Adjuvanted-SARS-CoV-2 Spike Protein-Based Microparticulate Vaccine Delivered by Dissolving Microneedles Induces Humoral, Mucosal, and Cellular Immune Responses in Mice
Abstract
:1. Introduction
2. Results
2.1. Formulation and Characterization of Spike RBD, Adjuvant Microparticles and Dissolvable Microneedles
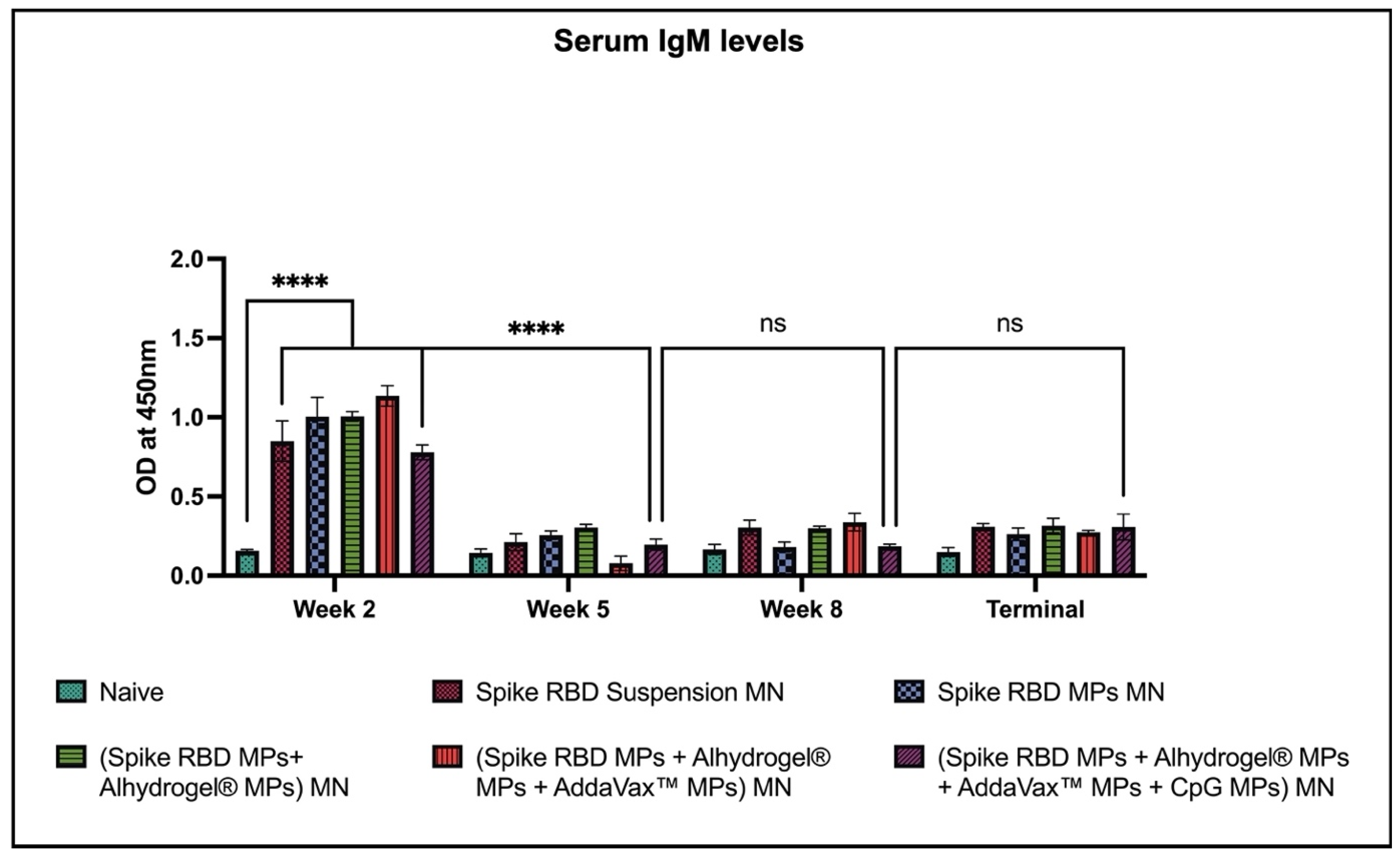
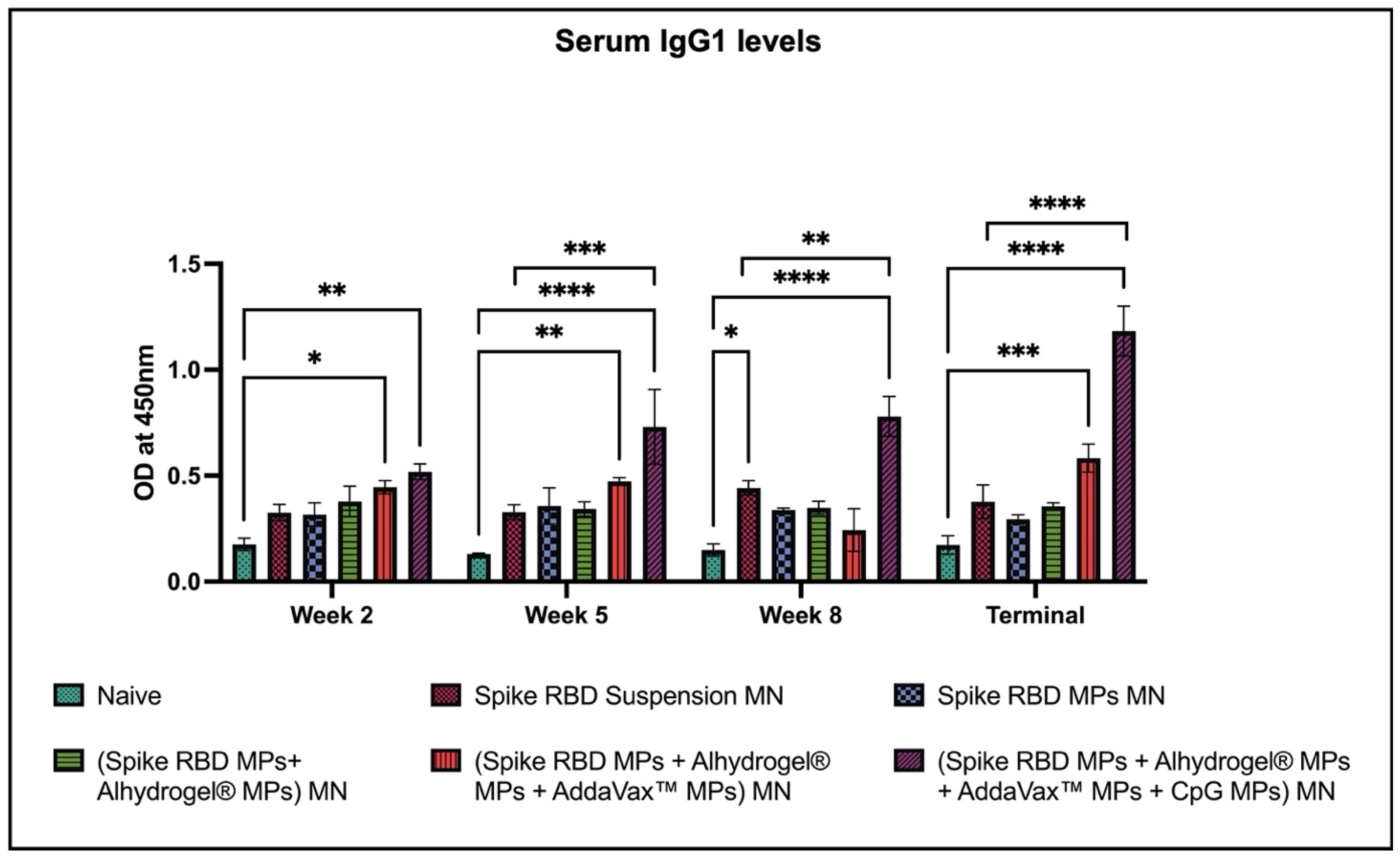
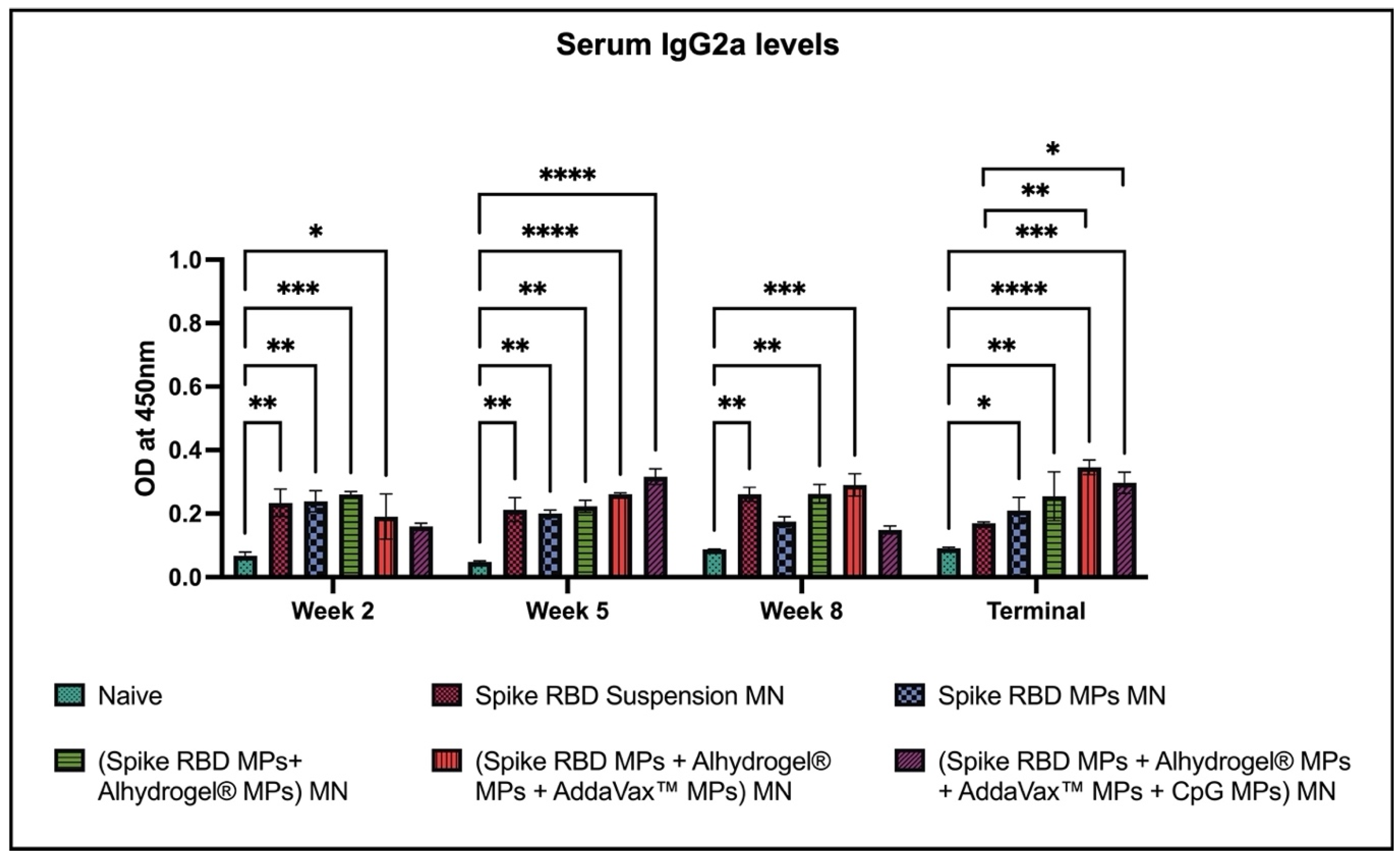
2.2. Spike RBD Specific Antibodies Detected in Sera of Vaccinated Mice
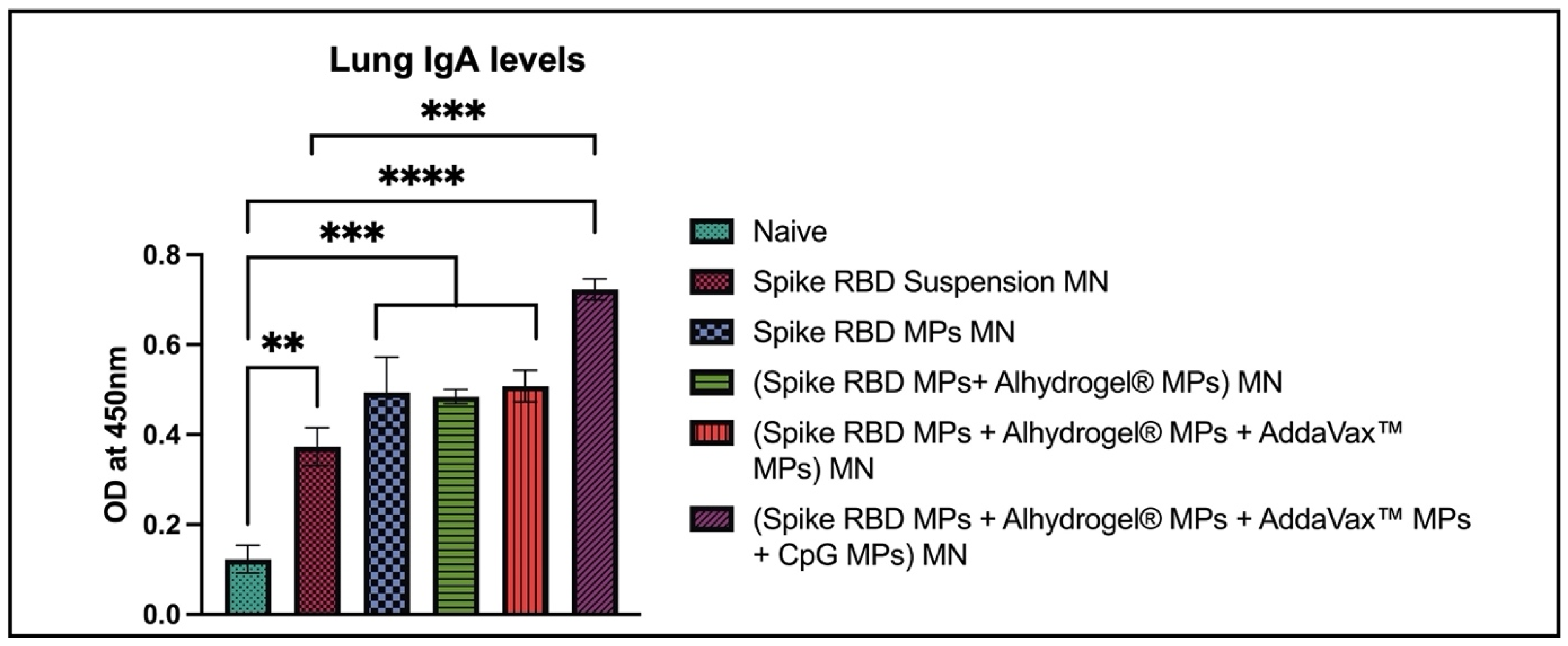
2.3. Spike RBD Specific IgA Antibodies Detected in Lung Homogenates of Vaccinated Mice
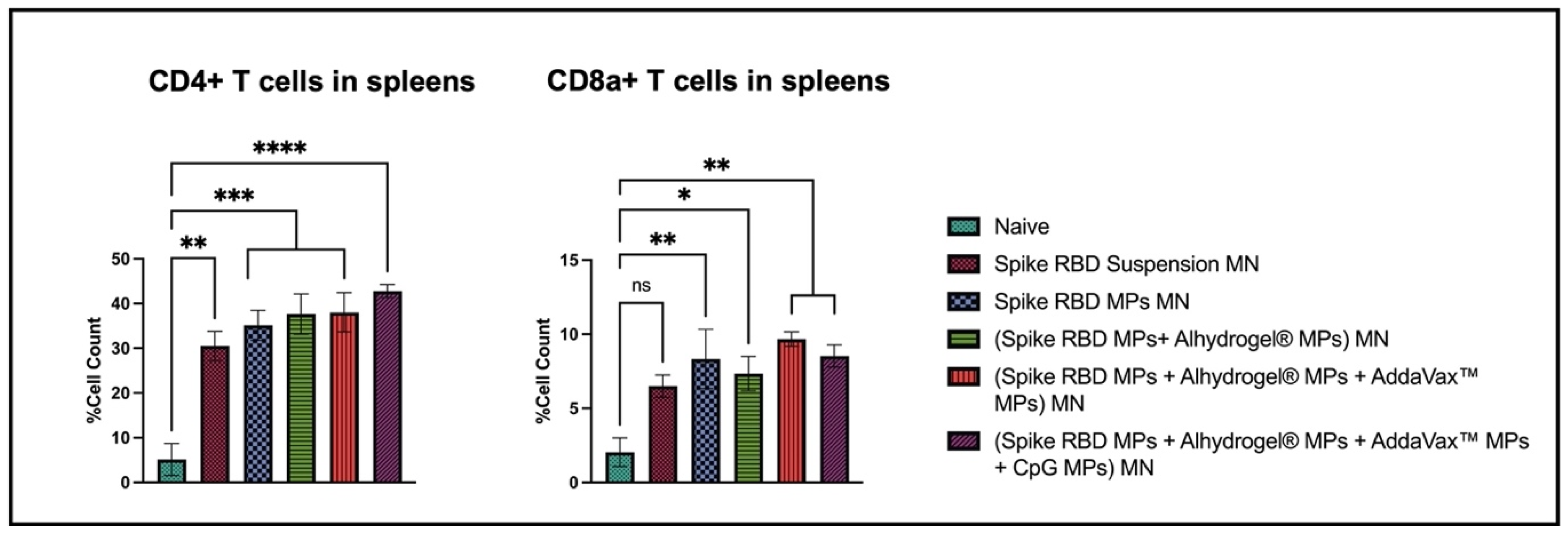
2.4. Elevated Percentage of Cells Expressing CD4 and CD8a Molecules in Spleen of Vaccinated Mice
2.5. Elevated Percentage of Cells Expressing CD4 and CD8a Molecules in Lymph Nodes of Vaccinated Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation and Characterization of Spike RBD Microparticles along with Adjuvant Microparticles
4.2.2. Formulation of Dissolvable Microneedle-Loaded with Vaccine and Adjuvant Microparticles
4.2.3. In Vivo Immunization with Dissolvable Microneedles Loaded with Vaccine and Adjuvant Microparticles
4.2.4. Quantification of Spike RBD Specific Antibody Responses in Serum
4.2.5. Measurement of Spike RBD Specific IgA Responses in Lung Homogenates
4.2.6. Evaluation of Spike RBD Specific Helper and Cytotoxic T Cell Responses in Spleen and Lymph Nodes
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ochani, R.; Asad, A.; Yasmin, F.; Shaikh, S.; Khalid, H.; Batra, S.; Sohail, M.R.; Mahmood, S.F.; Ochani, R.; Arshad, M.H.; et al. COVID-19 pandemic: From origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. InfezMed 2021, 29, 20–36. [Google Scholar]
- COVID Live—Coronavirus Statistics—Worldometer. Available online: https://www.worldometers.info/coronavirus/ (accessed on 4 May 2023).
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; et al. Coronavirus Pandemic (COVID-19). Our World in Data. 2020. Available online: https://ourworldindata.org/covid-vaccinations?country=JPN~USA (accessed on 7 August 2022).
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef]
- Ten Health Issues WHO Will Tackle This Year. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 3 July 2023).
- Noel, M.; Taddio, A.; McMurtry, C.M.; Chambers, C.T.; Riddell, R.P.; Shah, V. HELPinKids&Adults Knowledge Synthesis of the Management of Vaccination Pain and High Levels of Needle Fear: Limitations of the Evidence and Recommendations for Future Research. Clin. J. Pain 2015, 31, S124–S131. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.-Y.; Zhao, R.; Gao, L.-J.; Gao, X.-F.; Wang, D.-P.; Cao, J.-M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell. Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.H.; Hashmi, Z.; Goel, A.; Ahmad, R.; Gupta, K.; Khan, N.; Alam, I.; Ahmed, F.; Ansari, M.A. COVID-19 Pandemic and Vaccines Update on Challenges and Resolutions. Front. Cell. Infect. Microbiol. 2021, 11, 690621. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [Green Version]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef] [Green Version]
- Bagwe, P.V.; Bagwe, P.V.; Ponugoti, S.S.; Joshi, S.V. Peptide-Based Vaccines and Therapeutics for COVID-19. Int. J. Pept. Res. Ther. 2022, 28, 94. [Google Scholar] [CrossRef]
- Samrat, S.K.; Tharappel, A.M.; Li, Z.; Li, H. Prospect of SARS-CoV-2 spike protein: Potential role in vaccine and therapeutic development. Virus Res. 2020, 288, 198141. [Google Scholar] [CrossRef]
- Patil, S.; Vijayanand, S.; Joshi, D.; Menon, I.; Gomes, K.B.; Kale, A.; Bagwe, P.; Yacoub, S.; Uddin, M.N.; D’Souza, M.J. Subunit microparticulate vaccine delivery using microneedles trigger significant SARS-spike-specific humoral and cellular responses in a preclinical murine model. Int. J. Pharm. 2023, 632, 122583. [Google Scholar] [CrossRef] [PubMed]
- Vijayanand, S.; Patil, S.; Joshi, D.; Menon, I.; Gomes, K.B.; Kale, A.; Bagwe, P.; Yacoub, S.; Uddin, M.N.; D’souza, M.J. Microneedle Delivery of an Adjuvanted Microparticulate Vaccine Induces High Antibody Levels in Mice Vaccinated against Coronavirus. Vaccines 2022, 10, 1491. [Google Scholar] [CrossRef] [PubMed]
- Gause, K.T.; Wheatley, A.K.; Cui, J.; Yan, Y.; Kent, S.J.; Caruso, F. Immunological Principles Guiding the Rational Design of Particles for Vaccine Delivery. ACS Nano 2017, 11, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Zabel, F.; Schnetzler, Y.; Titz, A.; Brombacher, F.; Bachmann, M.F. Innate Immunity Mediates Follicular Transport of Particulate but Not Soluble Protein Antigen. J. Immunol. 2012, 188, 3724–3733. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-I.; Kim, D.; Yu, K.-M.; Seo, H.D.; Lee, S.-A.; Casel, M.A.B.; Jang, S.-G.; Kim, S.; Jung, W.; Lai, C.-J.; et al. Development of Spike Receptor-Binding Domain Nanoparticles as a Vaccine Candidate against SARS-CoV-2 Infection in Ferrets. mBio 2021, 12, e00230-21. [Google Scholar] [CrossRef]
- Silva, A.L.; Soema, P.C.; Slütter, B.; Ossendorp, F.; Jiskoot, W. PLGA particulate delivery systems for subunit vaccines: Linking particle properties to immunogenicity. Hum. Vaccines Immunother. 2016, 12, 1056–1069. [Google Scholar] [CrossRef]
- Allahyari, M.; Mohit, E. Peptide/protein vaccine delivery system based on PLGA particles. Hum. Vaccines Immunother. 2015, 12, 806–828. [Google Scholar] [CrossRef] [Green Version]
- Storni, T.; Kündig, T.M.; Senti, G.; Johansen, P. Immunity in response to particulate antigen-delivery systems. Adv. Drug Deliv. Rev. 2005, 57, 333–355. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- He, Y.; Park, K. Effects of the Microparticle Shape on Cellular Uptake. Mol. Pharm. 2016, 13, 2164–2171. [Google Scholar] [CrossRef] [Green Version]
- Bagwe, P.; Bajaj, L.; Gala, R.P.; D‘souza, M.J.; Zughaier, S.M. Assessment of In Vitro Immunostimulatory Activity of an Adjuvanted Whole-Cell Inactivated Neisseria gonorrhoeae Microparticle Vaccine Formulation. Vaccines 2022, 10, 983. [Google Scholar] [CrossRef]
- Zablon, F.M. MHC Molecules, Antigen Processing and Presentation. The Biology Notes. 2021. Available online: https://thebiologynotes.com/mhc-molecules-antigen-processing-presentation/ (accessed on 13 September 2021).
- Hu, X.; Deng, Y.; Chen, X.; Zhou, Y.; Zhang, H.; Wu, H.; Yang, S.; Chen, F.; Zhou, Z.; Wang, M.; et al. Immune Response of a Novel ATR-AP205-001 Conjugate Anti-hypertensive Vaccine. Sci. Rep. 2017, 7, 12580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) As biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Bilati, U.; Allémann, E.; Doelker, E. Strategic approaches for overcoming peptide and protein instability within biodegradable nano- and microparticles. Eur. J. Pharm. Biopharm. 2005, 59, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, P.S.; O’hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- CDC. Adjuvants and Vaccines. Vaccine Safety. 2020. Available online: https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html (accessed on 22 September 2022).
- InvivoGen. Alhydrogel. Alum Vaccine Adjuvant for Research. Available online: https://www.invivogen.com/alhydrogel (accessed on 12 July 2022).
- InvivoGen. AddaVaxTM. 2016. Available online: https://www.invivogen.com/addavax (accessed on 12 July 2022).
- Liu, H.; Zhou, C.; An, J.; Song, Y.; Yu, P.; Li, J.; Gu, C.; Hu, D.; Jiang, Y.; Zhang, L.; et al. Development of recombinant COVID-19 vaccine based on CHO-produced, prefusion spike trimer and alum/CpG adjuvants. Vaccine 2021, 39, 7001–7011. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Yan, J.; Wu, W.; Tao, X.; Lu, X.; Liu, S.; Zhu, W. A CpG oligodeoxynucleotide enhances the immune response to rabies vaccination in mice. Virol. J. 2018, 15, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, D.; Chbib, C.; Uddin, M.N.; D’souza, M.J. Evaluation of Microparticulate (S)-4,5-Dihydroxy-2,3-pentanedione (DPD) as a Potential Vaccine Adjuvant. AAPS J. 2021, 23, 84. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Feng, S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): A multicentre, blinded, phase 2, randomised trial. Lancet Infect. Dis. 2022, 22, 1131–1141. [Google Scholar] [CrossRef]
- Streilein, J.W. Skin-Associated Lymphoid Tissues (SALT): Origins and Functions. J. Investig. Dermatol. 1983, 80, S12–S16. [Google Scholar] [CrossRef] [PubMed]
- Sparber, F.; Tripp, C.H.; Hermann, M.; Romani, N.; Stoitzner, P. Langerhans cells and dermal dendritic cells capture protein antigens in the skin: Possible targets for vaccination through the skin. Immunobiology 2010, 215, 770–779. [Google Scholar] [CrossRef] [Green Version]
- Prausnitz, M.R.; Mikszta, J.A.; Cormier, M.; Andrianov, A.K. Microneedle-Based Vaccines. Curr. Top. Microbiol. Immunol. 2009, 333, 369–393. [Google Scholar] [CrossRef] [Green Version]
- Leone, M.; Mönkäre, J.; Bouwstra, J.A.; Kersten, G. Dissolving Microneedle Patches for Dermal Vaccination. Pharm. Res. 2017, 34, 2223–2240. [Google Scholar] [CrossRef] [Green Version]
- Menon, I.; Bagwe, P.; Gomes, K.B.; Bajaj, L.; Gala, R.; Uddin, M.N.; D’souza, M.J.; Zughaier, S.M. Microneedles: A New Generation Vaccine Delivery System. Micromachines 2021, 12, 435. [Google Scholar] [CrossRef]
- Menon, I.; Kang, S.M.; D’Souza, M. Nanoparticle formulation of the fusion protein virus like particles of respiratory syncytial virus stimulates enhanced in vitro antigen presentation and autophagy. Int. J. Pharm. 2022, 623, 121919. [Google Scholar] [CrossRef] [PubMed]
- Oyewumi, M.O.; Kumar, A.; Cui, Z. Nano-microparticles as immune adjuvants: Correlating particle sizes and the resultant immune responses. Expert Rev. Vaccines 2010, 9, 1095–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, H.; Wei, W.; Yue, Z.; Lv, P.; Wang, L.; Ma, G.; Su, Z. Particle size affects the cellular response in macrophages. Eur. J. Pharm. Sci. 2010, 41, 650–657. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Chapter 3–Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 47–76. [Google Scholar] [CrossRef]
- Kale, A.; Joshi, D.; Menon, I.; Bagwe, P.; Patil, S.; Vijayanand, S.; Gomes, K.B.; Uddin, M.N.; D’souza, M.J. Zika Vaccine Microparticles (MPs)-Loaded Dissolving Microneedles (MNs) Elicit a Significant Immune Response in a Pre-Clinical Murine Model. Vaccines 2023, 11, 583. [Google Scholar] [CrossRef]
- Sheng, T.; Luo, B.; Zhang, W.; Ge, X.; Yu, J.; Zhang, Y.; Gu, Z. Microneedle-Mediated Vaccination: Innovation and Translation. Adv. Drug Deliv. Rev. 2021, 179, 113919. [Google Scholar] [CrossRef]
- RePORT. RePORTER. Available online: https://reporter.nih.gov/search/zLkEsG2LhUKQWqvXy4rTtg/project-details/10147381 (accessed on 12 July 2022).
- Gunn, B.M.; Alter, G. Modulating Antibody Functionality in Infectious Disease and Vaccination. Trends Mol. Med. 2016, 22, 969–982. [Google Scholar] [CrossRef]
- Mazzini, L.; Martinuzzi, D.; Hyseni, I.; Benincasa, L.; Molesti, E.; Casa, E.; Lapini, G.; Piu, P.; Trombetta, C.M.; Marchi, S.; et al. Comparative analyses of SARS-CoV-2 binding (IgG, IgM, IgA) and neutralizing antibodies from human serum samples. J. Immunol. Methods 2021, 489, 112937. [Google Scholar] [CrossRef]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M.; et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Protoc. 2020, 15, 3699–3715. [Google Scholar] [CrossRef] [PubMed]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed]
- Mountford, A.P.; Fisher, A.; Wilson, R.A. The profile of IgG1 and IgG2a antibody responses in mice exposed to Schistosoma mansoni. Parasite Immunol. 1994, 16, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Aleebrahim-Dehkordi, E.; Molavi, B.; Mokhtari, M.; Deravi, N.; Fathi, M.; Fazel, T.; Mohebalizadeh, M.; Koochaki, P.; Shobeiri, P.; Hasanpour-Dehkordi, A. T helper type (Th1/Th2) responses to SARS-CoV-2 and influenza A (H1N1) virus: From cytokines produced to immune responses. Transpl. Immunol. 2022, 70, 101495. [Google Scholar] [CrossRef]
- Carty, S.A.; Riese, M.J.; Koretzky, G.A. Chapter 21–T-Cell Immunity. In Hematology, 7th ed.; Hoffman, R., Benz, E.J., Silberstein, L.E., Heslop, H.E., Weitz, J.I., Anastasi, J., Salama, M.E., Abutalib, S.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 221–239. [Google Scholar] [CrossRef]
- Zimmer, C.; Corum, J.; Wee, S.-L.; Kristoffersen, M. Coronavirus Vaccine Tracker. The New York Times. 2020. Available online: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html (accessed on 3 July 2023).
- Institute for Health Metrics and Evaluation. COVID-19 Vaccine Efficacy Summary. 2021. Available online: https://www.healthdata.org/covid/covid-19-vaccine-efficacy-summary (accessed on 3 July 2023).
- Peacocke, E.F.; Heupink, L.F.; Frønsdal, K.; Dahl, E.H.; Chola, L. Global access to COVID-19 vaccines: A scoping review of factors that may influence equitable access for low and middle-income countries. BMJ Open 2021, 11, e049505. [Google Scholar] [CrossRef]
- Fedson, D.S.; Dunnill, P. Commentary: From Scarcity to Abundance: Pandemic Vaccines and Other Agents for “Have Not” Countries. J. Public Health Policy 2007, 28, 322–340. [Google Scholar] [CrossRef]
- Gayle, H.; Foege, W.; Brown, L.; Kahn, B. (Eds.) Framework for Equitable Allocation of COVID-19 Vaccine; National Academies Press: Washington, DC, USA, 2020. [Google Scholar] [CrossRef]
- Gomes, K.B.; D’Souza, B.; Vijayanand, S.; Menon, I.; D’Souza, M.J. A dual-delivery platform for vaccination using antigen-loaded nanoparticles in dissolving microneedles. Int. J. Pharm. 2022, 613, 121393. [Google Scholar] [CrossRef]
- Kale, A.; Joshi, D.; Menon, I.; Bagwe, P.; Patil, S.; Vijayanand, S.; Gomes, K.B.; D’Souza, M. Novel Microparticulate Zika Vaccine Induces a Significant Immune Response in a Preclinical Murine Model after Intramuscular Administration. Int. J. Pharm. 2022, 624, 121975. [Google Scholar] [CrossRef]
- Menon, I.; Patil, S.; Bagwe, P.; Vijayanand, S.; Kale, A.; Gomes, K.B.; Kang, S.M.; D’souza, M. Dissolving Microneedles Loaded with Nanoparticle Formulation of Respiratory Syncytial Virus Fusion Protein Virus-like Particles (F-VLPs) Elicits Cellular and Humoral Immune Responses. Vaccines 2023, 11, 866. [Google Scholar] [CrossRef] [PubMed]




| Vaccine Group | Description of Vaccine Received | Route of Vaccination |
|---|---|---|
| Naïve | No treatment | - |
| entry 2 | data | data |
| Spike RBD Suspension MN | Spike RBD suspension only in MNs | Intradermal |
| Spike RBD MPs MN | Spike RBD PLGA MPs in MNs | Intradermal |
| (Spike RBD MPs + Alhyrogel® MPs + AddaVax™) MN | Spike RBD PLGA MPs, Alhyrogel® PLGA MPs and Addavax™ PLGA MPs in MN | Intradermal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, S.; Vijayanand, S.; Menon, I.; Gomes, K.B.; Kale, A.; Bagwe, P.; Yacoub, S.; Uddin, M.N.; D’Souza, M.J. Adjuvanted-SARS-CoV-2 Spike Protein-Based Microparticulate Vaccine Delivered by Dissolving Microneedles Induces Humoral, Mucosal, and Cellular Immune Responses in Mice. Pharmaceuticals 2023, 16, 1131. https://doi.org/10.3390/ph16081131
Patil S, Vijayanand S, Menon I, Gomes KB, Kale A, Bagwe P, Yacoub S, Uddin MN, D’Souza MJ. Adjuvanted-SARS-CoV-2 Spike Protein-Based Microparticulate Vaccine Delivered by Dissolving Microneedles Induces Humoral, Mucosal, and Cellular Immune Responses in Mice. Pharmaceuticals. 2023; 16(8):1131. https://doi.org/10.3390/ph16081131
Chicago/Turabian StylePatil, Smital, Sharon Vijayanand, Ipshita Menon, Keegan Braz Gomes, Akanksha Kale, Priyal Bagwe, Shadi Yacoub, Mohammad N. Uddin, and Martin J. D’Souza. 2023. "Adjuvanted-SARS-CoV-2 Spike Protein-Based Microparticulate Vaccine Delivered by Dissolving Microneedles Induces Humoral, Mucosal, and Cellular Immune Responses in Mice" Pharmaceuticals 16, no. 8: 1131. https://doi.org/10.3390/ph16081131
APA StylePatil, S., Vijayanand, S., Menon, I., Gomes, K. B., Kale, A., Bagwe, P., Yacoub, S., Uddin, M. N., & D’Souza, M. J. (2023). Adjuvanted-SARS-CoV-2 Spike Protein-Based Microparticulate Vaccine Delivered by Dissolving Microneedles Induces Humoral, Mucosal, and Cellular Immune Responses in Mice. Pharmaceuticals, 16(8), 1131. https://doi.org/10.3390/ph16081131












