Tamarind Seed Polysaccharide Hydrolysate Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis via Regulating the Gut Microbiota
Abstract
1. Introduction
2. Results
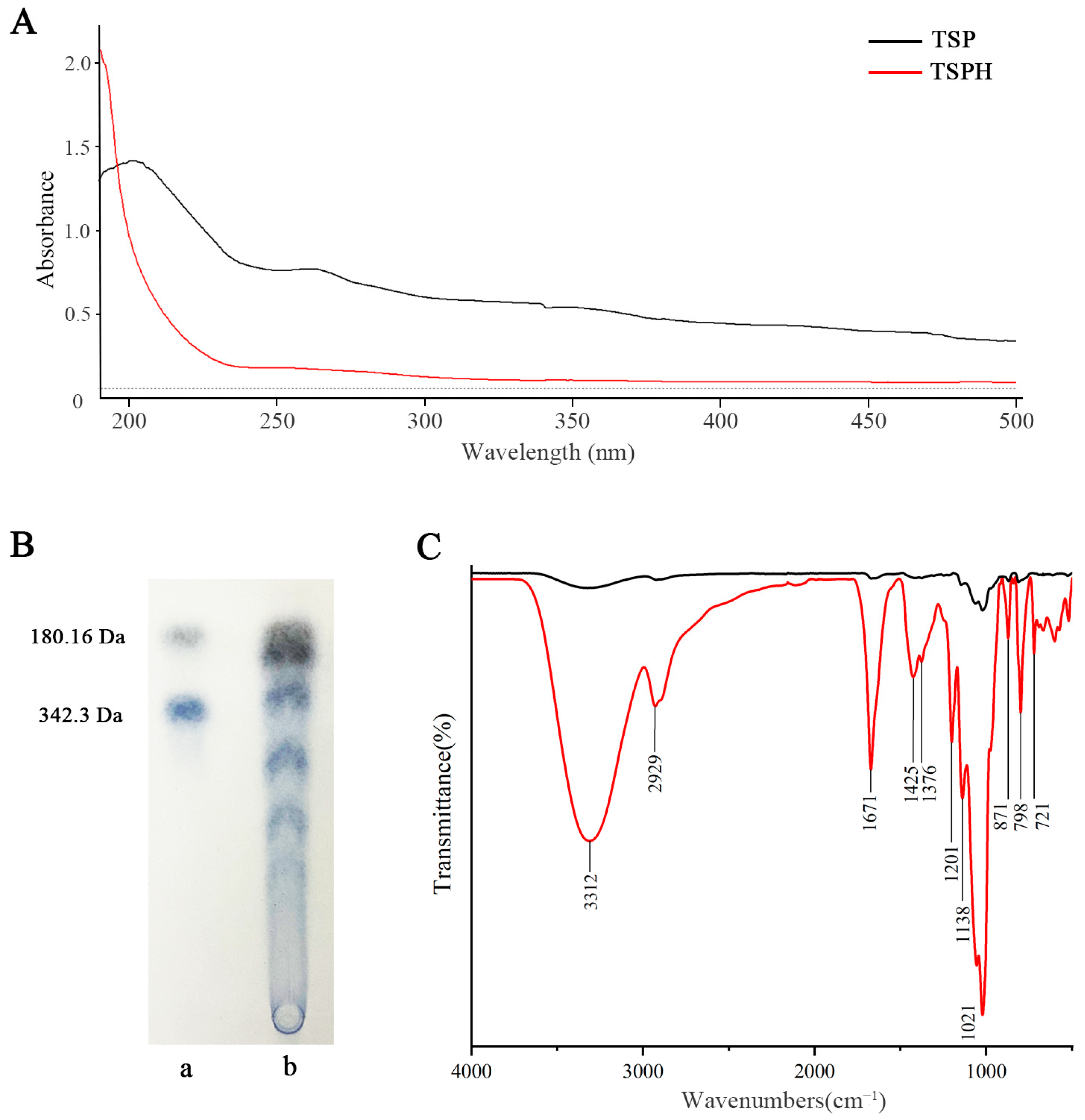
2.1. Structural Composition Analysis
2.2. TLC Analysis
2.3. FT-IR Spectra Analysis
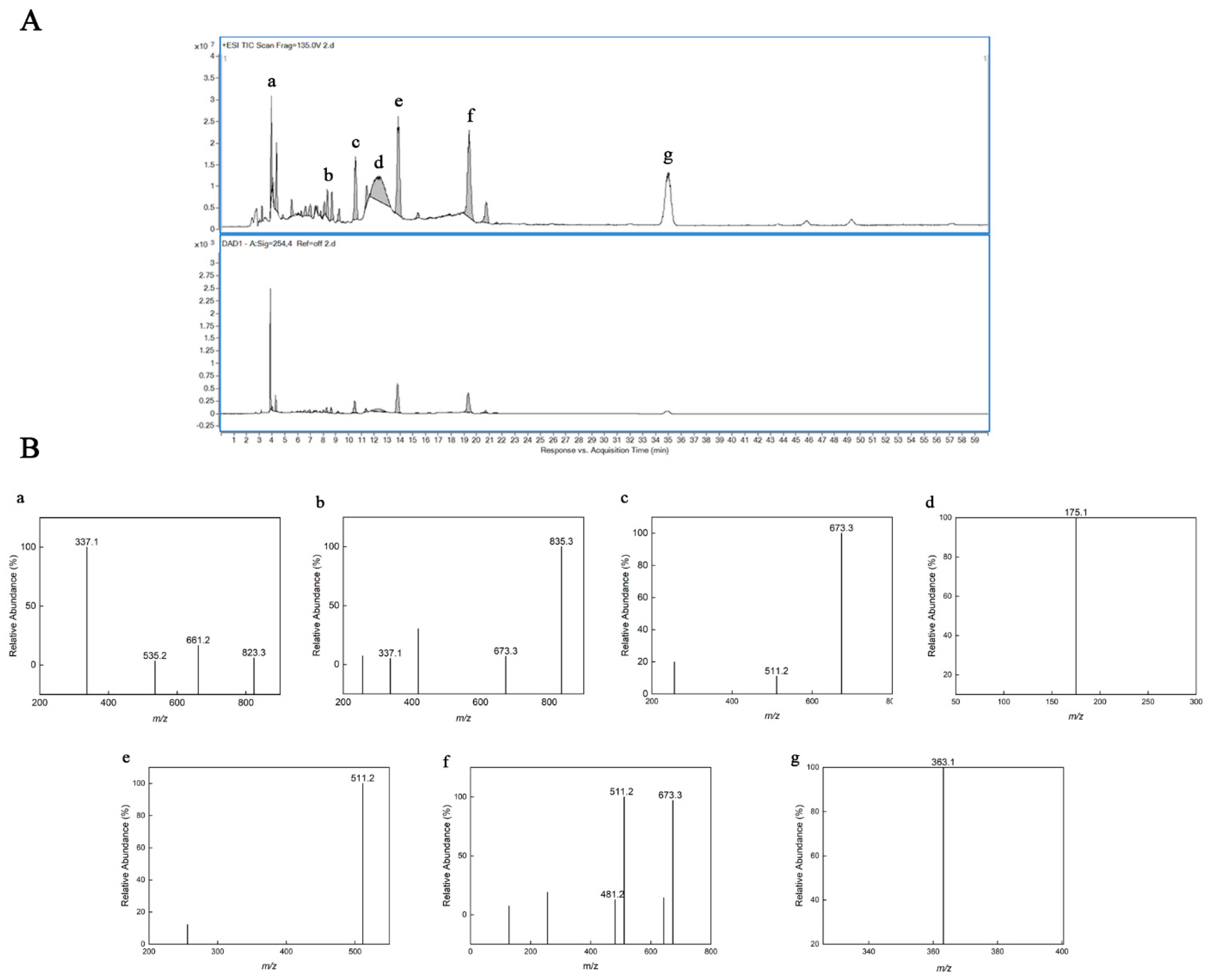
2.4. High Performance Liquid Chromatography and Electrospray Ionization Mass Spectrometry (HPLC–ESI/MS) Analysis
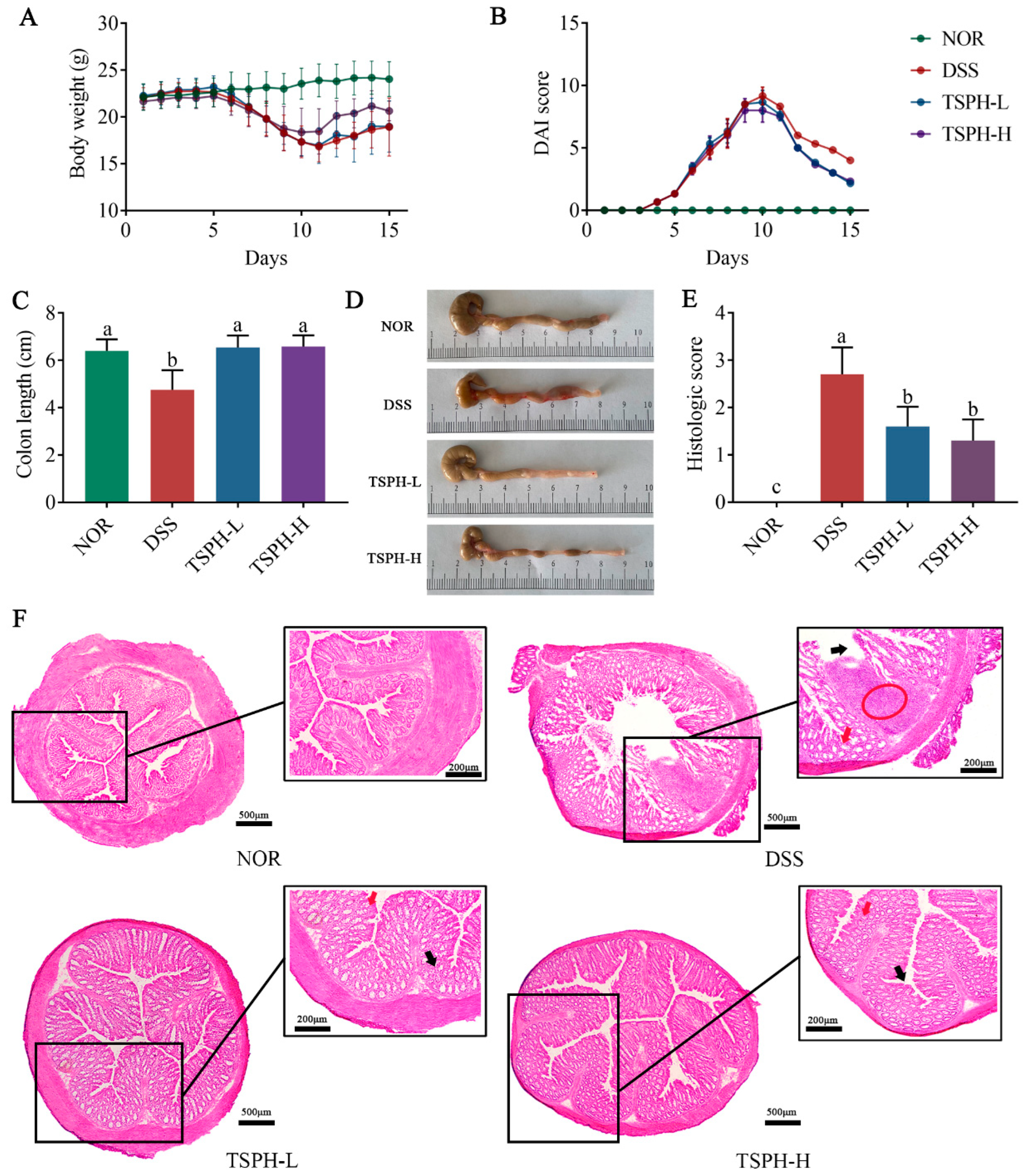
2.5. TSPH Ameliorated Colitis Symptoms in DSS-Treated Mice
2.6. TSPH Reduced the Histological Injury of Colon
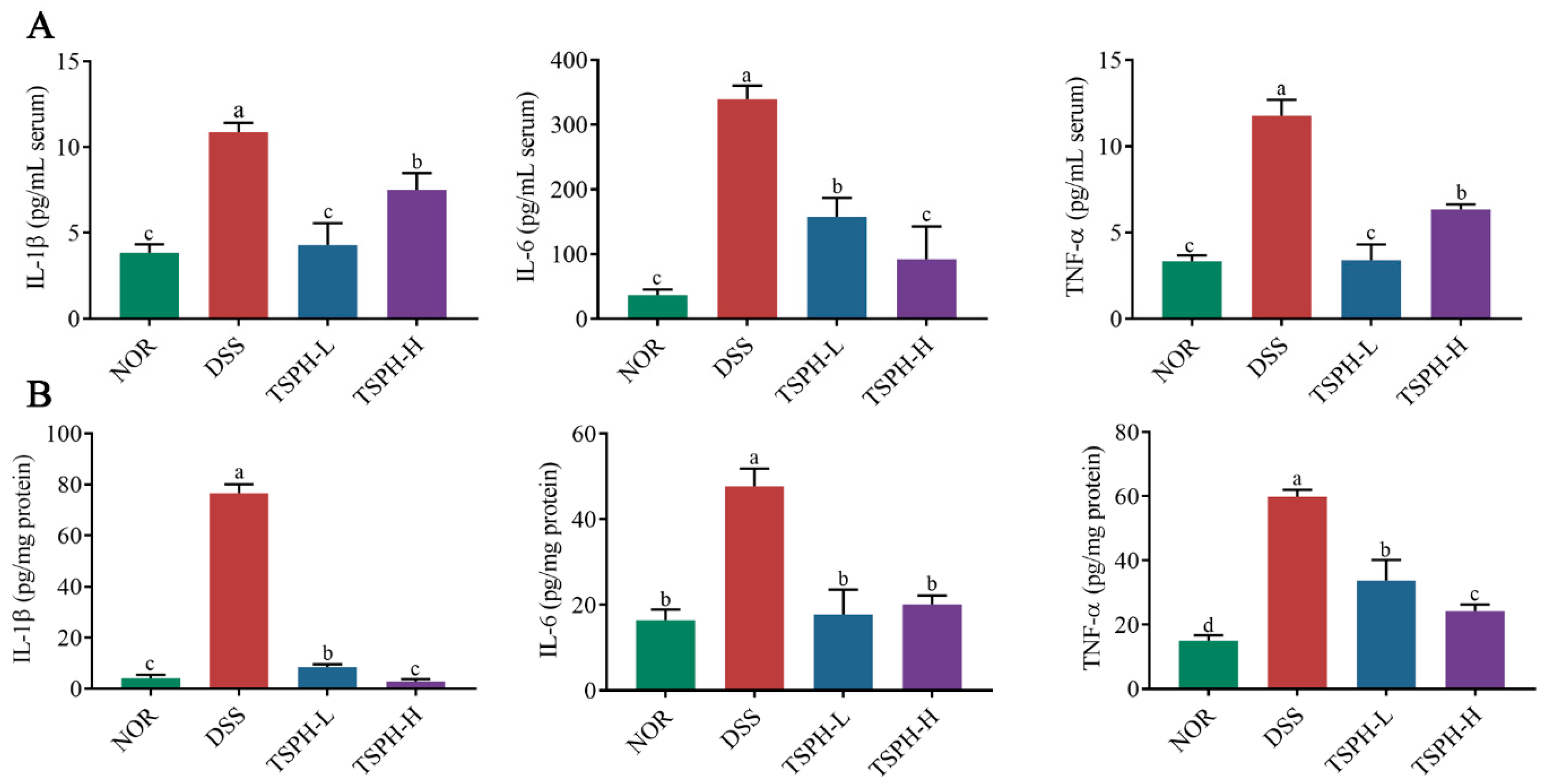
2.7. TSPH Inhibited Inflammatory Cytokine Secretion
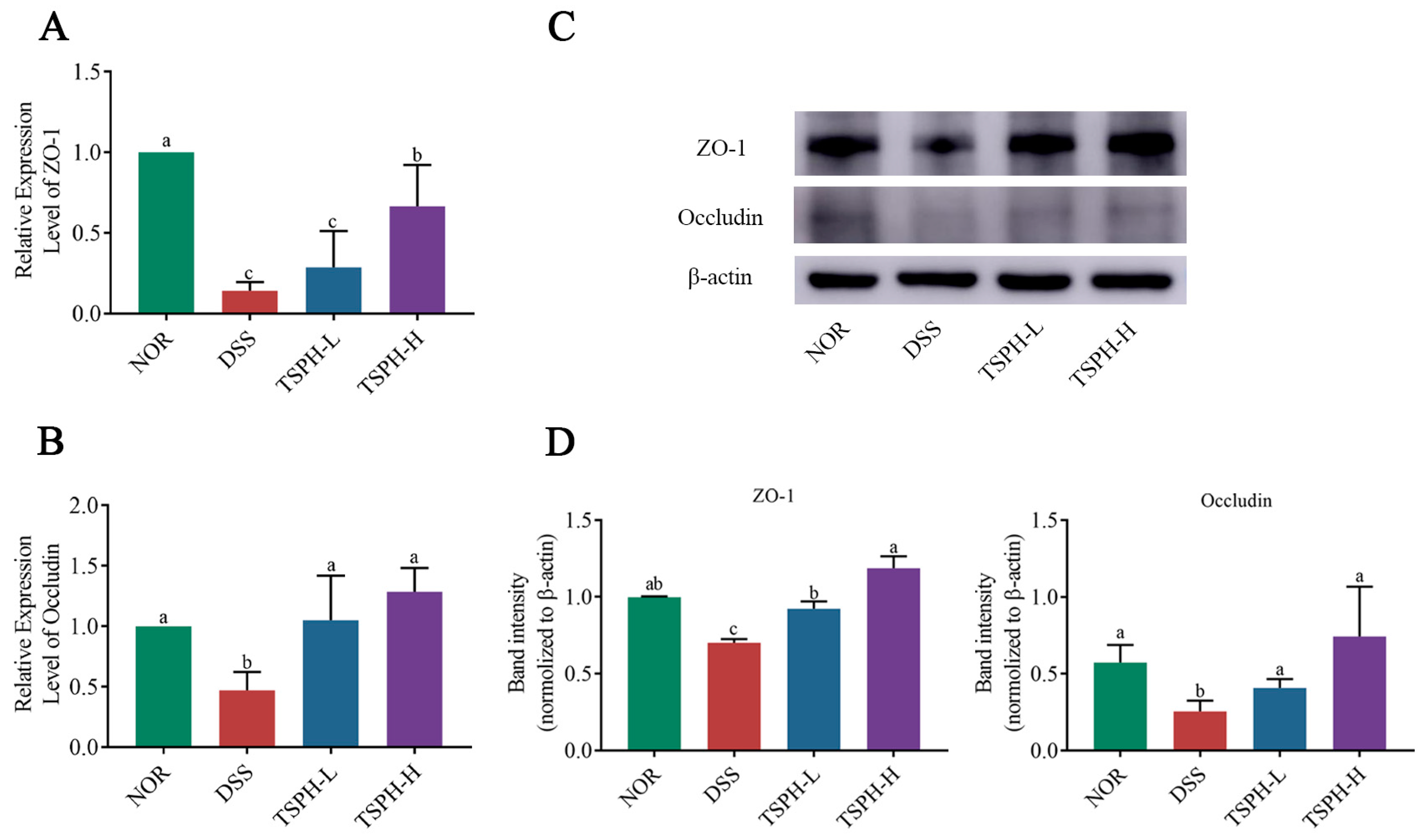
2.8. TSPH Regulates Aberrant Expression of Tight Junction(TJ) Proteins
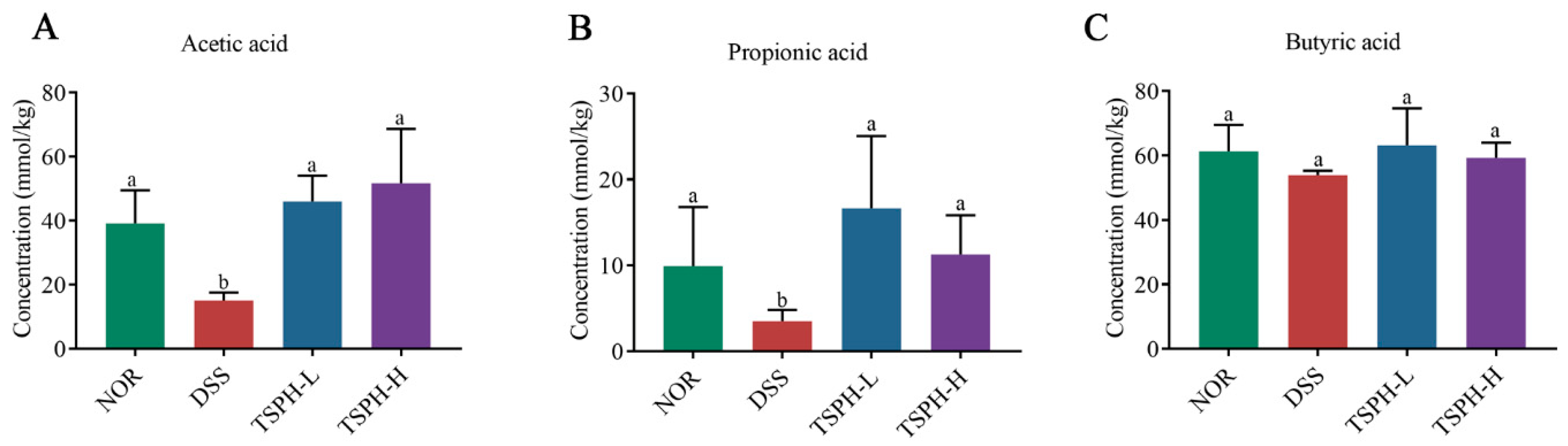
2.9. TSPH Promoted the Production of SCFAs
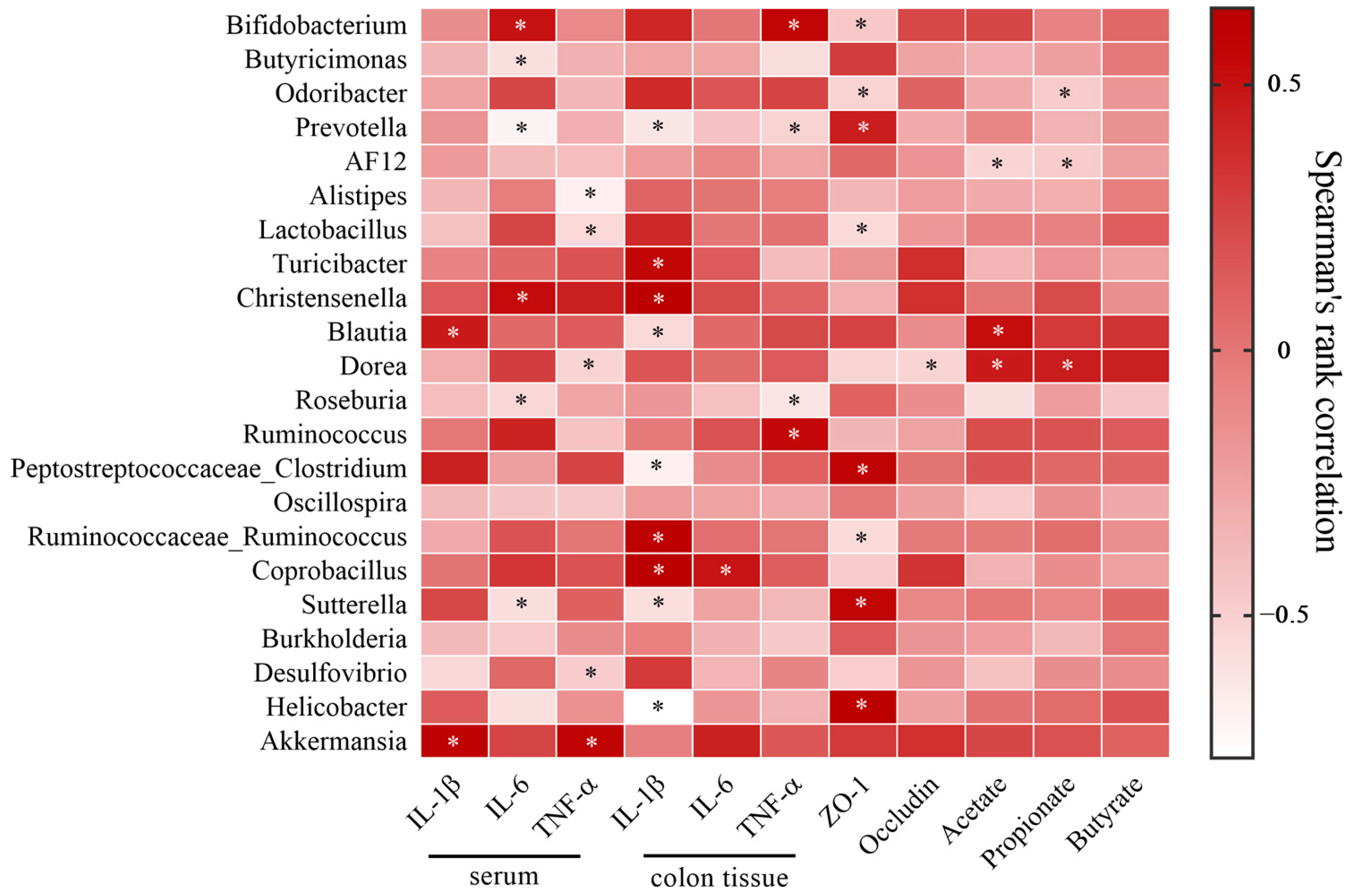
2.10. LBGH Regulated Gut Microbiota
3. Discussion
4. Materials and Methods
4.1. Hydrolysis and Purification of Oligosaccharide
4.2. UV Analysis
4.3. TLC Analysis
4.4. FT-IR Spectra Analysis
4.5. HPLC–ESI/MS Analysis
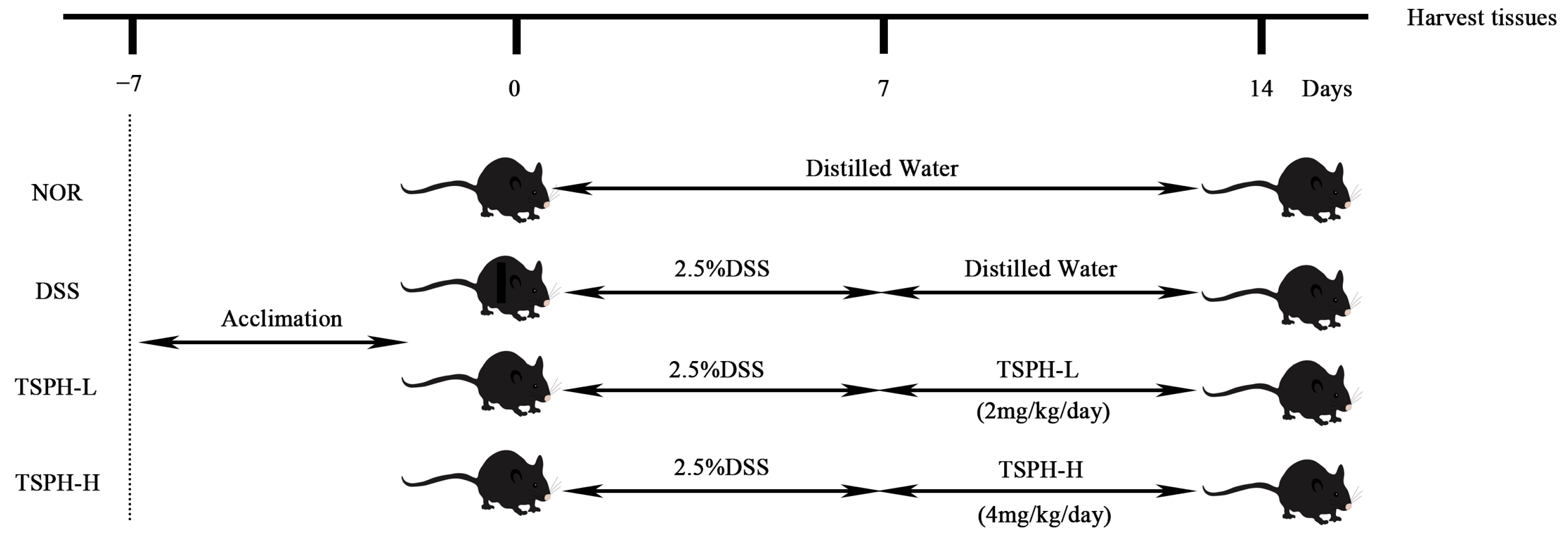
4.6. Animal Experiments
4.7. Evaluation of the Colon Histological
4.8. Measurement of Cytokines
4.9. Determination of TJ Proteins Related Gene Expression
4.10. Assessment of TJ Proteins Expression
4.11. Determination of SCFAs
4.12. Gut Microbiota Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wilks, S. Morbid appearances in the intestine of Miss Bankes. Med. Times Gaz. 1859, 2, 264–265. [Google Scholar]
- Khan, S.; Waliullah, S.; Godfrey, V.; Khan, M.A.W.; Ramachandran, R.A.; Cantarel, B.L.; Behrendt, C.; Peng, L.; Hooper, L.V.; Zaki, H. Dietary simple sugars alter microbial ecology in the gut and promote colitis in mice. Sci. Transl. Med. 2020, 12, eaay6218. [Google Scholar] [CrossRef]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Coward, S.; Clement, F.; Benchimol, E.I.; Bernstein, C.N.; Avina-Zubieta, J.A.; Bitton, A.; Carroll, M.W.; Hazlewood, G.; Jacobson, K.; Jelinski, S.; et al. Past and Future Burden of Inflammatory Bowel Diseases Based on Modeling of Population-Based Data. Gastroenterology 2019, 156, 1345–1353.e4. [Google Scholar] [CrossRef] [PubMed]
- Larabi, A.; Barnich, N.; Nguyen, H.T.T. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 2020, 16, 38–51. [Google Scholar] [CrossRef]
- Aviello, G.; Singh, A.K.; O’Neill, S.; Conroy, E.; Gallagher, W.; D’Agostino, G.; Walker, A.W.; Bourke, B.; Scholz, D.; Knaus, U.G. Colitis susceptibility in mice with reactive oxygen species deficiency is mediated by mucus barrier and immune defense defects. Mucosal Immunol. 2019, 12, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Sugihara, K.; Gillilland, M.G.; Jon, S.; Kamada, N.; Moon, J.J. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 2020, 19, 118–126. [Google Scholar] [CrossRef]
- Tang, Y.-H.; Liu, H.-C.; Song, G.; Wu, T.-T.; Zhao, Y.; Shi, L.-J. A case-control study on the association of intestinal flora with ulcerative colitis. AMB Express 2021, 11, 106. [Google Scholar] [CrossRef]
- Dai, Z.F.; Ma, X.Y.; Yang, R.L.; Wang, H.C.; Xu, D.D.; Yang, J.N.; Guo, X.B.; Meng, S.S.; Xu, R.; Li, Y.X.; et al. Intestinal flora alterations in patients with ulcerative colitis and their association with inflammation. Exp. Ther. Med. 2021, 22, 1322. [Google Scholar] [CrossRef]
- Assa, A.; Butcher, J.; Li, J.; Elkadri, A.; Sherman, P.M.; Muise, A.M.; Stintzi, A.; Mack, D. Mucosa-Associated Ileal Microbiota in New-Onset Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 1533–1539. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopec, P.; Slizewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Dai, S.H.; Pan, M.F.; El-Nezami, H.S.; Wan, J.M.F.; Wang, M.F.; Habimana, O.; Lee, J.C.Y.; Louie, J.C.Y.; Shah, N.P. Effects of Lactic Acid Bacteria-Fermented Soymilk on Isoflavone Metabolites and Short-Chain Fatty Acids Excretion and Their Modulating Effects on Gut Microbiota. J. Food Sci. 2019, 84, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef]
- Hung, K.-Y.; Wu, S.-Y.; Pao, H.-P.; Liao, W.-I.; Chu, S.-J. Acetate, a gut bacterial product, ameliorates ischemia-reperfusion induced acute lung injury in rats. Int. Immunopharmacol. 2022, 111, 109136. [Google Scholar] [CrossRef]
- Nakano, T.; Uchiyama, K.; Ushiroda, C.; Kashiwagi, S.; Toyokawa, Y.; Mizushima, K.; Inoue, K.; Dohi, O.; Okayama, T.; Yoshida, N.; et al. Promotion of wound healing by acetate in murine colonic epithelial cell via c-Jun N-terminal kinase activation. J. Gastroenterol. Hepatol. 2020, 35, 1171–1179. [Google Scholar] [CrossRef]
- Mishiro, T.; Kusunoki, R.; Otani, A.; Ansary, M.M.U.; Tongu, M.; Harashima, N.; Yamada, T.; Sato, S.; Amano, Y.; Itoh, K.; et al. Butyric acid attenuates intestinal inflammation in murine DSS-induced colitis model via milk fat globule-EGF factor 8. Lab. Investig. 2013, 93, 834–843. [Google Scholar] [CrossRef]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; Stangl, G.I.; Eilers, E.; Bader, V.; Haase, S.; Kaisler, J.; David, C.; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067–1080.e16. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Liu, J.M.; Ling, Z.X. Short-chain fatty acids-producing probiotics: A novel source of psychobiotics. Crit. Rev. Food Sci. Nutr. 2022, 62, 7929–7959. [Google Scholar] [CrossRef]
- Zhang, N.; Jin, M.L.; Wang, K.M.; Zhang, Z.H.; Shah, N.P.; Wei, H. Functional oligosaccharide fermentation in the gut: Improving intestinal health and its determinant factors—A review. Carbohydr. Polym. 2022, 284, 119043. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, H.; Lee, J.S.; Yamagishi, S.; Shinoki, A.; Lang, W.; Thawornkuno, C.; Kang, H.K.; Kumagai, Y.; Suzuki, S.; Kitamura, S.; et al. The Delay in the Development of Experimental Colitis from Isomaltosyloligosaccharides in Rats Is Dependent on the Degree of Polymerization. PLoS ONE 2012, 7, e50658. [Google Scholar] [CrossRef]
- Hamman, H.; Steenekamp, J.; Hamman, J. Use of Natural Gums and Mucilages as Pharmaceutical Excipients. Curr. Pharm. Des. 2015, 21, 4775–4797. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.S.; Mohammadzadeh, V.; Yazdi, M.E.T.; Barani, M.; Rahdar, A.; Kyzas, G.Z. Plant-Based Gums and Mucilages Applications in Pharmacology and Nanomedicine: A Review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D. Functionalization of Tamarind Gum for Drug Delivery. In Functional Biopolymers; Thakur, V.K., Thakur, M.K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 25–56. [Google Scholar]
- Goyal, P.; Kumar, V.; Sharma, P. Carboxymethylation of Tamarind kernel powder. Carbohydr. Polym. 2007, 69, 251–255. [Google Scholar] [CrossRef]
- GRAS Notice (GRN) No. 503. Determination of the GRAS Status of the Addition of Tamarind Seed Polysaccharide to Conventional Foods as a Stabilizer and Thickener. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=503 (accessed on 17 June 2021).
- Nagar, C.K.; Dash, S.K.; Rayaguru, K. Tamarind seed: Composition, applications, and value addition: A comprehensive review. J. Food Process. Preserv. 2022, 46, e16872. [Google Scholar] [CrossRef]
- Sreelekha, T.T.; Vijayakumar, T.; Ankanthil, R.; Vijayan, K.K.; Nair, M.K. Immunomodulatory effects of a polysaccharide from Tamarindus indica. Anti-Cancer Drugs 1993, 4, 209–212. [Google Scholar] [CrossRef]
- Aravind, S.R.; Joseph, M.M.; Varghese, S.; Balaram, P.; Sreelekha, T.T. Antitumor and Immunopotentiating Activity of Polysaccharide PST001 Isolated from the Seed Kernel of Tamarindus indica: An In Vivo Study in Mice. Sci. World J. 2012, 2012, 361382. [Google Scholar] [CrossRef]
- Qi, X.; Tester, R.F. Utilisation of dietary fibre (non-starch polysaccharide and resistant starch) molecules for diarrhoea therapy: A mini-review. Int. J. Biol. Macromol. 2019, 122, 572–577. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. X-ray diffraction, IR spectroscopy and thermal characterization of partially hydrolyzed guar gum. Int. J. Biol. Macromol. 2012, 50, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, N.J.F.H. Structural elucidation of a water-soluble polysaccharide isolated from Balangu shirazi (Lallemantia royleana) seeds. Food Hydrocoll. 2017, 72, 263–270. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.L. Extraction and antioxidant activities of cushaw polysaccharide. Int. J. Biol. Macromol. 2018, 120, 1646–1649. [Google Scholar] [CrossRef]
- Fan, B.; Li, T.; Song, X.; Wu, C.; Qian, C. A rapid, accurate and sensitive method for determination of monosaccharides in different varieties of Osmanthus fragrans Lour by pre-column derivatization with HPLC-MS/MS. Int. J. Biol. Macromol. 2019, 125, 221–231. [Google Scholar] [CrossRef]
- Ponzini, E.; Natalello, A.; Usai, F.; Bechmann, M.; Peri, F.; Mueller, N.; Grandori, R. Structural characterization of aerogels derived from enzymatically oxidized galactomannans of fenugreek, sesbania and guar gums. Carbohydr. Polym. 2019, 207, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, T.L.; Wang, J.Q.; Xia, Y.J.; Song, Z.B.; Ai, L.Z. An amendment to the fine structure of galactoxyloglucan from Tamarind (Tamarindus indica L.) seed. Int. J. Biol. Macromol. 2020, 149, 1189–1197. [Google Scholar] [CrossRef]
- Aravind, S.R.; Joseph, M.M.; George, S.K.; Dileep, K.V.; Varghese, S.; Rose-James, A.; Balaram, P.; Sadasivan, C.; Sreelekha, T.T. TRAIL-based tumor sensitizing galactoxyloglucan, a novel entity for targeting apoptotic machinery. Int. J. Biochem. Cell Biol. 2015, 59, 153–166. [Google Scholar] [CrossRef]
- Pott, J.; Kabat, A.M.; Maloy, K.J. Intestinal Epithelial Cell Autophagy Is Required to Protect against TNF-Induced Apoptosis during Chronic Colitis in Mice. Cell Host Microbe 2018, 23, 191–202.e4. [Google Scholar] [CrossRef]
- Ludwiczek, O.; Vannier, E.; Borggraefe, I.; Kaser, A.; Siegmund, B.; Dinarello, C.A.; Tilg, H. Imbalance between interleukin-1 agonists and antagonists: Relationship to severity of inflammatory bowel disease. Clin. Exp. Immunol. 2004, 138, 323–329. [Google Scholar] [CrossRef]
- Neurath, M.F.; Becker, C.; Barbulescu, K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut 1998, 43, 856–860. [Google Scholar] [CrossRef]
- Neurath, M.F.; Fuss, I.; Schurmann, G.; Pettersson, S.; Arnold, K.; Muller-Lobeck, H.; Strober, W.; Herfarth, C.; Buschenfelde, K.H. Cytokine gene transcription by NF-kappa B family members in patients with inflammatory bowel disease. Ann. N. Y. Acad. Sci. 1998, 859, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Denizot, J.; Sivignon, A.; Barreau, F.; Darcha, C.; Chan, H.F.C.; Stanners, C.P.; Hofman, P.; Darfeuille-Michaud, A.; Barnich, N. Adherent-invasive Escherichia coli induce claudin-2 expression and barrier defect in CEABAC10 mice and Crohn’s disease patients. Inflamm. Bowel Dis. 2012, 18, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, R.; Tan, J.; Peng, L.; Wang, P.; Liu, J.; Xiong, H.; Jiang, B.; Chen, Y. Syndecan-1 Acts in Synergy with Tight Junction Through Stat3 Signaling to Maintain Intestinal Mucosal Barrier and Prevent Bacterial Translocation. Inflamm. Bowel Dis. 2015, 21, 1894–1907. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Anderson, J.M. Architecture of tight junctions and principles of molecular composition. Semin. Cell Dev. Biol. 2014, 36, 157–165. [Google Scholar] [CrossRef]
- Stevenson, B.R.; Siliciano, J.D.; Mooseker, M.S.; Goodenough, D.A. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986, 103, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; O’Riordan, M.X.D. Regulation of Bacterial Pathogenesis by Intestinal Short-Chain Fatty Acids. Adv. Appl. Microbiol. 2013, 85, 93–118. [Google Scholar]
- Osaka, T.; Moriyama, E.; Arai, S.; Date, Y.; Yagi, J.; Kikuchi, J.; Tsuneda, S. Meta-Analysis of Fecal Microbiota and Metabolites in Experimental Colitic Mice during the Inflammatory and Healing Phases. Nutrients 2017, 9, 1329. [Google Scholar] [CrossRef]
- Turnbaugh, P.J. Microbes and Diet-Induced Obesity: Fast, Cheap, and Out of Control. Cell Host Microbe 2017, 21, 278–281. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving our Microbial Self: The Deleterious Consequences of a Diet Deficient in Microbiota-Accessible Carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Tseng, S.-F.; Wu, T.-R.; Chen, Y.-Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2017, 8, 16130. [Google Scholar] [CrossRef]
- Willing, B.P.; Dicksved, J.; Halfvarson, J.; Andersson, A.F.; Lucio, M.; Zheng, Z.; Jarnerot, G.; Tysk, C.; Jansson, J.K.; Engstrand, L. A Pyrosequencing Study in Twins Shows That Gastrointestinal Microbial Profiles Vary With Inflammatory Bowel Disease Phenotypes. Gastroenterology 2010, 139, 1844–1854.e1. [Google Scholar] [CrossRef]
- Liu, M.J.; Yang, J.Y.; Yan, Z.H.; Hu, S.; Li, J.Q.; Xu, Z.X.; Jian, Y.P. Recent findings in Akkermansia muciniphila-regulated metabolism and its role in intestinal diseases. Clin. Nutr. 2022, 41, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Khalil, A.A.; Rahman, U.-U.; Khalid, A.; Naz, S.; Shariati, M.A.; Rebezov, M.; Urtecho, E.Z.; de Albuquerque, R.D.D.G.; Anwar, S.; et al. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6034–6054. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, Z.Y. Polysaccharides isolated from Nostoc commune Vaucher inhibit colitis-associated colon tumorigenesis in mice and modulate gut microbiota. Food Funct. 2019, 10, 6873–6881. [Google Scholar] [CrossRef]
- Guo, C.L.; Wang, Y.Q.; Zhang, S.A.; Zhang, X.Q.; Du, Z.Y.; Li, M.X.; Ding, K. Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and SCFAs metabolism. Int. J. Biol. Macromol. 2021, 181, 357–368. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef]
- Mao, Y.H.; Xu, Y.X.; Li, Y.H.; Cao, J.; Song, F.L.; Zhao, D.; Zhao, Y.M.; Wang, Z.M.; Yang, Y. Effects of konjac glucomannan with different molecular weights on gut microflora with antibiotic perturbance in in vitro fecal fermentation. Carbohydr. Polym. 2021, 273, 118546. [Google Scholar] [CrossRef]
- Malgas, S.; Pletschke, B.I. The effect of an oligosaccharide reducing-end xylanase, BhRex8A, on the synergistic degradation of xylan backbones by an optimised xylanolytic enzyme cocktail. Enzym. Microb. Technol. 2019, 122, 74–81. [Google Scholar] [CrossRef]
- Lu, J.; Ai, C.; Guo, L.; Fu, Y.; Cao, C.; Song, S. Characteristic oligosaccharides released from acid hydrolysis for the structural analysis of chondroitin sulfate. Carbohydr. Res. 2017, 449, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.L.; Wen, C.R.; Lu, J.J.; Teng, N.; Song, S.; Zhu, B.W. Characterization of acidic polysaccharides from the mollusks through acid hydrolysis. Carbohydr. Polym. 2015, 130, 268–274. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, B.; Wu, Y.; Liu, Y.; Gu, X.; Zhang, H.; Wang, C.; Cao, H.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of kappa-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015, 178, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ma, Y.; Xiao, J.; You, L.; Pedisic, S.; Liao, L. The possible mechanism of the protective effect of a sulfated polysaccharide from Gracilaria Lemaneiformis against colitis induced by dextran sulfate sodium in mice. Food Chem. Toxicol. 2021, 149, 112001. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cong, Q.; Du, Z.; Liao, W.; Zhang, L.; Yao, Y.; Ding, K. Sulfated fucoidan FP08S2 inhibits lung cancer cell growth in vivo by disrupting angiogenesis via targeting VEGFR2/VEGF and blocking VEGFR2/Erk/VEGF signaling. Cancer Lett. 2016, 382, 44–52. [Google Scholar] [CrossRef]
- Zhao, G.; Nyman, M.; Jonsson, J.A. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, K.; Wang, D.; Su, L.; Liu, X.; Yue, Q.; Zhang, S.; Zhao, L. Tamarind Seed Polysaccharide Hydrolysate Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis via Regulating the Gut Microbiota. Pharmaceuticals 2023, 16, 1133. https://doi.org/10.3390/ph16081133
Jiang K, Wang D, Su L, Liu X, Yue Q, Zhang S, Zhao L. Tamarind Seed Polysaccharide Hydrolysate Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis via Regulating the Gut Microbiota. Pharmaceuticals. 2023; 16(8):1133. https://doi.org/10.3390/ph16081133
Chicago/Turabian StyleJiang, Kangjia, Duo Wang, Le Su, Xinli Liu, Qiulin Yue, Song Zhang, and Lin Zhao. 2023. "Tamarind Seed Polysaccharide Hydrolysate Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis via Regulating the Gut Microbiota" Pharmaceuticals 16, no. 8: 1133. https://doi.org/10.3390/ph16081133
APA StyleJiang, K., Wang, D., Su, L., Liu, X., Yue, Q., Zhang, S., & Zhao, L. (2023). Tamarind Seed Polysaccharide Hydrolysate Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis via Regulating the Gut Microbiota. Pharmaceuticals, 16(8), 1133. https://doi.org/10.3390/ph16081133





