Phytochemical Investigation of Lepionurus sylvestris Blume and Their Anti-Diabetes Effects via Anti-Alpha Glucosidase and Insulin Secretagogue Activities Plus Molecular Docking
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Investigation from Ethanolic Leaf Extract of L. sylvestris
2.1.1. Interruptin A (1)
2.1.2. Interruptin C (2)
2.1.3. Ergosterol (3)
2.1.4. Diglycerol (4)
2.1.5. The New Diterpene Derivative: 15-16-epoxy-neo-cleoda-3,7(20),13(16),14-tetraene-12,17:18,19-diolide (Lepionurodiolide) (5)
2.2. Total Phenolic and Total Flavonoid Contents
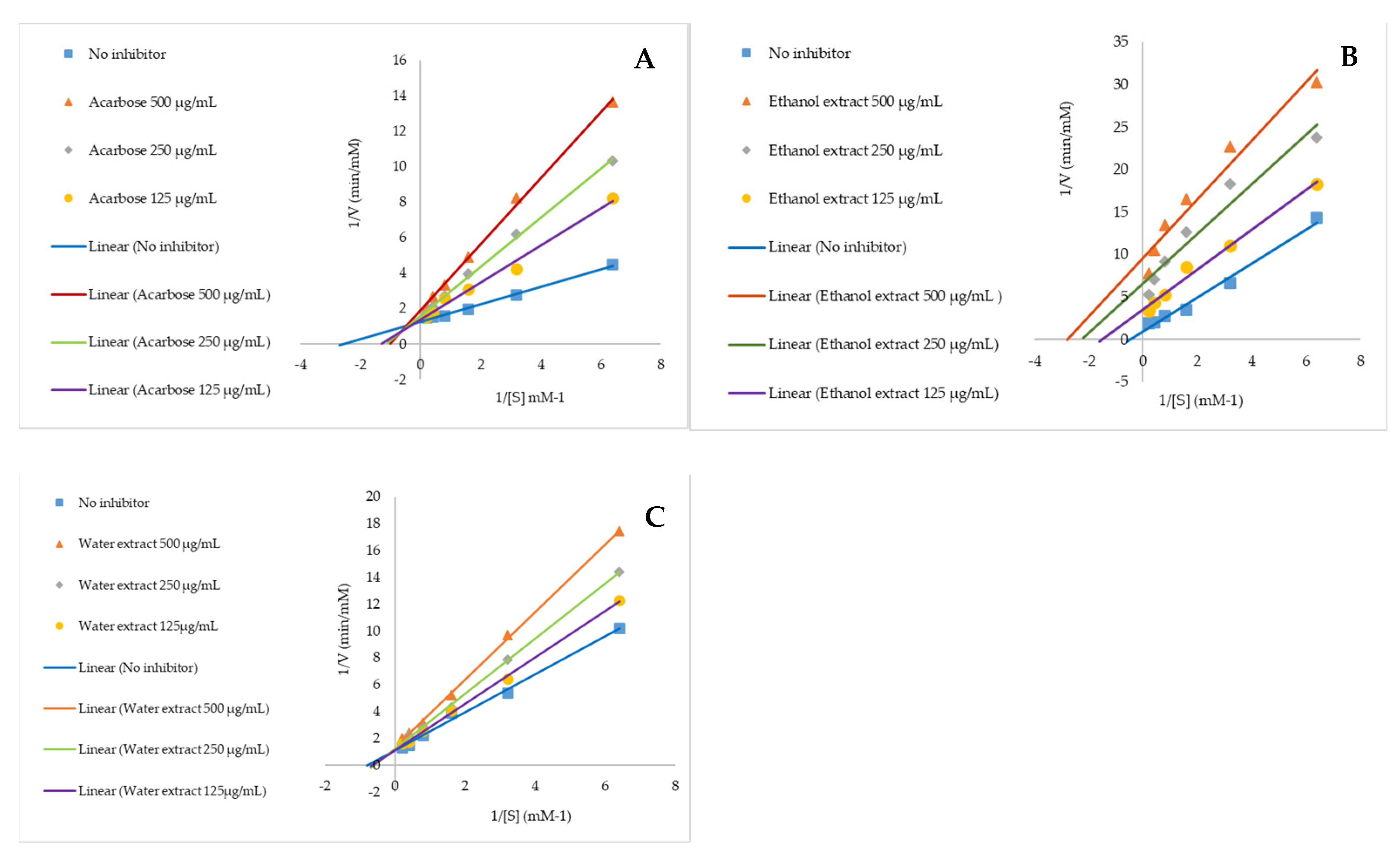
2.3. Anti-Alpha Glucosidase and Mechanism of Action
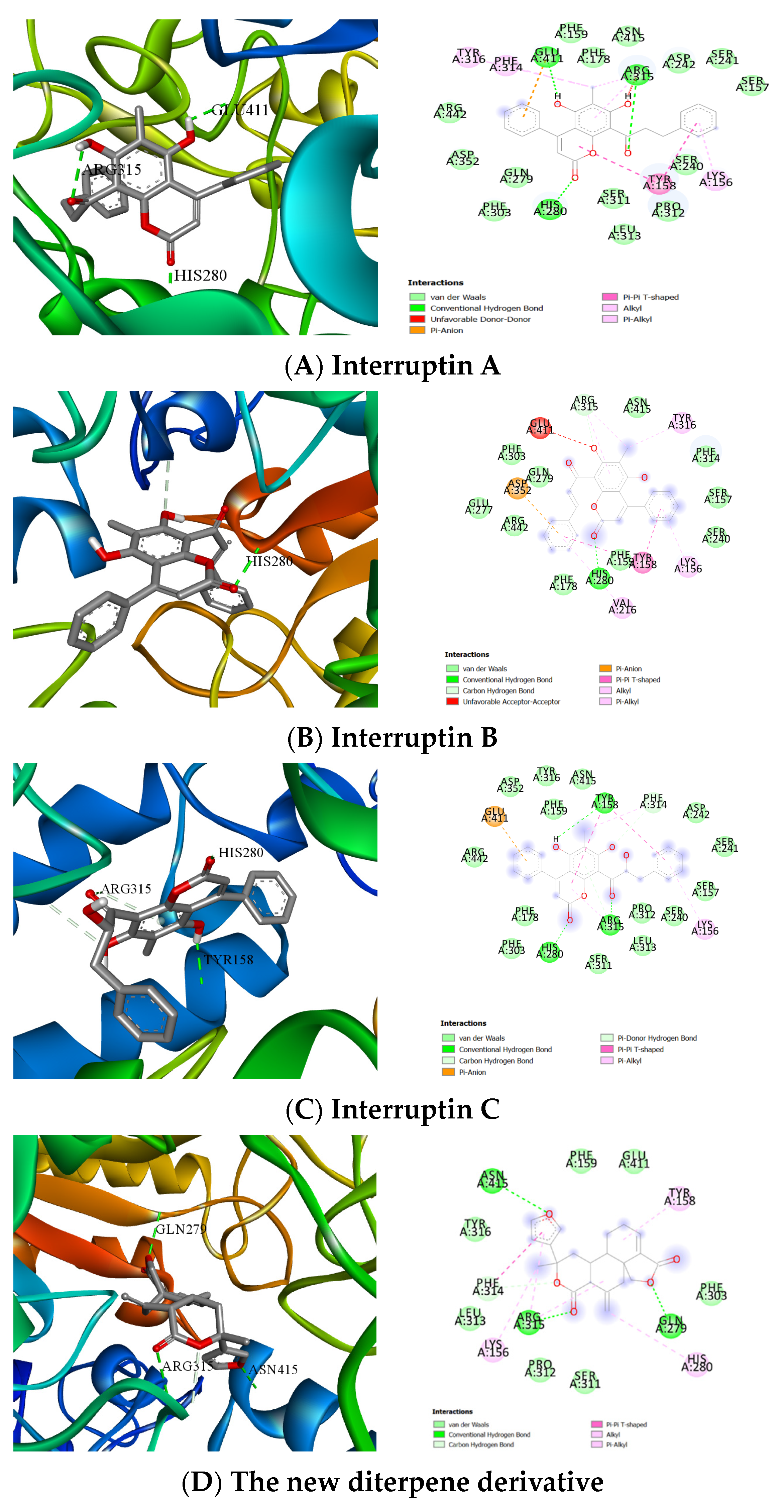
2.4. Computer Molecular Docking of the Effective Compounds
2.5. Insulin Secretagogue Activity
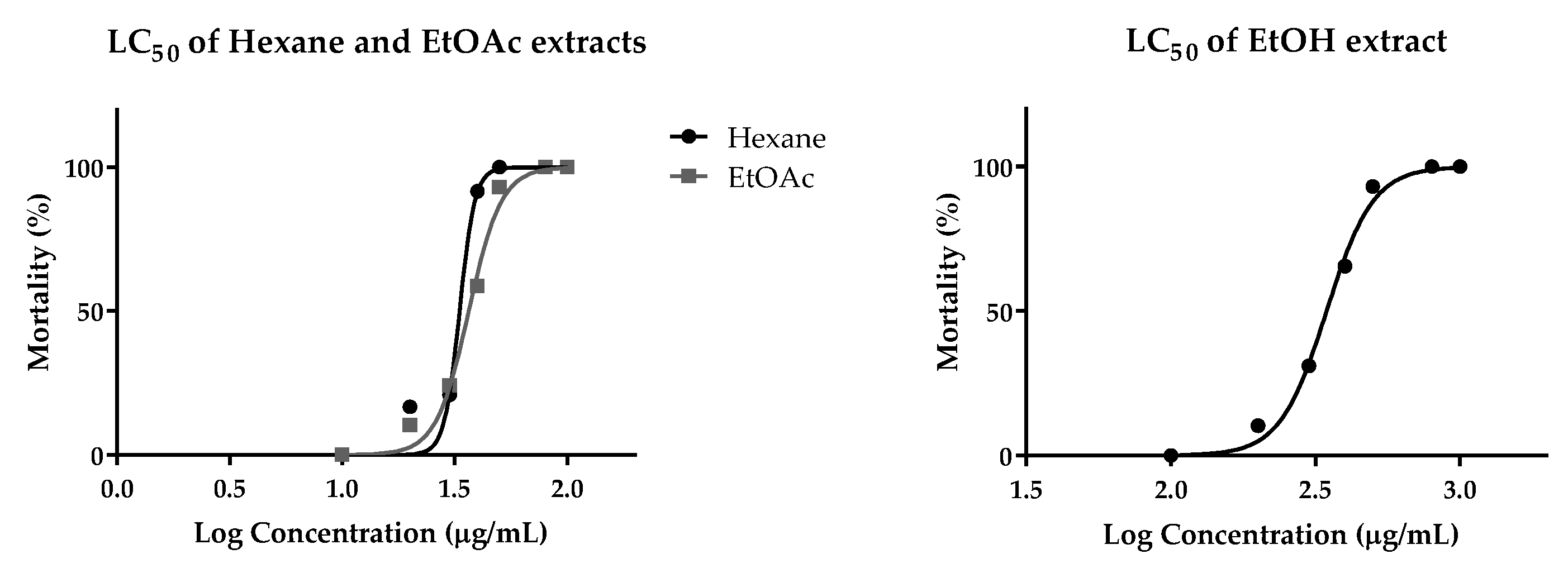
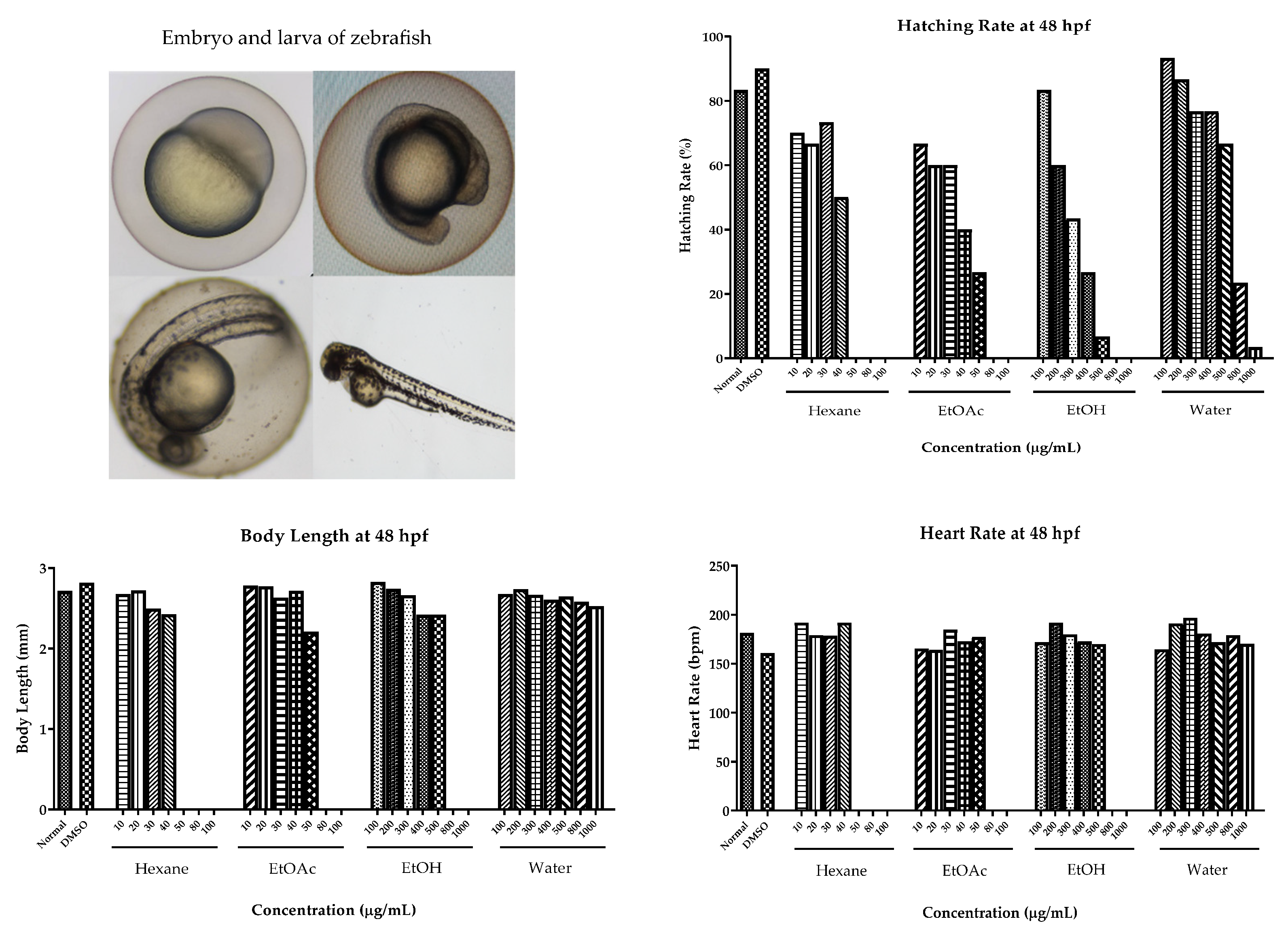
2.6. Cytotoxic Effect on Human Cancer Cells and Toxicity in Zebrafish Model
3. Discussion
4. Materials and Methods
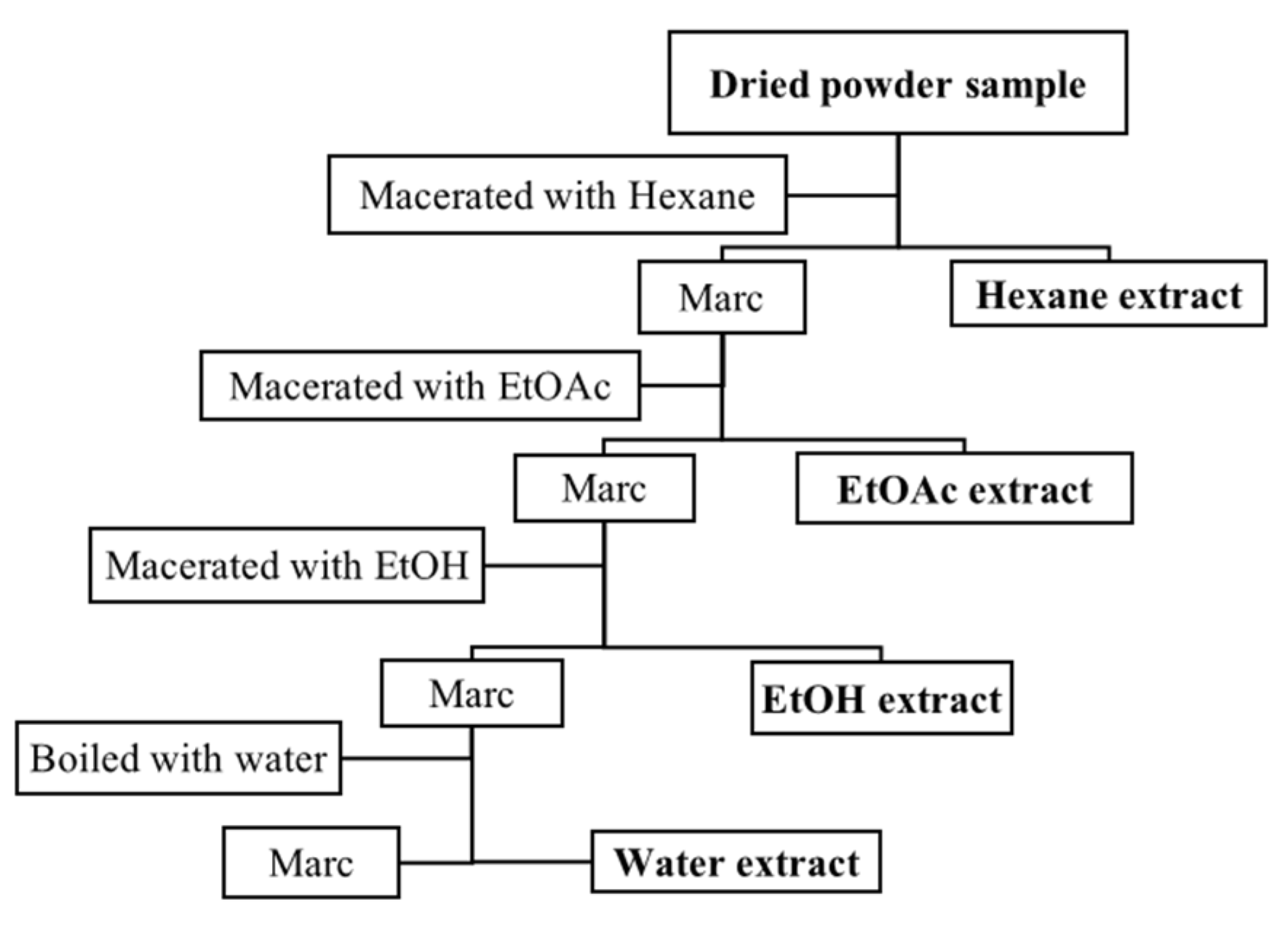
4.1. Plant Material and Extraction Method
4.2. Phytochemical Investigation and Structure Elucidation Techniques
4.3. Total Phenolics and Total Flavonoids Content
4.4. Anti-Alpha Glucosidase Activity and Mechanism of Action
4.5. Molecular Docking
4.6. Insulin Secretagogue Activity
4.7. Cytotoxic Effects on Human Cancer Cells and Toxicity in Zebrafish Model
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meerungrueang, W.; Panichayupakaranant, P. Antimicrobial activities of some Thai traditional medical longevity formulations from plants and antibacterial compounds from Ficus foveolata. Pharm. Biol. 2014, 52, 1104–1110. [Google Scholar] [PubMed] [Green Version]
- Chanthasri, W.; Jo Aan, G.; Singkonpong, N.; Sudkhaw, T.; Maneenoon, K.; Limsuwan, S.; Sanpinit, S.; Wetchakul, P.; Chusri, S. Antioxidant and lifespan-extending effects of a rejuvenating Thai traditional polyherbal remedy (Phy-Blica-O) in Caenorhabditis elegans. Trop. J. Nat. Prod. Res. 2021, 5, 1554–1568. [Google Scholar]
- Chaithada, P.; Lamlert, U.; Ketmunee, C.; Kongnoom, T. Relationship of total phenolic content and total flavonoid content affecting antioxidant Activity of Momordica subangulata Bl. and Lepionurus sylvestris Bl. Wichcha J. Nakhon Si Thammarat Rajabhat Univ. 2009, 38, 120–134. [Google Scholar]
- Tantikarnkul, A.; Rukleng, S.; Insatsapong, P.; Itharat, A.; Keawpradub, N. A Preliminary Study on Biologial Activities of Lepionurus sylvestris Bl. (In Thai); Faculty of Pharmaceutical Sciences, Prince of Songkla University: Songkhla, Thailand, 1999. [Google Scholar]
- Wirasathien, L. Anti-Helicobacter Pylori Activity of Local Edible Plants (In Thai); Faculty of Pharmacy, Srinakharinwirot University: Bangkok, Thailand, 2009. [Google Scholar]
- Quadri-Spinelli, T.; Heilmann, J.; Rali, T.; Sticher, O. Bioactive coumarin derivatives from the fern Cyclosorus interruptus. Planta Med. 2000, 66, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.C.; Zee, S.D.; Cho, S.Y.; Choi, S.U.; Lee, K.R. Cytotoxic ergosterols from Paecilomyces sp. J300. Arch. Pharm. Res. 2002, 25, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Tryznowski, M.; Świderska, A.; Żołek-Tryznowska, Z.; Gołofit, T.; Parzuchowski, P.G. Data on synthesis and characterization of new diglycerol based environmentally friendly non-isocyanate poly (hydroxyurethanes). Data Brief. 2016, 6, 77–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes response on antimicrobial atelocollagen substrates: Extent of cytotoxicity, cell viability and proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [PubMed] [Green Version]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E.; et al. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure-activity relationship study. J. Enzyme. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as α-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food. Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [PubMed]
- Chang, C.; Yang, M.-H.; Wen, H.-M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Ramamoorthy, P.k.; Bono, A. Antioxidant activity, total phenolic and flavonoid content of Morinda citrifolia fruit extracts from various extraction processes. J. Eng. Sci. Technol. 2007, 2, 70–80. [Google Scholar]
- Phoopha, S.; Wattanapiromsakul, C.; Pitakbut, T.; Dej-adisai, S. Chemical constituents of Litsea elliptica and their alpha-glucosidase inhibition with molecular docking. Pharmacog. Mag. 2020, 16, 327–334. [Google Scholar]
- Kazeem, M.I.; Adamson, J.O.; Ogunwande, I.A. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed Res. Int. 2013, 2013, 527570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, M.T.; Seong, S.H.; Nguyen, T.D.; Cho, W.K.; Ah, K.J.; Ma, J.Y.; Woo, M.H.; Choi, J.S.; Min, B.S. Chalcone derivatives from the root bark of Morus alba L. act as inhibitors of PTP1B and α-glucosidase. Phytochemistry 2018, 155, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Nuankaew, W.; Heemman, A.; Wattanapiromsakul, C.; Shim, J.H.; Kim, N.W.; Yasmin, T.; Jeong, S.Y.; Nam, Y.H.; Hong, B.N.; Dej-Adisai, S.; et al. Anti-insulin resistance effect of constituents from Senna siamea on zebrafish model, its molecular docking, and structure-activity relationships. J. Nat. Med. 2021, 75, 520–531. [Google Scholar] [CrossRef]
- Merglen, A.; Theander, S.; Rubi, B.; Chaffard, G.; Wollheim, C.B.; Maechler, P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology 2004, 145, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Dej-adisai, S.; Phoopha, S.; Puripattanavong, J. Phytochemical investigation and bioactivities of Alternanthera ramosissima (Mart.) Chodat and Hassl. Pharmacog. Mag. 2018, 14, 346–351. [Google Scholar] [CrossRef]
- Ko, J.H.; Nam, Y.H.; Joo, S.W.; Kim, H.G.; Lee, Y.G.; Kang, T.H.; Baek, N.I. Flavonoid 8-O-glucuronides from the aerial parts of Malva verticillata and their recovery effects on alloxan-induced pancreatic islets in Zebrafish. Molecules 2018, 23, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Development, O.f.E.C.-o.a. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2013. [Google Scholar]




| Part | Extract | Alpha Glucosidase Inhibition at 2 mg/mL | IC50 μg/mL |
| Leaf | Hexane | 13.27 ± 2.40 | 10,620 |
| Ethyl acetate | 39.89 ± 1.51 | 2630 | |
| Ethanol | 95.77 ± 0.63 | 30 | |
| Water | 57.99 ± 1.42 | 1460 | |
| Acarbose | 88.18 ± 0.53 | 210 | |
| Sample | Compound | Alpha Glucosidase Inhibition at 400 μg/mL | IC50 μg/mL (mM) |
| Isolated compounds | Interruptin A | 30.31 ± 1.17 | 478.73 (1.085) |
| Interruptin C | 58.17 ± 0.83 | 293.05 (0.732) | |
| Diglycerol | 6.23 ± 1.84 | 2052.80 (12.341) | |
| Ergosterol | 18.63 ± 1.42 | 971.50 (2.449) | |
| The new diterpene (Lepionurodiolide) | 58.48 ± 0.48 | 203.71 (0.598) | |
| Acarbose | 62.14 ± 0.37 | 237.08 (0.367) |
| Part | Cytotoxic Effect at 25 µg/mL (% Inhibition) ± SD | |||
|---|---|---|---|---|
| A549 | MCF-7 | HeLa | HGF | |
| Hexane | 15.40 ± 2.75 | 53.54 ± 1.81 | 83.89 ± 4.31 | 48.23 ± 2.96 |
| Ethyl acetate | 8.61 ± 1.33 | 72.72 ± 1.05 | 91.94 ± 0.07 | 52.19 ± 1.81 |
| Ethanol | 25.34 ± 0.26 | 33.41 ± 2.86 | 78.51 ± 2.87 | 13.46 ± 3.25 |
| Water | 28.23 ± 1.68 | 10.59 ± 3.65 | 74.05 ± 2.79 | 9.78 ± 2.83 |
| Camptothecin | 87.74 ± 1.78 | 92.95 ± 0.33 | 85.20 ± 1.23 | 7.35 ± 1.34 |
| Solvent Extract | Embryonic Toxicity on Zebrafish |
|---|---|
| LD50 (µg/mL) | |
| Hexane | 33.26 |
| Ethyl acetate | 36.55 |
| Ethanol | 345.9 |
| Water | >1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phoopha, S.; Sangkaew, S.; Wattanapiromsakul, C.; Nuankaew, W.; Kang, T.H.; Dej-adisai, S. Phytochemical Investigation of Lepionurus sylvestris Blume and Their Anti-Diabetes Effects via Anti-Alpha Glucosidase and Insulin Secretagogue Activities Plus Molecular Docking. Pharmaceuticals 2023, 16, 1132. https://doi.org/10.3390/ph16081132
Phoopha S, Sangkaew S, Wattanapiromsakul C, Nuankaew W, Kang TH, Dej-adisai S. Phytochemical Investigation of Lepionurus sylvestris Blume and Their Anti-Diabetes Effects via Anti-Alpha Glucosidase and Insulin Secretagogue Activities Plus Molecular Docking. Pharmaceuticals. 2023; 16(8):1132. https://doi.org/10.3390/ph16081132
Chicago/Turabian StylePhoopha, Sathianpong, Surat Sangkaew, Chatchai Wattanapiromsakul, Wanlapa Nuankaew, Tong Ho Kang, and Sukanya Dej-adisai. 2023. "Phytochemical Investigation of Lepionurus sylvestris Blume and Their Anti-Diabetes Effects via Anti-Alpha Glucosidase and Insulin Secretagogue Activities Plus Molecular Docking" Pharmaceuticals 16, no. 8: 1132. https://doi.org/10.3390/ph16081132
APA StylePhoopha, S., Sangkaew, S., Wattanapiromsakul, C., Nuankaew, W., Kang, T. H., & Dej-adisai, S. (2023). Phytochemical Investigation of Lepionurus sylvestris Blume and Their Anti-Diabetes Effects via Anti-Alpha Glucosidase and Insulin Secretagogue Activities Plus Molecular Docking. Pharmaceuticals, 16(8), 1132. https://doi.org/10.3390/ph16081132







