Singlet Oxygen in Photodynamic Therapy
Abstract
1. Introduction
2. Properties of Singlet Oxygen
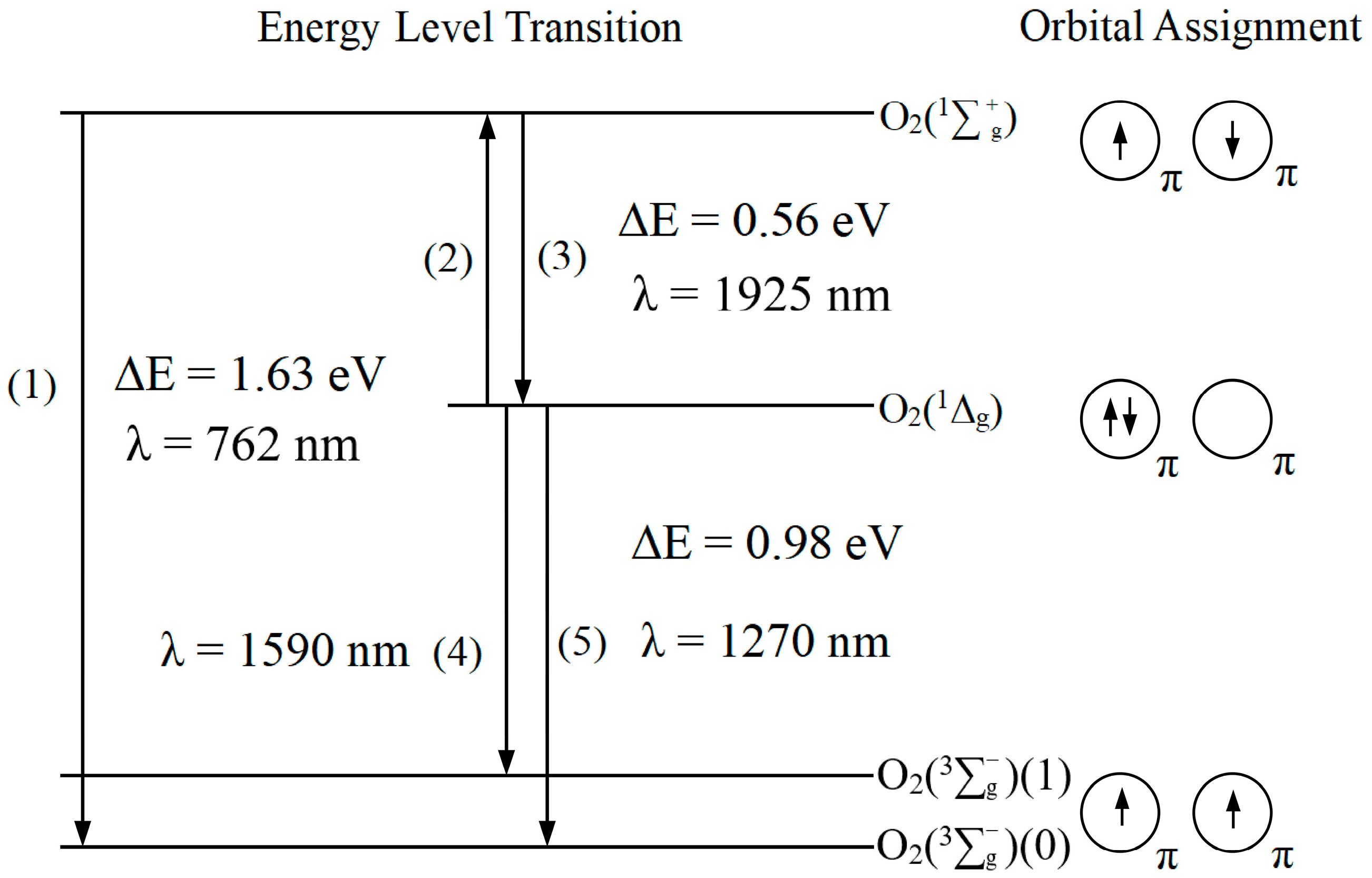
2.1. Electronic Structure and Leaps in Singlet Oxygen
2.2. Physical Chemistry Properties of Singlet Oxygen
| Solvent | kq (M−1s−1) | ke (M−1s−1) | τ∆ (μs) |
|---|---|---|---|
| H2O | 2.9 × 109 a | 0.25 b | 3.1 a,b,c |
| CH3OH | 1.2 × 109 c | 0.81 b | 9.5 a |
| C6H14 | 5.5 × 108 a | N/A | 23.4 a |
| C6H6 | 3.9 × 108 a | 4.5 b | 30 a |
| C5H12 | 4.5 × 108 a | N/A | 34.7 a |
| D2O | 3.7 × 108 a | 0.22 b | 68 a,b |
| CD3OD | N/A | 0.79 b | 270 b |
2.3. Oxidizing Activity of Singlet Oxygen
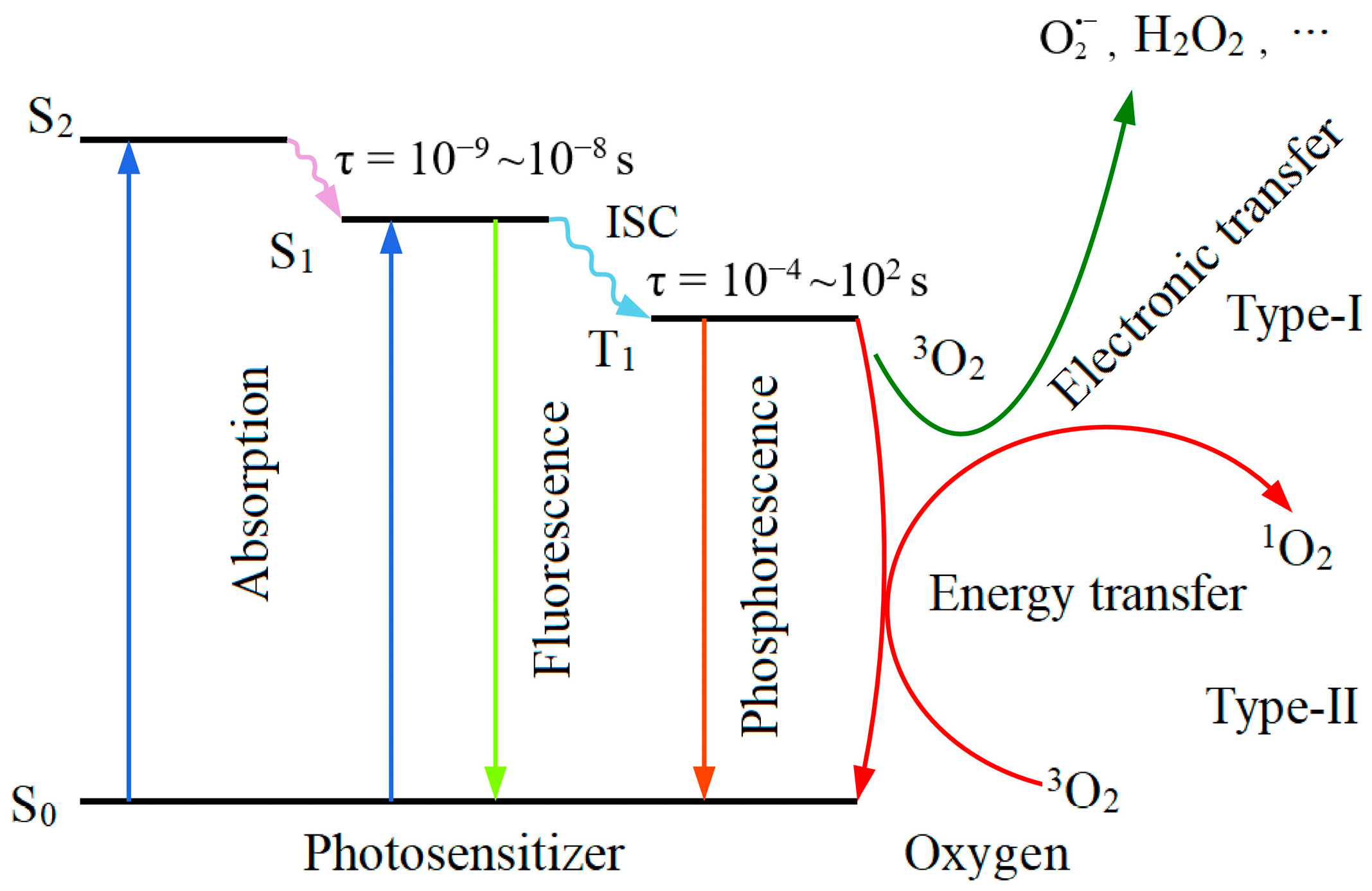
3. Singlet Oxygen Production in PDT Process
4. Singlet Oxygen Detection
4.1. Direct Detection of Singlet Oxygen
4.2. Indirect Detection of Singlet Oxygen
4.2.1. Electron Paramagnetic Resonance
4.2.2. Fluorescence Photometry
4.2.3. Spectrophotometry
4.2.4. Chemiluminescence
5. Available Singlet Oxygen Data of Regulatory-Approved PS
6. Conclusive Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Patrizia, A.; Kristian, B.; Keith, A.C.; Thomas, H.F.; Albert, W.G.; Sandra, O.G.; Stephen, M.H.; Michael, R.H.; Asta, J.; David, K.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar]
- Kamanli, A.F.; Çetinel, G.; Yıldız, M.Z. A New Handheld Singlet Oxygen Detection System (SODS) and NIR Light Source Based Phantom Environment for Photodynamic Therapy Applications. Photodiagnosis Photodyn. Ther. 2020, 29, 101577. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Huang, Z.; Dallimore, I.; Moghissi, K. Tools of Clinical Photodynamic Therapy (PDT): A Mini Compendium. Photodiagnosis Photodyn. Ther. 2024, 46, 104058. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Pustimbara, A.; Li, C.; Ogura, S.I. Hemin Enhance s the 5-aminolevulinic Acid-Photodynamic Therapy Effect Through the Changes of Cellular Iron Homeostasis. Photodiagnosis Photodyn. Ther. 2024, 48, 104253. [Google Scholar] [CrossRef]
- Pierre, G.; Jean-François, S.; Georges, W.; Mizeret, J.; Woodtli, A.; Jean-François, T.; Fontolliet, C.; Hubert, V.; Ph, M. Tetra(m-hydroxyphenyl)chlorin Clinical Photodynamic Therapy of Early Bronchial and Oesophageal Cancers. Laser Med. Sci. 1996, 11, 227–235. [Google Scholar]
- David, K. Pharmacokinetics of N-aspartyl Chlorin e6 in Cancer Patients. J. Photochem. Photobiol. B 1997, 39, 81–83. [Google Scholar]
- Ming, L.; Changhua, L. Recent Advances in Activatable Organic Photosensitizers for Specific Photodynamic Therapy. ChemPlusChem 2020, 85, 948–957. [Google Scholar]
- Ochsner, M. Photophysical and Photobiological Processes in the Photodynamic Therapy of Tumours. J. Photochem. Photobiol. B 1997, 39, 1–18. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part Two-Cellular Signaling, Cell Metabolism and Modes of Cell Death. Photodiagnosis Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Weishaupt, K.R.; Gomer, C.J.; Dougherty, T.J. Identification of Singlet Oxygen as the Cytotoxic Agent in Photoinactivation of a Murine Tumor. Cancer Res. 1976, 36, 2326–2329. [Google Scholar] [PubMed]
- Murotomi, K.; Umeno, A.; Shichiri, M.; Tanito, M.; Yoshida, Y. Significance of Singlet Oxygen Molecule in Pathologies. Int. J. Mol. Sci. 2023, 24, 2739. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized Singlet Oxygen and its Applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Foote, C.S. Mechanisms of Photosensitized Oxidation. There are Several Different Types of Photosensitized Oxidation Which May be Important in Biological Systems. Science 1968, 162, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Salokhiddinov, K.I.; Dzhagarov, B.M.; Byteva, I.M.; Gurinovich, G.P. Photosensitized Luminescence of Singlet Oxygen in Solutions at 1588 nm. Chem. Phys. Lett. 1980, 76, 85–87. [Google Scholar] [CrossRef]
- Macpherson, A.N.; Truscott, T.G.; Turner, P.H. Fourier-Transform Luminescence Spectroscopy of Solvated Singlet Oxygen. J. Chem. Soc. Faraday Trans. 1994, 90, 1065–1072. [Google Scholar] [CrossRef]
- Spiller, W.; Kliesch, H.; Wöhrle, D.; Hackbarth, S.; Röder, B.; Schnurpfeil, G. Singlet Oxygen Quantum Yields of Different Photosensitizers in Polar Solvents and Micellar Solutions. J. Porphyr. Phthalocya 1998, 2, 145–158. [Google Scholar] [CrossRef]
- Darwent, J.R.; Douglas, P.; Harriman, A.; Porter, G.; Richoux, M.C. Metal Phthalocyanines and Porphyrins as Photosensitizers for Reduction of Water to Hydrogen. Coord. Chem. Rev. 1982, 44, 83–126. [Google Scholar] [CrossRef]
- Gunduz, H.; Kolemen, S.; Akkaya, E.U. Singlet Oxygen Probes: Diversity in Signal Generation Mechanisms Yields a Larger Color Palette. Coord. Chem. Rev. 2021, 429, 213641. [Google Scholar] [CrossRef]
- Losev, A.P.; Nichiporovich, I.N.; Byteva, I.M.; Drozdov, N.N.; Jghgami, I.F.A. The Perturbing Effect of Solvents on the Luminescence Rate Constant of Singlet Molecular Oxygen. Chem. Phys. Lett. 1991, 181, 45–50. [Google Scholar] [CrossRef]
- Kearns, D.R. Physical and Chemical Properties of Singlet Molecular Oxygen. Chem. Rev. 1971, 71, 395–427. [Google Scholar] [CrossRef]
- Kramarenko, G.G.; Hummel, S.G.; Martin, S.M.; Buettner, G.R. Ascorbate Reacts with Singlet Oxygen to Produce Hydrogen Peroxide. Photochem. Photobiol. 2006, 82, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, C.; Schmidt, R. Physical Mechanisms of Generation and Deactivation of Singlet Oxygen. Chem. Rev. 2003, 103, 1685–1757. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, K.; Schenck, G.O. Mechanism and Stereoselectivity of Photosensitized Oxygen Transfer Reactions. Pure Appl. Chem. 1964, 9, 507–526. [Google Scholar] [CrossRef]
- Foote, C.S.; Denny, R.W. Chemistry of Singlet Oxygen. VII. Quenching by Beta.-Carotene. J. Am. Chem. Soc. 1968, 90, 6233–6235. [Google Scholar] [CrossRef]
- Hall, R.D.; Chignell, C.F. Steady-State Near-Infrared Detection of Singlet Molecular Oxygen: A Stern-Volmer Quenching Experiment with Sodium Azide. Photochem. Photobiol. 1987, 45, 459–464. [Google Scholar] [CrossRef]
- Gorman, A.A.; Ian, R.G.; Hamblett, I.; Standen, M.C. Reversible Exciplex Formation Between Singlet Oxygen, 1.DELTA.g, and Vitamin E. Solvent and Temperature Effects. J. Am. Chem. Soc. 1984, 106, 6956–6959. [Google Scholar] [CrossRef]
- Michaeli, A.; Feitelson, J. Reactivity of Singlet Oxygen Toward Amino Acids and Peptides. Photochem. Photobiol. 1994, 59, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Matheson, I.B.; Etheridge, R.D.; Kratowich, N.R.; Lee, J. The Quenching of Singlet Oxygen by Amino Acids and Proteins. Photochem. Photobiol. 1975, 21, 165–171. [Google Scholar] [CrossRef]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef]
- Moan, J. On the Diffusion Length of Singlet Oxygen in Cells and Tissues. J. Photochem. 1990, 6, 343–344. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The Role of Porphyrin Chemistry in Tumor Imaging and Photodynamic Therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Klaper, M.; Linker, T. New Singlet Oxygen Donors Based on Naphthalenes: Synthesis, Physical Chemical Data, and Improved Stability. Chemistry 2015, 21, 8569–8577. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Reconciling the Chemistry and Biology of Reactive Oxygen Species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Schröder, P.; Biesalski, H.K. Low Molecular Weight Antioxidants. In Reactions, Processes: Oxidants and Antioxidant Defense Systems; Grune, T., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2005; pp. 77–90. [Google Scholar]
- Kruk, J.; Szymańska, R. Singlet Oxygen Oxidation Products of Carotenoids, Fatty Acids and Phenolic Prenyllipids. J. Photochem. Photobiol. B 2021, 216, 112148. [Google Scholar] [CrossRef]
- Kim, J.; Rodriguez, M.E.; Guo, M.; Kenney, M.E.; Oleinick, N.L.; Anderson, V.E. Oxidative Modification of Cytochrome c by Singlet Oxygen. Free Radic. Biol. Med. 2008, 44, 1700–1711. [Google Scholar] [CrossRef][Green Version]
- Marques, E.F.; Medeiros, M.H.G.; Di Mascio, P. Lysozyme Oxidation by Singlet Molecular Oxygen: Peptide Characterization Using [(18) O]-Labeling Oxygen and nLC-MS/MS. J. Mass. Spectrom. 2017, 52, 739–751. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Rougee, M.; Bensasson, R.V.; Land, E.J.; Pariente, R. Deactivation of Singlet Molecular Oxygen by Thiols and Related Compounds, Possible Protectors Against Skin Photosensitivity. Photochem. Photobiol. 1988, 47, 485–489. [Google Scholar] [CrossRef]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Formation and Repair of Oxidatively Generated Damage in Cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Kamali, M.; Zhang, X.; Feijoo, S.; Al-Salem, S.M.; Dewil, R.; Appels, L. Biochar in Hydroxyl Radical-Based Electrochemical Advanced Oxidation Processes (eAOPs)—Mechanisms and Prospects. Chem. Eng. J. 2023, 467, 143291. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Sies, H. Oxidative Stress: Oxidants and Antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry Behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Vani, R.; Masannagari, P.; Aastha, C.; Chaitra, B.; Prerana, B.; Ranjithvishal; Shruthi, L.; Sudharshan, N. Reactive Oxygen Species and Antioxidant Interactions in Erythrocytes. In The Erythrocyte; Vani, R., Ed.; IntechOpen: Rijeka, Croatia, 2022; Chapter 1. [Google Scholar]
- Zhang, H.; Limphong, P.; Pieper, J.; Liu, Q.; Rodesch, C.K.; Christians, E.; Benjamin, I.J. Glutathione-Dependent Reductive Stress Triggers Mitochondrial Oxidation and Cytotoxicity. FASEB J. 2012, 26, 1442–1451. [Google Scholar] [CrossRef]
- Fujii, J.; Soma, Y.; Matsuda, Y. Biological Action of Singlet Molecular Oxygen from the Standpoint of Cell Signaling, Injury and Death. Molecules 2023, 28, 4085. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, H.; Meyers, A.D.; Musani, A.I.; Wang, L.; Tagg, R.; Barqawi, A.B.; Chen, Y.K. Photodynamic Therapy for Treatment of Solid Tumors--Potential and Technical Challenges. Technol. Cancer Res. Treat. 2008, 7, 309–320. [Google Scholar] [CrossRef]
- Uzdensky, A.B.; Berezhnaya, E.; Kovaleva, V.; Neginskaya, M.; Rudkovskii, M.; Sharifulina, S. Photodynamic Therapy: A Review of Applications in Neurooncology and Neuropathology. J. Biomed. Opt. 2015, 20, 61108. [Google Scholar] [CrossRef]
- Yang, W.; Rastogi, V.; Sun, H.; Sharma, D.; Wilson, B.C.; Hadfield, R.H.; Zhu, T.C. Multispectral Singlet Oxygen Luminescent Dosimetry (MSOLD) for Photofrin-mediated Photodynamic Therapy. Proc. SPIE Int. Soc. Opt. Eng. 2023, 12359, 1235908. [Google Scholar]
- Li, B.; Shen, Y.; Lin, H.; Wilson, B.C. Correlation of in Vitro Cell Viability and Cumulative Singlet Oxygen Luminescence from Protoporphyrin IX in Mitochondria and Plasma Membrane. Photodiagnosis Photodyn. Ther. 2024, 46, 104080. [Google Scholar] [CrossRef]
- Gemmell, N.R.; McCarthy, A.; Kim, M.M.; Veilleux, I.; Zhu, T.C.; Buller, G.S.; Wilson, B.C.; Hadfield, R.H. A Compact Fiber-Optic Probe-Based Singlet Oxygen Luminescence Detection System. J. Biophotonics 2017, 10, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Gemmell, N.R.; McCarthy, A.; Liu, B.; Tanner, M.G.; Dorenbos, S.D.; Zwiller, V.; Patterson, M.S.; Buller, G.S.; Wilson, B.C.; Hadfield, R.H. Singlet Oxygen Luminescence Detection with a Fiber-Coupled Superconducting Nanowire Single-Photon Detector. Opt. Express 2013, 21, 5005–5013. [Google Scholar] [CrossRef]
- Cui, S.; Ke, C.; Peng, W.; You, L.; Zhang, X.; Huang, Z. Optimizing Single Photon Detection Based on Superconducting Strip Photon Detector (SSPD) for Singlet Oxygen Luminescence Detection. Proc. SPIE 2023, 12770, 127702S1–127702S8. [Google Scholar]
- McIntosh, A.R.; Bolton, J.R. Triplet State Involvement in Primary Photochemistry of Photosynthetic Photosystem II. Nature 1976, 263, 443–445. [Google Scholar] [CrossRef]
- Nishide, N.; Miyoshi, N. Singlet Oxygen Trapping by DRD156 in Micellar Solutions. Life Sci. 2002, 72, 321–328. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence Probes Used for Detection of Reactive Oxygen Species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Cho, S.; Han, Y.; You, Y.; Nam, W. Ratiometric Fluorescent Probes for Detection of Intracellular Singlet Oxygen. Org. Lett. 2013, 15, 3582–3585. [Google Scholar] [CrossRef]
- Umezawa, N.; Tanaka, K.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Nagano, T. Novel Fluorescent Probes for Singlet Oxygen. Angew. Chem. Int. Ed. Engl. 1999, 38, 2899–2901. [Google Scholar] [CrossRef]
- Tanaka, K.; Miura, T.; Umezawa, N.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Nagano, T. Rational Design of Fluorescein-Based Fluorescence Probes. Mechanism-Based Design of a Maximum Fluorescence Probe for Singlet Oxygen. J. Am. Chem. Soc. 2001, 123, 2530–2536. [Google Scholar] [CrossRef]
- Arnab, M. Photophysical Detection of Singlet Oxygen. In Reactive Oxygen Species; Ahmad, R., Surguchov, A., Eds.; IntechOpen: London, UK, 2022; Chapter 2; pp. 1–20. 2022. [Google Scholar]
- Ragàs, X.; Jiménez-Banzo, A.; Sánchez-García, D.; Batllori, X.; Nonell, S. Singlet Oxygen Photosensitisation by the Fluorescent Probe Singlet Oxygen Sensor Green. Chem Commun (Camb) 2009, 20, 2920–2922. [Google Scholar] [CrossRef]
- Murotomi, K.; Umeno, A.; Sugino, S.; Yoshida, Y. Quantitative Kinetics of Intracellular Singlet Oxygen Generation Using a Fluorescence Probe. Sci. Rep. 2020, 10, 10616. [Google Scholar] [CrossRef] [PubMed]
- Lindig, B.A.; Rodgers, M.A.J.; Schaap, A.P. Determination of the Lifetime of Singlet Oxygen in Water-d2 Using 9,10-anthracenedipropionic Acid, a Water-Soluble Probe. J. Am. Chem. Soc. 1980, 102, 5590–5593. [Google Scholar] [CrossRef]
- Song, B.; Wang, G.; Yuan, J. Measurement and Characterization of Singlet Oxygen Production in Copper ion-Catalyzed Aerobic Oxidation of Ascorbic Acid. Talanta 2007, 72, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.J.; Monson, E.; Reddy, R.G.; Rehemtulla, A.; Ross, B.D.; Philbert, M.; Schneider, R.J.; Kopelman, R. Production of Singlet Oxygen by Ru(dpp(SO3)2)3 Incorporated in Polyacrylamide PEBBLES. Sens. Actuat B-Chem. 2003, 90, 82–89. [Google Scholar] [CrossRef]
- Yasuta, N.; Takenaka, N.; Takemura, T. Mechanism of Photosensitized Chemiluminescence of 2-Methyl-6-phenylimidazo[1,2-a]pyrazin-3(7H)-one (CLA) in Aqueous Solution. Chem. Lett. 2003, 28, 451–452. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Ma, H.; Zhang, D.; Li, J.; Zhu, D. 4,5-Dimethylthio-4′-[2-(9-anthryloxy)ethylthio]tetrathiafulvalene, a Highly Selective and Sensitive Chemiluminescence Probe for Singlet Oxygen. J. Am. Chem. Soc. 2004, 126, 11543–11548. [Google Scholar] [CrossRef]
- Bresolí-Obach, R.; Torra, J.; Zanocco, R.P.; Zanocco, A.L.; Nonell, S. Singlet Oxygen Quantum Yield Determination Using Chemical Acceptors. Methods Mol. Biol. 2021, 2202, 165–188. [Google Scholar]
- Tanielian, C.; Wolff, C.; Esch, M. Singlet Oxygen Production in Water: Aggregation and Charge-Transfer Effects. J. Phys. Chem. 1996, 100, 6555–6560. [Google Scholar] [CrossRef]
- Fernandez, J.M.; Bilgin, M.D.; Grossweiner, L.I. Singlet Oxygen Generation by Photodynamic Agents. J. Photochem. Photobiol. B 1997, 37, 131–140. [Google Scholar] [CrossRef]
- Hadjur, C.; Wagnieres, G.; Monnier, P.; van den Bergh, H. EPR and Spectrophotometric Studies of Free Radicals (O2−, •OH, BPD-MA) and Singlet Oxygen (1O2) Generated by Irradiation of Benzoporphyrin Derivative Monoacid Ring A. Photochem. Photobiol. 1997, 65, 818–827. [Google Scholar] [CrossRef]
- Nishimura, T.; Hara, K.; Honda, N.; Okazaki, S.; Hazama, H.; Awazu, K. Determination and Analysis of Singlet Oxygen Quantum Yields of Talaporfin Sodium, Protoporphyrin IX, and Lipidated Protoporphyrin IX Using Near-Infrared Luminescence Spectroscopy. Lasers Med. Sci. 2020, 35, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Bregnhøj, M.; Thorning, F.; Ogilby, P.R. Singlet Oxygen Photophysics: From Liquid Solvents to Mammalian Cells. Chem. Rev. 2024, in press. [Google Scholar] [CrossRef]
- Tanielian, C.; Schweitzer, C.; Mechin, R.; Wolff, C. Quantum Yield of Singlet Oxygen Production by Monomeric and Aggregated Forms of Hematoporphyrin Derivative. Free Radical Biol. Med. 2001, 30, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Sanchez, A.; Kasselouri, A.; Desroches, M.C.; Blais, J.; Maillard, P.; de Oliveira, D.M.; Tedesco, A.C.; Prognon, P.; Delaire, J. Photophysical Properties of Glucoconjugated Chlorins and Porphyrins and their Associations with Cyclodextrins. J. Photochem. Photobiol. B 2005, 81, 154–162. [Google Scholar] [CrossRef]
- Spikes, J.D.; Bommer, J.C. Photosensitizing Properties of Mono-L-Aspartyl Chlorin e6 (NPe6): A Candidate Sensitizer for the Photodynamic Therapy of Tumors. J. Photochem. Photobiol. B 1993, 17, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Aveline, B.; Hasan, T.; Redmond, R.W. Photophysical and Photosensitizing Properties of Benzoporphyrin Derivative Monoacid Ring A (BPD-MA)*. Photochem. Photobiol. 1994, 59, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in Photosensitizers and Light Delivery for Photodynamic Therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef]
- Przygoda, M.; Bartusik-Aebisher, D.; Dynarowicz, K.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Cellular Mechanisms of Singlet Oxygen in Photodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 16890. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, R.; Zhang, X.F.; Liu, J.; Luo, L. Halogenated BODIPY Photosensitizers: Photophysical Processes for Generation of Excited Triplet State, Excited Singlet State and Singlet Oxygen. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 272, 120965. [Google Scholar] [CrossRef]
- Li, N.; Cui, S.; Yang, A.; Xiao, B.; Cao, Y.; Yang, X.; Lin, C. Sequence-Dependent Effects of Hematoporphyrin Derivatives (HPD) Photodynamic Therapy and Cisplatin on Lung Adenocarcinoma Cells. Photodiagnosis Photodyn. Ther. 2024, 47, 104102. [Google Scholar] [CrossRef]
- Wang, S.; Bromley, E.; Xu, L.; Chen, J.C.; Keltner, L. Talaporfin sodium. Expert. Opin. Pharmacother. 2010, 11, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.D.; He, Q.X.; Li, X.; Yoon, J.; Huang, J.D. Phthalocyanines as Contrast Agents for Photothermal Therapy. Coord. Chem. Rev. 2021, 426, 213548. [Google Scholar] [CrossRef]
- Hurley, D.J.; Gallagher, D.; Petronzi, V.; O’Rourke, M.; Kinsella, F.; Townley, D. Examining the Efficacy of Verteporfin Photo-Dynamic Therapy (PDT) at Different Dose & Fluence Levels. Photodiagnosis Photodyn. Ther. 2023, 44, 103848. [Google Scholar] [PubMed]
- Pihl, C.; Lerche, C.M.; Andersen, F.; Bjerring, P.; Haedersdal, M. Improving the Efficacy of Photodynamic Therapy for Actinic Keratosis: A Comprehensive Review of Pharmacological Pretreatment Strategies. Photodiagnosis Photodyn. Ther. 2023, 43, 103703. [Google Scholar] [CrossRef]
| Photosensitizer | Quencher | Solvent | kq (M−1s−1) | Type of Quenching | Reference |
|---|---|---|---|---|---|
| Chlorophyll | β-Carotene (C40H56) | Benzene | 1.3 × 1010 | Physical | [25] |
| Methanol | 9.3 × 109 | Physical | |||
| Rose Bengal and Eosin Y | Sodium azide (NaN3) | Water | 6 × 108 | Physical | [26] |
| 2-Acetonaphthone | Vitamin E (α-Tocopherol) | Methanol | 3 × 108 | Physical | [27] |
| Toluene | 2.2 × 108 | Physical | |||
| Rose Bengal and Porphyrin | Histidine (C6H9N3O2) | Water | 4.6 × 107 | Physical and Chemical | [28] |
| Rose Bengal and Porphyrin | Tryptophan | Water | 3.2 × 107 | Physical and Chemical | [28] |
| Methylene Blue | Tyrosine | water | 7 × 106 | Chemical | [29] |
| Photosensitizer | λmax (nm)/ Ɛmax (M−1cm−1) | Solvent/(Standard) | Φ∆ | Method | Reference |
|---|---|---|---|---|---|
| Porfimer Sodium | 632/3000 | PBS/TX100 (RB:0.75) | 0.89 | Lysozyme inactivation | [73] |
| Hematoporphyrin derivative (HpD) | 630 | Methanol | 0.64 | Oxygen depletion | [77] |
| Methanol-D | 0.76 | Oxygen depletion | [72] | ||
| Protoporphyrin IX (PpIX) | 630/3480 | D-PBS/TX100 (RB:0.75) | 0.78 | TRIL | [75] |
| PBS/TX100 (RB:0.75) | 0.8 | TRIL | [75] | ||
| PBS/TX100 (MB:0.52) | 0.56 | Lysozyme inactivation | [73] | ||
| Meta-tetra(hydroxyl pheny) chlorin (m-THPC) | 652/35,000 | Ethanol (Pheophorbide-a:0.52) | 0.42 | TRIL | [78] |
| N-aspartyl chlorin e6 (NPe6) | 664/40,000 | D2O (RB:0.75) | 0.66 | Oxygen depletion | [79] |
| PBS (MB:0.52) | 0.64 | Lysozyme inactivation | [80] | ||
| Benzoporphyrin derivative monoacid ring A (BPD-MA) | 689/34,000 | Ethanol | 0.81 | Cytochrome C reduction | [74] |
| Methanol | 0.78 | Cytochrome C reduction | [74] | ||
| Benzene | 0.77 | TRIL | [80] | ||
| Talaporfin sodium (mono-L-aspartyl chlorine e6) | 654/40,000 | D-PBS (RB:0.75) | 0.53 | TRIL | [75] |
| D-PBS/TX100 (RB:0.75) | 0.59 | TRIL | [73] | ||
| Aluminum phthalocyanine tetrasulfonate (ALPcS4) | 676/200,000 | PBS/TX100 | 0.38 | Lysozyme inactivation | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, S.; Guo, X.; Wang, S.; Wei, Z.; Huang, D.; Zhang, X.; Zhu, T.C.; Huang, Z. Singlet Oxygen in Photodynamic Therapy. Pharmaceuticals 2024, 17, 1274. https://doi.org/10.3390/ph17101274
Cui S, Guo X, Wang S, Wei Z, Huang D, Zhang X, Zhu TC, Huang Z. Singlet Oxygen in Photodynamic Therapy. Pharmaceuticals. 2024; 17(10):1274. https://doi.org/10.3390/ph17101274
Chicago/Turabian StyleCui, Shengdong, Xingran Guo, Sen Wang, Zhe Wei, Deliang Huang, Xianzeng Zhang, Timothy C. Zhu, and Zheng Huang. 2024. "Singlet Oxygen in Photodynamic Therapy" Pharmaceuticals 17, no. 10: 1274. https://doi.org/10.3390/ph17101274
APA StyleCui, S., Guo, X., Wang, S., Wei, Z., Huang, D., Zhang, X., Zhu, T. C., & Huang, Z. (2024). Singlet Oxygen in Photodynamic Therapy. Pharmaceuticals, 17(10), 1274. https://doi.org/10.3390/ph17101274






