Automated Synthesis of [68Ga]Ga-FAPI-46 on a Scintomics GRP Synthesizer
Abstract
:1. Introduction
2. Results
2.1. Radiolabelling
2.2. Quality Control
2.3. Stability Testing
3. Discussion
4. Materials and Methods
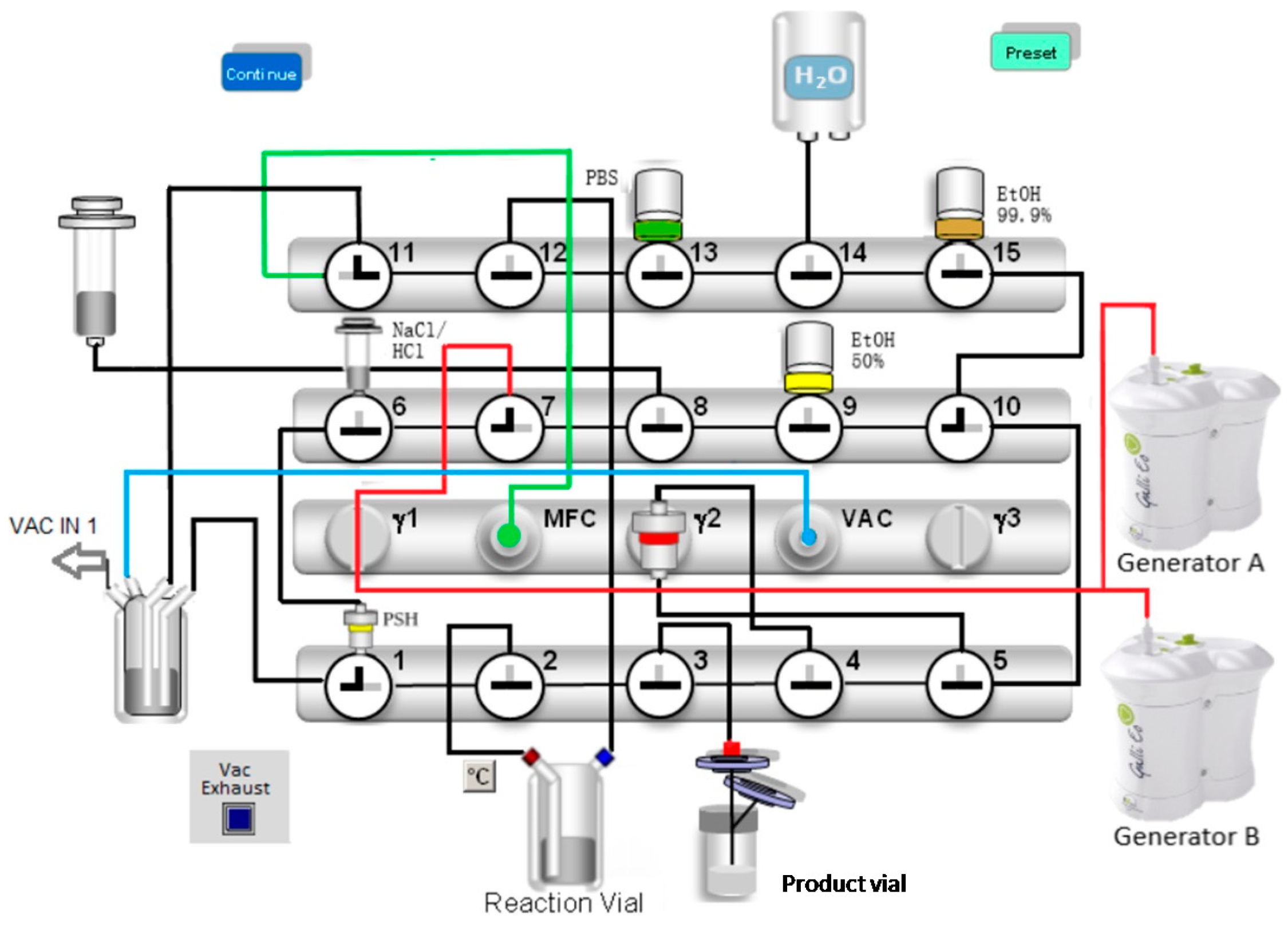
4.1. Radiolabelling
4.2. Radionuclide Identity and Half-Life Determination
4.3. Radiochemical Purity Testing by Radio-HPLC
4.4. Radiochemical Purity Testing by Radio-TLC
4.5. Evaluation of the pH Value
4.6. Post-Release Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Koczorowska, M.M.; Tholen, S.; Bucher, F.; Lutz, L.; Kizhakkedathu, J.N.; De Wever, O.; Wellner, U.F.; Biniossek, M.L.; Stahl, A.; Lassmann, S.; et al. Fibroblast activation protein-α, a stromal cell surface protease, shapes key features of cancer associated fibroblasts through proteome and degradome alterations. Mol. Oncol. 2016, 10, 40–58. [Google Scholar] [CrossRef] [Green Version]
- Altmann, A.; Haberkorn, U.; Siveke, J. The Latest Developments in Imaging of Fibroblast Activation Protein. J. Nucl. Med. 2021, 62, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Gascard, P.; Tlsty, T.D. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef] [Green Version]
- Park, J.E.; Lenter, M.C.; Zimmermann, R.N.; Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Fibroblast Activation Protein, a Dual Specificity Serine Protease Expressed in Reactive Human Tumor Stromal Fibroblasts. J. Biol. Chem. 1999, 274, 36505–36512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom. Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef]
- Zi, F.; He, J.; He, D.; Li, Y.; Yang, L.; Cai, Z. Fibroblast activation protein α in tumor microenvironment: Recent progression and implications (review). Mol. Med. Rep. 2015, 11, 3203–3211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 4, 16. [Google Scholar] [CrossRef]
- Imlimthan, S.; Moon, E.S.; Rathke, H.; Afshar-Oromieh, A.; Rösch, F.; Rominger, A.; Gourni, E. New Frontiers in Cancer Imaging and Therapy Based on Radiolabeled Fibroblast Activation Protein Inhibitors: A Rational Review and Current Progress. Pharmaceuticals 2021, 14, 1023. [Google Scholar] [CrossRef] [PubMed]
- Meletta, R.; Müller Herde, A.; Chiotellis, A.; Isa, M.; Rancic, Z.; Borel, N.; Ametamey, S.M.; Krämer, S.D.; Schibli, R. Evaluation of the radiolabeled boronic acid-based FAP inhibitor MIP-1232 for atherosclerotic plaque imaging. Molecules 2015, 20, 2081–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, K.; Heirbaut, L.; Cheng, J.D.; Joossens, J.; Ryabtsova, O.; Cos, P.; Maes, L.; Lambeir, A.M.; De Meester, I.; Augustyns, K.; et al. Selective Inhibitors of Fibroblast Activation Protein (FAP) with a (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine Scaffold. ACS Med. Chem. Lett. 2013, 4, 491–496. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuyumcu, S.; Kovan, B.; Sanli, Y.; Buyukkaya, F.; Has Simsek, D.; Özkan, Z.G.; Isik, E.G.; Ekenel, M.; Turkmen, C. Safety of Fibroblast Activation Protein-Targeted Radionuclide Therapy by a Low-Dose Dosimetric Approach Using 177Lu-FAPI04. Clin. Nucl. Med. 2021, 46, 641–646. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef]
- Lindner, T.; Giesel, F.L.; Kratochwil, C.; Serfling, S.E. Radioligands Targeting Fibroblast Activation Protein (FAP). Cancers 2021, 13, 5744. [Google Scholar] [CrossRef] [PubMed]
- Spreckelmeyer, S.; Balzer, M.; Poetzsch, S.; Brenner, W. Fully-automated production of [68Ga]Ga-FAPI-46 for clinical application. EJNMMI Radiopharm. Chem. 2020, 5, 31. [Google Scholar] [CrossRef]
- Da Pieve, C.; Costa Braga, M.; Turton, D.R.; Valla, F.A.; Cakmak, P.; Plate, K.H.; Kramer-Marek, G. New Fully Automated Preparation of High Apparent Molar Activity 68Ga-FAPI-46 on a TrasisAiO Platform. Molecules 2022, 27, 675. [Google Scholar] [CrossRef]
- Alfteimi, A.; Lützen, U.; Helm, A.; Jüptner, M.; Marx, M.; Zhao, Y.; Zuhayra, M. Automated synthesis of [68Ga]Ga-FAPI-46 without pre-purification of the generator eluate on three common synthesis modules and two generator types. EJNMMI Radiopharm. Chem. 2022, 7, 20. [Google Scholar] [CrossRef]
- Gillings, N.; Hjelstuen, O.; Ballinger, J.; Behe, M.; Decristoforo, C.; Elsinga, P.; Ferrari, V.; Kolenc Peitl, P.; Koziorowski, J.; Laverman, P.; et al. Guideline on current good radiopharmacy practice (cGRPP) for the small-scale preparation of radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2021, 6, 8. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines (EDQM). Monograph: 2482, Gallium (68Ga) edotreotide injection. In European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2022. [Google Scholar]
- European Directorate for the Quality of Medicines (EDQM). Monograph: 3044, Gallium (68Ga) PSMA-11 injection. In European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2022. [Google Scholar]
- Kvaternik, H.; Hausberger, D.; Zink, C.; Rumpf, B.; Aigner, R.M. 68Ga-peptide preparation with the use of two 68Ge/68Ga-generators. EJNMMI Radiopharm. Chem. 2016, 1 (Suppl. 1), 36. [Google Scholar] [CrossRef] [Green Version]
- Hörmann, A.A.; Plhak, E.; Klingler, M.; Rangger, C.; Pfister, J.; Schwach, G.; Kvaternik, H.; von Guggenberg, E. Automated Synthesis of 68Ga-Labeled DOTA-MGS8 and Preclinical Characterization of Cholecystokinin-2 Receptor Targeting. Molecules 2022, 27, 2034. [Google Scholar] [CrossRef]
- IRE-Elit. Summary of Product Characteristics for Galli Ad, 0.74–1.85 GBq, Radionuclide Generator. Available online: https://www.ire.eu/medias/295/UK.pdf (accessed on 8 August 2023).
- Antunes, I.F.; Franssen, G.M.; Zijlma, R.; van der Woude, G.L.K.; Yim, C.B.; Laverman, P.; Boersma, H.H.; Elsinga, P.H. New Sensitive Method for HEPES Quantification in 68Ga-Radiopharmaceuticals. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, S407–S408. [Google Scholar] [CrossRef] [PubMed]
- Kvaternik, H.; Plhak, E.; Rumpf, B.; Hausberger, D.; Aigner, R.M. Assay of Bacterial Endotoxins in Radiopharmaceuticals by Microplate Reader. EJNMMI Radiopharm. Chem. 2018, 3 (Suppl. 1), 7. [Google Scholar] [CrossRef] [Green Version]
- European Directorate for the Quality of Medicines (EDQM). Monograph: 50400 Residual Solvents. In European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2022. [Google Scholar]



| Quality Control | Method | Criteria | Result (n = 10) |
|---|---|---|---|
| Appearance | visual inspection | clear and colourless | conforms |
| pH value | pH indicator strips | 4–8 | 7.0 |
| Radioactivity concentration | dose calibrator | 50.8 ± 7.6 MBq/mL | |
| Radionuclide identity | gamma spectrometry | 511 keV, 1077 keV | conforms |
| Approximate half-life | dose calibrator | 62–74 min | conforms |
| Identity of [68Ga]Ga-FAPI-46 | Radio-HPLC | Retention time compared with cold reference | 8.5 ± 0.04 min |
| Colloidal gallium-68 | Radio-TLC | ≤3.0% | 0.2 ± 0.1% |
| Free [68Ga]GaCl3 | Radio-HPLC | ≤2.0% | 0.6 ± 0.2% |
| Radiochemical purity of [68Ga]Ga-FAPI-46 | Radio-HPLC | ≥95.0% | 97.6 ± 0.3% |
| [68Ga]Ga-FAPI-46, FAPI-46 and related substances | HPLC | ≤5 µg/mL | 4.1 ± 0.4 µg/mL |
| Unspecific impurities | HPLC | ≤5 µg/mL | 0.3 ± 0.1 µg/mL |
| Ethanol content | gas chromatography | ≤10.0% (v/v) | ≤6% |
| HEPES content | HPLC | ≤500 µg/V | ≤200 µg/V |
| Bacterial endotoxins | LAL test | ≤175 IU/V | ≤0.05 IU/mL (detection limit) |
| Sterility | Ph. Eur. | sterile | conforms |
| Stability Testing | Acceptance Criteria | EOS | 1 h | 2 h | 3 h |
|---|---|---|---|---|---|
| [68Ga]Ga-FAPI-46 (HPLC) [%] | ≥95% | 97.4 ± 0.4 | 96.2 ± 0.6 | 95.7 ± 0.7 | 95.3 ± 0.8 |
| [68Ga]GaCl3 (HPLC) [%] | ≤2% | 0.6 ± 0.2 | 1.1 ± 0.3 | 1.2 ± 0.2 | 1.4 ± 0.5 |
| Gallium-68 in colloidal form (TLC) [%] | ≤3% | 0.4 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.2 | 1.0 ± 0.3 |
| This Work | Da Pieve et al. [16] | |
|---|---|---|
| Synthesis Module | Scintomics GRP 3 V | Trasis AiO |
| Radionuclide source | Galli Ad Direct eluation | Galli Ad Delivery vial |
| Purification of generator eluate | SCX | SCX |
| Labelling buffer | HEPES 1.5 M | Acetate 0.2 M/ 1 mg sodium ascorbate |
| Labelling temperature/time | 125 °C/6 min | 95 °C/10 min |
| Purification of the Product | Sep-Pak C18 | Oasis HLB + Sep-Pak QMA |
| RCY d.c. (%) | 72.6 ± 4.9 | 66.0 ± 7.6 |
| 1. | Preconditioning of the Sep-Pak Light C18 cartridge with 5 mL ethanol (99.9%) and 19 mL water. |
| 2. | Drying of valve benches and the Sep-Pak Light C18 cartridge with nitrogen gas (N2). |
| 3. | Preparing the elution of the generators by building up the vacuum until the pressure drops below 200 mbar. |
| 4. | Simultaneous automatic elution of the Galli Ad generators over the PSH+ cartridge. |
| 5. | Lifting the negative pressure by gently filling with N2. |
| 6. | Eluting the gallium-68 from the PSH+ cartridge to the reactor vial with 1.5 mL 5 M NaCl (5 M)/150 µL HCl (6 M) using the syringe pump. |
| 7. | Labelling at 125 °C/6 min in HEPES buffer (1.5 M). |
| 8. | Transfer of the reaction mixture from the reaction vial onto the Sep-Pak Light C18 cartridge. |
| 9. | Washing of Sep-Pak Light C18 cartridge with 14 mL water followed by a drying step with N2. |
| 10. | Washing of the reaction vial with 10 mL water and drying with N2. |
| 11. | Washing of Sep-Pak Light C18 Cartridge with 10 mL water followed by drying with N2. |
| 12. | Elution of [68Ga]Ga-FAPI-46 to the product vial with 2 mL ethanol/water (1/1). |
| 13. | Dilution of the product with 15 mL PBS. |
| 14. | Washing of the valve benches with 10 mL PBS buffer and 10 mL water followed by a drying step with N2. |
| 15. | Closing all valves—end of synthesis (EOS). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plhak, E.; Pichler, C.; Dittmann-Schnabel, B.; Gößnitzer, E.; Aigner, R.M.; Stanzel, S.; Kvaternik, H. Automated Synthesis of [68Ga]Ga-FAPI-46 on a Scintomics GRP Synthesizer. Pharmaceuticals 2023, 16, 1138. https://doi.org/10.3390/ph16081138
Plhak E, Pichler C, Dittmann-Schnabel B, Gößnitzer E, Aigner RM, Stanzel S, Kvaternik H. Automated Synthesis of [68Ga]Ga-FAPI-46 on a Scintomics GRP Synthesizer. Pharmaceuticals. 2023; 16(8):1138. https://doi.org/10.3390/ph16081138
Chicago/Turabian StylePlhak, Elisabeth, Christopher Pichler, Björn Dittmann-Schnabel, Edith Gößnitzer, Reingard M. Aigner, Susanne Stanzel, and Herbert Kvaternik. 2023. "Automated Synthesis of [68Ga]Ga-FAPI-46 on a Scintomics GRP Synthesizer" Pharmaceuticals 16, no. 8: 1138. https://doi.org/10.3390/ph16081138
APA StylePlhak, E., Pichler, C., Dittmann-Schnabel, B., Gößnitzer, E., Aigner, R. M., Stanzel, S., & Kvaternik, H. (2023). Automated Synthesis of [68Ga]Ga-FAPI-46 on a Scintomics GRP Synthesizer. Pharmaceuticals, 16(8), 1138. https://doi.org/10.3390/ph16081138





