Potential Anti-Obesity Effect of Hazel Leaf Extract in Mice and Network Pharmacology of Selected Polyphenols
Abstract
:1. Introduction
2. Results
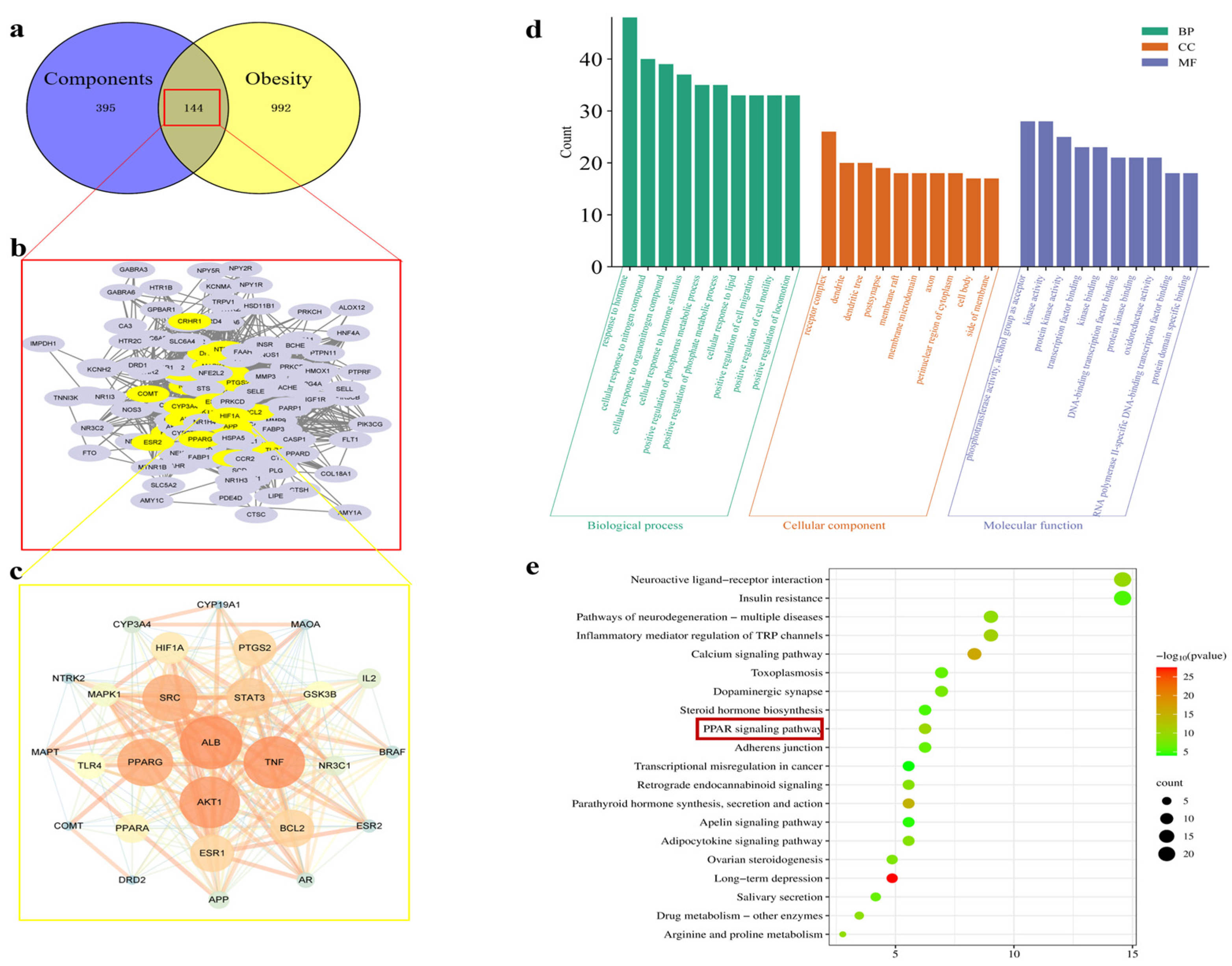
2.1. Results of Network Pharmacology
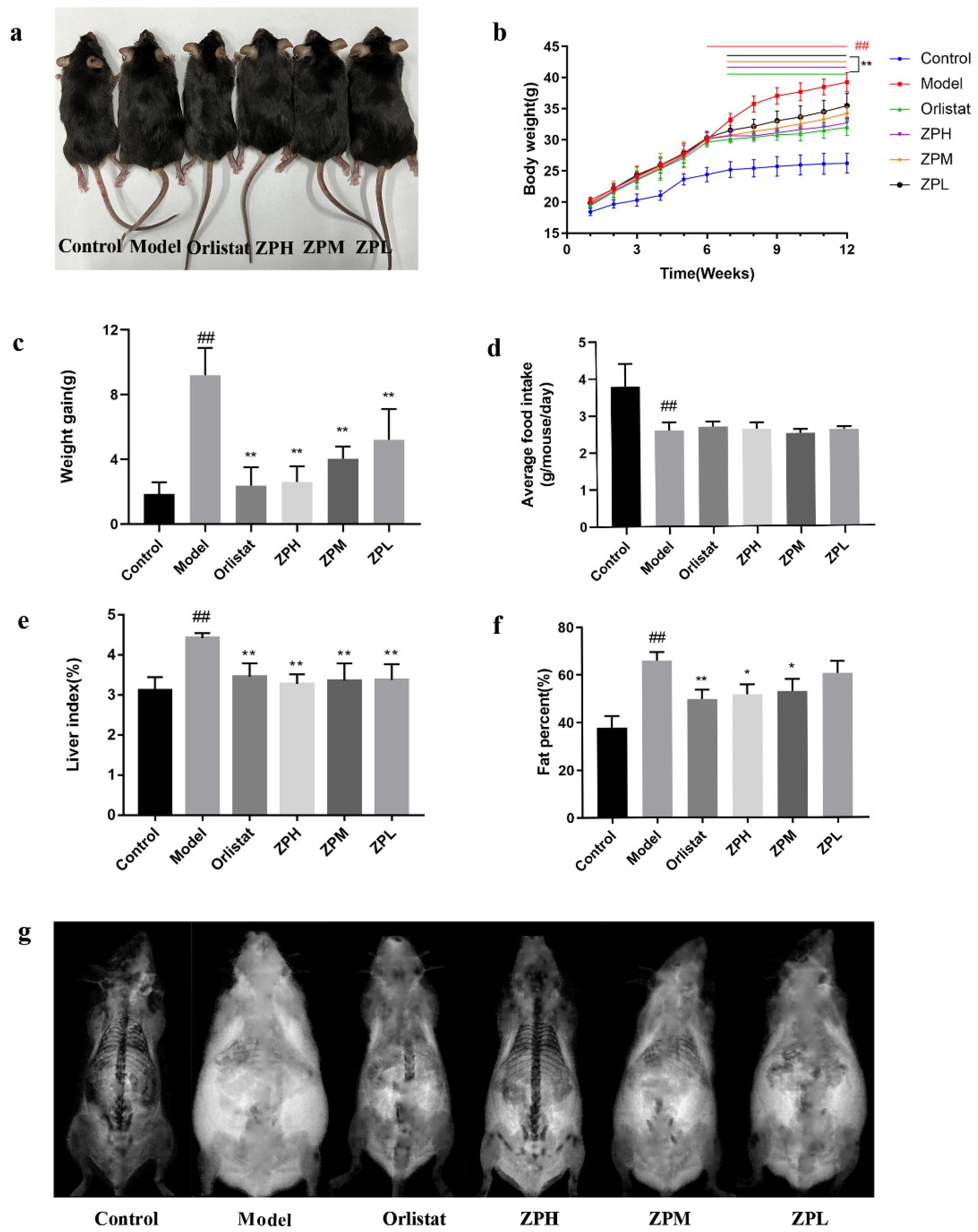
2.2. Effects of ZP on Weight Gain, Food Intake, Liver Index, and Fat Content in Obese Mice
2.3. Effects of ZP on Blood Lipid Levels in Obese Mice
2.4. Effects of ZP on ALT and AST in Obese Mice
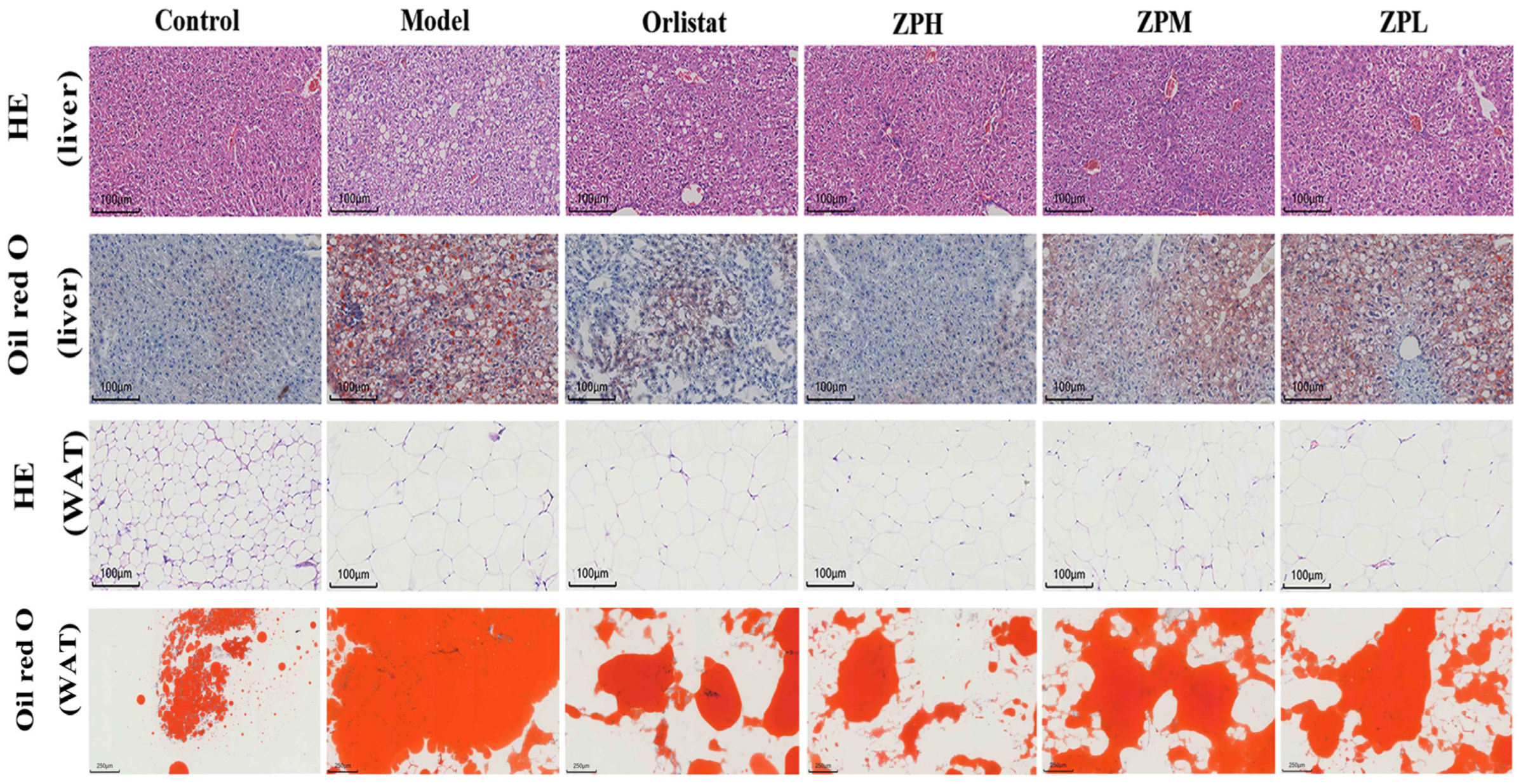
2.5. Effects of ZP on Histological in Obese Mice
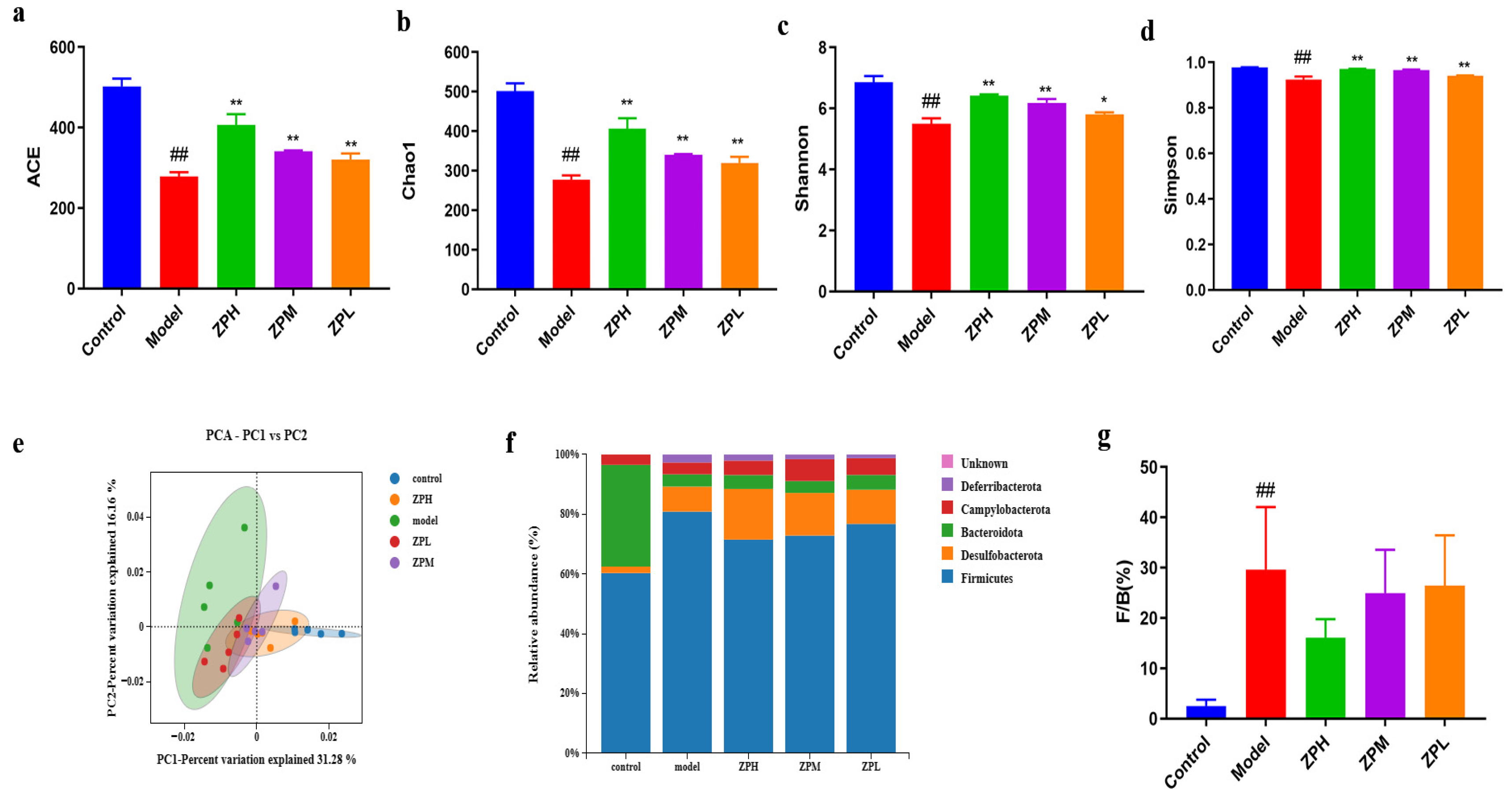
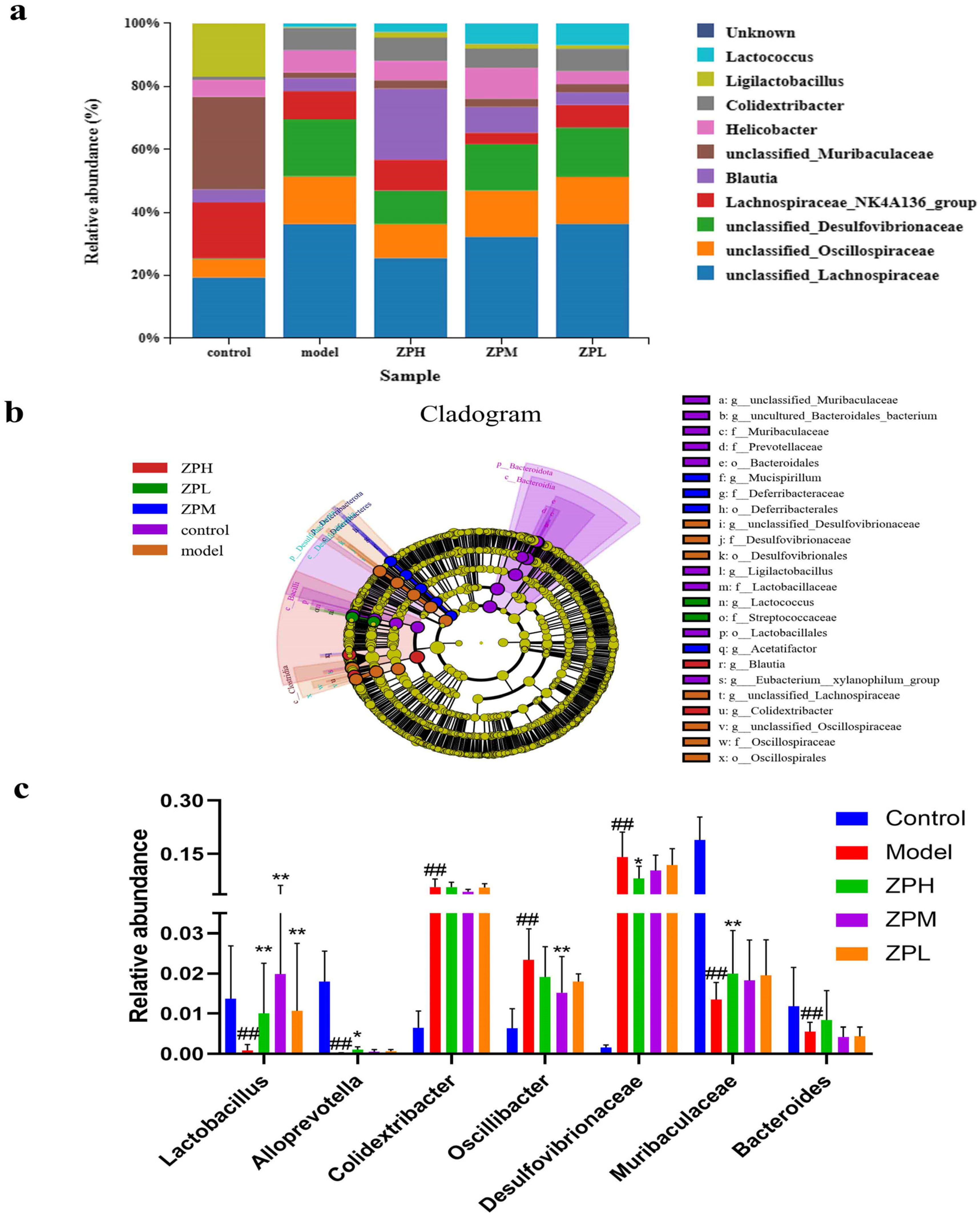
2.6. Effects of ZP on Gut Microbes in Obese Mice
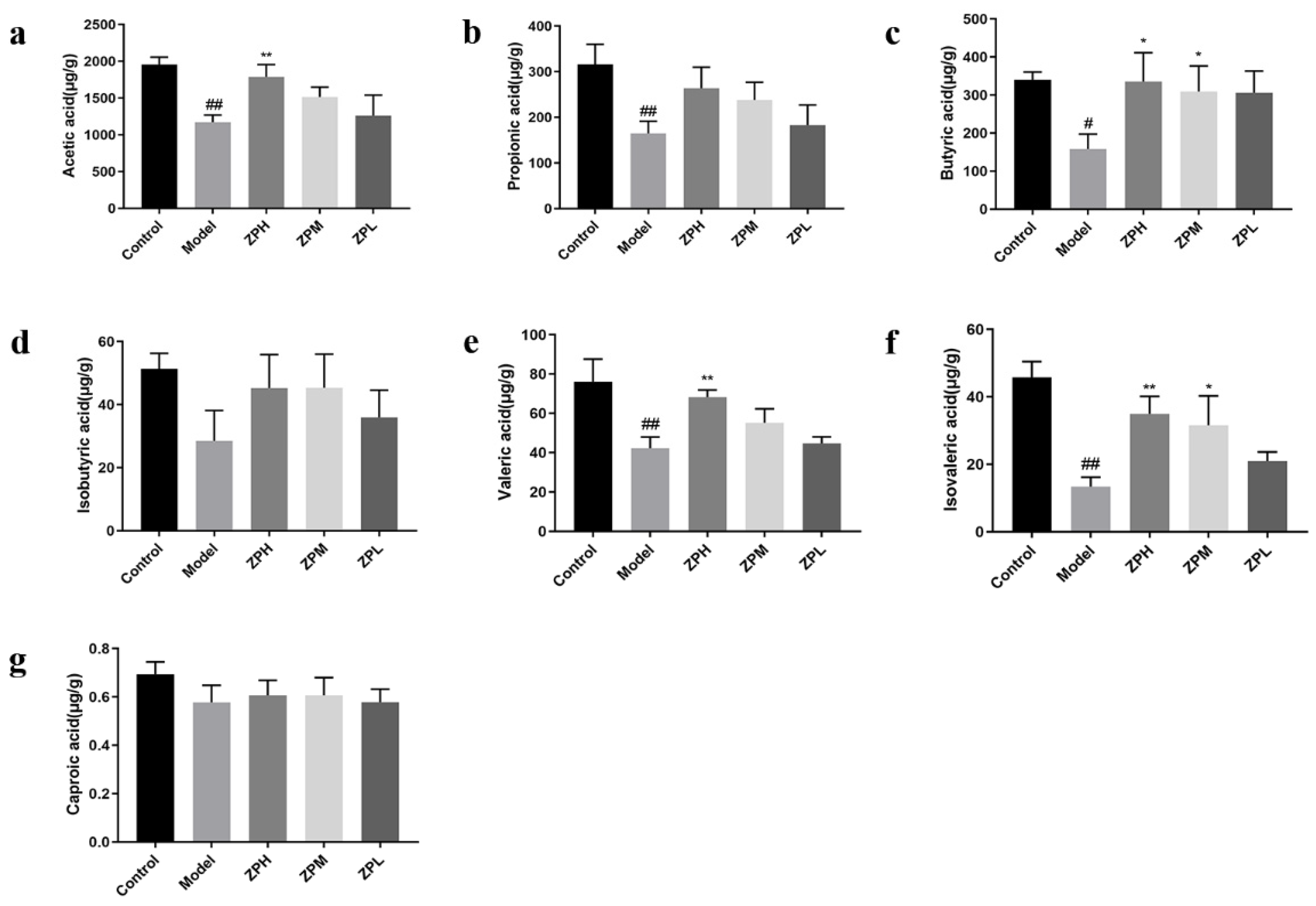
2.7. Effects of ZP on SCFAs Content in Obese Mice
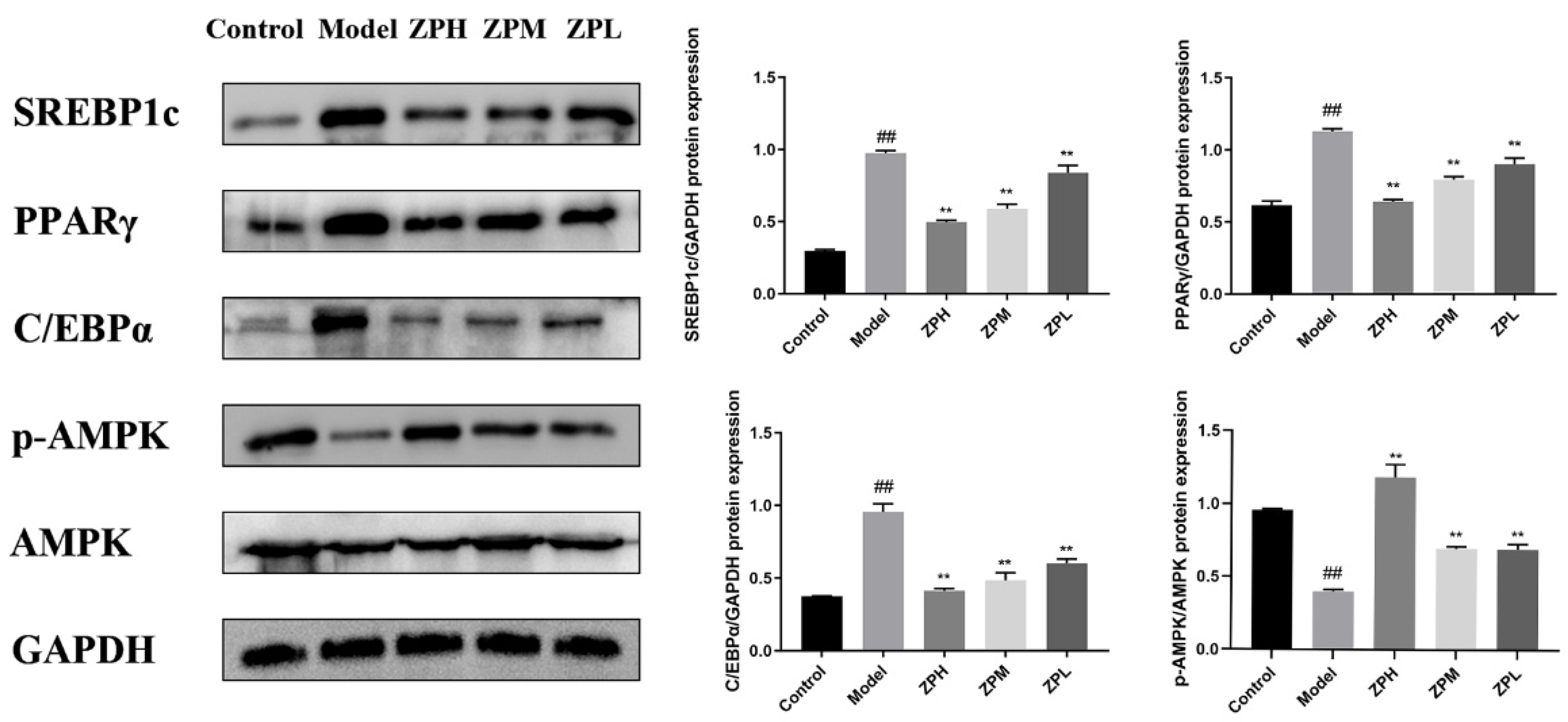
2.8. Effects of ZP on White Adipose Tissue Protein Expression
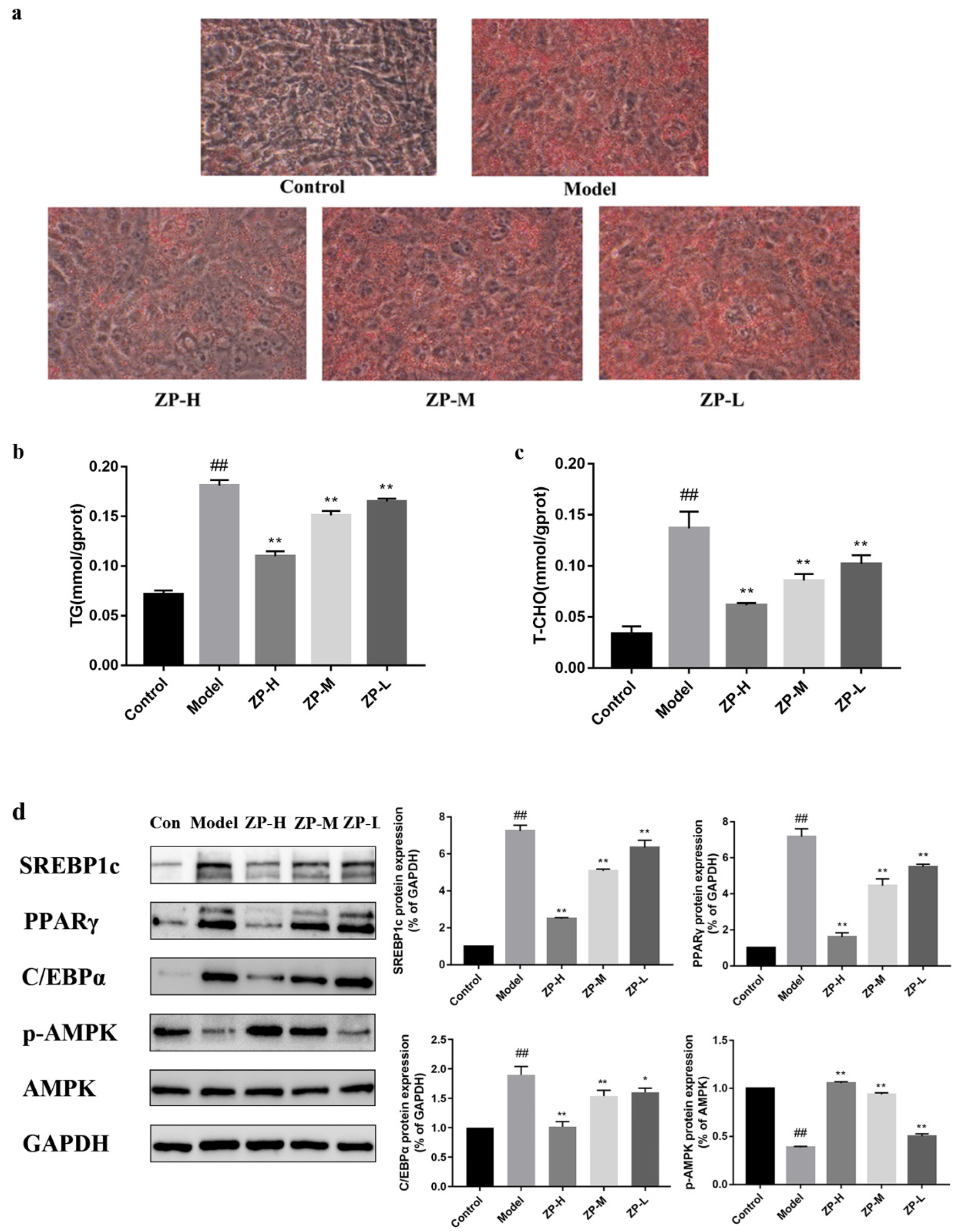
2.9. Effect of ZP on Lipid Levels in 3T3-L1 Cells
2.10. Molecular Docking Analysis
3. Discussion and Limitation
3.1. Discussion
3.2. Limitation
4. Materials and Methods
4.1. Materials and Reagents
4.2. Network Pharmacology Analysis
4.3. Animals and the Experimental Design
4.4. Serum Biochemical Analysis
4.5. Histological Analysis
4.6. Gut Microbial Analysis
4.7. Determination of Short-Chain Fatty Acids (SCFAs)
4.8. Cell Culture and Treat
4.9. Cell Oil Red O Staining
4.10. Western Blotting
4.11. Molecular Docking Method
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight/ (accessed on 14 September 2024).
- Ginsberg, H.N.; MacCallum, P.R. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J. Cardiometab. Syndr. 2009, 4, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Jegal, J.; Kim, Y.N.; Heo, J.D.; Rho, J.R.; Yang, M.H.; Jeong, E.J. Chokeberry Extract and Its Active Polyphenols Suppress Adipogenesis in 3T3-L1 Adipocytes and Modulates Fat Accumulation and Insulin Resistance in Diet-Induced Obese Mice. Nutrients 2018, 10, 1734. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, Y.; Huang, Q.; Deng, R.; Zheng, X. Averrhoa carambola L. fruit polyphenols ameliorate hyperlipidemia, hepatic steatosis, and hyperglycemia by modulating lipid and glucose metabolism in mice with obesity. J. Sci. Food Agric. 2023, 103, 6531–6539. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Choi, H.J.; Ryu, G.H.; Lee, J.W.; Beak, J.K.; Koh, E.J.; Jeong, J.B. Paeonia lactiflora root decreases lipid accumulation through the induction of lipolysis and thermogenesis via AMPK activation in 3T3-L1 cells. Int. J. Mol. Med. 2023, 52, 65. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira e Silva, A.M.; Pereira, R.O.; Oliveira, A.K.d.S.; Harris, F.S.; de Melo, I.L.P.; Almeida-Souza, T.H.; Yoshime, L.T.; dos Santos Melo, C.; Lopes dos Santos, J.; de Andrade-Wartha, E.R.S.; et al. Ameliorative effects of aqueous extract from rosemary on oxidative stress and inflammation pathways caused by a high-fat diet in C57BL/6 mice. Appl. Physiol. Nutr. Metab. 2024, 49, 459–472. [Google Scholar] [CrossRef]
- Ramadan, N.S.; El-Sayed, N.H.; El-Toumy, S.A.; Mohamed, D.A.; Aziz, Z.A.; Marzouk, M.S.; Esatbeyoglu, T.; Farag, M.A.; Shimizu, K. Anti-Obesity Evaluation of Averrhoa carambola L. Leaves and Assessment of Its Polyphenols as Potential alpha-Glucosidase Inhibitors. Molecules 2022, 27, 5159. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Q.; Wang, X.; Wang, H. Mulberry leaf polyphenols alleviated high-fat diet-induced obesity in mice. Front. Nutr. 2022, 9, 979058. [Google Scholar] [CrossRef]
- Samuel Wu, Y.-H.; Chiu, C.-H.; Yang, D.-J.; Lin, Y.-L.; Tseng, J.-K.; Chen, Y.-C. Inhibitory effects of litchi (Litchi chinensis Sonn.) flower-water extracts on lipase activity and diet-induced obesity. J. Funct. Foods 2013, 5, 923–929. [Google Scholar] [CrossRef]
- Amorim, R.P.; Daher, I.P.; Arcangelo, F.P.D.; Bellini, M.F.; Orcini, W.A.; Sarria, G.R.; Peruquetti, R.L. Short-term effects of green tea chronotherapy on the metabolic homeostasis of mice on different diets. Genet. Mol. Res. 2017, 16, gmr16029605. [Google Scholar] [CrossRef]
- Seo, D.B.; Jeong, H.W.; Kim, Y.J.; Kim, S.; Kim, J.; Lee, J.H.; Joo, K.; Choi, J.K.; Shin, S.S.; Lee, S.J. Fermented green tea extract exhibits hypolipidaemic effects through the inhibition of pancreatic lipase and promotion of energy expenditure. Br. J. Nutr. 2017, 117, 177–186. [Google Scholar] [CrossRef]
- Wu, D.; Ma, X.; Tian, W. Pomegranate husk extract, punicalagin and ellagic acid inhibit fatty acid synthase and adipogenesis of 3T3-L1 adipocyte. J. Funct. Foods 2013, 5, 633–641. [Google Scholar] [CrossRef]
- Ágnes Alberti, E.R. Szabolcs Béni and Ágnes Kéry, Evaluation of Radical Scavenging Activity of Sempervivum tectorum and Corylus avellana Extracts with Different Phenolic Composition. Nat. Prod. Commun. 2016, 11, 1934578X1601100412. [Google Scholar]
- Oliveira, I.; Sousa, A.; Valentao, P.; Andrade, P.; Ferreira, I.; Ferreres, F.; Bento, A.; Seabra, R.; Estevinho, L.; Pereira, J. Hazel (Corylus avellana L.) leaves as source of antimicrobial and antioxidative compounds. Food Chem. 2007, 105, 1018–1025. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Wang, Y.; Lv, G.; Lin, H.; Lin, Z. UPLC-MS/MS profiling, antioxidant and anti-inflammatory activities, and potential health benefits prediction of phenolic compounds in hazel leaf. Front. Nutr. 2023, 10, 1092071. [Google Scholar] [CrossRef]
- Lin, G.; Liu, X.; Yan, X.; Liu, D.; Yang, C.; Liu, B.; Huang, Y.; Zhao, C. Role of Green Macroalgae Enteromorpha Prolifera Polyphenols in the Modulation of Gene Expression and Intestinal Microflora Profiles in Type 2 Diabetic Mice. Int. J. Mol. Sci. 2018, 20, 25. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Guo, X.; Tang, R.; Yang, S.; Lu, Y.; Luo, J.; Liu, Z. Rutin and Its Combination With Inulin Attenuate Gut Dysbiosis, the Inflammatory Status and Endoplasmic Reticulum Stress in Paneth Cells of Obese Mice Induced by High-Fat Diet. Front. Microbiol. 2018, 9, 2651. [Google Scholar] [CrossRef]
- Hee, B.v.d.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [PubMed]
- Wang, Y.; Zhao, L.; Gao, L.; Pan, A.; Xue, H. Health policy and public health implications of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 446–461. [Google Scholar] [CrossRef]
- Kim, K.-H.; Park, Y. Food Components with Anti-Obesity Effect. Annu. Rev. Food Sci. Technol. 2011, 2, 237–257. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Z.; Brennan, M.A.; Wang, J.; Sun, J.; Fang, H.; Kang, M.; Brennan, C.S.; Mu, J. The effect of extruded multigrain powder on metabolism and intestinal flora of high-fat-diet induced C57BL/6J mice. Food Res. Int. 2023, 169, 112878. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Prifti, E.; Belda, E.; Ichou, F.; Kayser, B.D.; Dao, M.C.; Verger, E.O.; Hedjazi, L.; Bouillot, J.L.; Chevallier, J.M.; et al. Major microbiota dysbiosis in severe obesity: Fate after bariatric surgery. Gut 2019, 68, 70–82. [Google Scholar] [CrossRef]
- Sun, L.; Ma, L.; Ma, Y.; Zhang, F.; Zhao, C.; Nie, Y. Insights into the role of gut microbiota in obesity: Pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell 2018, 9, 397–403. [Google Scholar] [CrossRef]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, W.; Wang, J.; Yu, J.; Yang, L.Q. Intermittent Fasting Improves Lipid Metabolism Through Changes in Gut Microbiota in Diet-Induced Obese Mice. Med. Sci. Monit. 2020, 26, e926789. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Paone, P.; Suriano, F.; Jian, C.; Korpela, K.; Delzenne, N.M.; Van Hul, M.; Salonen, A.; Cani, P.D. Prebiotic oligofructose protects against high-fat diet-induced obesity by changing the gut microbiota, intestinal mucus production, glycosylation and secretion. Gut Microbes 2022, 14, 2152307. [Google Scholar] [CrossRef]
- Zhao, Q.; Fu, Y.; Zhang, F.; Wang, C.; Yang, X.; Bai, S.; Xue, Y.; Shen, Q. Heat-Treated Adzuki Bean Protein Hydrolysates Reduce Obesity in Mice Fed a High-Fat Diet via Remodeling Gut Microbiota and Improving Metabolic Function. Mol. Nutr. Food Res. 2022, 66, 2100907. [Google Scholar] [CrossRef]
- Requena, T.; Martínez-Cuesta, M.C.; Peláez, C. Diet and microbiota linked in health and disease. Food Funct. 2018, 9, 688–704. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Munoz-Garach, A.; Clemente-Postigo, M.; Tinahones, F.J. Importance of gut microbiota in obesity. Eur. J. Clin. Nutr. 2019, 72 (Suppl. S1), 26–37. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Avagliano, C.; Calignano, A.; Berni Canani, R. The protective role of butyrate against obesity and obesity-related diseases. Molecules 2021, 26, 682. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Kong, L.; Shan, M.; Lu, Z.; Lu, Y. Protective and ameliorating effects of probiotics against diet-induced obesity: A review. Food Res. Int. 2021, 147, 110490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kong, L.; Zhong, Z.; Lin, L.; Li, J.; Zheng, G. Short chain fatty acids increase fat oxidation and promote browning through β3-adrenergic receptor/AMP-activated protein kinase α signaling pathway in 3T3-L1 adipocytes. J. Funct. Foods 2023, 103, 105488. [Google Scholar] [CrossRef]
- Shimano, H.; Yahagi, N.; Amemiya-Kudo, M.; Hasty, A.H.; Osuga, J.; Tamura, Y.; Shionoiri, F.; Iizuka, Y.; Ohashi, K.; Harada, K.; et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J. Biol. Chem. 1999, 274, 35832–35839. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef]
- Zhang, G.; Li, N.; Tong, Y.; Li, P.; Han, H.; Song, Q.; Yang, B.; Cui, L. Artemisinin derivatives inhibit adipogenic differentiation of 3T3-L1 preadipocytes through upregulation of CHOP. Biochem. Res Commun. 2021, 557, 309–315. [Google Scholar] [CrossRef]
- Liu, S.; Chang, X.; Yu, J.; Xu, W. Cerasus humilis Cherry Polyphenol Reduces High-Fat Diet-Induced Obesity in C57BL/6 Mice by Mitigating Fat Deposition, Inflammation, and Oxidation. J. Agric. Food Chem. 2020, 68, 4424–4436. [Google Scholar] [CrossRef]
- Fang, C.; Kim, H.; Noratto, G.; Sun, Y.; Talcott, S.T.; Mertens-Talcott, S.U. Gallotannin derivatives from mango (Mangifera indica L.) suppress adipogenesis and increase thermogenesis in 3T3-L1 adipocytes in part through the AMPK pathway. J. Funct. Foods 2018, 46, 101–109. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Wu, J.; Liu, W.; Li, D.; Xu, J. Harmane ameliorates obesity though inhibiting lipid accumulation and inducing adipocyte browning. RSC Adv. 2020, 10, 4397–4403. [Google Scholar] [CrossRef]
- Rao, Y.; Yu, H.; Gao, L.; Lu, Y.T.; Xu, Z.; Liu, H.; Gu, L.Q.; Ye, J.M.; Huang, Z.S. Natural alkaloid bouchardatine ameliorates metabolic disorders in high-fat diet-fed mice by stimulating the sirtuin 1/liver kinase B-1/AMPK axis. Br. J. Pharmacol. 2017, 174, 2457–2470. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short chain fatty acids: Key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Lednovich, K.; Xu, K.; Nnyamah, C.; Layden, B.T.; Xu, P. Central and peripheral regulations mediated by short-chain fatty acids on energy homeostasis. Transl. Res. 2022, 248, 128–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Li, F.; Ma, S.; Zhao, L.; Ge, P.; Chen, H. Mechanisms of Qingyi Decoction in Severe Acute Pancreatitis Associated Acute Lung Injury via Gut Microbiota: Targeting the Short-Chain Fatty Acids-Mediated AMPK/NF-kB/NLRP3 Pathway. Microbiol. Spectr. 2023, 11, e03664-22. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. Swiss Target Prediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Scardoni, G.; Petterlini, M.; Laudanna, C. Analyzing biological network parameters with CentiScaPe. Bioinformatics 2009, 25, 2857–2859. [Google Scholar] [CrossRef]
- Madikyzy, M.; Tilegen, M.; Nazarbek, G.; Mu, C.; Kutzhanova, A.; Li, X.; Ma, C.; Xie, Y. Honghua extract mediated potent inhibition of COVID-19 host cell pathways. Sci. Rep. 2022, 12, 14296. [Google Scholar] [CrossRef]
- Vilar, S.; Cozza, G.; Moro, S. Medicinal Chemistry and the Molecular Operating Environment (MOE): Application of QSAR and Molecular Docking to Drug Discovery. Curr. Top. Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef]

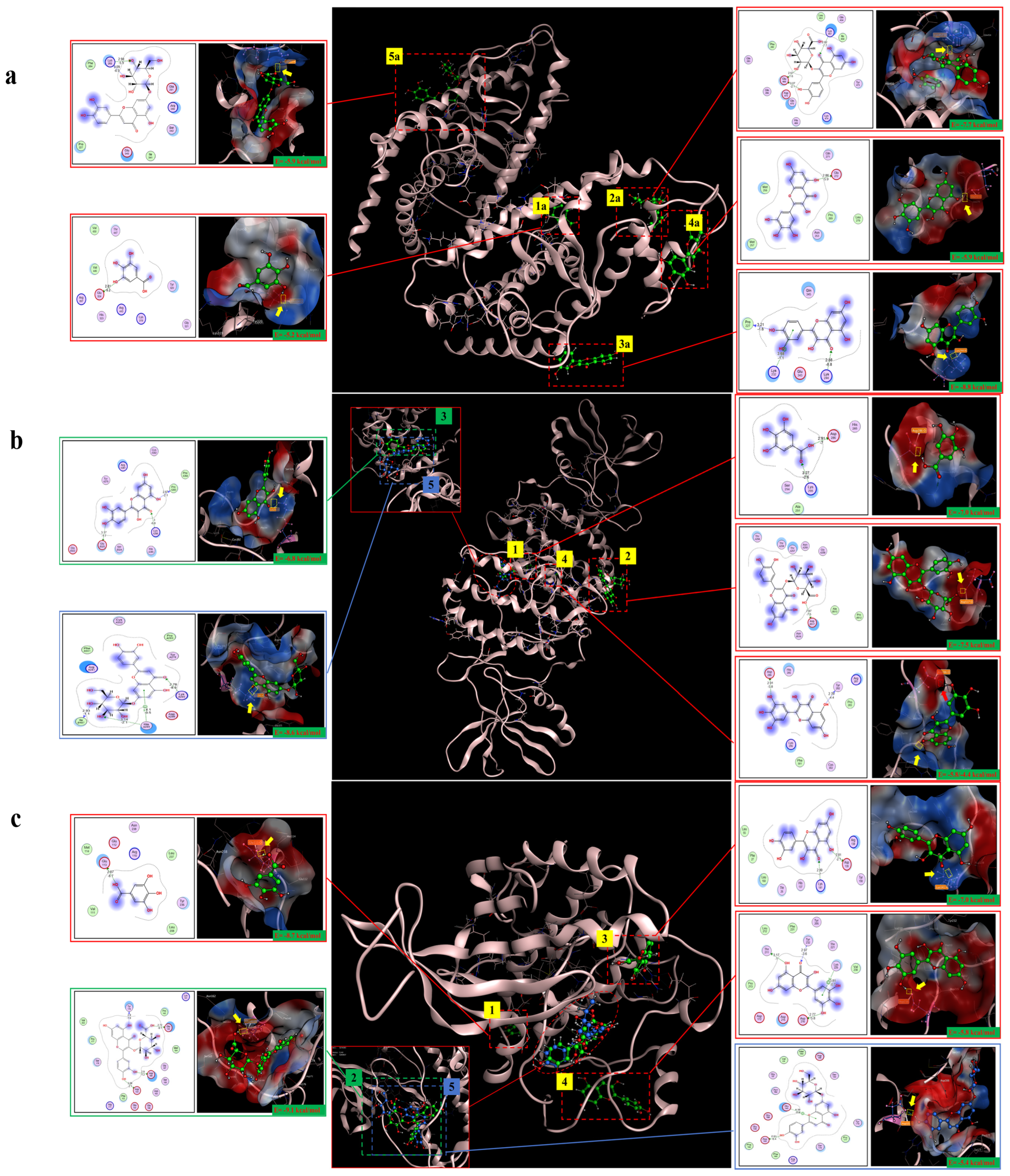
| Target | PDB ID | Target Structure | Compound | Affinity (kcal/mol) | Interaction | Receptor | Distance |
|---|---|---|---|---|---|---|---|
| PPARγ | 2I4J | 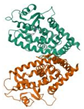 | Gallic acid | −5.2 | H-donor | Glu 324-OE2 | 2.81 |
| Quercetin-3-O-beta-D-glucopyranoside | −7.7 | H-acceptor | Lys 457-HZ2 | 2.82 | |||
| Quercetin | −8.8 | H-acceptor | Lys 244-HZ2 | 2.88 | |||
| Myricetin | −5.9 | H-donor | Glu 272-OE1 | 2.86 | |||
| Luteolin-7-O-glucoside | −5.9 | H-acceptor | Lys 263-HZ1 | 2.98 | |||
| C/EBPα | 6DC0 |  | Gallic acid | −7.0 | H-donor | Asp 286-OD2 | 2.91 |
| Quercetin-3-O-beta-D-glucopyranoside | −7.5 | H-donor | Asp B517-OD1 | 2.87 | |||
| Quercetin | −6.8 | H-acceptor | Lys A295-HZ1 | 3.00 | |||
| Myricetin | −5.8 | H-donor | Asp 286-OD2 | 2.91 | |||
| −4.4 | H-donor | Tyr 282-O | 2.76 | ||||
| Gallic acid | −8.6 | H-acceptor | Lys A295-HZ1 | 4.10 | |||
| AMPK | 3AQV | 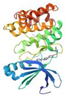 | Gallic acid | −8.7 | H-donor | Glu 113-OE1 | 2.87 |
| Quercetin-3-O-beta-D-glucopyranoside | −9.1 | H-donor | Glu 143-OE2 | 2.72 | |||
| Quercetin | −7.0 | H-acceptor | Lys 141-HZ3 | 2.99 | |||
| Myricetin | −5.8 | H-donor | Asp 216-OD2 | 2.77 | |||
| Luteolin-7-O-glucoside | −5.4 | H-donor | Asp 103-OD2 | 2.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Alimu, A.; Li, Y.; Lin, Z.; Li, J.; Wang, X.; Wang, Y.; Lv, G.; Lin, H.; Lin, Z. Potential Anti-Obesity Effect of Hazel Leaf Extract in Mice and Network Pharmacology of Selected Polyphenols. Pharmaceuticals 2024, 17, 1349. https://doi.org/10.3390/ph17101349
Zhao J, Alimu A, Li Y, Lin Z, Li J, Wang X, Wang Y, Lv G, Lin H, Lin Z. Potential Anti-Obesity Effect of Hazel Leaf Extract in Mice and Network Pharmacology of Selected Polyphenols. Pharmaceuticals. 2024; 17(10):1349. https://doi.org/10.3390/ph17101349
Chicago/Turabian StyleZhao, Jiarui, Aikebaier Alimu, Yvmo Li, Zhi Lin, Jun Li, Xinhe Wang, Yuchen Wang, Guangfu Lv, He Lin, and Zhe Lin. 2024. "Potential Anti-Obesity Effect of Hazel Leaf Extract in Mice and Network Pharmacology of Selected Polyphenols" Pharmaceuticals 17, no. 10: 1349. https://doi.org/10.3390/ph17101349






