Chemical Adjustment of Fibrinolysis
Abstract
:1. Introduction
2. Fibrinolysis
3. Fibrinolytic System Pathologies and Targeting
3.1. Bleeding
3.2. Thromboses
4. In Silico Approaches to Identifying New Targets in the Fibrinolytic System
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivanciu, L.; Stalker, T.J. Spatiotemporal regulation of coagulation and platelet activation during the hemostatic response in vivo. J. Thromb. Haemost. 2015, 13, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.; Escaig, R.; Nicolai, L. Hemostasis without clot formation: How platelets guard the vasculature in inflammation, infection, and malignancy. Blood 2023, 142, 1413–1425. [Google Scholar] [CrossRef]
- Kaiser, R.; Escaig, R.; Kranich, J.; Hoffknecht, M.L.; Anjum, A.; Polewka, V.; Mader, M.; Hu, W.; Belz, L.; Gold, C.; et al. Procoagulant platelet sentinels prevent inflammatory bleeding through GPIIBIIIA and GPVI. Blood 2022, 140, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Seelam, S.; Bumrah, K.; Sherif, A.; Shrestha, U. Systemic thrombolysis with newer thrombolytics vs anticoagulation in acute intermediate risk pulmonary embolism: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 482. [Google Scholar] [CrossRef]
- Wyseure, T.; Cooke, E.J.; Declerck, P.J.; Behrendt, N.; Meijers, J.C.M.; von Drygalski, A.; Mosnier, L.O. Defective TAFI activation in hemophilia A mice is a major contributor to joint bleeding. Blood 2018, 132, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Broze, G.J.; Higuchi, D.A. Coagulation-dependent inhibition of fibrinolysis: Role of carboxypeptidase-U and the premature lysis of clots from hemophilic plasma. Blood 1996, 88, 3815–3823. [Google Scholar] [CrossRef] [PubMed]
- Izcovich, A.; Criniti, J.M.; Popoff, F.; Lu, L.; Wu, J.; Ageno, W.; Witt, D.M.; Jaff, M.R.; Schulman, S.; Manja, V.; et al. Thrombolytics for venous thromboembolic events: A systematic review with meta-analysis. Blood Adv. 2020, 4, 1539–1553. [Google Scholar] [CrossRef]
- Podoplelova, N.A.; Nechipurenko, D.Y.D.; Ignatova, A.A.A.; Sveshnikova, A.N.; MA, P.; Panteleev, M.A. Procoagulant Platelets: Mechanisms of Generation and Action. Hamostaseologie 2021, 41, 146–153. [Google Scholar] [CrossRef]
- Heemskerk, J.W.M.; Bevers, E.M.; Lindhout, T. Platelet activation and blood coagulation. Thromb. Haemost. 2002, 88, 186–193. [Google Scholar]
- Carson, S.D.; Brozna, J.P. The role of tissue factor in the production of thrombin. Blood Coagul. Fibrinolysis 1993, 4, 281–292. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Mechanisms of fibrin polymerization and clinical implications. Blood 2013, 121, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Rijken, D.C.; Hoylaerts, M.; Collen, D. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J. Biol. Chem. 1982, 257, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Zhalyalov, A.S.; Panteleev, M.A.; Gracheva, M.A.; Ataullakhanov, F.I.; Shibeko, A.M. Co-ordinated spatial propagation of blood plasma clotting and fibrinolytic fronts. PLoS ONE 2017, 12, e0180668. [Google Scholar] [CrossRef]
- Levin, E.G.; Marzec, U.; Anderson, J.; Harker, L.A. Thrombin stimulates tissue plasminogen activator release from cultured human endothelial cells. J. Clin. Investig. 1984, 74, 1988–1995. [Google Scholar] [CrossRef]
- Collet, J.P.; Lesty, C.; Montalescot, G.; Weisel, J.W. Dynamic changes of fibrin architecture during fibrin formation and intrinsic fibrinolysis of fibrin-rich clots. J. Biol. Chem. 2003, 278, 21331–21335. [Google Scholar] [CrossRef]
- Bajzar, L.; Manuel, R.; Nesheim, M.E. Purification and characterization of TAFI, a thrombin-activable fibrinolysis inhibitor. J. Biol. Chem. 1995, 270, 14477–14484. [Google Scholar] [CrossRef] [PubMed]
- Kotova, Y.N.; Podoplelova, N.A.; Obydennyy, S.I.; Kostanova, E.A.; Ryabykh, A.A.; Demyanova, A.S.; Biriukova, M.I.; Rosenfeld, M.A.; Sokolov, A.V.; Chambost, H.; et al. Binding of Coagulation Factor XIII Zymogen to Activated Platelet Subpopulations: Roles of Integrin alphaIIbbeta3 and Fibrinogen. Thromb. Haemost. 2019, 119, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Whyte, C.S.; Mitchell, J.L.; Mutch, N.J. Platelet-Mediated Modulation of Fibrinolysis. Semin. Thromb. Hemost. 2017, 43, 115–128. [Google Scholar] [CrossRef]
- Saes, J.L.; Schols, S.E.M.; van Heerde, W.L.; Nijziel, M.R. Hemorrhagic disorders of fibrinolysis: A clinical review. J. Thromb. Haemost. 2018, 16, 1498–1509. [Google Scholar] [CrossRef]
- Woodman, R.; Harker, L. Bleeding Complications Associated With Cardiopulmonary Bypass. Blood 1990, 76, 1680–1697. [Google Scholar] [CrossRef]
- Coffey, A.; Pittmam, J.; Halbrook, H.; Fehrenbacher, J.; Beckman, D.; Hormuth, D.; Moorman, D.; Edwards, J.R. The use of tranexamic acid to reduce postoperative bleeding following cardiac surgery: A double-blind randomized trial. Am. Surg. 1995, 61, 566–568. [Google Scholar] [PubMed]
- Forsgren, M.; Råden, B.; Israelsson, M.; Larsson, K.; Hedén, L.O. Molecular cloning and characterization of a full-length cDNA clone for human plasminogen. FEBS Lett. 1987, 213, 254–260. [Google Scholar] [CrossRef]
- Tordai, H.; Bányai, L.; Patthy, L. The PAN module: The N-terminal domains of plasminogen and hepatocyte growth factor are homologous with the apple domains of the prekallikrein family and with a novel domain found in numerous nematode proteins. FEBS Lett. 1999, 461, 63–67. [Google Scholar] [CrossRef] [PubMed]
- THORSEN, S. The mechanism of plasminogen activation and the variability of the fibrin effector during tissue-type plasminogen activator-mediated fibrinolysis. Ann. N. Y. Acad. Sci. 1992, 667, 52–63. [Google Scholar] [CrossRef]
- Krishnamurti, C.; Vukelja, S.J.; Alving, B.M. Inhibitory effects of lysine analogues on t-PA induced whole blood clot lysis. Thromb. Res. 1994, 73, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Casati, V.; Guzzon, D.; Oppizzi, M.; Cossolini, M.; Torri, G.; Calori, G.; Alfieri, O. Hemostatic effects of aprotinin, tranexamic acid and epsilon-aminocaproic acid in primary cardiac surgery. Ann. Thorac. Surg. 1999, 68, 2252–2256. [Google Scholar] [CrossRef]
- Martin, K.; Knorr, J.; Breuer, T.; Gertler, R.; MacGuill, M.; Lange, R.; Tassani, P.; Wiesner, G. Seizures After Open Heart Surgery: Comparison of ε-Aminocaproic Acid and Tranexamic Acid. J. Cardiothorac. Vasc. Anesth. 2011, 25, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Fillingham, Y.A.; Ramkumar, D.B.; Jevsevar, D.S.; Yates, A.J.; Shores, P.; Mullen, K.; Bini, S.A.; Clarke, H.D.; Schemitsch, E.; Johnson, R.L.; et al. The Efficacy of Tranexamic Acid in Total Knee Arthroplasty: A Network Meta-Analysis. J. Arthroplasty 2018, 33, 3090–3098.e1. [Google Scholar] [CrossRef]
- Boylan, J.F.; Klinck, J.R.; Sandler, A.N.; Arellano, R.; Greig, P.D.; Nierenberg, H.; Roger, S.L.; Glynn, M.F.X. Tranexamic acid reduces blood loss, transfusion requirements, and coagulation factor use in primary orthotopic liver transplantation. Anesthesiology 1996, 85, 1043–1048. [Google Scholar] [CrossRef]
- Karkouti, K.; Beattie, W.S.; Dattilo, K.M.; McCluskey, S.A.; Ghannam, M.; Hamdy, A.; Wijeysundera, D.N.; Fedorko, L.; Yau, T.M. A propensity score case-control comparison of aprotinin and tranexamic acid in high-transfusion-risk cardiac surgery. Transfusion 2006, 46, 327–338. [Google Scholar] [CrossRef]
- Ker, K.; Edwards, P.; Perel, P.; Shakur, H.; Roberts, I. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ 2012, 344, e3054. [Google Scholar] [CrossRef] [PubMed]
- Bargehr, C.; Knöfler, R.; Streif, W. Treatment of Inherited Platelet Disorders: Current Status and Future Options. Hamostaseologie 2023, 43, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Chornenki, N.L.J.; Um, K.J.; Mendoza, P.A.; Samienezhad, A.; Swarup, V.; Chai-Adisaksopha, C.; Siegal, D.M. Risk of venous and arterial thrombosis in non-surgical patients receiving systemic tranexamic acid: A systematic review and meta-analysis. Thromb. Res. 2019, 179, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Lawati, K.A.; Sharif, S.; Al Maqbali, S.; Rimawi, H.A.; Petrosoniak, A.; Belley-Cote, E.P.; Sharma, S.V.; Morgenstern, J.; Fernando, S.M.; Owen, J.J.; et al. Efficacy and safety of tranexamic acid in acute traumatic brain injury: A systematic review and meta-analysis of randomized-controlled trials. Intensive Care Med. 2021, 47, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Xin, Y.; Chen, X.; Song, Z.; He, Z.; Zhao, Y. Tranexamic Acid in Cerebral Hemorrhage: A Meta-Analysis and Systematic Review. CNS Drugs 2019, 33, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Daglas, M.; Galle, A.; Draxler, D.F.; Ho, H.; Liu, Z.; Sashindranath, M.; Medcalf, R.L. Sex-dependent effects of tranexamic acid on blood-brain barrier permeability and the immune response following traumatic brain injury in mice. J. Thromb. Haemost. 2020, 18, 2658–2671. [Google Scholar] [CrossRef]
- Takada, A.; Sugawara, Y.; Takada, Y. Enhancement of the activation of Glu-plasminogen by urokinase in the simultaneous presence of tranexamic acid or fibrin. Haemostasis 1989, 19, 26–31. [Google Scholar] [CrossRef]
- Wu, T.B.; Orfeo, T.; Moore, H.B.; Sumislawski, J.J.; Cohen, M.J.; Petzold, L.R. Computational model of tranexamic acid on urokinase mediated fibrinolysis. PLoS ONE 2020, 15, e0233640. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Sawamura, A.; Gando, S.; Jesmin, S.; Naito, S.; Ieko, M. A low TAFI activity and insufficient activation of fibrinolysis by both plasmin and neutrophil elastase promote organ dysfunction in disseminated intravascular coagulation associated with sepsis. Thromb. Res. 2012, 130, 906–913. [Google Scholar] [CrossRef]
- Moore, H.B.; Moore, E.E.; Gonzalez, E.; Chapman, M.P.; Chin, T.L.; Silliman, C.C.; Banerjee, A.; Sauaia, A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: The spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J. Trauma Acute Care Surg. 2014, 77, 811–817. [Google Scholar] [CrossRef]
- Okamoto, K.; Tamura, T.; Sawatsubashi, Y. Sepsis and disseminated intravascular coagulation. J. Intensive Care 2016, 4, 23. [Google Scholar] [CrossRef]
- Wada, H. Disseminated intravascular coagulation. Clin. Chim. Acta 2004, 344, 13–21. [Google Scholar] [CrossRef]
- Hou, P.C.; Filbin, M.R.; Wang, H.; Ngo, L.; Aird, W.C.; Shapiro, N.I.; Wang, H.; Huang, D.T.; Angus, D.C.; Kellum, J.A.; et al. Endothelial Permeability and Hemostasis in Septic Shock: Results From the ProCESS Trial. Chest 2017, 152, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Voss, R.; Matthias, F.R.; Borkowski, G.; Reitz, D. Activation and inhibition of fibrinolysis in septic patients in an internal intensive care unit. Br. J. Haematol. 1990, 75, 99–105. [Google Scholar] [CrossRef] [PubMed]
- De Fouw, N.J.; Van Hinsbergh, V.W.M.; De Jong, Y.F.; Haverkate, F.; Bertina, R.M. The interaction of activated protein C and thrombin with the plasminogen activator inhibitor released from human endothelial cells. Thromb. Haemost. 1987, 57, 176–182. [Google Scholar] [CrossRef]
- Fourrier, F.; Chopin, C.; Goudemand, J.; Hendrycx, S.; Caron, C.; Rime, A.; Marey, A.; Lestavel, P. Septic Shock, Multiple Organ Failure, and Disseminated Intravascular Coagulation: Compared Patterns of Antithrombin III, Protein C, and Protein S Deficiencies. Chest 1992, 101, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Bachler, M.; Bösch, J.; Stürzel, D.P.; Hell, T.; Giebl, A.; Ströhle, M.; Klein, S.J.; Schäfer, V.; Lehner, G.F.; Joannidis, M.; et al. Impaired fibrinolysis in critically ill COVID-19 patients. Br. J. Anaesth. 2021, 126, 590–598. [Google Scholar] [CrossRef]
- Juhan-Vague, I.; Valadier, J.; Alessi, M.C.; Aillaud, M.F.; Ansaldi, J.; Philip-Joet, C.; Holvoet, P.; Serradimigni, A.; Collen, D. Deficient t-PA release and elevated PA inhibitor levels in patients with spontaneous or recurrent deep venous thrombosis. Thromb. Haemost. 1987, 57, 67–72. [Google Scholar] [CrossRef]
- Nilsson, I.M.; Ljungn, H.; Tengborn, L. Two different mechanisms in patients with venous thrombosis and defective fibrinolysis: Low concentration of plasminogen activator or increased concentration of plasminogen activator inhibitor. Br. Med. J. (Clin. Res. Ed). 1985, 290, 1453. [Google Scholar] [CrossRef]
- Oolofesson, B.O.; Dahlèn, G.; Nilsson, T.K. Evidence for increased levels of plasminogen activator inhibitor and tissue plasminogen activator in plasma of patients with angiographically verified coronary artery disease. Eur. Heart J. 1989, 10, 77–82. [Google Scholar] [CrossRef]
- Zuo, Y.; Warnock, M.; Harbaugh, A.; Yalavarthi, S.; Gockman, K.; Zuo, M.; Madison, J.A.; Knight, J.S.; Kanthi, Y.; Lawrence, D.A. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci. Rep. 2021, 11, 1580. [Google Scholar] [CrossRef] [PubMed]
- Folsom, A.R.; Cushman, M.; Heckbert, S.R.; Rosamond, W.D.; Aleksic, N. Prospective study of fibrinolytic markers and venous thromboembolism. J. Clin. Epidemiol. 2003, 56, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Van De Craen, B.; Declerck, P.J.; Gils, A. The Biochemistry, Physiology and Pathological roles of PAI-1 and the requirements for PAI-1 inhibition in vivo. Thromb. Res. 2012, 130, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Carrell, R.; Travis, J. α1-Antitrypsin and the serpins: Variation and countervariation. Trends Biochem. Sci. 1985, 10, 20–24. [Google Scholar] [CrossRef]
- Vaughan, D.E. PAI-1 and atherothrombosis. J. Thromb. Haemost. 2005, 3, 1879–1883. [Google Scholar] [CrossRef]
- Izuhara, Y.; Takahashi, S.; Nangaku, M.; Takizawa, S.; Ishida, H.; Kurokawa, K.; Van Ypersele De Strihou, C.; Hirayama, N.; Miyata, T. Inhibition of Plasminogen Activator Inhibitor-1. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 672–677. [Google Scholar] [CrossRef]
- Sillen, M.; Declerck, P.J. Targeting PAI-1 in Cardiovascular Disease: Structural Insights Into PAI-1 Functionality and Inhibition. Front. Cardiovasc. Med. 2020, 7, 622473. [Google Scholar] [CrossRef]
- Yamaoka, N.; Kawano, Y.; Izuhara, Y.; Miyata, T.; Meguro, K. Structure–Activity Relationships of New 2-Acylamino-3-thiophenecarboxylic Acid Dimers as Plasminogen Activator Inhibitor-1 Inhibitors. Chem. Pharm. Bull. 2010, 58, 615–619. [Google Scholar] [CrossRef]
- Izuhara, Y.; Yamaoka, N.; Kodama, H.; Dan, T.; Takizawa, S.; Hirayama, N.; Meguro, K.; Van Ypersele De Strihou, C.; Miyata, T. A novel inhibitor of plasminogen activator inhibitor-1 provides antithrombotic benefits devoid of bleeding effect in nonhuman primates. J. Cereb. Blood Flow Metab. 2010, 30, 904–912. [Google Scholar] [CrossRef]
- Noguchi, R.; Kaji, K.; Namisaki, T.; Moriya, K.; Kawaratani, H.; Kitade, M.; Takaya, H.; Aihara, Y.; Douhara, A.; Asada, K.; et al. Novel oral plasminogen activator inhibitor-1 inhibitor TM5275 attenuates hepatic fibrosis under metabolic syndrome via suppression of activated hepatic stellate cells in rats. Mol. Med. Rep. 2020, 22, 2948–2956. [Google Scholar] [CrossRef]
- Jeong, B.Y.; Uddin, M.J.; Park, J.H.; Lee, J.H.; Lee, H.B.; Miyata, T.; Ha, H. Novel Plasminogen Activator Inhibitor-1 Inhibitors Prevent Diabetic Kidney Injury in a Mouse Model. PLoS ONE 2016, 11, e0157012. [Google Scholar] [CrossRef]
- Yamaoka, N.; Murano, K.; Kodama, H.; Maeda, A.; Dan, T.; Nakabayashi, T.; Miyata, T.; Meguro, K. Identification of novel plasminogen activator inhibitor-1 inhibitors with improved oral bioavailability: Structure optimization of N-acylanthranilic acid derivatives. Bioorg. Med. Chem. Lett. 2018, 28, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Bishop, N.; Li, Z.; Cipolla, M.J. Inhibition of PAI (plasminogen activator inhibitor)-1 improves brain collateral perfusion and injury after acute ischemic stroke in aged hypertensive rats. Stroke 2018, 49, 1969–1976. [Google Scholar] [CrossRef]
- Piao, L.; Jung, I.; Huh, J.Y.; Miyata, T.; Ha, H. A novel plasminogen activator inhibitor-1 inhibitor, TM5441, protects against high-fat diet-induced obesity and adipocyte injury in mice. Br. J. Pharmacol. 2016, 173, 2622–2632. [Google Scholar] [CrossRef]
- Pelisch, N.; Dan, T.; Ichimura, A.; Sekiguchi, H.; Vaughan, D.E.; Van Ypersele De Strihou, C.; Miyata, T. Plasminogen Activator Inhibitor-1 Antagonist TM5484 Attenuates Demyelination and Axonal Degeneration in a Mice Model of Multiple Sclerosis. PLoS ONE 2015, 10, e0124510. [Google Scholar] [CrossRef] [PubMed]
- Dunster, J.L.; Panteleev, M.A.; Gibbins, J.M.; Sveshnikova, A.N. Mathematical Techniques for Understanding Platelet Regulation and the Development of New Pharmacological Approaches. Methods Mol. Biol. 2018, 1812, 255–279. [Google Scholar] [CrossRef] [PubMed]
- Shibeko, A.M.; Panteleev, M.A. Untangling the complexity of blood coagulation network: Use of computational modelling in pharmacology and diagnostics. Brief. Bioinform. 2016, 17, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.P.; Wang, J.; Zhang, Y.; Wang, L.; Jiang, X. Quantitative Systems Pharmacology for Rare Disease Drug Development. J. Pharm. Sci. 2023, 112, 2313–2320. [Google Scholar] [CrossRef]
- Shibeko, A.M.; Lobanova, E.S.; Panteleev, M.A.; Ataullakhanov, F.I. Blood flow controls coagulation onset via the positive feedback of factor VII activation by factor Xa. BMC Syst. Biol. 2010, 4, 5. [Google Scholar] [CrossRef]
- Panteleev, M.A.; Balandina, A.N.; Lipets, E.N.; Ovanesov, M.V.; Ataullakhanov, F.I. Task-oriented modular decomposition of biological networks: Trigger mechanism in blood coagulation. Biophys. J. 2010, 98, 1751–1761. [Google Scholar] [CrossRef]
- Rauch, N.; Rukhlenko, O.S.; Kolch, W.; Kholodenko, B.N. MAPK kinase signalling dynamics regulate cell fate decisions and drug resistance. Curr. Opin. Struct. Biol. 2016, 41, 151–158. [Google Scholar] [CrossRef]
- Panteleev, M.A.; Dashkevich, N.M.; Ataullakhanov, F.I. Hemostasis and thrombosis beyond biochemistry: Roles of geometry, flow and diffusion. Thromb. Res. 2015, 136, 699–711. [Google Scholar] [CrossRef]
- Shibeko, A.M.; Chopard, B.; Hoekstra, A.G.; Panteleev, M.A. Redistribution of TPA Fluxes in the Presence of PAI-1 Regulates Spatial Thrombolysis. Biophys. J. 2020, 119, 638–651. [Google Scholar] [CrossRef]
- Huisse, M.G.; Ajzenberg, N.; Feldman, L.; Guillin, M.C.; Steg, P.G. Microparticle-linked tissue factor activity and increased thrombin activity play a potential role in fibrinolysis failure in ST-segment elevation myocardial infarction. Thromb. Haemost. 2009, 101, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Tzekaki, E.E.; Geromichalos, G.; Lavrentiadou, S.N.; Tsantarliotou, M.P.; Pantazaki, A.A.; Papaspyropoulos, A. Oleuropein is a natural inhibitor of PAI-1-mediated proliferation in human ER-/PR- breast cancer cells. Breast Cancer Res. Treat. 2021, 186, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, X.; Shen, J.; Sun, Q.; Guo, X.; Yang, M.; Leng, J. Integrative identification of human serpin PAI-1 inhibitors from Dracaena dragon blood and molecular implications for inhibitor-induced PAI-1 allosterism. Biotechnol. Appl. Biochem. 2022, 69, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gu, B.; Xu, X.Y. In Silico Study of Different Thrombolytic Agents for Fibrinolysis in Acute Ischemic Stroke. Pharmaceutics 2023, 15, 797. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Piebalgs, A.; Huang, Y.; Longstaff, C.; Hughes, A.D.; Chen, R.; Thom, S.A.; Xu, X.Y. Mathematical Modelling of Intravenous Thrombolysis in Acute Ischaemic stroke: Effects of Dose Regimens on Levels of Fibrinolytic Proteins and Clot Lysis Time. Pharmaceutics 2019, 11, 111. [Google Scholar] [CrossRef] [PubMed]
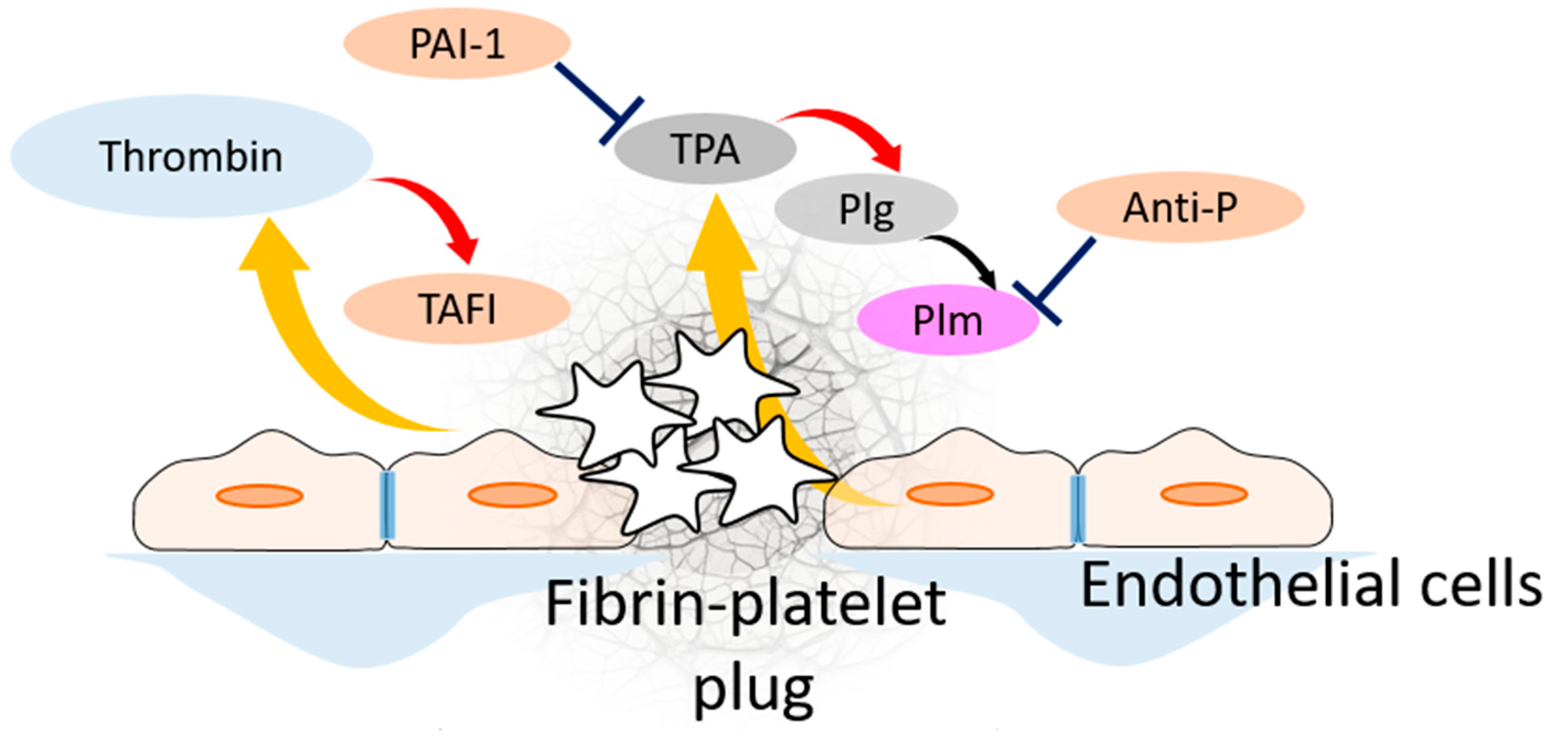

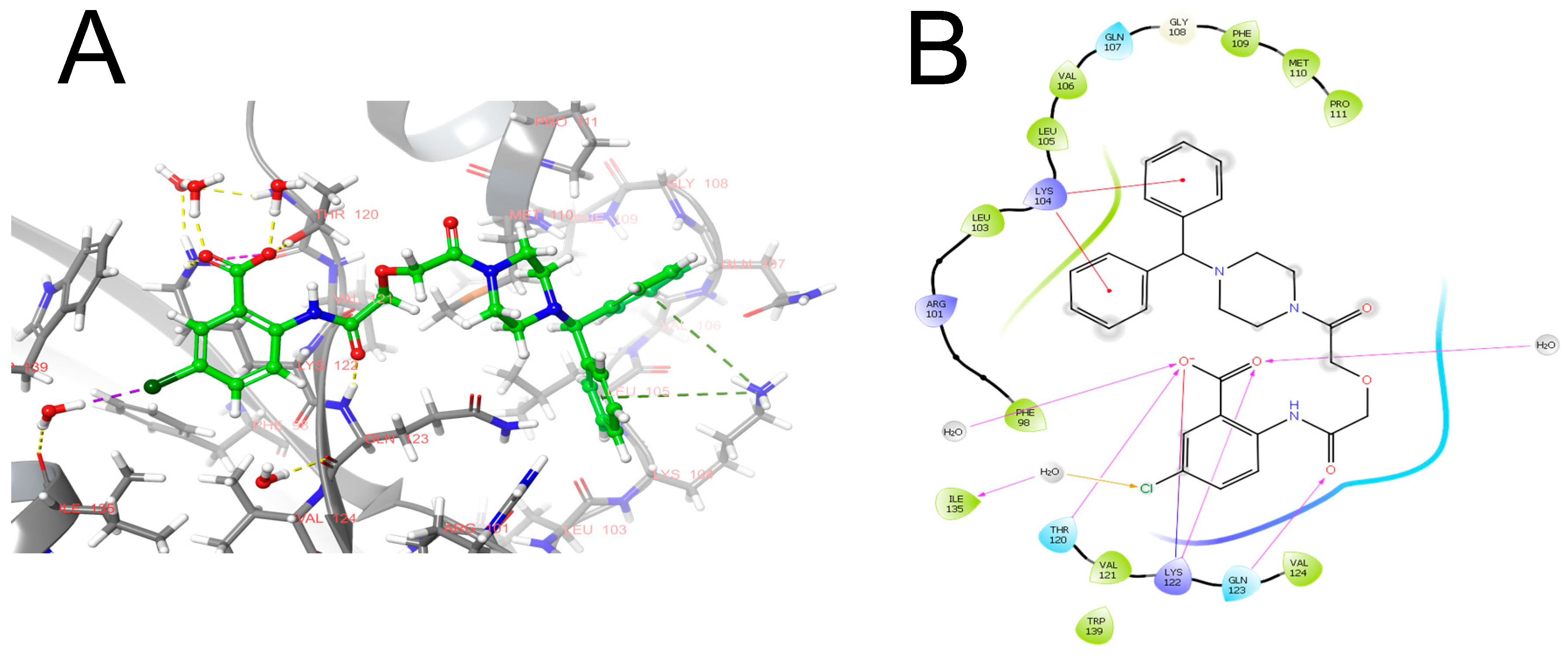
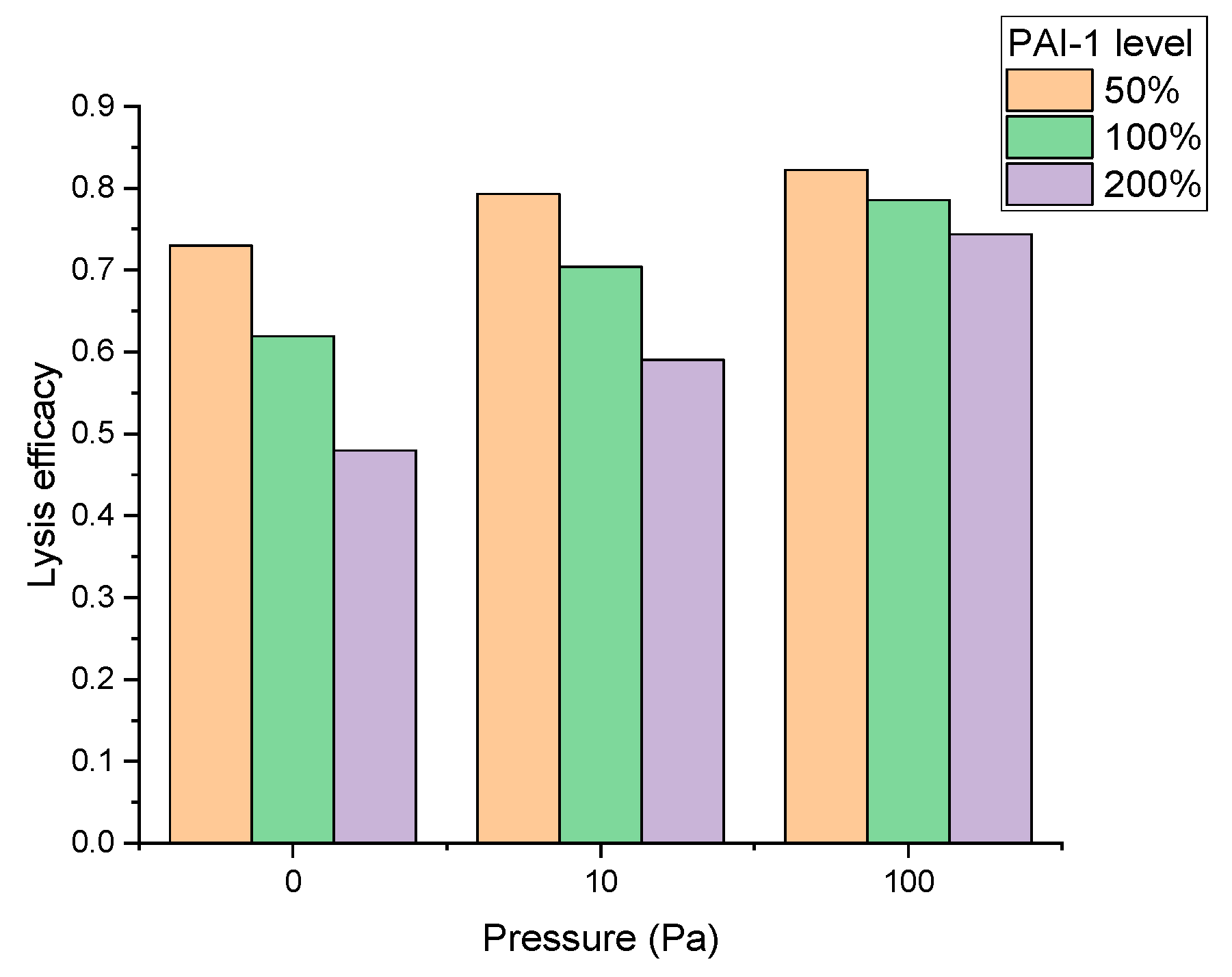
| Participant of Fibrinolytic System | Its Source | It Is Activated by | Its Target | It Is Inhibited by |
|---|---|---|---|---|
| TPA | Endothelial cells | plasmin | Activates plasminogen | PAI-1 |
| UPA | Present in blood plasma | Activates plasminogen | PAI-1 | |
| PAI-1 | Platelets, present in blood plasma | Inhibits TPA and UPA | Activated protein C, autoinactivation | |
| Plasmin(ogen) | Present in blood plasma | UPA, TPA, TPA bound with fibrin | Cleaves fibrin | Antiplasmin, a2-macroglobulin |
| Fibrin(ogen) | Present in blood plasma | Thrombin | ||
| TAFI | Present in blood plasma | Thrombin–thrombomodulin complex | Cleaves C-terminal lysines on fibrin molecules, which prevents TPA and plasmin(ogen) binding | autoinactivation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibeko, A.M.; Ilin, I.S.; Podoplelova, N.A.; Sulimov, V.B.; Panteleev, M.A. Chemical Adjustment of Fibrinolysis. Pharmaceuticals 2024, 17, 92. https://doi.org/10.3390/ph17010092
Shibeko AM, Ilin IS, Podoplelova NA, Sulimov VB, Panteleev MA. Chemical Adjustment of Fibrinolysis. Pharmaceuticals. 2024; 17(1):92. https://doi.org/10.3390/ph17010092
Chicago/Turabian StyleShibeko, Alexey M., Ivan S. Ilin, Nadezhda A. Podoplelova, Vladimir B. Sulimov, and Mikhail A. Panteleev. 2024. "Chemical Adjustment of Fibrinolysis" Pharmaceuticals 17, no. 1: 92. https://doi.org/10.3390/ph17010092
APA StyleShibeko, A. M., Ilin, I. S., Podoplelova, N. A., Sulimov, V. B., & Panteleev, M. A. (2024). Chemical Adjustment of Fibrinolysis. Pharmaceuticals, 17(1), 92. https://doi.org/10.3390/ph17010092







