Topical Biocomposites Based on Collagen, Hyaluronic Acid and Metronidazole as Periodontitis Treatment
Abstract
1. Introduction
2. Results and Discussion
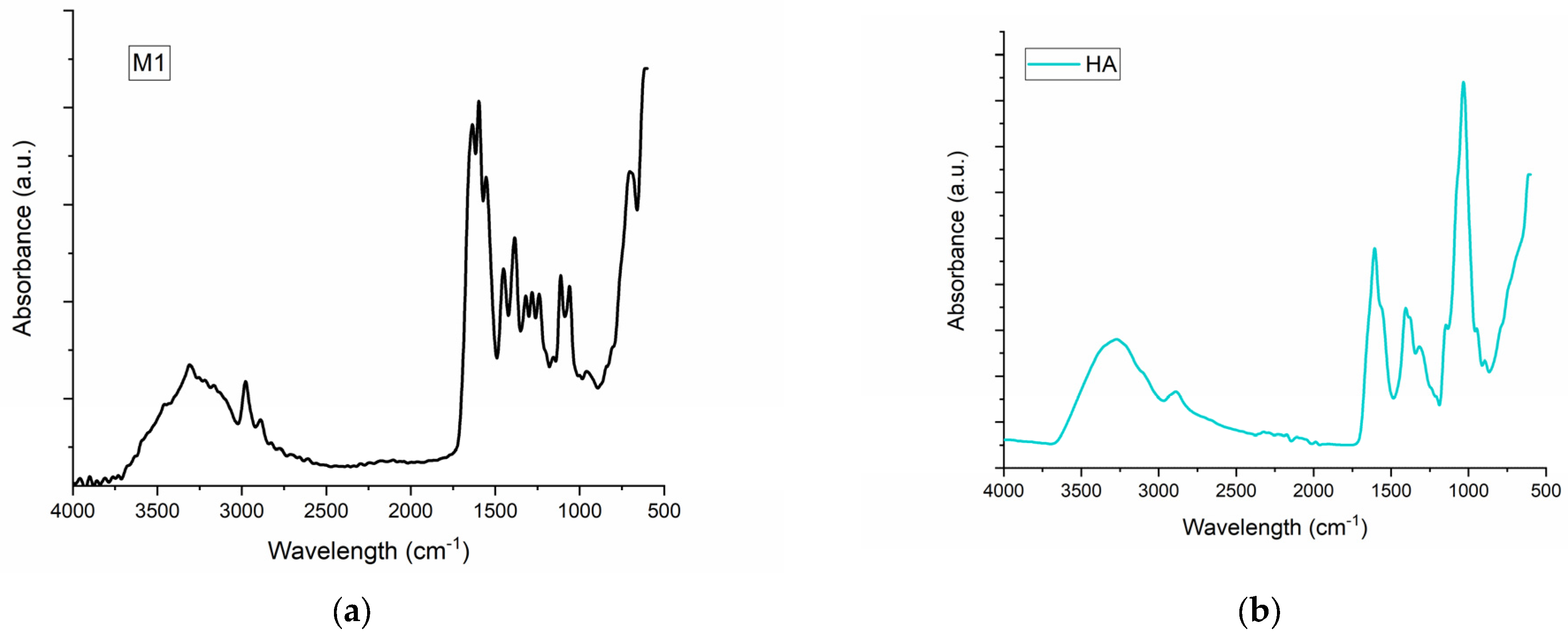
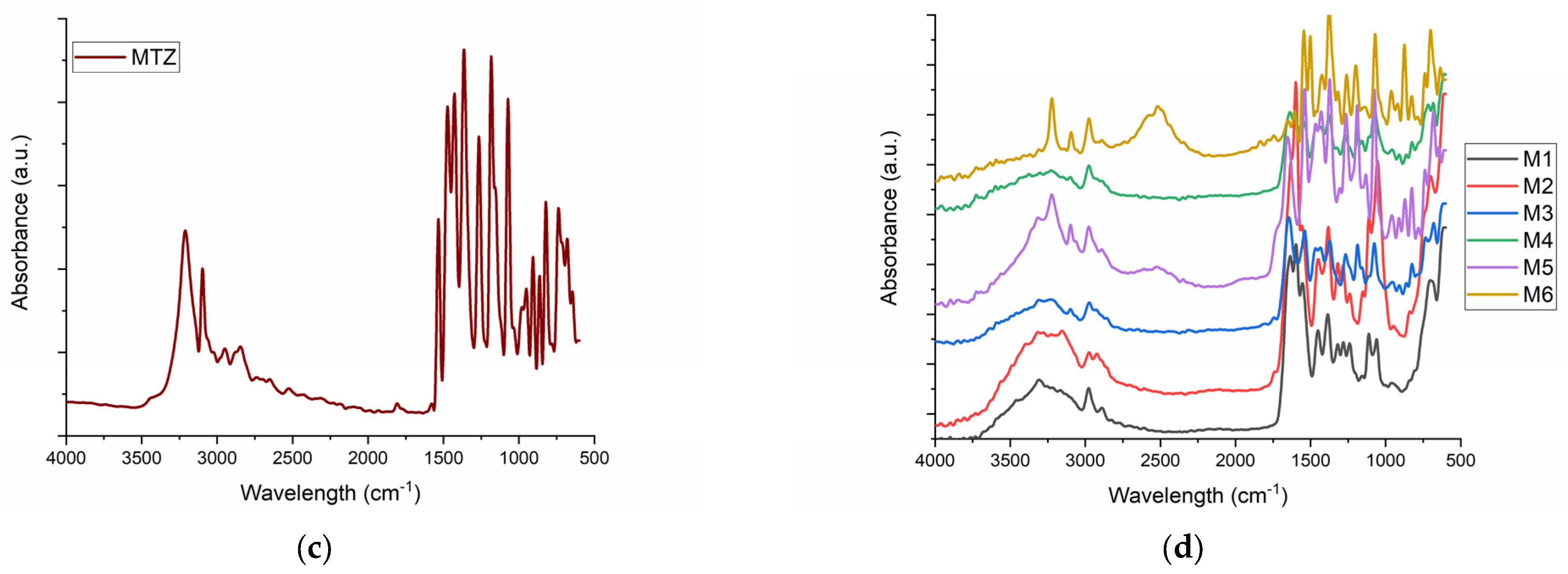
2.1. FT-IR Spectroscopy for M1–M6 Sponges
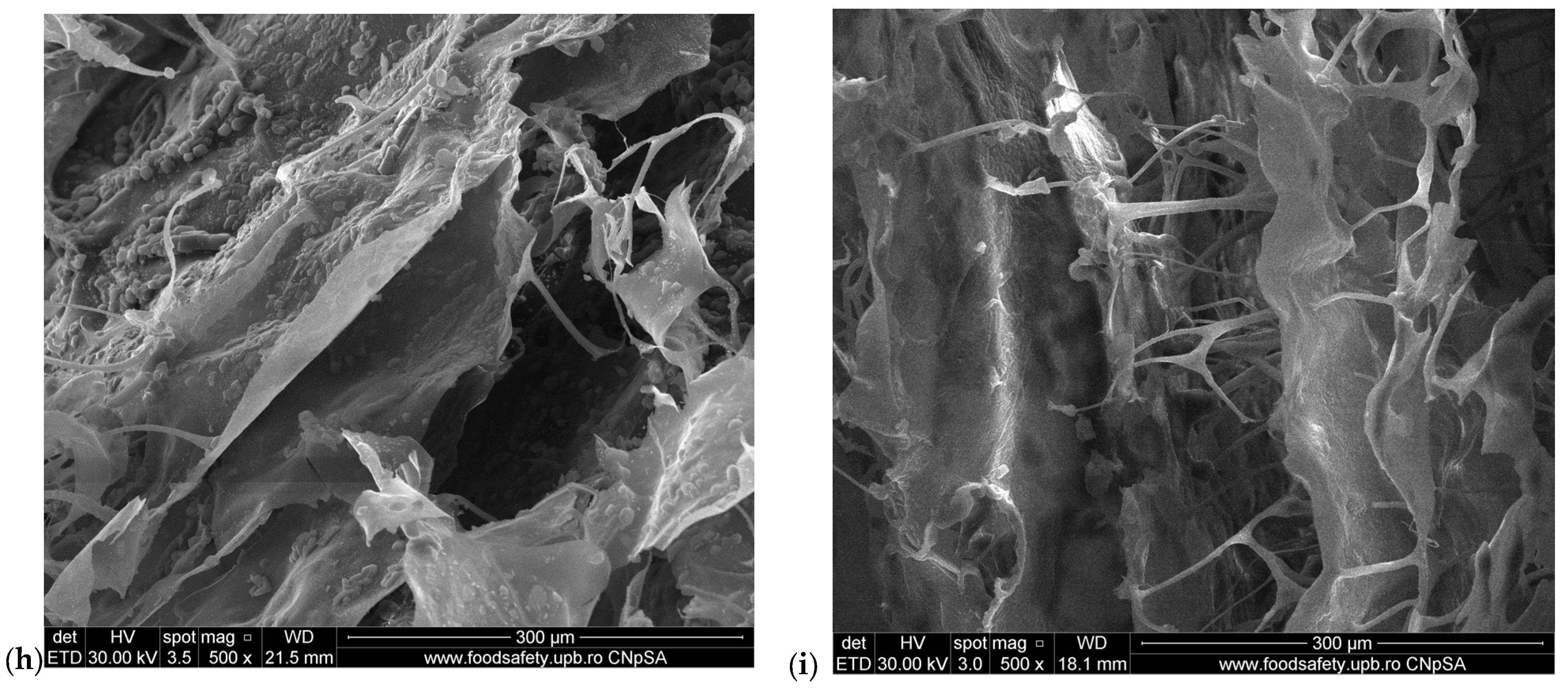
2.2. Scanning Electron Microscopy (SEM) of M1–M6 Sponges
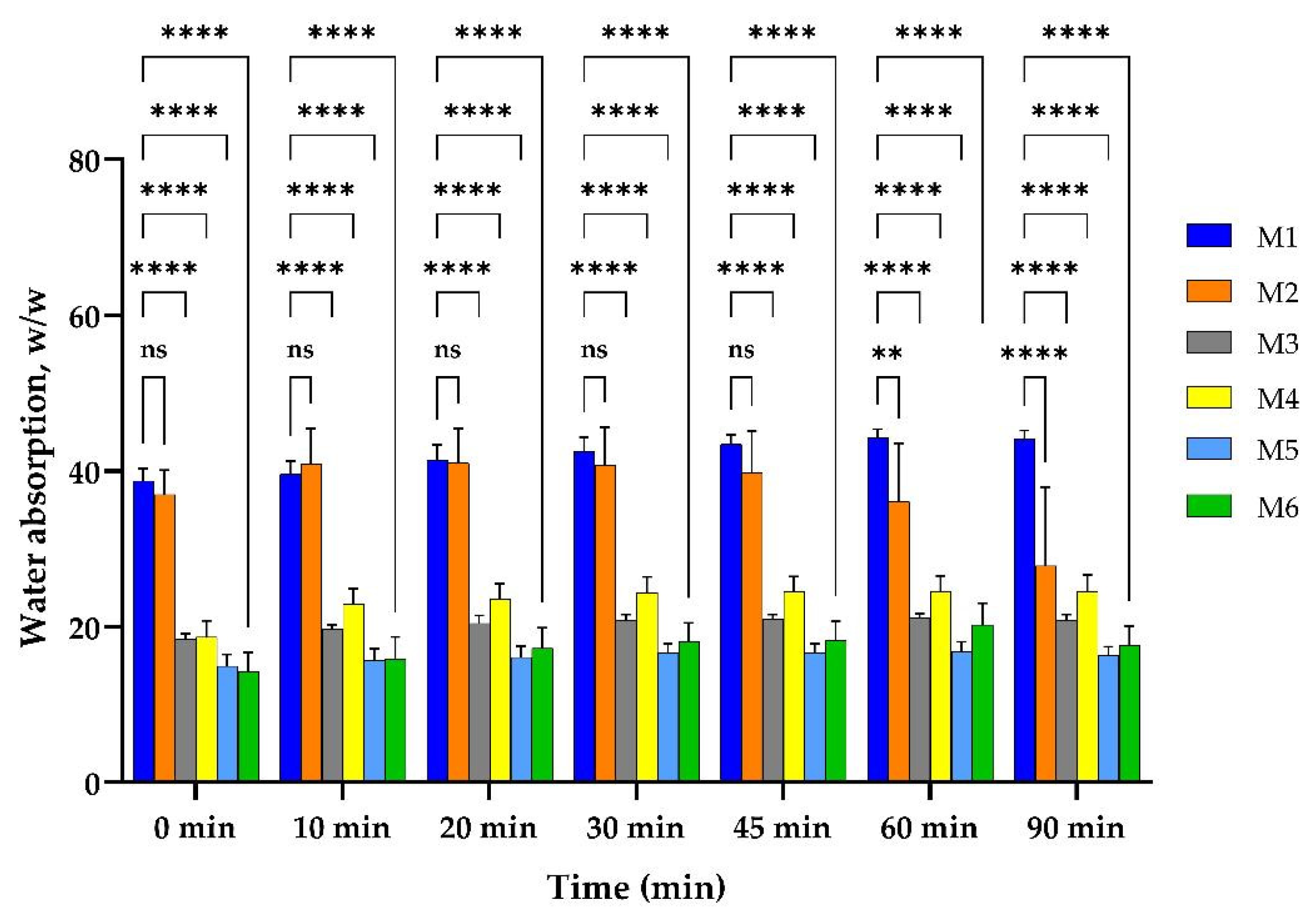
2.3. Water Uptake Ability of M1–M6 Sponges
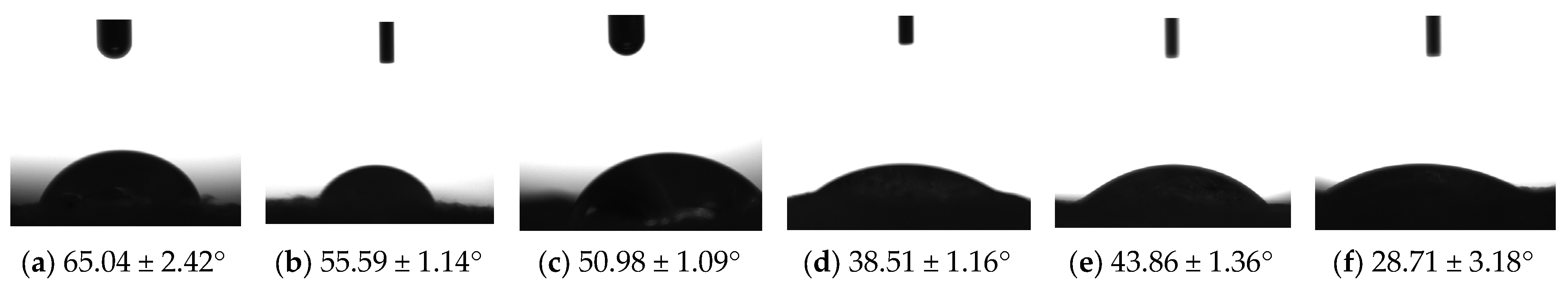
2.4. Contact Angle Evaluation of M1–M6 Sponges
2.5. In Vitro Drug Release Analysis from Sponges and Data Modeling
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Sponges by Freeze-Drying Hydrogels
3.2.2. Fourier-Transform Infrared Spectrometry (FTIR) of Sponges
3.2.3. Scanning Electron Microscopy of Sponges
3.2.4. Water Uptake of Sponges
3.2.5. Sponge Contact Angle Evaluation
3.2.6. In Vitro Drug Release Analysis from Sponges and Data Modeling
3.2.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loesche, W. Dental Caries and Periodontitis: Contrasting Two Infections That Have Medical Implications. Infect. Dis. Clin. N. Am. 2007, 21, 471–502. [Google Scholar] [CrossRef] [PubMed]
- Tatakis, D.N.; Kumar, P.S. Etiology and Pathogenesis of Periodontal Diseases. Dent. Clin. N. Am. 2005, 49, 491–516. [Google Scholar] [CrossRef] [PubMed]
- Offenbacher, S.; Barros, S.P.; Singer, R.E.; Moss, K.; Williams, R.C.; Beck, J.D. Periodontal Disease at the Biofilm–Gingival Interface. J. Periodontol. 2007, 78, 1911–1925. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Mittal, N. Periodontal Diseases—A Brief Review. Int. J. Oral Health Dent. 2020, 6, 177–187. [Google Scholar] [CrossRef]
- Sanz, M.; D’Aiuto, F.; Deanfield, J.; Fernandez-Aviles, F. European Workshop in Periodontal Health and Cardiovascular Disease—Scientific Evidence on the Association between Periodontal and Cardiovascular Diseases: A Review of the Literature. Eur. Heart J. Suppl. 2010, 12, B3–B12. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Periodontitis in 1990–2010. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Marcenes, W.; Kassebaum, N.J.; Bernabé, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C.J.L. Global Burden of Oral Conditions in 1990–2010. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; Abyu, G.Y.; Alsharif, U.; et al. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of Periodontitis in Dentate People between 2011 and 2020: A Systematic Review and Meta-analysis of Epidemiological Studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A New Classification Scheme for Periodontal and Peri-implant Diseases and Conditions—Introduction and Key Changes from the 1999 Classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef]
- Deas, D.E.; Moritz, A.J.; Sagun, R.S.; Gruwell, S.F.; Powell, C.A. Scaling and Root Planing vs. Conservative Surgery in the Treatment of Chronic Periodontitis. Periodontol. 2000 2016, 71, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, R.A.; Wang, H.L.; Eber, R.; Oh, T.J. Systemic Chemotherapeutic Agents as Adjunctive Periodontal Therapy: A Narrative Review and Suggested Clinical Recommendations. J. Int. Acad. Periodontol. 2015, 17, 123–134. [Google Scholar] [PubMed]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Kao, R.T.; Nares, S.; Reynolds, M.A. Periodontal Regeneration—Intrabony Defects: A Systematic Review From the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S77–S104. [Google Scholar] [CrossRef]
- Hämmerle, C.H.F.; Jung, R.E.; Feloutzis, A. A Systematic Review of the Survival of Implants in Bone Sites Augmented with Barrier Membranes (Guided Bone Regeneration) in Partially Edentulous Patients. J. Clin. Periodontol. 2002, 29, 226–231. [Google Scholar] [CrossRef]
- Jain, N.; Jain, G.K.; Javed, S.; Iqbal, Z.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K. Recent Approaches for the Treatment of Periodontitis. Drug Discov. Today 2008, 13, 932–943. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Wei, Y.; Deng, Y.; Ma, S.; Ran, M.; Jia, Y.; Meng, J.; Han, F.; Gou, J.; Yin, T.; He, H.; et al. Local Drug Delivery Systems as Therapeutic Strategies against Periodontitis: A Systematic Review. J. Control. Release 2021, 333, 269–282. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Yu, D.-G.; Bligh, S.-W.A. Alginate-Based Electrospun Nanofibers and the Enabled Drug Controlled Release Profiles: A Review. Biomolecules 2024, 14, 789. [Google Scholar] [CrossRef]
- Dong, R.; Gong, W.; Guo, Q.; Liu, H.; Yu, D.-G. Synergistic Effects of Radical Distributions of Soluble and Insoluble Polymers within Electrospun Nanofibers for an Extending Release of Ferulic Acid. Polymers 2024, 16, 2614. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gong, W.; Zhang, Z.; Zhou, J.; Yu, D.-G.; Yi, T. Reverse Gradient Distributions of Drug and Polymer Molecules within Electrospun Core–Shell Nanofibers for Sustained Release. Int. J. Mol. Sci. 2024, 25, 9524. [Google Scholar] [CrossRef]
- Rajeshwari, H.R.; Dhamecha, D.; Jagwani, S.; Rao, M.; Jadhav, K.; Shaikh, S.; Puzhankara, L.; Jalalpure, S. Local Drug Delivery Systems in the Management of Periodontitis: A Scientific Review. J. Control. Release 2019, 307, 393–409. [Google Scholar] [CrossRef]
- Mandlik, V.; Jha, A. Periochip. Med. J. Armed Forces India 2007, 63, 368–369. [Google Scholar] [CrossRef][Green Version]
- John, P. Adjunctive Effects of A Piscean Collagen-Based Controlled-Release Chlorhexidine Chip in the Treatment of Chronic Periodontitis: A Clinical and Microbiological Study. J. Clin. Diagn. Res. 2015, 9, ZC70–ZC74. [Google Scholar] [CrossRef] [PubMed]
- Paolantonio, M.; D’Angelo, M.; Grassi, R.F.; Perinetti, G.; Piccolomini, R.; Pizzo, G.; Annunziata, M.; D’Archivio, D.; D’Ercole, S.; Nardi, G.; et al. Clinical and Microbiologic Effects of Subgingival Controlled-Release Delivery of Chlorhexidine Chip in the Treatment of Periodontitis: A Multicenter Study. J. Periodontol. 2008, 79, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Vancea, C.-V.; Grosu-Bularda, A.; Cretu, A.; Hodea, F.-V.; Al-Falah, K.; Stoian, A.; Chiotoriu, A.L.; Mihai, C.; Hariga, C.S.; Lascar, I.; et al. Therapeutic Strategies for Nerve Injuries: Current Findings and Future Perspectives. Are Textile Technologies a Potential Solution? Ind. Textila 2022, 73, 704–712. [Google Scholar] [CrossRef]
- Melcher, A.H. On the Repair Potential of Periodontal Tissues. J. Periodontol. 1976, 47, 256–260. [Google Scholar] [CrossRef]
- Ali, M.; Yang, F.; Plachokova, A.S.; Jansen, J.A.; Walboomers, X.F. Application of Specialized Pro-resolving Mediators in Periodontitis and Peri-implantitis: A Review. Eur. J. Oral Sci. 2021, 129, e12759. [Google Scholar] [CrossRef]
- Binlateh, T.; Thammanichanon, P.; Rittipakorn, P.; Thinsathid, N.; Jitprasertwong, P. Collagen-Based Biomaterials in Periodontal Regeneration: Current Applications and Future Perspectives of Plant-Based Collagen. Biomimetics 2022, 7, 34. [Google Scholar] [CrossRef]
- Embery, G.; Waddington, R.J.; Hall, R.C.; Last, K.S. Connective Tissue Elements as Diagnostic Aids in Periodontology. Periodontol 2000 2000, 24, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Gontiya, G.; Galgali, S. Effect of Hyaluronan on Periodontitis: A Clinical and Histological Study. J. Indian Soc. Periodontol. 2012, 16, 184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zheng, K.; Sui, B.; Boccaccini, A.R.; Sun, J. In Vitro Evaluation of Poly (Vinyl Alcohol)/Collagen Blended Hydrogels for Regulating Human Periodontal Ligament Fibroblasts and Gingival Fibroblasts. Int. J. Biol. Macromol. 2020, 163, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Srithep, Y.; Akkaprasa, T.; Pholharn, D.; Morris, J.; Liu, S.-J.; Patrojanasophon, P.; Ngawhirunpat, T. Metronidazole-Loaded Polylactide Stereocomplex Electrospun Nanofiber Mats for Treatment of Periodontal Disease. J. Drug Deliv. Sci. Technol. 2021, 64, 102582. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, K.; Xia, Y.; Qian, C.; Yu, D.-G.; Xie, Y.; Liao, Y. Electrospun Trilayer Eccentric Janus Nanofibers for a Combined Treatment of Periodontitis. Adv. Fiber Mater. 2024, 6, 1053–1073. [Google Scholar] [CrossRef]
- Simonca, A.G.; Kaya, M.G.A.; Rău, I.; Marin, M.M.; Dinu-Pîrvu, C.-E.; Ghica, M.V. The Influence of the Formula-Tion Factors on the Design and Characterization of Some Collagen-Based Hydrogels with Metronidazole. Sci. Bull. -Univ. Politeh. Buchar. Ser. B 2023, 85, 43–52. [Google Scholar]
- Manju, S.; Sreenivasan, K. Conjugation of Curcumin onto Hyaluronic Acid Enhances Its Aqueous Solubility and Stability. J. Colloid. Interface Sci. 2011, 359, 318–325. [Google Scholar] [CrossRef]
- Kumar Trivedi, M. Spectroscopic Characterization of Biofield Treated Metronidazole and Tinidazole. Med. Chem. 2015, 5, 7. [Google Scholar] [CrossRef]
- Bahman, M.; Jalili, H.; Etesam, M.; Amrane, A. Investigation of Pharmaceutical Compounds (Metronidazole, Rosuvastatin and Codeine Phosphate) Removal by Synechocystis Sp. PCC6803 Microalga. J. Water Process Eng. 2022, 47, 102820. [Google Scholar] [CrossRef]
- Krainer, S.; Hirn, U. Contact Angle Measurement on Porous Substrates: Effect of Liquid Absorption and Drop Size. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126503. [Google Scholar] [CrossRef]
- Menzies, K.L.; Jones, L. The Impact of Contact Angle on the Biocompatibility of Biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.V.; Albu, M.G.; Popa, L.; Moisescu, S. Response Surface Methodology and Taguchi Approach to Assess the Combined Effect of Formulation Factors on Minocycline Delivery from Collagen Sponges. Die Pharm. Int. J. Pharm. Sci. 2013, 68, 340–348. [Google Scholar]
- Xia, G.; Zhai, D.; Sun, Y.; Hou, L.; Guo, X.; Wang, L.; Li, Z.; Wang, F. Preparation of a Novel Asymmetric Wettable Chitosan-Based Sponge and Its Role in Promoting Chronic Wound Healing. Carbohydr. Polym. 2020, 227, 115296. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, A.Z.; Nelson-Filho, P.; Romano, F.L.; da Silva, R.A.; Saraiva, M.C.; da Silva, L.A.; Matsumoto, M.A. Gingival Crevicular Fluid Volume and Periodontal Parameters Alterations after Use of Conventional and Self-Ligating Brackets. J. Orthod. 2016, 43, 260–267. [Google Scholar] [CrossRef]
- Drummond, S.; Canavarro, C.; Perinetti, G.; Teles, R.; Capelli, J. The Monitoring of Gingival Crevicular Fluid Volume during Orthodontic Treatment: A Longitudinal Randomized Split-Mouth Study. Eur. J. Orthod. 2012, 34, 109–113. [Google Scholar] [CrossRef]
- Holm, R.; Borkenfelt, S.; Allesø, M.; Andersen, J.E.T.; Beato, S.; Holm, P. Investigation of Surface Porosity Measurements and Compaction Pressure as Means to Ensure Consistent Contact Angle Determinations. Int. J. Pharm. 2016, 498, 355–361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, J.; Wei, Y.; Xiong, J.; Liu, H.; Lv, G.; Zhao, J.; He, H.; Gou, J.; Yin, T.; Tang, X.; et al. A Multiple Controlled-Release Hydrophilicity Minocycline Hydrochloride Delivery System for the Efficient Treatment of Periodontitis. Int. J. Pharm. 2023, 636, 122802. [Google Scholar] [CrossRef]
- Tihan, G.T.; Rău, I.; Zgârian, R.G.; Ungureanu, C.; Barbaresso, R.C.; Kaya, M.G.A.; Dinu-Pîrvu, C.; Ghica, M.V. Oxytetracycline versus Doxycycline Collagen Sponges Designed as Potential Carrier Supports in Biomedical Applications. Pharmaceutics 2019, 11, 363. [Google Scholar] [CrossRef]
- Ioan, D.-C.; Rău, I.; Albu Kaya, M.G.; Radu, N.; Bostan, M.; Zgârian, R.G.; Tihan, G.T.; Dinu-Pîrvu, C.-E.; Lupuliasa, A.; Ghica, M.V. Ciprofloxacin-Collagen-Based Materials with Potential Oral Surgical Applications. Polymers 2020, 12, 1915. [Google Scholar] [CrossRef]
- Barat, R.; Srinatha, A.; Pandit, J.; Anupurba, S.; Mittal, N. Chitosan Inserts for Periodontitis: Influence of Drug Loading, Plasticizer and Crosslinking on in Vitro Metronidazole Release. Acta Pharm. 2007, 57, 469–477. [Google Scholar] [CrossRef]
- Ghavami-Lahiji, M.; Shafiei, F.; Najafi, F.; Erfan, M. Drug-Loaded Polymeric Films as a Promising Tool for the Treatment of Periodontitis. J. Drug Deliv. Sci. Technol. 2019, 52, 122–129. [Google Scholar] [CrossRef]
- Kida, D.; Karolewicz, B.; Junka, A.; Sender-Janeczek, A.; Duś, I.; Marciniak, D.; Szulc, M. Metronidazole-Loaded Porous Matrices for Local Periodontitis Treatment: In Vitro Evaluation and In Vivo Pilot Study. Appl. Sci. 2019, 9, 4545. [Google Scholar] [CrossRef]
- Pandey, S.; Das, U.; Patil, A. Formulation and Ex-Vivo Evaluation of Metronidazole Microemulsion Loaded Hydrogel for Prevention of Periodontitis. J. Pharm. Investig. 2014, 44, 225–236. [Google Scholar] [CrossRef]
- Ho, H.N.; Le, H.H.; Le, T.G.; Duong, T.H.A.; Ngo, V.Q.T.; Dang, C.T.; Nguyen, V.M.; Tran, T.H.; Nguyen, C.N. Formulation and Characterization of Hydroxyethyl Cellulose-Based Gel Containing Metronidazole-Loaded Solid Lipid Nanoparticles for Buccal Mucosal Drug Delivery. Int. J. Biol. Macromol. 2022, 194, 1010–1018. [Google Scholar] [CrossRef]
- Jitrangsri, K.; Lertsuphotvanit, N.; Kabthong, N.; Phaechamud, T. Metronidazole-Loaded Camphor-Based In Situ Forming Matrix for Periodontitis Treatment. AAPS PharmSciTech 2023, 24, 185. [Google Scholar] [CrossRef]
- Khan, G.; Yadav, S.K.; Patel, R.R.; Nath, G.; Bansal, M.; Mishra, B. Development and Evaluation of Biodegradable Chitosan Films of Metronidazole and Levofloxacin for the Management of Periodontitis. AAPS PharmSciTech 2016, 17, 1312–1325. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.J.; Kharrazi, H.; Chang, H.-Y.; Bodycombe, D.; Lemke, K.; Weiner, J.P. Exploring the Use of Machine Learning for Risk Adjustment: A Comparison of Standard and Penalized Linear Regression Models in Predicting Health Care Costs in Older Adults. PLoS ONE 2019, 14, e0213258. [Google Scholar] [CrossRef]
- Saffron, C.M.; Park, J.-H.; Dale, B.E.; Voice, T.C. Kinetics of Contaminant Desorption from Soil: Comparison of Model Formulations Using the Akaike Information Criterion. Environ. Sci. Technol. 2006, 40, 7662–7667. [Google Scholar] [CrossRef]
- Romero, A.I.; Villegas, M.; Cid, A.G.; Parentis, M.L.; Gonzo, E.E.; Bermúdez, J.M. Validation of Kinetic Modeling of Progesterone Release from Polymeric Membranes. Asian J. Pharm. Sci. 2018, 13, 54–62. [Google Scholar] [CrossRef]
- Albu, M.G. Collagen Gels and Matrices for Biomedical Applications; Lambert Academic Publishing: Saarbrücken, Germany, 2011. [Google Scholar]
- Sleiman, L.; Lazăr (Popa), A.-D.; Albu-Kaya, M.; Marin, M.M.; Kaya, D.A.; Vasile, O.-R.; Dinescu, S. Development and Investigation of an Innovative 3D Biohybrid Based on Collagen and Silk Sericin Enriched with Flavonoids for Potential Wound Healing Applications. Polymers 2024, 16, 1627. [Google Scholar] [CrossRef]
- Popa, L.; Ghica, M.V.; Albu, M.G.; Ortan, A.; Dinu-Pîrvu, C.E. Hysteresis of contact angle. Dynamic wettability studies of collagen and doxycycline porous matrices crosslinked with tannic acid. Dig. J. Nanomater. Biostruct. 2013, 8, 937–943. [Google Scholar]
- Askarizadeh, M.; Esfandiari, N.; Honarvar, B.; Sajadian, S.A.; Azdarpour, A. Kinetic Modeling to Explain the Release of Medicine from Drug Delivery Systems. ChemBioEng Rev. 2023, 10, 1006–1049. [Google Scholar] [CrossRef]
- Ștefania, M.; Albu Kaya, M.G.; Ghica, M.V.; Dinu-Pîrvu, C.; Popa, L.; Udeanu, D.I.; Mihai, G.; Enachescu, M. Collagen-Polyvinyl Alcohol-Indomethacin Biohybrid Matrices as Wound Dressings. Pharmaceutics 2018, 10, 224. [Google Scholar] [CrossRef] [PubMed]


| Sponges | Zero-Order Model | Higuchi Model | Power Law Model | Release Exponent | Kinetic Constant (1/min) | MTZ Released (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | Adj R2 | AICc | R | Adj R2 | AICc | R | Adj R2 | AICc | ||||
| M3 | 0.8701 | 0.7085 | −46.18 | 0.9698 | 0.9290 | −64.53 | 0.9841 | 0.9622 | −72.74 | 0.37 | 0.077 | 72.36 |
| M4 | 0.8796 | 0.7286 | −42.07 | 0.9742 | 0.9390 | −61.48 | 0.9862 | 0.9672 | −69.55 | 0.37 | 0.089 | 88.59 |
| M5 | 0.8295 | 0.6260 | −41.27 | 0.9504 | 0.8842 | −56.51 | 0.9845 | 0.9633 | −71.47 | 0.30 | 0.131 | 80.98 |
| M6 | 0.8511 | 0.6695 | −38.97 | 0.9621 | 0.9109 | −56.02 | 0.9897 | 0.9757 | −72.90 | 0.31 | 0.141 | 95.57 |
| Sample Code | Coll, % | MTZ, % | HA, % | GA, % |
|---|---|---|---|---|
| M1 | 1 | 0 | 0 | 0.2 |
| M2 | 1 | 0 | 0.8 | 0.2 |
| M3 | 1 | 1.5 | 0 | 0.2 |
| M4 | 1 | 1.5 | 0.8 | 0.2 |
| M5 | 1 | 2.0 | 0 | 0.2 |
| M6 | 1 | 2.0 | 0.8 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, M.G.A.; Simonca, A.G.; Rau, I.; Coman, A.E.; Marin, M.M.; Popa, L.; Trusca, R.; Dinu-Pirvu, C.-E.; Ghica, M.V. Topical Biocomposites Based on Collagen, Hyaluronic Acid and Metronidazole as Periodontitis Treatment. Pharmaceuticals 2024, 17, 1336. https://doi.org/10.3390/ph17101336
Kaya MGA, Simonca AG, Rau I, Coman AE, Marin MM, Popa L, Trusca R, Dinu-Pirvu C-E, Ghica MV. Topical Biocomposites Based on Collagen, Hyaluronic Acid and Metronidazole as Periodontitis Treatment. Pharmaceuticals. 2024; 17(10):1336. https://doi.org/10.3390/ph17101336
Chicago/Turabian StyleKaya, Madalina Georgiana Albu, Alice Geanina Simonca, Ileana Rau, Alina Elena Coman, Minodora Maria Marin, Lacramioara Popa, Roxana Trusca, Cristina-Elena Dinu-Pirvu, and Mihaela Violeta Ghica. 2024. "Topical Biocomposites Based on Collagen, Hyaluronic Acid and Metronidazole as Periodontitis Treatment" Pharmaceuticals 17, no. 10: 1336. https://doi.org/10.3390/ph17101336
APA StyleKaya, M. G. A., Simonca, A. G., Rau, I., Coman, A. E., Marin, M. M., Popa, L., Trusca, R., Dinu-Pirvu, C.-E., & Ghica, M. V. (2024). Topical Biocomposites Based on Collagen, Hyaluronic Acid and Metronidazole as Periodontitis Treatment. Pharmaceuticals, 17(10), 1336. https://doi.org/10.3390/ph17101336











