Inhibitory Effects of Decursin Derivative against Lipopolysaccharide-Induced Inflammation
Abstract
:1. Introduction
2. Results
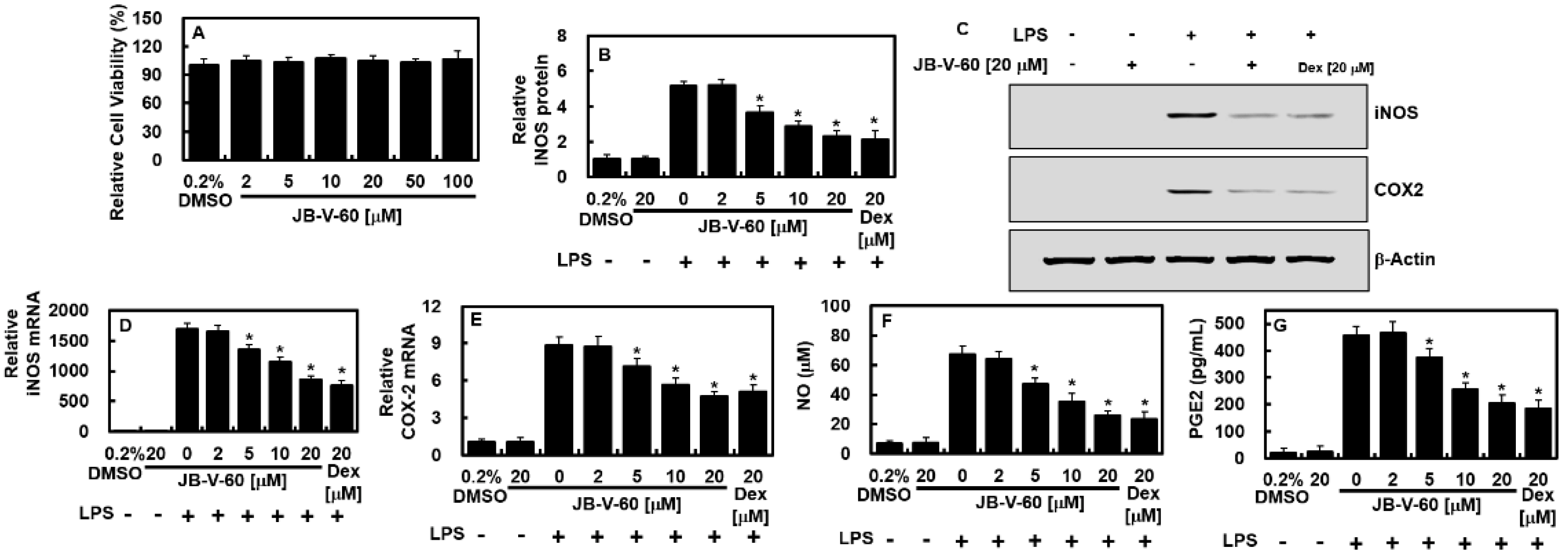
2.1. Effect of JB-V-60 on iNOS and COX-2 Expression in LPS-Stimulated HPAECs
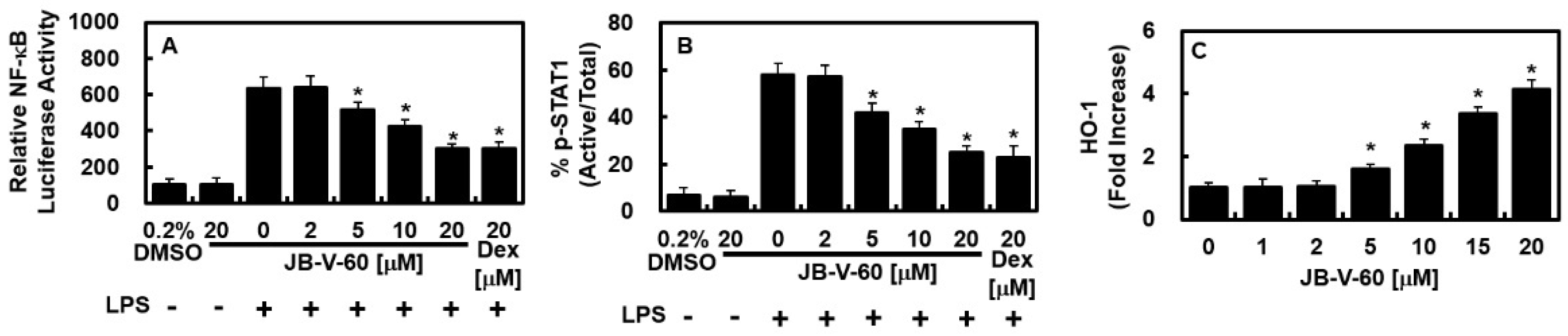
2.2. Effect of JB-V-60 on NF-κB Activity, STAT-1 Phosphorylation, and HO-1 Protein Levels in LPS-Stimulated HPAECs
2.3. Effects of JB-V-60 on Nrf2 Nuclear Translocation, ARE Reporter Activity, and Anti-Inflammatory Mechanisms in LPS-Stimulated HPAECs
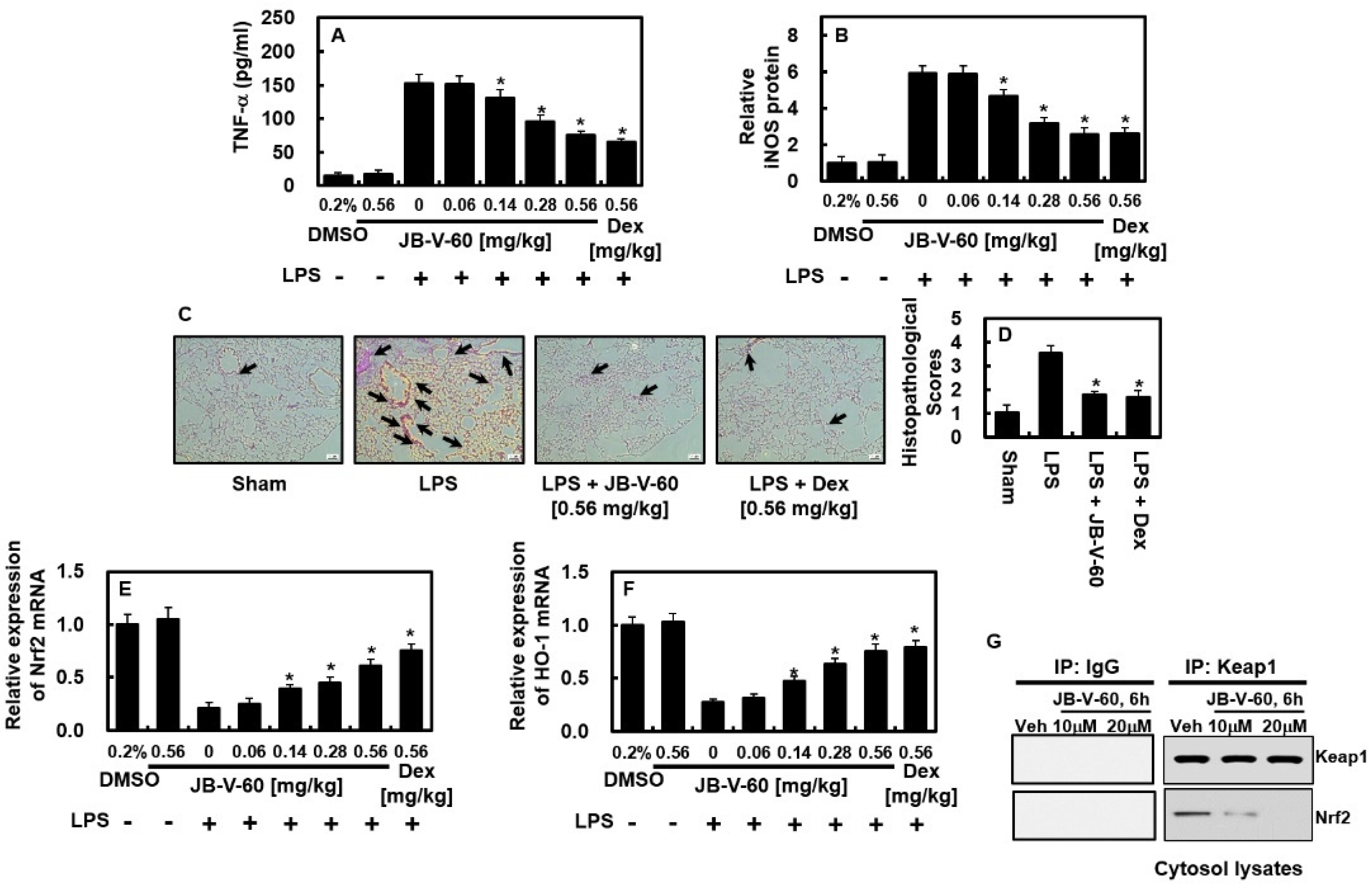
2.4. Effects of JB-V-60 on TNF-α and iNOS Protein Expression in a Mouse Model of LPS-Induced Lung Injury
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Substances
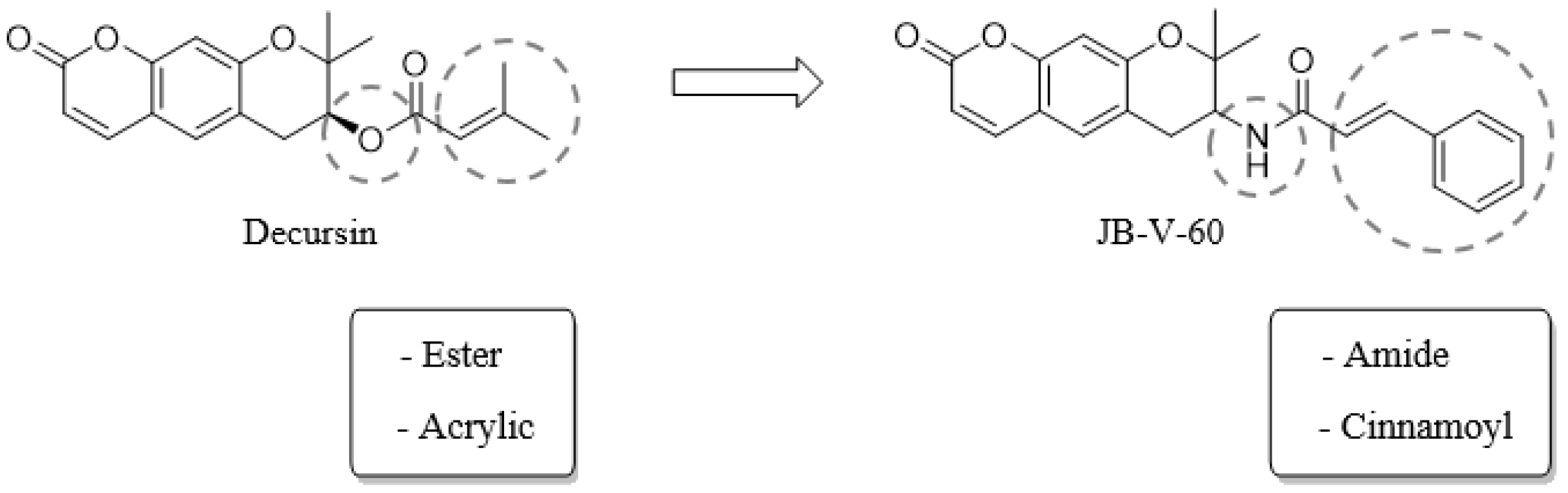
4.2. General Information of JB-V-60
Method for the Synthesis of N-(8,8-dimethyl-2-oxo-7,8-dihydro-2H,6H-pyrano[3,2-g]chromen-7-yl)cinnamamide (JB-V-60) (Scheme 1)
4.3. In Vitro Studies
4.3.1. Cell Viability Assay
4.3.2. Enzyme-Linked Immunosorbent Assays (ELISAs)
4.3.3. Nitrite Levels
4.3.4. Intracellular Fractionation, Co-Immunoprecipitation (Co-IP), and Immunoblotting
4.3.5. Quantitative Real-Time Polymerase Chain Reaction
4.3.6. Transfection
4.3.7. ARE Luciferase Reporter Assay
4.4. In Vivo Studies
4.4.1. Mouse Model of LPS-Induced Lung Injury
4.4.2. Histopathological Analysis
4.4.3. Evaluation of Oxidative Stress Markers
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chau, L.Y. Heme oxygenase-1: Emerging target of cancer therapy. J. Biomed. Sci. 2015, 22, 22. [Google Scholar]
- Waza, A.A.; Hamid, Z.; Ali, S.; Bhat, S.A.; Bhat, M.A. A review on heme oxygenase-1 induction: Is it a necessary evil. Inflamm. Res. 2018, 67, 579–588. [Google Scholar] [PubMed]
- Tsoyi, K.; Lee, T.Y.; Lee, Y.S.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Chang, K.C. Heme-oxygenase-1 induction and carbon monoxide-releasing molecule inhibit lipopolysaccharide (lps)-induced high-mobility group box 1 release in vitro and improve survival of mice in lps- and cecal ligation and puncture-induced sepsis model in vivo. Mol. Pharmacol. 2009, 76, 173–182. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018, 17, 297–314. [Google Scholar]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting nf-kappab pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target Ther. 2020, 5, 209. [Google Scholar]
- Druszczynska, M.; Godkowicz, M.; Kulesza, J.; Wawrocki, S.; Fol, M. Cytokine receptors-regulators of antimycobacterial immune response. Int. J. Mol. Sci. 2022, 23, 1112. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, X.; Yu, S.; Kou, J. Molecular mechanisms in lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Int. Immunopharmacol. 2015, 29, 937–946. [Google Scholar]
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar]
- Cho, J.H.; Kwon, J.E.; Cho, Y.; Kim, I.; Kang, S.C. Anti-inflammatory effect of angelica gigas via heme oxygenase (ho)-1 expression. Nutrients 2015, 7, 4862–4874. [Google Scholar] [CrossRef]
- He, L.; Pan, Y.; Yu, J.; Wang, B.; Dai, G.; Ying, X. Decursin alleviates the aggravation of osteoarthritis via inhibiting pi3k-akt and nf-kb signal pathway. Int. Immunopharmacol. 2021, 97, 107657. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Kim, H.J.; Shin, J.S.; Kim, D.H.; Cho, H.J.; Lee, S.S.; Ahn, S.K.; Yun-Choi, H.S.; Lee, J.H.; Seo, H.G.; et al. Ho-1 and jak-2/stat-1 signals are involved in preferential inhibition of inos over cox-2 gene expression by newly synthesized tetrahydroisoquinoline alkaloid, ckd712, in cells activated with lipopolysacchride. Cell. Signal. 2008, 20, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Nizamutdinova, I.T.; Jang, H.J.; Mun, L.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Chang, K.C. Carbon monoxide from corm-2 reduces hmgb1 release through regulation of ifn-beta/jak2/stat-1/inos/no signaling but not cox-2 in tlr-activated macrophages. Shock 2010, 34, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, D.; Lee, Y.; Lee, T.; Song, K.S.; Yang, E.J.; Bae, J.S. Isolation, synthesis, and antisepsis effects of a c-methylcoumarinochromone isolated from abronia nana cell culture. J. Nat. Prod. 2018, 81, 1173–1182. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.Y.; Yoo, Y.; Kim, S.Y.; Kim, J.E.; Kim, S.W.; Seo, Y.K.; Park, E.K.; Kim, I.S.; Bae, J.S. Macrophagic stabilin-1 restored disruption of vascular integrity caused by sepsis. Thromb. Haemost. 2018, 118, 1776–1789. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Gaddi, A.; Cicero, A.F.; Pedro, E.J. Clinical perspectives of anti-inflammatory therapy in the elderly: The lipoxigenase (lox)/cycloxigenase (cox) inhibition concept. Arch. Gerontol. Geriatr. 2004, 38, 201–212. [Google Scholar] [CrossRef]
- Wullaert, A.; Bonnet, M.C.; Pasparakis, M. Nf-kappab in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011, 21, 146–158. [Google Scholar]
- Lee, I.-C.; Ryu, C.-W.; Bae, J.-S. Novel herbal medicine c-kok suppresses the inflammatory gene inos via the inhibition of p-stat-1 and nf-κb. Biotechnol. Bioproc. Eng. 2020, 25, 536–542. [Google Scholar]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Lee, I.-C.; Bae, J.-S. Hepatic protective effects of jujuboside b through the modulation of inflammatory pathways. Biotechnol. Bioproc. Eng. 2022, 27, 336–343. [Google Scholar] [CrossRef]
- Lee, W.H.; Choo, S.; Sim, H.; Bae, J.S. Inhibitory activities of ononin on particulate matter-induced oxidative stress. Biotechnol. Bioproc. Eng. 2021, 26, 208–215. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, W.; Yang, S.; Cho, S.H.; Baek, M.C.; Song, G.Y.; Bae, J.S. Suppressive effects of rare ginsenosides, rk1 and rg5, on hmgb1-mediated septic responses. Food Chem. Toxicol. 2019, 124, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.H.; Kim, G.O.; Choi, H.J.; Yun, M.Y.; Park, D.H.; Song, G.Y.; Bae, J.S. Inhibitory activities of gdx-365 on hmgb1-mediated septic responses. Biotechnol. Bioproc. Eng. 2023, 28, 623–631. [Google Scholar] [CrossRef]
- Kim, C.; Ryu, S.H.; Choi, H.; Park, D.H.; Bae, J.S. The inhibitory functions of sparstolonin b against ambient fine particulate matter induced lung injury. Biotechnol. Bioproc. Eng. 2022, 27, 949–960. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Kim, J.E.; Cho, G.E.; Song, G.Y.; Bae, J.S. Pulmonary protective functions of rare ginsenoside rg4 on particulate matter-induced inflammatory responses. Biotechnol. Bioproc. Eng. 2019, 24, 445–453. [Google Scholar] [CrossRef]


| SOD (U/mg Protein) | CAT (U/mg Protein) | |
|---|---|---|
| DMSO | 1.05 ± 0.03 | 4.03 ± 0.15 |
| JB-V-60 (0.56 mg/kg) | 1.04 ± 0.02 | 4.11 ± 0.11 |
| LPS | 0.57 ± 0.03 | 2.63 ± 0.23 |
| LPS + JB-V-60 (0.06 mg/kg) | 0.52 ± 0.04 | 2.71 ± 0.12 |
| LPS + JB-V-60 (0.14 mg/kg) | 0.65 ± 0.04 * | 3.18 ± 0.15 * |
| LPS + JB-V-60 (0.28 mg/kg) | 0.72 ± 0.03 * | 3.73 ± 0.21 * |
| LPS + JB-V-60 (0.56 mg/kg) | 0.91 ± 0.04 * | 3.95 ± 0.21 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Heo, J.-B.; Cho, S.; Ryu, C.-W.; Heo, H.-J.; Yun, M.-Y.; Nam, G.; Song, G.-Y.; Bae, J.-S. Inhibitory Effects of Decursin Derivative against Lipopolysaccharide-Induced Inflammation. Pharmaceuticals 2024, 17, 1337. https://doi.org/10.3390/ph17101337
Lee J, Heo J-B, Cho S, Ryu C-W, Heo H-J, Yun M-Y, Nam G, Song G-Y, Bae J-S. Inhibitory Effects of Decursin Derivative against Lipopolysaccharide-Induced Inflammation. Pharmaceuticals. 2024; 17(10):1337. https://doi.org/10.3390/ph17101337
Chicago/Turabian StyleLee, Jinhee, Jong-Beom Heo, Sanghee Cho, Chang-Woo Ryu, Hae-Joon Heo, Mi-Young Yun, Gaewon Nam, Gyu-Yong Song, and Jong-Sup Bae. 2024. "Inhibitory Effects of Decursin Derivative against Lipopolysaccharide-Induced Inflammation" Pharmaceuticals 17, no. 10: 1337. https://doi.org/10.3390/ph17101337







