astaxanthin to inhibit B16F10 metastasis. Food Chem. Toxicol. 2020, 135, 110993. [Google Scholar] [CrossRef]
- Valderrama, B.; Ruiz, J.J.; Gutiérrez, M.S.; Alveal, K.; Caruffo, M.; Oliva, M.; Flores, H.; Silva, A.; Toro, M.; Reyes-Jara, A.; et al. Cultivable yeast microbiota from the marine fish species Genypterus chilensis and Seriolella violacea. J. Fungi 2021, 7, 515. [Google Scholar] [CrossRef]
- Tian, B.C.; Liu, G.L.; Chi, Z.; Hu, Z.; Chi, Z.M. Occurrence and distribution of strains of Saccharomyces cerevisiae in China Seas. J. Mar. Sci. Eng. 2021, 9, 590. [Google Scholar] [CrossRef]
- Wasana, W.P.; Senevirathne, A.; Nikapitiya, C.; Eom, T.Y.; Lee, Y.; Lee, J.S.; Kang, D.H.; Oh, C.; De Zoysa, M. A novel Pseudoalteromonas xiamenensis marine isolate as a potential probiotic: Anti-inflammatory and innate immune modulatory effects against thermal and pathogenic stresses. Mar. Drugs 2021, 19, 707. [Google Scholar] [CrossRef] [PubMed]
- Vidya, S.; Thiruneelakandan, G. Probiotic potentials of lactobacillus and its anti cancer activity. Int. J. Curr. Res. 2015, 7, 20680–20684. [Google Scholar]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The role of probiotics in cancer prevention. Cancers 2020, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Dongare, P.N.; Motule, A.S.; More, M.P.; Patinge, P.A.; Bakal, R. An overview on anti-cancer drugs from marine source. World J. Pharm. Res. 2021, 10, 950–956. [Google Scholar]
- Singh, A.; Krishna, S. Immunomodulatory and Therapeutic Potential of Marine Flora Products in the Treatment of Cancer. In Bioactive Natural Products for the Management of Cancer: From Bench to Bedside; Sharma, A.K., Ed.; Springer: Singapore, 2019; pp. 139–166. Available online: http://link.springer.com/10.1007/978-981-13-7607-8_7 (accessed on 20 March 2023).
- Dharmaraj, S. Marine Streptomyces as a novel source of bioactive substances. World J. Microbiol. Biotechnol. 2010, 26, 2123–2139. [Google Scholar] [CrossRef]
- Malik, S.S.; Saeed, A.; Baig, M.; Asif, N.; Masood, N.; Yasmin, A. Anticarcinogenecity of microbiota and probiotics in breast cancer. Int. J. Food Prop. 2018, 21, 655–666. [Google Scholar] [CrossRef]
- Sankarapandian, V.; Venmathi Maran, B.A.; Rajendran, R.L.; Jogalekar, M.P.; Gurunagarajan, S.; Krishnamoorthy, R.; Gangadaran, P.; Ahn, B.C. An update on the effectiveness of probiotics in the prevention and treatment of cancer. Life 2022, 12, 59. [Google Scholar] [CrossRef]
- Shenderov, B.A. Metabiotics: Novel idea or natural development of probiotic conception. Microb. Ecol. Health Dis. 2013, 12, 24. [Google Scholar] [CrossRef]
- Hradicka, P.; Beal, J.; Kassayova, M.; Foey, A.; Demeckova, V. A novel lactic acid bacteria mixture: Macrophage-targeted prophylactic intervention in colorectal cancer management. Microorganisms 2020, 8, 387. [Google Scholar] [CrossRef]
- Yu, A.Q.; Li, L. The potential role of probiotics in cancer prevention and treatment. Nutr. Cancer 2016, 68, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Sithranga Boopathy, N.; Kathiresan, K. Anticancer drugs from marine flora: An overview. J. Oncol. 2010, 2010, 214186. [Google Scholar] [CrossRef] [PubMed]
- Pereira, W.A.; Piazentin, A.C.M.; de Oliveira, R.C.; Mendonça, C.M.N.; Tabata, Y.A.; Mendes, M.A.; Fock, R.A.; Makiyama, E.N.; Corrêa, B.; Vallejo, M.; et al. Bacteriocinogenic probiotic bacteria isolated from an aquatic environment inhibit the growth of food and fish pathogens. Sci. Rep. 2022, 12, 5530. [Google Scholar] [CrossRef] [PubMed]
- Sarika, A.; Lipton, A.; Aishwarya, M.; Mol, R.R. Lactic Acid Bacteria from Marine Fish: Antimicrobial Resistance and Production of Bacteriocin Effective against L. monocytogenes In Situ. J. Food Microbiol. Saf. Hyg. 2018, 3. Available online: https://www.omicsonline.org/open-access/lactic-acid-bacteria-from-marine-fish-antimicrobial-resistance-and-production-of-bacteriocin-effective-against-l-monocytogenes-in-2476-2059-1000128-96294.html (accessed on 20 March 2023).
- Chbel, A.; Rodriguez-Castro, J.; Quinteiro, J.; Rey-Méndez, M.; Delgado, A.S.; Soukri, A.; Khalfi, B.E. Isolation, Molecular Identification and Antibacterial Potential of Marine Bacteria from Deep Atlantic Ocean of Morocco. Avicenna J. Med. Biotechnol. 2022. Available online: https://publish.kne-publishing.com/index.php/AJMB/article/view/9827 (accessed on 20 March 2023).
- Alonso, S.; Carmen Castro, M.; Berdasco, M.; de la Banda, I.G.; Moreno-Ventas, X.; de Rojas, A.H. Isolation and partial characterization of lactic acid bacteria from the gut microbiota of marine fishes for potential application as probiotics in aquaculture. Probiotics Antimicrob. Proteins 2019, 11, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Kaktcham, P.M.; Temgoua, J.B.; Ngoufack Zambou, F.; Diaz-Ruiz, G.; Wacher, C.; Pérez-Chabela, M.d.L. Quantitative analyses of the bacterial microbiota of rearing environment, tilapia and common carp cultured in earthen ponds and inhibitory activity of its lactic acid bacteria on fish spoilage and pathogenic bacteria. World J. Microbiol. Biotechnol. 2017, 33, 32. [Google Scholar] [CrossRef]
- Sironi, M.; Cagliani, R.; Forni, D.; Clerici, M. Evolutionary insights into host–pathogen interactions from mammalian sequence data. Nat. Rev. Genet. 2015, 16, 224–236. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.S. Effect of probiotics enriched diet on Paralichthys olivaceus infected with lymphocystis disease virus (LCDV). Fish Shellfish Immunol. 2010, 29, 868–874. [Google Scholar] [CrossRef]
- Liu, C.H.; Chiu, C.H.; Wang, S.W.; Cheng, W. Dietary administration of the probiotic, Bacillus subtilis E20, enhances the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coioides. Fish Shellfish Immunol. 2012, 33, 699–706. [Google Scholar] [CrossRef]
- Balcázar, J.L.; Vendrell, D.; de Blas, I.; Ruiz-Zarzuela, I.; Gironés, O.; Múzquiz, J.L. In vitro competitive adhesion and production of antagonistic compounds by lactic acid bacteria against fish pathogens. Vet. Microbiol. 2007, 122, 373–380. [Google Scholar] [CrossRef]
- Chattaraj, S.; Ganguly, A.; Mandal, A.; Das Mohapatra, P.K. A review of the role of probiotics for the control of viral diseases in aquaculture. Aquac. Int. 2022, 30, 2513–2539. [Google Scholar] [CrossRef]
- Sundararaman, A.; Ray, M.; Ravindra, P.V.; Halami, P.M. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 8089–8104. [Google Scholar] [CrossRef] [PubMed]
- Zaharuddin, L.; Mokhtar, N.M.; Muhammad, N.K.N.; Raja, A.R.A. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Lidon, F.; Silva, M. An overview on applications and side effects of antioxidant food additives. Emir. J. Food Agric. 2016, 28, 823. [Google Scholar] [CrossRef]
- Husain, F.; Duraisamy, S.; Balakrishnan, S.; Ranjith, S.; Chidambaram, P.; Kumarasamy, A. Phenotypic assessment of safety and probiotic potential of native isolates from marine fish Moolgarda seheli towards sustainable aquaculture. Biologia 2022, 77, 775–790. [Google Scholar] [CrossRef]
- Angulo, M.; Reyes-Becerril, M.; Cepeda-Palacios, R.; Tovar-Ramírez, D.; Esteban, M.Á.; Angulo, C. Probiotic effects of marine Debaryomyces hansenii CBS 8339 on innate immune and antioxidant parameters in newborn goats. Appl. Microbiol. Biotechnol. 2019, 103, 2339–2352. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine natural products: A source of novel anticancer drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef]
- Saeed, A.F.U.H.; Su, J.; Ouyang, S. Marine-derived drugs: Recent advances in cancer therapy and immune signaling. Biomed. Pharmacother. 2021, 134, 111091. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. Marine microbial-derived antibiotics and biosurfactants as potential new agents against catheter-associated urinary tract infections. Mar. Drugs 2021, 19, 255. [Google Scholar] [CrossRef]
- Fenical, W. Marine microbial natural products: The evolution of a new field of science. J. Antibiot. 2020, 73, 481–487. [Google Scholar] [CrossRef]
- Montuori, E.; de Pascale, D.; Lauritano, C. Recent discoveries on marine organism immunomodulatory activities. Mar. Drugs 2022, 20, 422. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, M.A.; Tammam, M.A.; El-Demerdash, A.; Atanasov, A.G. Insights about clinically approved and preclinically investigated marine natural products. Curr. Res. Biotechnol. 2020, 2, 88–102. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Ng, H.J.; Webb, H.K. The family pseudoalteromonadaceae. Prokaryotes 2014, 1, 575–582. [Google Scholar]
- Beleneva, I.A.; Kharchenko, U.V.; Kukhlevsky, A.D.; Boroda, A.V.; Izotov, N.V.; Gnedenkov, A.S.; Egorkin, V.S. Biogenic synthesis of selenium and tellurium nanoparticles by marine bacteria and their biological activity. World. J. Microbiol. Biotechnol. 2022, 38, 188. [Google Scholar] [CrossRef]
- Rao, A.S.; Nair, A.; Salu, H.A.; Pooja, K.R.; Nandyal, N.A.; Joshi, V.S.; More, V.S.; Francois, N.; Anantharaju, K.S.; More, S.S. Carbohydrases: A class of all-pervasive industrial biocatalysts. Biotechnol. Microb. Enzym. 2023, 497–523. [Google Scholar]
- Chen, X.L.; Wang, Y.; Wang, P.; Zhang, Y.Z. Proteases from the marine bacteria in the genus Pseudoalteromonas: Diversity, characteristics, ecological roles, and application potentials. Mar. Life Sci. Technol. 2020, 2, 309–323. [Google Scholar] [CrossRef]
- Li, B.; Wang, P.; Zeng, Z.; Cai, X.; Wang, G.; Wang, X. Complete genome sequence of Pseudoalteromonas rubra SCSIO 6842, harboring a putative conjugative plasmid pMBL6842. J. Biotech. 2016, 224, 66–67. [Google Scholar] [CrossRef]
- Pandey, A.K.; Verma, S. Combination drug therapy for multimodal treatment of cancer by targeting mitochondrial transcriptional pathway: An in-silico approach. Med. Hypotheses 2020, 143, 110075. [Google Scholar] [CrossRef]
- Gao, S.; Pan, L.; Huang, F.; Song, M.; Tian, C.; Zhang, M. Metagenomic insights into the structure and function of intestinal microbiota of the farmed Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2019, 499, 109–118. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, X.; Xu, H.; Liu, C.; Li, R.; Zhang, Y.; Miao, J.; Qu, C. Optimization of κ-Selenocarrageenase Production by Pseudoalteromonas sp. Xi13 and its immobilization. Molecules 2022, 27, 7716. [Google Scholar] [CrossRef]
- Wang, X.; Liang, C.; Yang, X.; Chen, F.; Li, R.; Qu, C.; Miao, J. Complete genome sequence of Pseudoalteromonas sp. Xi13 capable of degrading κ-selenocarrageenan isolated from the floating ice of Southern Ocean. Mar. Genom. 2022, 61, 100917. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Long, L.; Sang, Y.; Jiang, Z.; Ni, H.; Zheng, M.; Li, L.; Li, Q.; Zhu, Y. Simultaneous enhancement of thermostability and catalytic activity of κ-carrageenase from Pseudoalteromonas tetraodonis by rational design. Enz. Microb. Technol. 2023, 167, 110241. [Google Scholar] [CrossRef]
- Zhao, Y.; Chi, Z.; Xu, Y.; Shi, N.; Chi, Z.; Liu, G. High-level extracellular expression of κ-carrageenase in Brevibacillus choshinensis for the production of a series of κ-carrageenan oligosaccharides. Process Biochem. 2018, 64, 83–92. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, B.; Zhang, Y.; Sun, W.; Pu, Z.; Bao, Y. Purification and characterization of a cold-adapted κ-carrageenase from Pseudoalteromonas sp. ZDY3. Protein Expr. Purif. 2021, 178, 105768. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, H.; Qiao, C.; Li, L.; Jiang, Z.; Xiao, A.; Ni, H. Characterization of an arylsulfatase from a mutant library of Pseudoalteromonas carrageenovora arylsulfatase. Int. J. Biol. Macromol. 2017, 96, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Soliev, A.B.; Hosokawa, K.; Enomoto, K. Effects of prodigiosin family compounds from Pseudoalteromonas sp. 1020R on the activities of protein phosphatases and protein kinases. J. Enzy. Inhib. Med. Chem. 2015, 30, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Di Guida, R.; Casillo, A.; Stellavato, A.; Kawai, S.; Ogawa, T.; Di Meo, C.; Kawamoto, J.; Kurihara, T.; Schiraldi, C.; Corsaro, M.M. Capsular polysaccharide from a fish-gut bacterium induces/promotes apoptosis of colon cancer cells in vitro through Caspases’ pathway activation. Carbohydr. Polym. 2022, 278, 118908. [Google Scholar] [CrossRef]
- Ueoka, R.; Shinzato, N.; Kagaya, N.; Suenaga, H.; Shin-ya, K. Pseudoalteropeptide A, a novel lipopeptide from the marine bacterium Pseudoalteromonas piscicida SWA4_PA4 isolated from marine seaweed. J. Antibiot. 2021, 74, 105–110. [Google Scholar] [CrossRef]
- Sannino, F.; Sansone, C.; Galasso, C.; Kildgaard, S.; Tedesco, P.; Fani, R.; Marino, G.; de Pascale, D.; Ianora, A.; Parrilli, E.; et al. Pseudoalteromonas haloplanktis TAC125 produces 4-hydroxybenzoic acid that induces pyroptosis in human A459 lung adenocarcinoma cells. Sci. Rep. 2018, 8, 1190. [Google Scholar] [CrossRef]
- Daboor, S.M.; Raudonis, R.; Cohen, A.; Rohde, J.R.; Cheng, Z. Marine bacteria, a source for alginolytic enzyme to disrupt Pseudomonas aeruginosa biofilms. Mar. Drugs 2019, 17, 307. [Google Scholar] [CrossRef]
- Daboor, S.M.; Rohde, J.R.; Cheng, Z. Disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate lyase enhances pathogen eradication by antibiotics. J. Cyst. Fibros. 2021, 20, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Thøgersen, M.S.; Delpin, M.W.; Melchiorsen, J.; Kilstrup, M.; Månsson, M.; Bunk, B. Production of the bioactive compounds violacein and indolmycin is conditional in a maeA mutant of Pseudoalteromonas luteoviolacea S4054 lacking the malic enzyme. Front. Microbiol. 2016, 7, 1461. [Google Scholar] [CrossRef] [PubMed]
- Choe, H.; Lee, S.H.; Kim, S.G.; Park, D.S.; Nasir, A.; Kim, K.M. Complete genome of Pseudoalteromonas phenolica KCTC 12086T (= O-BC30T), a marine bacterium producing polybrominated aromatic compounds. J. Biotechnol. 2016, 218, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Handayani, D.P.; Isnansetyo, A.; Istiqomah, I.; Jumina, J. New report: Genome mining untaps the antibiotics biosynthetic gene cluster of Pseudoalteromonas xiamenensis STKMTI.2 from a mangrove soil sediment. Mar. Biotechnol. 2022, 24, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Sorieul, L.; Wabete, N.; Ansquer, D.; Mailliez, J.R.; Pallud, M.; Zhang, C.; Lindivat, M.; Boulo, V.; Pham, D. Survival improvement conferred by the Pseudoalteromonas sp. NC201 probiotic in Litopenaeus stylirostris exposed to Vibrio nigripulchritudo infection and salinity stress. Aquaculture 2018, 495, 888–898. [Google Scholar] [CrossRef]
- Sorieul, L.; Rückert, C.; Al-Dilaimi, A.; Winkler, A.; Kalinowski, J.; Pham, D.; Wabete, N.; Boulo, V. Whole-Genome Sequence of Pseudoalteromonas sp. NC201, a probiotic Strain for Litopenaeus stylirostris Hatcheries in New Caledonia. Microbio. Res. Announce. 2019, 8, e00477-19. [Google Scholar] [CrossRef]
- Marchetti, P.M.; Kelly, V.; Simpson, J.P.; Ward, M.; Campopiano, D.J. The carbon chain-selective adenylation enzyme TamA: The missing link between fatty acid and pyrrole natural product biosynthesis. Org. Biomol. Chem. 2018, 16, 2735–2740. [Google Scholar] [CrossRef]
- Maansson, M.; Vynne, N.G.; Klitgaard, A.; Nybo, J.L.; Melchiorsen, J.; Nguyen, D.D.; Sanchez, L.M.; Ziemert, N.; Dorrestein, P.C.; Andersen, M.R.; et al. An integrated metabolomic and genomic mining workflow to uncover the biosynthetic potential of bacteria. mSystems 2016, 1, e00028-15. [Google Scholar] [CrossRef]
- de Rond, T.; Stow, P.; Eigl, I.; Johnson, R.E.; Chan, L.J.G.; Goyal, G.; Baidoo, E.E.K.; Hillson, N.J.; Petzold, C.J.; Sarpong, R.; et al. Oxidative cyclization of prodigiosin by an alkylglycerol monooxygenase-like enzyme. Nat. Chem. Biol. 2017, 13, 1155–1157. [Google Scholar] [CrossRef]
- Yu, M.; Tang, K.; Liu, J.; Shi, X.; Gulder, T.A.; Zhang, X.H. Genome analysis of Pseudoalteromonas flavipulchra JG1 reveals various survival advantages in marine environment. BMC Genom. 2013, 14, 707. [Google Scholar] [CrossRef]
- Sánchez-Díaz, R.; Molina-Garza, Z.J.; Cruz-Suárez, L.E.; Selvin, J.; Kiran, G.S.; Ibarra-Gámez, J.C.; Gómez-Gil, B.; Galaviz-Silva, L. Draft genome sequence of Pseudoalteromonas piscicida strain 36Y_RITHPW, a hypersaline seawater isolate from the south coast of Sonora, Mexico. J. Glob. Antimicrob. Resist. 2019, 16, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.P.; Needleman, D.S.; Watson, M.A.; Polson, S.W. Whole-genome sequences of two Pseudoalteromonas piscicida strains, DE1-A and DE2-A, with strong antibacterial activity against Vibrio vulnificus. Microbiol. Resour. Announc. 2019, 8, e01451-18. [Google Scholar] [CrossRef] [PubMed]
- Jouault, A.; Gobet, A.; Simon, M.; Portier, E.; Perennou, M.; Corre, E.; Gaillard, F.; Vallenet, D.; Michel, G.; Fleury, Y.; et al. Alterocin, an antibiofilm protein secreted by Pseudoalteromonas sp. Strain 3J6. Appl. Environ. Microbiol. 2020, 86, e00893-20. [Google Scholar] [CrossRef] [PubMed]
- Defer, D.; Desriac, F.; Henry, J.; Bourgougnon, N.; Baudy-Floc’h, M.; Brillet, B.; Le Chevalier, P.; Fleury, Y. Antimicrobial peptides in oyster hemolymph: The bacterial connection. Fish Shellfish Immunol. 2013, 34, 1439–1447. [Google Scholar] [CrossRef]
- Desriac, F.; El Harras, A.; Simon, M.; Bondon, A.; Brillet, B.; Le Chevalier, P.; Pugnière, M.; Got, P.; Destoumieux-Garzón, D.; Fleury, Y. Alterins produced by oyster-associated Pseudoalteromonas are antibacterial cyclolipopeptides with LPS-binding activity. Mar. Drugs 2020, 18, 630. [Google Scholar] [CrossRef]
- Offret, C.; Cuny, H.; Bodet, P.E.; Desriac, F.; Jegou, C.; Bazire, A.; Chevrot, R.; Thiery, V.; Brillet, B.; Fleury, Y. Alterins, a new family of marine antibacterial cyclolipopeptides. Int. J. Antimicrob. Agents 2022, 59, 106514. [Google Scholar] [CrossRef]
- Fukami, K.; Takagi, F.; Shimizu, S.; Ishigo, K.; Takahashi, M.; Horikawa, T. Isolation of bacteria able to degrade poly-hydroxybutyrate-co-hydroxyhexanoate, and the inhibitory effects of the degradation products on shrimp pathogen Vibrio penaeicida. Microb. Pathog. 2021, 160, 105167. [Google Scholar] [CrossRef]
- Kozuma, S.; Hirota-Takahata, Y.; Fukuda, D.; Kuraya, N.; Nakajima, M.; Ando, O. Identification and biological activity of ogipeptins, novel LPS inhibitors produced by marine bacterium. J. Antibiot. 2017, 70, 79–83. [Google Scholar] [CrossRef]
- Wasana, W.P.; Senevirathne, A.; Nikapitiya, C.; Lee, J.S.; Kang, D.H.; Kwon, K.K.; Oh, C.; De Zoysa, M. Probiotic effects of Pseudoalteromonas ruthenica: Antibacterial, immune stimulation and modulation of gut microbiota composition. Fish Shellfish Immunol. 2022, 131, 229–243. [Google Scholar] [CrossRef]
- Maynard, A.; Butler, N.L.; Ito, T.; José Da Silva, A.; Murai, M.; Chen, T.; Koffas, M.A.G.; Miyoshi, H.; Barquera, B. Antibiotic korormicin a kills bacteria by producing reactive oxygen species. J. Bacteriol. 2019, 201, 718–736. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Tang, Y.; Sun, B.; Huang, J.; Song, X. Pseudoalteromonas probiotics as potential biocontrol agents improve the survival of Penaeus vannamei challenged with acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus. Aquaculture 2018, 494, 30–36. [Google Scholar] [CrossRef]
- Wang, H.; Sun, B.; Xie, G.; Wan, X.; Huang, J.; Song, X. Spotlight on a novel bactericidal mechanism and a novel SXT/R391-like integrative and conjugative element, carrying multiple antibiotic resistance genes, in Pseudoalteromonas flavipulchra strain CDM8. Microbiol. Res. 2021, 1, 242. [Google Scholar] [CrossRef]
- Paulsen, S.S.; Isbrandt, T.; Kirkegaard, M.; Buijs, Y.; Strube, M.L.; Sonnenschein, E.C.; Larsen, T.O.; Gram, L. Production of the antimicrobial compound tetrabromopyrrole and the Pseudomonas quinolone system precursor, 2-heptyl-4-quinolone, by a novel marine species Pseudoalteromonas galatheae sp. nov. Sci. Rep. 2020, 10, 21630. [Google Scholar] [CrossRef] [PubMed]
- Desriac, F.; Le Chefvalier, P.; Brillet, B.; Leguerinel, I.; Thuillier, B.; Paillard, C.; Fleury, Y. Exploring the hologenome concept in marine bivalvia: Haemolymph microbiota as a pertinent source of probiotics for aquaculture. FEMS Microbiol. Lett. 2013, 350, 107–116. [Google Scholar] [CrossRef]
- Offret, C.; Rochard, V.; Laguerre, H.; Mounier, J.; Huchette, S.; Brillet, B.; Le Chevalier, P.; Fleury, Y. Protective Efficacy of a Pseudoalteromonas Strain in European Abalone, Haliotis tuberculata, infected with Vibrio harveyi ORM4. Probiotics Antimicrob. Proteins 2019, 11, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Tangestani, M.; Broady, P.; Varsani, A. An investigation of antibacterial activity of New Zealand seaweed-associated marine bacteria. Future Microbiol. 2021, 6, 1167–1179. [Google Scholar] [CrossRef]
- Papa, R.; Parrilli, E.; Sannino, F.; Barbato, G.; Tutino, M.L.; Artini, M.; Selan, L. Anti-biofilm activity of the Antarctic marine bacterium Pseudoalteromonas haloplanktis TAC125. Res Microbiol. 2013, 164, 450–456. [Google Scholar] [CrossRef]
- Sannino, F.; Parrilli, E.; Apuzzo, G.A.; de Pascale, D.; Tedesco, P.; Maida, I.; Perrin, E.; Fondi, M.; Fani, R.; Marino, G.; et al. Pseudoalteromonas haloplanktis produces methylamine, a volatile compound active against Burkholderia cepacia complex strains. New Biotechnol. 2017, 35, 13–18. [Google Scholar] [CrossRef]
- Venuti, I.; Ceruso, M.; D’angelo, C.; Casillo, A.; Pepe, T. Antimicrobial activity evaluation of pure compounds obtained from Pseudoalteromonas haloplanktis against Listeria monocytogenes: Preliminary results. Ital. J. Food Saf. 2022, 11, 10320. [Google Scholar] [CrossRef]
- Casillo, A.; Papa, R.; Ricciardelli, A.; Sannino, F.; Ziaco, M.; Tilotta, M.; Selan, L.; Marino, G.; Corsaro, M.M.; Tutino, M.L.; et al. Anti-biofilm activity of a long-chain fatty aldehyde from antarctic Pseudoalteromonas haloplanktis TAC125 against Staphylococcus epidermidis biofilm. Front. Cell. Infect. Microbiol. 2017, 7, 46. [Google Scholar] [CrossRef]
- Ayuningrum, D.; Liu, Y.; Sibero, M.T.; Kristiana, R.; Asagabaldan, M.A.; Wuisan, Z.G.; Trianto, A.; Radjasa, O.K.; Sabdono, A.; Schäberle, T.F. Tunicate-associated bacteria show a great potential for the discovery of antimicrobial compounds. PLoS ONE 2019, 14, e0213797. [Google Scholar] [CrossRef] [PubMed]
- Tebben, J.; Motti, C.; Tapiolas, D.; Thomas-Hall, P.; Harder, T. A coralline algal-associated bacterium, Pseudoalteromonas strain J010, yields five new korormicins and a bromopyrrole. Mar. Drugs 2014, 12, 2802–2815. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, E.C.; Stierhof, M.; Goralczyk, S.; Vabre, F.M.; Pellissier, L.; Hanssen, K.Ø.; de la Cruz, M.; Díaz, C.; de Witte, P.; Copmans, D.; et al. Pseudochelin A, a siderophore of Pseudoalteromonas piscicida S2040. Tetrahedron 2017, 73, 2633–2637. [Google Scholar] [CrossRef]
- Eliseikina, M.G.; Beleneva, I.A.; Kukhlevsky, A.D.; Shamshurina, E.V. Identification and analysis of the biological activity of the new strain of Pseudoalteromonas piscicida isolated from the hemal fluid of the bivalve Modiolus kurilensis (F. R. Bernard, 1983). Arch. Microbiol. 2021, 203, 4461–4473. [Google Scholar] [CrossRef] [PubMed]
- Feliatra, F.; Setiaji, J.; Teruna, H.Y.; Lukistyowati, I.; Suharman, I.; Muchlisin, Z.A.; Johan, T.I. Antibacterial activity in secondary metabolite extracts of heterotrophic bacteria against Vibrio alginolyticus, Aeromonas hydrophila, and Pseudomonas aeruginosa. F1000Research 2020, 9, 1491. [Google Scholar] [CrossRef]
- Amoah, K.; Dong, X.H.; Tan, B.P.; Zhang, S.; Kuebutornye, F.K.A.; Chi, S.Y.; Yang, Q.; Liu, H.; Zhang, H.; Yang, Y. In vitro assessment of the safety and potential probiotic characteristics of three bacillus strains isolated from the intestine of hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Front. Vet. Sci. 2021, 8, 675962. [Google Scholar] [CrossRef]
- Zaghloul, E.H.; Abuohashish, H.M.; El Sharkawy, A.S.; Abbas, E.M.; Ahmed, M.M.; Al-Rejaie, S.S. Probiotic potential of the marine isolate Enterococcus faecium EA9 and in vivo evaluation of its antisepsis action in rats. Mar. Drugs 2023, 21, 45. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Zoumpopoulou, G.; Foligné, B.; Alexandraki, V.; Kazou, M.; Pot, B.; Tsakalidou, E. Discovering probiotic microorganisms: In vitro, in vivo, genetic and omics approaches. Front. Microbiol. 2015, 6, 58. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Lacroix, C.; Yildirim, S. Fermentation technologies for the production of probiotics with high viability and functionality. Curr. Opin. Biotechnol. 2007, 18, 176–183. [Google Scholar] [CrossRef]
- Selvamani, S.; Ramli, S.; Dailin, D.J.; Natasya, K.H.; Varzakas, T.; Abomoelak, B.; Sukmawati, D.; Nurjayadi, M.; Liu, S.; Gupta, V.K.; et al. Extractive fermentation as a novel strategy for high cell mass production of hetero-fermentative probiotic strain Limosilactobacillus reuteri. Fermentation 2022, 8, 527. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Rønhave Laursen, R.; Ouwehand, A.C. The production and delivery of probiotics: A review of a practical approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef]
- Kiepś, J.; Dembczyński, R. Current trends in the production of probiotic formulations. Foods 2022, 11, 2330. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, C.; Høj, L.; Bourne, D.G. Probiotics for coral aquaculture: Challenges and considerations. Curr. Opin. Biotechnol. 2022, 73, 380–386. [Google Scholar] [CrossRef]
- Bhatnagar, I.; Vasagan, M.; Bramhachari, P.V. Opening avenues in marine probiotics: Present and future. In Marine Microorganisms; CRC Press: Boca Raton, FL, USA, 2016; pp. 143–160. [Google Scholar]
- Pereira, R.B.; Evdokimov, N.M.; Lefranc, F.; Valentão, P.; Kornienko, A.; Pereira, D.M.; Andrade, P.B.; Gomes, N.G.M. Marine-derived anticancer agents: Clinical benefits, innovative mechanisms, and new targets. Mar. Drugs 2019, 17, 329. [Google Scholar] [CrossRef]
- Gu, S.; Li, J.; Chen, B.; Sun, T.; Liu, Q.; Xiao, D.; Tian, C. Metabolic engineering of the thermophilic filamentous fungus Myceliophthora thermophila to produce fumaric acid. Biotechnol. Biofuels 2018, 11, 323. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Zhang, Z.; Qin, G.; Chen, T.; Tian, S. Molecular basis and regulation of pathogenicity and patulin biosynthesis in Penicillium expansum. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3416–3438. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, M.W.; Siddique, R.; Nabi, G.; Manan, S.; Yousaf, M.; Hou, H. Role of recombinant DNA technology to improve life. Int. J. Genom. 2016, 2016, 2405954. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).