Drugs Targeting Sirtuin 2 Exhibit Broad-Spectrum Anti-Infective Activity
Abstract
1. Introduction
2. SIRT2 Impacts the Growth of Intracellular Pathogens
3. Biochemistry of SIRT2 Modulators
3.1. SIRT2 Isoforms
3.2. SIRT2 Catalytic Mechanism
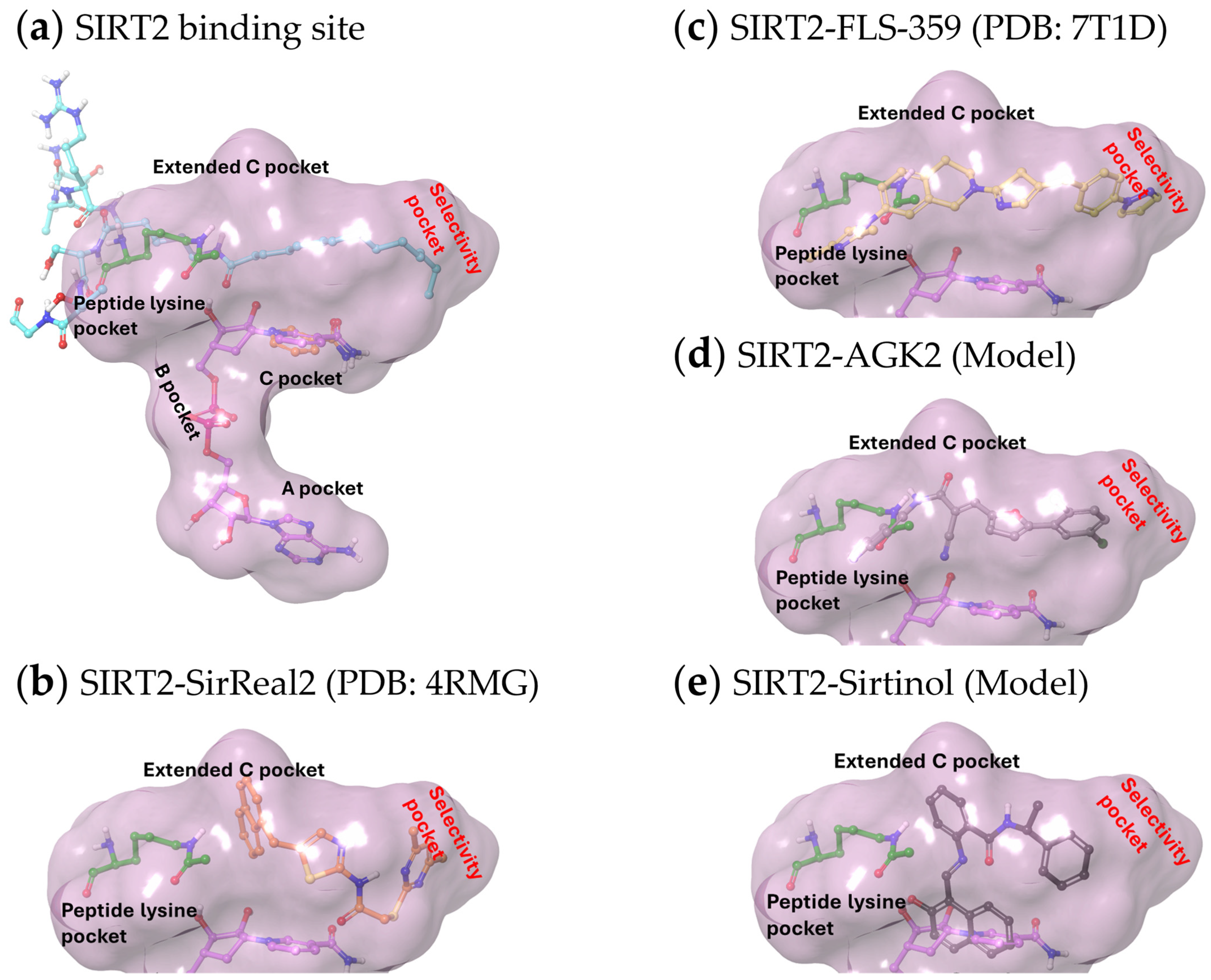
3.3. Structures of SIRT2 Modulators Bound to the Enzyme
3.4. SIRT2 Modulators Demonstrating Anti-Infective Activity Are Allosteric Partial Modulators
3.5. SIRT2 Partial Allosteric Modulators Are Acyl-Substrate Selective
4. Tolerability and Pharmacology of SIRT2 Modulation
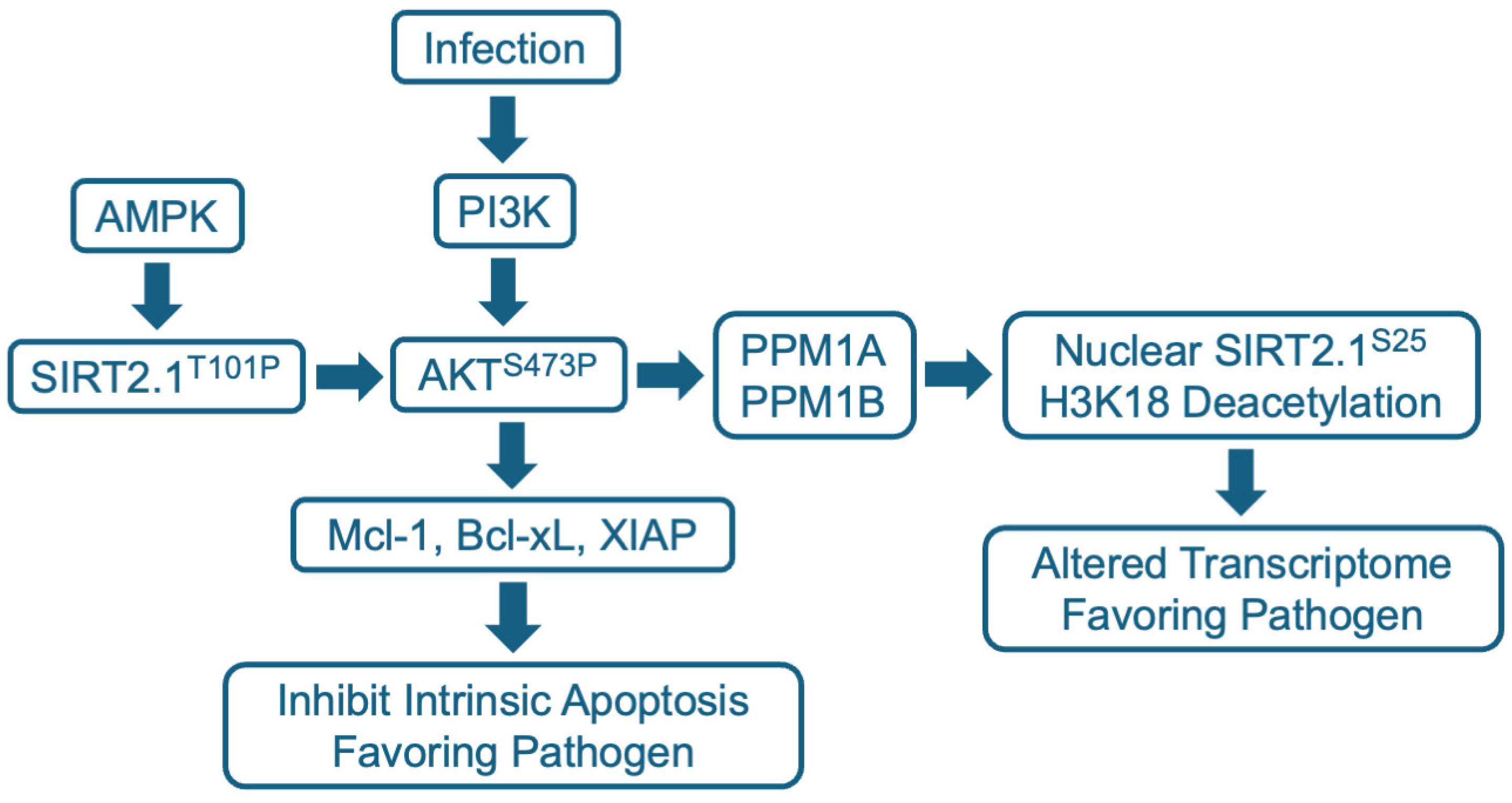
5. Anti-Infective Mechanisms of SIRT2 Modulators
5.1. Microtubule Activity
5.2. Innate Defense
5.3. Intracellular Signaling
5.4. Host Cell and Viral Transcription
5.5. Central Carbon Metabolism and Lipid Metabolism
5.6. Acetylation of Viral Proteins
6. Potential Therapeutic Utility of SIRT2 Modulators as Anti-Infective Agents
6.1. Combined Cell Autonomous Effects of SIRT2 Modulation
6.2. Immune Modulation
6.3. Inflammation
6.4. Sepsis
7. Some Interesting Questions
7.1. Why Does FLS-359 More Potently Inhibit the Production of HCMV Progeny than It Inhibits the Activity of Purified SIRT2?
7.2. Which SIRT2-Modulated Processes Must Be Targeted to Generate Anti-Infective Activity?
7.3. What Is the Potential for Combination Therapies of SIRT2 Modulators and Direct-Acting Therapeutics?
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lieberman-Blum, S.S.; Fung, H.B.; Bandres, J.C. Maraviroc: A CCR5-Receptor Antagonist for the Treatment of HIV-1 Infection. Clin. Ther. 2008, 30, 1228–1250. [Google Scholar] [CrossRef] [PubMed]
- Radoshitzky, S.R.; Iversen, P.; Lu, X.; Zou, J.; Kaptein, S.J.F.; Stuthman, K.S.; Van Tongeren, S.A.; Steffens, J.; Gong, R.; Truong, H.; et al. Expanded Profiling of Remdesivir as a Broad-Spectrum Antiviral and Low Potential for Interaction with Other Medications in Vitro. Sci. Rep. 2023, 13, 3131. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sharma, S.; Kumar, R.; Tripathi, B.N.; Barua, S.; Ly, H.; Rouse, B.T. Host-Directed Antiviral Therapy. Clin. Microbiol. Rev. 2020, 33, e00168-19. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, J.; Gao, H.; Liu, C.; Zhan, P.; Liu, X. Broad-Spectrum Antiviral Strategy: Host-Targeting Antivirals against Emerging and Re-Emerging Viruses. Eur. J. Med. Chem. 2024, 265, 116069. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Song, K.; Ye, J.; Li, W.; Zhong, Y.; Feng, Z.; Liang, S.; Cai, Z.; Xu, K. A Broad Antiviral Strategy: Inhibitors of Human DHODH Pave the Way for Host-Targeting Antivirals against Emerging and Re-Emerging Viruses. Viruses 2022, 14, 928. [Google Scholar] [CrossRef]
- Dwek, R.A.; Bell, J.I.; Feldmann, M.; Zitzmann, N. Host-Targeting Oral Antiviral Drugs to Prevent Pandemics. Lancet 2022, 399, 1381–1382. [Google Scholar] [CrossRef]
- Tsai, K.; Cullen, B.R. Epigenetic and Epitranscriptomic Regulation of Viral Replication. Nat. Rev. Microbiol. 2020, 18, 559–570. [Google Scholar] [CrossRef]
- Wang, X.; Xia, H.; Liu, S.; Cao, L.; You, F. Epigenetic Regulation in Antiviral Innate Immunity. Eur. J. Immunol. 2021, 51, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Svinkina, T.; Gu, H.; Silva, J.C.; Mertins, P.; Qiao, J.; Fereshetian, S.; Jaffe, J.D.; Kuhn, E.; Udeshi, N.D.; Carr, S.A. Deep, Quantitative Coverage of the Lysine Acetylome Using Novel Anti-Acetyl-Lysine Antibodies and an Optimized Proteomic Workflow. Mol. Cell. Proteomics 2015, 14, 2429–2440. [Google Scholar] [CrossRef]
- Narita, T.; Weinert, B.T.; Choudhary, C. Functions and Mechanisms of Non-Histone Protein Acetylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 156–174. [Google Scholar] [CrossRef]
- Feehley, T.; O’Donnell, C.W.; Mendlein, J.; Karande, M.; McCauley, T. Drugging the Epigenome in the Age of Precision Medicine. Clin. Epigenetics 2023, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Holdgate, G.A.; Bardelle, C.; Lanne, A.; Read, J.; O’Donovan, D.H.; Smith, J.M.; Selmi, N.; Sheppard, R. Drug Discovery for Epigenetics Targets. Drug Discov. Today 2022, 27, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mahur, P.; Muthukumaran, J.; Singh, A.K.; Jain, M. Shedding Light on Structure, Function and Regulation of Human Sirtuins: A Comprehensive Review. 3 Biotech 2023, 13, 29. [Google Scholar] [CrossRef]
- Feldman, J.L.; Dittenhafer-Reed, K.E.; Denu, J.M. Sirtuin Catalysis and Regulation. J. Biol. Chem. 2012, 287, 42419–42427. [Google Scholar] [CrossRef]
- Bursch, K.L.; Goetz, C.J.; Smith, B.C. Current Trends in Sirtuin Activator and Inhibitor Development. Molecules 2024, 29, 1185. [Google Scholar] [CrossRef]
- de Freitas e Silva, R.; Bassi, G.; Câmara, N.O.S.; Moretti, N.S. Sirtuins: Key Pieces in the Host Response to Pathogens’ Puzzle. Mol. Immunol. 2023, 160, 150–160. [Google Scholar] [CrossRef]
- Wang, J.; Qin, X.; Huang, Y.; Zhang, G.; Liu, Y.; Cui, Y.; Wang, Y.; Pei, J.; Ma, S.; Song, Z.; et al. Sirt1 Negatively Regulates Cellular Antiviral Responses by Preventing the Cytoplasmic Translocation of Interferon-Inducible Protein 16 in Human Cells. J. Virol. 2023, 97, e0197522. [Google Scholar] [CrossRef]
- Eskandarian, H.A.; Impens, F.; Nahori, M.-A.; Soubigou, G.; Coppee, J.-Y.; Cossart, P.; Hamon, M.A. A Role for SIRT2-Dependent Histone H3K18 Deacetylation in Bacterial Infection. Science 2013, 341, 1238858. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, M.J.G.; Hamon, M.A. Histone H3 Deacetylation Promotes Host Cell Viability for Efficient Infection by Listeria Monocytogenes. PLoS Pathog. 2021, 17, e1010173. [Google Scholar] [CrossRef]
- Yu, H.-B.; Cheng, S.-T.; Ren, F.; Chen, Y.; Shi, X.-F.; Wong, V.K.W.; Law, B.Y.K.; Ren, J.-H.; Zhong, S.; Chen, W.-X.; et al. SIRT7 Restricts HBV Transcription and Replication through Catalyzing Desuccinylation of Histone H3 Associated with CccDNA Minichromosome. Clin. Sci. 2021, 135, 1505–1522. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Gutierrez, N.M.; Marzuki, M.B.; Lu, X.; Foreman, T.W.; Paleja, B.; Lee, B.; Balachander, A.; Chen, J.; Tsenova, L.; et al. Host Sirtuin 1 Regulates Mycobacterial Immunopathogenesis and Represents a Therapeutic Target against Tuberculosis. Sci. Immunol. 2017, 2, eaaj1789. [Google Scholar] [CrossRef] [PubMed]
- Roche, K.L.; Remiszewski, S.; Todd, M.J.; Kulp, J.L.; Tang, L.; Welsh, A.V.; Barry, A.P.; De, C.; Reiley, W.W.; Wahl, A.; et al. An Allosteric Inhibitor of Sirtuin 2 Deacetylase Activity Exhibits Broad-Spectrum Antiviral Activity. J. Clin. Investig. 2023, 133, e158978. [Google Scholar] [CrossRef] [PubMed]
- Outeiro, T.F.; Kontopoulos, E.; Altmann, S.M.; Kufareva, I.; Strathearn, K.E.; Amore, A.M.; Volk, C.B.; Maxwell, M.M.; Rochet, J.C.; McLean, P.J.; et al. Sirtuin 2 Inhibitors Rescue α-Synuclein-Mediated Toxicity in Models of Parkinson’s Disease. Science 2007, 317, 516–519. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Fox, L.M.; Rozkalne, A.; Pitstick, R.; Carlson, G.A.; Kazantsev, A.G. Inhibition of Sirtuin 2 with Sulfobenzoic Acid Derivative AK1 Is Non-Toxic and Potentially Neuroprotective in a Mouse Model of Frontotemporal Dementia. Front. Pharmacol. 2012, 3, 42. [Google Scholar] [CrossRef]
- Taylor, D.M.; Balabadra, U.; Xiang, Z.; Woodman, B.; Meade, S.; Amore, A.; Maxwell, M.M.; Reeves, S.; Bates, G.P.; Luthi-Carter, R.; et al. A Brain-Permeable Small Molecule Reduces Neuronal Cholesterol by Inhibiting Activity of Sirtuin 2 Deacetylase. ACS Chem. Biol. 2011, 6, 540–546. [Google Scholar] [CrossRef]
- Khanfar, M.A.; Quinti, L.; Wang, H.; Choi, S.H.; Kazantsev, A.G.; Silverman, R.B. Development and Characterization of 3-(Benzylsulfonamido)Benzamides as Potent and Selective SIRT2 Inhibitors. Eur. J. Med. Chem. 2014, 76, 414–426. [Google Scholar] [CrossRef]
- Grozinger, C.M.; Chao, E.D.; Blackwell, H.E.; Moazed, D.; Schreiber, S.L. Identification of a Class of Small Molecule Inhibitors of the Sirtuin Family of NAD-Dependent Deacetylases by Phenotypic Screening. J. Biol. Chem. 2001, 276, 38837–38843. [Google Scholar] [CrossRef] [PubMed]
- Lain, S.; Hollick, J.J.; Campbell, J.; Staples, O.D.; Higgins, M.; Aoubala, M.; McCarthy, A.; Appleyard, V.; Murray, K.E.; Baker, L.; et al. Discovery, In Vivo Activity, and Mechanism of Action of a Small-Molecule P53 Activator. Cancer Cell 2008, 13, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Hu, J.; He, B.; Negrón Abril, Y.L.; Stupinski, J.; Weiser, K.; Carbonaro, M.; Chiang, Y.-L.; Southard, T.; Giannakakou, P.; et al. A SIRT2-Selective Inhibitor Promotes c-Myc Oncoprotein Degradation and Exhibits Broad Anticancer Activity. Cancer Cell 2016, 29, 297–310. [Google Scholar] [CrossRef]
- Singh, A.P.; Nigam, L.; Yadav, Y.; Shekhar, S.; Subbarao, N.; Dey, S. Design and in Vitro Analysis of SIRT2 Inhibitor Targeting Parkinson’s Disease. Mol. Divers. 2021, 25, 2261–2270. [Google Scholar] [CrossRef]
- Hong, J.Y.; Cassel, J.; Yang, J.; Lin, H.; Weiser, B.P. High-Throughput Screening Identifies Ascorbyl Palmitate as a SIRT2 Deacetylase and Defatty-Acylase Inhibitor. ChemMedChem 2021, 16, 3484–3494. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.L.; Rajabi, N.; Kudo, N.; Lundø, K.; Moreno-Yruela, C.; Bæk, M.; Fontenas, M.; Lucidi, A.; Madsen, A.S.; Yoshida, M.; et al. Mechanism-Based Inhibitors of SIRT2: Structure–Activity Relationship, X-Ray Structures, Target Engagement, Regulation of α-Tubulin Acetylation and Inhibition of Breast Cancer Cell Migration. RSC Chem. Biol. 2021, 2, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Lei, J.; Zhang, T.; Lu, P.; Cui, D.; Yang, B.; Zhao, G.; Peng, F.; Cao, Z.; Peng, C.; et al. Isobavachalcone, a Natural Sirtuin 2 Inhibitor, Exhibits anti-Riple-negative Breast Cancer Efficacy in Vitro and in Vivo. Phyther. Res. 2024, 38, 1815–1829. [Google Scholar] [CrossRef]
- Tang, L.; Remiszewski, S.; Snedeker, A.; Chiang, L.W.; Shenk, T. An Allosteric Inhibitor of Sirtuin 2 Blocks Hepatitis B Virus Covalently Closed Circular DNA Establishment and Its Transcriptional Activity. Antiviral Res. 2024, 226, 105888. [Google Scholar] [CrossRef]
- Piracha, Z.Z.; Kwon, H.; Saeed, U.; Kim, J.; Jung, J.; Chwae, Y.-J.; Park, S.; Shin, H.-J.; Kim, K. Sirtuin 2 Isoform 1 Enhances Hepatitis B Virus RNA Transcription and DNA Synthesis through the AKT/GSK-3β/β-Catenin Signaling Pathway. J. Virol. 2018, 92, e00955-18. [Google Scholar] [CrossRef]
- Yu, H.-B.; Jiang, H.; Cheng, S.-T.; Hu, Z.-W.; Ren, J.-H.; Chen, J. AGK2, A SIRT2 Inhibitor, Inhibits Hepatitis B Virus Replication In Vitro And In Vivo. Int. J. Med. Sci. 2018, 15, 1356–1364. [Google Scholar] [CrossRef]
- Li, Y.; Bie, J.; Song, C.; Li, Y.; Zhang, T.; Li, H.; Zhao, L.; You, F.; Luo, J. SIRT2 Negatively Regulates the CGAS-STING Pathway by Deacetylating G3BP1. EMBO Rep. 2023, 24, e57500. [Google Scholar] [CrossRef]
- Kanda, T.; Sasaki, R.; Nakamoto, S.; Haga, Y.; Nakamura, M.; Shirasawa, H.; Okamoto, H.; Yokosuka, O. The Sirtuin Inhibitor Sirtinol Inhibits Hepatitis A Virus (HAV) Replication by Inhibiting HAV Internal Ribosomal Entry Site Activity. Biochem. Biophys. Res. Commun. 2015, 466, 567–571. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, W.; Zhang, J.; Li, L.; Wan, Y.; Tang, X.; Chen, X.; Liu, S.; Yao, X. Tenovin-1 Inhibited Dengue Virus Replication through SIRT2. Eur. J. Pharmacol. 2021, 907, 174264. [Google Scholar] [CrossRef]
- Hackett, B.A.; Dittmar, M.; Segrist, E.; Pittenger, N.; To, J.; Griesman, T.; Gordesky-Gold, B.; Schultz, D.C.; Cherry, S. Sirtuin Inhibitors Are Broadly Antiviral against Arboviruses. mBio 2019, 10, e01446-19. [Google Scholar] [CrossRef]
- Duran-Castells, C.; Llano, A.; Kawana-Tachikawa, A.; Prats, A.; Martinez-Zalacain, I.; Kobayashi-Ishihara, M.; Oriol-Tordera, B.; Peña, R.; Gálvez, C.; Silva-Arrieta, S.; et al. Sirtuin-2, NAD-Dependent Deacetylase, Is a New Potential Therapeutic Target for HIV-1 Infection and HIV-Related Neurological Dysfunction. J. Virol. 2023, 97, e0165522. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, A.; Kumar, S.; Khan, M.Z.; Singh, A.; Dwivedi, V.P.; Nandicoori, V.K. Host Sirtuin 2 as an Immunotherapeutic Target against Tuberculosis. Elife 2020, 9, 106644. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, M.; Chandra, K.; Sarikhani, M.; Ramani, R.; Sundaresan, N.R.; Chakravortty, D. Salmonella Escapes Adaptive Immune Response via SIRT2 Mediated Modulation of Innate Immune Response in Dendritic Cells. PLOS Pathog. 2018, 14, e1007437. [Google Scholar] [CrossRef] [PubMed]
- Ladds, M.J.G.W.; Popova, G.; Pastor-Fernández, A.; Kannan, S.; van Leeuwen, I.M.M.; Håkansson, M.; Walse, B.; Tholander, F.; Bhatia, R.; Verma, C.S.; et al. Exploitation of Dihydroorotate Dehydrogenase (DHODH) and P53 Activation as Therapeutic Targets: A Case Study in Polypharmacology. J. Biol. Chem. 2020, 295, 17935–17949. [Google Scholar] [CrossRef] [PubMed]
- Rack, J.G.M.; VanLinden, M.R.; Lutter, T.; Aasland, R.; Ziegler, M. Constitutive Nuclear Localization of an Alternatively Spliced Sirtuin-2 Isoform. J. Mol. Biol. 2014, 426, 1677–1691. [Google Scholar] [CrossRef]
- North, B.J.; Verdin, E. Interphase Nucleo-Cytoplasmic Shuttling and Localization of SIRT2 during Mitosis. PLoS ONE 2007, 2, e784. [Google Scholar] [CrossRef]
- Vaquero, A. SirT2 Is a Histone Deacetylase with Preference for Histone H4 Lys 16 during Mitosis. Genes Dev. 2006, 20, 1256–1261. [Google Scholar] [CrossRef]
- Feldman, J.L.; Baeza, J.; Denu, J.M. Activation of the Protein Deacetylase SIRT6 by Long-Chain Fatty Acids and Widespread Deacylation by Mammalian Sirtuins. J. Biol. Chem. 2013, 288, 31350–31356. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, X.; Wang, X.; Zheng, Y.; Qiu, J.; Peng, Y.; Xu, J.; Chai, Z.; Liu, C. Multiple Roles of SIRT2 in Regulating Physiological and Pathological Signal Transduction. Genet. Res. 2022, 2022, 9282484. [Google Scholar] [CrossRef]
- Delaney, K.; Tan, M.; Zhu, Z.; Gao, J.; Dai, L.; Kim, S.; Ding, J.; He, M.; Halabelian, L.; Yang, L.; et al. Histone Lysine Methacrylation Is a Dynamic Post-Translational Modification Regulated by HAT1 and SIRT2. Cell Discov. 2021, 7, 122. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, L.; Wang, R.; Wang, J.; Li, J.; Xu, W.; Li, Y.; Yao, S.Q.; Zhang, L.; Hao, Q.; et al. Chemical Probes Reveal Sirt2’s New Function as a Robust “Eraser” of Lysine Lipoylation. J. Am. Chem. Soc. 2019, 141, 18428–18436. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, D.; Wang, Y.; Perez-Neut, M.; Han, Z.; Zheng, Y.G.; Hao, Q.; Zhao, Y. Lysine Benzoylation Is a Histone Mark Regulated by SIRT2. Nat. Commun. 2018, 9, 3374. [Google Scholar] [CrossRef] [PubMed]
- Jennings, E.Q.; Ray, J.D.; Zerio, C.J.; Trujillo, M.N.; McDonald, D.M.; Chapman, E.; Spiegel, D.A.; Galligan, J.J. Sirtuin 2 Regulates Protein LactoylLys Modifications. Chembiochem 2021, 22, 2102–2106. [Google Scholar] [CrossRef]
- Jin, J.; He, B.; Zhang, X.; Lin, H.; Wang, Y. SIRT2 Reverses 4-Oxononanoyl Lysine Modification on Histones. J. Am. Chem. Soc. 2016, 138, 12304–12307. [Google Scholar] [CrossRef]
- Sauve, A.A.; Celic, I.; Avalos, J.; Deng, H.; Boeke, J.D.; Schramm, V.L. Chemistry of Gene Silencing: The Mechanism of NAD + -Dependent Deacetylation Reactions. Biochemistry 2001, 40, 15456–15463. [Google Scholar] [CrossRef]
- Sauve, A.A.; Youn, D.Y. Sirtuins: NAD+-Dependent Deacetylase Mechanism and Regulation. Curr. Opin. Chem. Biol. 2012, 16, 535–543. [Google Scholar] [CrossRef]
- Feldman, J.L.; Dittenhafer-Reed, K.E.; Kudo, N.; Thelen, J.N.; Ito, A.; Yoshida, M.; Denu, J.M. Kinetic and Structural Basis for Acyl-Group Selectivity and NAD(+) Dependence in Sirtuin-Catalyzed Deacylation. Biochemistry 2015, 54, 3037–3050. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fung, Y.M.E.; Zhang, W.; He, B.; Chung, M.W.H.; Jin, J.; Hu, J.; Lin, H.; Hao, Q. Deacylation Mechanism by SIRT2 Revealed in the 1′-SH-2′-O-Myristoyl Intermediate Structure. Cell Chem. Biol. 2017, 24, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Avalos, J.L.; Bever, K.M.; Wolberger, C. Mechanism of Sirtuin Inhibition by Nicotinamide: Altering the NAD+ Cosubstrate Specificity of a Sir2 Enzyme. Mol. Cell 2005, 17, 855–868. [Google Scholar] [CrossRef]
- Gertz, M.; Fischer, F.; Nguyen, G.T.T.; Lakshminarasimhan, M.; Schutkowski, M.; Weyand, M.; Steegborn, C. Ex-527 Inhibits Sirtuins by Exploiting Their Unique NAD + -Dependent Deacetylation Mechanism. Proc. Natl. Acad. Sci. 2013, 110, E2772–E2781. [Google Scholar] [CrossRef]
- Teng, Y.-B.; Jing, H.; Aramsangtienchai, P.; He, B.; Khan, S.; Hu, J.; Lin, H.; Hao, Q. Efficient Demyristoylase Activity of SIRT2 Revealed by Kinetic and Structural Studies. Sci. Rep. 2015, 5, 8529. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, T.; Schiedel, M.; Karaman, B.; Roessler, C.; North, B.J.; Lehotzky, A.; Oláh, J.; Ladwein, K.I.; Schmidtkunz, K.; Gajer, M.; et al. Selective Sirt2 Inhibition by Ligand-Induced Rearrangement of the Active Site. Nat. Commun. 2015, 6, 6263. [Google Scholar] [CrossRef] [PubMed]
- Schiedel, M.; Rumpf, T.; Karaman, B.; Lehotzky, A.; Gerhardt, S.; Ovádi, J.; Sippl, W.; Einsle, O.; Jung, M. Structure-Based Development of an Affinity Probe for Sirtuin 2. Angew. Chemie Int. Ed. 2016, 55, 2252–2256. [Google Scholar] [CrossRef]
- Schiedel, M.; Rumpf, T.; Karaman, B.; Lehotzky, A.; Oláh, J.; Gerhardt, S.; Ovádi, J.; Sippl, W.; Einsle, O.; Jung, M. Aminothiazoles as Potent and Selective Sirt2 Inhibitors: A Structure-Activity Relationship Study. J. Med. Chem. 2016, 59, 1599–1612. [Google Scholar] [CrossRef] [PubMed]
- Sundriyal, S.; Moniot, S.; Mahmud, Z.; Yao, S.; Di Fruscia, P.; Reynolds, C.R.; Dexter, D.T.; Sternberg, M.J.E.; Lam, E.W.F.; Steegborn, C.; et al. Thienopyrimidinone Based Sirtuin-2 (SIRT2)-Selective Inhibitors Bind in the Ligand Induced Selectivity Pocket. J. Med. Chem. 2017, 60, 1928–1945. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Quinti, L.; Casale, M.; Moniot, S.; Pais, T.F.; Van Kanegan, M.J.; Kaltenbach, L.S.; Pallos, J.; Lim, R.G.; Naidu, S.D.; Runne, H.; et al. SIRT2- and NRF2-Targeting Thiazole-Containing Compound with Therapeutic Activity in Huntington’s Disease Models. Cell Chem. Biol. 2016, 23, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Moniot, S.; Schutkowski, M.; Steegborn, C. Crystal Structure Analysis of Human Sirt2 and Its ADP-Ribose Complex. J. Struct. Biol. 2013, 182, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Lin, H. Substrate-Selective Small-Molecule Modulators of Enzymes: Mechanisms and Opportunities. Curr. Opin. Chem. Biol. 2022, 72, 102231. [Google Scholar] [CrossRef]
- Koyuncu, E.; Budayeva, H.G.; Miteva, Y.V.; Ricci, D.P.; Silhavy, T.J.; Shenk, T.; Cristea, I.M. Sirtuins Are Evolutionarily Conserved Viral Restriction Factors. mBio 2014, 5, e02249-14. [Google Scholar] [CrossRef]
- Ciarlo, E.; Heinonen, T.; Théroude, C.; Herderschee, J.; Mombelli, M.; Lugrin, J.; Pfefferlé, M.; Tyrrell, B.; Lensch, S.; Acha-Orbea, H.; et al. Sirtuin 2 Deficiency Increases Bacterial Phagocytosis by Macrophages and Protects from Chronic Staphylococcal Infection. Front. Immunol. 2017, 8, 1037. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, T.; Ciarlo, E.; Rigoni, E.; Regina, J.; Le Roy, D.; Roger, T. Dual Deletion of the Sirtuins SIRT2 and SIRT3 Impacts on Metabolism and Inflammatory Responses of Macrophages and Protects From Endotoxemia. Front. Immunol. 2019, 10, 2713. [Google Scholar] [CrossRef]
- Zhang, L.; Kim, S.; Ren, X. The Clinical Significance of SIRT2 in Malignancies: A Tumor Suppressor or an Oncogene? Front. Oncol. 2020, 10, 01721. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Vassilopoulos, A.; Wang, R.-H.; Lahusen, T.; Xiao, Z.; Xu, X.; Li, C.; Veenstra, T.D.; Li, B.; Yu, H.; et al. SIRT2 Maintains Genome Integrity and Suppresses Tumorigenesis through Regulating APC/C Activity. Cancer Cell 2011, 20, 487–499. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.-F.; Wang, N.-Y.; Wang, X.-M.; Liang, S.-T.; Zheng, W.; Lu, Y.-B.; Zhao, X.; Hao, D.-L.; Zhang, Z.-Q.; et al. SIRT2 Acts as a Cardioprotective Deacetylase in Pathological Cardiac Hypertrophy. Circulation 2017, 136, 2051–2067. [Google Scholar] [CrossRef]
- Lantier, L.; Williams, A.S.; Hughey, C.C.; Bracy, D.P.; James, F.D.; Ansari, M.A.; Gius, D.; Wasserman, D.H. SIRT2 Knockout Exacerbates Insulin Resistance in High Fat-Fed Mice. PLoS ONE 2018, 13, e0208634. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, S.K.; Cars, O. Optimizing Drug Exposure to Minimize Selection of Antibiotic Resistance. Clin. Infect. Dis. 2007, 45, S129–S136. [Google Scholar] [CrossRef]
- Raymond, B. Five Rules for Resistance Management in the Antibiotic Apocalypse, a Road Map for Integrated Microbial Management. Evol. Appl. 2019, 12, 1079–1091. [Google Scholar] [CrossRef]
- Bantia, S.; Parker, C.D.; Ananth, S.L.; Horn, L.L.; Andries, K.; Chand, P.; Kotian, P.L.; Dehghani, A.; El-Kattan, Y.; Lin, T.; et al. Comparison of the Anti-Influenza Virus Activity of RWJ-270201 with Those of Oseltamivir and Zanamivir. Antimicrob. Agents Chemother. 2001, 45, 1162–1167. [Google Scholar] [CrossRef]
- He, G.; Massarella, J.; Ward, P. Clinical Pharmacokinetics of the Prodrug Oseltamivir and Its Active Metabolite Ro 64-0802. Clin. Pharmacokinet. 1999, 37, 471–484. [Google Scholar] [CrossRef]
- Davies, B.E. Pharmacokinetics of Oseltamivir: An Oral Antiviral for the Treatment and Prophylaxis of Influenza in Diverse Populations. J. Antimicrob. Chemother. 2010, 65, ii5–ii10. [Google Scholar] [CrossRef] [PubMed]
- Bravo, F.J.; Cardin, R.D.; Bernstein, D.I. A Model of Human Cytomegalovirus Infection in Severe Combined Immunodeficient Mice. Antiviral Res. 2007, 76, 104–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lischka, P.; Hewlett, G.; Wunberg, T.; Baumeister, J.; Paulsen, D.; Goldner, T.; Ruebsamen-Schaeff, H.; Zimmermann, H. InVitro and In Vivo Activities of the Novel Anticytomegalovirus Compound AIC246. Antimicrob. Agents Chemother. 2010, 54, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.; De, C.; Abad Fernandez, M.; Lenarcic, E.M.; Xu, Y.; Cockrell, A.S.; Cleary, R.A.; Johnson, C.E.; Schramm, N.J.; Rank, L.M.; et al. Precision Mouse Models with Expanded Tropism for Human Pathogens. Nat. Biotechnol. 2019, 37, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.; Gralinski, L.E.; Johnson, C.E.; Yao, W.; Kovarova, M.; Dinnon, K.H.; Liu, H.; Madden, V.J.; Krzystek, H.M.; De, C.; et al. SARS-CoV-2 Infection Is Effectively Treated and Prevented by EIDD-2801. Nature 2021, 591, 451–457. [Google Scholar] [CrossRef]
- Li, S.; Guo, L. The Role of Sirtuin 2 in Liver—An Extensive and Complex Biological Process. Life Sci. 2024, 339, 122431. [Google Scholar] [CrossRef]
- Kaya, S.G.; Eren, G. Selective Inhibition of SIRT2: A Disputable Therapeutic Approach in Cancer Therapy. Bioorg. Chem. 2024, 143, 107038. [Google Scholar] [CrossRef]
- Zheng, M.; Hu, C.; Wu, M.; Chin, Y. Emerging Role of SIRT2 in Non-Small Cell Lung Cancer (Review). Oncol. Lett. 2021, 22, 731. [Google Scholar] [CrossRef]
- Roshdy, E.; Mustafa, M.; Shaltout, A.E.-R.; Radwan, M.O.; Ibrahim, M.A.A.; Soliman, M.E.; Fujita, M.; Otsuka, M.; Ali, T.F.S. Selective SIRT2 Inhibitors as Promising Anticancer Therapeutics: An Update from 2016 to 2020. Eur. J. Med. Chem. 2021, 224, 113709. [Google Scholar] [CrossRef]
- Chen, G.; Huang, P.; Hu, C. The Role of SIRT2 in Cancer: A Novel Therapeutic Target. Int. J. Cancer 2020, 147, 3297–3304. [Google Scholar] [CrossRef]
- North, B.J.; Marshall, B.L.; Borra, M.T.; Denu, J.M.; Verdin, E. The Human Sir2 Ortholog, SIRT2, Is an NAD+-Dependent Tubulin Deacetylase. Mol. Cell 2003, 11, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hubbert, C.; Guardiola, A.; Shao, R.; Kawaguchi, Y.; Ito, A.; Nixon, A.; Yoshida, M.; Wang, X.-F.; Yao, T.-P. HDAC6 Is a Microtubule-Associated Deacetylase. Nature 2002, 417, 455–458. [Google Scholar] [CrossRef]
- Skoge, R.H.; Ziegler, M. SIRT2 Inactivation Reveals a Subset of Hyperacetylated Perinuclear Microtubules Inaccessible to HDAC6. J. Cell Sci. 2016, 129, 2972–2982. [Google Scholar] [CrossRef]
- Janke, C.; Montagnac, G. Causes and Consequences of Microtubule Acetylation. Curr. Biol. 2017, 27, R1287–R1292. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.S.; Naghavi, M.H. Microtubules and Viral Infection. Adv. Virus Res. 2023, 115, 87–134. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, V.; Greis, K.D.; Sztul, E.; Britt, W.J. Accumulation of Virion Tegument and Envelope Proteins in a Stable Cytoplasmic Compartment during Human Cytomegalovirus Replication: Characterization of a Potential Site of Virus Assembly. J. Virol. 2000, 74, 975–986. [Google Scholar] [CrossRef]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The CGAS–STING Pathway as a Therapeutic Target in Inflammatory Diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef]
- Liu, Z.-S.; Cai, H.; Xue, W.; Wang, M.; Xia, T.; Li, W.-J.; Xing, J.-Q.; Zhao, M.; Huang, Y.-J.; Chen, S.; et al. G3BP1 Promotes DNA Binding and Activation of CGAS. Nat. Immunol. 2019, 20, 18–28. [Google Scholar] [CrossRef]
- Zhao, M.; Xia, T.; Xing, J.; Yin, L.; Li, X.; Pan, J.; Liu, J.; Sun, L.; Wang, M.; Li, T.; et al. The Stress Granule Protein G3BP1 Promotes Pre-condensation of CGAS to Allow Rapid Responses to DNA. EMBO Rep. 2022, 23, e53166. [Google Scholar] [CrossRef]
- Jayabalan, A.K.; Griffin, D.E.; Leung, A.K.L. Pro-Viral and Anti-Viral Roles of the RNA-Binding Protein G3BP1. Viruses 2023, 15, 449. [Google Scholar] [CrossRef]
- Kim, S.S.-Y.; Sze, L.; Lam, K.-P. The Stress Granule Protein G3BP1 Binds Viral DsRNA and RIG-I to Enhance Interferon-β Response. J. Biol. Chem. 2019, 294, 6430–6438. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ru, Y.; Ren, J.; Bai, J.; Wei, J.; Fu, S.; Liu, X.; Li, D.; Zheng, H. G3BP1 Inhibits RNA Virus Replication by Positively Regulating RIG-I-Mediated Cellular Antiviral Response. Cell Death Dis. 2019, 10, 946. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt Signal Transduction for Cancer Therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Ramakrishnan, G.; Davaakhuu, G.; Kaplun, L.; Chung, W.-C.; Rana, A.; Atfi, A.; Miele, L.; Tzivion, G. Sirt2 Deacetylase Is a Novel AKT Binding Partner Critical for AKT Activation by Insulin. J. Biol. Chem. 2014, 289, 6054–6066. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chan, A.W.H.; To, K.-F.; Chen, W.; Zhang, Z.; Ren, J.; Song, C.; Cheung, Y.-S.; Lai, P.B.S.; Cheng, S.-H.; et al. SIRT2 Overexpression in Hepatocellular Carcinoma Mediates Epithelial to Mesenchymal Transition by Protein Kinase B/Glycogen Synthase Kinase-3β/β-Catenin Signaling. Hepatology 2013, 57, 2287–2298. [Google Scholar] [CrossRef]
- Bellacosa, A.; Testa, J.R.; Staal, S.P.; Tsichlis, P.N. A Retroviral Oncogene, Akt, Encoding a Serine-Threonine Kinase Containing an SH2-like Region. Science 1991, 254, 274–277. [Google Scholar]
- Blanco, J.; Cameirao, C.; López, M.C.; Muñoz-Barroso, I. Phosphatidylinositol-3-Kinase-Akt Pathway in Negative-Stranded RNA Virus Infection: A Minireview. Arch. Virol. 2020, 165, 2165–2176. [Google Scholar] [CrossRef]
- Liu, X.; Cohen, J.I. The Role of PI3K/Akt in Human Herpesvirus Infection: From the Bench to the Bedside. Virology 2015, 479–480, 568–577. [Google Scholar] [CrossRef]
- Bossler, F.; Hoppe-Seyler, K.; Hoppe-Seyler, F. PI3K/AKT/MTOR Signaling Regulates the Virus/Host Cell Crosstalk in HPV-Positive Cervical Cancer Cells. Int. J. Mol. Sci. 2019, 20, 2188. [Google Scholar] [CrossRef]
- Rawat, S.; Bouchard, M.J. The Hepatitis B Virus (HBV) HBx Protein Activates AKT To Simultaneously Regulate HBV Replication and Hepatocyte Survival. J. Virol. 2015, 89, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.; Coombs, K.M.; Kobasa, D. Comprehending a Killer: The Akt/MTOR Signaling Pathways Are Temporally High-Jacked by the Highly Pathogenic 1918 Influenza Virus. EBioMedicine 2018, 32, 142–163. [Google Scholar] [CrossRef] [PubMed]
- Basile, M.S.; Cavalli, E.; McCubrey, J.; Hernández-Bello, J.; Muñoz-Valle, J.F.; Fagone, P.; Nicoletti, F. The PI3K/Akt/MTOR Pathway: A Potential Pharmacological Target in COVID-19. Drug Discov. Today 2022, 27, 848–856. [Google Scholar] [CrossRef]
- Raja, R.; Ronsard, L.; Lata, S.; Trivedi, S.; Banerjea, A.C. HIV-1 Tat Potently Stabilises Mdm2 and Enhances Viral Replication. Biochem. J. 2017, 474, 2449–2464. [Google Scholar] [CrossRef]
- Deregibus, M.C.; Cantaluppi, V.; Doublier, S.; Brizzi, M.F.; Deambrosis, I.; Albini, A.; Camussi, G. HIV-1-Tat Protein Activates Phosphatidylinositol 3-Kinase/ AKT-Dependent Survival Pathways in Kaposi’s Sarcoma Cells. J. Biol. Chem. 2002, 277, 25195–25202. [Google Scholar] [CrossRef]
- Cheung, J.; Remiszewski, S.; Chiang, L.W.; Ahmad, E.; Pal, M.; Rahman, S.A.; Nikolovska-Coleska, Z.; Chan, G.C. Inhibition of SIRT2 Promotes Death of Human Cytomegalovirus-Infected Peripheral Blood Monocytes via Apoptosis and Necroptosis. Antiviral Res. 2023, 217, 105698. [Google Scholar] [CrossRef]
- Chan, G.; Bivins-Smith, E.R.; Smith, M.S.; Smith, P.M.; Yurochko, A.D. Transcriptome Analysis Reveals Human Cytomegalovirus Reprograms Monocyte Differentiation toward an M1 Macrophage. J. Immunol. 2008, 181, 698–711. [Google Scholar] [CrossRef]
- Elder, E.; Krishna, B.; Williamson, J.; Aslam, Y.; Farahi, N.; Wood, A.; Romashova, V.; Roche, K.; Murphy, E.; Chilvers, E.; et al. Monocytes Latently Infected with Human Cytomegalovirus Evade Neutrophil Killing. iScience 2019, 12, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Nogalski, M.T.; Bentz, G.L.; Smith, M.S.; Parmater, A.; Yurochko, A.D. PI3K-Dependent Upregulation of Mcl-1 by Human Cytomegalovirus Is Mediated by Epidermal Growth Factor Receptor and Inhibits Apoptosis in Short-Lived Monocytes. J. Immunol. 2010, 184, 3213–3222. [Google Scholar] [CrossRef]
- Cojohari, O.; Peppenelli, M.A.; Chan, G.C. Human Cytomegalovirus Induces an Atypical Activation of Akt To Stimulate the Survival of Short-Lived Monocytes. J. Virol. 2016, 90, 6443–6452. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Pillai, V.B.; Wolfgeher, D.; Samant, S.; Vasudevan, P.; Parekh, V.; Raghuraman, H.; Cunningham, J.M.; Gupta, M.; Gupta, M.P. The Deacetylase SIRT1 Promotes Membrane Localization and Activation of Akt and PDK1 During Tumorigenesis and Cardiac Hypertrophy. Sci. Signal. 2011, 4, ra46. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.M.; Chevalier, C.; Chaze, T.; Gianetto, Q.; Impens, F.; Matondo, M.; Cossart, P.; Hamon, M.A. Infection Reveals a Modification of SIRT2 Critical for Chromatin Association. Cell Rep. 2018, 23, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Wapenaar, H.; Dekker, F.J. Experimental Approaches Toward Histone Acetyltransferase Inhibitors as Therapeutics. In Medical Epigenetics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 685–704. [Google Scholar]
- Chu, X.; Wu, B.; Fan, H.; Hou, J.; Hao, J.; Hu, J.; Wang, B.; Liu, G.; Li, C.; Meng, S. PTD-Fused P53 as a Potential Antiviral Agent Directly Suppresses HBV Transcription and Expression. Antiviral Res. 2016, 127, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Peuget, S.; Selivanova, G. P53-Dependent Repression: DREAM or Reality? Cancers 2021, 13, 4850. [Google Scholar] [CrossRef]
- Reed, S.; Quelle, D. P53 Acetylation: Regulation and Consequences. Cancers 2014, 7, 30–69. [Google Scholar] [CrossRef]
- Kalle, A.M.; Mallika, A.; Badiger, J.; Alinakhi; Talukdar, P. Sachchidanand Inhibition of SIRT1 by a Small Molecule Induces Apoptosis in Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2010, 401, 13–19. [Google Scholar] [CrossRef]
- Peck, B.; Chen, C.Y.; Ho, K.K.; Di Fruscia, P.; Myatt, S.S.; Coombes, R.C.; Fuchter, M.J.; Hsiao, C.D.; Lam, E.W.F. SIRT Inhibitors Induce Cell Death and P53 Acetylation through Targeting Both SIRT1 and SIRT2. Mol. Cancer Ther. 2010, 9, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, G.; Breitenbücher, F.; Schuler, M.; Ehrenhofer-Murray, A.E. A Novel Sirtuin 2 (SIRT2) Inhibitor with P53-Dependent pro-Apoptotic Activity in Non-Small Cell Lung Cancer. J. Biol. Chem. 2014, 289, 5208–5216. [Google Scholar] [CrossRef]
- Wu, D.-Q.; Ding, Q.-Y.; Tao, N.-N.; Tan, M.; Zhang, Y.; Li, F.; Zhou, Y.-J.; Dong, M.-L.; Cheng, S.-T.; Ren, F.; et al. SIRT2 Promotes HBV Transcription and Replication by Targeting Transcription Factor P53 to Increase the Activities of HBV Enhancers and Promoters. Front. Microbiol. 2022, 13, 836446. [Google Scholar] [CrossRef]
- Hamaidi, I.; Zhang, L.; Kim, N.; Wang, M.-H.; Iclozan, C.; Fang, B.; Liu, M.; Koomen, J.M.; Berglund, A.E.; Yoder, S.J.; et al. Sirt2 Inhibition Enhances Metabolic Fitness and Effector Functions of Tumor-Reactive T Cells. Cell Metab. 2020, 32, 420–436.e12. [Google Scholar] [CrossRef]
- Hamaidi, I.; Kim, S. Sirtuins Are Crucial Regulators of T Cell Metabolism and Functions. Exp. Mol. Med. 2022, 54, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Li, M.; Hou, T.; Gao, T.; Zhu, W.; Yang, Y. Sirtuins in Glucose and Lipid Metabolism. Oncotarget 2017, 8, 1845–1859. [Google Scholar] [CrossRef]
- Girdhar, K.; Powis, A.; Raisingani, A.; Chrudinová, M.; Huang, R.; Tran, T.; Sevgi, K.; Dogus Dogru, Y.; Altindis, E. Viruses and Metabolism: The Effects of Viral Infections and Viral Insulins on Host Metabolism. Annu. Rev. Virol. 2021, 8, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Hensley, L.; McKnight, K.L.; Hu, F.; Madden, V.; Ping, L.; Jeong, S.-H.; Walker, C.; Lanford, R.E.; Lemon, S.M. A Pathogenic Picornavirus Acquires an Envelope by Hijacking Cellular Membranes. Nature 2013, 496, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Shiota, T.; Li, Z.; Chen, G.-Y.; McKnight, K.L.; Shirasaki, T.; Yonish, B.; Kim, H.; Fritch, E.J.; Sheahan, T.P.; Muramatsu, M.; et al. Hepatoviruses Promote Very-Long-Chain Fatty Acid and Sphingolipid Synthesis for Viral RNA Replication and Quasi-Enveloped Virus Release. Sci. Adv. 2023, 9, eadj4198. [Google Scholar] [CrossRef]
- Purdy, J.G.; Shenk, T.; Rabinowitz, J.D. Fatty Acid Elongase 7 Catalyzes Lipidome Remodeling Essential for Human Cytomegalovirus Replication. Cell Rep. 2015, 10, 1375–1385. [Google Scholar] [CrossRef]
- Murray, L.A.; Sheng, X.; Cristea, I.M. Orchestration of Protein Acetylation as a Toggle for Cellular Defense and Virus Replication. Nat. Commun. 2018, 9, 4967. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Feng, T.; Chen, Z.; Yan, Y.; Chen, Z.; Dai, J. Protein Acetylation Going Viral: Implications in Antiviral Immunity and Viral Infection. Int. J. Mol. Sci. 2022, 23, 11308. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A. Infection in Organ Transplantation. Am. J. Transplant. 2017, 17, 856–879. [Google Scholar] [CrossRef]
- Taneja, A.; Chewning, J.H.; Saad, A. Viral Infections after Allogeneic Hematopoietic Stem Cell Transplant. Adv. CELL GENE Ther. 2019, 2, e43. [Google Scholar] [CrossRef]
- Manansala, M.; Baughman, R.; Novak, R.; Judson, M.; Sweiss, N. Management of Immunosuppressants in the Era of Coronavirus Disease-2019. Curr. Opin. Pulm. Med. 2021, 27, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Liu, X.; Li, X.; Kong, H.; Tian, L.; Chen, Y. T-Cell Exhaustion in Chronic Hepatitis B Infection: Current Knowledge and Clinical Significance. Cell Death Dis. 2015, 6, e1694. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, P.; Boni, C.; Barili, V.; Laccabue, D.; Ferrari, C. Strategies to Overcome HBV-Specific T Cell Exhaustion: Checkpoint Inhibitors and Metabolic Re-Programming. Curr. Opin. Virol. 2018, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, A.; Pahuja, I.; Negi, K.; Verma, A.; Ghoshal, A.; Mathew, B.; Tripathi, G.; Maras, J.S.; Chaturvedi, S.; Dwivedi, V.P. SIRT2 Inhibition by AGK2 Enhances Mycobacteria-Specific Stem Cell Memory Responses by Modulating Beta-Catenin and Glycolysis. iScience 2023, 26, 106644. [Google Scholar] [CrossRef]
- O’Sullivan, D. The Metabolic Spectrum of Memory T Cells. Immunol. Cell Biol. 2019, 97, 636–646. [Google Scholar] [CrossRef]
- Tsogtbaatar, E.; Landin, C.; Minter-Dykhouse, K.; Folmes, C.D.L. Energy Metabolism Regulates Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 87. [Google Scholar] [CrossRef]
- Rapaka, R.R.; Cross, A.S.; McArthur, M.A. Using Adjuvants to Drive T Cell Responses for Next-Generation Infectious Disease Vaccines. Vaccines 2021, 9, 820. [Google Scholar] [CrossRef]
- Rothgiesser, K.M.; Erener, S.; Waibel, S.; Luscher, B.; Hottiger, M.O. SIRT2 Regulates NF- B-Dependent Gene Expression through Deacetylation of P65 Lys310. J. Cell Sci. 2010, 123, 4251–4258. [Google Scholar] [CrossRef]
- Lugrin, J.Ô.; Ciarlo, E.; Santos, A.; Grandmaison, G.; Dos Santos, I.; Le Roy, D.; Roger, T. The Sirtuin Inhibitor Cambinol Impairs MAPK Signaling, Inhibits Inflammatory and Innate Immune Responses and Protects from Septic Shock. Biochim. Biophys. Acta—Mol. Cell Res. 2013, 1833, 1498–1510. [Google Scholar] [CrossRef]
- Yuan, F.; Xu, Z.; Lu, L.; Nie, H.; Ding, J.; Ying, W.; Tian, H. SIRT2 Inhibition Exacerbates Neuroinflammation and Blood–Brain Barrier Disruption in Experimental Traumatic Brain Injury by Enhancing NF-κB P65 Acetylation and Activation. J. Neurochem. 2016, 136, 581–593. [Google Scholar] [CrossRef]
- Lee, A.S.; Jung, Y.J.; Kim, D.; Nguyen-Thanh, T.; Kang, K.P.; Lee, S.; Park, S.K.; Kim, W. SIRT2 Ameliorates Lipopolysaccharide-Induced Inflammation in Macrophages. Biochem. Biophys. Res. Commun. 2014, 450, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Anoopkumar-Dukie, S.; Mallik, S.B.; Davey, A.K. SIRT1 and SIRT2 Modulators Reduce LPS-Induced Inflammation in HAPI Microglial Cells and Protect SH-SY5Y Neuronal Cells in Vitro. J. Neural Transm. 2021, 128, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Wang, Y.; Zhang, W.; Zhang, H.; Chen, Q.; Wang, L.; Shi, C.; Gong, Z. AGK2 Alleviates Lipopolysaccharide Induced Neuroinflammation through Regulation of Mitogen-Activated Protein Kinase Phosphatase-1. J. Neuroimmune Pharmacol. 2020, 15, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Ji, H.; Wu, D. SIRT2 Plays Complex Roles in Neuroinflammation Neuroimmunology-Associated Disorders. Front. Immunol. 2023, 14, 1174180. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef]
- van der Poll, T.; Shankar-Hari, M.; Wiersinga, W.J. The Immunology of Sepsis. Immunity 2021, 54, 2450–2464. [Google Scholar] [CrossRef]
- Rello, J.; Valenzuela-Sánchez, F.; Ruiz-Rodriguez, M.; Moyano, S. Sepsis: A Review of Advances in Management. Adv. Ther. 2017, 34, 2393–2411. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-Induced Immunosuppression: From Cellular Dysfunctions to Immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Torres, L.K.; Pickkers, P.; van der Poll, T. Sepsis-Induced Immunosuppression. Annu. Rev. Physiol. 2022, 84, 157–181. [Google Scholar] [CrossRef]
- Herminghaus, A.; Osuchowski, M.F. How Sepsis Parallels and Differs from COVID-19. eBioMedicine 2022, 86, 104355. [Google Scholar] [CrossRef]
- Deitch, E.A. RODENT MODELS OF INTRA-ABDOMINAL INFECTION. Shock 2005, 24, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Raven, K. Rodent Models of Sepsis Found Shockingly Lacking. Nat. Med. 2012, 18, 998. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.; Seymour, C.W.; Rosengart, M.R. Current Murine Models of Sepsis. Surg. Infect. 2016, 17, 385–393. [Google Scholar] [CrossRef]
- Doi, K.; Leelahavanichkul, A.; Yuen, P.S.T.; Star, R.A. Animal Models of Sepsis and Sepsis-Induced Kidney Injury. J. Clin. Investig. 2009, 119, 2868–2878. [Google Scholar] [CrossRef]
- Zhao, T.; Alam, H.B.; Liu, B.; Bronson, R.T.; Nikolian, V.C.; Wu, E.; Chong, W.; Li, Y. Selective Inhibition of SIRT2 Improves Outcomes in a Lethal Septic Model. Curr. Mol. Med. 2015, 15, 634–641. [Google Scholar] [CrossRef]
- Wang, X.; Buechler, N.L.; Martin, A.; Wells, J.; Yoza, B.; McCall, C.E.; Vachharajani, V. Sirtuin-2 Regulates Sepsis Inflammation in Ob/Ob Mice. PLoS ONE 2016, 11, e0160431. [Google Scholar] [CrossRef] [PubMed]
- Buechler, N.; Wang, X.; Yoza, B.K.; McCall, C.E.; Vachharajani, V. Sirtuin 2 Regulates Microvascular Inflammation during Sepsis. J. Immunol. Res. 2017, 2017, 2648946. [Google Scholar] [CrossRef]
- Akinnusi, M.E.; Pineda, L.A.; El Solh, A.A. Effect of Obesity on Intensive Care Morbidity and Mortality: A Meta-Analysis*. Crit. Care Med. 2008, 36, 151–158. [Google Scholar] [CrossRef]
- Krishnan, J.; Danzer, C.; Simka, T.; Ukropec, J.; Walter, K.M.; Kumpf, S.; Mirtschink, P.; Ukropcova, B.; Gasperikova, D.; Pedrazzini, T.; et al. Dietary Obesity-Associated Hif1α Activation in Adipocytes Restricts Fatty Acid Oxidation and Energy Expenditure via Suppression of the Sirt2-NAD + System. Genes Dev. 2012, 26, 259–270. [Google Scholar] [CrossRef]
- Marandu, T.; Dombek, M.; Cook, C.H. Impact of Cytomegalovirus Load on Host Response to Sepsis. Med. Microbiol. Immunol. 2019, 208, 295–303. [Google Scholar] [CrossRef]
- Walton, A.H.; Muenzer, J.T.; Rasche, D.; Boomer, J.S.; Sato, B.; Brownstein, B.H.; Pachot, A.; Brooks, T.L.; Deych, E.; Shannon, W.D.; et al. Reactivation of Multiple Viruses in Patients with Sepsis. PLoS ONE 2014, 9, e98819. [Google Scholar] [CrossRef]
- Stein, J.; Volk, H.-D.; Liebenthal, C.; Kruger, D.H.; Prosch, S. Tumour Necrosis Factor Stimulates the Activity of the Human Cytomegalovirus Major Immediate Early Enhancer/Promoter in Immature Monocytic Cells. J. Gen. Virol. 1993, 74, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Limaye, A.P.; Kirby, K.A.; Rubenfeld, G.D.; Leisenring, W.M.; Bulger, E.M.; Neff, M.J.; Gibran, N.S.; Huang, M.L.; Santo Hayes, T.K.; Corey, L.; et al. Cytomegalovirus Reactivation in Critically Ill Immunocompetent Patients. JAMA 2008, 300, 413. [Google Scholar] [CrossRef]
- Lachance, P.; Chen, J.; Featherstone, R.; Sligl, W.I. Association Between Cytomegalovirus Reactivation and Clinical Outcomes in Immunocompetent Critically Ill Patients: A Systematic Review and Meta-Analysis. Open Forum Infect. Dis. 2017, 4, ofx029. [Google Scholar] [CrossRef]
- Mansfield, S.A.; Cook, C.H. Antiviral Prophylaxis of Cytomegalovirus Reactivation in Immune Competent Patients—The Jury Remains Out. J. Thorac. Dis. 2017, 9, 2221–2223. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, H.; Lee, S.H.; Jin, Y.-H.; Lee, K.Y. Src Regulates the Activity of SIRT2. Biochem. Biophys. Res. Commun. 2014, 450, 1120–1125. [Google Scholar] [CrossRef]
- North, B.J.; Verdin, E. Mitotic Regulation of SIRT2 by Cyclin-Dependent Kinase 1-Dependent Phosphorylation. J. Biol. Chem. 2007, 282, 19546–19555. [Google Scholar] [CrossRef] [PubMed]
- Budayeva, H.G.; Cristea, I.M. Human Sirtuin 2 Localization, Transient Interactions, and Impact on the Proteome Point to Its Role in Intracellular Trafficking. Mol. Cell. Proteomics 2016, 15, 3107–3125. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.; Ambrosy, B.; Planz, O.; Schloer, S.; Rescher, U.; Ludwig, S. The MEK1/2 Inhibitor ATR-002 (Zapnometinib) Synergistically Potentiates the Antiviral Effect of Direct-Acting Anti-SARS-CoV-2 Drugs. Pharmaceutics 2022, 14, 1776. [Google Scholar] [CrossRef]
- Ren, J.-H.; Tao, Y.; Zhang, Z.-Z.; Chen, W.-X.; Cai, X.-F.; Chen, K.; Ko, B.C.B.; Song, C.-L.; Ran, L.-K.; Li, W.-Y.; et al. Sirtuin 1 Regulates Hepatitis B Virus Transcription and Replication by Targeting Transcription Factor AP-1. J. Virol. 2014, 88, 2442–2451. [Google Scholar] [CrossRef]
- Deng, J.-J.; Kong, K.-Y.E.; Gao, W.-W.; Tang, H.-M.V.; Chaudhary, V.; Cheng, Y.; Zhou, J.; Chan, C.-P.; Wong, D.K.-H.; Yuen, M.-F.; et al. Interplay between SIRT1 and Hepatitis B Virus X Protein in the Activation of Viral Transcription. Biochim. Biophys. Acta—Gene Regul. Mech. 2017, 1860, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Yamai, T.; Hikita, H.; Fukuoka, M.; Fukutomi, K.; Murai, K.; Nakabori, T.; Yamada, R.; Miyakawa, K.; Watashi, K.; Ryo, A.; et al. SIRT1 Enhances Hepatitis Virus B Transcription Independent of Hepatic Autophagy. Biochem. Biophys. Res. Commun. 2020, 527, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Meng, C.; Liu, Y.; Cheng, Y.; Liu, Y.; Long, Y.; Sun, S.; Feng, F. Silencing SIRT1 Promotes the Anti-HBV Action of IFN-α by Regulating Pol Expression and Activating the JAK-STAT Signaling Pathway. Int. Immunopharmacol. 2023, 124, 110939. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, M.; Zong, R.; Fan, C.; Ren, F.; Wu, W.; Li, C. Deacetylation of Notch1 by SIRT1 Contributes to HBsAg- and HBeAg-Mediated M2 Macrophage Polarization. Am. J. Physiol. Liver Physiol. 2022, 322, G459–G471. [Google Scholar] [CrossRef]
- Pagans, S.; Pedal, A.; North, B.J.; Kaehlcke, K.; Marshall, B.L.; Dorr, A.; Hetzer-Egger, C.; Henklein, P.; Frye, R.; McBurney, M.W.; et al. SIRT1 Regulates HIV Transcription via Tat Deacetylation. PLoS Biol 2005, 3, e41. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Wang, L.; Aliyari, S.; Cheng, G. SARS-CoV-2 Virus NSP14 Impairs NRF2/HMOX1 Activation by Targeting Sirtuin 1. Cell. Mol. Immunol. 2022, 19, 872–882. [Google Scholar] [CrossRef]
- Walter, M.; Chen, I.P.; Vallejo-Gracia, A.; Kim, I.-J.; Bielska, O.; Lam, V.L.; Hayashi, J.M.; Cruz, A.; Shah, S.; Soveg, F.W.; et al. SIRT5 Is a Proviral Factor That Interacts with SARS-CoV-2 Nsp14 Protein. PLoS Pathog. 2022, 18, e1010811. [Google Scholar] [CrossRef]
- Diner, B.A.; Lum, K.K.; Toettcher, J.E.; Cristea, I.M. Viral DNA Sensors IFI16 and Cyclic GMP-AMP Synthase Possess Distinct Functions in Regulating Viral Gene Expression, Immune Defenses, and Apoptotic Responses during Herpesvirus Infection. MBio 2016, 7, e01553-16. [Google Scholar] [CrossRef]
- Almine, J.F.; O’Hare, C.A.J.; Dunphy, G.; Haga, I.R.; Naik, R.J.; Atrih, A.; Connolly, D.J.; Taylor, J.; Kelsall, I.R.; Bowie, A.G.; et al. IFI16 and CGAS Cooperate in the Activation of STING during DNA Sensing in Human Keratinocytes. Nat. Commun. 2017, 8, 14392. [Google Scholar] [CrossRef]
- Broussy, S.; Laaroussi, H.; Vidal, M. Biochemical Mechanism and Biological Effects of the Inhibition of Silent Information Regulator 1 (SIRT1) by EX-527 (SEN0014196 or Selisistat). J. Enzyme Inhib. Med. Chem. 2020, 35, 1124–1136. [Google Scholar] [CrossRef]

| Infectious Agent | SIRT2 Modulator | Assay (Cell Type) | IC50 (µM) a | CC50 (µM) b | Reference |
|---|---|---|---|---|---|
| DNA viruses | |||||
| Human cytomegalovirus, strain TB40/E (herpesvirus) | FLS-359 | Cell-to-cell spread (MRC-5) | 0.5 ± 0.2 | >15.8 | [22] |
| Hepatitis B virus genotype D (hepadnavirus) | FLS-359 AGK2 | cccDNA establishment (C3A-NTCP) Virus yield (PHH c) Viral RNAs and proteins (Huh7; HepAD38; HepG2-NTCP) | <0.6 4.8 ND d | >10 >10 ND | [34] [22] [35,36] |
| Epstein–Barr virus, strain Akata (herpesvirus) | FLS-359 | EBV gp350 expression (Akata) | 3.8 | >100 | [22] |
| Herpes simplex 1 (herpesvirus) | AGK2 | Virus yield (THP-1 and HeLa) | ~5.0 | ND | [37] |
| RNA viruses | |||||
| SARS-CoV-2 (coronavirus) | FLS-359 | Virus yield (Calu-3) | 0.3 | 15.8 | [22] |
| Zika DAK-41525 strain (flavivirus) | FLS-359 | Virus yield (HFF e) | 0.4 | 41.6 | [22] |
| Influenza A H1N1 (orthomyxovirus) | FLS-359 | Virus yield (dNHBE f) | 1.2 g | >100 | [22] |
| OC43 (coronavirus) | FLS-359 | CPE reduction (MRC-5) | 1.7 | >50 | [22] |
| Junin, Candid 1 strain (arenavirus) | FLS-359 | Virion antigen reduction (HFF e) | 3.2 | >25 | [22] |
| Respiratory syncytial virus, long strain (orthopneumovirus) | FLS-359 | Virion antigen reduction (MRC-5) | 6.7 | >12.5 | [22] |
| Hepatitis A virus strain HA11-1299 (picornavirus) | Sirtinol h | Intracellular viral RNA (Huh7) | ND | ND | [38] |
| Dengue types 1-4 (flavivirus) | Tenovin-1 h | Virus yield (BHK-21) | 1.0–3.8 | ND | [39] |
| West Nile virus strain Kunjin (flavivirus); Rift Valley fever virus strain MP12 (bunyavirus) | Tenovin-1 h Sirtinol h | Intracellular viral RNA (U2OS) | Tenovin-1: 0.4–2.0 Sirtinol: 8.0–40 i | Tenovin-1: >10 Sirtinol: >200 | [40] |
| Retroviruses | |||||
| HIV NL4-3 | AK-1 | Reduce viral p24 (T cells; MDMs) Reactivate from latency (J-LAT; primary glial) | ND d | >50 | [41] |
| Bacteria | |||||
| Listeria monocytogenes | AGK2 | Reduce bacterial colony forming units per infected cell (Caco2) and in mice | ND d | >20 | [18] |
| Mycobacterium tuberculosis | AGK2 | Reduce bacterial colony forming units per infected macrophage and in mice | ND d | ND | [42] |
| Salmonella typhimurium | AK-7 | Different in vivo versus cultured cell result j | ND | ND | [43] |
| Small Molecule | Structure | Chemical Name [Reference] a |
|---|---|---|
| SIRT2 selective | ||
| FLS-359 |  | 7-(2,4-dimethyl-1H-imidazol-1-yl)-2-(5-{[4-(1H-pyrazol-1-yl)phenyl]methyl}-1,3-thiazol-2-yl)-1,2,3,4-tetrahydroisoquinoline [22] |
| AGK2 |  | 2-cyano-3-[5-(2,5-dichlorophenyl)-2-furanyl]-N-5-quinolinyl-2-propenamide [23] |
| AK-1 |  | 3-(azepan-1-ylsulfonyl)-N-(3-nitrophenyl)benzamide [24] |
| AK-7 | 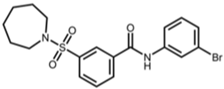 | 3-(azepan-1-ylsulfonyl)-N-(3-bromophenyl)benzamide [25] |
| SIRT1 and SIRT2 | ||
| Sirtinol |  | 2-[(2-hydroxynaphthalen-1-ylmethylene)amino]-N-(1-phenethyl)benzamide [27] |
| Tenovin-1 | 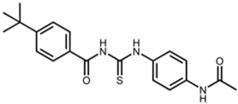 | N-[(4-acetamidophenyl)carbamothioyl]-4-tert-butylbenzamide [28] |
| Lysine Modification | Peptide a [Reference] |
|---|---|
| Acetyl, Propionyl, Butyryl, Hexanoyl, Octanoyl, Decanoyl, Dodecanyl, Myristoyl, Crotonyl | H3K9 [48] |
| Methacryl | H3K18 [50] |
| Lipoyl | PDH-E2K259 b [51] |
| Benzoyl | H2BK5 [52] |
| Lactoyl | PKM2K305 c [53] |
| 4-Oxononanoyl | H3K27 [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shenk, T.; Kulp III, J.L.; Chiang, L.W. Drugs Targeting Sirtuin 2 Exhibit Broad-Spectrum Anti-Infective Activity. Pharmaceuticals 2024, 17, 1298. https://doi.org/10.3390/ph17101298
Shenk T, Kulp III JL, Chiang LW. Drugs Targeting Sirtuin 2 Exhibit Broad-Spectrum Anti-Infective Activity. Pharmaceuticals. 2024; 17(10):1298. https://doi.org/10.3390/ph17101298
Chicago/Turabian StyleShenk, Thomas, John L. Kulp III, and Lillian W. Chiang. 2024. "Drugs Targeting Sirtuin 2 Exhibit Broad-Spectrum Anti-Infective Activity" Pharmaceuticals 17, no. 10: 1298. https://doi.org/10.3390/ph17101298
APA StyleShenk, T., Kulp III, J. L., & Chiang, L. W. (2024). Drugs Targeting Sirtuin 2 Exhibit Broad-Spectrum Anti-Infective Activity. Pharmaceuticals, 17(10), 1298. https://doi.org/10.3390/ph17101298







