Histone Modification of Colorectal Cancer by Natural Products
Abstract
:1. Introduction
2. The Role of Histone Modifications in CRC
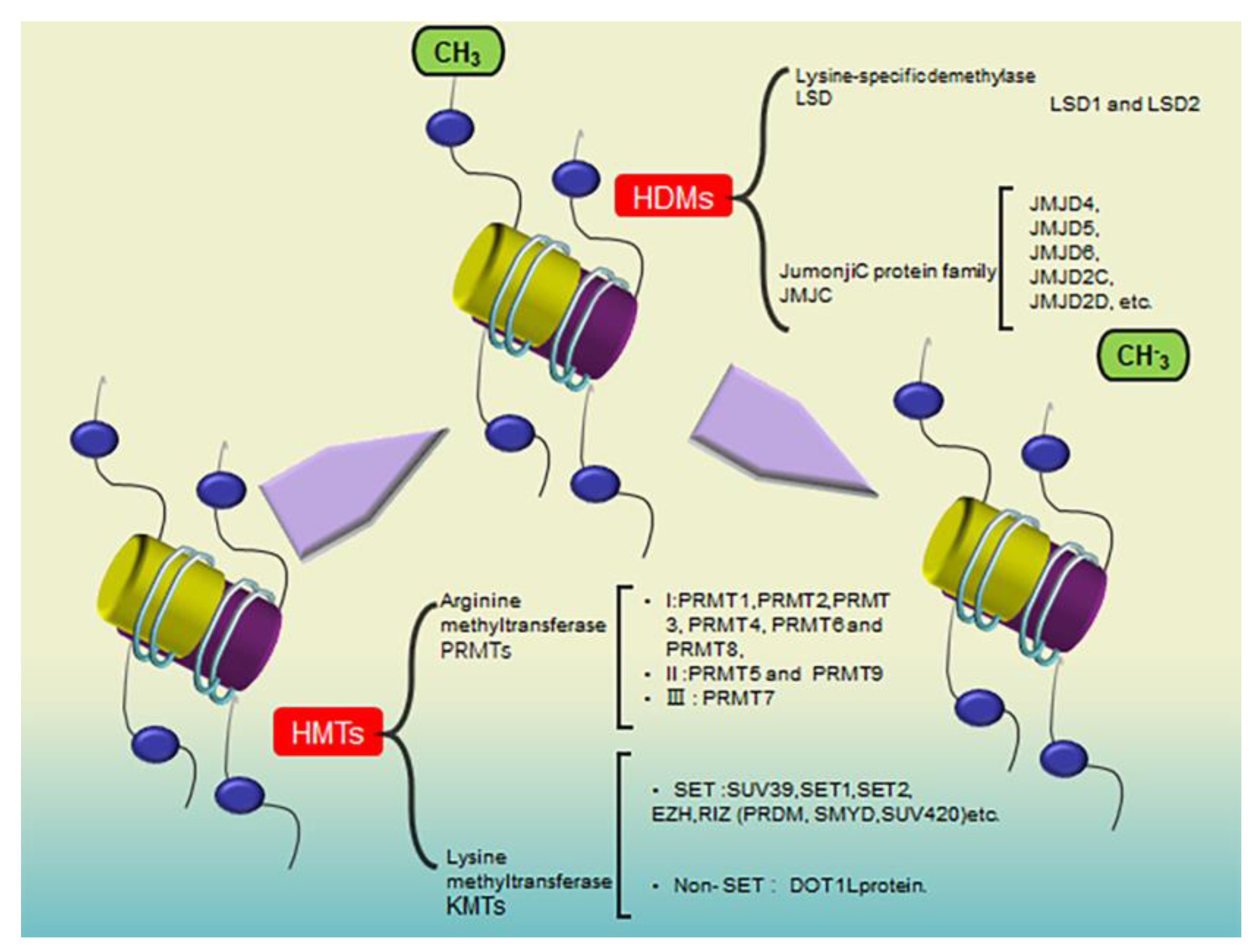
3. Histone Methylation Regulates CRC
4. Histone Acetylation Regulates CRC
5. Histone Phosphorylation Regulates CRC
6. Discussion
7. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swain, B.C.; Subadini, S.; Rout, J.; Mishra, P.P.; Sahoo, H.; Tripathy, U.; Sakshi. Biophysical study on complex formation between β-Lactoglobulin and vitamin B12. Food Chem. 2020, 312, 126064. [Google Scholar] [CrossRef]
- Miceli, M.; Bontempo, P.; Nebbioso, A.; Altucci, L. Natural compounds in epigenetics: A current view. Food Chem. Toxicol. 2014, 73, 71–83. [Google Scholar] [CrossRef]
- Allis, C.D.; Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.H.; Tawbi, H. Role of histone deacetylases and their inhibitors in cancer biology and treatment. Curr. Clin. Pharmacol. 2010, 5, 196–208. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Tycko, B. The history of cancer epigenetics. Nat. Rev. Cancer 2004, 4, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, D.; Sun, X.; Yang, K.; Yao, J.; Cheng, C.; Wang, C.; Zheng, J. CDX2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing Wnt/β-catenin signaling via transactivation of GSK-3β and Axin2 expression. Cell Death Dis. 2019, 10, 26. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhan, X.R.; Liu, X.M.; Wang, X.C. CREB is a regulatory target for the protein kinase Akt/PKB in the differentiation of pancreatic ductal cells into islet β-cells mediated by hepatocyte growth factor. Biochem. Biophys. Res. Commun. 2011, 404, 711–716. [Google Scholar] [CrossRef]
- Cao, H.; Song, S.; Zhang, H.; Zhang, Y.; Qu, R.; Yang, B.; Jing, Y.; Hu, T.; Yan, F.; Wang, B. Chemopreventive effects of berberine on intestinal tumor development in Apcmin/+ mice. BMC Gastroenterol. 2013, 13, 163. [Google Scholar] [CrossRef] [Green Version]
- Sandig, B.V.; Brand, K.; Herwig, S.; Lukas, J.; Bartek, J.; Strauss, M. p16 and p53 genes transferred with the help of adenovirus to induce apoptic tumor cell death. Ugeskr. Laeger 1997, 159, 6825–6830. [Google Scholar]
- Lee, C.W.; Ito, K.; Ito, Y. Role of RUNX3 in bone morphogenetic protein signaling in colorectal cancer. Cancer Res. 2010, 70, 4243–4252. [Google Scholar] [CrossRef] [Green Version]
- Aladhraei, M.; Al-Salami, E.; Poungvarin, N.; Suwannalert, P. The roles of p53 and XPO1 on colorectal cancer progression in Yemeni patients. J. Gastrointest. Oncol. 2019, 10, 437–444. [Google Scholar] [CrossRef]
- Cedar, H.; Bergman, Y. Linking DNA methylation and histone modification: Patterns and paradigms. Nat. Rev. Genet. 2009, 10, 295–304. [Google Scholar] [CrossRef]
- Irshad, R.; Husain, M. Natural products in the reprogramming of cancer epigenetics. Toxicol. Appl. Pharmacol. 2021, 417, 115467. [Google Scholar] [CrossRef] [PubMed]
- Kanherkar, R.R.; Bhatia-Dey, N.; Csoka, A.B. Epigenetics across the human lifespan. Front. Cell Dev. Biol. 2014, 2, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, O.; Imamura, H.; Shimizu, T.; Kinoshita, J.; Okabe, T.; Hirano, A.; Yoshimatsu, K.; Konno, S.; Aiba, M.; Ogawa, K. Expression of twist and wnt in human breast cancer. Anticancer. Res. 2004, 24, 3851–3856. [Google Scholar]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A.; Shi, Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Sakane, C.; Okitsu, T.; Wada, A.; Sagami, H.; Shidoji, Y. Inhibition of lysine-specific demethylase 1 by the acyclic diterpenoid geranylgeranoic acid and its derivatives. Biochem. Biophys. Res. Commun. 2014, 444, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Rao, C.M. Epigenetic tools (The Writers, The Readers and The Erasers) and their implications in cancer therapy. Eur. J. Pharmacol. 2018, 837, 8–24. [Google Scholar] [CrossRef]
- Murray-Zmijewski, F.; Slee, E.A.; Lu, X. A complex barcode underlies the heterogeneous response of p53 to stress. Nat. Rev. Mol. Cell Biol. 2008, 9, 702–712. [Google Scholar] [CrossRef]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Vousden, K.H.; Lane, D.P. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 275–283. [Google Scholar] [CrossRef]
- Liebl, M.C.; Hofmann, T.G. The Role of p53 Signaling in Colorectal Cancer. Cancers 2021, 13, 2125. [Google Scholar] [CrossRef]
- Li, J.X.; Liu, H.L. The relationship of DNA methylation and histone methylation. Hereditas 2004, 26, 267–270. [Google Scholar]
- Alelu-Paz, R.; Ashour, N.; Gonzalez-Corpas, A.; Ropero, S. DNA methylation, histone modifications, and signal transduction pathways: A close relationship in malignant gliomas pathophysiology. J. Signal Transduct. 2012, 2012, 956958. [Google Scholar] [CrossRef] [Green Version]
- Rose, N.R.; Klose, R.J. Understanding the relationship between DNA methylation and histone lysine methylation. Biochim. Biophys. Acta 2014, 1839, 1362–1372. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Xu, X.; Chen, D.; Zhao, F.; Wang, W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed. Pharmacother. 2019, 110, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Lomenick, B.; Jung, G.; Wohlschlegel, J.A.; Huang, J. Target identification using drug affinity responsive target stability (DARTS). Curr. Protoc. Chem. Biol. 2011, 3, 163–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomenick, B.; Hao, R.; Jonai, N.; Chin, R.M.; Aghajan, M.; Warburton, S.; Wang, J.; Wu, R.P.; Gomez, F.; Loo, J.A.; et al. Target identification using drug affinity responsive target stability (DARTS). Proc. Natl. Acad. Sci. USA 2009, 106, 21984–21989. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.M.; Kim, S.H.; Kim, M.S.; Kim, D.C.; Lee, E.H.; Lee, J.S.; Lee, S.H.; Kim, Y.Z. Epigenetic Role of Histone Lysine Methyltransferase and Demethylase on the Expression of Transcription Factors Associated with the Epithelial-to-Mesenchymal Transition of Lung Adenocarcinoma Metastasis to the Brain. Cancers 2020, 12, 3632. [Google Scholar] [CrossRef]
- Chatterjee, B.; Ghosh, K.; Suresh, L.; Kanade, S.R. Curcumin ameliorates PRMT5-MEP50 arginine methyltransferase expression by decreasing the Sp1 and NF-YA transcription factors in the A549 and MCF-7 cells. Mol. Cell Biochem. 2019, 455, 73–90. [Google Scholar] [CrossRef]
- Hampsey, M.; Reinberg, D. Tails of intrigue: Phosphorylation of RNA polymerase II mediates histone methylation. Cell 2003, 113, 429–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, W.D.; Arora, S.; Busby, J.; Balasubramanian, S.; Gehling, V.S.; Nasveschuk, C.G.; Vaswani, R.G.; Yuan, C.C.; Hatton, C.; Zhao, F.; et al. EZH2 inhibitor efficacy in non-Hodgkin’s lymphoma does not require suppression of H3K27 monomethylation. Chem. Biol. 2014, 21, 1463–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kung, P.P.; Rui, E.; Bergqvist, S.; Bingham, P.; Braganza, J.; Collins, M.; Cui, M.; Diehl, W.; Dinh, D.; Fan, C.; et al. Correction to Design and Synthesis of Pyridone-Containing 3,4-Dihydroisoquinoline-1(2H)-ones as a Novel Class of Enhancer of Zeste Homolog 2 (EZH2) Inhibitors. J. Med. Chem. 2016, 59, 11196. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo-Torres, E.; Hernandez-Oliveras, A.; Meneses-Morales, I.; Rodriguez, G.; Fuentes-Garcia, G.; Zarain-Herzberg, A. Resveratrol up-regulates ATP2A3 gene expression in breast cancer cell lines through epigenetic mechanisms. Int. J. Biochem. Cell Biol. 2019, 113, 37–47. [Google Scholar] [CrossRef]
- Rice, J.C.; Allis, C.D. Histone methylation versus histone acetylation: New insights into epigenetic regulation. Curr. Opin. Cell Biol. 2001, 13, 263–273. [Google Scholar] [CrossRef]
- Campbell, J.E.; Kuntz, K.W.; Knutson, S.K.; Warholic, N.M.; Keilhack, H.; Wigle, T.J.; Raimondi, A.; Klaus, C.R.; Rioux, N.; Yokoi, A.; et al. EPZ011989, A Potent, Orally-Available EZH2 Inhibitor with Robust in Vivo Activity. ACS Med. Chem. Lett. 2015, 6, 491–495. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Ma, W.; Wang, B. The landscape of histone acetylation involved in epithelial-mesenchymal transition in lung cancer. J. Cancer Res. Ther. 2013, 9 (Suppl. S2), S86–S91. [Google Scholar] [CrossRef]
- He, W.; Yu, Y.; Huang, W.; Feng, G.; Li, J. The Pseudogene DUXAP8 Promotes Colorectal Cancer Cell Proliferation, Invasion, and Migration by Inducing Epithelial-Mesenchymal Transition Through Interacting with EZH2 and H3K27me3. Onco Targets Ther. 2020, 13, 11059–11070. [Google Scholar] [CrossRef] [PubMed]
- Wissmann, M.; Yin, N.; Muller, J.M.; Greschik, H.; Fodor, B.D.; Jenuwein, T.; Vogler, C.; Schneider, R.; Gunther, T.; Buettner, R.; et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat. Cell Biol. 2007, 9, 347–353. [Google Scholar] [CrossRef]
- Ruthenburg, A.J.; Allis, C.D.; Wysocka, J. Methylation of lysine 4 on histone H3: Intricacy of writing and reading a single epigenetic mark. Mol. Cell 2007, 25, 15–30. [Google Scholar] [CrossRef]
- Hossain, S.; Liu, Z.; Wood, R.J. Association between histone deacetylase activity and vitamin D-dependent gene expressions in relation to sulforaphane in human colorectal cancer cells. J. Sci. Food Agric. 2021, 101, 1833–1843. [Google Scholar] [CrossRef]
- Chung, J.W.; Noh, E.J.; Zhao, H.L.; Sim, J.S.; Ha, Y.W.; Shin, E.M.; Lee, E.B.; Cheong, C.S.; Kim, Y.S. Anti-inflammatory activity of prosapogenin methyl ester of platycodin D via nuclear factor-kappaB pathway inhibition. Biol. Pharm. Bull. 2008, 31, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.H.; Liu, L.Z. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv. Cancer Res. 2009, 102, 19–65. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Sun, L.Y.; Zhang, Y.Q. A Hopeful Natural Product, Pristimerin, Induces Apoptosis, Cell Cycle Arrest, and Autophagy in Esophageal Cancer Cells. Anal Cell Pathol 2019, 2019, 6127169. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Li, Z.Y.; Liu, W.P.; Zhao, M.R. Crosstalk between Wnt/β-catenin and Hedgehog/Gli signaling pathways in colon cancer and implications for therapy. Cancer Biol. Ther. 2015, 16, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Zhang, Y.; Wang, S.; Liu, Y.; Zheng, L.; Yang, J.; Huang, W.; Ye, Y.; Luo, W.; Xiao, D. Hes1 is involved in the self-renewal and tumourigenicity of stem-like cancer cells in colon cancer. Sci. Rep. 2014, 4, 3963. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.C.; Tsao, T.C.; Hsu, S.R.; Wang, H.C.; Tsai, T.C.; Kao, J.Y.; Way, T.D. EGCG inhibits transforming growth factor-β-mediated epithelial-to-mesenchymal transition via the inhibition of Smad2 and Erk1/2 signaling pathways in nonsmall cell lung cancer cells. J. Agric. Food Chem. 2012, 60, 9863–9873. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Duan, Z.J.; Chang, J.Y.; Zhang, Z.F.; Chu, R.; Li, Y.L.; Dai, K.H.; Mo, G.Q.; Chang, Q.Y. Retraction: Sinomenine Sensitizes Multidrug-Resistant Colon Cancer Cells (Caco-2) to Doxorubicin by Downregulation of MDR-1 Expression. PLoS ONE 2019, 14, e0215388. [Google Scholar] [CrossRef]
- Wang, K.; Liu, W.; Xu, Q.; Gu, C.; Hu, D. Tenacissoside G synergistically potentiates inhibitory effects of 5-fluorouracil to human colorectal cancer. Phytomedicine 2021, 86, 153553. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Cai, M.; Ma, C.; Xiang, Y.; Hu, W.; Zhou, B.; Zhao, C.; Dai, X.; Li, X.; et al. Cinobufagin suppresses colorectal cancer growth via STAT3 pathway inhibition. Am. J. Cancer Res. 2021, 11, 200–214. [Google Scholar] [PubMed]
- Wang, K.; Huang, W.; Sang, X.; Wu, X.; Shan, Q.; Tang, D.; Xu, X.; Cao, G. Atractylenolide I inhibits colorectal cancer cell proliferation by affecting metabolism and stemness via AKT/mTOR signaling. Phytomedicine 2020, 68, 153191. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Taniguchi, M. Geniposide prevents tumor growth by inhibiting colonic interleukin-1β and monocyte chemoattractant protein-1 via down-regulated expression of cyclooxygenase-2 and thymocyte selection-associated high mobility box proteins TOX/TOX2 in azoxymethane/dextran sulfate sodium-treated mice. Int. Immunopharmacol. 2023, 118, 110077. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jin, J.; Zhang, Z.; Zuo, L.; Jiang, M.; Xie, C. Shikonin exerts antitumor activity by causing mitochondrial dysfunction in hepatocellular carcinoma through PKM2-AMPK-PGC1alpha signaling pathway. Biochem. Cell Biol. 2019, 97, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Hu, H. Costunolide Induces Apoptosis of K562/ADR Cells through PI3K/AKT Pathway. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2021, 29, 68–71. [Google Scholar] [CrossRef]
- He, F.; Xiong, W.; Wang, Y.; Li, L.; Liu, C.; Yamagami, T.; Taketo, M.M.; Zhou, C.; Chen, Y. Epithelial Wnt/β-catenin signaling regulates palatal shelf fusion through regulation of Tgfβ3 expression. Dev. Biol. 2011, 350, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Y.; Feng, Y.Y.; Dan, W.; Pan, D.; Zhang, G.F.; Wang, X.L.; Hou, G.J. Study on the influence of curcumin on chemosensitivity of nephroblastoma cells. Asian Pac. J. Trop. Med. 2016, 9, 801–805. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Yang, Q.; Mu, Y.; Zhou, L.; Liu, Y.; Zhou, Q.; He, B. Berberine inhibits the proliferation of colon cancer cells by inactivating Wnt/β-catenin signaling. Int. J. Oncol. 2012, 41, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Luo, H.; Lee, S.; Jin, F.; Yang, J.S.; Montellier, E.; Buchou, T.; Cheng, Z.; Rousseaux, S.; Rajagopal, N.; et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 2011, 146, 1016–1028. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Yang, Y.; Wang, S.; He, X.; Liu, M.; Bai, B.; Tian, C.; Sun, R.; Yu, T.; Chu, X. Role of acetylation in doxorubicin-induced cardiotoxicity. Redox Biol. 2021, 46, 102089. [Google Scholar] [CrossRef]
- Marks, P.; Rifkind, R.A.; Richon, V.M.; Breslow, R.; Miller, T.; Kelly, W.K. Histone deacetylases and cancer: Causes and therapies. Nat. Rev. Cancer 2001, 1, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peterson, Y.K.; Inks, E.S.; Himes, R.A.; Li, J.; Zhang, Y.; Kong, X.; Chou, C.J. Class I HDAC Inhibitors Display Different Antitumor Mechanism in Leukemia and Prostatic Cancer Cells Depending on Their p53 Status. J. Med. Chem. 2018, 61, 2589–2603. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Zhang, G.; Hwa, Y.L.; Li, J.; Dowdy, S.C.; Jiang, S.W. Nonhistone protein acetylation as cancer therapy targets. Expert. Rev. Anticancer. Ther. 2010, 10, 935–954. [Google Scholar] [CrossRef] [Green Version]
- Mottamal, M.; Zheng, S.; Huang, T.L.; Wang, G. Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules 2015, 20, 3898–3941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Cheng, L.; Gao, Y.; Zhang, B.; Zheng, X.; Wang, L.; Li, P.; Sun, Q.; Li, H. Plant HP1 protein ADCP1 links multivalent H3K9 methylation readout to heterochromatin formation. Cell Res. 2019, 29, 54–66. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhang, H.; Cong, J.; Cui, M.; Ma, M.; Zhang, F.; Sun, H.; Chen, C. H3K27 acetylation-induced lncRNA EIF3J-AS1 improved proliferation and impeded apoptosis of colorectal cancer through miR-3163/YAP1 axis. J. Cell Biochem. 2020, 121, 1923–1933. [Google Scholar] [CrossRef]
- Wang, S.; Zang, C.; Xiao, T.; Fan, J.; Mei, S.; Qin, Q.; Wu, Q.; Li, X.; Xu, K.; He, H.H.; et al. Modeling cis-regulation with a compendium of genome-wide histone H3K27ac profiles. Genome Res. 2016, 26, 1417–1429. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef] [Green Version]
- Salvador, L.A.; Luesch, H. Discovery and mechanism of natural products as modulators of histone acetylation. Curr. Drug Targets 2012, 13, 1029–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Zhang, F.; Ding, J.; Liang, Y.; Zhan, Z.; Zhan, Y.; Chen, L.H.; Ding, Y. Histone Methyltransferase SETDB1 Promotes the Progression of Colorectal Cancer by Inhibiting the Expression of TP53. J. Cancer 2017, 8, 3318–3330. [Google Scholar] [CrossRef] [Green Version]
- Mitani, Y.; Oue, N.; Hamai, Y.; Aung, P.P.; Matsumura, S.; Nakayama, H.; Kamata, N.; Yasui, W. Histone H3 acetylation is associated with reduced p21(WAF1/CIP1) expression by gastric carcinoma. J. Pathol. 2005, 205, 65–73. [Google Scholar] [CrossRef]
- Couture, J.F.; Trievel, R.C. Histone-modifying enzymes: Encrypting an enigmatic epigenetic code. Curr. Opin. Struct. Biol. 2006, 16, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Dent, S.Y. Histone modifying enzymes and cancer: Going beyond histones. J. Cell Biochem. 2005, 96, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Audia, J.E.; Campbell, R.M. Histone Modifications and Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019521. [Google Scholar] [CrossRef]
- Kuo, M.H.; Allis, C.D. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays 1998, 20, 615–626. [Google Scholar] [CrossRef]
- Danielsen, S.A.; Eide, P.W.; Nesbakken, A.; Guren, T.; Leithe, E.; Lothe, R.A. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim. Biophys. Acta 2015, 1855, 104–121. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Yuan, C.; Wang, C.; Gao, T.; Zheng, Z. MiR-489 inhibited the development of gastric cancer via regulating HDAC7 and PI3K/AKT pathway. World J. Surg. Oncol. 2020, 18, 73. [Google Scholar] [CrossRef] [Green Version]
- Yarushkin, A.A.; Mazin, M.E.; Yunusova, A.Y.; Korchagina, K.V.; Pustylnyak, Y.A.; Prokopyeva, E.A.; Pustylnyak, V.O. CAR-mediated repression of Cdkn1a(p21) is accompanied by the Akt activation. Biochem. Biophys. Res. Commun. 2018, 504, 361–366. [Google Scholar] [CrossRef]
- Nural-Guvener, H.; Zakharova, L.; Feehery, L.; Sljukic, S.; Gaballa, M. Anti-Fibrotic Effects of Class I HDAC Inhibitor, Mocetinostat Is Associated with IL-6/Stat3 Signaling in Ischemic Heart Failure. Int. J. Mol. Sci. 2015, 16, 11482–11499. [Google Scholar] [CrossRef] [Green Version]
- Akone, S.H.; Ntie-Kang, F.; Stuhldreier, F.; Ewonkem, M.B.; Noah, A.M.; Mouelle, S.E.M.; Muller, R. Natural Products Impacting DNA Methyltransferases and Histone Deacetylases. Front. Pharmacol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Yoshida, M.; Kijima, M.; Akita, M.; Beppu, T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 1990, 265, 17174–17179. [Google Scholar] [CrossRef] [PubMed]
- Plumb, J.A.; Finn, P.W.; Williams, R.J.; Bandara, M.J.; Romero, M.R.; Watkins, C.J.; La Thangue, N.B.; Brown, R. Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol. Cancer Ther. 2003, 2, 721–728. [Google Scholar] [PubMed]
- Scuto, A.; Kirschbaum, M.; Kowolik, C.; Kretzner, L.; Juhasz, A.; Atadja, P.; Pullarkat, V.; Bhatia, R.; Forman, S.; Yen, Y.; et al. The novel histone deacetylase inhibitor, LBH589, induces expression of DNA damage response genes and apoptosis in Ph- acute lymphoblastic leukemia cells. Blood 2008, 111, 5093–5100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arts, J.; King, P.; Marien, A.; Floren, W.; Belien, A.; Janssen, L.; Pilatte, I.; Roux, B.; Decrane, L.; Gilissen, R.; et al. JNJ-26481585, a novel "second-generation" oral histone deacetylase inhibitor, shows broad-spectrum preclinical antitumoral activity. Clin. Cancer Res. 2009, 15, 6841–6851. [Google Scholar] [CrossRef] [Green Version]
- Phiel, C.J.; Zhang, F.; Huang, E.Y.; Guenther, M.G.; Lazar, M.A.; Klein, P.S. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001, 276, 36734–36741. [Google Scholar] [CrossRef] [Green Version]
- Aviram, A.; Zimrah, Y.; Shaklai, M.; Nudelman, A.; Rephaeli, A. Comparison between the effect of butyric acid and its prodrug pivaloyloxymethylbutyrate on histones hyperacetylation in an HL-60 leukemic cell line. Int. J. Cancer 1994, 56, 906–909. [Google Scholar] [CrossRef]
- DiGiuseppe, J.A.; Weng, L.J.; Yu, K.H.; Fu, S.; Kastan, M.B.; Samid, D.; Gore, S.D. Phenylbutyrate-induced G1 arrest and apoptosis in myeloid leukemia cells: Structure-function analysis. Leukemia 1999, 13, 1243–1253. [Google Scholar] [CrossRef] [Green Version]
- Giannini, G.; Vesci, L.; Battistuzzi, G.; Vignola, D.; Milazzo, F.M.; Guglielmi, M.B.; Barbarino, M.; Santaniello, M.; Fanto, N.; Mor, M.; et al. ST7612AA1, a thioacetate-ω(γ-lactam carboxamide) derivative selected from a novel generation of oral HDAC inhibitors. J. Med. Chem. 2014, 57, 8358–8377. [Google Scholar] [CrossRef]
- Hu, E.; Dul, E.; Sung, C.M.; Chen, Z.; Kirkpatrick, R.; Zhang, G.F.; Johanson, K.; Liu, R.; Lago, A.; Hofmann, G.; et al. Identification of novel isoform-selective inhibitors within class I histone deacetylases. J. Pharmacol. Exp. Ther. 2003, 307, 720–728. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, P.; Martel, F. Butyrate and colorectal cancer: The role of butyrate transport. Curr. Drug Metab. 2013, 14, 994–1008. [Google Scholar] [CrossRef]
- De Souza, C.; Chatterji, B.P. HDAC Inhibitors as Novel Anti-Cancer Therapeutics. Recent. Pat. Anticancer. Drug Discov. 2015, 10, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Sun, G.; Zhong, M.; Yu, Y.; Brewer, M.A. Anticancer efficacy of cisplatin and trichostatin A or 5-aza-2’-deoxycytidine on ovarian cancer. Br. J. Cancer 2013, 108, 579–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saijo, K.; Imamura, J.; Narita, K.; Oda, A.; Shimodaira, H.; Katoh, T.; Ishioka, C. Biochemical, biological and structural properties of romidepsin (FK228) and its analogs as novel HDAC/PI3K dual inhibitors. Cancer Sci. 2015, 106, 208–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcu, M.G.; Jung, Y.J.; Lee, S.; Chung, E.J.; Lee, M.J.; Trepel, J.; Neckers, L. Curcumin is an inhibitor of p300 histone acetylatransferase. Med. Chem. 2006, 2, 169–174. [Google Scholar] [CrossRef]
- Collins, H.M.; Abdelghany, M.K.; Messmer, M.; Yue, B.; Deeves, S.E.; Kindle, K.B.; Mantelingu, K.; Aslam, A.; Winkler, G.S.; Kundu, T.K.; et al. Differential effects of garcinol and curcumin on histone and p53 modifications in tumour cells. BMC Cancer 2013, 13, 37. [Google Scholar] [CrossRef] [Green Version]
- Kuttan, R.; Sudheeran, P.C.; Josph, C.D. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987, 73, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Santer, F.R.; Hoschele, P.P.; Oh, S.J.; Erb, H.H.; Bouchal, J.; Cavarretta, I.T.; Parson, W.; Meyers, D.J.; Cole, P.A.; Culig, Z. Inhibition of the acetyltransferases p300 and CBP reveals a targetable function for p300 in the survival and invasion pathways of prostate cancer cell lines. Mol. Cancer Ther. 2011, 10, 1644–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoni, M.; Massari, F.; Del Re, M.; Ciccarese, C.; Piva, F.; Principato, G.; Montironi, R.; Santini, D.; Danesi, R.; Tortora, G.; et al. Investigational therapies targeting signal transducer and activator of transcription 3 for the treatment of cancer. Expert. Opin. Investig. Drugs 2015, 24, 809–824. [Google Scholar] [CrossRef]
- Lao, V.V.; Grady, W.M. Epigenetics and colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 686–700. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Chen, L.; Sun, Q.; Zhao, H.; Yang, H.; Ren, S.; Liu, M.; Meng, X.; Xu, H. Scutellarin ameliorates colitis-associated colorectal cancer by suppressing Wnt/β-catenin signaling cascade. Eur. J. Pharmacol. 2021, 906, 174253. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, T.; Ye, H.; Shen, Y.; Li, Z.; Chen, L.; Wang, C.; Chen, X.; Zhao, H.; Xiang, Y.; et al. Gracillin shows potent efficacy against colorectal cancer through inhibiting the STAT3 pathway. J. Cell Mol. Med. 2021, 25, 801–812. [Google Scholar] [CrossRef]
- Baker, S.P.; Phillips, J.; Anderson, S.; Qiu, Q.; Shabanowitz, J.; Smith, M.M.; Yates, J.R., 3rd; Hunt, D.F.; Grant, P.A. Histone H3 Thr 45 phosphorylation is a replication-associated post-translational modification in S. cerevisiae. Nat. Cell Biol. 2010, 12, 294–298. [Google Scholar] [CrossRef] [Green Version]
- Thorsness, P.E.; Koshland, D.E., Jr. Inactivation of isocitrate dehydrogenase by phosphorylation is mediated by the negative charge of the phosphate. J. Biol. Chem. 1987, 262, 10422–10425. [Google Scholar] [CrossRef]
- North, J.A.; Simon, M.; Ferdinand, M.B.; Shoffner, M.A.; Picking, J.W.; Howard, C.J.; Mooney, A.M.; van Noort, J.; Poirier, M.G.; Ottesen, J.J. Histone H3 phosphorylation near the nucleosome dyad alters chromatin structure. Nucleic Acids Res. 2014, 42, 4922–4933. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Cui, L.; Ahmed, S.; Zainab, F.; Wu, Q.; Wang, X.; Yuan, Z. An overview of epigenetic agents and natural nutrition products targeting DNA methyltransferase, histone deacetylases and microRNAs. Food Chem. Toxicol. 2019, 123, 574–594. [Google Scholar] [CrossRef] [PubMed]
- Verdone, L.; Agricola, E.; Caserta, M.; Di Mauro, E. Histone acetylation in gene regulation. Brief. Funct. Genom. Proteomic 2006, 5, 209–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.Q.; Su, S.B. An overview of epigenetics in Chinese medicine researches. Chin. J. Integr. Med. 2017, 23, 714–720. [Google Scholar] [CrossRef]
- Liu, D.; Yan, H.; Kong, Y.; You, Y.; Li, Y.; Wang, L.; Tong, Y.; Wang, J. Preparation of Colon-Targeted Acetylharpagide Tablets and its Release Properties in vivo and in vitro. Front. Pharmacol. 2018, 9, 832. [Google Scholar] [CrossRef]
- Yurasakpong, L.; Nantasenamat, C.; Nobsathian, S.; Chaithirayanon, K.; Apisawetakan, S. Betulinic Acid Modulates the Expression of HSPA and Activates Apoptosis in Two Cell Lines of Human Colorectal Cancer. Molecules 2021, 26, 6377. [Google Scholar] [CrossRef]
- Yan, W.; Bai, Z.; Wang, J.; Li, X.; Chi, B.; Chen, X. ANP32A modulates cell growth by regulating p38 and Akt activity in colorectal cancer. Oncol. Rep. 2017, 38, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Liu, Y.; Guan, S.; Qiu, Z.; Liu, D. Natural products exert anti-tumor effects by regulating exosomal ncRNA. Front. Oncol. 2022, 12, 1006114. [Google Scholar] [CrossRef]
- Wang, H.; Geng, Q.R.; Wang, L.; Lu, Y. Curcumin potentiates antitumor activity of L-asparaginase via inhibition of the AKT signaling pathway in acute lymphoblastic leukemia. Leuk. Lymphoma 2012, 53, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Hanikoglu, A.; Hanikoglu, F.; Ozben, T. Natural Product Inhibitors of Histone Deacetylases as New Anticancer Agents. Curr. Protein Pept. Sci. 2018, 19, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, T.; Luo, H.; Liu, Y.; Liu, D. Targeting Epigenetic Regulatory Enzymes for Cancer Therapeutics: Novel Small-Molecule Epidrug Development. Front. Oncol. 2022, 12, 848221. [Google Scholar] [CrossRef] [PubMed]
- Cuttini, E.; Goi, C.; Pellarin, E.; Vida, R.; Brancolini, C. HDAC4 in cancer: A multitasking platform to drive not only epigenetic modifications. Front. Mol. Biosci. 2023, 10, 1116660. [Google Scholar] [CrossRef]
- Baylin, S.B.; Jones, P.A. A decade of exploring the cancer epigenome—Biological and translational implications. Nat. Rev. Cancer 2011, 11, 726–734. [Google Scholar] [CrossRef]
- Baba, Y.; Murata, A.; Watanabe, M.; Baba, H. Clinical implications of the LINE-1 methylation levels in patients with gastrointestinal cancer. Surg. Today 2014, 44, 1807–1816. [Google Scholar] [CrossRef]
- Tan, S.H.; Barker, N. Wnt Signaling in Adult Epithelial Stem Cells and Cancer. Prog. Mol. Biol. Transl. Sci. 2018, 153, 21–79. [Google Scholar] [CrossRef]
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Ghate, N.B.; Kim, S.; Spiller, E.; Kim, S.; Shin, Y.; Rhie, S.K.; Smbatyan, G.; Lenz, H.J.; Mumenthaler, S.M.; An, W. VprBP directs epigenetic gene silencing through histone H2A phosphorylation in colon cancer. Mol. Oncol. 2021, 15, 2801–2817. [Google Scholar] [CrossRef]
- Pabla, B.; Bissonnette, M.; Konda, V.J. Colon cancer and the epidermal growth factor receptor: Current treatment paradigms, the importance of diet, and the role of chemoprevention. World J. Clin. Oncol. 2015, 6, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cui, G.H.; Liu, F.; Wu, Q.L.; Chen, Y. Inhibitory effect of triptolide on lymph node metastasis in patients with non-Hodgkin lymphoma by regulating SDF-1/CXCR4 axis in vitro. Acta Pharmacol. Sin. 2006, 27, 1438–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, L.H.; Zhang, H.; Hayward, L.; Takemura, H.; Shao, R.G.; Pommier, Y. Tetrandrine induces early G1 arrest in human colon carcinoma cells by down-regulating the activity and inducing the degradation of G1-S-specific cyclin-dependent kinases and by inducing p53 and p21Cip1. Cancer Res. 2004, 64, 9086–9092. [Google Scholar] [CrossRef] [Green Version]
- He, B.C.; Gao, J.L.; Luo, X.; Luo, J.; Shen, J.; Wang, L.; Zhou, Q.; Wang, Y.T.; Luu, H.H.; Haydon, R.C.; et al. Ginsenoside Rg3 inhibits colorectal tumor growth through the down-regulation of Wnt/ss-catenin signaling. Int. J. Oncol. 2011, 38, 437–445. [Google Scholar] [CrossRef]
- Wu, K.; Wang, C.Z.; Yuan, C.S.; Huang, W.H. Oplopanax horridus: Phytochemistry and Pharmacological Diversity and Structure-Activity Relationship on Anticancer Effects. Evid. Based Complement. Altern. Med. 2018, 2018, 9186926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; Wang, X.; Zheng, L.; Zhan, Y.; Zhang, D.; Zhang, J.; Zhang, Y. Brucine suppresses colon cancer cells growth via mediating KDR signalling pathway. J. Cell Mol. Med. 2013, 17, 1316–1324. [Google Scholar] [CrossRef]
- Chien, C.C.; Wu, M.S.; Shen, S.C.; Ko, C.H.; Chen, C.H.; Yang, L.L.; Chen, Y.C. Activation of JNK contributes to evodiamine-induced apoptosis and G2/M arrest in human colorectal carcinoma cells: A structure-activity study of evodiamine. PLoS ONE 2014, 9, e99729. [Google Scholar] [CrossRef]
- Jiang, J.; Griffin, J.D. Wnt/β-catenin Pathway Modulates the Sensitivity of the Mutant FLT3 Receptor Kinase Inhibitors in a GSK-3β Dependent Manner. Genes. Cancer 2010, 1, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kim, H.J.; Kim, H.R.; Lee, S.H.; Cho, S.D.; Choi, C.S.; Nam, J.S.; Jung, J.Y. Antitumor actions of baicalein and wogonin in HT-29 human colorectal cancer cells. Mol. Med. Rep. 2012, 6, 1443–1449. [Google Scholar] [CrossRef] [Green Version]
- Taye, N.; Alam, A.; Ghorai, S.; Chatterji, D.G.; Parulekar, A.; Mogare, D.; Singh, S.; Sengupta, P.; Chatterjee, S.; Bhat, M.K.; et al. SMAR1 inhibits Wnt/β-catenin signaling and prevents colorectal cancer progression. Oncotarget 2018, 9, 21322–21336. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Jia, L.; Huang, C.; Qi, Q.; Jahangir, A.; Xia, Y.; Liu, W.; Shi, R.; Tang, L.; Chen, Z. Polyphyllin I Promotes Autophagic Cell Death and Apoptosis of Colon Cancer Cells via the ROS-Inhibited AKT/mTOR Pathway. Int. J. Mol. Sci. 2022, 23, 9368. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Pan, B.; Yang, X.; Tang, W. Polyphyllin VII suppresses cell proliferation, the cell cycle and cell migration in colorectal cancer. Oncol. Lett. 2021, 21, 25. [Google Scholar] [CrossRef]
- Mandlik, D.S.; Mandlik, S.K.; Patel, S. Protective effect of sarsasapogenin in TNBS induced ulcerative colitis in rats associated with downregulation of pro-inflammatory mediators and oxidative stress. Immunopharmacol. Immunotoxicol. 2021, 43, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Won, S.B.; Kwon, Y.H. Luteolin Induces Apoptosis and Autophagy in HCT116 Colon Cancer Cells via p53-Dependent Pathway. Nutr. Cancer 2022, 74, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Liu, X.; Fu, X.; Zhang, L.; Sui, H.; Zhou, L.; Sun, J.; Cai, J.; Qin, J.; Ren, J.; et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. PLoS ONE 2013, 8, e78700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.G.; Li, Y.C.; Lee, Y.M.; Lin, J.P.; Cheng, K.C.; Chang, W.C. Aloe-emodin inhibited N-acetylation and DNA adduct of 2-aminofluorene and arylamine N-acetyltransferase gene expression in mouse leukemia L 1210 cells. Leuk Res 2003, 27, 831–840. [Google Scholar] [CrossRef]
- Gao, F.H.; Hu, X.H.; Li, W.; Liu, H.; Zhang, Y.J.; Guo, Z.Y.; Xu, M.H.; Wang, S.T.; Jiang, B.; Liu, F.; et al. Oridonin induces apoptosis and senescence in colorectal cancer cells by increasing histone hyperacetylation and regulation of p16, p21, p27 and c-myc. BMC Cancer 2010, 10, 610. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Z.; Wu, K.; Huang, J.; Liu, Y.; Wang, X.; Meng, Z.J.; Yuan, S.X.; Wang, D.X.; Luo, J.Y.; Zuo, G.W.; et al. The PTEN/PI3K/Akt and Wnt/β-catenin signaling pathways are involved in the inhibitory effect of resveratrol on human colon cancer cell proliferation. Int. J. Oncol. 2014, 45, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Riganti, C.; Doublier, S.; Viarisio, D.; Miraglia, E.; Pescarmona, G.; Ghigo, D.; Bosia, A. Artemisinin induces doxorubicin resistance in human colon cancer cells via calcium-dependent activation of HIF-1alpha and P-glycoprotein overexpression. Br. J. Pharmacol. 2009, 156, 1054–1066. [Google Scholar] [CrossRef] [Green Version]



| Serials | Natural Products | Cas | Origin | Signal Pathways | References |
|---|---|---|---|---|---|
| 1 | Butane | 4478-93-7 | Thioglucoside is hydrolyzed by black mustard enzyme in plants | Inhibition of HDACs activity | [42] |
| 2 | Platycodin D | 58479-68-8 | Extract of Platycodon grandiflorus, family Platycodonaceae | p38 | [43] |
| 3 | Triptolide; | 38748-32-2 | The roots, leaves and flowers of the Weeping Vine | PI3K/Akt | [44] |
| 4 | pristimerin; | 1258-84-0 | One of the active ingredients of the roots of the Weeping Maple | Wnt/β-catenin | [45] |
| 5 | Peiminine; | 18059-10-4 | Phyllostachys spp. extract | Hedgehog | [46] |
| 6 | Rutin | 153-18-4 | Rutinoside, a natural flavonoid glycoside | Notch | [47] |
| 7 | Hederagenin; | 465-99-6 | Herb extracts such as Willingham | EMT | [48] |
| 8 | Andrographolide | 5508-58-7 | The active ingredients of Andrographis paniculata, family Siracaceae | Hedgehog | [47] |
| 9 | Sinomenine; | 115-53-7 | The roots and stems of the green vine of the family Fabaceae | NF-κB | [49] |
| 10 | Tenacissoside G; | 191729-43-8 | One of the components of the total saponin of Tongguan vine, family Lauraceae | ATM-CHK2-p53 | [50] |
| 11 | Desacetylcinobufagin; | 4026-95-3 | One of the main bioactive components of the skin and parotid gland behind the ear of the Chinese toad | Wnt/β-catenin | [51] |
| 12 | Magnoflorine | 6681-18-1 | Apomorphine-like alkaloids in medicinal plants of the family Fabaceae | ROS/KRAS/AMPK | [52] |
| 13 | Geniposide;GEN | 24512-63-8 | A cyclic alkane glycoside extracted from Gardenia jasminoides, family Cyperaceae | Akt/MDM2/p53 | [53] |
| 14 | shikonin, Shi | 517-89-5 | Comfrey root extract | Oxidative stress | [54] |
| 15 | Costunolide, CT | 553-21-9 | Sesquiterpene lactone compounds in the Chinese herb Mucuna pruriens | Up-regulation of Caspase-3 and Caspase-9 protein expression | [55] |
| 16 | Asterolide | 73069-14-4 | Extracts of natural products such as Atractylodes macrocephala | Inhibition of tumour cell stemness | [52] |
| 17 | Ursolic acid | 77-52-1 | Extracts of the leaves of Mignonette and Bearberry of the family Rhododendronaceae | Hedgehog, Wnt/β-catenin | [56] |
| 18 | Curcumin | 458-37-7 | A polyphenolic compound in the rhizome of Turmeric, family Gingeraceae | NF-KB, EMT, Wnt/β-catenin | [57] |
| 19 | 5,6-Dihydro-9,10-dimethoxybenz | 141433-60-5 | An isoquinoline alkaloid from the buttercup plant Huanglian | EGFR, NF-κB, Wnt/β-catenin | [58] |
| Serials | Cas | Natural Products | Categorisation | Origin | Related Targets | References |
|---|---|---|---|---|---|---|
| 1 | 520-18-3 | Kaempferol | flavonoid | Polyphenol anti-inflammatory antioxidants in fruits and vegetables | Inhibited the expression of DNMTs and HDACs in HCT-116 and HT-29 colon cancer cells, thereby reducing A4CT2 methylation levels, promoting gene transcription, significantly down-regulating the expression of key genes in the Wnt signaling pathway downstream of A4CT2, and down-regulating the expression of Wnt/β-catenin signaling pathway proteins in HCT-116 cells, thereby blocking cells in the G1 phase and inducing apoptosis. | [121] |
| 2 | 38748-32-2 | Triptolide | diterpene lactone | Triterpenoids and epoxyditerpene lactones from Radix Rehmanniae, family Weigelaeaceae | Inhibits β-catenin entry into the nucleus in the Wnt/β-catenin signaling pathway, reduces downstream target gene LEF/TCF expression, inhibits cell transcription and translation, causes G1 phase block in colon cancer HT29 and SW480 cells, and inhibits cell proliferation in a dose- and time-dependent manner | [122] |
| 3 | 518-34-3 | 6,6′,7,12-Tetramethoxy-2,2′-di | Bisbenzy-isoquinoline alkaloids | A dibenzylisoquinoline alkaloid of the plant Fangqi, family Fangqiidae. | Inhibition of LEF/TCF4 and c-Myc/Max transcriptional activity and β-catenin protein expression in the Wnt/β-catenin signaling pathway, thereby inhibiting the proliferation of colon cancer HCT116 cells | [123] |
| 4 | 14197-60-5 | Ginsenoside Rg3 | tetracyclic triterpenoid saponin | A tetracyclic triterpenoid saponin from Ginseng, family Ginseng | It inhibits β-catenin/TCF4 transcriptional activity and downstream c-Myc protein expression by blocking the nuclear translocation of β-catenin, thereby inhibiting the proliferation of colon cancer HCT116 and SW480 cells, inducing apoptosis, reducing β-catenin phosphorylation and decreasing β-catenin and c-Myc protein expression. | [124] |
| 5 | 55297-87-5 | Falcarindiol | polyacetylene oxide | A high content of kynurenine compounds in Northeastern prickly ginseng of the genus Pentaphyllaceae. | Inhibition of cell proliferation of colon cancer HCT116 cells in the G2/M phase by suppressing the expression of β-catenin, cyclinD1 and c-Myc in the Wnt/β-catenin pathway | [125] |
| 6 | 57-24-9 | Strychnine | alkaloid | An alkaloid isolated from strychnine | Inhibition of cell proliferation of colon cancer HCT116 cells in the G2/M phase by suppressing the expression of β-catenin, cyclinD1 and c-Myc in the Wnt/β-catenin pathway | [126] |
| 7 | 518-17-2 | Evodiamine | alkaloid | An alkaloid from Cornus officinalis, family Rutaceae | Reduced expression of Wnt and β-catenin mRNA and consequent inhibition of Wnt/β-catenin downstream of vascular endothelial growth factor | [127] |
| 8 | 528-43-8 | Magnolol | Dimeric phenylpropanoids | A new lignan-like compound from the Magnoliaceae thicket | Enhances the expression of caspase 3 protein and promotes apoptosis | [128] |
| 9 | 632-85-9 | Wogonin | flavonoid | A flavonoid from plants such as Scutellaria baicalensis | Modulation of the expression of Wnt/β-catenin signal pathway-related proteins β-catenin, survivin, GSK-3β and Bcl-2-related X protein blocks cells in the G1 phase. | [129] |
| 10 | 20958-18-3 | Dihydrotanshinone | Fat-soluble phenanthrenequinone compounds | Salvia Miltiorrhiza Root Extract | Inhibition of Wnt/β-catenin signaling by down-regulation of β-catenin and/or c-Myc expression to suppress proliferation of colon cancer cells. | [130] |
| 11 | 2086-83-1 | Berberine | Quaternary isoquinoline alkaloids | A quaternary ammonium alkaloid from the Chinese medicine Huanglian | Inhibition of Wnt/β-catenin signaling by down-regulation of β-catenin and/or c-Myc expression to suppress proliferation of colon cancer cells. | [58] |
| 12 | 76296-75-8 | Polyphyllin VII | steroidal saponin | Steroidal saponins extracted from the lily family Chrysanthemum. | Induces apoptosis by upregulating the expression of activated caspase-3, caspase-8, caspase-9 and Bcl-2-related X proteins and downregulating Bcl-2 expression | [131] |
| 13 | 2752-65-0 | GAMBOGIC ACID | flavonoid | The main active ingredient of Garcinia Cambogia, family Garciniaceae. | Induced apoptosis by up-regulation of activated caspase-3, activated caspase-9, Beclin 1, microtubule-associated protein 1 light chain 3-II/microtubule-associated protein 1 light chain 3-I levels and down-regulation of β-catenin, c-Myc levels | [132] |
| 14 | 126-19-2 | Sarsasapogenin | saponin | Saponin elements in the Chinese medicine Zhi Mu | Decrease the relative expression of β-catenin, c-Myc and TCF4 proteins and increase the relative expression of epithelial calmodulin protein, inhibit cell proliferation and induce apoptosis | [133] |
| 15 | 491-70-3 | Luteolin | flavonoid | A flavonoid found in plants such as honeysuckle and fruits and vegetables such as peppers | Enhanced expression of epithelial calcineurin, inhibition of Twist, β-catenin, TCF4 and MMP-2 expression, and inhibition of cell invasion and metastasis. | [134] |
| 16 | 520-36-5 | Apigenin | flavonoid | A flavonoid found in fruits and vegetables such as celery. | Reduces β-catenin, mRNA and protein levels, promotes β-catenin phosphorylation, inhibits β-catenin nuclear translocation, which in turn reduces the expression of Wnt/β-catenin downstream metastasis-related protein cyclooxygenase 2, human filament protein formation enhancer 1, and MMP-2, thereby inhibiting colorectal cancer value-added. | [135] |
| 17 | 518-82-1 | Emodin; | flavonoid | A mono-anthracene-nuclear dihydroxyanthraquinone derivative from rhubarb and other plants | Inhibition of graft tumour growth and microangiogenesis through downregulation of pro-angiogenic factor expression in the Wnt/β-catenin signal pathway. | [136] |
| 18 | 28957-04-2 | Oridonin | Kaurane-type diterpenoids | Extracts of the plant Camellia sinensis, family Labiatae | Inhibition of Wnt/β-catenin signaling by down-regulation of β-catenin and/or c-Myc expression to suppress proliferation of colon cancer cells. | [137] |
| 19 | 501-36-0 | Resveratrol | Non-flavonoid polypheolic organic compounds | Polyphenols from plants such as grapes | It can inhibit the transcriptional activity of TCF4/LEF reporter plasmid and reduce the expression of β-catenin protein and mRNA. Reduced phosphorylated GSK-3β protein levels, β-catenin mRNA expression levels and β-catenin/TCF4 transcriptional activity inhibited proliferation of colon cancer HCT116 cells, blocked cells in G1 phase and promoted apoptosis. | [138] |
| 20 | 88495-63-0 | Artesunate | diterpene lactone | A derivative of artemisinin from Artemisia annua, family Asteraceae | Inhibition of Wnt/β-catenin signaling by down-regulation of β-catenin and/or c-Myc expression to suppress proliferation of colon cancer cells. | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, Z.; Chen, M.; Yu, Q.; Guo, S.; Chen, T.; Liu, D. Histone Modification of Colorectal Cancer by Natural Products. Pharmaceuticals 2023, 16, 1095. https://doi.org/10.3390/ph16081095
Geng Z, Chen M, Yu Q, Guo S, Chen T, Liu D. Histone Modification of Colorectal Cancer by Natural Products. Pharmaceuticals. 2023; 16(8):1095. https://doi.org/10.3390/ph16081095
Chicago/Turabian StyleGeng, Zijun, Meiqi Chen, Qixuan Yu, Shuoxi Guo, Tianli Chen, and Da Liu. 2023. "Histone Modification of Colorectal Cancer by Natural Products" Pharmaceuticals 16, no. 8: 1095. https://doi.org/10.3390/ph16081095





