The Endocannabinoid System of the Nervous and Gastrointestinal Systems Changes after a Subnoxious Cisplatin Dose in Male Rats
Abstract
:1. Introduction
2. Results
2.1. Body Weight and Intakes
2.2. Semiquantitative Analysis of Gastrointestinal Motor Function
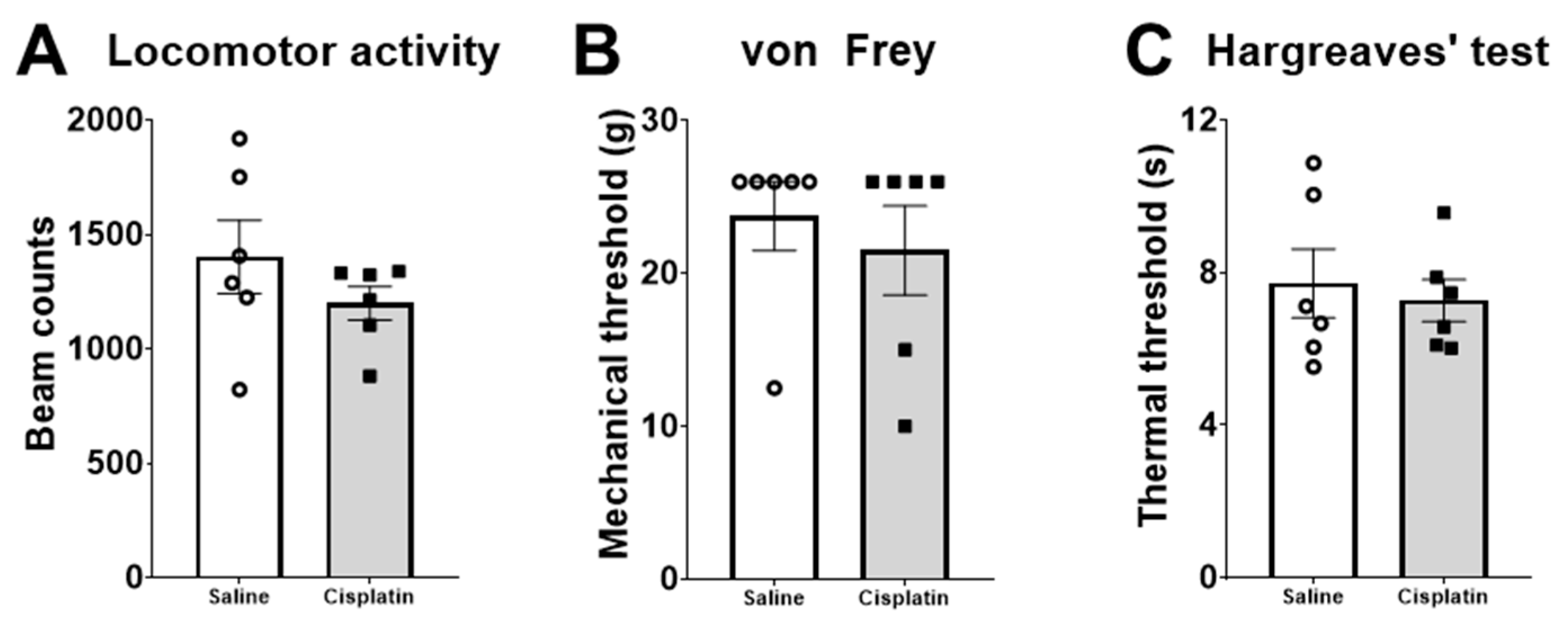
2.3. Behaviour
2.4. Weight and Macroscopic Analysis of Organs
2.5. Levels of Endocannabinoid Ligands and Related N-Acylethanolamines
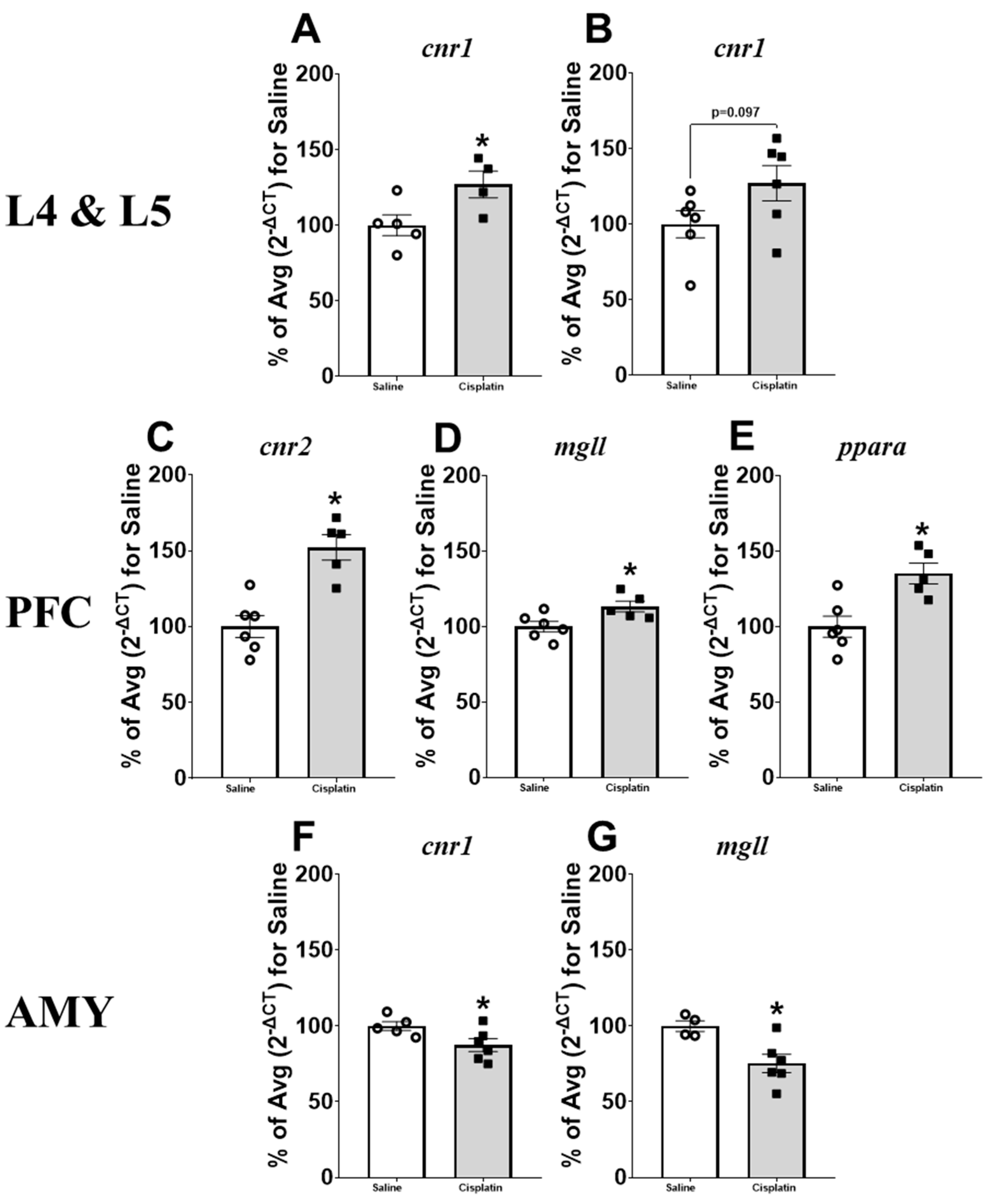
2.6. Gene Expression Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drug Preparation and Dose Selection
4.3. Experiment Outline
4.4. Radiographic Analysis of Gastrointestinal Motility
4.5. Macroscopic Analysis and Weight of Organs of Interest
4.6. Behavioural Tests
4.6.1. Von Frey Test
4.6.2. Hargreaves’ Test
4.6.3. Locomotor Activity
4.7. Liquid Chromatography Coupled to Tandem Mass Spectrometry (LC-MS/MS)
4.7.1. Tissue Lipid Extraction
4.7.2. Plasma Lipid Extraction
4.7.3. Standard Curve
4.7.4. LC-MS/MS
4.8. Real-Time PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, M.B.; Haldar Neer, A.H. Chemotherapy. Cancer Treat. Res. 2023, 185, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Bagues, A.; López-Tofiño, Y.; Llorente-Berzal, Á.; Abalo, R. Cannabinoid Drugs against Chemotherapy-Induced Adverse Effects: Focus on Nausea/Vomiting, Peripheral Neuropathy and Chemofog in Animal Models. Behav. Pharmacol. 2022, 33, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Blanton, H.L.; Brelsfoard, J.; DeTurk, N.; Pruitt, K.; Narasimhan, M.; Morgan, D.J.; Guindon, J. Cannabinoids: Current and Future Options to Treat Chronic and Chemotherapy-Induced Neuropathic Pain. Drugs 2019, 79, 969–995. [Google Scholar] [CrossRef] [PubMed]
- Sleurs, C.; Deprez, S.; Emsell, L.; Lemiere, J.; Uyttebroeck, A. Chemotherapy-Induced Neurotoxicity in Pediatric Solid Non-CNS Tumor Patients: An Update on Current State of Research and Recommended Future Directions. Crit. Rev. Oncol. Hematol. 2016, 103, 37–48. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Shahid, F.; Farooqui, Z.; Khan, F. Cisplatin-Induced Gastrointestinal Toxicity: An Update on Possible Mechanisms and on Available Gastroprotective Strategies. Eur. J. Pharmacol. 2018, 827, 49–57. [Google Scholar] [CrossRef]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Rabik, C.A.; Dolan, M.E. Molecular Mechanisms of Resistance and Toxicity Associated with Platinating Agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef]
- FDA; CDER CISplatin Injection. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/018057s092lbl.pdf (accessed on 3 May 2024).
- Breglio, A.M.; Rusheen, A.E.; Shide, E.D.; Fernandez, K.A.; Spielbauer, K.K.; McLachlin, K.M.; Hall, M.D.; Amable, L.; Cunningham, L.L. Cisplatin Is Retained in the Cochlea Indefinitely Following Chemotherapy. Nat. Commun. 2017, 8, 1654. [Google Scholar] [CrossRef]
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Avilia, G.; Bifulco, M.; Laezza, C. Cannabinoids: Therapeutic Use in Clinical Practice. Int. J. Mol. Sci. 2022, 23, 3344. [Google Scholar] [CrossRef] [PubMed]
- Massa, F.; Storr, M.; Lutz, B. The Endocannabinoid System in the Physiology and Pathophysiology of the Gastrointestinal Tract. J. Mol. Med. 2005, 83, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Cuddihey, H.; MacNaughton, W.K.; Sharkey, K.A. Role of the Endocannabinoid System in the Regulation of Intestinal Homeostasis. Cell Mol. Gastroenterol. Hepatol. 2022, 14, 947–963. [Google Scholar] [CrossRef] [PubMed]
- Osafo, N.; Yeboah, O.K.; Antwi, A.O. Endocannabinoid System and Its Modulation of Brain, Gut, Joint and Skin Inflammation. Mol. Biol. Rep. 2021, 48, 3665–3680. [Google Scholar] [CrossRef]
- Sharkey, K.A.; Wiley, J.W. The Role of the Endocannabinoid System in the Brain-Gut Axis. Gastroenterology 2016, 151, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef]
- Okine, B.N.; Rea, K.; Olango, W.M.; Price, J.; Herdman, S.; Madasu, M.K.; Roche, M.; Finn, D.P.; Finn, D.P. A Role for PPARα in the Medial Prefrontal Cortex in Formalin-Evoked Nociceptive Responding in Rats. Br. J. Pharmacol. 2014, 171, 1462–1471. [Google Scholar] [CrossRef]
- Hansen, H.; Vana, V. Non-Endocannabinoid N-Acylethanolamines and 2-Monoacylglycerols in the Intestine. Pharmacol. Br. J. Pharmacol. 2019, 176, 1443. [Google Scholar] [CrossRef]
- Nayebi, A.M.; Sharifi, H.; Ramadzani, M.; Rezazadeh, H. Effect of Acute and Chronic Administration of Carbamazepine on Cisplatin-Induced Hyperalgesia in Rats. Jundishapur J. Nat. Pharm. Prod. 2012, 7, 27–30. [Google Scholar] [CrossRef]
- Ben Ayed, W.; Ben Said, A.; Hamdi, A.; Mokrani, A.; Masmoudi, Y.; Toukabri, I.; Limayem, I.; Yahyaoui, Y. Toxicity, risk factors and management of cisplatin-induced toxicity: A prospective study. J. Oncol. Pharm. Pract. 2020, 26, 1621–1629. [Google Scholar] [CrossRef]
- Cabezos, P.A.; Vera, G.; Martín-Fontelles, M.I.; Fernández-Pujol, R.; Abalo, R. Cisplatin-Induced Gastrointestinal Dysmotility Is Aggravated after Chronic Administration in the Rat. Comparison with Pica. Neurogastroenterol. Motil. 2010, 22, 797-e225. [Google Scholar] [CrossRef] [PubMed]
- Cabezos, P.A.; Vera, G.; Castillo, M.; Fernández-Pujol, R.; Martín, M.I.; Abalo, R. Radiological Study of Gastrointestinal Motor Activity after Acute Cisplatin in the Rat. Temporal Relationship with Pica. Auton. Neurosci. 2008, 141, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Alonso Domínguez, T.; Civera Andrés, M.; Santiago Crespo, J.A.; García Malpartida, K.; Botella Romero, F. Digestive Toxicity in Cancer Treatments. Bibliographic Review. Influence on Nutritional Status. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2023, 70, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Leung, N. Cisplatin Nephrotoxicity: A Review of the Literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef]
- Motwani, S.S.; Kaur, S.S.; Kitchlu, A. Cisplatin Nephrotoxicity: Novel Insights Into Mechanisms and Preventative Strategies. Semin. Nephrol. 2022, 42, 151341. [Google Scholar] [CrossRef]
- Martín-Ruíz, M.; Uranga, J.A.; Mosinska, P.; Fichna, J.; Nurgali, K.; Martín-Fontelles, M.I.; Abalo, R. Alterations of Colonic Sensitivity and Gastric Dysmotility after Acute Cisplatin and Granisetron. Neurogastroenterol. Motil. 2019, 31, e13499. [Google Scholar] [CrossRef]
- Uranga, J.A.; García-Martínez, J.M.; García-Jiménez, C.; Vera, G.; Martín-Fontelles, M.I.; Abalo, R. Alterations in the Small Intestinal Wall and Motor Function after Repeated Cisplatin in Rat. Neurogastroenterol. Motil. 2017, 29, e13047. [Google Scholar] [CrossRef]
- Hassan, M.A.M.; Wahdan, S.A.; El-Naga, R.N.; Abdelghany, T.M.; El-Demerdash, E. Ondansetron Attenuates Cisplatin-Induced Behavioral and Cognitive Impairment through Downregulation of NOD-like Receptor Inflammasome Pathway. Toxicol. Appl. Pharmacol. 2024, 485, 116875. [Google Scholar] [CrossRef]
- Yoshiya, T.; Mimae, T.; Ito, M.; Sasada, S.; Tsutani, Y.; Satoh, K.; Masuda, T.; Miyata, Y.; Hattori, N.; Okada, M. Prospective, Randomized, Cross-over Pilot Study of the Effects of Rikkunshito, a Japanese Traditional Herbal Medicine, on Anorexia and Plasma-Acylated Ghrelin Levels in Lung Cancer Patients Undergoing Cisplatin-Based Chemotherapy. Investig. New Drugs 2020, 38, 485–492. [Google Scholar] [CrossRef]
- Rapoport, B.L. Delayed Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Incidence, and Current Management. Front. Pharmacol. 2017, 8, 19. [Google Scholar] [CrossRef]
- Andrews, P.L.R.; Horn, C.C. Signals for Nausea and Emesis: Implications for Models of Upper Gastrointestinal Diseases. Auton. Neurosci. 2006, 125, 100–115. [Google Scholar] [CrossRef] [PubMed]
- De Jonghe, B.C.; Horn, C.C. Chemotherapy-Induced Pica and Anorexia Are Reduced by Common Hepatic Branch Vagotomy in the Rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R756–R765. [Google Scholar] [CrossRef]
- Liu, Y.L.; Malik, N.M.; Sanger, G.J.; Andrews, P.L.R. Ghrelin Alleviates Cancer Chemotherapy-Associated Dyspepsia in Rodents. Cancer Chemother. Pharmacol. 2006, 58, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Sanger, G.J.; Andrews, P.L.R. A History of Drug Discovery for Treatment of Nausea and Vomiting and the Implications for Future Research. Front. Pharmacol. 2018, 9, 913. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Yoshinaga, N.; Waku, K. Rapid generation of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, in rat brain after decapitation. Neurosci. Lett. 2001, 297, 175–178. [Google Scholar] [CrossRef]
- Brose, S.A.; Golovko, S.A.; Golovko, M.Y. Brain 2-Arachidonoylglycerol Levels Are Dramatically and Rapidly Increased Under Acute Ischemia-Injury Which Is Prevented by Microwave Irradiation. Lipids 2016, 51, 487–495. [Google Scholar] [CrossRef]
- Abalo, R.; Chen, C.; Vera, G.; Fichna, J.; Thakur, G.A.; López-Pérez, A.E.; Makriyannis, A.; Martín-Fontelles, M.I.; Storr, M. In Vitro and Non-Invasive in Vivo Effects of the Cannabinoid-1 Receptor Agonist AM841 on Gastrointestinal Motor Function in the Rat. Neurogastroenterol. Motil. 2015, 27, 1721–1735. [Google Scholar] [CrossRef]
- Keenan, C.M.; Storr, M.A.; Thakur, G.A.; Wood, J.T.; Wager-Miller, J.; Straiker, A.; Eno, M.R.; Nikas, S.P.; Bashashati, M.; Hu, H.; et al. AM841, a Covalent Cannabinoid Ligand, Powerfully Slows Gastrointestinal Motility in Normal and Stressed Mice in a Peripherally Restricted Manner. Br. J. Pharmacol. 2015, 172, 2406–2418. [Google Scholar] [CrossRef]
- Vera, G.; Castillo, M.; Cabezos, P.A.; Chiarlone, A.; Martín, M.I.; Gori, A.; Pasquinelli, G.; Barbara, G.; Stanghellini, V.; Corinaldesi, R.; et al. Enteric neuropathy evoked by repeated cisplatin in the rat. Neurogastroenterol. Motil. 2011, 23, 370–378. [Google Scholar] [CrossRef]
- Capasso, R.; Matias, I.; Lutz, B.; Borrelli, F.; Capasso, F.; Marsicano, G.; Mascolo, N.; Petrosino, S.; Monory, K.; Valenti, M.; et al. Fatty Acid Amide Hydrolase Controls Mouse Intestinal Motility in Vivo. Gastroenterology 2005, 129, 941–951. [Google Scholar] [CrossRef]
- Feng, C.C.; Yan, X.J.; Chen, X.; Wang, E.M.; Liu, Q.; Zhang, L.Y.; Chen, J.; Fang, J.Y.; Chen, S.L. Vagal Anandamide Signaling via Cannabinoid Receptor 1 Contributes to Luminal 5-HT Modulation of Visceral Nociception in Rats. Pain 2014, 155, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- López-Tofiño, Y.; Barragán del Caz, L.F.; Benítez-Álvarez, D.; Molero-Mateo, P.; Nurgali, K.; Vera, G.; Bagües, A.; Abalo, R. Contractility of Isolated Colonic Smooth Muscle Strips from Rats Treated with Cancer Chemotherapy: Differential Effects of Cisplatin and Vincristine. Front. Neurosci. 2023, 17, 1304609. [Google Scholar] [CrossRef] [PubMed]
- Vera, G.; Cabezos, P.A.; Martín, M.I.; Abalo, R. Characterization of Cannabinoid-Induced Relief of Neuropathic Pain in a Rat Model of Cisplatin-Induced Neuropathy. Pharmacol. Biochem. Behav. 2013, 105, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Khasabova, I.A.; Yao, X.; Paz, J.; Lewandowski, C.T.; Lindberg, A.E.; Coicou, L.; Burlakova, N.; Simone, D.A.; Seybold, V.S. JZL184 Is Anti-Hyperalgesic in a Murine Model of Cisplatin-Induced Peripheral Neuropathy. Pharmacol. Res. 2014, 90, 67–75. [Google Scholar] [CrossRef]
- Guindon, J.; Lai, Y.; Takacs, S.M.; Bradshaw, H.B.; Hohmann, A.G. Alterations in Endocannabinoid Tone Following Chemotherapy-Induced Peripheral Neuropathy: Effects of Endocannabinoid Deactivation Inhibitors Targeting Fatty-Acid Amide Hydrolase and Monoacylglycerol Lipase in Comparison to Reference Analgesics Following Cisplatin Treatment. Pharmacol. Res. 2013, 67, 94–109. [Google Scholar] [CrossRef]
- Mitrirattanakul, S.; Ramakul, N.; Guerrero, A.V.; Matsuka, Y.; Ono, T.; Iwase, H.; Mackie, K.; Faull, K.F.; Spigelman, I. Site-Specific Increases in Peripheral Cannabinoid Receptors and Their Endogenous Ligands in a Model of Neuropathic Pain. Pain 2006, 126, 102–114. [Google Scholar] [CrossRef]
- McLaughlin, R.J.; Gobbi, G. Cannabinoids and Emotionality: A Neuroanatomical Perspective. Neuroscience 2012, 204, 134–144. [Google Scholar] [CrossRef]
- Neugebauer, V. Amygdala Pain Mechanisms. Handb. Exp. Pharmacol. 2015, 227, 261–284. [Google Scholar] [CrossRef]
- Woodhams, S.G.; Chapman, V.; Finn, D.P.; Hohmann, A.G.; Neugebauer, V. The Cannabinoid System and Pain. Neuropharmacology 2017, 124, 105–120. [Google Scholar] [CrossRef]
- Kiritoshi, T.; Ji, G.; Neugebauer, V. Rescue of Impaired MGluR5-Driven Endocannabinoid Signaling Restores Prefrontal Cortical Output to Inhibit Pain in Arthritic Rats. J. Neurosci. 2016, 36, 837–850. [Google Scholar] [CrossRef]
- Vuic, B.; Milos, T.; Tudor, L.; Konjevod, M.; Nikolac Perkovic, M.; Jazvinscak Jembrek, M.; Nedic Erjavec, G.; Svob Strac, D. Cannabinoid CB2 Receptors in Neurodegenerative Proteinopathies: New Insights and Therapeutic Potential. Biomedicines 2022, 10, 3000. [Google Scholar] [CrossRef]
- Kasatkina, L.A.; Rittchen, S.; Sturm, E.M. Neuroprotective and Immunomodulatory Action of the Endocannabinoid System under Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 5431. [Google Scholar] [CrossRef] [PubMed]
- Mounier, N.M.; Abdel-Maged, A.E.S.; Wahdan, S.A.; Gad, A.M.; Azab, S.S. Chemotherapy-Induced Cognitive Impairment (CICI): An Overview of Etiology and Pathogenesis. Life Sci. 2020, 258, 118071. [Google Scholar] [CrossRef] [PubMed]
- Ongnok, B.; Chattipakorn, N.; Chattipakorn, S.C. Doxorubicin and Cisplatin Induced Cognitive Impairment: The Possible Mechanisms and Interventions. Exp. Neurol. 2020, 324, 113118. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, Y.; Gargouri, B.; Kebieche, M.; Fetoui, H. Naringin Abrogates Cisplatin-Induced Cognitive Deficits and Cholinergic Dysfunction Through the Down-Regulation of AChE Expression and INOS Signaling Pathways in Hippocampus of Aged Rats. J. Mol. Neurosci. 2015, 56, 349–362. [Google Scholar] [CrossRef] [PubMed]
- La Porta, C.; Bura, S.A.; Negrete, R.; Maldonado, R. Involvement of the Endocannabinoid System in Osteoarthritis Pain. Eur. J. Neurosci. 2014, 39, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, I.; Hauer, D.; Huge, V.; Vogeser, M.; Campolongo, P.; Chouker, A.; Thiel, M.; Schelling, G. Enhanced Anandamide Plasma Levels in Patients with Complex Regional Pain Syndrome Following Traumatic Injury: A Preliminary Report. Eur. Surg. Res. 2009, 43, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, I.; Schelling, G.; Eisner, C.; Richter, H.P.; Krauseneck, T.; Vogeser, M.; Hauer, D.; Campolongo, P.; Chouker, A.; Beyer, A.; et al. Anandamide and Neutrophil Function in Patients with Fibromyalgia. Psychoneuroendocrinology 2008, 33, 676–685. [Google Scholar] [CrossRef]
- Kurlyandchik, I.; Lauche, R.; Tiralongo, E.; Warne, L.N.; Schloss, J. Plasma and interstitial levels of endocannabinoids and N-acylethanolamines in patients with chronic widespread pain and fibromyalgia: A systematic review and meta-analysis. Pain Rep. 2022, 7, e1045. [Google Scholar] [CrossRef]
- Cupini, L.M.; Bari, M.; Battista, N.; Argirò, G.; Finazzi-Agrò, A.; Calabresi, P.; MacCarrone, M. Biochemical Changes in Endocannabinoid System Are Expressed in Platelets of Female but Not Male Migraineurs. Cephalalgia 2006, 26, 277–281. [Google Scholar] [CrossRef]
- Torimoto, K.; Ueda, T.; Gotoh, D.; Kano, K.; Miyake, M.; Nakai, Y.; Hori, S.; Morizawa, Y.; Onishi, K.; Shimizu, T.; et al. Serum Anandamide and Lipids Associated with Linoleic Acid Can Distinguish Interstitial Cystitis/Bladder Pain Syndrome from Overactive Bladder: An Exploratory Study. LUTS Low. Urin. Tract Symptoms 2023, 15, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Bashashati, M.; Leishman, E.; Bradshaw, H.; Sigaroodi, S.; Tatro, E.; Bright, T.; McCallum, R.; Sarosiek, I. Plasma Endocannabinoids and Cannabimimetic Fatty Acid Derivatives Are Altered in Gastroparesis: A Sex- and Subtype-Dependent Observation. Neurogastroenterol. Motil. 2021, 33, e13961. [Google Scholar] [CrossRef]
- Llorente-Berzal, A.; McGowan, F.; Gaspar, J.C.; Rea, K.; Roche, M.; Finn, D.P. Sexually Dimorphic Expression of Fear-Conditioned Analgesia in Rats and Associated Alterations in the Endocannabinoid System in the Periaqueductal Grey. Neuroscience 2022, 480, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.M.; Downey, L.; Conboy, M.; Finn, D.P.; Roche, M. Alterations in the Endocannabinoid System in the Rat Valproic Acid Model of Autism. Behav. Brain Res. 2013, 249, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Concannon, R.M.; Okine, B.N.; Finn, D.P.; Dowd, E. Upregulation of the cannabinoid CB2 receptor in environmental and viral inflammation-driven rat models of Parkinson’s disease. Exp. Neurol. 2016, 283 Pt A, 204–212. [Google Scholar] [CrossRef]
- Moore, D.; McGabe, G.; Craig, B. Introduction to the Practice of Statistics, 6th ed.; Greenberg, B., Ed.; WH Freeman and Company: Austin, TX, USA, 2009. [Google Scholar]





| Saline | Cisplatin | ||
|---|---|---|---|
| Weight of animals at sacrifice (g) | 400.7 ± 11.6 | 366.0 ± 6.7 * | |
| Weight of organ at sacrifice (g) | Stomach | 6.72 ± 1.17 | 3.10 ± 0.18 * |
| Full small intestine | 12.09 ± 0.54 | 9.51 ± 0.41 * | |
| Empty small intestine | 8.92 ± 0.30 | 7.51 ± 0.22 * | |
| Milking | 3.01 ± 0.33 | 1.87 ± 0.26 * | |
| Caecum | 5.45 ± 0.27 | 5.17 ± 0.46 | |
| Full colorectum | 4.36 ± 0.26 | 4.19 ± 0.40 | |
| Empty colorectum | 2.23 ± 0.09 | 2.04 ± 0.11 | |
| Kidneys | 2.54 ± 0.11 | 2.80 ± 0.20 | |
| Area and length of organs at sacrifice | Stomach (cm2) | 7.05 ± 0.84 | 4.45 ± 0.28 * |
| Caecum (cm2) | 6.54 ± 0.39 | 5.83 ± 0.57 | |
| Small intestine (cm) | 58.53 ± 1.63 | 58.01 ± 2.13 | |
| Colorectum (cm) | 13.10 ± 0.36 | 12.06 ± 0.44 | |
| AEA (nmol/g) Saline vs. Cisplatin | 2-AG (nmol/g) Saline vs. Cisplatin | PEA (nmol/g) Saline vs. Cisplatin | OEA (nmol/g) Saline vs. Cisplatin | ||
|---|---|---|---|---|---|
| Gastrointestinal tissue | Antrum | 0.025 ± 0.009 vs. 0.016 ± 0.008 | 74.87 ± 36.73 vs. 32.73 ± 16.41 | 0.111 ± 0.020 vs. 0.113 ± 0.006 | 0.736 ± 0.069 vs. 0.621 ± 0.028 |
| Fundus | 0.026 ± 0.012 vs. 0.011 ± 0.002 | 4.16 ± 1.35 vs. 3.22 ± 0.39 | 1.872 ± 0.412 vs. 2.044 ± 0.476 | 3.326 ± 0.482 vs. 3.477 ± 0.273 | |
| Ileum | 0.046 ± 0.005 vs. 0.068 ± 0.008 * | 90.19 ± 12.01 vs. 82.57 ± 10.70 | 0.974 ± 0.061 vs. 0.947 ± 0.039 | 5.435 ± 0.855 vs. 3.987 ± 0.261 | |
| Distal colon | 0.028 ± 0.003 vs. 0.023 ± 0.003 | 23.03 ± 1.87 vs. 19.82 ± 3.00 | 0.489 ± 0.053 vs. 0.437 ± 0.045 | 3.31 ± 0.32 vs. 2.69 ± 0.40 | |
| Central nervous tissue | Prefrontal cortex | 0.014 ± 0.005 vs. 0.017 ± 0.001 | 12.05 ± 3.12 vs. 11.39 ± 2.03 | 0.061 ± 0.017 vs. 0.060 ± 0.008 | 0.087 ± 0.018 vs. 0.088 ± 0.006 |
| Periaqueductal grey | 0.016 ± 0.002 vs. 0.015 ± 0.003 | 19.17 ± 3.84 vs. 26.97 ± 5.47 | 0.736 ± 0.105 vs. 0.626 ± 0.113 | 0.485 ± 0.051 vs. 0.444 ± 0.074 | |
| Amygdala | 0.027 ± 0.002 vs. 0.022 ± 0.002 | 29.05 ± 2.27 vs. 26.40 ± 4.07 | 0.091 ± 0.007 vs. 0.097 ± 0.008 | 0.134 ± 0.006 vs. 0.133 ± 0.008 | |
| AEA (pmol/mL) Saline vs. Cisplatin | 2-AG (pmol/mL) Saline vs. Cisplatin | PEA (pmol/mL) Saline vs. Cisplatin | OEA (pmol/mL) Saline vs. Cisplatin | ||
| Plasma | 0.760 ± 0.245 vs. 1.113 ± 0.158 | 0.878 ± 0.250 vs. 2.269 ± 0.526 | 9.18 ± 2.32 vs. 12.33 ± 0.66 | 15.22 ± 4.11 vs. 23.34 ± 1.51 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Tofiño, Y.; Hopkins, M.A.; Bagues, A.; Boullon, L.; Abalo, R.; Llorente-Berzal, Á. The Endocannabinoid System of the Nervous and Gastrointestinal Systems Changes after a Subnoxious Cisplatin Dose in Male Rats. Pharmaceuticals 2024, 17, 1256. https://doi.org/10.3390/ph17101256
López-Tofiño Y, Hopkins MA, Bagues A, Boullon L, Abalo R, Llorente-Berzal Á. The Endocannabinoid System of the Nervous and Gastrointestinal Systems Changes after a Subnoxious Cisplatin Dose in Male Rats. Pharmaceuticals. 2024; 17(10):1256. https://doi.org/10.3390/ph17101256
Chicago/Turabian StyleLópez-Tofiño, Yolanda, Mary A. Hopkins, Ana Bagues, Laura Boullon, Raquel Abalo, and Álvaro Llorente-Berzal. 2024. "The Endocannabinoid System of the Nervous and Gastrointestinal Systems Changes after a Subnoxious Cisplatin Dose in Male Rats" Pharmaceuticals 17, no. 10: 1256. https://doi.org/10.3390/ph17101256
APA StyleLópez-Tofiño, Y., Hopkins, M. A., Bagues, A., Boullon, L., Abalo, R., & Llorente-Berzal, Á. (2024). The Endocannabinoid System of the Nervous and Gastrointestinal Systems Changes after a Subnoxious Cisplatin Dose in Male Rats. Pharmaceuticals, 17(10), 1256. https://doi.org/10.3390/ph17101256








