Natural Products for Cancer Prevention and Interception: Preclinical and Clinical Studies and Funding Opportunities
Abstract
:1. Introduction
2. Single Agents
2.1. Ingenol Mebutate
2.2. n-3 Fatty Acids (n-3FA)
2.3. Allium Compounds
2.4. Vitamin D
2.5. Carotenoids
Lycopene
2.6. Perillyl Alcohol (PA)
2.7. Melatonin
2.8. Sulforaphane
2.9. Tea (Green, Black)
Polyphenol E (Poly E)
2.10. Isoflavones
2.11. Curcumin
2.12. Selenium
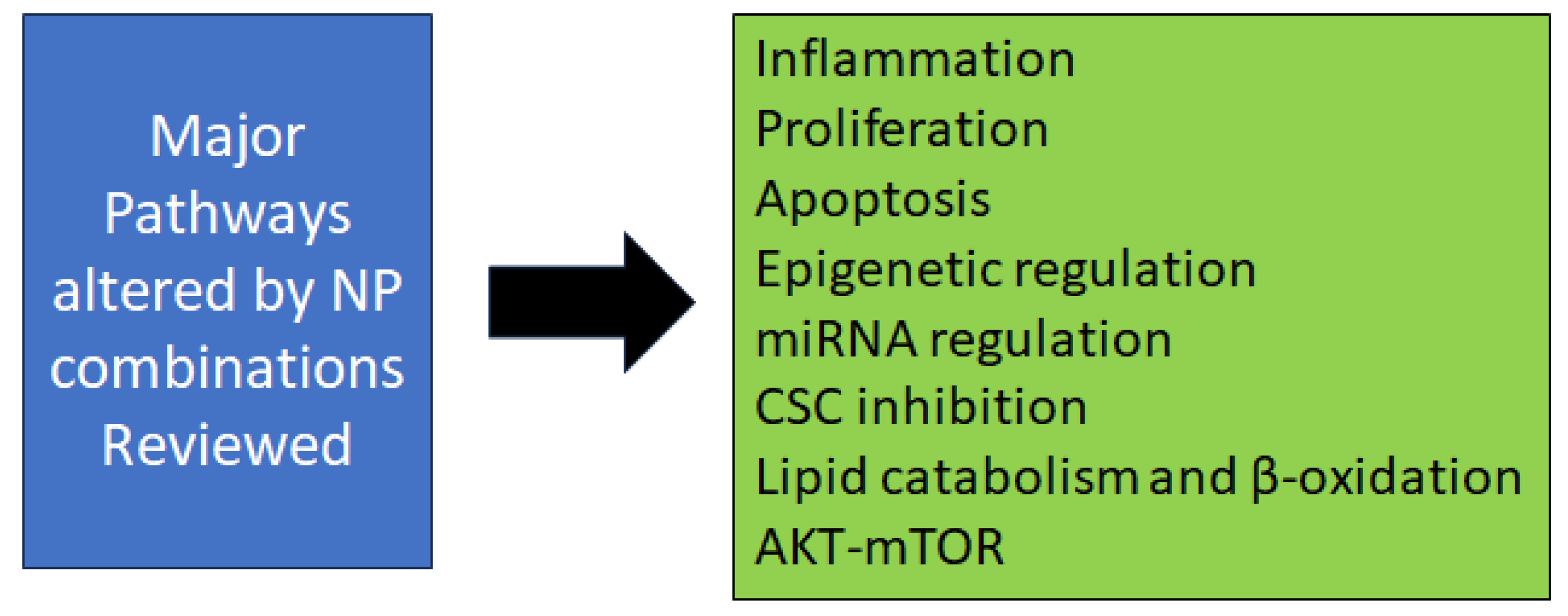
3. Combination Strategies
3.1. Preclinical Animal Model Studies for Combination Chemoprevention
3.1.1. Colon Cancer
3.1.2. Head and Neck Cancer
| Cancer Type | Agent Combination | Animal Model | Efficacy | Potential Mechanisms/Targets | Reference |
|---|---|---|---|---|---|
| Colon | Quercetin (8 mg/kg) + Resveratrol (10 mg/kg) | AOM-induced rat colon cancer | High-grade crypt abnormality in control: 73%, resveratrol: 45%, quercetin: 36%, combination tx: 27% | ↑ apoptosis, ↓ cell proliferation | [29] |
| Colon | Selenium (1 ppm) + Green Tea Extract (0.5%) | AOM-induced rat colon cancer | Combination of tx-inhibited large ACF, tumor incidence, multiplicity, and size (p < 0.01) | ↓ cell proliferation, cyclin D1, DNMT, restoration of SFRP5 mRNA, ↑ histone H3 acetylation | [30] |
| Intestine: multiple sites | EGCG (0.1%) + Sulindac (0.03%) | APC min mice | Tumor#/mouse in untreated control, EGCG, and Sulindac groups were 76, 57, and 49, respectively The combination tx group had only 32 tumors (~66% reduction, p < 0.05) | ND | [31] |
| Intestine: multiple sites | Fish Oil (12%) + EGCG (0.16%) | APC min mice | Combination tx reduced total tumor multiplicity by 53%, p < 0.05 | ↑ apoptosis ↓ PGE2 levels | [32] |
| Colon | Curcumin (0.1%) + Catechin (0.1%) | DMH-induced rat colon cancer | ACF number and colon tumor incidence decreased, respectively, by 57% and 53% in the combination tx group compared to untreated control | ↓ proliferative index ↑ apoptosis | [33] |
| Colon | Garlic (2%) + Tomato (2%) | AOM-induced rat colon cancer | Tx resulted in a significant reduction in ACF by 45% in garlic, 68% in tomato, and 72% in the combination tx groups | ↓ cell proliferation ↑ apoptosis ↓ COX-2 expression | [34] |
| Gastric | S-allylcysteine (100 mg/kg) + Lycopene (1.25 mg/kg) | MNNG and S-NaCl-induced gastric carcinogenesis in rats | Combination tx reduced tumor incidence from 100 to 17% with the tumor burden lowered from 148 to 24 mm | ↓ Bcl-2, ↑ Bax, ↑ Bim ↑ caspase 8 | [35] |
| Colon | Fish Oil (11.5%) + Pectin (6%) | AOM-induced rat colon cancer | Combination tx had a significantly lower colon tumor incidence (51%) compared with those receiving the control diet (76%) (p = 0.016) | ↑ Bcl-2 promoter methylation ↑ apoptosis | [36] |
| Colon | Fish Oil (11.5%) + Pectin (6%) | AOM-induced rat colon cancer | Combination tx protected the colon from the carcinogen-induced dysregulation of multiple miRNAs | differential expression of miRNAs (Let-7d, miR-15b, miR-107, miR-191, miR-324-5p) | [37] |
| Colon | Fish Oil (11.5%) + Pectin (6%) | AOM-induced colon cancer in Lgr5-EGFP-IRES-creERT2 mice | Total ACF in the control vs. tx group: 44 vs. 28 (p < 0.05), multi-crypt ACF 6 vs. 4 (p = 0.06) | ↑ miR-19b, miR-26b, miR-203 in Lgr5high cells | [38] |
| Colon | Fish Oil (11.5%) + Pectin (6%) | AOM-induced rat colon cancer | Combination tx vs. control significantly reduced high multiplicity aberrant crypt foci from 63.2 to 26.7 | upregulation of lipid catabolism and beta-oxidation-associated genes | [39] |
| Intestinal tumorigenesis | Sulforaphane (300 ppm) + Dibenzoylmethane (0.5%) | APC min mice | Combination tx inhibited intestinal polyp formation by 57% (p < 0.001) and completely prevented tumor development (p = 0.002) | ↓ PGE2, ↓ LTB4 | [40] |
| Oral squamous cell carcinoma | Green Tea (6 mg/mL) ingested orally + Curcumin (10 mmol) applied topically | DMBA-induced buccal pouch carcinoma in hamsters | Green tea and curcumin combination inhibited oral tumorigenesis and induced apoptosis | ↓ cancer stem cell markers (CD133, CD44) | [42] |
| Oral squamous cell carcinoma | Green Tea (6 mg/mL) ingested orally + Curcumin (10 mmol) applied topically | DMBA-induced oral carcinogenesis in hamsters | Combination tx decreased precancer and SCC lesion numbers by over 50% and lesion volume by one-third for precancers and two-thirds for cancers | ↑ apoptosis ↓ proliferation | [43] |
| Head and neck | Resveratrol (30 mg/kg) + EGCG (125 mg/kg) | Tu212 xenograft model | Tumor weight and volume were significantly reduced by combination tx | ↓ AKT-mTOR pathway ↑ apoptosis | [44] |
| Prostate | Vitamin E (800 IU) + Selenium (200 µg) + Lycopene (50 mg) | Lady (12T-10) transgenic mouse model | Combination tx reduced the incidence of PCa by >80% | ↑ apoptosis ↓ proliferation | [45] |
| Prostate | Curcumin (6 μmol i.p.) + PEITC (5 μmol i.p.) | PC-3 PCa xenograft model | Combination tx significantly reduced tumor volume vs. individual tx and control groups | ↓ proliferation ↑ apoptosis | [46] |
| Prostate | Tomato (5%) + Broccoli (5%) | Dunning R3327-H PCa rat model | Combination tx decreased the tumor weight by 52% (p < 0.001) | ↓ proliferation ↑ apoptosis | [47] |
| Lung | I3C (10 μmol/g diet) + Silibinin (7 μmol/g diet) | NNK-induced lung cancer in A/J mice | Lung adenocarcinoma presence and tumor number were reduced by 60% and 95%, respectively | ↓ p-Akt, ↓ p-ERK ↓ cyclin D1 ↑ apoptosis | [48] |
| Breast | SFN-enriched Broccoli Sprouts (13% in diet) + Genistein (250 mg/kg diet) | C3(1) SV40 Tag transgenic mouse model | Combination tx was more effective at reducing tumor incidence and volume compared to the control and either single treatment | ND | [49] |
| Breast | Genistein (250 mg/kg) + Tamoxifen (25 mg/pellet) implanted subcutaneously | C3(1)-SV40 Tag transgenic mouse model | The tumor growth rate was reduced by combination tx | ↓ tumor cell proliferation | [50] |
| Pancreas | Curcumin (2000 ppm) + Fish Oil (15%) | BxPC-3 pancreatic cancer xenograft model | Combination tx reduced tumor volume > 72% | ↓ COX-2, ↓ iNOS ↓ 5-LOX ↑ p21 | [51] |
3.1.3. PCa
3.1.4. Lung Cancer
3.1.5. Breast Cancer
3.1.6. Pancreatic Cancer
3.2. Clinical Studies for Natural Product Combination Chemoprevention
4. Challenges When Conducting NP Studies
5. Potential Opportunities for the Discovery and Development of NPs for Cancer Prevention and Interception
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Sauter, E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert. Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Khalfe, Y.; Rosen, T. Ingenol Mebutate: Expanded Utility. J. Drugs Dermatol. 2020, 19, 156–161. [Google Scholar] [CrossRef] [PubMed]
- MacLean, C.H.; Newberry, S.J.; Mojica, W.A.; Khanna, P.; Issa, A.M.; Suttorp, M.J.; Lim, Y.W.; Traina, S.B.; Hilton, L.; Garland, R.; et al. Effects of omega-3 fatty acids on cancer risk: A systematic review. JAMA 2006, 295, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Mukhtar, H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp. Dermatol. 2006, 15, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed 2014, 4, 1–14. [Google Scholar]
- Zhou, Y.; Zhuang, W.; Hu, W.; Liu, G.J.; Wu, T.X.; Wu, X.T. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology 2011, 141, 80–89. [Google Scholar] [CrossRef]
- Galeone, C.; Pelucchi, C.; Levi, F.; Negri, E.; Franceschi, S.; Talamini, R.; Giacosa, A.; La Vecchia, C. Onion and garlic use and human cancer. Am. J. Clin. Nutr. 2006, 84, 1027–1032. [Google Scholar] [CrossRef]
- McCullough, M.L.; Zoltick, E.S.; Weinstein, S.J.; Fedirko, V.; Wang, M.; Cook, N.R.; Eliassen, A.H.; Zeleniuch-Jacquotte, A.; Agnoli, C.; Albanes, D.; et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. J. Natl. Cancer Inst. 2019, 111, 158–169. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Wen, X.; Zhang, Y.; Wei, X.; Liu, T. Vitamin D Deficiency and Increased Risk of Bladder Carcinoma: A Meta-Analysis. Cell Physiol. Biochem. 2015, 37, 1686–1692. [Google Scholar] [CrossRef]
- Travis, R.C.; Perez-Cornago, A.; Appleby, P.N.; Albanes, D.; Joshu, C.E.; Lutsey, P.L.; Mondul, A.M.; Platz, E.A.; Weinstein, S.J.; Layne, T.M.; et al. A Collaborative Analysis of Individual Participant Data from 19 Prospective Studies Assesses Circulating Vitamin D and Prostate Cancer Risk. Cancer Res. 2019, 79, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Rowles, J.L.; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Beynon, R.; Richmond, R.C.; Santos Ferreira, D.L.; Ness, A.R.; May, M.; Smith, G.D.; Vincent, E.E.; Adams, C.; Ala-Korpela, M.; Wurtz, P.; et al. Investigating the effects of lycopene and green tea on the metabolome of men at risk of prostate cancer: The ProDiet randomised controlled trial. Int. J. Cancer 2019, 144, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Zhou, Y.; Ment, X.; Zhang, J.-J.; Xu, D.-P.; Li, H.-B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef]
- Kuran, D.; Pgorzelska, A.; Wiktorska, K. Breast ancer prevention-is there a future for sulforaphane and its analogs? Nutrients 2021, 12, 1559. [Google Scholar] [CrossRef]
- Atwell, L.L.; Zhang, Z.; Mori, M.; Farris, P.; Vetto, J.T.; Naik, A.M.; Oh, K.Y.; Thuillier, P.; Ho, E.; Shannon, J. Sulforaphane Bioavailability and Chemopreventive Activity in Women Scheduled for Breast Biopsy. Cancer Prev. Res. 2015, 8, 1184–1191. [Google Scholar] [CrossRef]
- Filippini, T.; Malavolti, M.; Borrelli, F.; Izzo, A.A.; Fairweather-Tait, S.J.; Horneber, M.; Vinceti, M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2020, 3, CD005004. [Google Scholar]
- Singh, B.N.; Rawat, A.K.; Bhagat, R.M.; Singh, B.R. Black tea: Phytochemicals, cancer chemoprevention, and clinical studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 1394–1410. [Google Scholar] [CrossRef]
- Nair, H.; Alex, V.V.; Anto, R.J. Significance of Nutraceuticals in Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Sinicrope, F.A.; Viggiano, T.R.; Buttar, N.S.; Song, L.; Schroeder, K.W.; Kraichely, R.E.; Larson, M.V.; Sedlack, R.E.; Kisiel, J.B.; Gostout, C.J.; et al. Randomized Phase II Trial of Polyphenon E versus Placebo in Patients at High Risk of Recurrent Colonic Neoplasia. Cancer Prev. Res. 2021, 14, 573–580. [Google Scholar] [CrossRef]
- Qin, W.; Shi, J.; Zhu, W.; Hewett, J.; Ruhlen, R.; MacDonald, R.; Rottinghaus, G.E.; Chen, Y.-C. Sauter, ER Soy isoflavones have an antiestrogenic effect and alter mammary promoter hypermethylation in healthy premenopausal women. Nutr. Cancer 2009, 61, 238–244. [Google Scholar] [CrossRef]
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2022, 80, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, J.; Bonilla, C.; Haycock, P.C.; Langdon, R.J.Q.; Lotta, L.A.; Langenberg, C.; Relton, C.L.; Lewis, S.J.; Evans, D.M.; Consortium, P.; et al. Circulating Selenium and Prostate Cancer Risk: A Mendelian Randomization Analysis. J. Natl. Cancer Inst. 2018, 110, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018, 1, CD005195. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Mason, A.M.; Carter, P.; Vithayathil, M.; Kar, S.; Burgess, S.; Larsson, S.C. Selenium and cancer risk: Wide-angled Mendelian randomization analysis. Int. J. Cancer 2022, 150, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Ignatenko, N.A.; Besselsen, D.G.; Stringer, D.E.; Blohm-Mangone, K.A.; Cui, H.; Gerner, E.W. Combination chemoprevention of intestinal carcinogenesis in a murine model of familial adenomatous polyposis. Nutr. Cancer 2008, 60 (Suppl. S1), 30–35. [Google Scholar] [CrossRef]
- Samadder, N.J.; Neklason, D.W.; Boucher, K.M.; Byrne, K.R.; Kanth, P.; Samowitz, W.; Jones, D.; Tavtigian, S.V.; Done, M.W.; Berry, T.; et al. Effect of Sulindac and Erlotinib vs Placebo on Duodenal Neoplasia in Familial Adenomatous Polyposis: A Randomized Clinical Trial. JAMA 2016, 315, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Ulusan, A.M.; Rajendran, P.; Dashwood, W.M.; Yavuz, O.F.; Kapoor, S.; Gustafson, T.A.; Savage, M.I.; Brown, P.H.; Sei, S.; Mohammed, A.; et al. Optimization of Erlotinib Plus Sulindac Dosing Regimens for Intestinal Cancer Prevention in an Apc-Mutant Model of Familial Adenomatous Polyposis (FAP). Cancer Prev Res 2021, 14, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Tezerji, S.; Abdolazimi, H.; Fallah, A.; Talaei, B. The effect of resveratrol and quercetin intervention on azoxymethane-induced colon cancer in rats model. Clin. Nutr. Open Sci. 2022, 45, 91–102. [Google Scholar] [CrossRef]
- Hu, Y.; McIntosh, G.H.; Le Leu, R.K.; Nyskohus, L.S.; Woodman, R.J.; Young, G.P. Combination of selenium and green tea improves the efficacy of chemoprevention in a rat colorectal cancer model by modulating genetic and epigenetic biomarkers. PLoS ONE 2013, 8, e64362. [Google Scholar] [CrossRef]
- Suganuma, M.; Ohkura, Y.; Okabe, S.; Fujiki, H. Combination cancer chemoprevention with green tea extract and sulindac shown in intestinal tumor formation in Min mice. J. Cancer Res. Clin. Oncol. 2001, 127, 69–72. [Google Scholar] [CrossRef]
- Bose, M.; Hao, X.; Ju, J.; Husain, A.; Park, S.; Lambert, J.D.; Yang, C.S. Inhibition of tumorigenesis in ApcMin/+ mice by a combination of (-)-epigallocatechin-3-gallate and fish oil. J. Agric. Food Chem. 2007, 55, 7695–7700. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Ren, G.; Xu, X.; Yuan, H.; Wang, Z.; Kang, L.; Yu, W.; Tian, K. Combination of curcumin and green tea catechins prevents dimethylhydrazine-induced colon carcinogenesis. Food Chem. Toxicol. 2010, 48, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Ghosh, S.; Das, S. Modulatory influence of garlic and tomato on cyclooxygenase-2 activity, cell proliferation and apoptosis during azoxymethane induced colon carcinogenesis in rat. Cancer Lett. 2004, 208, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.; Mani, A.; Nagini, S. Combination of S-allylcysteine and lycopene induces apoptosis by modulating Bcl-2, Bax, Bim and caspases during experimental gastric carcinogenesis. Eur. J. Cancer Prev. 2005, 14, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Turner, N.D.; Davidson, L.A.; Chapkin, R.S.; Carroll, R.J.; Lupton, J.R. A chemoprotective fish oil/pectin diet enhances apoptosis via Bcl-2 promoter methylation in rat azoxymethane-induced carcinomas. Exp. Biol. Med. 2012, 237, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.A.; Wang, N.; Shah, M.S.; Lupton, J.R.; Ivanov, I.; Chapkin, R.S. n-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis 2009, 30, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.S.; Kim, E.; Davidson, L.A.; Knight, J.M.; Zoh, R.S.; Goldsby, J.S.; Callaway, E.S.; Zhou, B.; Ivanov, I.; Chapkin, R.S. Comparative effects of diet and carcinogen on microRNA expression in the stem cell niche of the mouse colonic crypt. Biochim. Biophys. Acta 2016, 1862, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Triff, K.; McLean, M.W.; Callaway, E.; Goldsby, J.; Ivanov, I.; Chapkin, R.S. Dietary fat and fiber interact to uniquely modify global histone post-translational epigenetic programming in a rat colon cancer progression model. Int. J. Cancer 2018, 143, 1402–1415. [Google Scholar] [CrossRef]
- Shen, G.; Khor, T.O.; Hu, R.; Yu, S.; Nair, S.; Ho, C.T.; Reddy, B.S.; Huang, M.T.; Newmark, H.L.; Kong, A.N. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007, 67, 9937–9944. [Google Scholar] [CrossRef]
- Siddappa, G.; Kulsum, S.; Ravindra, D.R.; Kumar, V.V.; Raju, N.; Raghavan, N.; Sudheendra, H.V.; Sharma, A.; Sunny, S.P.; Jacob, T.; et al. Curcumin and metformin-mediated chemoprevention of oral cancer is associated with inhibition of cancer stem cells. Mol. Carcinog. 2017, 56, 2446–2460. [Google Scholar] [CrossRef]
- Saleh, M.M.; Darwish, Z.E.; El Nouaem, M.I.; Fayed, N.A.; Mourad, G.M.; Ramadan, O.R. The potential preventive effect of dietary phytochemicals In Vivo. BDJ Open 2023, 9, 30. [Google Scholar] [CrossRef]
- Li, N.; Chen, X.; Liao, J.; Yang, G.; Wang, S.; Josephson, Y.; Han, C.; Chen, J.; Huang, M.T.; Yang, C.S. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis 2002, 23, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Wang, D.; Nannapaneni, S.; Lamichhane, R.; Chen, Z.G.; Shin, D.M. Combination of resveratrol and green tea epigallocatechin gallate induces synergistic apoptosis and inhibits tumor growth in vivo in head and neck cancer models. Oncol. Rep. 2021, 45, 87. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, V.; Fleshner, N.E.; Sugar, L.M.; Klotz, L.H. Antioxidants block prostate cancer in lady transgenic mice. Cancer Res. 2004, 64, 5891–5896. [Google Scholar] [CrossRef] [PubMed]
- Khor, T.O.; Keum, Y.S.; Lin, W.; Kim, J.H.; Hu, R.; Shen, G.; Xu, C.; Gopalakrishnan, A.; Reddy, B.; Zheng, X.; et al. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer Res. 2006, 66, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Canene-Adams, K.; Lindshield, B.L.; Wang, S.; Jeffery, E.H.; Clinton, S.K.; Erdman, J.W., Jr. Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res. 2007, 67, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Dagne, A.; Melkamu, T.; Schutten, M.M.; Qian, X.; Upadhyaya, P.; Luo, X.; Kassie, F. Enhanced inhibition of lung adenocarcinoma by combinatorial treatment with indole-3-carbinol and silibinin in A/J mice. Carcinogenesis 2011, 32, 561–567. [Google Scholar] [CrossRef]
- Paul, B.; Li, Y.; Tollefsbol, T.O. The Effects of Combinatorial Genistein and Sulforaphane in Breast Tumor Inhibition: Role in Epigenetic Regulation. Int. J. Mol. Sci. 2018, 19, 1754. [Google Scholar] [CrossRef]
- Li, Y.; Meeran, S.M.; Patel, S.N.; Chen, H.; Hardy, T.M.; Tollefsbol, T.O. Epigenetic reactivation of estrogen receptor-alpha (ERalpha) by genistein enhances hormonal therapy sensitivity in ERalpha-negative breast cancer. Mol. Cancer 2013, 12, 9. [Google Scholar] [CrossRef]
- Swamy, M.V.; Citineni, B.; Patlolla, J.M.; Mohammed, A.; Zhang, Y.; Rao, C.V. Prevention and treatment of pancreatic cancer by curcumin in combination with omega-3 fatty acids. Nutr. Cancer 2008, 60 (Suppl. S1), 81–89. [Google Scholar] [CrossRef]
- Ledesma, M.C.; Jung-Hynes, B.; Schmit, T.L.; Kumar, R.; Mukhtar, H.; Ahmad, N. Selenium and vitamin E for prostate cancer: Post-SELECT (Selenium and Vitamin E Cancer Prevention Trial) status. Mol. Med. 2011, 17, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Albanes, D.; Heinonen, O.P.; Huttunen, J.K.; Taylor, P.R.; Virtamo, J.; Edwards, B.K.; Haapakoski, J.; Rautalahti, M.; Hartman, A.M.; Palmgren, J. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am. J. Clin. Nutr. 1995, 62 (Suppl. S6), 1427S–1430S. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.A.; Sprange, K.; Hepburn, T.; Tan, W.; Shafayat, A.; Rees, C.J.; Clifford, G.; Logan, R.F.; Loadman, P.M.; Williams, E.A.; et al. Eicosapentaenoic acid and aspirin, alone and in combination, for the prevention of colorectal adenomas (seAFOod Polyp Prevention trial): A multicentre, randomised, double-blind, placebo-controlled, 2 × 2 factorial trial. Lancet 2018, 392, 2583–2594. [Google Scholar] [CrossRef] [PubMed]
- Neetha, M.C.; Pattabhiramasastry, S.; Shivaprasad, N.V.; Venkatesh, U.G. Chemopreventive Synergism between Green Tea Extract and Curcumin in Patients with Potentially Malignant Oral Disorders: A Double-blind, Randomized Preliminary Study. J. Contemp. Dent. Prac. 2020, 21, 521–531. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Vaishampayan, U.; Hussain, M.; Banerjee, M.; Seren, S.; Sarkar, F.H.; Fontana, J.; Forman, J.D.; Cher, M.L.; Powell, I.; Pontes, J.E.; et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr. Cancer 2007, 59, 1–7. [Google Scholar] [CrossRef]
- Li, W.Q.; Zhang, J.Y.; Ma, J.L.; Li, Z.X.; Zhang, L.; Zhang, Y.; Guo, Y.; Zhou, T.; Li, J.Y.; Shen, L.; et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: Follow-up of a randomized intervention trial. BMJ 2019, 366, l5016. [Google Scholar] [CrossRef]
- Zhou, P.; Cheng, S.W.; Yang, R.; Wang, B.; Liu, J. Combination chemoprevention: Future direction of colorectal cancer prevention. Eur. J. Cancer Prev. 2012, 21, 231–240. [Google Scholar] [CrossRef]
- Pandit, A.P.; Dalal, P.S.; Patole, V.C. Curcumin as a permeability enhancer enhanced the antihyperlipidemic activity of dietary green tea extract. BMC Complement. Alter. Med. 2019, 19, 129. [Google Scholar] [CrossRef]
- Johnson, J.J.; Nihal, M.; Siddiqui, I.A.; Scarlett, C.O.; Bailey, H.H.; Mukhtar, H.; Ahmad, N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011, 55, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Wang, X.; Di, X.; Liu, Y. Effect of piperine on the bioavailability and pharmacokinetics of emodin in rats. J. Pharm. Biomed. Anal. 2015, 115, 144–149. [Google Scholar] [CrossRef] [PubMed]
| Disease Endpoint | Gender | Interventions | Intervention Frequency/Length | Results (Efficacy, Targets, Mechanisms) |
|---|---|---|---|---|
| PCa prevention | ♂ (≥50-AA; ≥55-others) | vitE 400 IU Selenium 200 mcg Placebo | Daily/7–12 years | ↑ risk of PCa cancer: 1.6/1000 person-years for vitE, 0.8 for selenium, 0.4 with the combination vs. control |
| Lung and other cancer prevention in smokers | ♂ 50–69 | vitE β carotene Placebo | Daily/5–8 years | vitE had no effect on lung cancer incidence vs. control, while a lower incidence of PCa and colorectum was observed. Those receiving β carotene had an ↑ incidence of lung, prostate, and stomach cancer |
| High risk for esophageal and gastric cancer | ♂ + ♀ 40–69 | Retinol, zinc, riboflavin niacin, ascorbate molybdenum, vitE, β carotene, selenium, placebo | Daily/63 months | vitE (50 mg) + β carotene (15 mg) + selenium (50 mcg) ↓ mortality due to gastric cancer by 21% and total cancer mortality by 13%. Other nutrients: no significant effect |
| High risk for colorectal cancer | ♂ + ♀ (55–73) | 2 g EPA-free FA, 300 mg aspirin, both, or placebo | Daily/12 months | Neither EPA nor aspirin reduced colorectal adenomas |
| Oral potentially malignant disorders | ♂ + ♀ | Green tea extract (topical + 800 mg/d systemic, curcumin topical + 950 mg/d systemic, or both | Daily/3 months | Response (lower p53, Ki67, cyclin D1) ↑ in the combination group (65%) vs. curcumin (55%) or green tea extract (35%) (p < 0.01) |
| APC | ♂ + ♀ | 480 mg curcumin 20 mg quercetin | Thrice daily/6 months | Combination tx led to ↓ polyp number and size (p < 0.05) after tx vs. baseline |
| PCa | ♂ | 15 mg lycopene, 40 mg soy isoflavone, or both | Twice daily/6 months | Lycopene and combination tx led to stable PSA in 95% and 67%, respectively, in patients with previously rising PSA |
| PCa | ♂ ≥50 | Lycopene 30 mg Fish oil 1 g Placebo | Daily/3 months | No genes were significantly associated with a high intake of fish oil or lycopene at baseline or after 3 months of study |
| Gastric cancer prevention in an area where gastric cancer is endemic | ♂ + ♀ 35–64 | H pylori tx, garlic, vitamin C, E, selenium | Twice daily/7.3 years | Each tx: H pylori, garlic, vitamins C, E, selenium significantly ↓ gastric cancer mortality, incidence decreased with vitamin but not garlic supplements |
| Cancer and cardiovascular (CV) incidence and mortality | ♂ 45–60 + ♂ + ♀ 35–60 | vitC 120 mcg vitE 30 mg β carotene 6 mg selenium 100 mcg Zinc 20 mg Placebo | Daily/7.5 years | A 31% ↓ total cancer incidence and 37% reduction in all-cause mortality in men but not women vs. control |
| Prostatic intraepithelial neoplasia and suspicious prostate findings | ♂ ≥21 | Green tea extract Fish oil Placebo | Twice daily/up to 20 weeks | No significant ∆ in FA synthase or cell proliferation with green tea extract, fish oil, or the combination vs. control |
| Colorectal adenoma recurrence | ♂ ≥50-AA; ≥55-others | vitE 400 IU Selenium 200 mcg Placebo | Daily/7–12 years | Neither selenium nor vitE affected adenoma recurrence vs. control |
| Smokers, former smokers, and workers exposed to asbestos | ♂ + ♀ 45–69 | β carotene 30 mg vitA 25,000 IU Placebo | Daily/4 years | β carotene and vitA may ↑ the risk of death from lung cancer, CV disease, and other causes |
| Postmenopausal women | ♀ Post | CaCO3 1000 mg vitD 400 IU Placebo | Daily/7 years | Ca and vitD: no effect on colorectal cancer incidence |
| Prevention of cancer and CV disease | ♂ ≥50; ♀ ≥55 | 2000 IU vitD n-3 FA | Daily/5.3 years | Neither vitD nor marine n-3 FA significantly ↓ cancer or CV risk vs. control |
| Lung cancer prevention in former smokers | ♂ + ♀ 40–80 | Green tea beverage Polyphenon E Placebo | Daily/6 months | There was no significant effect on urinary 8-OHdG or 8-F2 isoprostanes with either treatment or control |
| NCI Program or Title of the Funding Opportunity | Notice of Funding Opportunity (Hyperlinks) | Funding Type | Submission Dates |
|---|---|---|---|
| DDNP-CIP program | RFA-CA-23-028 | UG3/UH3 | June 2023–2025 |
| PREVENT program | PREVENT Concept Application | Contract | Twice a year, the second Monday in January and July |
| Dietary Effects on Nutrient Sensing Pathways in Tumor Etiology and Prevention | NOT-CA-21-121 | NOSI | Various, NOSI expires September 2024 |
| Administrative Supplements for Validation Studies of Analytical Methods for Dietary Supplement Constituents | NOT-OD-22-202 | NOSI | Various, NOSI expires April 2025 |
| NCI Clinical and Translational Exploratory/Developmental Studies | PAR-22-216 | R21 Clinical Trial Optional | October/November 2023–2024; February/March 2023–2025; June/July 2023–2025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauter, E.R.; Mohammed, A. Natural Products for Cancer Prevention and Interception: Preclinical and Clinical Studies and Funding Opportunities. Pharmaceuticals 2024, 17, 136. https://doi.org/10.3390/ph17010136
Sauter ER, Mohammed A. Natural Products for Cancer Prevention and Interception: Preclinical and Clinical Studies and Funding Opportunities. Pharmaceuticals. 2024; 17(1):136. https://doi.org/10.3390/ph17010136
Chicago/Turabian StyleSauter, Edward R., and Altaf Mohammed. 2024. "Natural Products for Cancer Prevention and Interception: Preclinical and Clinical Studies and Funding Opportunities" Pharmaceuticals 17, no. 1: 136. https://doi.org/10.3390/ph17010136
APA StyleSauter, E. R., & Mohammed, A. (2024). Natural Products for Cancer Prevention and Interception: Preclinical and Clinical Studies and Funding Opportunities. Pharmaceuticals, 17(1), 136. https://doi.org/10.3390/ph17010136









