Molecular Docking, Bioinformatic Analysis, and Experimental Verification for the Effect of Naringin on ADHD: Possible Inhibition of GSK-3β and HSP90
Abstract
:1. Introduction
2. Results
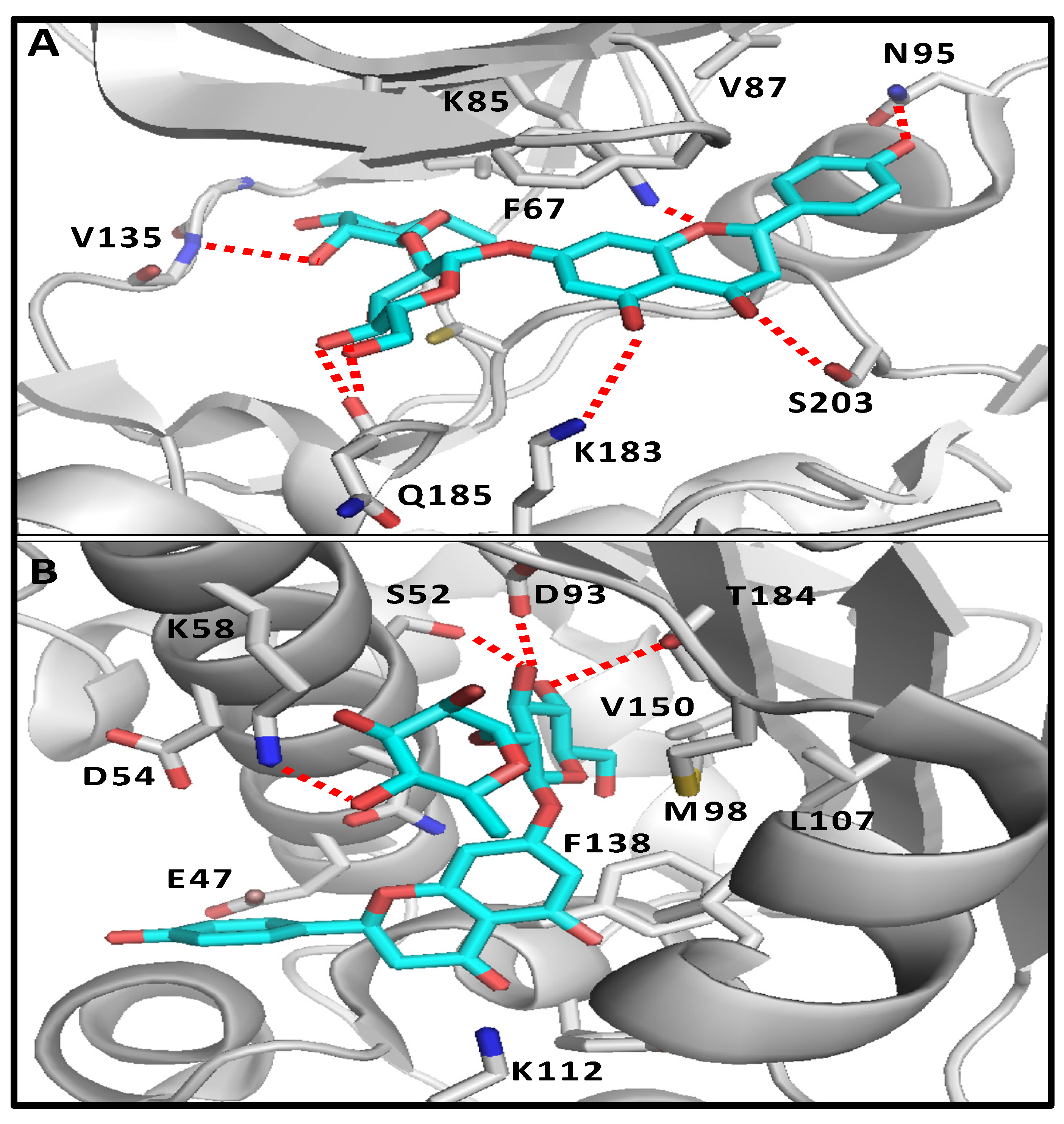
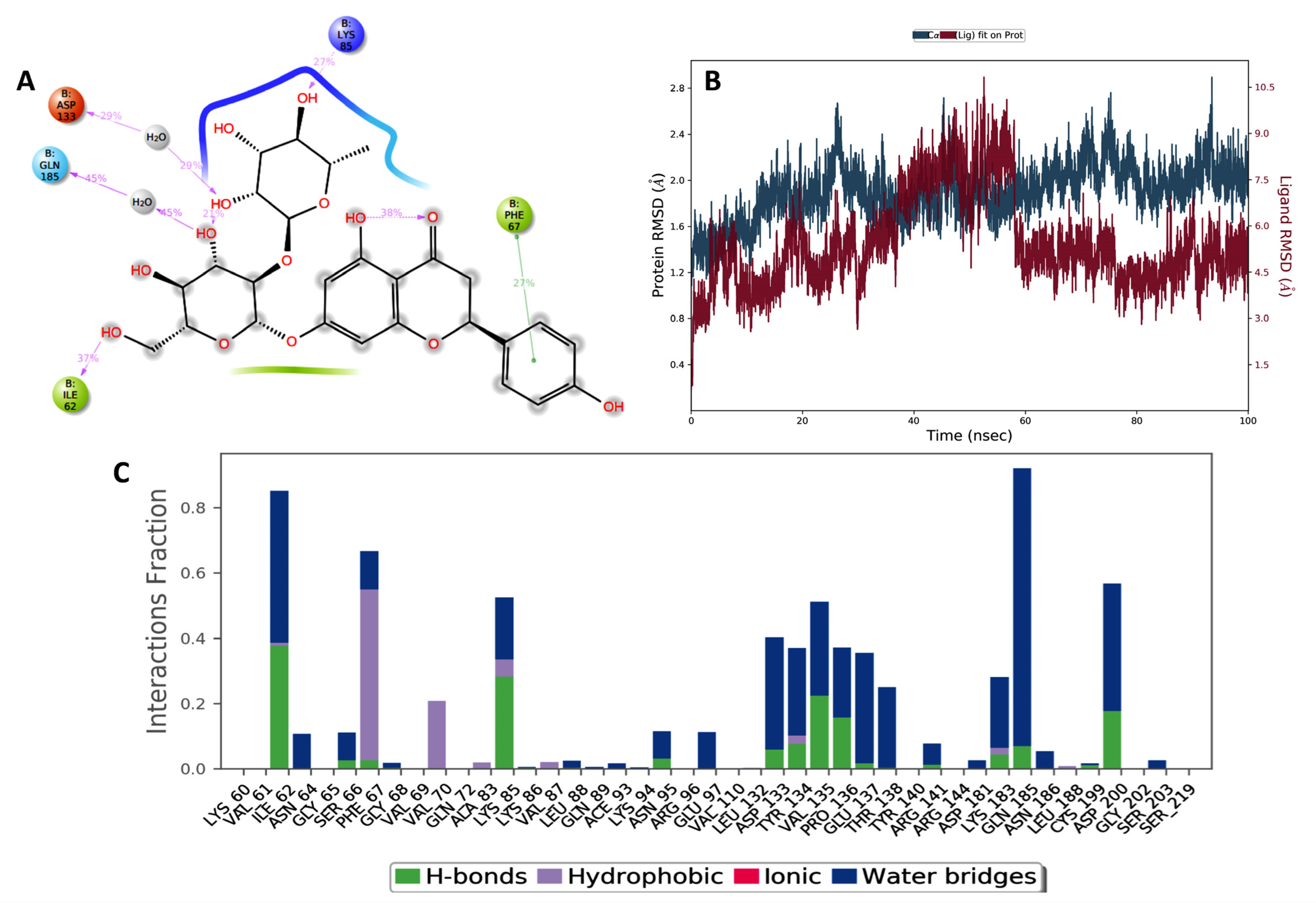
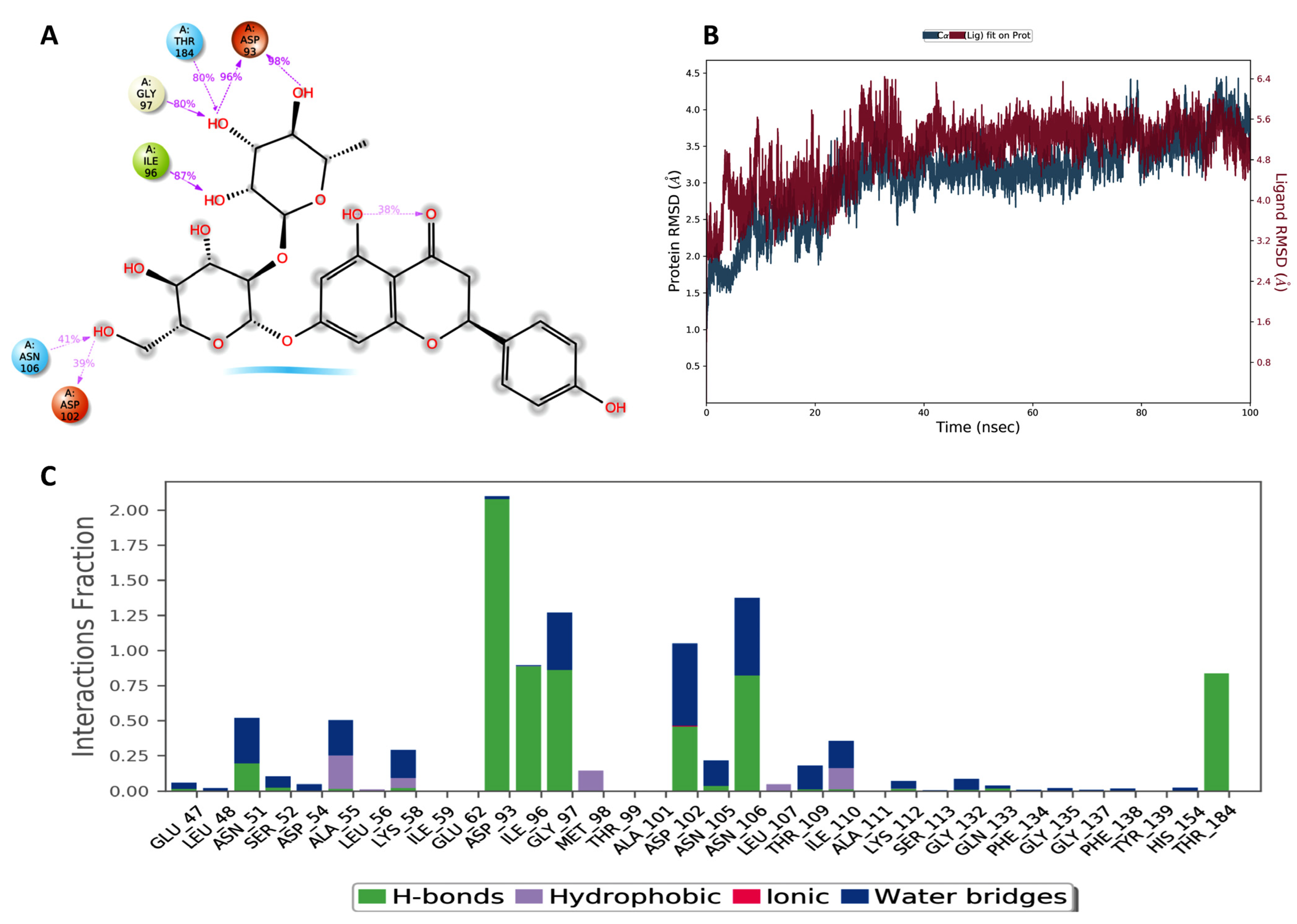
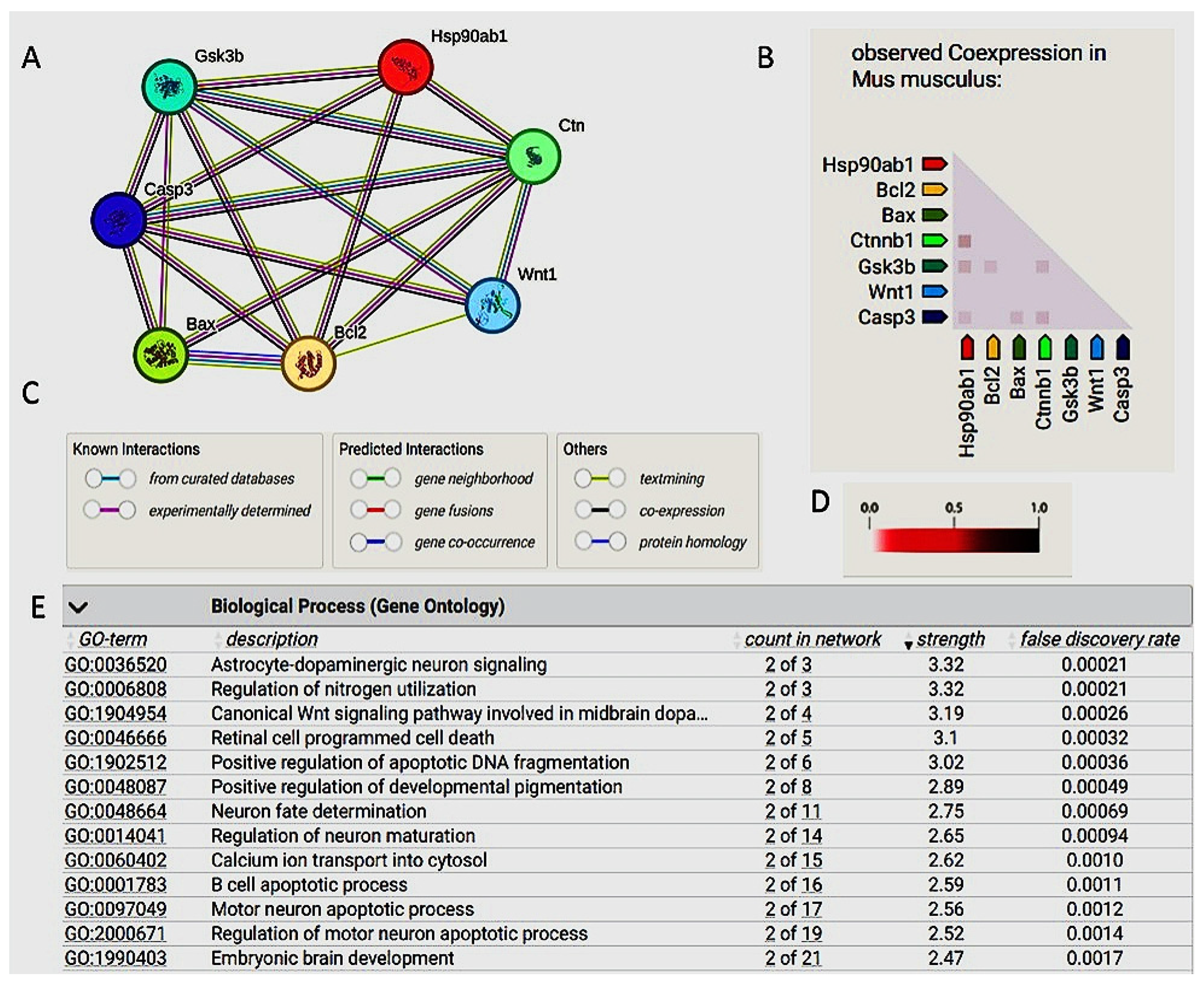
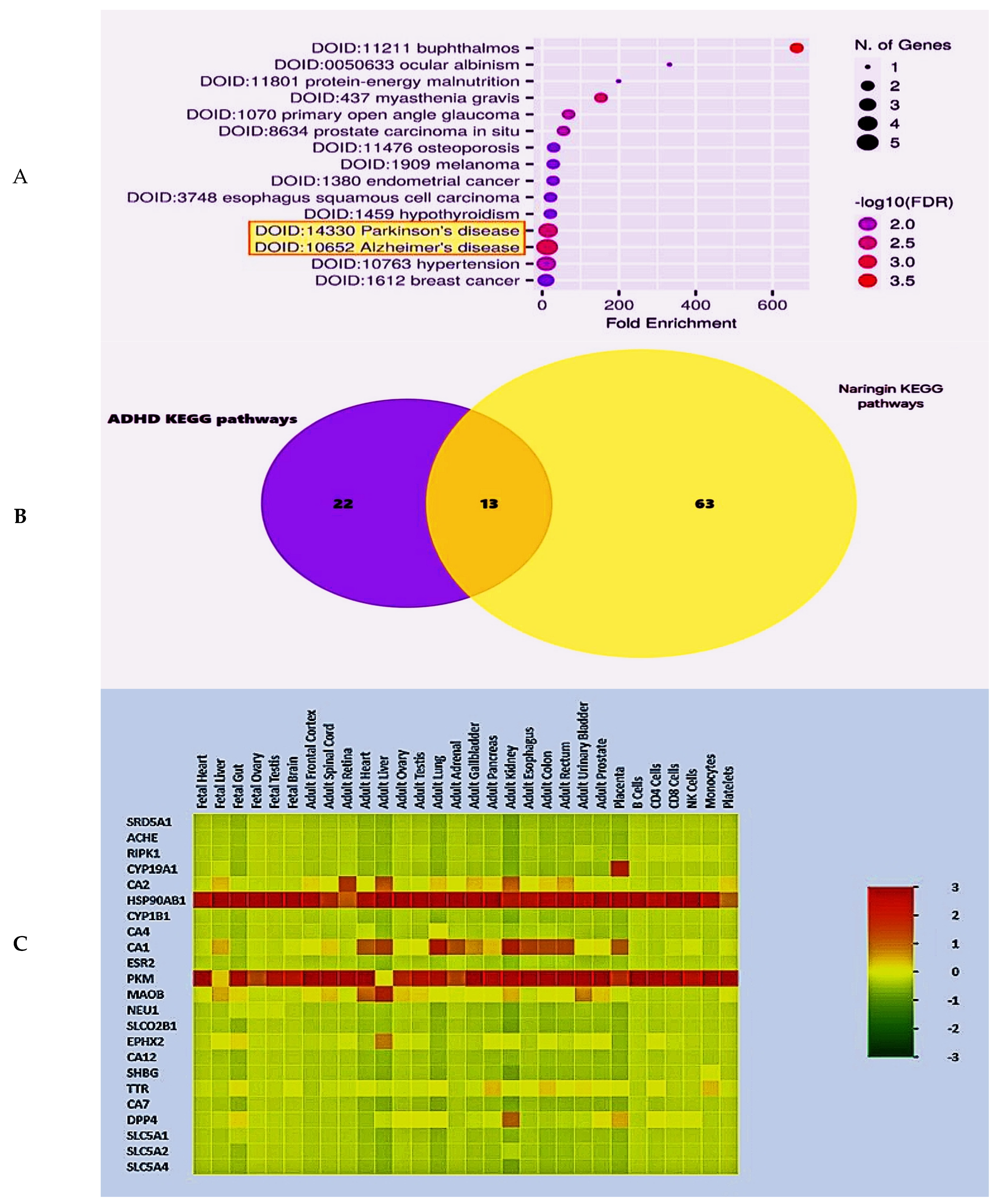
2.1. Molecular Docking and Bioinformatic Results
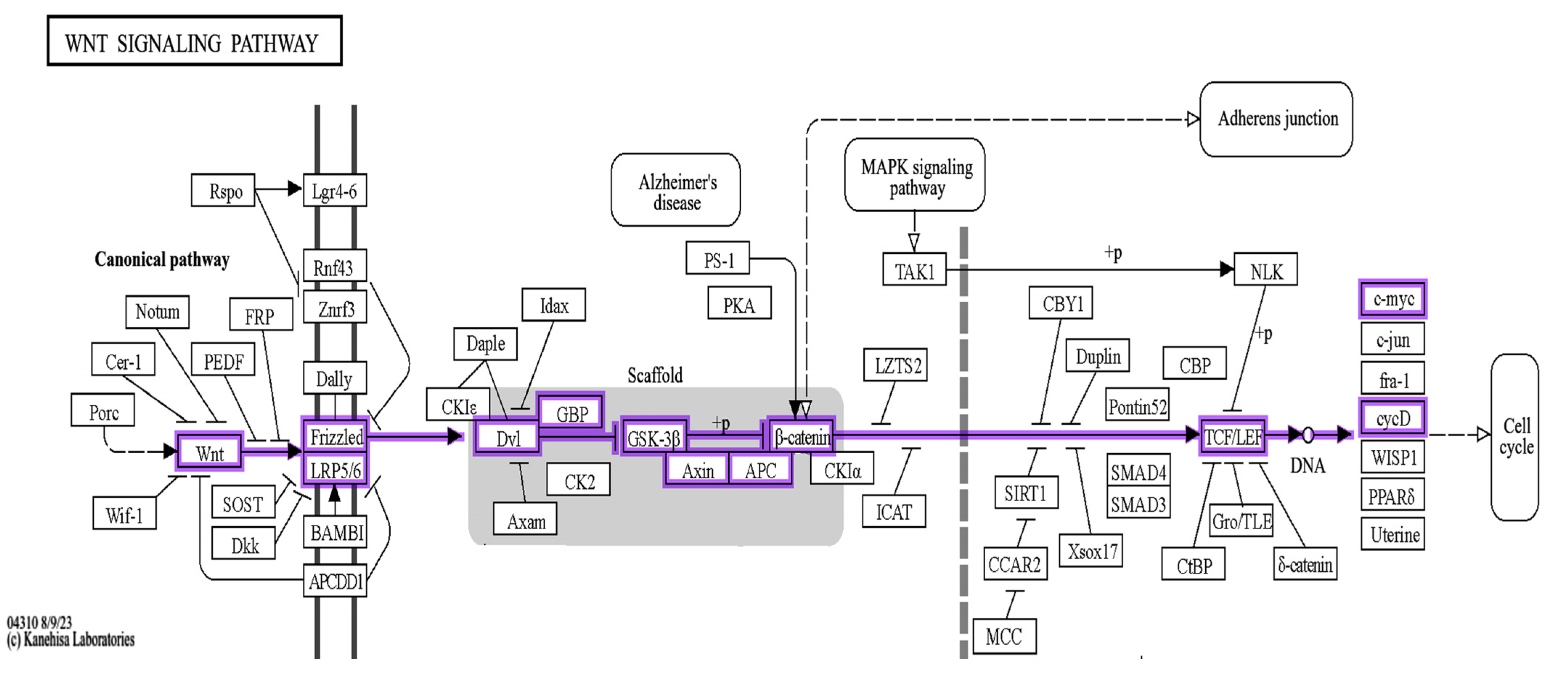
2.2. The Bioinformatic Results
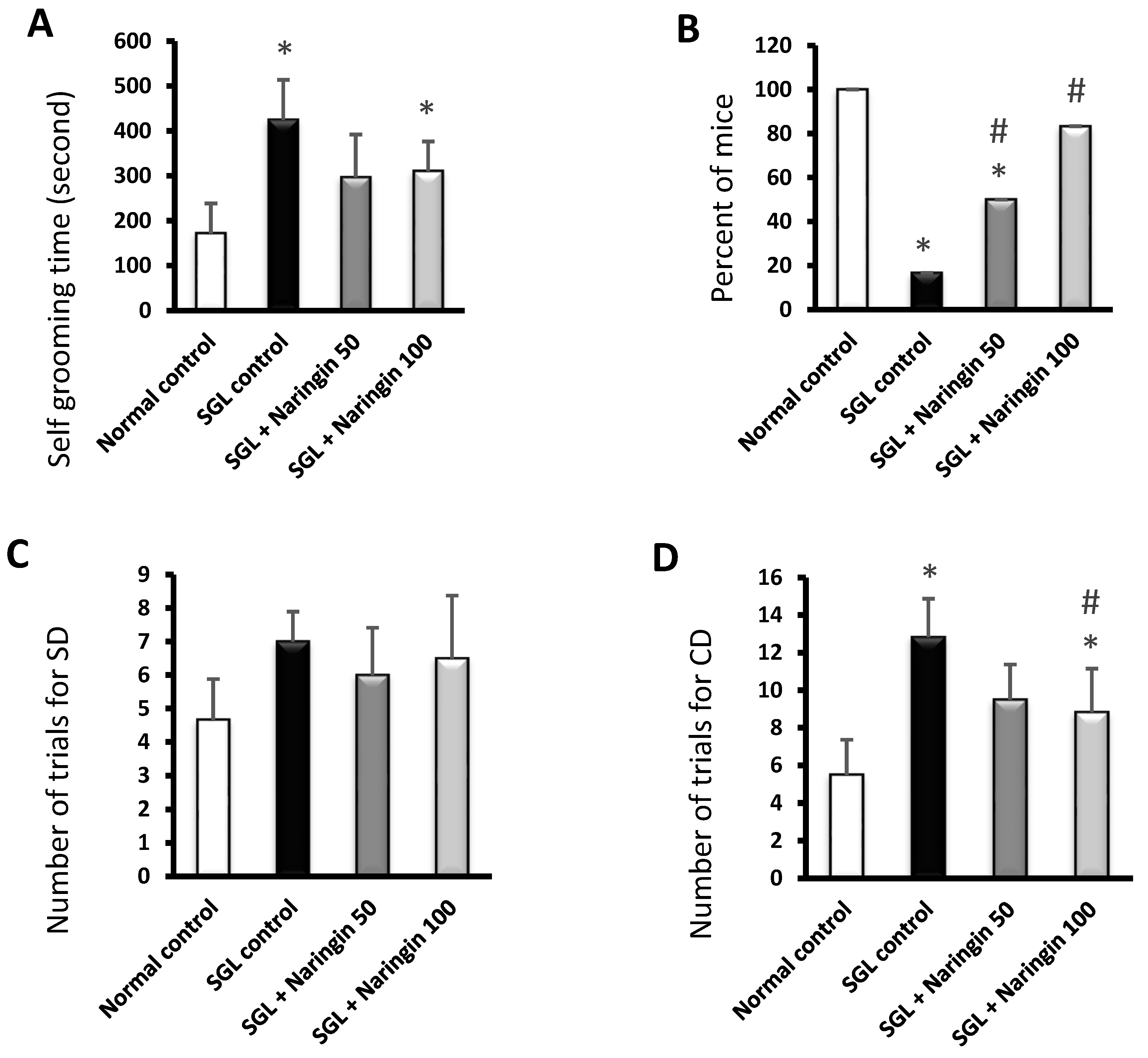
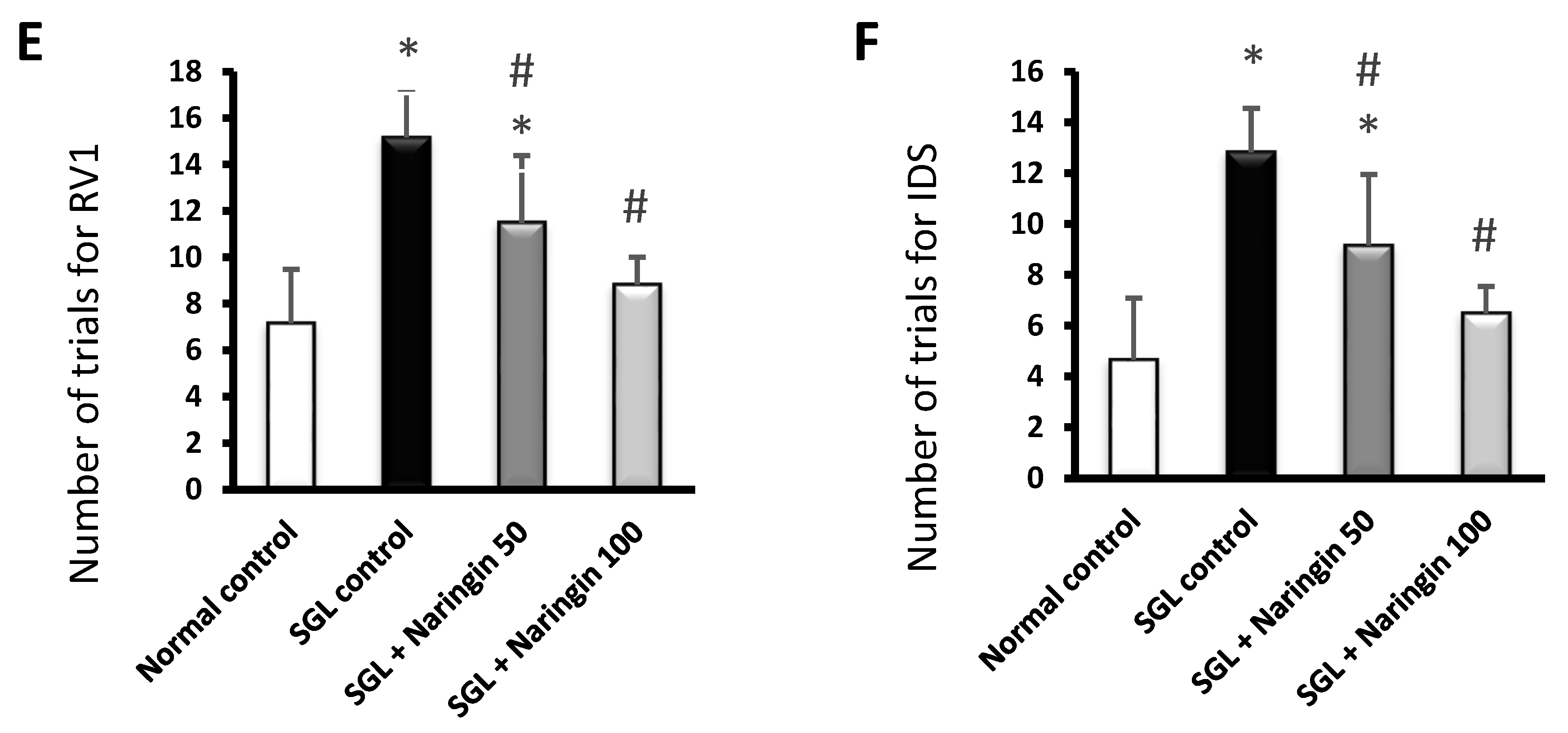
2.3. Mouse Study Results
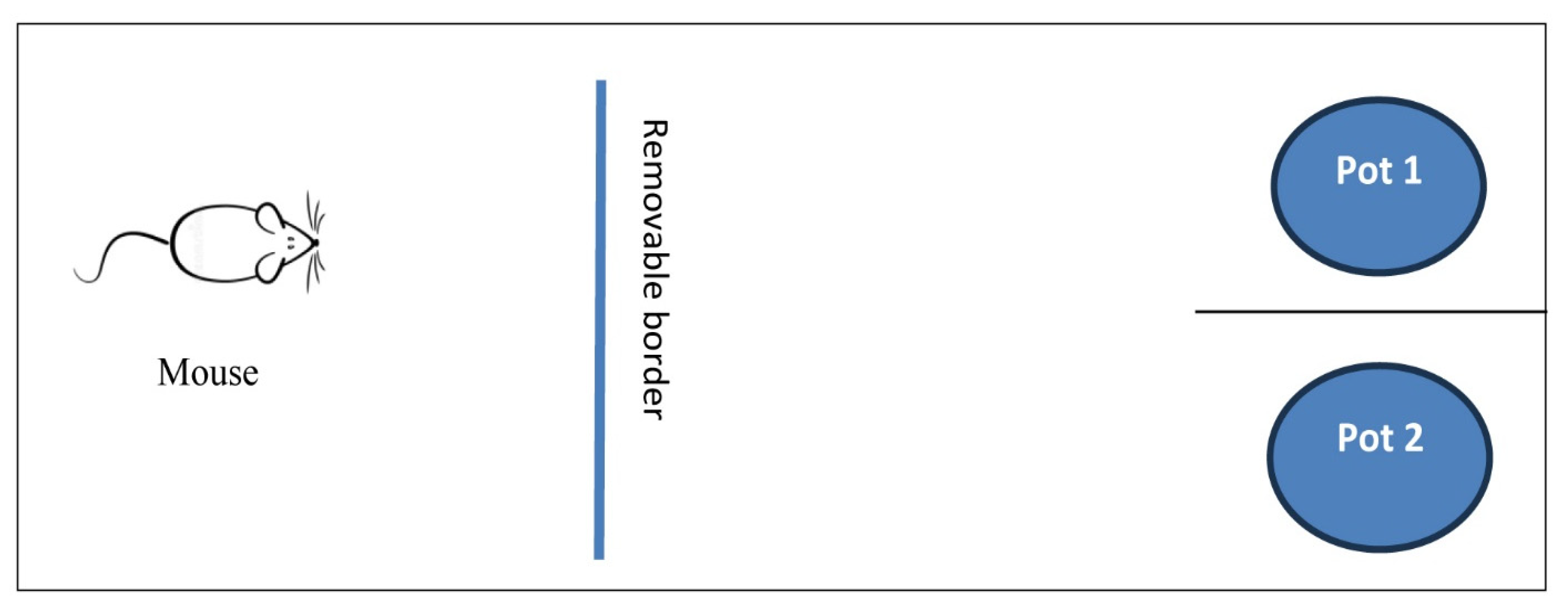
2.3.1. Self-Grooming and Rope Crawling Tests
2.3.2. ASST
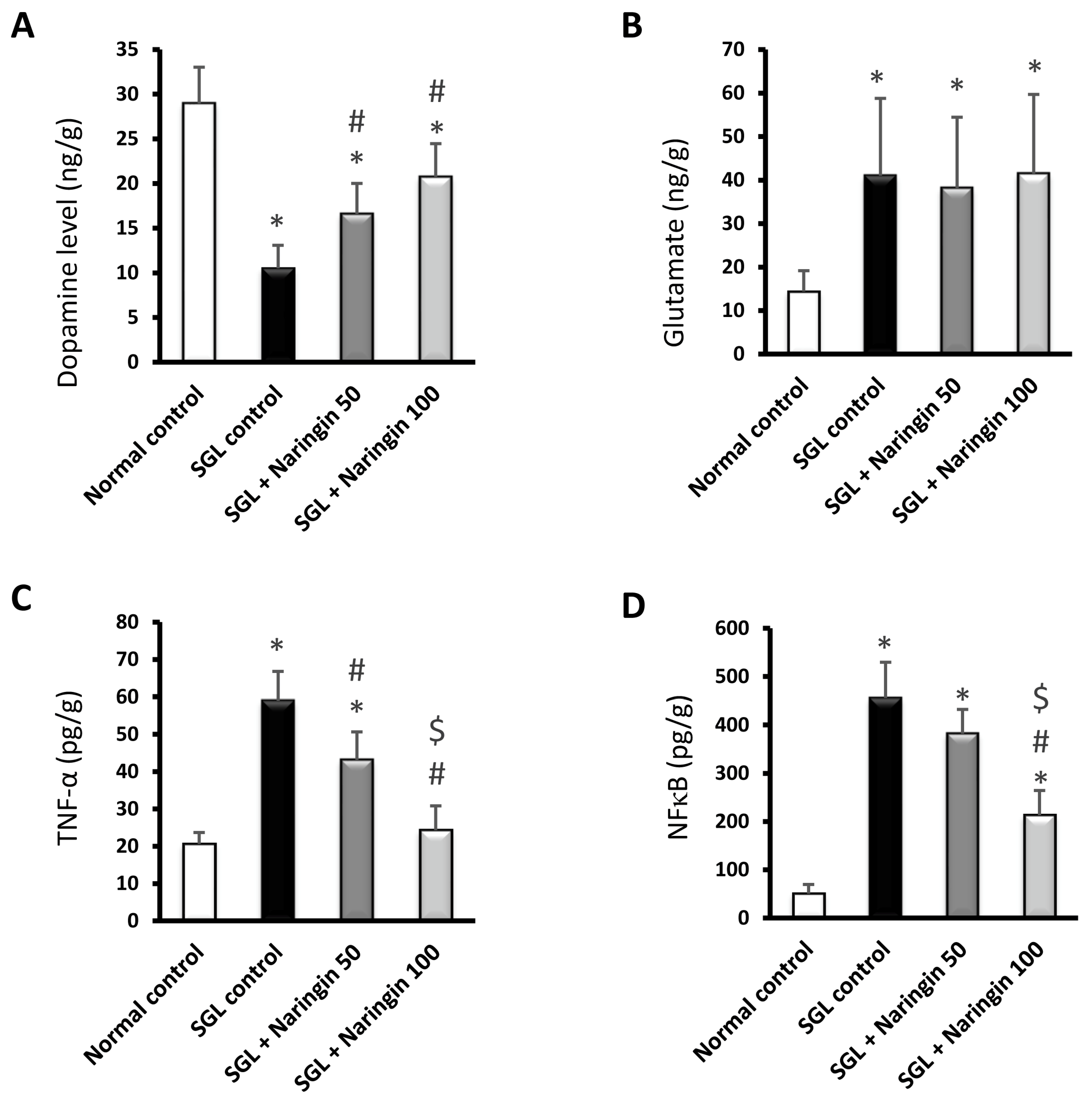
2.3.3. ELISA Assays
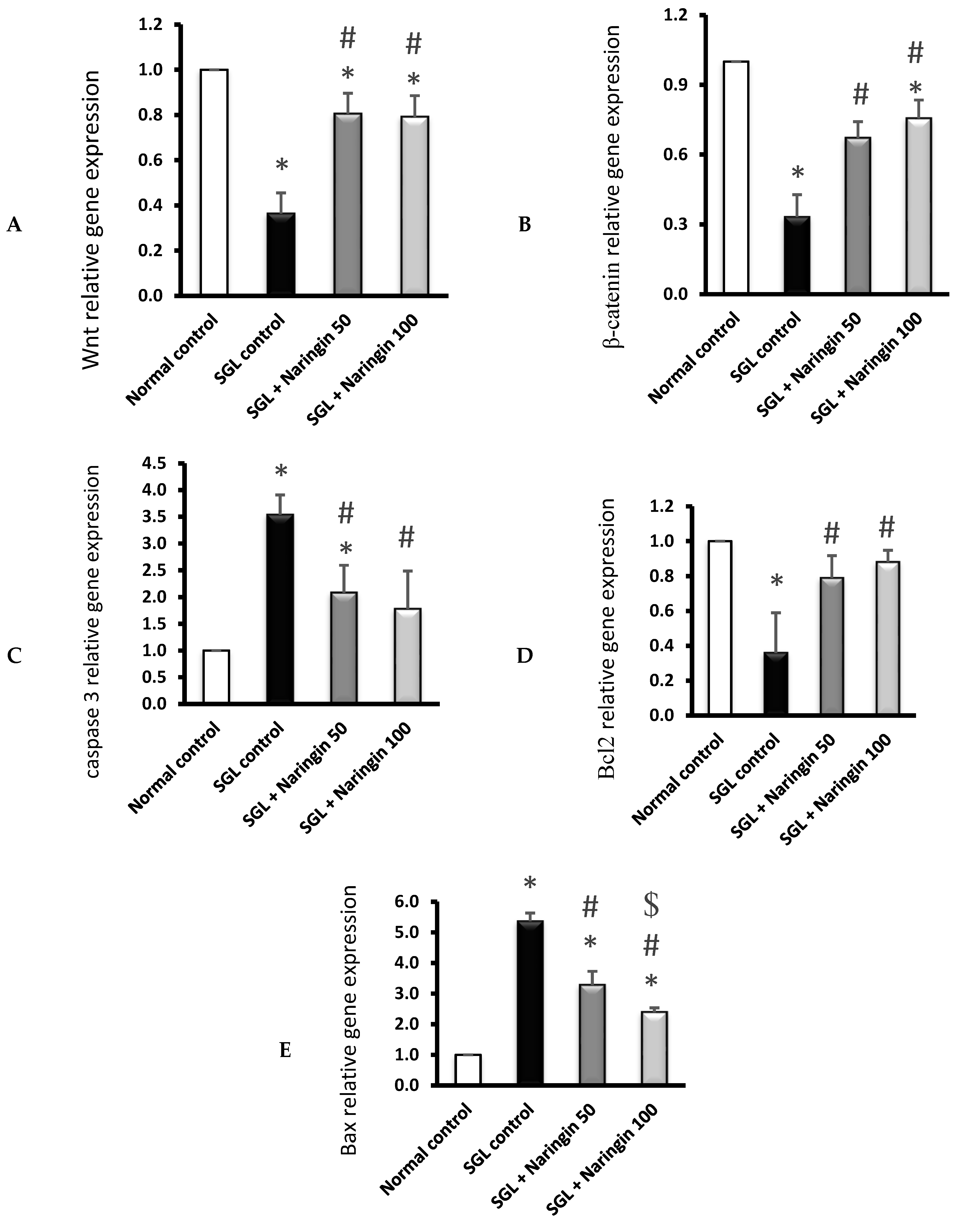
2.3.4. RT-PCR Analysis
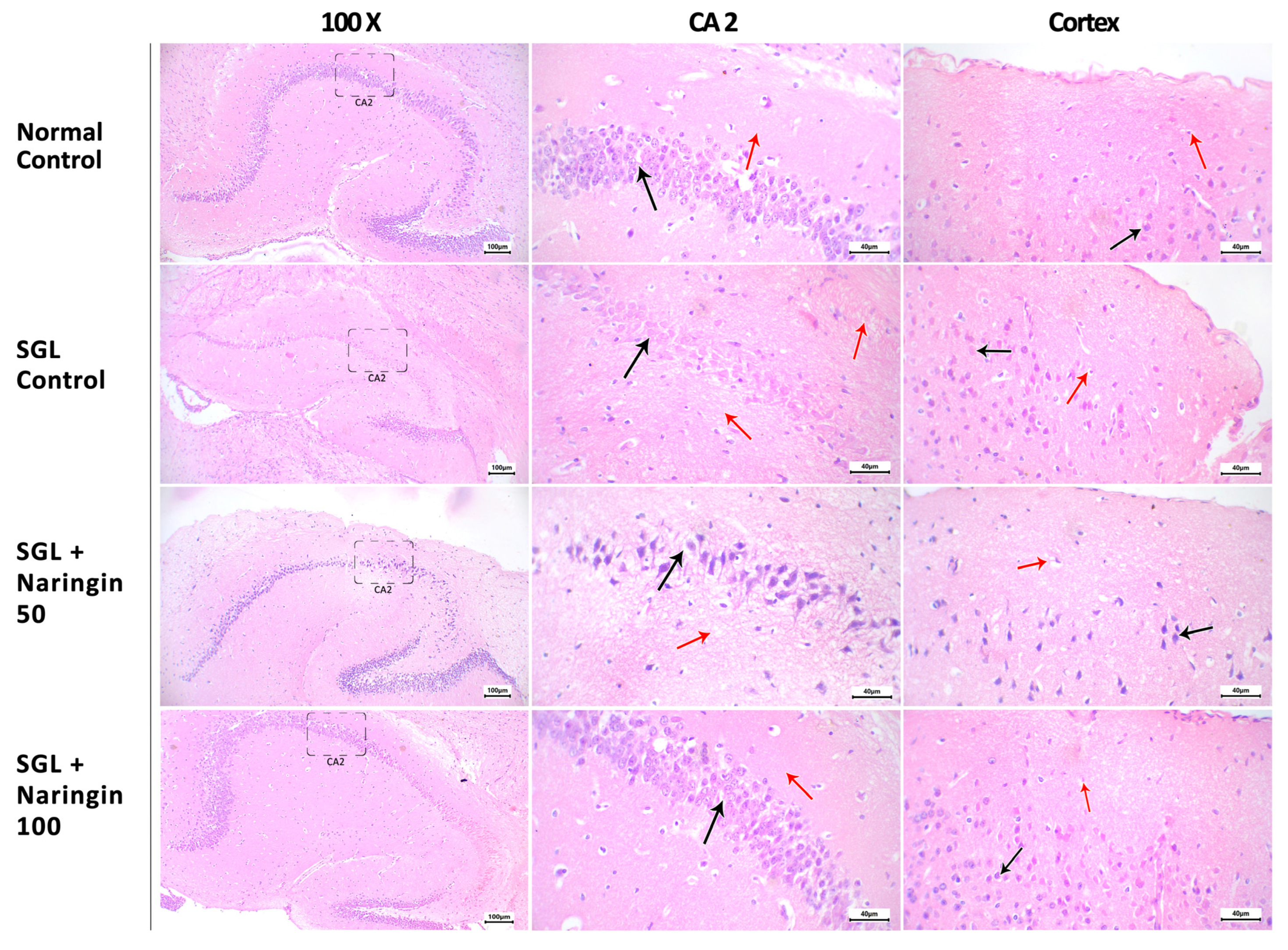
2.3.5. Hematoxylin and Eosin Staining
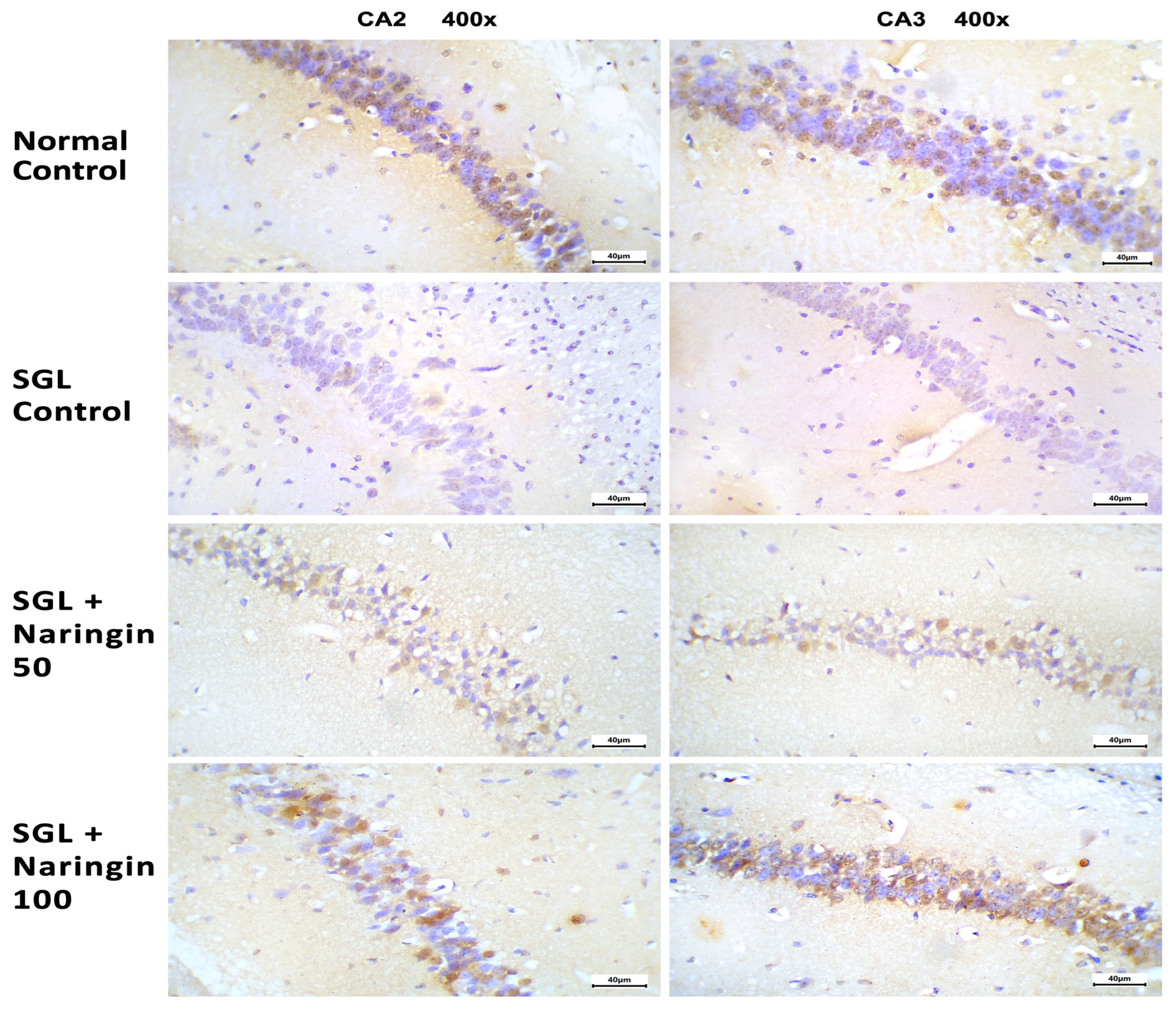
2.3.6. Immunohistochemical Staining for Bcl2
3. Discussion
4. Materials and Methods
4.1. Investigation by Molecular Docking and Molecular Dynamic Simulation
4.1.1. Molecular Docking
4.1.2. Molecular Dynamic Simulation
4.2. The Utilization of Bioinformatic Tools to Correlate the Target Protiens
4.3. The Mouse In Vivo Study
4.3.1. Chemical Preparation
4.3.2. Mouse Environment and Housing Conditions
4.3.3. Experimental Design
4.3.4. Evaluation of Mouse Psychomotor Activity by Behavior Tasks
Self-Grooming Test
Rope Crawling Test
Attentional Set-Shifting Task (ASST)
- The Habituation Phase (5 Days)
- The Training Phase (2 Days)
- Experimentation Phase (1 Day)
4.3.5. Mouse Scarification
4.3.6. Molecular Analysis
4.3.7. Assessment of Inflammatory Mediators
4.3.8. Histopathology, Immunohistochemical Staining, and Examination
4.3.9. Statistical Analysis and Data Manipulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, P.; Zha, M.; Yang, Q.; Zhang, Y.; Li, X.; Rudan, I. The Prevalence of Adult Attention-Deficit Hyperactivity Disorder: A Global Systematic Review and Meta-Analysis. J. Glob. Health 2021, 11, 4009. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadis, H.-M.; Lunghi, C.; Rahme, E.; Rochette, L.; Gignac, M.; Massamba, V.; Diallo, F.B.; Fansi, A.; Cortese, S.; Lesage, A. ADHD Medications Use and Risk of Mortality and Unintentional Injuries: A Population-Based Cohort Study. Transl. Psychiatry 2024, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Couture, J. A Review of the Pathophysiology, Etiology, and Treatment of Attention-Deficit Hyperactivity Disorder (ADHD). Ann. Pharmacother. 2014, 48, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulou, I.; Pagida, M.A.; Briana, D.D.; Panayotacopoulou, M.T. Perinatal Hypoxia as a Risk Factor for Psychopathology Later in Life: The Role of Dopamine and Neurotrophins. Hormones 2018, 17, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Soliva, J.C. Neuroimaging in the Diagnosis of ADHD: Where We Are and Where We Are Going. Expert Opin. Med. Diagn. 2011, 5, 307–318. [Google Scholar] [CrossRef]
- Minzenberg, M.J. Pharmacotherapy for Attention-Deficit/Hyperactivity Disorder: From Cells to Circuits. Neurotherapeutics 2012, 9, 610–621. [Google Scholar] [CrossRef]
- Rosa-Neto, P.; Lou, H.C.; Cumming, P.; Pryds, O.; Karrebaek, H.; Lunding, J.; Gjedde, A. Methylphenidate-Evoked Changes in Striatal Dopamine Correlate with Inattention and Impulsivity in Adolescents with Attention Deficit Hyperactivity Disorder. NeuroImage 2005, 25, 868–876. [Google Scholar] [CrossRef]
- Sun, H.; Wu, M.; Wang, M.; Zhang, X.; Zhu, J. The Regulatory Role of Endoplasmic Reticulum Chaperone Proteins in Neurodevelopment. Front. Neurosci. 2022, 16, 1032607. [Google Scholar] [CrossRef]
- Chang, P.-K.; Chu, J.; Tsai, Y.-T.; Lai, Y.-H.; Chen, J.-C. Dopamine D3 Receptor and GSK3β Signaling Mediate Deficits in Novel Object Recognition Memory within Dopamine Transporter Knockdown Mice. J. Biomed. Sci. 2020, 27, 16. [Google Scholar] [CrossRef]
- Fu, C.; Lei, Y.; Liang, L.; Jiang, J.; Qin, Y.; Lao, Y.; Tan, Z.; Wang, Y.; Liu, Q. Characterization of HSP90 Expression and Function Following CNS Injury. Neurosci. Lett. 2024, 836, 137875. [Google Scholar] [CrossRef]
- McFarland, N.R.; Dimant, H.; Kibuuka, L.; Ebrahimi-Fakhari, D.; Desjardins, C.A.; Danzer, K.M.; Danzer, M.; Fan, Z.; Schwarzschild, M.A.; Hirst, W.; et al. Chronic Treatment with Novel Small Molecule Hsp90 Inhibitors Rescues Striatal Dopamine Levels but Not α-Synuclein-Induced Neuronal Cell Loss. PLoS ONE 2014, 9, e86048. [Google Scholar] [CrossRef] [PubMed]
- Putcha, P.; Danzer, K.M.; Kranich, L.R.; Scott, A.; Silinski, M.; Mabbett, S.; Hicks, C.D.; Veal, J.M.; Steed, P.M.; Hyman, B.T.; et al. Brain-Permeable Small-Molecule Inhibitors of Hsp90 Prevent α-Synuclein Oligomer Formation and Rescue α-Synuclein-Induced Toxicity. J. Pharmacol. Exp. Ther. 2010, 332, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Y.; Huang, L.; Chen, J.; Li, J.J.; Wang, R.; Kim, E.; Chen, Y.; Justicia, C.; Sakata, K.; et al. A CNS-Permeable Hsp90 Inhibitor Rescues Synaptic Dysfunction and Memory Loss in APP-Overexpressing Alzheimer’s Mouse Model via an HSF1-Mediated Mechanism. Mol. Psychiatry 2017, 22, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Aguilà, M.; Bevilacqua, D.; McCulley, C.; Schwarz, N.; Athanasiou, D.; Kanuga, N.; Novoselov, S.S.; Lange, C.A.K.; Ali, R.R.; Bainbridge, J.W.; et al. Hsp90 Inhibition Protects against Inherited Retinal Degeneration. Hum. Mol. Genet. 2014, 23, 2164–2175. [Google Scholar] [CrossRef]
- Stothert, A.R.; Suntharalingam, A.; Tang, X.; Crowley, V.M.; Mishra, S.J.; Webster, J.M.; Nordhues, B.A.; Huard, D.J.E.; Passaglia, C.L.; Lieberman, R.L.; et al. Isoform-Selective Hsp90 Inhibition Rescues Model of Hereditary Open-Angle Glaucoma. Sci. Rep. 2017, 7, 17951. [Google Scholar] [CrossRef]
- Jaworski, T.; Banach-Kasper, E.; Gralec, K. GSK-3 β at the Intersection of Neuronal Plasticity and Neurodegeneration. Neural Plast. 2019, 2019, 4209475. [Google Scholar] [CrossRef]
- Li, Y.-C.; Gao, W.-J. GSK-3β Activity and Hyperdopamine-Dependent Behaviors. Neurosci. Biobehav. Rev. 2011, 35, 645–654. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Li, W.; Zhang, H. Activation of Wnt/β-Catenin Signalling via GSK3 Inhibitors Direct Differentiation of Human Adipose Stem Cells into Functional Hepatocytes. Sci. Rep. 2017, 7, 40716. [Google Scholar] [CrossRef]
- Bharathy, N.; Svalina, M.N.; Settelmeyer, T.P.; Cleary, M.M.; Berlow, N.E.; Airhart, S.D.; Xiang, S.; Keck, J.; Hayden, J.B.; Shern, J.F.; et al. Preclinical Testing of the Glycogen Synthase Kinase-3β Inhibitor Tideglusib for Rhabdomyosarcoma. Oncotarget 2017, 8, 62976–62983. [Google Scholar] [CrossRef]
- Arciniegas Ruiz, S.M.; Eldar-Finkelman, H. Glycogen Synthase Kinase-3 Inhibitors: Preclinical and Clinical Focus on CNS-A Decade Onward. Front. Mol. Neurosci. 2022, 14, 792364. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Wang, C.; Hong, X.; Miao, J.; Liao, Y.; Zhou, L.; Liu, Y. An Essential Role for Wnt/β-Catenin Signaling in Mediating Hypertensive Heart Disease. Sci. Rep. 2018, 8, 8996. [Google Scholar] [CrossRef] [PubMed]
- Custodio, R.J.P.; Kim, H.J.; Kim, J.; Ortiz, D.M.; Kim, M.; Buctot, D.; Sayson, L.V.; Lee, H.J.; Kim, B.-N.; Yi, E.C.; et al. Hippocampal Dentate Gyri Proteomics Reveals Wnt Signaling Involvement in the Behavioral Impairment in the THRSP-Overexpressing ADHD Mouse Model. Commun. Biol. 2023, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Grünblatt, E.; Bartl, J.; Walitza, S. Methylphenidate Enhances Neuronal Differentiation and Reduces Proliferation Concomitant to Activation of Wnt Signal Transduction Pathways. Transl. Psychiatry 2018, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Grünblatt, E.; Nemoda, Z.; Werling, A.M.; Roth, A.; Angyal, N.; Tarnok, Z.; Thomsen, H.; Peters, T.; Hinney, A.; Hebebrand, J.; et al. The Involvement of the Canonical Wnt-signaling Receptor LRP5 and LRP6 Gene Variants with ADHD and Sexual Dimorphism: Association Study and Meta-analysis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2019, 180, 365–376. [Google Scholar] [CrossRef]
- Walter, N.M.; Yde Ohki, C.M.; Rickli, M.; Smigielski, L.; Walitza, S.; Grünblatt, E. An Investigation on the Alterations in Wnt Signaling in ADHD across Developmental Stages. Neurosci. Appl. 2024, 3, 104070. [Google Scholar] [CrossRef]
- Liang, E.F.; Lim, S.Z.; Tam, W.W.; Ho, C.S.; Zhang, M.W.; McIntyre, R.S.; Ho, R.C. The Effect of Methylphenidate and Atomoxetine on Heart Rate and Systolic Blood Pressure in Young People and Adults with Attention-Deficit Hyperactivity Disorder (ADHD): Systematic Review, Meta-Analysis, and Meta-Regression. Int. J. Environ. Res. Public Health 2018, 15, 1789. [Google Scholar] [CrossRef]
- Daughton, J.M.; Kratochvil, C.J. Review of ADHD Pharmacotherapies: Advantages, Disadvantages, and Clinical Pearls. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 240–248. [Google Scholar] [CrossRef]
- Pliszka, S. Practice Parameter for the Assessment and Treatment of Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 894–921. [Google Scholar] [CrossRef]
- Biederman, J.; Spencer, T.J. Psychopharmacological Interventions. Child Adolesc. Psychiatr. Clin. N. Am. 2008, 17, 439–458. [Google Scholar] [CrossRef]
- Krishna Veni, N.; Karthika, D.; Surya Devi, M.; Rubini, M.F.; Vishalini, M.; Pradeepa, Y.J. Analysis of Monosodium L-Glutamate in Food Products by High-Performance Thin Layer Chromatography. J. Young Pharm. 2010, 2, 297–300. [Google Scholar] [CrossRef]
- Shivasharan, B.D.; Nagakannan, P.; Thippeswamy, B.S.; Veerapur, V.P. Protective Effect of Calendula officinalis L. Flowers Against Monosodium Glutamate Induced Oxidative Stress and Excitotoxic Brain Damage in Rats. Indian J. Clin. Biochem. 2013, 28, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, H.I.; Abd El-Fadeal, N.M.; El-Sayed, R.M.; Hegazy, A.; El-Kherbetawy, M.K.; Hamad, A.G.; ElSayed, M.H.; Zaitone, S.A. Computational Analysis and Experimental Data Exploring the Role of Hesperetin in Ameliorating ADHD and SIRT1/Nrf2/Keap1/OH-1 Signaling. Int. J. Mol. Sci. 2024, 25, 9284. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.A.; Elsherbiny, N.; Alzahrani, S.; Alshareef, H.M.; Abd Elmageed, Z.Y.; Ajwah, S.M.; Hamdan, A.M.E.; Abdou, Y.S.; Galal, O.O.; El Azazy, M.K.A.; et al. Neuroprotective Effect of Morin Hydrate against Attention-Deficit/Hyperactivity Disorder (ADHD) Induced by MSG and/or Protein Malnutrition in Rat Pups: Effect on Oxidative/Monoamines/Inflammatory Balance and Apoptosis. Pharmaceuticals 2022, 15, 1012. [Google Scholar] [CrossRef] [PubMed]
- Kesherwani, R.; Bhoumik, S.; Kumar, R.; Rizvi, S.I. Monosodium Glutamate Even at Low Dose May Affect Oxidative Stress, Inflammation and Neurodegeneration in Rats 2022. Indian J. Clin. Biochem. 2024, 39, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Asejeje, F.O.; Abiola, M.A.; Adeyemo, O.A.; Ogunro, O.B.; Ajayi, A.M. Exogenous Monosodium Glutamate Exacerbates Lipopolysaccharide-Induced Neurobehavioral Deficits, Oxidative Damage, Neuroinflammation, and Cholinergic Dysfunction in Rat Brain. Neurosci. Lett. 2024, 825, 137710. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Reddy, T.K. The Grapefruit Flavanone Naringin Protects against the Radiation-Induced Genomic Instability in the Mice Bone Marrow: A Micronucleus Study. Mutat. Res. Toxicol. Environ. Mutagen. 2002, 519, 37–48. [Google Scholar] [CrossRef]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muñiz, P. Antioxidant Properties, Radical Scavenging Activity and Biomolecule Protection Capacity of Flavonoid Naringenin and Its Glycoside Naringin: A Comparative Study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- Eom, S.; Lee, B.-B.; Lee, S.; Park, Y.; Yeom, H.D.; Kim, T.-H.; Nam, S.-H.; Lee, J.H. Antioxidative and Analgesic Effects of Naringin through Selective Inhibition of Transient Receptor Potential Vanilloid Member 1. Antioxidants 2021, 11, 64. [Google Scholar] [CrossRef]
- Long, J.; Chen, J.; Liao, Y.; Zhou, Y.; Liang, B.; Zhou, Y. Naringin Provides Neuroprotection in CCL2-Induced Cognition Impairment by Attenuating Neuronal Apoptosis in the Hippocampus. Behav. Brain Funct. 2020, 16, 4. [Google Scholar] [CrossRef]
- Han, Y.; Su, J.; Liu, X.; Zhao, Y.; Wang, C.; Li, X. Naringin Alleviates Early Brain Injury after Experimental Subarachnoid Hemorrhage by Reducing Oxidative Stress and Inhibiting Apoptosis. Brain Res. Bull. 2017, 133, 42–50. [Google Scholar] [CrossRef]
- Cui, J.; Wang, G.; Kandhare, A.D.; Mukherjee-Kandhare, A.A.; Bodhankar, S.L. Neuroprotective Effect of Naringin, a Flavone Glycoside in Quinolinic Acid-Induced Neurotoxicity: Possible Role of PPAR-γ, Bax/Bcl-2, and Caspase-3. Food Chem. Toxicol. 2018, 121, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Kim, H.H.; Preethi, V.; Moniruzzaman, M.; Lee, K.H.; Kalaiselvi, S.; Kim, G.S.; Min, T. Assessment of Anti-Inflammatory and Antioxidant Effects of Citrus Unshiu Peel (CUP) Flavonoids on LPS-Stimulated RAW 264.7 Cells. Plants 2021, 10, 2209. [Google Scholar] [CrossRef] [PubMed]
- Akamo, A.J.; Akinloye, D.I.; Ugbaja, R.N.; Adeleye, O.O.; Dosumu, O.A.; Eteng, O.E.; Antiya, M.C.; Amah, G.; Ajayi, O.A.; Faseun, S.O. Naringin Prevents Cyclophosphamide-Induced Erythrocytotoxicity in Rats by Abrogating Oxidative Stress. Toxicol. Rep. 2021, 8, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Khan, H.; Aschner, M.; Hasan, M.M.; Hassan, S.T.S. Therapeutic Potential of Naringin in Neurological Disorders. Food Chem. Toxicol. 2019, 132, 110646. [Google Scholar] [CrossRef] [PubMed]
- Gentile, G.; Merlo, G.; Pozzan, A.; Bernasconi, G.; Bax, B.; Bamborough, P.; Bridges, A.; Carter, P.; Neu, M.; Yao, G.; et al. 5-Aryl-4-Carboxamide-1, 3-Oxazoles: Potent and Selective GSK-3 Inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 1989–1994. [Google Scholar] [CrossRef]
- Tassone, G.; Mazzorana, M.; Mangani, S.; Petricci, E.; Cini, E.; Giannini, G.; Pozzi, C.; Maramai, S. Structural Characterization of Human Heat Shock Protein 90 N-Terminal Domain and Its Variants K112R and K112A in Complex with a Potent 1,2,3-Triazole-Based Inhibitor. Int. J. Mol. Sci. 2022, 23, 9458. [Google Scholar] [CrossRef]
- Li, J.; Sun, L.; Xu, C.; Yu, F.; Zhou, H.; Zhao, Y.; Zhang, J.; Cai, J.; Mao, C.; Tang, L.; et al. Structure Insights into Mechanisms of ATP Hydrolysis and the Activation of Human Heat-Shock Protein 90. Acta Biochim. Biophys. Sin. 2012, 44, 300–306. [Google Scholar] [CrossRef]
- Wang, H.; Liang, J.; Wang, Y.; Zheng, J.; Liu, Y.; Zhao, Y.; Ma, Y.; Chen, P.; Yang, X. Exploring the Effects of Naringin on Oxidative Stress-Impaired Osteogenic Differentiation via the Wnt/β-Catenin and PI3K/Akt Pathways. Sci. Rep. 2024, 14, 14047. [Google Scholar] [CrossRef]
- Backe, S.J.; Heritz, J.A.; Mollapour, M. The HSP90 Chaperone Code Regulates the Crosstalk between Proteostasis and Autophagy. Autophagy 2024, 20, 1689–1691. [Google Scholar] [CrossRef]
- Tharamelveliyil Rajendran, A.; Dheeraj Rajesh, G.; Ashtekar, H.; Sairam, A.; Kumar, P.; Vadakkepushpakath, A.N. Uncovering Naringin’s Anticancer Mechanisms in Glioblastoma via Molecular Docking and Network Pharmacology Approaches. Sci. Rep. 2024, 14, 21486. [Google Scholar] [CrossRef]
- Viswanathan, S.; Sivaraj, R.; Vasanthi, A.H.R.; Subramanian, K.; Ramesh, V. An In-Silico Approach—Molecular Docking Analysis of Flavonoids against GSK-3β and TNF-α Targets in Alzheimer’s Disease. J. Recept. Signal Transduct. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rosso, S.B.; Inestrosa, N.C. WNT Signaling in Neuronal Maturation and Synaptogenesis. Front. Cell. Neurosci. 2013, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Grove, E.A.; Tole, S.; Limon, J.; Yip, L.; Ragsdale, C.W. The Hem of the Embryonic Cerebral Cortex Is Defined by the Expression of Multiple Wnt Genes and Is Compromised in Gli3-Deficient Mice. Development 1998, 125, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Machon, O.; Van Den Bout, C.J.; Backman, M.; Kemler, R.; Krauss, S. Role of β-Catenin in the Developing Cortical and Hippocampal Neuroepithelium. Neuroscience 2003, 122, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Chodelkova, O.; Masek, J.; Korinek, V.; Kozmik, Z.; Machon, O. Tcf7L2 Is Essential for Neurogenesis in the Developing Mouse Neocortex. Neural Develop. 2018, 13, 8. [Google Scholar] [CrossRef]
- Draganova, K.; Zemke, M.; Zurkirchen, L.; Valenta, T.; Cantù, C.; Okoniewski, M.; Schmid, M.-T.; Hoffmans, R.; Götz, M.; Basler, K.; et al. Wnt/β-Catenin Signaling Regulates Sequential Fate Decisions of Murine Cortical Precursor Cells. Stem Cells 2015, 33, 170–182. [Google Scholar] [CrossRef]
- Ishii, K.; Furuta, T.; Kasuya, Y. Determination of Naringin and Naringenin in Human Plasma by High-Performance Liquid Chromatography. J. Chromatogr. B. Biomed. Sci. App. 1996, 683, 225–229. [Google Scholar] [CrossRef]
- Heisler, J.M.; Morales, J.; Donegan, J.J.; Jett, J.D.; Redus, L.; O’Connor, J.C. The Attentional Set Shifting Task: A Measure of Cognitive Flexibility in Mice. J. Vis. Exp. 2015, 96, 51944. [Google Scholar] [CrossRef]
- Swamy, R.S.; Kumar, N.; Shenoy, S.; Cheruku, S.P.; Rao, V.; Kumar, N.; Kumar, S.; Ravichandiran, V. Neuroprotective Effect by Naringin against Fluorosis-Induced Neurodegeneration in Adult Wistar Rats. NeuroReport 2023, 34, 449–456. [Google Scholar] [CrossRef]
- Dai, X.; Jia, Y.; Cao, R.; Zhou, M. Naringin Prevents Cognitive Dysfunction in Aging Rats by Inhibiting Toll-Like Receptor 4 (TLR4)/NF-κB Pathway and Endoplasmic Reticulum Stress. Evid.-Based Complement. Alternat. Med. 2023, 2023, 2919811. [Google Scholar] [CrossRef]
- Bauer, J.; Werner, A.; Kohl, W.; Kugel, H.; Shushakova, A.; Pedersen, A.; Ohrmann, P. Hyperactivity and Impulsivity in Adult Attention-Deficit/Hyperactivity Disorder Is Related to Glutamatergic Dysfunction in the Anterior Cingulate Cortex. World J. Biol. Psychiatry 2018, 19, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, M.; Zhang, Q.; Chen, X.; Wu, J. The Role of Glutamate Receptors in Attention-Deficit/Hyperactivity Disorder: From Physiology to Disease. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2019, 180, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Alomar, S.Y.; Gheit, R.E.A.E.; Enan, E.T.; El-Bayoumi, K.S.; Shoaeir, M.Z.; Elkazaz, A.Y.; Al Thagfan, S.S.; Zaitone, S.A.; El-Sayed, R.M. Novel Mechanism for Memantine in Attenuating Diabetic Neuropathic Pain in Mice via Downregulating the Spinal HMGB1/TRL4/NF-kB Inflammatory Axis. Pharmaceuticals 2021, 14, 307. [Google Scholar] [CrossRef]
- Alomar, S.Y.; Barakat, B.M.; Eldosoky, M.; Atef, H.; Mohamed, A.S.; Elhawary, R.; El-Shafey, M.; Youssef, A.M.; Elkazaz, A.Y.; Gabr, A.M.; et al. Protective Effect of Metformin on Rat Diabetic Retinopathy Involves Suppression of Toll-like Receptor 4/Nuclear Factor-k B Expression and Glutamate Excitotoxicity. Int. Immunopharmacol. 2021, 90, 107193. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, A.; Shi, M.Y.; Yan, Z. Disrupted Glutamatergic Transmission in Prefrontal Cortex Contributes to Behavioral Abnormality in an Animal Model of ADHD. Neuropsychopharmacology 2017, 42, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elfotuh, K.; Abdel-Sattar, S.A.; Abbas, A.N.; Mahran, Y.F.; Alshanwani, A.R.; Hamdan, A.M.E.; Atwa, A.M.; Reda, E.; Ahmed, Y.M.; Zaghlool, S.S.; et al. The Protective Effect of Thymoquinone or/and Thymol against Monosodium Glutamate-Induced Attention-Deficit/Hyperactivity Disorder (ADHD)-like Behavior in Rats: Modulation of Nrf2/HO-1, TLR4/NF-κB/NLRP3/Caspase-1 and Wnt/β-Catenin Signaling Pathways in Rat Model. Biomed. Pharmacother. 2022, 155, 113799. [Google Scholar] [CrossRef]
- Ilgin, N.; Senol, S.; Gucuyener, K.; Gokcora, N.; Atavci, S.; Sener, S. Is Increased D2 Receptor Availability Associated with Response to Stimulant Medication in ADHD. Dev. Med. Child Neurol. 2001, 43, 755. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Newcorn, J.; Fowler, J.S.; Telang, F.; Solanto, M.V.; Logan, J.; Wong, C.; Ma, Y.; Swanson, J.M.; et al. Brain Dopamine Transporter Levels in Treatment and Drug Naïve Adults with ADHD. NeuroImage 2007, 34, 1182–1190. [Google Scholar] [CrossRef]
- Stahl, S.M.; Pradko, J.; Haight, B.R.; Modell, J.G.; Rockett, C.B.; Learned-Coughlin, S. A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor. Prim. Care Companion CNS Disord. 2004, 6, 159. [Google Scholar] [CrossRef]
- Zimmer, L. Contribution of Clinical Neuroimaging to the Understanding of the Pharmacology of Methylphenidate. Trends Pharmacol. Sci. 2017, 38, 608–620. [Google Scholar] [CrossRef]
- Faraone, S.V. The Pharmacology of Amphetamine and Methylphenidate: Relevance to the Neurobiology of Attention-Deficit/Hyperactivity Disorder and Other Psychiatric Comorbidities. Neurosci. Biobehav. Rev. 2018, 87, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Llorente, A.M.; Voigt, R.G.; Jensen, C.L.; Berretta, M.C.; Kennard Fraley, J.; Heird, W.C. Performance on a Visual Sustained Attention and Discrimination Task Is Associated with Urinary Excretion of Norepineprhine Metabolite in Children with Attention-Deficit/Hyperactivity Disorder (AD/HD). Clin. Neuropsychol. 2006, 20, 133–144. [Google Scholar] [CrossRef]
- Weißflog, L.; Scholz, C.-J.; Jacob, C.P.; Nguyen, T.T.; Zamzow, K.; Groß-Lesch, S.; Renner, T.J.; Romanos, M.; Rujescu, D.; Walitza, S.; et al. KCNIP4 as a Candidate Gene for Personality Disorders and Adult ADHD. Eur. Neuropsychopharmacol. 2013, 23, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Wiese, K.E.; Nusse, R.; Van Amerongen, R. Wnt Signalling: Conquering Complexity. Development 2018, 145, dev165902. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Rubio, C.; Rosiles-Abonce, A.; Trejo-Solis, C.; Rubio-Osornio, M.; Mendoza, C.; Custodio, V.; Martinez-Lazcano, J.C.; Gonzalez, E.; Paz, C. Increase Signaling of Wnt/β-Catenin Pathway and Presence of Apoptosis in Cerebellum of Kindled Rats. CNS Neurol. Disord.-Drug Targets 2017, 16, 772–780. [Google Scholar] [CrossRef]
- De Ferrari, G.V.; Chacón, M.A.; Barría, M.I.; Garrido, J.L.; Godoy, J.A.; Olivares, G.; Reyes, A.E.; Alvarez, A.; Bronfman, M.; Inestrosa, N.C. Activation of Wnt Signaling Rescues Neurodegeneration and Behavioral Impairments Induced by β-Amyloid Fibrils. Mol. Psychiatry 2003, 8, 195–208. [Google Scholar] [CrossRef]
- Ye, X.; Zerlanko, B.; Kennedy, A.; Banumathy, G.; Zhang, R.; Adams, P.D. Downregulation of Wnt Signaling Is a Trigger for Formation of Facultative Heterochromatin and Onset of Cell Senescence in Primary Human Cells. Mol. Cell 2007, 27, 183–196. [Google Scholar] [CrossRef]
- Acebron, S.P.; Niehrs, C. β-Catenin-Independent Roles of Wnt/LRP6 Signaling. Trends Cell Biol. 2016, 26, 956–967. [Google Scholar] [CrossRef]
- Caracci, M.O.; Avila, M.E.; Espinoza-Cavieres, F.A.; López, H.R.; Ugarte, G.D.; De Ferrari, G.V. Wnt/β-Catenin-Dependent Transcription in Autism Spectrum Disorders. Front. Mol. Neurosci. 2021, 14, 764756. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A Mechanistic Review on Its Biological Activities and Health Benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ma, J.; Liu, Z.; Lu, Y.; Hu, B.; Yu, H. Effect of Naringenin on Brain Insulin Signaling and Cognitive Functions in ICV-STZ Induced Dementia Model of Rats. Neurol. Sci. 2014, 35, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Hu, Z.; Zhang, Z.; Liu, G.; Wang, Y.; Ren, Y.; Wu, X.; Geng, F. Protective Role Of Naringenin Against Aβ25-35-Caused Damage via ER and PI3K/Akt-Mediated Pathways. Cell. Mol. Neurobiol. 2018, 38, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, S.; Joghataei, M.-T.; Mohseni, S.; Baluchnejadmojarad, T.; Bagheri, M.; Khamse, S.; Roghani, M. Naringenin Improves Learning and Memory in an Alzheimer’s Disease Rat Model: Insights into the Underlying Mechanisms. Eur. J. Pharmacol. 2015, 764, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Affany, A.; Salvayre, R.; Douste-Blazy, L. Comparison of the Protective Effect of Various Flavonoids Against Lipid Peroxidation of Erythrocyte Membranes (Induced by Cumene Hydroperoxide). Fundam. Clin. Pharmacol. 1987, 1, 451–457. [Google Scholar] [CrossRef]
- Korkina, L.G.; Afanas’Ev, I.B. Antioxidant and Chelating Properties of Flavonoids. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 1996; Volume 38, pp. 151–163. ISBN 978-0-12-032939-7. [Google Scholar]
- Chen, Y.; Zheng, R.; Jia, Z.; Ju, Y. Flavonoids as Superoxide Scavengers and Antioxidants. Free Radic. Biol. Med. 1990, 9, 19–21. [Google Scholar] [CrossRef]
- Russo, A.; Acquaviva, R.; Campisi, A.; Sorrenti, V.; Di Giacomo, C.; Virgata, G.; Barcellona, M.L.; Vanella, A. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biol. Toxicol. 2000, 16, 91–98. [Google Scholar] [CrossRef]
- Alvarez-González, I.; Madrigal-Bujaidar, E.; Dorado, V.; Espinosa-Aguirre, J.J. Inhibitory Effect of Naringin on the Micronuclei Induced by Ifosfamide in Mouse, and Evaluation of Its Modulatory Effect on the Cyp3a Subfamily. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 480–481, 171–178. [Google Scholar] [CrossRef]
- Abd-Elmawla, M.A.; Essam, R.M.; Ahmed, K.A.; Abdelmonem, M. Implication of Wnt/GSK-3β/β-Catenin Signaling in the Pathogenesis of Mood Disturbances Associated with Hyperthyroidism in Rats: Potential Therapeutic Effect of Naringin. ACS Chem. Neurosci. 2023, 14, 2035–2048. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, Z. The Effect of Naringin on the Apoptosis of Degenerative Nucleus Pulposus Cells: A Study on the Function and Mechanism. Drug Des. Devel. Ther. 2022, 16, 499–508. [Google Scholar] [CrossRef]
- McGann, M. FRED Pose Prediction and Virtual Screening Accuracy. J. Chem. Inf. Model. 2011, 51, 578–596. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic acids research 2016, 44, D457–D462. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, A.Y.; Odetunde, I.; Akintola, A.S.; Ogundeji, M.O.; Ajao, A.; Obelawo, A.Y.; Onaolapo, O.J. Dietary Composition Modulates Impact of Food-Added Monosodium Glutamate on Behaviour, Metabolic Status and Cerebral Cortical Morphology in Mice. Biomed. Pharmacother. 2019, 109, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Termkwancharoen, C.; Malakul, W.; Phetrungnapha, A.; Tunsophon, S. Naringin Ameliorates Skeletal Muscle Atrophy and Improves Insulin Resistance in High-Fat-Diet-Induced Insulin Resistance in Obese Rats. Nutrients 2022, 14, 4120. [Google Scholar] [CrossRef] [PubMed]
- Meikle, L.; Pollizzi, K.; Egnor, A.; Kramvis, I.; Lane, H.; Sahin, M.; Kwiatkowski, D.J. Response of a Neuronal Model of Tuberous Sclerosis to Mammalian Target of Rapamycin (mTOR) Inhibitors: Effects on mTORC1 and Akt Signaling Lead to Improved Survival and Function. J. Neurosci. 2008, 28, 5422–5432. [Google Scholar] [CrossRef] [PubMed]
- Soltani, Z.; Shariatpanahi, M.; Aghsami, M.; Owliaey, H.; Kheradmand, A. Investigating the Effect of Exposure to Monosodium Glutamate during Pregnancy on Development of Autism in Male Rat Offspring. Food Chem. Toxicol. 2024, 185, 114464. [Google Scholar] [CrossRef]
- Yu, T.; Zhao, Y.; Shi, W.; Ma, R.; Yu, L. Effects of Maternal Oral Administration of Monosodium Glutamate at a Late Stage of Pregnancy on Developing Mouse Fetal Brain. Brain Res. 1997, 747, 195–206. [Google Scholar] [CrossRef]
- Birrell, J.M.; Brown, V.J. Medial Frontal Cortex Mediates Perceptual Attentional Set Shifting in the Rat. J. Neurosci. 2000, 20, 4320–4324. [Google Scholar] [CrossRef]
- El-Sherbeeny, N.A.; Soliman, N.; Youssef, A.M.; Abd El-Fadeal, N.M.; El-Abaseri, T.B.; Hashish, A.A.; Abdelbasset, W.K.; El-Saber Batiha, G.; Zaitone, S.A. The Protective Effect of Biochanin A against Rotenone-Induced Neurotoxicity in Mice Involves Enhancing of PI3K/Akt/mTOR Signaling and Beclin-1 Production. Ecotoxicol. Environ. Saf. 2020, 205, 111344. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Abdel-Mottaleb, Y.; Elkazaz, A.Y.; Atef, H.; Lashine, R.M.; Youssef, A.M.; Ezzat, W.; El-Ghaiesh, S.H.; Elshaer, R.E.; El-Shafey, M.; et al. Carbamazepine Alleviates Retinal and Optic Nerve Neural Degeneration in Diabetic Mice via Nerve Growth Factor-Induced PI3K/Akt/mTOR Activation. Front. Neurosci. 2019, 13, 1089. [Google Scholar] [CrossRef] [PubMed]
- Bahr, H.I.; Toraih, E.A.; Mohammed, E.A.; Mohammad, H.M.F.; Ali, E.A.I.; Zaitone, S.A. Chemopreventive Effect of Leflunomide against Ehrlich’s Solid Tumor Grown in Mice: Effect on EGF and EGFR Expression and Tumor Proliferation. Life Sci. 2015, 141, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Zaitone, S.A.; Moustafa, Y.M. Boswellic Acids Synergize Antitumor Activity and Protect against the Cardiotoxicity of Doxorubicin in Mice Bearing Ehrlich’s Carcinoma. Can. J. Physiol. Pharmacol. 2015, 93, 695–708. [Google Scholar] [CrossRef] [PubMed]
| Number | Name | Applied Diet | Treatment |
|---|---|---|---|
| Group 1 | Normal control | Normal chow diet * | Distilled water |
| Group 2 | SGL control | SGL diet † [94] | Distilled water |
| Group 3 | SGL + naringin-50 mg/kg | SGL diet | Naringin 50 mg/kg [95] |
| Group 4 | SGL + naringin-100 mg/kg | SGL diet | Naringin 100 mg/kg [59,60] |
| GenesPrimers | Sequences | Accession Number |
|---|---|---|
| Wnt | Forward 5′GCCTGTGAAGGACTCAGAACTTG3′ Reverse 5′AGCTGTCACTGCCGTTGGAAGT3′ | NM_001285794.1 |
| β-Catenin | Forward 5′GTTCGCCTTCATTATGGACTGCC3′ Reverse 5′ATAGCACCCTGTTCCCGCAAAG3′ | NM_007614.3 |
| Caspase-3 | Forward 5′GGAGTCTGACTGGAAAGCCGAA3′ Reverse 5′CTTCTGGCAAGCCATCTCCTCA3′ | NM_009810.3 |
| Bcl2 | Forward 5′CCTGTGGATGACTGAGTACCTG 3′ Reverse 5′AGCCAGGAGAAATCAAACAGAGG3′ | NM_009741.3 |
| BAX | Forward 5′AGGATGCGTCCACCAAGAAGCT3′ Reverse 5′TCCGTGTCCACGTCAGCAATCA3′ | NM_007527.3 |
| β-actin | Forward 5′TCCTCCTGAGCGCAAGTACTCT3′ Reverse 5′GCTCAGTAACAGTCCGCCTAGAA3′ | NM_007393.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, H.I.; Zaitone, S.A.; El-Sayed, K.; Lashine, R.M.; Ahmed, N.; Moursi, S.M.M.; Shehata, S.A.; Aldahish, A.A.; Helal, M.A.; El-Kherbetawy, M.K.; et al. Molecular Docking, Bioinformatic Analysis, and Experimental Verification for the Effect of Naringin on ADHD: Possible Inhibition of GSK-3β and HSP90. Pharmaceuticals 2024, 17, 1436. https://doi.org/10.3390/ph17111436
Mokhtar HI, Zaitone SA, El-Sayed K, Lashine RM, Ahmed N, Moursi SMM, Shehata SA, Aldahish AA, Helal MA, El-Kherbetawy MK, et al. Molecular Docking, Bioinformatic Analysis, and Experimental Verification for the Effect of Naringin on ADHD: Possible Inhibition of GSK-3β and HSP90. Pharmaceuticals. 2024; 17(11):1436. https://doi.org/10.3390/ph17111436
Chicago/Turabian StyleMokhtar, Hatem I., Sawsan A. Zaitone, Karima El-Sayed, Rehab M. Lashine, Nada Ahmed, Suzan M. M. Moursi, Shaimaa A. Shehata, Afaf A. Aldahish, Mohamed A. Helal, Mohamed K. El-Kherbetawy, and et al. 2024. "Molecular Docking, Bioinformatic Analysis, and Experimental Verification for the Effect of Naringin on ADHD: Possible Inhibition of GSK-3β and HSP90" Pharmaceuticals 17, no. 11: 1436. https://doi.org/10.3390/ph17111436
APA StyleMokhtar, H. I., Zaitone, S. A., El-Sayed, K., Lashine, R. M., Ahmed, N., Moursi, S. M. M., Shehata, S. A., Aldahish, A. A., Helal, M. A., El-Kherbetawy, M. K., Fawzy, M. S., & Abd El-Fadeal, N. M. (2024). Molecular Docking, Bioinformatic Analysis, and Experimental Verification for the Effect of Naringin on ADHD: Possible Inhibition of GSK-3β and HSP90. Pharmaceuticals, 17(11), 1436. https://doi.org/10.3390/ph17111436










