The Potential Role of Boron in the Modulation of Gut Microbiota Composition: An In Vivo Pilot Study
Abstract
1. Introduction
1.1. Boron
1.2. Boron and Microbiota: Exploring Links
2. Results
2.1. In Silico Toxicity Assay
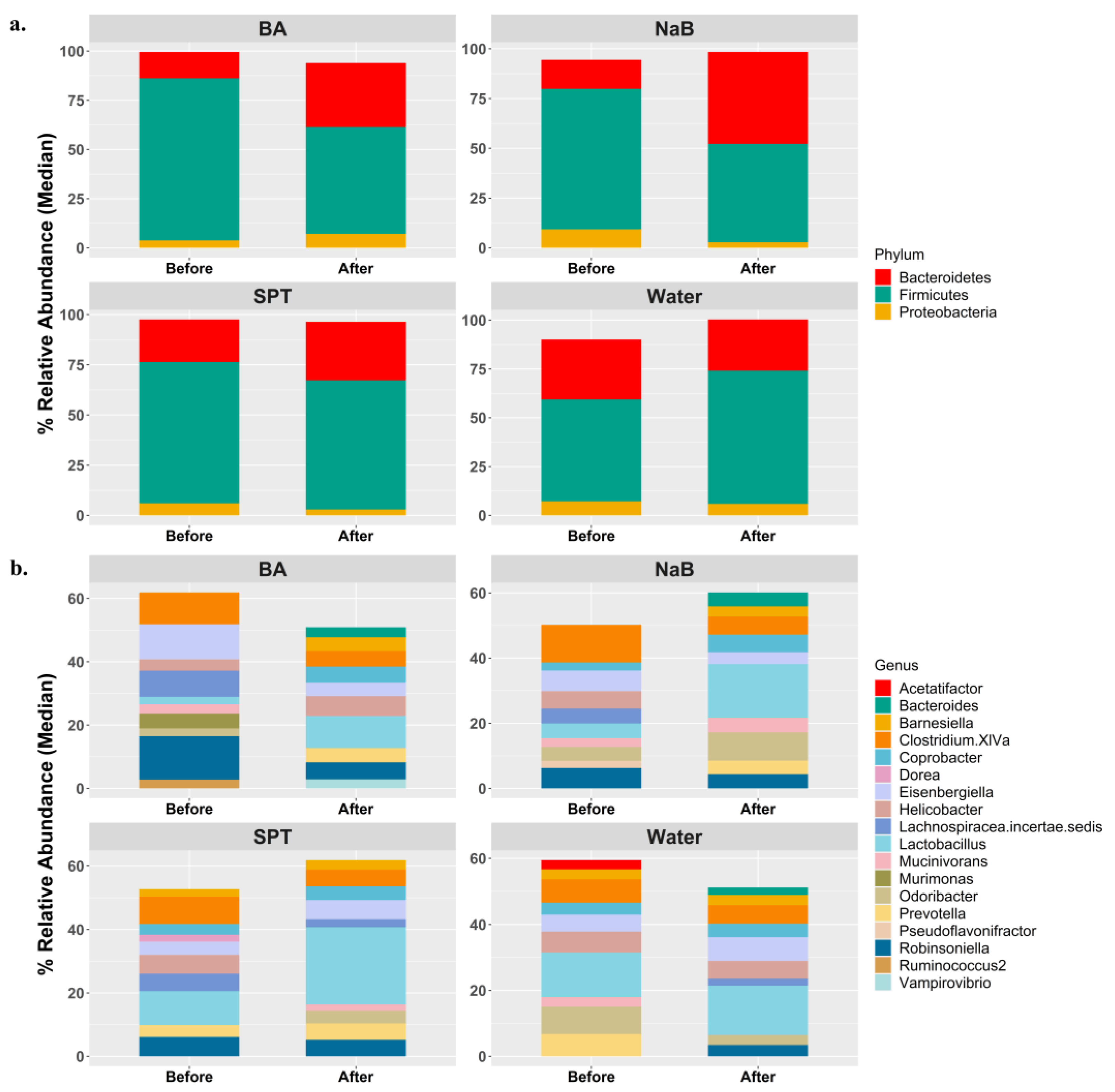
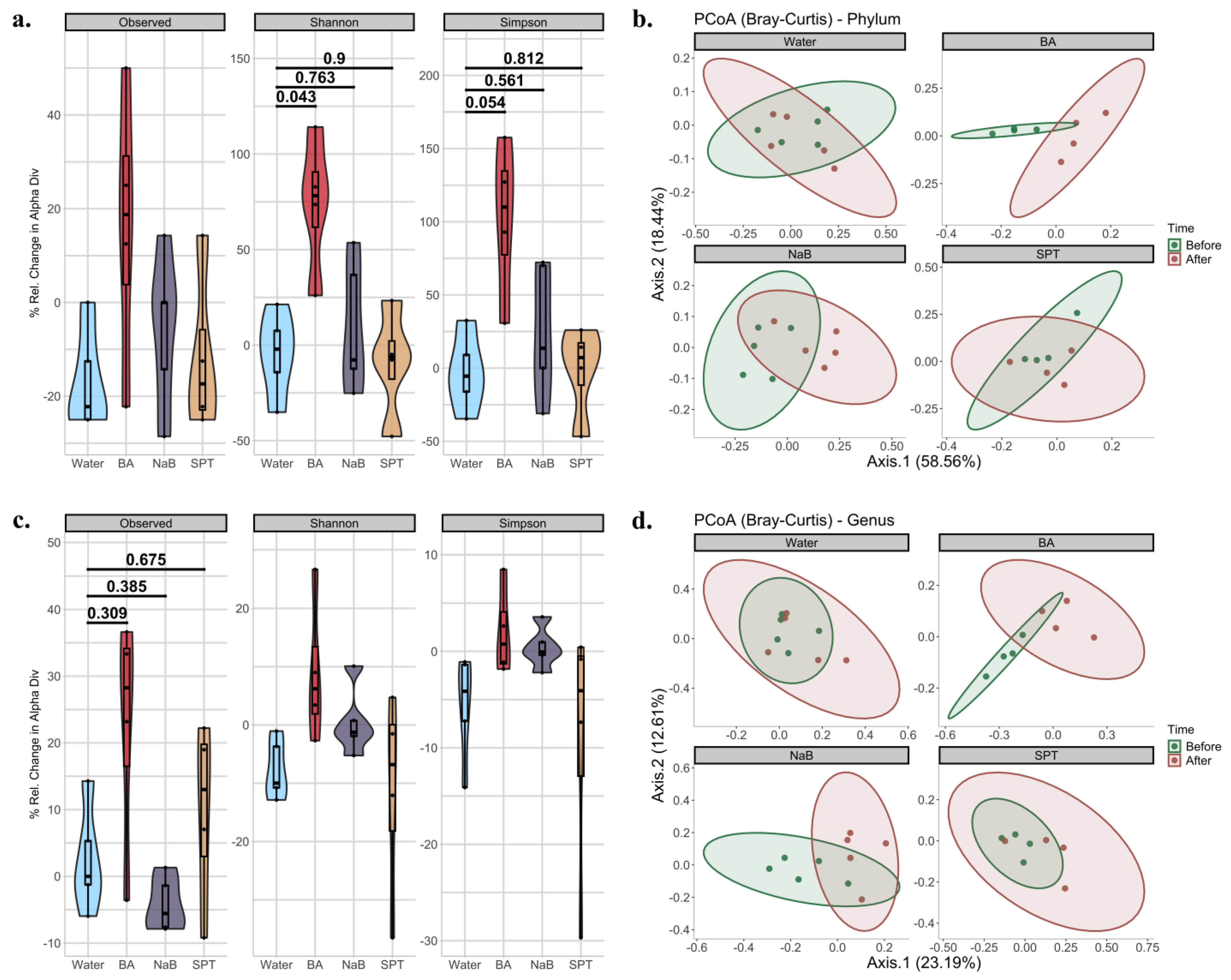
2.2. Gut Bacterial Diversity in Mice Treated with Different Boron Compounds
2.3. Alterations in Gut Microbiota Compositions in Response to Boron
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Mouse Model Handling and Boron Application
4.3. DNA Isolation, Library Preparation, and Sequencing
4.4. In Silico Analysis
4.5. Bioinformatics and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The Role of the Gut Microbiome and Its Metabolites in Metabolic Diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Nakatsu, Y.; Kushiyama, A.; Yamamotoya, T.; Matsunaga, Y.; Inoue, M.-K.; Fujishiro, M.; Sakoda, H.; Ohno, H.; Yoneda, M.; et al. Gut Microbiota as a Therapeutic Target for Metabolic Disorders. Curr. Med. Chem. 2018, 25, 984–1001. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Goodrich, J.K.; Jackson, M.A.; Yet, I.; Davenport, E.R.; Vieira-Silva, S.; Debelius, J.; Pallister, T.; Mangino, M.; Raes, J.; et al. Heritable Components of the Human Fecal Microbiome Are Associated with Visceral Fat. Genome Biol. 2016, 17, 189. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, 36–44. [Google Scholar] [CrossRef]
- Baquero, F.; Nombela, C. The Microbiome as a Human Organ. Clin. Microbiol. Infect. 2012, 18, 2–4. [Google Scholar] [CrossRef]
- Hirschberg, S.; Gisevius, B.; Duscha, A.; Haghikia, A. Implications of Diet and the Gut Microbiome in Neuroinflammatory and Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3109. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Shan, Y.; Lee, M.; Chang, E.B. The Gut Microbiome and Inflammatory Bowel Diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of Gut Bacteria on Human Health and Diseases. Int. J. Mol. Sci. 2015, 16, 7493. [Google Scholar] [CrossRef]
- Kochar, B.; Orkaby, A.R.; Ananthakrishnan, A.N.; Ritchie, C.S. Frailty in Inflammatory Bowel Diseases: An Emerging Concept. Therap. Adv. Gastroenterol. 2021, 14, 17562848211025474. [Google Scholar] [CrossRef] [PubMed]
- Cardinelli, C.S.; Sala, P.C.; Alves, C.C.; Torrinhas, R.S.; Waitzberg, D.L. Influence of Intestinal Microbiota on Body Weight Gain: A Narrative Review of the Literature. Obes. Surg. 2015, 25, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-Balance Studies Reveal Associations between Gut Microbes, Caloric Load, and Nutrient Absorption in Humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyöty, H.; et al. Toward Defining the Autoimmune Microbiome for Type 1 Diabetes. ISME J. 2011, 5, 82–91. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Akbar, N.; Khan, N.A.; Muhammad, J.S.; Siddiqui, R. The Role of Gut Microbiome in Cancer Genesis and Cancer Prevention. Heal. Sci. Rev. 2022, 2, 100010. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Leblhuber, F.; Steiner, K.; Geisler, S.; Fuchs, D.; Gostner, J.M. On the Possible Relevance of Bottom-up Pathways in the Pathogenesis of Alzheimer’s Disease. Curr. Top. Med. Chem. 2020, 20, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Soldan, M.M.P.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple Sclerosis Patients Have a Distinct Gut Microbiota Compared to Healthy Controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain. Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Wu, S.; Yi, J.; Zhang, Y.G.; Zhou, J.; Sun, J. Leaky Intestine and Impaired Microbiome in an Amyotrophic Lateral Sclerosis Mouse Model. Physiol. Rep. 2015, 3, e12356. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Wu, S.; Yi, J.; Xia, Y.; Jin, D.; Zhou, J.; Sun, J. Target Intestinal Microbiota to Alleviate Disease Progression in Amyotrophic Sclerosis. Clin. Ther. 2017, 39, 322. [Google Scholar] [CrossRef]
- Blacher, E.; Bashiardes, S.; Shapiro, H.; Rothschild, D.; Mor, U.; Dori-Bachash, M.; Kleimeyer, C.; Moresi, C.; Harnik, Y.; Zur, M.; et al. Potential Roles of Gut Microbiome and Metabolites in Modulating ALS in Mice. Nature 2019, 572, 474–480. [Google Scholar] [CrossRef]
- World Health Organization; International Atomic Energy Agency; Food and Agriculture Organization of the United Nations. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Biţă, A.; Scorei, I.R.; Bălşeanu, T.A.; Ciocîlteu, M.V.; Bejenaru, C.; Radu, A.; Bejenaru, L.E.; Rău, G.; Mogoşanu, G.D.; Neamţu, J.; et al. New Insights into Boron Essentiality in Humans and Animals. Int. J. Mol. Sci. 2022, 23, 9147. [Google Scholar] [CrossRef]
- Cebeci, E.; Yüksel, B.; Şahin, F. Anti-Cancer Effect of Boron Derivatives on Small-Cell Lung Cancer. J. Trace Elem. Med. Biol. 2022, 70, 126923. [Google Scholar] [CrossRef] [PubMed]
- Devirian, T.; Volpe, S. The Physiological Effects of Dietary Boron. Crit. Rev. Food Sci. Nutr. 2003, 43, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Angenent, P. Advances in Boron-based Drugs in Medicinal Chemistry. Arch. Chem. Res. Adv. Boron-based Drugs Med. Chem. 2023, 7, 4657. [Google Scholar]
- Brittingham, A.; Wilson, W.A. The Antimicrobial Effect of Boric Acid on Trichomonas vaginalis. Sex. Transm. Dis. 2014, 41, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Trippier, P.; McGuigan, C. Boronic Acids in Medicinal Chemistry: Anticancer, Antibacterial and Antiviral Applications. Medchemcomm 2010, 1, 183–198. [Google Scholar] [CrossRef]
- Demirci, S.; Kaya, M.S.; Doğan, A.; Kalay, Ş.; Altin, N.Ö.; Yarat, A.; Akyüz, S.H.; Şahin, F. Antibacterial and Cytotoxic Properties of Boron-Containing Dental Composite. Turk. J. Biol. 2015, 39, 417–426. [Google Scholar] [CrossRef]
- Demirci, S.; Doğan, A.; Karakuş, E.; Halıcı, Z.; Topçu, A.; Demirci, E.; Sahin, F. Boron and Poloxamer (F68 and F127) Containing Hydrogel Formulation for Burn Wound Healing. Biol. Trace Elem. Res. 2015, 168, 169–180. [Google Scholar] [CrossRef]
- Doğan, A.; Demirci, S.; Çağlayan, A.B.; Kılıç, E.; Günal, M.Y.; Uslu, Ü.; Cumbul, A.; Şahin, F. Sodium Pentaborate Pentahydrate and Pluronic Containing Hydrogel Increases Cutaneous Wound Healing in Vitro and in Vivo. Biol. Trace Elem. Res. 2014, 162, 72–79. [Google Scholar] [CrossRef]
- Coskun, M. Success in Treating Wounds with Local Boric Acid: A Case Study. J. Wound Care 2023, 32, 686–690. [Google Scholar] [CrossRef]
- Nielsen, F.H. Update on Human Health Effects of Boron. J. Trace Elem. Med. Biol. 2014, 28, 383–387. [Google Scholar] [CrossRef]
- Sheng, M.H.; Janette Taper, L.; Veit, H.; Qian, H.; Ritchey, S.J.; William Lau, K.H. Dietary Boron Supplementation Enhanced the Action of Estrogen, but Not That of Parathyroid Hormone, to Improve Trabecular Bone Quality in Ovariectomized Rats. Biol. Trace Elem. Res. 2001, 82, 109–123. [Google Scholar] [CrossRef]
- Penland, J.G. Dietary Boron, Brain Function, and Cognitive Performance. Environ. Health Perspect. 1994, 102, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Penland, J.G. The Importance of Boron Nutrition for Brain and Psychological Function. Biol. Trace Elem. Res. 1998, 66, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Denny, W.; Dos Santos, J. Boron in Drug Design: Recent Advances in the Development of New Therapeutic Agents. Eur. J. Med. Chem. 2019, 179, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Thapa, P.; Karki, R.; Schinke, C.; Das, S.; Kambhampati, S.; Banerjee, S.K.; Van Veldhuizen, P.; Verma, A.; Weiss, L.M.; et al. Boron Chemicals in Diagnosis and Therapeutics. Future Med. Chem. 2013, 5, 653–676. [Google Scholar] [CrossRef]
- Baker, S.J.; Ding, C.Z.; Akama, T.; Zhang, Y.-K.; Hernandez, V.; Xia, Y. Therapeutic Potential of Boron-Containing Compounds. Future Med. Chem. 2009, 1, 1275–1288. [Google Scholar] [CrossRef]
- Barranco, W.T.; Kim, D.H.; Stella, S.L.; Eckhert, C.D. Boric Acid Inhibits Stored Ca2+ Release in DU-145 Prostate Cancer Cells. Cell Biol. Toxicol. 2009, 25, 309–320. [Google Scholar] [CrossRef]
- Adams, J.; Behnke, M.; Chen, S.; Cruickshank, A.A.; Dick, L.R.; Grenier, L.; Klunder, J.M.; Ma, Y.-T.; Plamondon, L.; Stein, R.L. Potent and Selective Inhibitors of the Proteasome: Dipeptidyl Boronic Acids. Bioorg. Med. Chem. Lett. 1998, 8, 333–338. [Google Scholar] [CrossRef]
- Scorei, R.; Popa, R. Boron-Containing Compounds as Preventive and Chemotherapeutic Agents for Cancer. Anticancer. Agents Med. Chem. 2010, 10, 346–351. [Google Scholar] [CrossRef]
- Adamczyk-Wozniak, A.; Borys, K.M.; Sporzynski, A. Recent Developments in the Chemistry and Biological Applications of Benzoxaboroles. Chem. Rev. 2015, 115, 5224–5247. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, D. An Upcoming Drug for Onychomycosis: Tavaborole. J. Pharmacol. Pharmacother. 2015, 6, 236–239. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, S. Recent Development of Leucyl-TRNA Synthetase Inhibitors as Antimicrobial Agents. Medchemcomm 2019, 10, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; S Hosmane, N.; Zhu, Y. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828. [Google Scholar] [CrossRef]
- Teixidor, F.; Núñez, R.; Viñas, C. Towards the Application of Purely Inorganic Icosahedral Boron Clusters in Emerging Nanomedicine. Molecules 2023, 28, 4449. [Google Scholar] [CrossRef] [PubMed]
- Ozansoy, M.; Altintaş, M.; Ozansoy, M.; Günay, N.; Kiliç, E.; Kiliç, Ü. Two Boron-Containing Compounds Affect the Cellular Viability of SH-SY5Y Cells in an in Vitro Amyloid-Beta Toxicity Model. Turk. J. Biol. 2020, 44, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Del Turco, S.; Genchi, G.G.; D’Alessandro, D.; Basta, G.; Mattoli, V. Transferrin-Conjugated Boron Nitride Nanotubes: Protein Grafting, Characterization, and Interaction with Human Endothelial Cells. Int. J. Pharm. 2012, 436, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Aysan, E.; Sahin, F.; Telci, D.; Erdem, M.; Muslumanoglu, M.; Yardmc, E.; Bektasoglu, H. Mechanism of Body Weight Reducing Effect of Oral Boric Acid Intake. Int. J. Endocrinol. 2013, 2013, 10–14. [Google Scholar] [CrossRef]
- Kucukkurt, I.; Akbel, E.; Karabag, F.; Ince, S. The Effects of Dietary Boron Compounds in Supplemented Diet on Hormonal Activity and Some Biochemical Parameters in Rats. Toxicol. Ind. Health 2015, 31, 255–260. [Google Scholar] [CrossRef]
- Doğan, A.; Demirci, S.; Apdik, H.; Faruk Bayrak, O.; Gulluoglu, S.; Can Tuysuz, E.; Gusev, O.; Rizvanov, A.A.; Nikerel, E.; Şahin, F. A New Hope for Obesity Management: Boron Inhibits Adipogenesis in Progenitor Cells through the Wnt/β-Catenin Pathway. Metabolism 2017, 69, 130–142. [Google Scholar] [CrossRef]
- Kuru, R.; Yilmaz, S.; Balan, G.; Tuzuner, B.A.; Tasli, P.N.; Akyuz, S.; Yener Ozturk, F.; Altuntas, Y.; Yarat, A.; Sahin, F. Boron-Rich Diet May Regulate Blood Lipid Profile and Prevent Obesity: A Non-Drug and Self-Controlled Clinical Trial. J. Trace Elem. Med. Biol. 2019, 54, 191–198. [Google Scholar] [CrossRef]
- Biţă, A.; Scorei, I.R.; Rangavajla, N.; Bejenaru, L.E.; Rău, G.; Bejenaru, C.; Ciocîlteu, M.V.; Dincă, L.; Neamţu, J.; Bunaciu, A.; et al. Diester Chlorogenoborate Complex: A New Naturally Occurring Boron-Containing Compound. Inorganics 2023, 11, 112. [Google Scholar] [CrossRef]
- Mitruţ, I.; Scorei, I.R.; Manolea, H.O.; Biţă, A.; Mogoantă, L.; Neamţu, J.; Bejenaru, L.E.; Ciocîlteu, M.V.; Bejenaru, C.; Rău, G.; et al. Boron-Containing Compounds in Dentistry: A Narrative Review. Rom. J. Morphol. Embryol. = Rev. Roum. Morphol. Embryol. 2022, 63, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Biţă, C.E.; Scorei, I.R.; Vreju, A.F.; Muşetescu, A.E.; Mogoşanu, G.D.; Biţă, A.; Dinescu, V.C.; Dinescu, Ş.C.; Criveanu, C.; Bărbulescu, A.L.; et al. Microbiota-Accessible Boron-Containing Compounds in Complex Regional Pain Syndrome. Medicina 2023, 59, 1965. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Oliveira, R.A.; Djukovic, A.; Ubeda, C.; Xavier, K.B. Manipulation of the Quorum Sensing Signal AI-2 Affects the Antibiotic-Treated Gut Microbiota. Cell Rep. 2015, 10, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.M.; Nemzer, B.V.; Rangavajla, N.; Biţă, A.; Rogoveanu, O.C.; Neamţu, J.; Scorei, I.R.; Bejenaru, L.E.; Rău, G.; Bejenaru, C. The Fructoborates: Part of a Family of Naturally Occurring Sugar–Borate Complexes—Biochemistry, Physiology, and Impact on Human Health: A Review. Biol. Trace Elem. Res. 2019, 188, 11–25. [Google Scholar] [CrossRef]
- Donoiu, I.; Militaru, C.; Obleagă, O.; Hunter, J.M.; Neamţu, J.; Biţă, A.; Scorei, I.R.; Rogoveanu, O.C. Effects of Boron-Containing Compounds on Cardiovascular Disease Risk Factors—A Review. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2018, 50, 47–56. [Google Scholar] [CrossRef]
- Attin, T.; Paqué, F.; Ajam, F.; Lennon, Á.M. Review of the Current Status of Tooth Whitening with the Walking Bleach Technique. Int. Endod. J. 2003, 36, 313–329. [Google Scholar] [CrossRef]
- Ji, C.; Svensson, F.; Zoufir, A.; Bender, A. EMolTox: Prediction of Molecular Toxicity with Confidence. Bioinformatics 2018, 34, 2508–2509. [Google Scholar] [CrossRef]
- Onder, F.C.; Siyah, P.; Durdagi, S.; Ay, M.; Ozpolat, B. Novel Etodolac Derivatives as Eukaryotic Elongation Factor 2 Kinase (EEF2K) Inhibitors for Targeted Cancer Therapy. RSC Med. Chem. 2022, 13, 840–849. [Google Scholar] [CrossRef]
- Creanza, T.M.; Delre, P.; Ancona, N.; Lentini, G.; Saviano, M.; Mangiatordi, G.F. Structure-Based Prediction of HERG-Related Cardiotoxicity: A Benchmark Study. J. Chem. Inf. Model. 2021, 61, 4758–4770. [Google Scholar] [CrossRef]
- Wagner, B.D.; Grunwald, G.K.; Zerbe, G.O.; Mikulich-Gilbertson, S.K.; Robertson, C.E.; Zemanick, E.T.; Harris, J.K. On the Use of Diversity Measures in Longitudinal Sequencing Studies of Microbial Communities. Front. Microbiol. 2018, 9, 1037. [Google Scholar] [CrossRef]
- Lee, J.; Song, X.; Hyun, B.; Jeon, C.O.; Hyun, S. Drosophila Gut Immune Pathway Suppresses Host Development-Promoting Effects of Acetic Acid Bacteria. Mol. Cells 2023, 46, 637–653. [Google Scholar] [CrossRef] [PubMed]
- McCuaig, B.; Goto, Y. Immunostimulating Commensal Bacteria and Their Potential Use as Therapeutics. Int. J. Mol. Sci. 2023, 24, 15644. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Nong, K.; Qin, X.; Liu, Z.; Wang, Z.; Wu, Y.; Zhang, B.; Chen, W.; Fang, X.; Liu, Y.; Wang, X.; et al. Potential Effects and Mechanism of Flavonoids Extract of Callicarpa nudiflora Hook on DSS-Induced Colitis in Mice. Phytomedicine 2024, 128, 155523. [Google Scholar] [CrossRef]
- Hsiao, A.; Ahmed, A.M.S.; Subramanian, S.; Griffin, N.W.; Drewry, L.L.; Petri, W.A.J.; Haque, R.; Ahmed, T.; Gordon, J.I. Members of the Human Gut Microbiota Involved in Recovery from Vibrio cholerae Infection. Nature 2014, 515, 423–426. [Google Scholar] [CrossRef]
- Thompson, J.A.; Oliveira, R.A.; Xavier, K.B. Chemical Conversations in the Gut Microbiota. Gut Microbes 2016, 7, 163–170. [Google Scholar] [CrossRef]
- Miljkovic, D.; Scorei, R.I.; Cimpoiaşu, V.M.; Scorei, I.D. Calcium Fructoborate: Plant-Based Dietary Boron for Human Nutrition. J. Diet. Suppl. 2009, 6, 211–226. [Google Scholar] [CrossRef]
- Hu, Y.; Quan, H.; Cui, J.; Luo, W.; Zeng, W.; Chen, D. Carbon Nanodot Modified N, O-Doped Porous Carbon for Solid-State Supercapacitor: A Comparative Study with Carbon Nanotube and Graphene Oxide. J. Alloys Compd. 2021, 877, 160237. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J.; Lee, K.Y.; Jung, I.; Park, M. Dispersion of Graphene-Based Nanocarbon Fillers in Polyamide 66 by Dry Processing and Its Effect on Mechanical Properties. Compos. Part B Eng. 2017, 114, 445–456. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A Relevant Minority for the Maintenance of Gut Homeostasis. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2018, 50, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiao, X.; Zhang, Q.; Zheng, J.; Li, M.; Wang, X.; Deng, M.; Zhai, X.; Liu, J. Gut Microbiota Might Be a Crucial Factor in Deciphering the Metabolic Benefits of Perinatal Genistein Consumption in Dams and Adult Female Offspring. Food Funct. 2019, 10, 4505–4521. [Google Scholar] [CrossRef] [PubMed]
- Wylie, K.M.; Truty, R.M.; Sharpton, T.J.; Mihindukulasuriya, K.A.; Zhou, Y.; Gao, H.; Sodergren, E.; Weinstock, G.M.; Pollard, K.S. Novel Bacterial Taxa in the Human Microbiome. PLoS ONE 2012, 7, e35294. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lang, T.; Shen, J.; Dai, J.; Tian, L.; Wang, X. Core Gut Bacteria Analysis of Healthy Mice. Front. Microbiol. 2019, 10, 887. [Google Scholar] [CrossRef]
- Ubeda, C.; Bucci, V.; Caballero, S.; Djukovic, A.; Toussaint, N.C.; Equinda, M.; Lipuma, L.; Ling, L.; Gobourne, A.; No, D.; et al. Intestinal Microbiota Containing Barnesiella Species Cures Vancomycin-Resistant Enterococcus faecium Colonization. Infect. Immun. 2013, 81, 965–973. [Google Scholar] [CrossRef]
- Wei, X.; Tao, J.; Xiao, S.; Jiang, S.; Shang, E.; Zhu, Z.; Qian, D.; Duan, J. Xiexin Tang Improves the Symptom of Type 2 Diabetic Rats by Modulation of the Gut Microbiota. Sci. Rep. 2018, 8, 3685. [Google Scholar] [CrossRef]
- Weiss, G.A.; Chassard, C.; Hennet, T. Selective Proliferation of Intestinal Barnesiella under Fucosyllactose Supplementation in Mice. Br. J. Nutr. 2014, 111, 1602–1610. [Google Scholar] [CrossRef]
- Li, M.; Wu, Y.; Hu, Y.; Zhao, L.; Zhang, C. Initial Gut Microbiota Structure Affects Sensitivity to DSS-Induced Colitis in a Mouse Model. Sci. China. Life Sci. 2018, 61, 762–769. [Google Scholar] [CrossRef]
- Ren, T.; Gao, Y.; Qiu, Y.; Jiang, S.; Zhang, Q.; Zhang, J.; Wang, L.; Zhang, Y.; Wang, L.; Nie, K. Gut Microbiota Altered in Mild Cognitive Impairment Compared With Normal Cognition in Sporadic Parkinson’s Disease. Front. Neurol. 2020, 11, 137. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered Gut Microbiota and Short Chain Fatty Acids in Chinese Children with Autism Spectrum Disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Rautio, M.; Eerola, E.; Väisänen-Tunkelrott, M.-L.; Molitoris, D.; Lawson, P.; Collins, M.D.; Jousimies-Somer, H. Reclassification of Bacteroides putredinis (Weinberg et Al., 1937) in a New Genus Alistipes Gen. Nov., as Alistipes putredinis Comb. Nov., and Description of Alistipes finegoldii sp. Nov., from Human Sources. Syst. Appl. Microbiol. 2003, 26, 182–188. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Shortt, C.; Hasselwander, O.; Meynier, A.; Nauta, A.; Fernández, E.N.; Putz, P.; Rowland, I.; Swann, J.; Türk, J.; Vermeiren, J.; et al. Systematic Review of the Effects of the Intestinal Microbiota on Selected Nutrients and Non-Nutrients. Eur. J. Nutr. 2018, 57, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Walker, A.; Pfitzner, B.; Harir, M.; Schaubeck, M.; Calasan, J.; Heinzmann, S.S.; Turaev, D.; Rattei, T.; Endesfelder, D.; Castell, W.Z.; et al. Sulfonolipids as Novel Metabolite Markers of Alistipes and Odoribacter Affected by High-Fat Diets. Sci. Rep. 2017, 7, 11047. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Xu, J.; Xue, C. Sphingolipids in Food and Their Critical Roles in Human Health. Crit. Rev. Food Sci. Nutr. 2021, 61, 462–491. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis Gut Microbiota Associated with Inflammation and Impaired Mucosal Immune Function in Intestine of Humans with Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2015, 5, 8096. [Google Scholar] [CrossRef]
- Mirhakkak, M.H.; Schäuble, S.; Klassert, T.E.; Brunke, S.; Brandt, P.; Loos, D.; Uribe, R.V.; Senne de Oliveira Lino, F.; Ni, Y.; Vylkova, S.; et al. Metabolic Modeling Predicts Specific Gut Bacteria as Key Determinants for Candida Albicans Colonization Levels. ISME J. 2021, 15, 1257–1270. [Google Scholar] [CrossRef]
- Dziarski, R.; Park, S.Y.; Kashyap, D.R.; Dowd, S.E.; Gupta, D. Pglyrp-Regulated Gut Microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Alistipes finegoldii Attenuates Colitis in Mice. PLoS ONE 2016, 11, e0146162. [Google Scholar] [CrossRef]
- Bharadia, L.; Agrawal, N.; Joshi, N. Development and Functions of the Infant Gut Microflora: Western vs. Indian Infants. Int. J. Pediatr. 2020, 2020, 7586264. [Google Scholar] [CrossRef]
- Boer, C.G.; Radjabzadeh, D.; Medina-Gomez, C.; Garmaeva, S.; Schiphof, D.; Arp, P.; Koet, T.; Kurilshikov, A.; Fu, J.; Ikram, M.A.; et al. Intestinal Microbiome Composition and Its Relation to Joint Pain and Inflammation. Nat. Commun. 2019, 10, 4881. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Lee, S.Y.; Kang, M.J.; Kim, B.S.; Lee, M.J.; Jung, S.S.; Yoon, J.S.; Cho, H.J.; Lee, E.; Yang, S.I.; et al. Imbalance of Gut Streptococcus, Clostridium, and Akkermansia Determines the Natural Course of Atopic Dermatitis in Infant. Allergy. Asthma Immunol. Res. 2020, 12, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Fukui, A.; Takagi, T.; Naito, Y.; Inoue, R.; Kashiwagi, S.; Mizushima, K.; Inada, Y.; Inoue, K.; Harusato, A.; Dohi, O.; et al. Higher Levels of Streptococcus in Upper Gastrointestinal Mucosa Associated with Symptoms in Patients with Functional Dyspepsia. Digestion 2020, 101, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Isenring, J.; Köhler, J.; Nakata, M.; Frank, M.; Jans, C.; Renault, P.; Danne, C.; Dramsi, S.; Kreikemeyer, B.; Oehmcke-Hecht, S. Streptococcus gallolyticus subsp. Gallolyticus endocarditis Isolate Interferes with Coagulation and Activates the Contact System. Virulence 2018, 9, 248–261. [Google Scholar] [CrossRef]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.-S.; Esmaillzadeh, A. Effect of Multispecies Probiotic Supplements on Metabolic Profiles, Hs-CRP, and Oxidative Stress in Patients with Type 2 Diabetes. Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef]
- Del Carmen, S.; de Moreno de LeBlanc, A.; Martin, R.; Chain, F.; Langella, P.; Bermúdez-Humarán, L.G.; LeBlanc, J.G. Genetically Engineered Immunomodulatory Streptococcus thermophilus Strains Producing Antioxidant Enzymes Exhibit Enhanced Anti-Inflammatory Activities. Appl. Environ. Microbiol. 2014, 80, 869–877. [Google Scholar] [CrossRef]
- Han, F.; Wu, G.; Zhang, Y.; Zheng, H.; Han, S.; Li, X.; Cai, W.; Liu, J.; Zhang, W.; Zhang, X.; et al. Streptococcus thermophilus Attenuates Inflammation in Septic Mice Mediated by Gut Microbiota. Front. Microbiol. 2020, 11, 598010. [Google Scholar] [CrossRef]
- Horino, H.; Fujita, T.; Tonouchi, A. Description of Anaerobacterium chartisolvens gen. nov., sp. nov., an Obligately Anaerobic Bacterium from Clostridium RRNA Cluster III Isolated from Soil of a Japanese Rice Field, and Reclassification of Bacteroides cellulosolvens Murray et al. 1984 as Pseudobacteroides cellulosolvens gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2014, 64, 1296–1303. [Google Scholar] [CrossRef]
- Deshpande, N.G.; Saxena, J.; Pesaresi, T.G.; Carrell, C.D.; Ashby, G.B.; Liao, M.-K.; Freeman, L.R. High Fat Diet Alters Gut Microbiota but Not Spatial Working Memory in Early Middle-Aged Sprague Dawley Rats. PLoS ONE 2019, 14, e0217553. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, C.; Han, J.; Ming, T.; Zhou, J.; Lu, C.; Li, Y.; Su, X. Novel High-Docosahexaenoic-Acid Tuna Oil Supplementation Modulates Gut Microbiota and Alleviates Obesity in High-Fat Diet Mice. Food Sci. Nutr. 2020, 8, 6513–6527. [Google Scholar] [CrossRef]
- Ma, C.; Huo, D.; You, Z.; Peng, Q.; Jiang, S.; Chang, H.; Zhang, J.; Zhang, H. Differential Pattern of Indigenous Microbiome Responses to Probiotic Bifidobacterium lactis V9 Consumption across Subjects. Food Res. Int. 2020, 136, 109496. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Quan, M.; Zhao, H.; Jia, J. Gut Microbiota Changes and Their Correlation with Cognitive and Neuropsychiatric Symptoms in Alzheimer’s Disease. J. Alzheimers. Dis. 2021, 81, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Morales, P.; Orellana, C.A.; Moutafis, G.; Moonen, G.; Rincon, G.; Nielsen, L.K.; Marcellin, E. Revisiting the Evolution and Taxonomy of Clostridia, a Phylogenomic Update. Genome Biol. Evol. 2019, 11, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Yutin, N.; Galperin, M.Y. A Genomic Update on Clostridial Phylogeny: Gram-Negative Spore Formers and Other Misplaced Clostridia. Environ. Microbiol. 2013, 15, 2631–2641. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Pindo, M.; Renzi, D.; et al. Altered Gut Microbiota in Rett Syndrome. Microbiome 2016, 4, 41. [Google Scholar] [CrossRef]
- Kelly, T.N.; Bazzano, L.A.; Ajami, N.J.; He, H.; Zhao, J.; Petrosino, J.F.; Correa, A.; He, J. Gut Microbiome Associates With Lifetime Cardiovascular Disease Risk Profile Among Bogalusa Heart Study Participants. Circ. Res. 2016, 119, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Tang, D.; Hou, P.; Shen, W.; Li, H.; Wang, T.; Liu, R. Dysbiosis of Gut Microbiota in Patients with Esophageal Cancer. Microb. Pathog. 2021, 150, 104709. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Fagan, A.; White, M.B.; Wade, J.B.; Hylemon, P.B.; Heuman, D.M.; Fuchs, M.; John, B.V.; Acharya, C.; Sikaroodi, M.; et al. Specific Gut and Salivary Microbiota Patterns Are Linked With Different Cognitive Testing Strategies in Minimal Hepatic Encephalopathy. Am. J. Gastroenterol. 2019, 114, 1080–1090. [Google Scholar] [CrossRef]
- Wang, J.; Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nat. 2012 4907418 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Till, H.; Castellani, C.; Moissl-Eichinger, C.; Gorkiewicz, G.; Singer, G. Disruptions of the Intestinal Microbiome in Necrotizing Enterocolitis, Short Bowel Syndrome, and Hirschsprung’s Associated Enterocolitis. Front. Microbiol. 2015, 6, 1154. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg Induction by a Rationally Selected Mixture of Clostridia Strains from the Human Microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium Species as Probiotics: Potentials and Challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Amir, I.; Bouvet, P.; Legeay, C.; Gophna, U.; Weinberger, A. Eisenbergiella tayi gen. nov., sp. nov., Isolated from Human Blood. Int. J. Syst. Evol. Microbiol. 2014, 64, 907–914. [Google Scholar] [CrossRef]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.B.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.-A.; Chevalier, S.; Shir, Y. Altered Microbiome Composition in Individuals with Fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Wu, H.; Wu, S.-D.; Lu, N.; Wang, Y.-T.; Liu, H.-N.; Dong, L.; Liu, T.-T.; Shen, X.-Z. Parasutterella, in Association with Irritable Bowel Syndrome and Intestinal Chronic Inflammation. J. Gastroenterol. Hepatol. 2018, 33, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, S.; Pechereau, F.; Leblanc, N.; Boubertakh, B.; Houde, A.; Martin, C.; Flamand, N.; Silvestri, C.; Raymond, F.; Di Marzo, V.; et al. Rapid and Concomitant Gut Microbiota and Endocannabinoidome Response to Diet-Induced Obesity in Mice. mSystems 2019, 4. [Google Scholar] [CrossRef]
- Luo, L.; Hu, M.; Li, Y.; Chen, Y.; Zhang, S.; Chen, J.; Wang, Y.; Lu, B.; Xie, Z.; Liao, Q. Association between Metabolic Profile and Microbiomic Changes in Rats with Functional Dyspepsia. RSC Adv. 2018, 8, 20166–20181. [Google Scholar] [CrossRef]
- Bao, J.; Zheng, H.; Wang, Y.; Zheng, X.; He, L.; Qi, W.; Wang, T.; Guo, B.; Guo, G.; Zhang, Z.; et al. Echinococcus granulosus Infection Results in an Increase in Eisenbergiella and Parabacteroides Genera in the Gut of Mice. Front. Microbiol. 2018, 9, 2890. [Google Scholar] [CrossRef]
- Baron, E.J.; Summanen, P.; Downes, J.; Roberts, M.C.; Wexler, H.; Finegold, S.M. Bilophila wadsworthia, Gen. Nov. and Sp. Nov., a Unique Gram-Negative Anaerobic Rod Recovered from Appendicitis Specimens and Human Faeces. J. Gen. Microbiol. 1989, 135, 3405–3411. [Google Scholar] [CrossRef]
- Schoenborn, L.; Abdollahi, H.; Tee, W.; Dyall-Smith, M.; Janssen, P.H. A Member of the Delta Subgroup of Proteobacteria from a Pyogenic Liver Abscess Is a Typical Sulfate Reducer of the Genus Desulfovibrio. J. Clin. Microbiol. 2001, 39, 787–790. [Google Scholar] [CrossRef]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.-L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila wadsworthia Aggravates High Fat Diet Induced Metabolic Dysfunctions in Mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef] [PubMed]
- Peck, S.C.; Denger, K.; Burrichter, A.; Irwin, S.M.; Balskus, E.P.; Schleheck, D. A Glycyl Radical Enzyme Enables Hydrogen Sulfide Production by the Human Intestinal Bacterium Bilophila wadsworthia. Proc. Natl. Acad. Sci. USA 2019, 116, 3171–3176. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Long, W.; Hao, B.; Ding, D.; Ma, X.; Zhao, L.; Pang, X. A Human Stool-Derived Bilophila wadsworthia Strain Caused Systemic Inflammation in Specific-Pathogen-Free Mice. Gut Pathog. 2017, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Salazar, E.O.; Ortiz-López, M.G.; Granados-Silvestre, M.d.L.Á.; Palacios-González, B.; Menjivar, M. Altered Gut Microbiota and Compositional Changes in Firmicutes and Proteobacteria in Mexican Undernourished and Obese Children. Front. Microbiol. 2018, 9, 2494. [Google Scholar] [CrossRef]
- Tang, X.J.; Fu, R.; Li, Q.; Liu, X.Q.; Hu, S.F. Differential Analysis of Intestinal Flora in Children with Different Clinical Phenotypes of Henoch-Schonlein Purpura of Different Clinical Phenotypes. Chin. J. Immunol. 2021, 37, 339–345. [Google Scholar]
- Palmieri, O.; Bossa, F.; Castellana, S.; Latiano, T.; Carparelli, S.; Martino, G.; Mangoni, M.; Corritore, G.; Nardella, M.; Guerra, M.; et al. Deciphering Microbial Composition in Patients with Inflammatory Bowel Disease: Implications for Therapeutic Response to Biologic Agents. Microorganisms 2024, 12, 1260. [Google Scholar] [CrossRef]
- Obregon-Tito, A.J.; Tito, R.Y.; Metcalf, J.; Sankaranarayanan, K.; Clemente, J.C.; Ursell, L.K.; Zech Xu, Z.; Van Treuren, W.; Knight, R.; Gaffney, P.M.; et al. Subsistence Strategies in Traditional Societies Distinguish Gut Microbiomes. Nat. Commun. 2015, 6, 6505. [Google Scholar] [CrossRef]
- Xiao, Y.; Cai, Y.; Bommineni, Y.R.; Fernando, S.C.; Prakash, O.; Gilliland, S.E.; Zhang, G. Identification and Functional Characterization of Three Chicken Cathelicidins with Potent Antimicrobial Activity. J. Biol. Chem. 2006, 281, 2858–2867. [Google Scholar] [CrossRef]
- Laviad-Shitrit, S.; Izhaki, I.; Lalzar, M.; Halpern, M. Comparative Analysis of Intestine Microbiota of Four Wild Waterbird Species. Front. Microbiol. 2019, 10, 1911. [Google Scholar] [CrossRef]
- Ali, I.; Liu, K.; Long, D.; Faisal, S.; Hilal, M.G.; Ali, I.; Huang, X.; Long, R. Ramadan Fasting Leads to Shifts in Human Gut Microbiota Structured by Dietary Composition. Front. Microbiol. 2021, 12, 642999. [Google Scholar] [CrossRef]
- Dam, B.; Misra, A.; Banerjee, S. Role of Gut Microbiota in Combating Oxidative Stress. In Oxidative Stress in Microbial Diseases; Springer: Berlin/Heidelberg, Germany, 2019; pp. 43–82. [Google Scholar]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in Health and Disease, a Driver or Just along for the Ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Annuk, H.; Shchepetova, J.; Kullisaar, T.; Songisepp, E.; Zilmer, M.; Mikelsaar, M. Characterization of Intestinal Lactobacilli as Putative Probiotic Candidates. J. Appl. Microbiol. 2003, 94, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Peng, J. Interaction between Boron and Other Elements in Plants. Genes 2023, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Arciniega-Martínez, I.M.; Romero-Aguilar, K.S.; Farfán-García, E.D.; García-Machorro, J.; Reséndiz-Albor, A.A.; Soriano-Ursúa, M.A. Diversity of Effects Induced by Boron-Containing Compounds on Immune Response Cells and on Antibodies in Basal State. J. Trace Elem. Med. Biol. 2022, 69, 126901. [Google Scholar] [CrossRef]
- Mei, S.; Yang, X.; Guo, H.; Gu, H.; Zha, L.; Cai, J.; Li, X.; Liu, Z.; Bennett, B.J.; He, L.; et al. A Small Amount of Dietary Carbohydrate Can Promote the HFD-Induced Insulin Resistance to a Maximal Level. PLoS ONE 2014, 9, e100875. [Google Scholar] [CrossRef]
- Siyah, P.; Akgol, S.; Durdagi, S.; Kocabas, F. Identification of First-in-Class Plasmodium OTU Inhibitors with Potent Anti-Malarial Activity. Biochem. J. 2021, 478, 3445–3466. [Google Scholar] [CrossRef]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.F.; Honig, B.; Shaw, D.E.; Friesner, R.A. A Hierarchical Approach to All-atom Protein Loop Prediction. Proteins Struct. Funct. Bioinform. 2004, 55, 351–367. [Google Scholar] [CrossRef]
- Bushnell, B. BBTools: A Suite of Fast, Multithreaded Bioinformatics Tools Designed for Analysis of DNA and RNA Sequence Data. Jt. Genome Inst. 2018. [Google Scholar]
- Boratyn, G.M.; Thierry-Mieg, J.; Thierry-Mieg, D.; Busby, B.; Madden, T.L. Magic-BLAST, an Accurate RNA-Seq Aligner for Long and Short Reads. BMC Bioinform. 2019, 20, 405. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Oksanen, A.J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; Hara, R.B.O.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, Version 2.6-8; The Comprehensive R Archive Network: Vienna, Austria, 2020. [Google Scholar]

| Groups | Species of Animal | Substance to Be Applied |
|---|---|---|
| Group I (n = 5) | BALB/c wild type | Control (water) |
| Group II (n = 4) | BALB/c wild type | Sodium pentaborate pentahydrate (NaB) |
| Group III (n = 4) | BALB/c wild type | Sodium perborate Tetrahydrate (SPT) |
| Group IV (n = 5) | BALB/c wild type | Boric acid (BA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sentürk, N.B.; Kasapoglu, B.; Sahin, E.; Ozcan, O.; Ozansoy, M.; Ozansoy, M.B.; Siyah, P.; Sezerman, U.; Sahin, F. The Potential Role of Boron in the Modulation of Gut Microbiota Composition: An In Vivo Pilot Study. Pharmaceuticals 2024, 17, 1334. https://doi.org/10.3390/ph17101334
Sentürk NB, Kasapoglu B, Sahin E, Ozcan O, Ozansoy M, Ozansoy MB, Siyah P, Sezerman U, Sahin F. The Potential Role of Boron in the Modulation of Gut Microbiota Composition: An In Vivo Pilot Study. Pharmaceuticals. 2024; 17(10):1334. https://doi.org/10.3390/ph17101334
Chicago/Turabian StyleSentürk, Nermin Basak, Burcu Kasapoglu, Eray Sahin, Orhan Ozcan, Mehmet Ozansoy, Muzaffer Beyza Ozansoy, Pinar Siyah, Ugur Sezerman, and Fikrettin Sahin. 2024. "The Potential Role of Boron in the Modulation of Gut Microbiota Composition: An In Vivo Pilot Study" Pharmaceuticals 17, no. 10: 1334. https://doi.org/10.3390/ph17101334
APA StyleSentürk, N. B., Kasapoglu, B., Sahin, E., Ozcan, O., Ozansoy, M., Ozansoy, M. B., Siyah, P., Sezerman, U., & Sahin, F. (2024). The Potential Role of Boron in the Modulation of Gut Microbiota Composition: An In Vivo Pilot Study. Pharmaceuticals, 17(10), 1334. https://doi.org/10.3390/ph17101334








