The Effective Synthesis of New Benzoquinoline Derivatives as Small Molecules with Anticancer Activity
Abstract
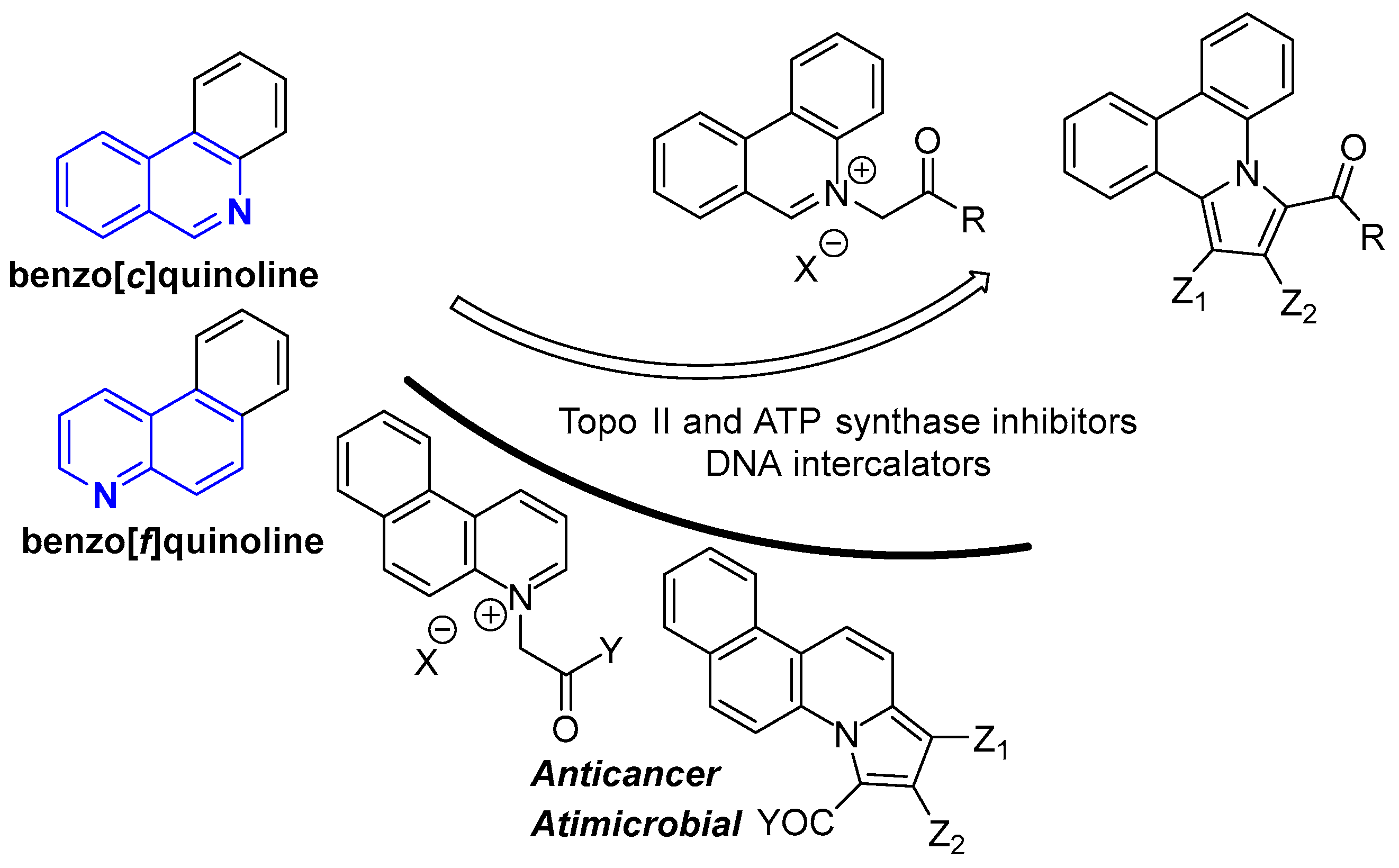
:1. Introduction
2. Results and Discussion
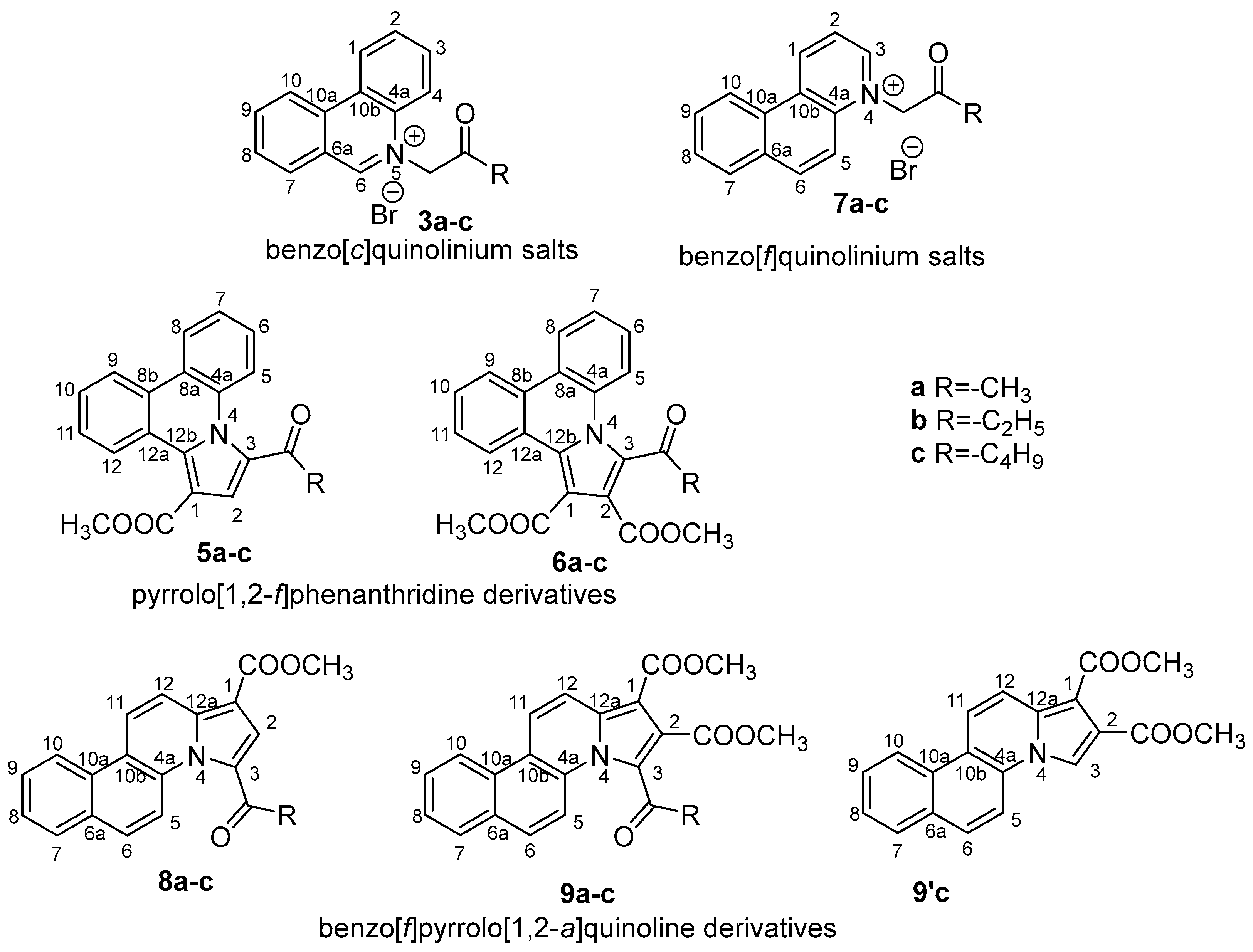
2.1. Design and Chemistry
2.2. Anticancer Activity
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ram, V.J.; Sethi, A.; Nath, M.; Pratap, R. Chapter 2. Six–Membered Heterocycles. In The Chemistry of Heterocycles: Chemistry of Six to Eight Membered N, O, S, P and Se; Dennis, S., McCloskey, E.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 101–106. [Google Scholar]
- Singh, R.; Panda, G. An overview of synthetic approaches for heterocyclic steroids. Tetrahedron 2013, 69, 2853–2884. [Google Scholar] [CrossRef]
- Antoci, V.; Oniciuc, L.; Amariucai–Mantu, D.; Moldoveanu, C.; Mangalagiu, V.; Amarandei, A.M.; Lungu, C.N.; Dunca, S.; Mangalagiu, I.I.; Zbancioc, G. Benzoquinoline Derivatives: A Straightforward and Efficient Route to Antibacterial and Antifungal Agents. Pharmaceuticals 2021, 14, 335. [Google Scholar] [CrossRef] [PubMed]
- Oniciuc, L.; Amăriucăi-Mantu, D.; Diaconu, D.; Mangalagiu, V.; Danac, R.; Antoci, V.; Mangalagiu, I.I. Benzoquinoline Derivatives: An Attractive Approach to Newly Small Molecules with Anticancer Activity. Int. J. Mol. Sci. 2023, 24, 8124. [Google Scholar] [CrossRef]
- Park, G.Y.; Wilson, J.J.; Song, Y.; Lippard, S.J. Phenanthriplatin, a monofunctional DNA–binding platinum anticancer drugcandidate with unusual potency and cellular activity profile. Proc. Natl. Acad. Sci. USA 2012, 109, 11987–11992. [Google Scholar] [CrossRef] [PubMed]
- Dikusar, E.A.; Kadutskii, A.P.; Kozlov, N.G.; Yuvchenko, A.P.; Mel’nichuk, L.A. Salts of spiro-derivatives of benzo[f]quinolineand several natural carboxylic acids. Chem. Nat. Compd. 2006, 42, 118–120. [Google Scholar] [CrossRef]
- Tietze, L.F.; Herzig, T.; Fecher, A. Novel Prodrugs Von 6–Hydroxy–2,3–dihydro–1H–indoles, 5–hydroxy–1,2–dihydro–3Hpyrrolo[3,2–E]indoles and 5–Hydroxy–1,2–dihydro–3H–benzo(e)indoles as Well as of 6–Hydroxy–1,2,3,4–tetrahydro benzo[f]quinoline Derivatives for Use in Selective Cancer Therapy. U.S. Patent 2004/0033962 A1, 7 August 2004. [Google Scholar]
- Sutherland, J.B.; Cross, E.L.; Heinze, T.M.; Freeman, J.P.; Moody, J.D. Fungal biotransformation of benzo[f]quinoline, benzo[h]quinoline, and phenanthridine. Appl. Microbiol. Biotechnol. 2005, 67, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Lintelmann, J.; França, M.H.; Hübner, E.; Matuschek, G. A liquid chromatography–atmospheric pressure photoionization tandem mass spectrometric method for the determination of azaarenes in atmospheric particulate matter. J. Chromatogr. A 2010, 1217, 1636–1646. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P.; Rosenfeld, P.E. Chapter 1. Wood–preserving chemicals. In Handbook of Pollution Prevention and Cleaner Production, Best Practices in the Wood and Paper Industries; Cheremisinoff, N.P., Rosenfeld, P.E., Eds.; Elsevier: Oxford, UK, 2010; pp. 1–26. [Google Scholar]
- Ma, C.Y.; Ho, C.; Caton, J.E.; Griest, W.H.; Guerin, M.R. Isolation and identification of benzoquinolines in natural and syntheticcrude oils. Fuel 1987, 66, 612–617. [Google Scholar] [CrossRef]
- Sikka, H.C.; Kandaswamia, C.; Kumar, S.; Rutkowski, J.P.; Dubey, S.K.; Earleyb, K.; Guptab, R.C. Hepatic DNA adduct formation in rats treated with benzo[f]quinoline. Cancer Lett. 1988, 43, 133–138. [Google Scholar] [CrossRef]
- Moldoveanu, C.; Mangalagiu, I.; Zbancioc, G. Fluorescent Azasteroids through Ultrasound Assisted Cycloaddition Reactions. Molecules 2021, 26, 5098. [Google Scholar] [CrossRef]
- Seo, J.; Park, S.R.; Kim, M.; Suh, M.C.; Lee, J. The role of electron-transporting Benzo[f]quinoline unit as an electron acceptor of new bipolar hosts for green PHOLEDs. Dyes Pigments 2019, 162, 959–966. [Google Scholar] [CrossRef]
- Park, S.R.; Seo, J.S.; Ahn, Y.; Lee, J.H.; Suh, M.C. Thermally stable benzo[f]quinoline based bipolar host materials for greenphosphorescent OLEDs. Org. Electron. 2018, 63, 194–199. [Google Scholar] [CrossRef]
- Kamatani, J.; Hashimoto, M.; Igawa, S.; Okada, S. Organic Metal Complex, and Organic Light Emitting Device and Display Apparatus Using the Same. U.S. Patent 2022/11342514 B2, 6 November 2022. [Google Scholar]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 25 September 2023).
- Abraham, J.; Staffurth, J. Hormonal therapy for cancer. Medicine 2016, 44, 30–33. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr Oncol. 2020, 27 (Suppl. S2), 87–97. [Google Scholar] [CrossRef]
- Czogała, W.; Czogała, M.; Kwiecinska, K.; Bik-Multanowski, M.; Tomasik, P.; Hałubiec, P.; Łazarczyk, A.; Miklusiak, K.; Skoczen, S. The Expression of Genes Related to Lipid Metabolism and Metabolic Disorders in Children before and after Hematopoietic StemCell Transplantation—A Prospective Observational Study. Cancers 2021, 13, 3614. [Google Scholar] [CrossRef]
- Kinch, M.S. An analysis of FDA–approved drugs for oncology. Drug Discov. Today 2014, 19, 1831–1835. [Google Scholar] [CrossRef]
- Magalhaes, L.G.; Ferreira, L.L.G.; Andricopulo, A.D. Recent Advances and Perspectives in Cancer Drug Design. An. Acad. Bras. Cienc. 2018, 90, 1233–1236. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Raimondi, M.V.; Tarantelli, C.; Spriano, F.; Bertoni, F.; Barraja, P.; Montalbano, A. Recurrence of the oxazolemotif in tubulin colchicine site inhibitors with anti-tumor activity. Eur. J. Med. Chem. Rep. 2021, 1, 100004. [Google Scholar]
- Barreca, M.; Spanò, V.; Rocca, R.; Bivacqua, R.; Abel, A.C.; Maruca, A.; Montalbano, A.; Raimondi, M.V.; Tarantelli, C.; Gaudio, E.; et al. Development of [1,2]oxazoloisoindoles tubulin polymerization inhibitors: Further chemical modifications and potential therapeutic effects against lymphomas. Eur. J. Med. Chem. 2022, 243, 114744. [Google Scholar] [CrossRef]
- Vieira, A.M.G.; Silvestre, O.F.; Silva, B.F.B.; Ferreira, C.J.O.; Lopes, I.; Gomes, A.C.; Espiña, B.; Sárria, M.P. pH-sensitive nanoliposomes for passive and CXCR-4-mediated marine yessotoxin delivery for cancer therapy. Nanomedicine 2022, 17, 717–739. [Google Scholar] [CrossRef] [PubMed]
- Rodriquez, M.; Taddei, M. Synthesis of Heterocycles via Microwave-Assisted Cycloadditions and Cyclocondensations. In Microwave-Assisted Synthesis of Heterocycle; Van der Eycken, E., Kappe, C.O., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 213–266. ISBN 978-3-54030-983-3. [Google Scholar]
- Perreux, L.; Loupy, A. Nonthermal Effects of Microwaves in Organic Synthesis. In Microwaves in Organic Synthesis, 2nd ed.; Loupy, A., Ed.; Wiley-VCH: Weinheim, Germany, 2006; pp. 134–218. ISBN 978-3-52731-452-2. [Google Scholar]
- Shingare, M.S.; Shingate, B.B. Ultrasound in Synthetic Applications and Organic Chemistry. In Handbook on Applications of Ultrasound: Sonochemistry for Sustainability, 1st ed.; Chen, D., Sharma, S.K., Mudhoo, A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-1-43984-206-5. [Google Scholar]
- Mason, T.J.; Peters, D. Practical Sonochemistry. Power Ultrasound Uses and Applications, 2nd ed.; Ellis Horwood: Chichester, UK, 2002; ISBN 978-1-89856-383-9. [Google Scholar]
- Zbancioc, G.; Huhn, T.; Groth, U.; Deleanu, C.; Mangalagiu, I.I. Pyrrolodiazine derivatives as blue organic luminophores: Synthesis and properties. Part 3. Tetrahedron 2010, 66, 4298–4306. [Google Scholar] [CrossRef]
- Xu, J. Microwave Irradiation and Selectivities in Organic Reactions. Prog. Chem. 2007, 19, 700–712. [Google Scholar]
- Xu, J. Selelectivities in microwave-assisted organic reactions. In Advances in Microwave Chemistry; Banik, B.K., Bandyopadhyay, D., Eds.; CRC Press: Boca Raton, FL, USA, 2018; Chapter 6; pp. 257–291. ISBN 978-0-81537-519-7. [Google Scholar]
- Li, X.; Xu, J. Effects of the Microwave Power on the Microwave-assisted Esterification. Curr. Microw. Chem. 2017, 4, 158–162. [Google Scholar] [CrossRef]
- Zbancioc, G.; Zbancioc, A.M.; Mangalagiu, I.I. Ultrasound and microwave assisted synthesis of dihydroxyacetophenone derivatives with or without 1,2-diazine skeleton. Ultrason. Sonochem. 2014, 21, 802–811. [Google Scholar] [CrossRef]
- Zbancioc, G.; Moldoveanu, C.; Mangalagiu, I.I. Ultrasound assisted synthesis of imidazolium salts: An efficient way to ionic liquids. Ultrason. Sonochem. 2015, 23, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.S. Green Chemistry with Microwave Energy. In Innovations in Green Chemistry and Green Engineering; Anastas, P.T., Zimmerman, J.B., Eds.; Springer: New York, NY, USA, 2013; ISBN 978-1-46145-816-6. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1988. [Google Scholar]
- Diaconu, D.; Antoci, V.; Mangalagiu, V.; Amariucai-Mantu, D.; Mangalagiu, I.I. Quinoline–imidazole/benzimidazole derivativesas dual–/multi–targeting hybrids inhibitors with anticancer and antimicrobial activity. Sci. Rep. 2022, 12, 16988. [Google Scholar] [CrossRef] [PubMed]
- Mantu, D.; Antoci, V.; Moldoveanu, C.; Zbancioc, G.; Mangalagiu, I.I. Hybrid imidazole (benzimidazole)/pyridine (quinoline)derivatives and evaluation of their anticancer and antimycobacterial activity. J. Enzym. Inhib. Med. Ch. 2016, 31 (Suppl. S2), 96–103. [Google Scholar] [CrossRef]
- Azad, I.; Ahmad, R.; Khan, T.; Saquib, M.; Hassan, F.; Akhter, Y.; Khan, A.; Nasibullah, M. Phenanthridine derivatives as promising new anticancer agents: Synthesis, biological evaluation and binding studies. Future Med. Chem. 2020, 12, 709–739. [Google Scholar] [CrossRef]
- Kubař, T.; Hanus, M.; Ryjáček, F.; Hobza, P. Binding of Cationic and Neutral Phenanthridine Intercalators to a DNA Oligomer Is Controlled by Dispersion Energy: Quantum Chemical Calculations and Molecular Mechanics Simulations. Chem.-A Eur. J. 2006, 12, 280–290. [Google Scholar] [CrossRef]
- Tumir, L.-M.; Radić Stojković, M.; Piantanida, I. Come-back of phenanthridine and phenanthridinium derivatives in the 21st century. Beilstein J. Org. Chem. 2014, 10, 2930–2954. [Google Scholar] [CrossRef] [PubMed]
- Lasák, P.; Motyka, K.; Kryštof, V.; Stýskala, J. Synthesis, Bacteriostatic and Anticancer Activity of Novel Phenanthridines Structurally Similar to Benzo[c]phenanthridine Alkaloids. Molecules 2018, 23, 2155. [Google Scholar] [CrossRef] [PubMed]
- Slaninová, I.; Pěnčíková, K.; Urbanová, J.; Slanina, J.; Táborská, E. Antitumour activities of sanguinarine and related alkaloids. Phytochem. Rev. 2014, 13, 51–68. [Google Scholar] [CrossRef]
- Nakanishi, T.; Masuda, A.; Suwa, M.; Akiyama, Y.; Hoshino-Abe, N.; Suzuki, M. Synthesis of derivatives of NK109, 7-OH Benzo[c]phenanthridine alkaloid, and evaluation of their cytotoxicities and reduction-resistant properties. Bioorg. Med. Chem. Lett. 2000, 10, 2321–2323. [Google Scholar] [CrossRef]
- Shoemaker, H.R. The NCI60 human tumor cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. Newcolorimetric cytotoxicity assay for anticancer–drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Boyd, R.B. The NCI In Vitro Anticancer Drug Discovery Screen. In Anticancer Drug Development Guide; Teicher, B.A., Ed.; Cancer Drug Discovery and Development; Humana Press: Totowa, NJ, USA, 1997; pp. 23–42. [Google Scholar]
- The COMPARE Program. Available online: http://dtp.nci.nih.gov/docs/compare/compare.html (accessed on 21 March 2023).
- Development. Available online: https://next.cancer.gov/developmentResources/default.htm (accessed on 14 March 2023).
- Cell Lines in the In Vitro Screen. Available online: https://dtp.cancer.gov/discovery_development/nci-60/cell_list.htm (accessed on 14 March 2023).
- The Standard NCI/DTP Methodology of the In Vitro Cancer Screen. Available online: https://dtp.cancer.gov/discovery_development/nci-60/methodology.html (accessed on 14 March 2023).

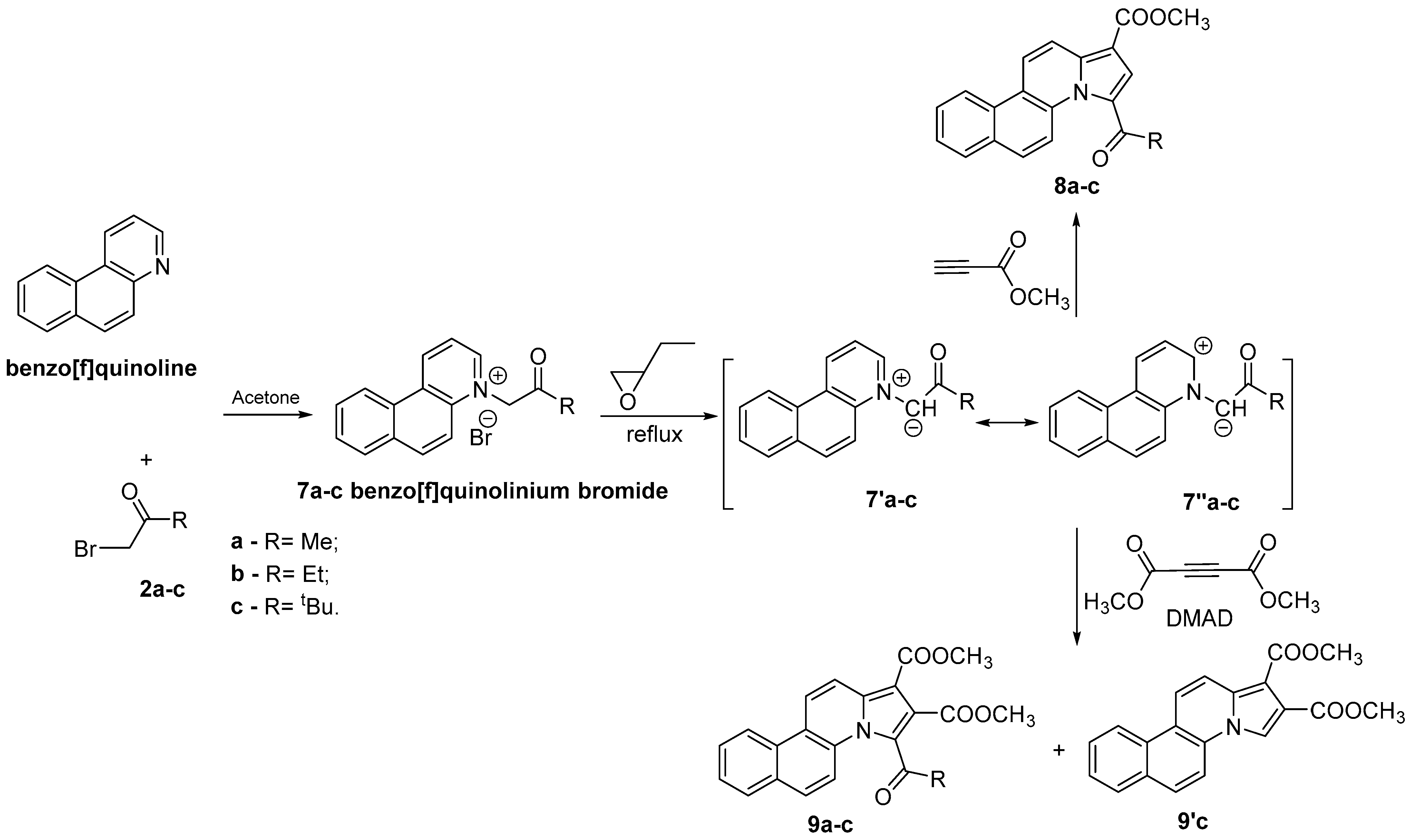
| Compd | Microwaves (MW) | Ultrasounds (US) | Conventional TH | |||
|---|---|---|---|---|---|---|
| Reaction Time, | Yield, % | Reaction Time, | Yield, % | Reaction Time, | Yield, % | |
| 3a | 10 min | 91 | 20 min | 87 | 36 h | 67 |
| 3b | 10 min | 93 | 20 min | 88 | 36 h | 70 |
| 3c | 10 min | 94 | 20 min | 90 | 36 h | 72 |
| 5a | 10 min | 78 | 20 min | 73 | 48 h | 57 |
| 5b | 10 min | 80 | 20 min | 75 | 48 h | 59 |
| 5c | 10 min | 82 | 20 min | 79 | 48 h | 61 |
| 6a | 15 min | 73 | 30 min | 68 | 48 h | 51 |
| 6b | 15 min | 75 | 30 min | 68 | 48 h | 49 |
| 6c | 15 min | 76 | 30 min | 71 | 48 h | 46 |
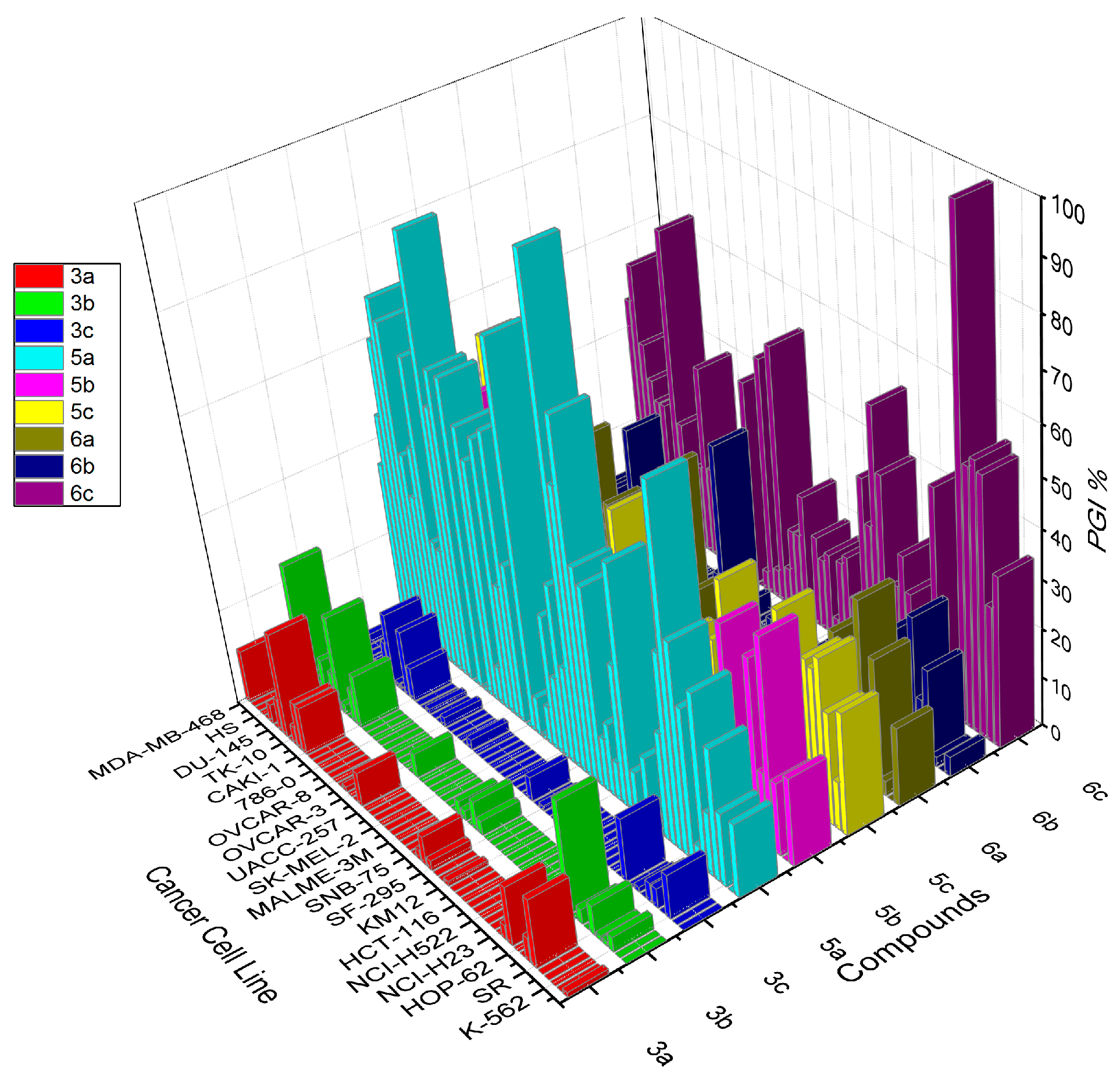
| Cell Type | Compound/Growth Inhibition Percent (PGI%) a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3a | 3b | 3c | 5a | 5b | 5c | 6a | 6b | 6c | |
| Leukemia | |||||||||
| CCRF–CEM | 1 | 0 | 0 | 15 | 18 | 25 | 16 | 4 | 35 |
| HL–60 (TB) | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 28 |
| K–562 | 0 | 3 | 9 | 15 | 15 | 23 | 3 | 2 | 53 |
| MOLT–4 | 0 | 1 | 0 | 27 | 43 | 33 | 27 | 19 | 55 |
| RPMI–8226 | 0 | 1 | 4 | 13 | 15 | 30 | 13 | 12 | 53 |
| SR | 14 | 6 | 4 | 36 | 37 | 31 | 37 | 28 | 100(17) b |
| Non–small Cell Lung Cancer | |||||||||
| A549/ATCC | 5 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| EKVX | 0 | 3 | 1 | 31 | 0 | 0 | 1 | 0 | 4 |
| HOP–62 | 0 | 0 | 0 | 43 | 0 | 0 | 0 | 0 | 0 |
| HOP–92 | 12 | 23 | 13 | 72 | 40 | 37 | 27 | 21 | 45 |
| NCI–H226 | 3 | 0 | 0 | 39 | 10 | 0 | 19 | 5 | 15 |
| NCI–H23 | 0 | 0 | 0 | 22 | 8 | 8 | 7 | 7 | 18 |
| NCI–H322M | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| NCI–460 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 |
| NCI–H522 | 0 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 27 |
| Colon Cancer | |||||||||
| COLO 205 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 |
| HCC–2998 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 15 |
| HCT–116 | 0 | 0 | 0 | 50 | 17 | 12 | 12 | 7 | 41 |
| HCT–15 | 0 | 0 | 0 | 29 | 28 | 35 | 15 | 11 | 54 |
| HT29 | 0 | 0 | 0 | 8 | 7 | 1 | 5 | 0 | 29 |
| KM12 | 1 | 0 | 0 | 15 | 13 | 21 | 4 | 0 | 38 |
| SW–620 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 0 |
| CNS Cancer | |||||||||
| SF–268 | 0 | 0 | 0 | 41 | 0 | 0 | 0 | 0 | 11 |
| SF–295 | 2 | 0 | 1 | 44 | 8 | 7 | 9 | 3 | 19 |
| SF–539 | 6 | 3 | 0 | 72 | 0 | 4 | 5 | 0 | 17 |
| SNB–19 | 2 | 6 | 5 | 58 | 0 | 0 | 0 | 0 | 4 |
| SNB–75 | 0 | 0 | 0 | 100(12) b | 0 | 16 | 0 | 0 | 17 |
| U251 | 0 | 2 | 0 | 20 | 1 | 3 | 0 | 0 | 15 |
| Melanoma | |||||||||
| LOX IMVI | 0 | 3 | 0 | 36 | 15 | 9 | 9 | 7 | 19 |
| MALME–3M | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 0 | 0 |
| M14 | 0 | 0 | 0 | 28 | 8 | 4 | 5 | 2 | 26 |
| MDA–MB–435 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 17 |
| SK–MEL–2 | 0 | 0 | 0 | 1 | 0 | 7 | 0 | 0 | 17 |
| SK–MEL–28 | 0 | 0 | 0 | 8 | 0 | 1 | 0 | 0 | 8 |
| SK–MEL–5 | 0 | 0 | 0 | 79 | 11 | 12 | 12 | 4 | 54 |
| UACC–257 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| UACC–62 | 7 | 5 | 2 | 33 | 38 | 34 | 37 | 37 | 50 |
| Ovarian Cancer | |||||||||
| IGROV1 | 0 | 0 | 0 | 59 | 7 | 14 | 4 | 9 | 4 |
| OVCAR–3 | 0 | 0 | 0 | 27 | 0 | 0 | 0 | 0 | 0 |
| OVCAR–4 | 1 | 0 | 0 | 56 | 13 | 15 | 18 | 5 | 43 |
| OVCAR–5 | 0 | 0 | 0 | 8 | 5 | 2 | 6 | 2 | 3 |
| OVCAR–8 | 0 | 0 | 2 | 50 | 3 | 2 | 0 | 0 | 10 |
| NCI/ADR–RES | 0 | 0 | 0 | 56 | 12 | 11 | 8 | 0 | 17 |
| SK–OV–3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Renal Cancer | |||||||||
| 786–0 | 0 | 0 | 1 | 64 | 0 | 8 | 0 | 0 | 14 |
| A498 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ACHN | 0 | 0 | 0 | 64 | 5 | 11 | 5 | 1 | 19 |
| CAKI–1 | 10 | 11 | 7 | 56 | 30 | 40 | 21 | 20 | 40 |
| RXF 393 | 10 | 7 | 14 | 89 | 10 | 9 | 8 | 3 | 18 |
| SN12C | 4 | 0 | 1 | 25 | 6 | 5 | 6 | 2 | 13 |
| TK–10 | 0 | 2 | 1 | 26 | 0 | 0 | 0 | 0 | 0 |
| UO–31 | 22 | 20 | 15 | 63 | 18 | 16 | 11 | 13 | 25 |
| Prostate Cancer | |||||||||
| PC–3 | 7 | 7 | 8 | 44 | 49 | 41 | 31 | 28 | 65 |
| DU–145 | 0 | 0 | 0 | 33 | 0 | 3 | 0 | 0 | 5 |
| Breast Cancer | |||||||||
| MCF7 | 4 | 8 | 2 | 41 | 16 | 14 | 11 | 6 | 27 |
| MDA–MB–231/ATCC | 0 | 0 | 0 | 67 | 22 | 21 | 16 | 13 | 27 |
| HS 578T | 0 | 0 | 0 | 71 | 8 | 1 | 4 | 0 | 31 |
| BT–549 | 0 | 0 | 0 | 62 | 0 | 0 | 0 | 0 | 38 |
| T–47D | 0 | 0 | 0 | 46 | 33 | 53 | 14 | 11 | 54 |
| MDA–MB–468 | 11 | 24 | 2 | 35 | 10 | 12 | 7 | 5 | 46 |
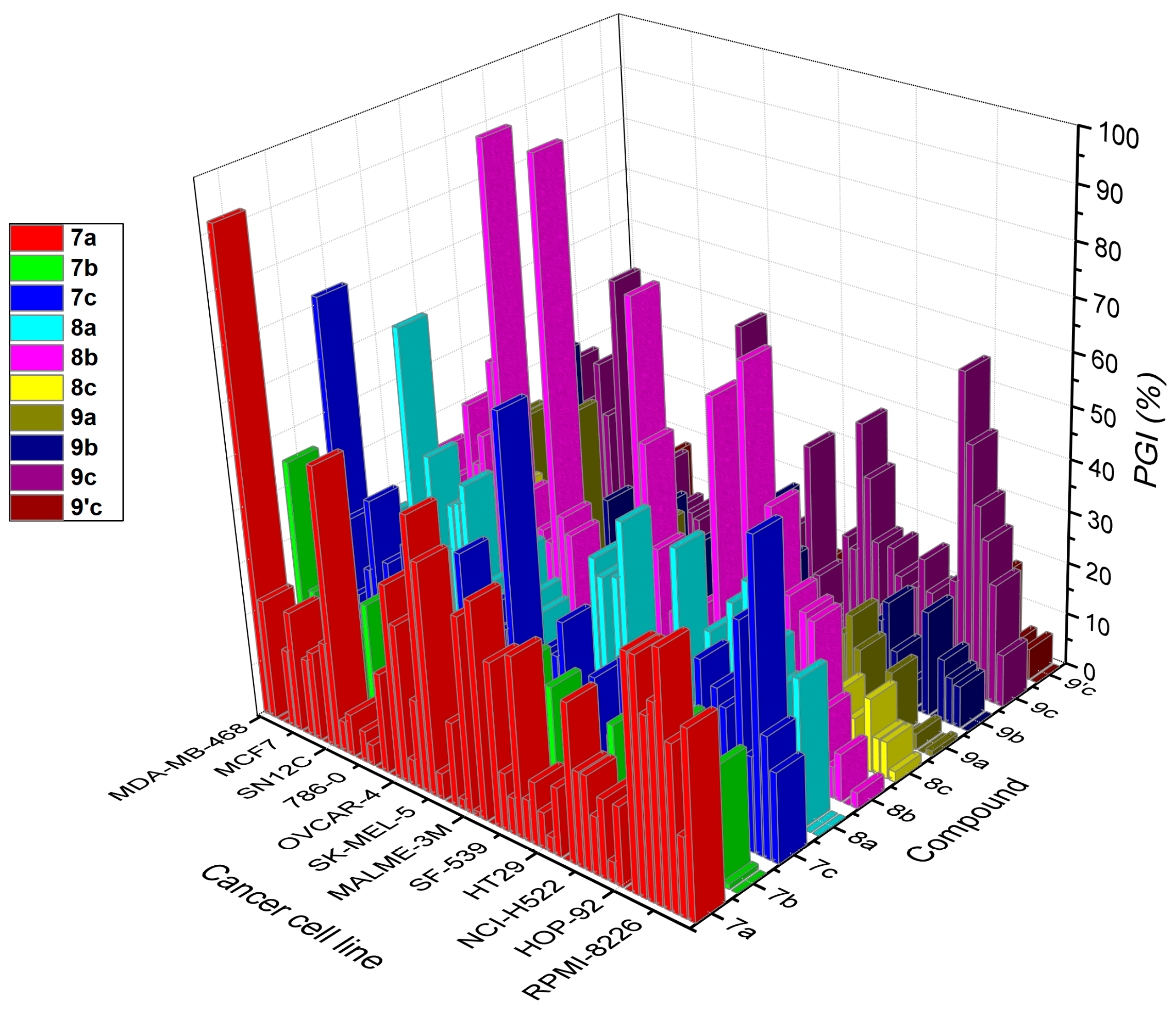
| Cell Type | Compound/Growth Inhibition Percent (PGI%) a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7a | 7b | 7c | 8a | 8b | 8c | 9a | 9b | 9c | 9′c | |
| Leukemia | ||||||||||
| CCRF–CEM | 35 | 0 | 17 | 0 | 3 | 2 | 1 | 0 | 10 | 0 |
| HL–60 (TB) | 15 | 1 | 23 | 0 | 0 | 7 | 0 | 8 | 23 | 6 |
| K–562 | 31 | 22 | 58 | 28 | 9 | 7 | 3 | 9 | 31 | 4 |
| MOLT–4 | 47 | 4 | 18 | 8 | 6 | 14 | 0 | 12 | 37 | 6 |
| RPMI–8226 | 37 | 0 | 42 | 0 | 16 | 0 | 0 | 0 | 48 | 0 |
| SR | 34 | 1 | 9 | 24 | 32 | 9 | 13 | 20 | 61 | 18 |
| Non–small Cell Lung Cancer | ||||||||||
| A549/ATCC | 44 | 23 | 25 | 31 | 33 | 0 | 0 | 0 | 21 | 0 |
| EKVX | 44 | 26 | 28 | 19 | 17 | 13 | 5 | 5 | 11 | 0 |
| HOP–62 | 0 | 2 | 3 | 42 | 35 | 0 | 0 | 2 | 10 | 0 |
| HOP–92 | 15 | 0 | 32 | 0 | 12 | 6 | 15 | 10 | 17 | 0 |
| NCI–H226 | 4 | 0 | 3 | 37 | 50 | 17 | 21 | 19 | 23 | 0 |
| NCI–H23 | 15 | 4 | 18 | 21 | 35 | 15 | 17 | 15 | 17 | 0 |
| NCI–H322M | 11 | 9 | 7 | 2 | 7 | 0 | 4 | 3 | 6 | 0 |
| NCI–460 | 18 | 2 | 16 | 30 | 74 | 0 | 0 | 0 | 18 | 0 |
| NCI–H522 | 18 | 7 | 30 | 9 | 0 | 4 | 4 | 5 | 23 | 0 |
| Colon Cancer | ||||||||||
| COLO 205 | 30 | 21 | 27 | 15 | 20 | 0 | 0 | 0 | 11 | 0 |
| HCC–2998 | 0 | 0 | 6 | 8 | 1 | 0 | 0 | 0 | 23 | 0 |
| HCT–116 | 13 | 0 | 8 | 43 | 66 | 2 | 11 | 0 | 35 | 2 |
| HCT–15 | 3 | 0 | 0 | 10 | 9 | 9 | 6 | 3 | 45 | 5 |
| HT29 | 7 | 0 | 26 | 0 | 27 | 1 | 0 | 0 | 20 | 4 |
| KM12 | 12 | 4 | 12 | 6 | 16 | 6 | 0 | 0 | 22 | 0 |
| SW–620 | 8 | 0 | 10 | 6 | 16 | 0 | 0 | 0 | 0 | 0 |
| CNS Cancer | ||||||||||
| SF–268 | 34 | 0 | 0 | 10 | 11 | 0 | 7 | 7 | 3 | 0 |
| SF–295 | 7 | 23 | 20 | 10 | 23 | 5 | 3 | 6 | 4 | 0 |
| SF–539 | 11 | 1 | 9 | 44 | 12 | 8 | 10 | 19 | 12 | 8 |
| SNB–19 | 31 | 15 | 14 | 33 | 34 | 0 | 43 | 31 | 37 | 0 |
| SNB–75 | 0 | 2 | 0 | 13 | 53 | 0 | 16 | 5 | 0 | 0 |
| U251 | 41 | 25 | 28 | 32 | 79 | 0 | 16 | 0 | 12 | 0 |
| Melanoma | ||||||||||
| LOX IMVI | 1 | 5 | 21 | 35 | 23 | 12 | 11 | 8 | 19 | 0 |
| MALME–3M | 37 | 1 | 22 | 11 | 29 | 8 | 7 | 10 | 16 | 7 |
| M14 | 2 | 0 | 13 | 0 | 0 | 0 | 4 | 13 | 19 | 0 |
| MDA–MB–435 | 16 | 0 | 0 | 1 | 7 | 0 | 0 | 2 | 7 | 2 |
| SK–MEL–2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SK–MEL–28 | 5 | 0 | 7 | 5 | 1 | 0 | 2 | 3 | 10 | 0 |
| SK–MEL–5 | 44 | 15 | 63 | 10 | 7 | 7 | 6 | 2 | 56 | 2 |
| UACC–257 | 52 | 7 | 22 | 0 | 1 | 0 | 0 | 0 | 10 | 0 |
| UACC–62 | 17 | 7 | 21 | 20 | 11 | 21 | 24 | 16 | 41 | 8 |
| Ovarian Cancer | ||||||||||
| IGROV1 | 5 | 0 | 5 | 23 | 30 | 6 | 0 | 0 | 1 | 0 |
| OVCAR–3 | 30 | 0 | 24 | 3 | 33 | 0 | 17 | 0 | 0 | 0 |
| OVCAR–4 | 37 | 0 | 18 | 30 | 99 | 6 | 0 | 12 | 12 | 0 |
| OVCAR–5 | 0 | 0 | 4 | 5 | 27 | 0 | 15 | 0 | 7 | 0 |
| OVCAR–8 | 19 | 15 | 33 | 21 | 29 | 0 | 10 | 21 | 14 | 0 |
| NCI/ADR–RES | 0 | 1 | 0 | 19 | 24 | 0 | 10 | 9 | 15 | 0 |
| SK–OV–3 | 4 | 0 | 3 | 22 | 0 | 0 | 0 | 0 | 3 | 0 |
| Renal Cancer | ||||||||||
| 786–0 | 6 | 7 | 5 | 0 | 34 | 0 | 11 | 0 | 15 | 2 |
| A498 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ACHN | 8 | 0 | 0 | 39 | 99 | 2 | 4 | 4 | 23 | 0 |
| CAKI–1 | 6 | 2 | 5 | 35 | 58 | 17 | 7 | 8 | 21 | 0 |
| RXF 393 | 54 | 0 | 1 | 34 | 18 | 0 | 40 | 0 | 20 | 0 |
| SN12C | 20 | 23 | 30 | 25 | 43 | 4 | 15 | 18 | 12 | 0 |
| TK–10 | 0 | 0 | 4 | 0 | 37 | 0 | 0 | 0 | 1 | 0 |
| UO–31 | 17 | 16 | 21 | 42 | 48 | 28 | 15 | 13 | 11 | 18 |
| Prostate Cancer | ||||||||||
| PC–3 | 15 | 8 | 25 | 12 | 8 | 15 | 3 | 9 | 57 | 3 |
| DU–145 | 0 | 0 | 0 | 5 | 7 | 0 | 0 | 0 | 0 | 0 |
| Breast Cancer | ||||||||||
| MCF7 | 23 | 9 | 36 | 65 | 20 | 33 | 14 | 11 | 29 | 10 |
| MDA–MB–231/ATCC | 15 | 0 | 22 | 24 | 31 | 20 | 36 | 9 | 39 | 0 |
| HS 578T | 0 | 9 | 11 | 18 | 37 | 6 | 36 | 44 | 16 | 0 |
| BT–549 | 0 | 21 | 16 | 3 | 0 | 0 | 5 | 0 | 0 | 0 |
| T–47D | 23 | 5 | 30 | 15 | 25 | 12 | 7 | 11 | 29 | 0 |
| MDA–MB–468 | 92 | 45 | 72 | 26 | 17 | 7 | 21 | 8 | 38 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zbancioc, G.; Mangalagiu, I.I.; Moldoveanu, C. The Effective Synthesis of New Benzoquinoline Derivatives as Small Molecules with Anticancer Activity. Pharmaceuticals 2024, 17, 52. https://doi.org/10.3390/ph17010052
Zbancioc G, Mangalagiu II, Moldoveanu C. The Effective Synthesis of New Benzoquinoline Derivatives as Small Molecules with Anticancer Activity. Pharmaceuticals. 2024; 17(1):52. https://doi.org/10.3390/ph17010052
Chicago/Turabian StyleZbancioc, Gheorghita, Ionel I. Mangalagiu, and Costel Moldoveanu. 2024. "The Effective Synthesis of New Benzoquinoline Derivatives as Small Molecules with Anticancer Activity" Pharmaceuticals 17, no. 1: 52. https://doi.org/10.3390/ph17010052
APA StyleZbancioc, G., Mangalagiu, I. I., & Moldoveanu, C. (2024). The Effective Synthesis of New Benzoquinoline Derivatives as Small Molecules with Anticancer Activity. Pharmaceuticals, 17(1), 52. https://doi.org/10.3390/ph17010052









