Antioxidant and Pro-Oxidant Properties of Selected Clinically Applied Antibiotics: Therapeutic Insights
Abstract
:1. Introduction
2. Results
3. Discussion
Limitations
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Measurement of Antioxidant and Pro-Oxidant Properties Using the Modified DPPH/FRAP Method
4.3. Molinspiration Calculation
- Octanol/water partition coefficient (LogP): calculated as a sum of fragment-based contributions and correction factors;
- Topological polar surface area (TPSA): calculated based on methodology of (https://molinspiration.com/ accessed on 17 September 2024) with summing fragment contributions fitted to the 3D volume of a training set of about 12,000, mostly drug-like, molecules. These geometries were optimized using the semi-empirical AM1 method;
- Rule of five: Most “drug-like” molecules have logP ≤ 5, molecular weight ≤ 500, ≤10 hydrogen bond acceptors, and ≤5 hydrogen bond donors;
- Number of rotatable bonds (nrotb): measures molecular flexibility and is a good descriptor of oral bioavailability and defined as any single non-ring bond bound to a non-terminal heavy atom, excluding amide C-N bonds due to their high rotational energy barrier.
4.4. Swiss Target Prediction Calculation
4.5. Optimization of Antibiotics Structure in Vacuum for Parameters Calculation
4.6. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Collaborating Centre for Drug Statistics Methodology. Purpose of the ATC/DDD System. Archived from the Original on 14 January 2010. Available online: https://atcddd.fhi.no/atc_ddd_methodology/purpose_of_the_atc_ddd_system/ (accessed on 6 July 2021).
- Reffat, N.; Schwei, R.J.; Griffin, M.; Pop-Vicas, A.; Schulz, L.T.; Michael, S.; Pulia, M.S. A Scoping Review of Bacterial Resistance Among Inpatients Amidst the COVID-19 Pandemic. J. Glob. Antimicrob. Resist. 2024, 38, 49–65. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Nunez, C.; Cesaro, A.; Hancock, R.E.W. Antibiotic failure: Beyond antimicrobial resistance. Drug Resist. Updat. 2023, 71, 101012. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Long, J.; Edelkamp, P.; Knafl, M.; Ahmed, S.; Khawaja, F.; Nastoupil, L.J.; Manzano, J.G.; Mulanovich, V.; Prabhakaran, S.; et al. Prediction of Mortality Following Cytokine Storm in Patients with Hematological Malignancies and COVID-19 Infections. Blood 2022, 140, 10907–10908. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Nick Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Lin, J.Y.; Zhu, Z.C.; Zhu, J.; Chen, L.; Hong Du, H. Antibiotic heteroresistance in Klebsiella pneumoniae: Definition, detection methods, mechanisms, and combination therapy. Microbiol. Res. 2024, 283, 127701. [Google Scholar] [CrossRef]
- Orthobullets. Available online: https://www.orthobullets.com/basic-science/9059/antibiotic-classification-and-mechanism (accessed on 15 June 2024).
- Saleem, N.; Ryckaert, F.; Snow, T.A.C.; Satta, G.; Singer, M.; Arulkumaran, N. Mortality and clinical cure rates for pneumonia: A systematic review, meta-analysis, and trial sequential analysis of randomized control trials comparing bactericidal and bacteriostatic antibiotic treatments. Clin. Microbiol. Infect. 2022, 28, 936–945. [Google Scholar] [CrossRef]
- Guillouzo, A.; Guguen-Guillouzo, C. Antibiotics-induced oxidative stress. Curr. Opin. Toxicol. 2020, 20–21, 23–28. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Wierzbowski, J.; Cottarel, G.; Collins, J.J. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell 2008, 135, 679–690. [Google Scholar] [CrossRef]
- Foti, J.J.; Devadoss, B.; Winkler, J.A.; Collins, J.J.; Walker, G.C. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science 2012, 336, 315–319. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X. Contribution of oxidative damage to antimicrobial lethality. Antimicrob. Agents Chemother. 2009, 53, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.; Coenye, T. The Role of Reactive Oxygen Species in Antibiotic—Mediated Killing of Bacteria. Trends Microbiol. 2017, 25, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Slauch, J.M. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol. 2011, 80, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Delghandi, P.S.; Soleimani, V.; Bazzaz, B.S.F.; Hosseinzadeh, H. A review on oxidant and antioxidant effects of antibacterial agents: Impacts on bacterial cell death and division and therapeutic effects or adverse reactions in humans. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 2667–2686. [Google Scholar] [CrossRef]
- Barreiro, C.; García-Estrada, C. Proteomics and Penicillium chrysogenum: Unveiling the secrets behind penicillin production. J. Proteom. 2019, 198, 119–131. [Google Scholar] [CrossRef]
- Pathak, A.; Nowell, R.W.; Wilson, C.G.; Ryan, M.J.; Barraclough, T.G. Comparative genomics of Alexander Fleming’s original Penicillium isolate (IMI 15378) reveals sequence divergence of penicillin synthesis genes. Sci. Rep. 2020, 10, 15705. [Google Scholar] [CrossRef]
- Pandey, N.; Cascella, M. Beta Lactam Antibiotics. In Stat Pearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Lin, X.; Kuck, U. Cephalosporins as key lead generation beta-lactam antibiotics. Appl. Microbiol. Biotechnol. 2022, 106, 8007–8020. [Google Scholar] [CrossRef]
- Tan, Q.; Qiu, J.; Luo, X.; Zhang, Y.; Liu, Y.; Chen, Y.; Yuan, J.; Liao, W. Progress in one-pot bioconversion of cephalosporin C to 7-Aminocephalosporanic acid. Curr. Pharm. Biotechnol. 2018, 19, 30–42. [Google Scholar] [CrossRef]
- Pickens, L.B.; Tang, Y. Oxytetracycline biosynthesis. J. Biol. Chem. 2010, 285, 27509–27515. [Google Scholar] [CrossRef]
- Jukes, T.H. Some historical notes on chlortetracycline. Rev. Infect. Dis. 1985, 7, 702–707. [Google Scholar] [CrossRef]
- Ni, H.; Mohsin, A.; Guo, M.; Chu, J.; Zhuang, Y. Two-component system AfrQ1Q2 involved in oxytetracycline biosynthesis of Streptomyces rimosus M4018 in a medium-dependent manner. J. Biosci. Bioeng. 2020, 129, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What we learn from cells culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Macáková, K.; Mladenka, P.; Filipsky, T.; Říha, M.; Jahodár, L.; Trejtnar, F.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. Iron reduction potentiates hydroxyl radical formation only in flavonols. Food Chem. 2012, 135, 2584–2592. [Google Scholar] [CrossRef] [PubMed]
- Sotler, R.; Poljšak, B.; Dahmane, R.; Jukić, T.; Jukić, D.P.; Rotim, C.; Trebše, P.; Starc, A. Prooxidant activities of antioxidants and their impact on health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef]
- Kutscher, A.H.; Lane, S.L. The clinical toxicity of antibacterial drugs: A comparative review of the literature based on 96,075 cases treated with the sulfonamides and antibiotics. Oral Surg. Oral Med. Oral Path. 1952, 5, 347–352. [Google Scholar] [CrossRef]
- Maliar, T.; Maliarová, M.; Blažková, M.; Kunštek, M.; Uváčková, Ľ.; Viskupičová, J.; Purdešová, A.; Beňovič, P. Simultaneously Determined Antioxidant and Pro-Oxidant Activity of Randomly Selected Plant Secondary Metabolites and Plant Extracts. Molecules 2023, 28, 6890. [Google Scholar] [CrossRef]
- Singh, S.; Khanna, D.; Kalra, S. Minocycline and Doxycycline: More Than Antibiotics. Curr. Mol. Pharmacol. 2021, 14, 1046–1065. [Google Scholar] [CrossRef]
- Pournaras, S.; Koumaki, V.; Spanakis, N.; Gennimata, V.; Tsakris, A. Current perspectives on tigecycline resistance in Enterobacteriaceae: Susceptibility testing issues and mechanisms of resistance. Int. J. Antimicrob. Agents 2016, 48, 11–18. [Google Scholar] [CrossRef]
- Vairo, C.; Vidal, M.V.; Hernandez, R.M.; Igartua, M.; Villullas, S. Colistin- and amikacin-loaded lipid-based drug delivery systems for resistant gram-negative lung and wound bacterial infections. Int. J. Pharm. 2023, 635, 122739. [Google Scholar] [CrossRef]
- Yang, M.; Yuan, F.; Guo, Y.; Wang, S. Efficacy of adding azithromycin to antibiotic prophylaxis in caesarean delivery: A meta-analysis and systematic review. Int. J. Antimicrob. Agents. 2022, 59, 106533. [Google Scholar] [CrossRef]
- Thottathil, S.; Puttaiahgowda, M.Y.; Kanth, S. Advancement and future perspectives on ampicillin-loaded antimicrobial polymers—A review. J. Drug Del. Scien. Technol. 2023, 81, 104227. [Google Scholar] [CrossRef]
- Sodhi, K.K.; Kumar, M.; Singh, D.K. Insight into the amoxicillin resistance, ecotoxicity, and remediation strategies. J. Water Proc, Eng. 2021, 39, 101858. [Google Scholar] [CrossRef]
- Pinart, M.; Kranz, J.; Jensen, K.; Proctor, T.; Naber, K.; Kunath, F.; Wagenlehner, F.; Schmidt, S. Optimal dosage and duration of pivmecillinam treatment for uncomplicated lower urinary tract infections: A systematic review and meta-analysis. Int. J. Infect. Dis. 2017, 58, 96–109. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, M.I.; Brahim, I.; Hisham, N.; Aladdin, R.; Mohammed, H.; Bahaaeldin, A. Recent updates of carbapenem antibiotics. Eur. J. Med. Chem. 2017, 131, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, D.E.; Tomas, M.; Miller, D.; Tomcsanyi, L.; Signorella, C.; Montepara, C.A.; Covvey, J.R.; Guarascio, A.J. Cephalosporins for the treatment of uncomplicated pyelonephritis: A systematic review. J. Am. Pharm. Assoc. 2023, 63, 1461–1471. [Google Scholar] [CrossRef]
- Verhoef, J.; Gillissen, A. Resistant Haemophilus influenzae in community-acquired respiratory tract infections: A role for cefixime. Int. J. Antimicrob. Agents 2003, 21, 501–509. [Google Scholar] [CrossRef]
- Falagas, M.E.; Trigkidis, K.K.; Vardakas, K.Z. Inhaled antibiotics beyond aminoglycosides, polymyxins and aztreonam: A systematic review. Int. J. Antimicrob. Agents 2015, 45, 221–233. [Google Scholar] [CrossRef]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Møller, N.; Muller, A.E.; Paccaud, J.P.; Mouton, J.W. Oral amoxicillin and amoxicillin–clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef]
- Ahmed, F.; Mahmud, M.T.; Naher, S.; Rana, M.J.; Ara, R.; Ur-Rahman, K.M.S. Effectiveness of Colistin in carbapenem resistant Acinetobacter baumannii—A systematic review. Health Sci. Rev. 2023, 8, 100113. [Google Scholar] [CrossRef]
- Tajik, S.; Shokri, F.; Rostamnezhad, M.; Khoshnood, S.; Mortazavi, S.M.; Mohammad Sholeh, M.; Kouhsari, M. Fosfomycin: A look at its various aspects. Gene Rep. 2020, 19, 100640. [Google Scholar] [CrossRef]
- Bobba, S.; Khader, S.A. Rifampicin drug resistance and host immunity in tuberculosis: More than meets the eye. Trends Imunnol. 2023, 44, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Zadeh, F.A.; Ebrahimzadeh, F.; Jafari-Sales, A.; Gholami, S. Globally Vibrio cholera antibiotics resistance to RNA and DNA effective antibiotics: A systematic review and meta-analysis. Microb. Pathog. 2022, 172, 105514. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Singh, R. Antibiotic resistance in food lactic acid bacteria—A review. Int. J. Food Microbiol. 2005, 105, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.K.; Singh, D.K. Insight into the fluoroquinolone resistance, sources, ecotoxicity, and degradation with special emphasis on ciprofloxacin. J. Water Proc. Eng. 2021, 43, 102218. [Google Scholar] [CrossRef]
- Dwyer, P.C.; Hogan, M.J.; Stewart, I. An integrated critical thinking framework for the 21st century. Think. Skills Creat. 2014, 12, 43–52. [Google Scholar] [CrossRef]
- Vaishampayan, A.; Grohmann, E. Antimicrobials Functioning through ROS-Mediated Mechanisms: Current Insights. Microorganisms 2021, 10, 61. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foti, J.R.; Bray, C.B.; Thompson, J.N.; Allgood, F.S. Know thy self, know thy leader: Contributions of a pattern-oriented approach to examining leader perceptions. Leadership Q. 2012, 23, 702–717. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, C.; Jang, H.J.; Kim, B.O.; Bae, H.W.; Chung, I.Y.; Kim, E.S.; Cho, Y.H. Antibacterial strategies inspired by the oxidative stress and response networks. J. Microbiol. 2019, 57, 203–212. [Google Scholar] [CrossRef]
- Sampson, T.R.; Liu, X.; Schroeder, M.R.; Kraft, C.S.; Burd, E.M.; Weiss, D.S. Rapid killing of Acinetobacter baumannii by polymyxins is mediated by a hydroxyl radical death pathway. Antimicrob. Agents Chemother. 2012, 56, 5642–5649. [Google Scholar] [CrossRef]
- Hoeksema, M.; Brul, S.; Ter Kuile, B.H. Influence of Reactive Oxygen Species on De Novo Acquisition of Resistance to Bactericidal Antibiotics. Antimicrob. Agents Chemother. 2018, 25, e02354-17. [Google Scholar] [CrossRef]
- Léger, L.; Budin-Verneuil, A.; Cacaci, M.; Benachour, A.; Hartke, A.; Verneuil, N. β-Lactam Exposure Triggers Reactive Oxygen Species Formation in Enterococcus faecalis via the Respiratory Chain Component DMK. Cell Rep. 2019, 29, 2184–2191.e3. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Ghotaslou, R.; Samiei, M.; Adibkia, K. Antimicrobial use of reactive oxygen therapy: Current insights. Infect. Drug Resis. 2018, 11, 567–576. [Google Scholar] [CrossRef]
- Imlay, J.A. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 2008, 77, 755–776. [Google Scholar] [CrossRef]
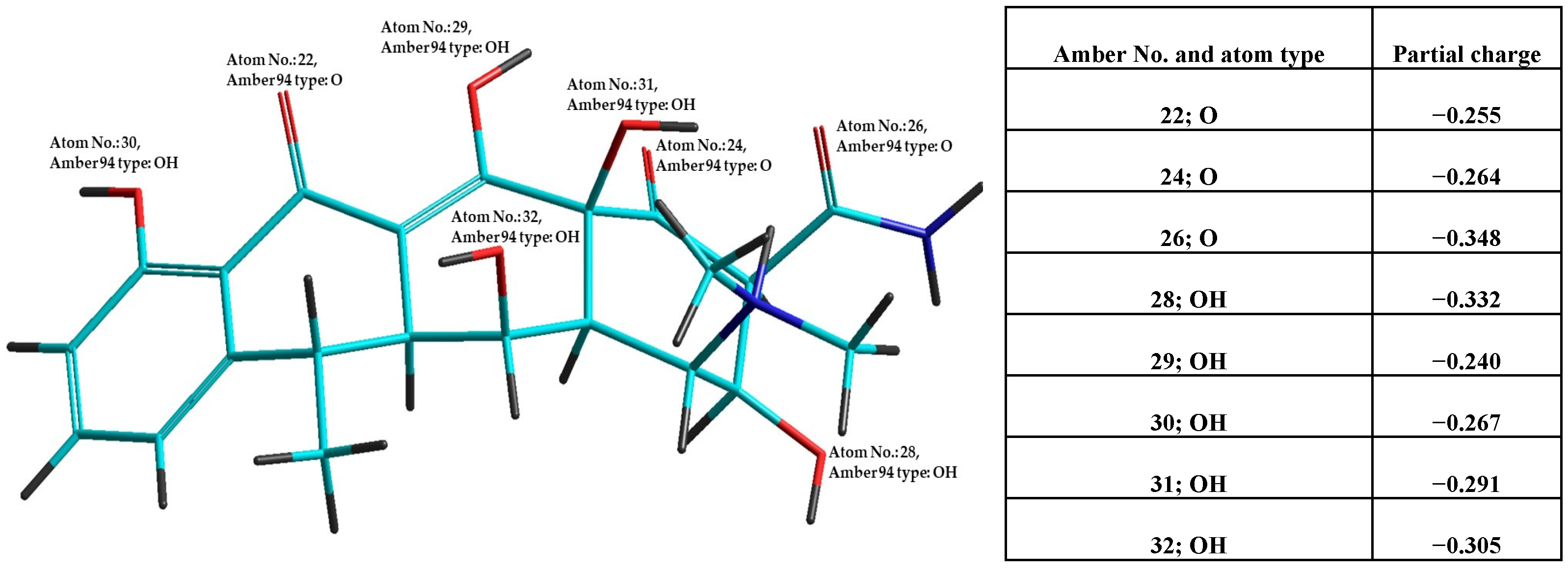
| Antibiotic | Antibiotic Category/ Mechanism | DPPH50 (μM) | r2 | FRAP50 (μM) | r2 | PABI |
|---|---|---|---|---|---|---|
| Doxycycline hydrochloride | TC’s/ PSI | 22.3 ± 0.6 | 0.992 | 5.9 ± 0.4 | 0.941 | 0.27 |
| Tigecycline | TCs/ PSI | 88.1 ± 2.9 | 0.962 | 91.6 ± 2.7 | 0.936 | 1.04 |
| Rifampicin | ANM’s/ NAB | 129 ± 6.8 | 0.989 | 33.1 ± 2.2 | 0.983 | 0.26 |
| Tebipenem | BLK’s/ BCED | 520.1 ± 40.6 | 0.988 | 3182.2 ± 69.87 | 0.923 | 6.12 |
| Cefuroxime | BLK’s/ BCED | 1533 ± 120 | 0.951 | 1919 ± 39 | 0.936 | 1.25 |
| Cefixime | BLK’s/ BCED | 2762 ± 220 | 0.934 | 3294 ± 46 | 0.955 | 1.19 |
| Clavulanate | BLK’s/ BCED | ND | N/A | 2625 ± 61 | 0.998 | ND |
| Colistin | P’s/ BCED | ND | N/A | ND | N/A | ND |
| Ampicillin | BLK’s/ BCED | ND | N/A | ND | N/A | ND |
| Amoxicillin | BLK’s/ BCED | ND | N/A | ND | N/A | ND |
| Amikacin | AG’s/ PSI | ND | N/A | ND | N/A | ND |
| Nalidixic acid | Q’s/ NAB | ND | N/A | ND | N/A | ND |
| Azithromycin | ML’s/ PSI | ND | N/A | ND | N/A | ND |
| Pipemidic acid trihydrate | PP’s/ NAB | ND | N/A | ND | N/A | ND |
| Pivmecillinam | BLK’s/ BCED | ND | N/A | ND | N/A | ND |
| Aztreonam | BLK’s/ BCED | ND | N/A | ND | N/A | ND |
| Fosfomycin sodium | PHA’s/ BCED | ND | N/A | ND | N/A | ND |
| Ciprofloxacin | FQ’s | ND | N/A | ND | N/A | ND |
| Antibiotic | Molinspiration Calculations | Hyperchem Calculations | ||||
|---|---|---|---|---|---|---|
| nVIOL * | AVG_Bioact ** | nOat | nSP2Oat | ∑p. ch. | AVG_p.ch. | |
| Doxycycline hydrochloride | 1 (nDHB) | −0.115 (PI; EI) | 8 | 2 | −2.59 | −0.288 |
| Tigecycline | 3 (MW, nDHB, nAHV) | −0.21 (EI) | 8 | 3 | −2.42 | −0.302 |
| Rifampicin | 3 (MW, nDHB, nAHB) | −2.11 | 13 | 3 | −3.59 | −0.299 |
| Tebipenem | 0 | −0.04 (PI; EI) | 6 | 0 | −1.80 | −0.300 |
| Cefuroxim | 1 (nAHB) | −0.28 (PI; EI) | 8 | 0 | −1.56 | −0.222 |
| Cefixim | 1 (AHB) | −0.23 (PI; EI) | 7 | 0 | 1.29 | −0.284 |
| Potassium clavulanate | 0 | −0.48 (PI; EI) | 5 | 0 | −1.37 | −0.273 |
| Colistin | 3 (MW, nDHB, nAHB) | −3.8 | 13 | 0 | −4.41 | −0.339 |
| Ampicilín | 0 | 0.04 (GPCRL; PI; EI) | 4 | 0 | −1.40 | −0.349 |
| Amoxicilín | 0 | 0.07 (GPCRL; PI; EI) | 5 | 0 | −1.67 | −0.335 |
| Amikacin | 3 (MW, nDHB, nAHB) | 0.33 (GPCRL; PI; EI) | 13 | 0 | −4.19 | −0.323 |
| Nalidixic acid | 0 | −0.16 (EI) | 3 | 0 | −0.95 | −0.317 |
| Azithromycin | 2 (MW, nAHB) | −0.59 | 12 | 0 | −3.4 | −0.309 |
| Pipemidic acid trihydrate | 0 | 0.22 (GPCRL; KI; EI) | 3 | 0 | −0.95 | −0.316 |
| Pivmecillinam | 1 (nRB) | 0.08 (GPCRL; PI; EI) | 5 | 0 | −1.47 | −0.295 |
| Aztreonam | 1 (nAHB) | 0.08 (GPCRL; PI; EI) | 8 | 0 | −3.5 | −0.438 |
| Fosfomycine sodium | 0 | −2.6 | 4 | 0 | −2.23 | −0.557 |
| Ciprofloxacin | 0 | 0.12 (GPCRL; EI) | 3 | 0 | −0.95 | −0.318 |
| Antibiotic | Target 1 (Probability) | Target 2 (Probability) | Target 3 (Probability) | Target with Highest Probability |
|---|---|---|---|---|
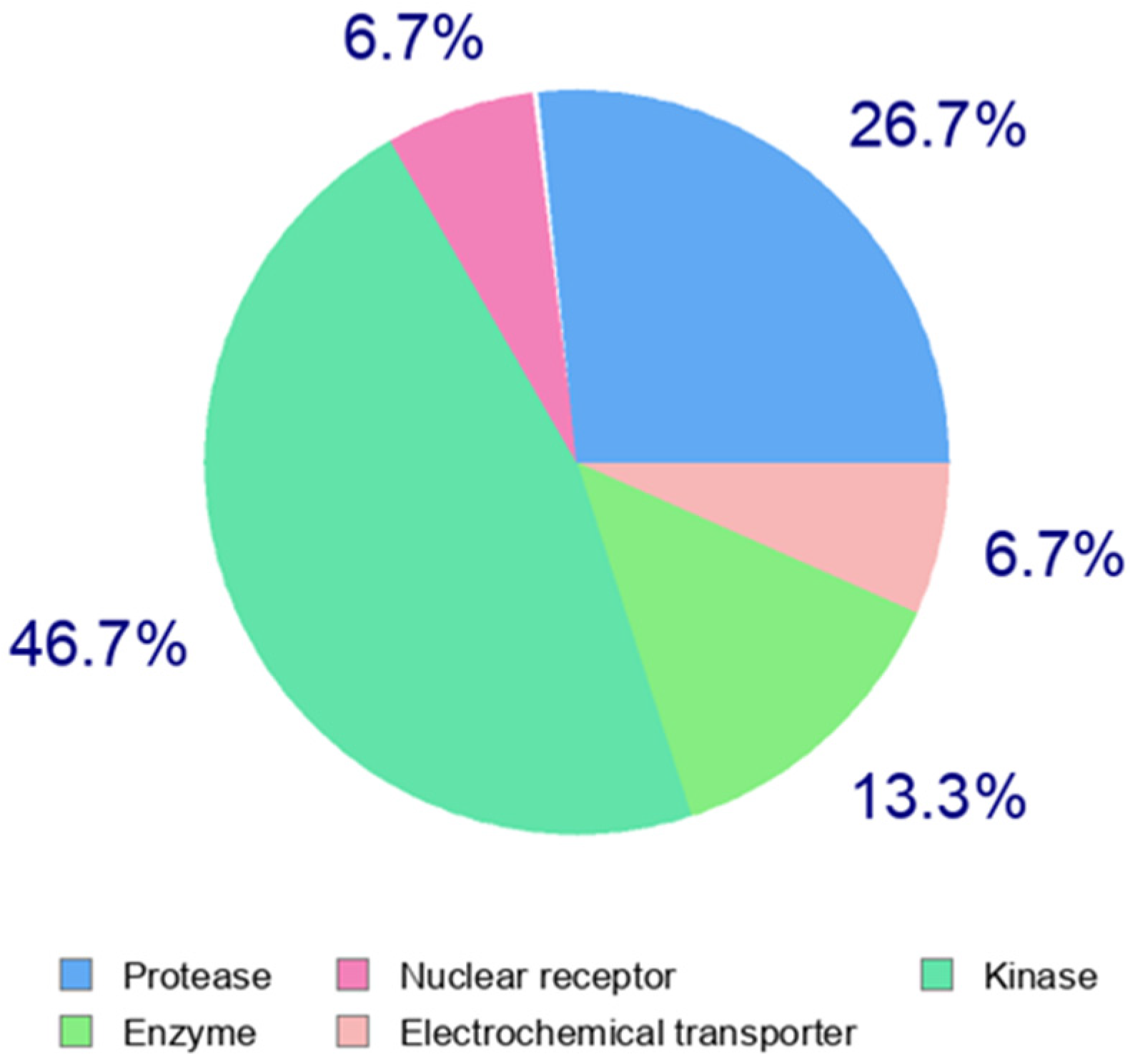
| Doxycycline hydrochloride | Kinases (46.7%) | Proteases (26.7%) | Enzymes in general (13.3%) | Matrix metalloproteinase 2 |
| Tigecycline | G coupled receptor, family E (46.7%) | Kinases (26.7%) | Protease (26.7%) | G protein-coupled receptor kinase 6 |
| Rifampicin | Kinases (46.7%) | Proteases (6.7%) | Enzymes in general (6.7%) | Bile salt export pump |
| Cefuroxime | Enzymes in general (26.7%) | Lyases (26.7%) | G coupled receptor, family A (46.7%) | PI3-kinase p110-gamma subunit |
| Cefixime | Enzymes in general (33%) | Kinases 20% | Proteases 13.3% | Dihydrofolate reductase |
| Potassium clavulanate | Enzymes in general (53.3%) | Proteases (26.7%) | Oxidoreductases (6.7%) | Leukocyte elastase |
| Colistin | Proteases (60%) | Kinases (6.7%) | Membrane receptors (6.7%) | Pepsinogen C |
| Ampicillin | Kinases (33%) | Proteases (26.7%) | Lyases (6.7%) | Integrin alpha-4/beta-1 |
| Amoxicillin | G coupled receptor, Family A (20%) | Adhesion (20%) | Lyases (6.7%) | Integrin alpha-4/beta-1 |
| Amikacin | G coupled receptor, Family A (33.3%) | Adhesion (20%) | Enzymes in general (6.7%) | Galectin-4 |
| Nalidixic acid | Erasers (26.7%) | Electrochemical transporter (13.3%) | Kinases (13.3%) | Serotonin transporter |
| Azithromycin | G coupled receptor, Family A (26.7%) | Enzymes in general (13.3%) | Electrochemical transporter (13.3%) | Human Ether-a-go-go-related Gene |
| Pipemidic acid trihydrate | Kinases (26.7%) | Enzymes in general (26.7%) | Proteases (13.3%) | Autotaxin |
| Pivmecillinam | Kinases (33.3%) | G coupled receptor, Family A (26.7%) | Enzymes in general (20%) | Phosphodiesterase 7A |
| Aztreonam | G coupled receptor, Family A (46.7%) | Enzymes in general (26.7%) | Proteases in general (13.3%) | Hypoxia-inducible factor prolyl 4-hydroxylase |
| Fosfomycin sodium | Family A, G coupled receptor, (50%) | Transferases (50%) | - | GABA-B receptor |
| Ciprofloxacin | Enzymes in general (33.3%) | Enzymes in general (26.7%) | Kinases (13.3%) | Glycogen synthase kinase-3 beta |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maliar, T.; Blažková, M.; Polák, J.; Maliarová, M.; Ürgeová, E.; Viskupičová, J. Antioxidant and Pro-Oxidant Properties of Selected Clinically Applied Antibiotics: Therapeutic Insights. Pharmaceuticals 2024, 17, 1257. https://doi.org/10.3390/ph17101257
Maliar T, Blažková M, Polák J, Maliarová M, Ürgeová E, Viskupičová J. Antioxidant and Pro-Oxidant Properties of Selected Clinically Applied Antibiotics: Therapeutic Insights. Pharmaceuticals. 2024; 17(10):1257. https://doi.org/10.3390/ph17101257
Chicago/Turabian StyleMaliar, Tibor, Marcela Blažková, Jaroslav Polák, Mária Maliarová, Eva Ürgeová, and Jana Viskupičová. 2024. "Antioxidant and Pro-Oxidant Properties of Selected Clinically Applied Antibiotics: Therapeutic Insights" Pharmaceuticals 17, no. 10: 1257. https://doi.org/10.3390/ph17101257
APA StyleMaliar, T., Blažková, M., Polák, J., Maliarová, M., Ürgeová, E., & Viskupičová, J. (2024). Antioxidant and Pro-Oxidant Properties of Selected Clinically Applied Antibiotics: Therapeutic Insights. Pharmaceuticals, 17(10), 1257. https://doi.org/10.3390/ph17101257






