Acute Kidney Injury Caused by Rhabdomyolysis Is Ameliorated by Serum Albumin-Based Supersulfide Donors through Antioxidative Pathways
Abstract
:1. Introduction
2. Results
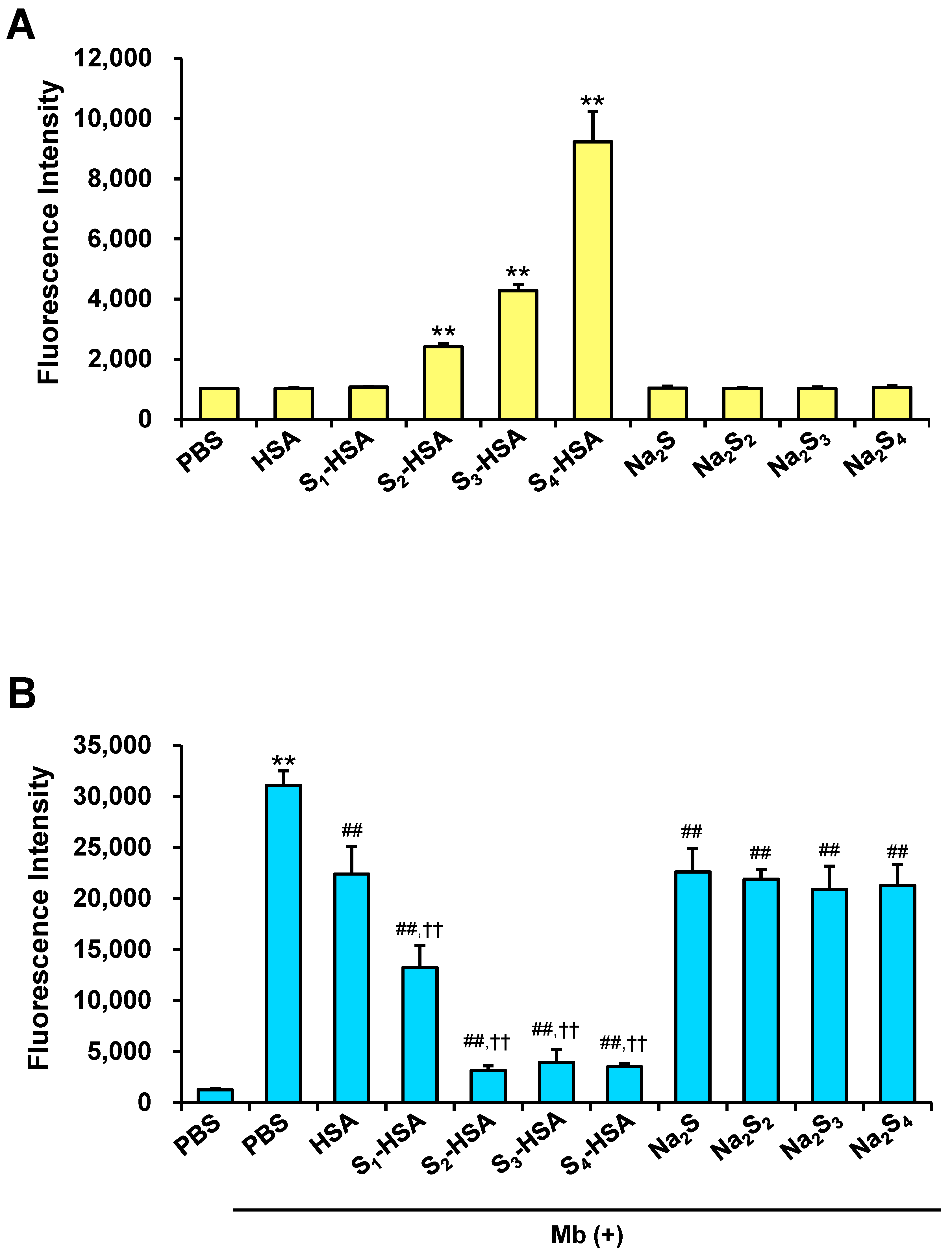
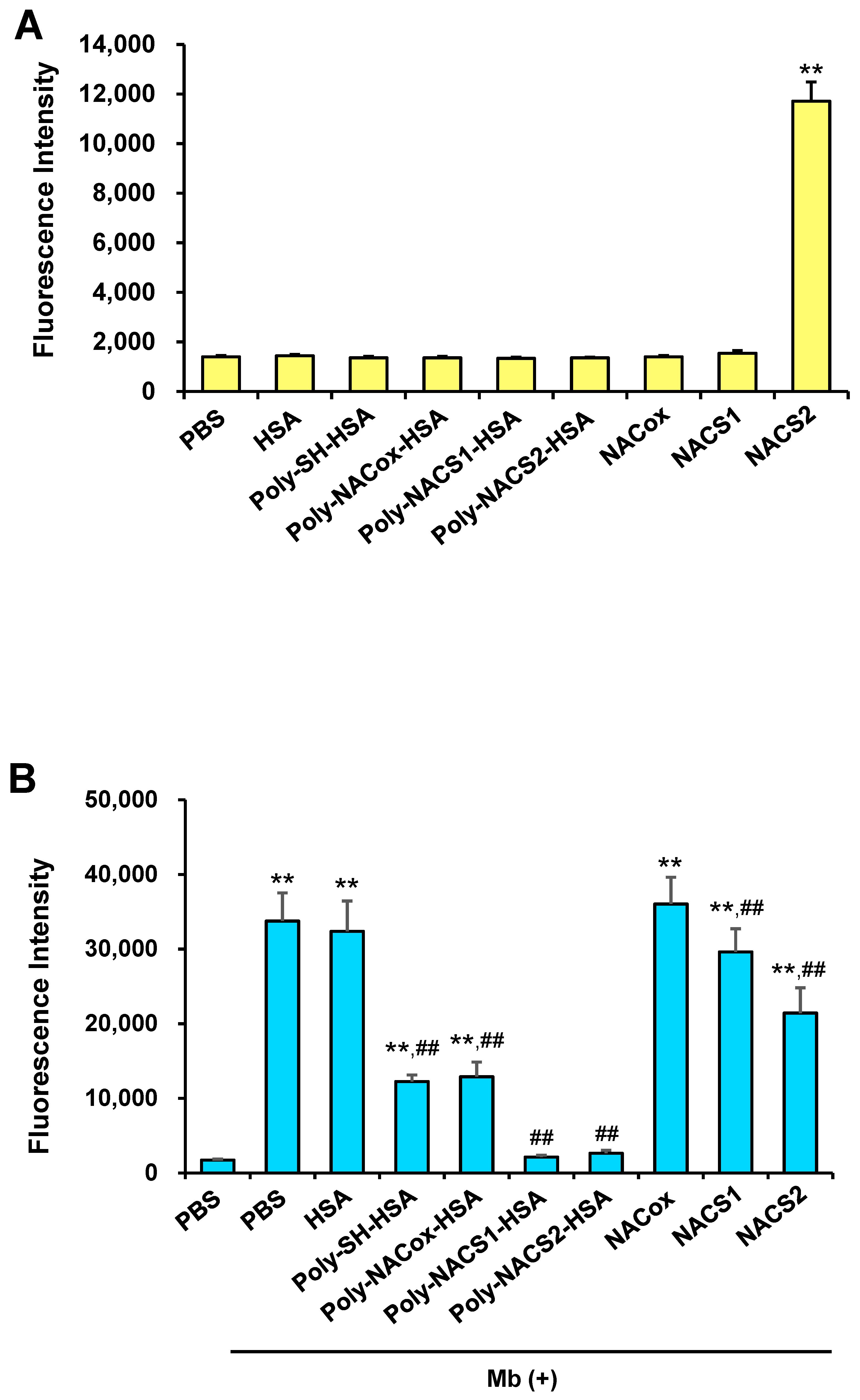
2.1. Development of a Novel Supersulfide-Bound Serum Albumin
2.2. Intracellular Uptake of Supersulfides and Antioxidative Activity
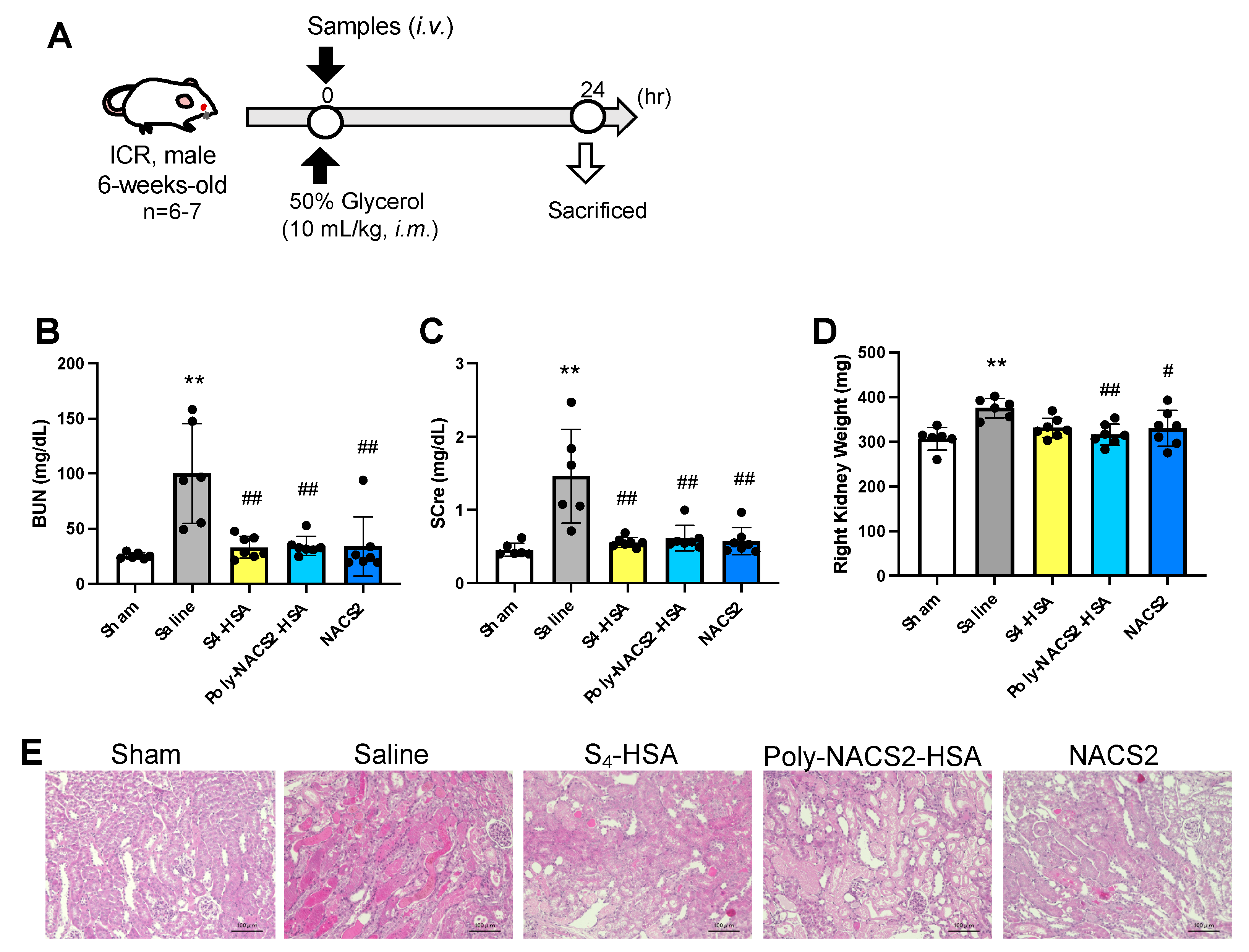
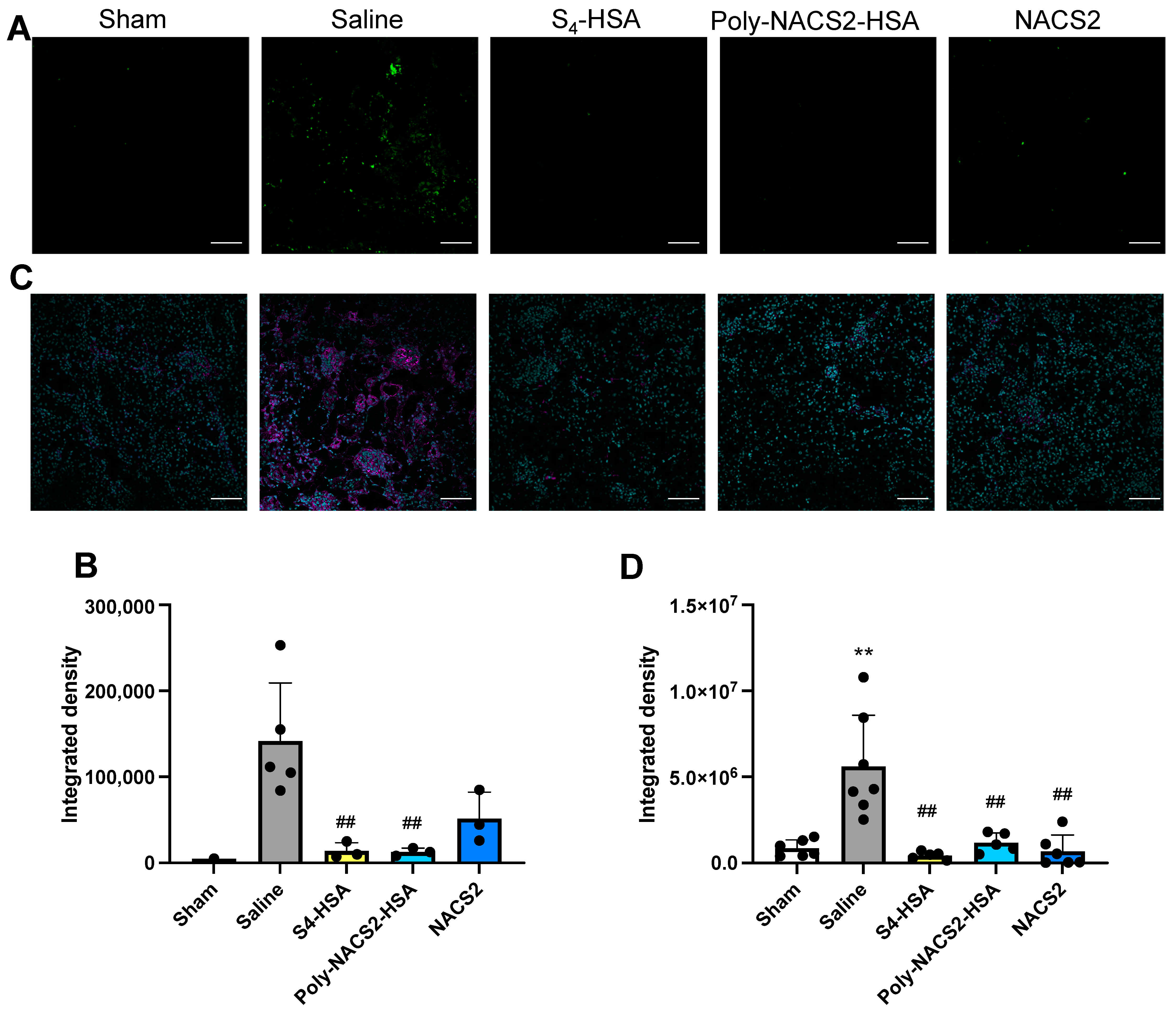
2.3. Effect of Supersulfide Donors on Glycerol-Induced AKI
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Sn-HSA
4.3. Preparation of Poly-NACSn-HSA
4.4. Measuring Polysulfides
4.5. Detection of Reactive Oxygen Species Induced by Myoglobin In Vitro
4.6. Cellular Uptake of Supersulfides in LLC-PK1 Cells
4.7. Glycerol-Induced Acute Kidney Injury Model In Vivo
4.8. Renal Histology
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stahl, K.; Rastelli, E.; Schoser, B. A systematic review on the definition of rhabdomyolysis. J. Neurol. 2020, 267, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Suwanwongse, K.; Shabarek, N. Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus 2020, 12, e7561. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.M. Factors predictive of acute renal failure in rhabdomyolysis. Arch. Intern. Med. 1988, 148, 1553–1557. [Google Scholar] [CrossRef]
- de Meijer, A.R.; Fikkers, B.G.; de Keijzer, M.H.; van Engelen, B.G.; Drenth, J.P. Serum creatine kinase as predictor of clinical course in rhabdomyolysis: A 5-year intensive care survey. Intensive Care Med. 2003, 29, 1121–1125. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, J.; Wang, X.; Wang, S.; Tang, Y.; Yang, L. Risk factors for severe acute kidney injury among patients with rhabdomyolysis. BMC Nephrol. 2020, 21, 498. [Google Scholar] [CrossRef]
- Holt, S.; Moore, K. Pathogenesis of renal failure in rhabdomyolysis: The role of myoglobin. Exp. Nephrol. 2000, 8, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Barayeu, U.; Sawa, T.; Nishida, M.; Wei, F.Y.; Motohashi, H.; Akaike, T. Supersulfide biology and translational medicine for disease control. Br. J. Pharmacol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Zhang, T.; Akaike, T.; Sawa, T. Redox Regulation of Xenobiotics by Reactive Sulfur and Supersulfide Species. Antioxid. Redox Signal. 2023. ahead of print. [Google Scholar] [CrossRef]
- Nishida, M.; Sawa, T.; Kitajima, N.; Ono, K.; Inoue, H.; Ihara, H.; Motohashi, H.; Yamamoto, M.; Suematsu, M.; Kurose, H. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat. Chem. Biol. 2012, 8, 714–724. [Google Scholar] [CrossRef]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef]
- Akaike, T.; Ida, T.; Wei, F.Y.; Nishida, M.; Kumagai, Y.; Alam, M.M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017, 8, 1177. [Google Scholar] [CrossRef]
- Kimura, Y.; Koike, S.; Shibuya, N.; Lefer, D.; Ogasawara, Y.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine-and glutathione-persulfide (Cys-SSH and GSSH) together with signaling molecules H2S2, H2S3 and H2S. Sci. Rep. 2017, 7, 10459. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Sano, H.; Takita, K.; Morita, M.; Yamanaka, S.; Ichikawa, T.; Numakura, T.; Ida, T.; Jung, M.; Ogata, S. Supersulphides provide airway protection in viral and chronic lung diseases. Nat. Commun. 2023, 14, 4476. [Google Scholar] [PubMed]
- Kanemaru, E.; Miyazaki, Y.; Marutani, E.; Ezaka, M.; Goto, S.; Ohshima, E.; Bloch, D.B.; Ichinose, F. Intranasal administration of polysulfide prevents neurodegeneration in spinal cord and rescues mice from delayed paraplegia after spinal cord ischemia. Redox Biol. 2023, 60, 102620. [Google Scholar] [CrossRef] [PubMed]
- Dillon, K.M.; Matson, J.B. A review of chemical tools for studying small molecule persulfides: Detection and delivery. ACS Chem. Biol. 2021, 16, 1128–1141. [Google Scholar] [PubMed]
- Yu, B.; Zheng, Y.; Yuan, Z.; Li, S.; Zhu, H.; De La Cruz, L.K.; Zhang, J.; Ji, K.; Wang, S.; Wang, B. Toward direct protein S-persulfidation: A prodrug approach that directly delivers hydrogen persulfide. J. Am. Chem. Soc. 2018, 140, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.R.; Dillon, K.M.; Wang, Y.; Carrazzone, R.J.; Matson, J.B. A persulfide donor responsive to reactive oxygen species: Insights into reactivity and therapeutic potential. Angew. Chem. Int. Ed. 2018, 57, 6324–6328. [Google Scholar] [CrossRef]
- Foster, J.C.; Radzinski, S.C.; Zou, X.; Finkielstein, C.V.; Matson, J.B. H2S-releasing polymer micelles for studying selective cell toxicity. Mol. Pharm. 2017, 14, 1300–1306. [Google Scholar]
- Tran, B.H.; Yu, Y.; Chang, L.; Tan, B.; Jia, W.; Xiong, Y.; Dai, T.; Zhong, R.; Zhang, W.; Le, V.M. A novel liposomal S-propargyl-cysteine: A sustained release of hydrogen sulfide reducing myocardial fibrosis via TGF-β1/Smad pathway. Int. J. Nanomed. 2019, 14, 10061–10077. [Google Scholar] [CrossRef]
- Dillon, K.M.; Carrazzone, R.J.; Wang, Y.; Powell, C.R.; Matson, J.B. Polymeric persulfide prodrugs: Mitigating oxidative stress through controlled delivery of reactive sulfur species. ACS Macro Lett. 2020, 9, 606–612. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Ishima, Y.; Kinoshita, R.; Chuang, V.T.; Tasaka, N.; Matsuo, N.; Watanabe, H.; Shimizu, T.; Ishida, T.; Otagiri, M. A novel S-sulfhydrated human serum albumin preparation suppresses melanin synthesis. Redox Biol. 2018, 14, 354–360. [Google Scholar] [CrossRef]
- Borgström, L.; Kågedal, B.; Paulsen, O. Pharmacokinetics of N-acetylcysteine in man. Eur. J. Clin. Pharmacol. 1986, 31, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ono, K.; Tsutsuki, H.; Ihara, H.; Islam, W.; Akaike, T.; Sawa, T. Enhanced Cellular Polysulfides Negatively Regulate TLR4 Signaling and Mitigate Lethal Endotoxin Shock. Cell Chem. Biol. 2019, 26, 686–698.e684. [Google Scholar] [CrossRef]
- Katayama, N.; Nakajou, K.; Komori, H.; Uchida, K.; Yokoe, J.-I.; Yasui, N.; Yamamoto, H.; Kai, T.; Sato, M.; Nakagawa, T. Design and evaluation of S-nitrosylated human serum albumin as a novel anticancer drug. J. Pharmacol. Exp. Ther. 2008, 325, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Ishima, Y.; Shibata, A.; Chuang, V.T.G.; Sawa, T.; Ihara, H.; Watanabe, H.; Xian, M.; Ouchi, Y.; Shimizu, T.; et al. Quantitative determination of polysulfide in albumins, plasma proteins and biological fluid samples using a novel combined assays approach. Anal. Chim. Acta 2017, 969, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Jiang, N.; Guo, L.; Ni, Z.; Al-Brakati, A.Y.; Othman, M.S.; Moneim, A.E.A.; Kassab, R.B. Oleuropein suppresses oxidative, inflammatory, and apoptotic responses following glycerol-induced acute kidney injury in rats. Life Sci. 2019, 232, 116634. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Coombes, J.S.; Bennett, N.; Johnson, D.W.; Gobe, G.C. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology 2012, 17, 311–321. [Google Scholar] [CrossRef]
- Cachofeiro, V.; Goicochea, M.; De Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease: New strategies to prevent cardiovascular risk in chronic kidney disease. Kidney Int. 2008, 74, S4–S9. [Google Scholar] [CrossRef]
- Ozbek, E. Induction of oxidative stress in kidney. Int. J. Nephrol. 2012, 2012, 465897. [Google Scholar]
- Pieniazek, A.; Bernasinska-Slomczewska, J.; Gwozdzinski, L. Uremic toxins and their relation with oxidative stress induced in patients with CKD. Int. J. Mol. Sci. 2021, 22, 6196. [Google Scholar] [CrossRef] [PubMed]
- Koning, A.M.; Frenay, A.-R.S.; Leuvenink, H.G.; van Goor, H. Hydrogen sulfide in renal physiology, disease and transplantation–the smell of renal protection. Nitric Oxide 2015, 46, 37–49. [Google Scholar] [CrossRef]
- Feliers, D.; Lee, H.J.; Kasinath, B.S. Hydrogen sulfide in renal physiology and disease. Antioxid. Redox Signal. 2016, 25, 720–731. [Google Scholar] [PubMed]
- Askari, H.; Seifi, B.; Kadkhodaee, M.; Sanadgol, N.; Elshiekh, M.; Ranjbaran, M.; Ahghari, P. Protective effects of hydrogen sulfide on chronic kidney disease by reducing oxidative stress, inflammation and apoptosis. EXCLI J. 2018, 17, 14. [Google Scholar]
- Cao, X.; Zhang, W.; Moore, P.K.; Bian, J. Protective smell of hydrogen sulfide and polysulfide in cisplatin-induced nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 313. [Google Scholar]
- Sun, H.-J.; Xiong, S.-P.; Cao, X.; Cao, L.; Zhu, M.-Y.; Wu, Z.-Y.; Bian, J.-S. Polysulfide-mediated sulfhydration of SIRT1 prevents diabetic nephropathy by suppressing phosphorylation and acetylation of p65 NF-κB and STAT3. Redox Biol. 2021, 38, 101813. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, S.; Teng, X.; Hu, Z.; Zhang, Z.; Qiu, X.; Tian, D.; Wu, Y. Hydrogen sulfide attenuates LPS-induced acute kidney injury by inhibiting inflammation and oxidative stress. Oxidative Med. Cell. Longev. 2018, 2018, 6717212. [Google Scholar] [CrossRef]
- Ikeda, M.; Ishima, Y.; Chuang, V.T.; Sakai, M.; Osafune, H.; Ando, H.; Shimizu, T.; Okuhira, K.; Watanabe, H.; Maruyama, T. Distribution of polysulfide in human biological fluids and their association with amylase and sperm activities. Molecules 2019, 24, 1689. [Google Scholar] [CrossRef]
- Shibata, A.; Ishima, Y.; Ikeda, M.; Sato, H.; Imafuku, T.; Chuang, V.T.; Ouchi, Y.; Abe, T.; Watanabe, H.; Ishida, T. Human serum albumin hydropersulfide is a potent reactive oxygen species scavenger in oxidative stress conditions such as chronic kidney disease. Biochem. Biophys. Res. Commun. 2016, 479, 578–583. [Google Scholar]
- Singh, A.P.; Muthuraman, A.; Jaggi, A.S.; Singh, N.; Grover, K.; Dhawan, R. Animal models of acute renal failure. Pharmacol. Rep. 2012, 64, 31–44. [Google Scholar]
- Shieh, M.; Ni, X.; Xu, S.; Lindahl, S.P.; Yang, M.; Matsunaga, T.; Flaumenhaft, R.; Akaike, T.; Xian, M. Shining a light on SSP4: A comprehensive analysis and biological applications for the detection of sulfane sulfurs. Redox Biol. 2022, 56, 102433. [Google Scholar] [CrossRef]
- Chen, W.; Liu, C.; Peng, B.; Zhao, Y.; Pacheco, A.; Xian, M. New fluorescent probes for sulfane sulfurs and the application in bioimaging. Chem. Sci. 2013, 4, 2892–2896. [Google Scholar] [CrossRef]
- Ishima, Y.; Hiroyama, S.; Kragh-Hansen, U.; Maruyama, T.; Sawa, T.; Akaike, T.; Kai, T.; Otagiri, M. One-step preparation of S-nitrosated human serum albumin with high biological activities. Nitric Oxide 2010, 23, 121–127. [Google Scholar]
- Ishima, Y.; Kragh-Hansen, U.; Maruyama, T.; Otagiri, M. Poly-s-nitrosated albumin as a safe and effective multifunctional antitumor agent: Characterization, biochemistry and possible future therapeutic applications. BioMed Res. Int. 2013, 2013, 353892. [Google Scholar] [CrossRef]
- Ishima, Y.; Yoshida, F.; Kragh-Hansen, U.; Watanabe, K.; Katayama, N.; Nakajou, K.; Akaike, T.; Kai, T.; Maruyama, T.; Otagiri, M. Cellular uptake mechanisms and responses to NO transferred from mono-and poly-S-nitrosated human serum albumin. Free. Radic. Res. 2011, 45, 1196–1206. [Google Scholar] [CrossRef]
- Zai, A.; Rudd, M.A.; Scribner, A.W.; Loscalzo, J. Cell-surface protein disulfide isomerase catalyzes transnitrosation and regulates intracellular transfer of nitric oxide. J. Clin. Investig. 1999, 103, 393–399. [Google Scholar] [CrossRef]
- Cui, S.; Verroust, P.; Moestrup, S.; Christensen, E. Megalin/gp330 mediates uptake of albumin in renal proximal tubule. Am. J. Physiol.-Ren. Physiol. 1996, 271, F900–F907. [Google Scholar] [CrossRef]
- Zhai, X.Y.; Nielsen, R.; Birn, H.; Drumm, K.; Mildenberger, S.; Freudinger, R.; Moestrup, S.K.; Verroust, P.J.; Christensen, E.I.; Gekle, M. Cubilin-and megalin-mediated uptake of albumin in cultured proximal tubule cells of opossum kidney. Kidney Int. 2000, 58, 1523–1533. [Google Scholar]
- Moraes, A.; Freire, D.; Habibi, H.; Lowe, J.; Magalhães, V. Cylindrospermopsin impairs tubular transport function in kidney cells LLC-PK1. Toxicol. Lett. 2021, 344, 26–33. [Google Scholar] [CrossRef]
- Alves, S.A.; Florentino, L.S.; Teixeira, D.E.; Silva-Aguiar, R.P.; Peruchetti, D.B.; Oliveira, A.C.; Scharfstein, J.; Marzolo, M.-P.; Pinheiro, A.A.S.; Caruso-Neves, C. Surface megalin expression is a target to the inhibitory effect of bradykinin on the renal albumin endocytosis. Peptides 2021, 146, 170646. [Google Scholar] [CrossRef]
- Boutaud, O.; Roberts II, L.J. Mechanism-based therapeutic approaches to rhabdomyolysis-induced renal failure. Free. Radic. Biol. Med. 2011, 51, 1062–1067. [Google Scholar] [CrossRef]
- Grivei, A.; Giuliani, K.T.; Wang, X.; Ungerer, J.; Francis, L.; Hepburn, K.; John, G.T.; Gois, P.F.; Kassianos, A.J.; Healy, H. Oxidative stress and inflammasome activation in human rhabdomyolysis-induced acute kidney injury. Free. Radic. Biol. Med. 2020, 160, 690–695. [Google Scholar] [CrossRef]
- Cuevasanta, E.; Lange, M.; Bonanata, J.; Coitino, E.L.; Ferrer-Sueta, G.; Filipovic, M.R.; Alvarez, B. Reaction of Hydrogen Sulfide with Disulfide and Sulfenic Acid to Form the Strongly Nucleophilic Persulfide. J. Biol. Chem. 2015, 290, 26866–26880. [Google Scholar] [CrossRef]
- Benchoam, D.; Semelak, J.A.; Cuevasanta, E.; Mastrogiovanni, M.; Grassano, J.S.; Ferrer-Sueta, G.; Zeida, A.; Trujillo, M.; Möller, M.N.; Estrin, D.A. Acidity and nucleophilic reactivity of glutathione persulfide. J. Biol. Chem. 2020, 295, 15466–15481. [Google Scholar] [CrossRef]
- Noguchi, N.; Saito, Y.; Niki, E. Actions of thiols, persulfides, and polysulfides as free radical scavenging antioxidants. Antioxid. Redox Signal. 2023, 39, 728–743. [Google Scholar]
- Chauvin, J.-P.R.; Griesser, M.; Pratt, D.A. The antioxidant activity of polysulfides: It’s radical! Chem. Sci. 2019, 10, 4999–5010. [Google Scholar]
- Álvarez, L.; Suarez Vega, V.; McGinity, C.; Khodade, V.S.; Toscano, J.P.; Nagy, P.; Lin, J.; Works, C.; Fukuto, J.M. The reactions of hydropersulfides (RSSH) with myoglobin. Arch. Biochem. Biophys 2020, 687, 108391. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. Protein sulfhydration. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 555, pp. 79–90. [Google Scholar]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell 2012, 45, 13–24. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 2013, 18, 1906–1919. [Google Scholar] [CrossRef] [PubMed]
- Vandiver, M.S.; Paul, B.D.; Xu, R.; Karuppagounder, S.; Rao, F.; Snowman, A.M.; Ko, H.S.; Lee, Y.I.; Dawson, V.L.; Dawson, T.M.; et al. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 2013, 4, 1626. [Google Scholar] [CrossRef]
- Kasamatsu, S.; Nishimura, A.; Morita, M.; Matsunaga, T.; Abdul Hamid, H.; Akaike, T. Redox signaling regulated by cysteine persulfide and protein polysulfidation. Molecules 2016, 21, 1721. [Google Scholar] [CrossRef]
- Iciek, M.; Kowalczyk-Pachel, D.; Bilska-Wilkosz, A.; Kwiecień, I.; Górny, M.; Włodek, L. S-sulfhydration as a cellular redox regulation. Biosci. Rep. 2016, 36, e00304. [Google Scholar] [CrossRef]
- Abiko, Y.; Yoshida, E.; Ishii, I.; Fukuto, J.M.; Akaike, T.; Kumagai, Y. Involvement of reactive persulfides in biological bismethylmercury sulfide formation. Chem. Res. Toxicol. 2015, 28, 1301–1306. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda-Imafuku, M.; Fukuta, T.; Tuan Giam Chuang, V.; Sawa, T.; Maruyama, T.; Otagiri, M.; Ishida, T.; Ishima, Y. Acute Kidney Injury Caused by Rhabdomyolysis Is Ameliorated by Serum Albumin-Based Supersulfide Donors through Antioxidative Pathways. Pharmaceuticals 2024, 17, 128. https://doi.org/10.3390/ph17010128
Ikeda-Imafuku M, Fukuta T, Tuan Giam Chuang V, Sawa T, Maruyama T, Otagiri M, Ishida T, Ishima Y. Acute Kidney Injury Caused by Rhabdomyolysis Is Ameliorated by Serum Albumin-Based Supersulfide Donors through Antioxidative Pathways. Pharmaceuticals. 2024; 17(1):128. https://doi.org/10.3390/ph17010128
Chicago/Turabian StyleIkeda-Imafuku, Mayumi, Tatsuya Fukuta, Victor Tuan Giam Chuang, Tomohiro Sawa, Toru Maruyama, Masaki Otagiri, Tatsuhiro Ishida, and Yu Ishima. 2024. "Acute Kidney Injury Caused by Rhabdomyolysis Is Ameliorated by Serum Albumin-Based Supersulfide Donors through Antioxidative Pathways" Pharmaceuticals 17, no. 1: 128. https://doi.org/10.3390/ph17010128
APA StyleIkeda-Imafuku, M., Fukuta, T., Tuan Giam Chuang, V., Sawa, T., Maruyama, T., Otagiri, M., Ishida, T., & Ishima, Y. (2024). Acute Kidney Injury Caused by Rhabdomyolysis Is Ameliorated by Serum Albumin-Based Supersulfide Donors through Antioxidative Pathways. Pharmaceuticals, 17(1), 128. https://doi.org/10.3390/ph17010128








