From Microcirculation to Aging-Related Diseases: A Focus on Endothelial SIRT1
Abstract
1. Introduction
2. Regulation of Microcirculation by Endothelial SIRT1
2.1. Regulation of Physiological Capillarization by SIRT1
2.2. SIRT1 and Diabetic Microangiopathy
2.3. SIRT1, Oxidative Stress and Mitochondrial Function
2.4. SIRT1 and Coronary Microvascular Function
2.5. SIRT1 and Brain Microvascular Function
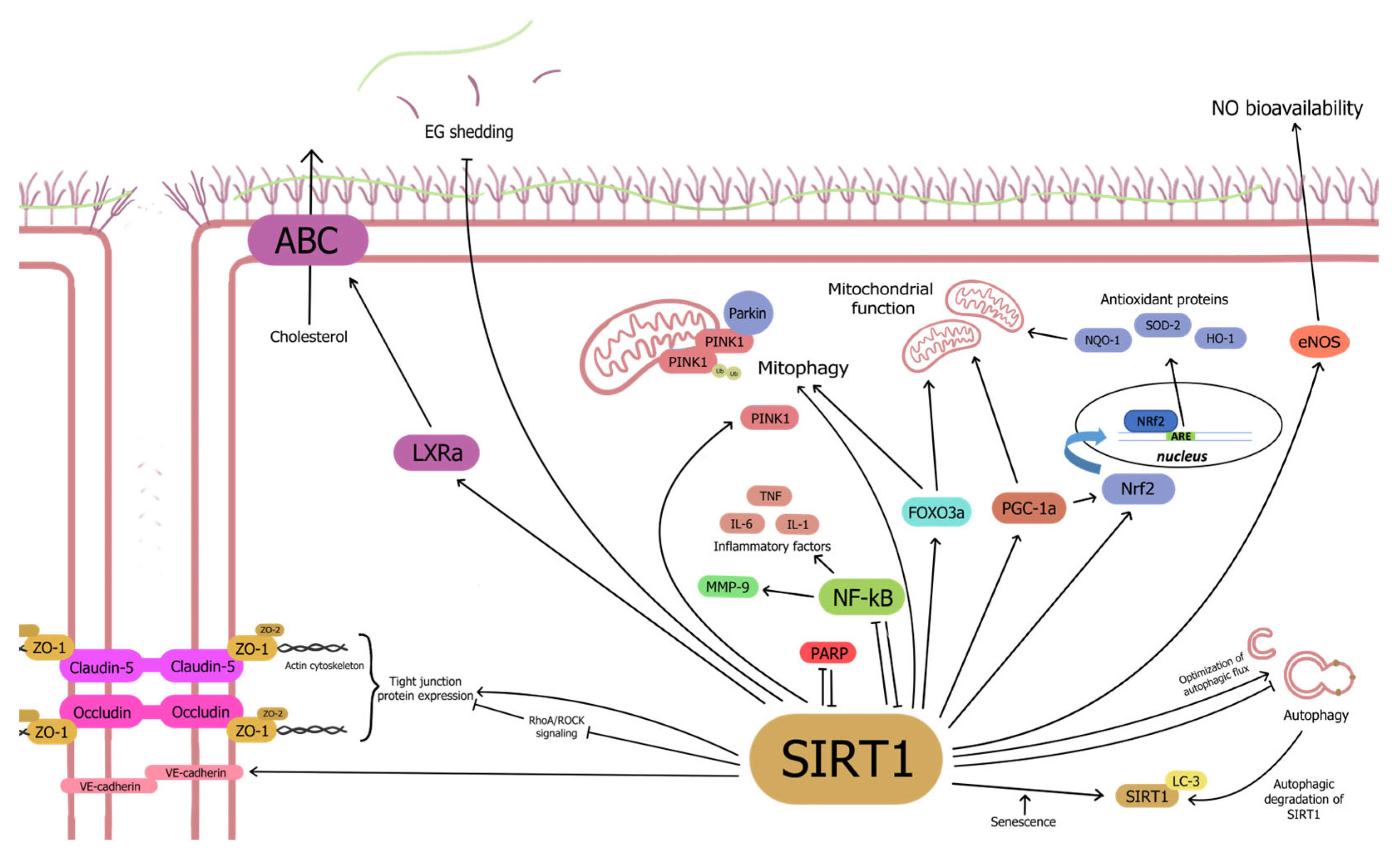
3. Mechanisms Underlying Regulation of Microcirculation by Endothelial SIRT1
3.1. SIRT1 Enhances Endothelium-Dependent Vasodilation in an eNOS-Dependent Manner
3.2. Anti-Senescence Activity of Endothelial SIRT1
3.2.1. SIRT1-NF-κB Interaction Dictates Microvascular Function and Inflammation
3.2.2. Senescence, Autophagy and SIRT1
3.2.3. SIRT1-PARP Has a Bidirectional Interaction in Regulating Microvascular Function
3.2.4. SIRT1 Regulates Oxidative Stress and Mitochondrial Dysfunction
3.3. SIRT1 Plays a Dual Role in Micro-Neovascularization
3.3.1. SIRT1 Promotes Angiogenesis by Inhibiting DLL4-Notch Signaling
3.3.2. SIRT1 Suppresses HIF-1α-Induced Pathological Angiogenesis
3.3.3. SIRT1-eNOS Axis Promotes Tissue Capillarization
3.4. SIRT1 Upregulates Tight Junction Proteins to Maintain Micro-Endothelial Cell-Cell Junctions
3.5. SIRT1 and Microcirculatory Thrombosis
3.5.1. SIRT1 Regulates Endothelial Glycocalyx Function
3.5.2. SIRT1 Interacts with Prostacyclin Signaling
3.5.3. SIRT1 Directly Regulates Platelet Activity and Lifespan
4. Targeting Microvascular SIRT1 in Aging-Related Disease
4.1. Natural Modulators of SIRT1
4.1.1. Resveratrol
4.1.2. Other Natural Modulators
4.2. Endogenous SIRT1 Modulators
4.2.1. Nicotinamide Adenine Dinucleotide (NAD+) Modulators
4.2.2. Hormones and Hormone-like Substances
4.2.3. Non-Coding RNA (ncRNA) Modulators of SIRT1
MicroRNA (miR)
Circular RNA (circRNA)
Long Non-Coding RNA
tRNA-Derived Stress-Induced RNA (tiRNAs)
4.3. Synthetic SIRT1 Modulators
4.3.1. SRT1720
4.3.2. SIRT1 Inhibitors
4.3.3. Other SIRT1 Modulators
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Munoz, C.J.; Lucas, A.; Williams, A.T.; Cabrales, P. A Review on Microvascular Hemodynamics: The Control of Blood Flow Distribution and Tissue Oxygenation. Crit Care Clin. 2020, 36, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Jin, K. A Microcirculatory Theory of Aging. Aging Dis. 2019, 10, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, B.; Ren, C.; Hu, J.; Greenberg, D.A.; Chen, T.; Xie, L.; Jin, K. Age-related Impairment of Vascular Structure and Functions. Aging Dis. 2017, 8, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Lovshin, J.A.; Bjornstad, P.; Lovblom, L.E.; Bai, J.W.; Lytvyn, Y.; Boulet, G.; Farooqi, M.A.; Santiago, S.; Orszag, A.; Scarr, D.; et al. Atherosclerosis and Microvascular Complications: Results From the Canadian Study of Longevity in Type 1 Diabetes. Diabetes Care 2018, 41, 2570–2578. [Google Scholar] [CrossRef]
- Spione, F.; Arevalos, V.; Gabani, R.; Sabaté, M.; Brugaletta, S. Coronary Microvascular Angina: A State-of-the-Art Review. Front. Cardiovasc. Med. 2022, 9, 800918. [Google Scholar] [CrossRef]
- Tissenbaum, H.A.; Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef]
- Lin, S.-J.; Defossez, P.-A.; Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 2000, 289, 2126–2128. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Mondaca-Ruff, D.; Singh, S.; Wang, Y. SIRT1 and Autophagy: Implications in Endocrine Disorders. Front. Endocrinol. 2022, 13, 930919. [Google Scholar] [CrossRef]
- Rodriguez-Miguelez, P.; Looney, J.; Thomas, J.; Harshfield, G.; Pollock, J.S.; Harris, R.A. Sirt1 during childhood is associated with microvascular function later in life. Am. J. Physiol. Circ. Physiol. 2020, 318, H1371–H1378. [Google Scholar] [CrossRef]
- Mengozzi, A.; Costantino, S.; Paneni, F.; Duranti, E.; Nannipieri, M.; Mancini, R.; Lai, M.; La Rocca, V.; Puxeddu, I.; Antonioli, L.; et al. Targeting SIRT1 Rescues Age- and Obesity-Induced Microvascular Dysfunction in Ex Vivo Human Vessels. Circ. Res. 2022, 131, 476–491. [Google Scholar] [CrossRef]
- Mariani, S.; di Giorgio, M.R.; Martini, P.; Persichetti, A.; Barbaro, G.; Basciani, S.; Contini, S.; Poggiogalle, E.; Sarnicola, A.; Genco, A.; et al. Inverse Association of Circulating SIRT1 and Adiposity: A Study on Underweight, Normal Weight, and Obese Patients. Front. Endocrinol. 2018, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Di Giorgio, M.R.; Rossi, E.; Tozzi, R.; Contini, S.; Bauleo, L.; Cipriani, F.; Toscano, R.; Basciani, S.; Barbaro, G.; et al. Blood SIRT1 Shows a Coherent Association with Leptin and Adiponectin in Relation to the Degree and Distribution of Adiposity: A Study in Obesity, Normal Weight and Anorexia Nervosa. Nutrients 2020, 12, 3506. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zeng, X.; Cheng, Y.; Tang, L.; Hong, D.; Yang, X. Lycopene ameliorates insulin resistance and increases muscle capillary density in aging via activation of SIRT1. J. Nutr. Biochem. 2022, 99, 108862. [Google Scholar] [CrossRef] [PubMed]
- Gonçalinho, G.H.F.; Kuwabara, K.L.; Faria, N.F.d.O.; Goes, M.F.d.S.; Roggerio, A.; Avakian, S.D.; Strunz, C.M.C.; Mansur, A.d.P. Sirtuin 1 and Vascular Function in Healthy Women and Men: A Randomized Clinical Trial Comparing the Effects of Energy Restriction and Resveratrol. Nutrients 2023, 15, 2949. [Google Scholar] [CrossRef] [PubMed]
- Elibol, B.; Kilic, U. High Levels of SIRT1 Expression as a Protective Mechanism Against Disease-Related Conditions. Front. Endocrinol. 2018, 9, 614. [Google Scholar] [CrossRef]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017, 18, 447–476. [Google Scholar] [CrossRef]
- Das, A.; Huang, G.X.; Bonkowski, M.S.; Longchamp, A.; Li, C.; Schultz, M.B.; Kim, L.-J.; Osborne, B.; Joshi, S.; Lu, Y.; et al. Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell 2018, 173, 74–89.e20. [Google Scholar] [CrossRef]
- Scarfò, G.; Daniele, S.; Chelucci, E.; Rizza, A.; Fusi, J.; Freggia, G.; Costa, B.; Taliani, S.; Artini, P.; Martini, C.; et al. Regular exercise delays microvascular endothelial dysfunction by regulating antioxidant capacity and cellular metabolism. Sci. Rep. 2023, 13, 17671. [Google Scholar] [CrossRef] [PubMed]
- Ringholm, S.; Biensø, R.S.; Kiilerich, K.; Guadalupe-Grau, A.; Aachmann-Andersen, N.J.; Saltin, B.; Plomgaard, P.; Lundby, C.; Wojtaszewski, J.F.P.; Calbet, J.A.; et al. Bed rest reduces metabolic protein content and abolishes exercise-induced mRNA responses in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E649–E658. [Google Scholar] [CrossRef]
- Xu, F.; Burk, D.; Gao, Z.; Yin, J.; Zhang, X.; Weng, J.; Ye, J. Angiogenic deficiency and adipose tissue dysfunction are associated with macrophage malfunction in SIRT1−/− mice. Endocrinology 2012, 153, 1706–1716. [Google Scholar] [CrossRef]
- Pang, C.; Gao, Z.; Yin, J.; Zhang, J.; Jia, W.; Ye, J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am. J. Physiol. Metab. 2008, 295, E313–E322. [Google Scholar] [CrossRef] [PubMed]
- Furuuchi, R.; Shimizu, I.; Yoshida, Y.; Katsuumi, G.; Suda, M.; Kubota, Y.; Walsh, K.; Minamino, T. Endothelial SIRT-1 has a critical role in the maintenance of capillarization in brown adipose tissue. iScience 2022, 25, 105424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, X.; Lu, L.; Deng, M.; Shi, X. Fibroblast growth factor 21 improves lipopolysaccharide-induced pulmonary microvascular endothelial cell dysfunction and inflammatory response through SIRT1-mediated NF-κB deacetylation. Can. J. Physiol. Pharmacol. 2022, 100, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Pang, J.; Alexander, J.S.; Rivera, C. The endotoxin/toll-like receptor-4 axis mediates gut microvascular dysfunction associated with post-prandial lipidemia. BMC Physiol. 2013, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Large, J.M.; Pang, D.K.; Robson, D.L.; Chan, P.; Toma, J.; Borradaile, N.M. Niacin receptor activation improves human microvascular endothelial cell angiogenic function during lipotoxicity. Atherosclerosis 2014, 237, 696–704. [Google Scholar] [CrossRef]
- Mai, H.; Liu, C.; Fu, B.; Ji, X.; Chen, M.; Zhang, Y.; Lin, Y.; Chen, J.; Song, Y.; Gu, S. Methylene Blue Reduces Retinal Cell Inflammation, Apoptosis, and Oxidative Stress in a Rat Model of Diabetic Retinopathy via Sirtuin 1 Activation. Altern. Ther. Health Med. 2023, 29, 156–165. [Google Scholar]
- Cui, C.; Li, Y.; Liu, Y. Down-regulation of miR-377 suppresses high glucose and hypoxia-induced angiogenesis and inflammation in human retinal endothelial cells by direct up-regulation of target gene SIRT1. Hum. Cell 2019, 32, 260–274. [Google Scholar] [CrossRef]
- Sohrab, G.; Nasrollahzadeh, J.; Tohidi, M.; Zand, H.; Nikpayam, O. Pomegranate Juice Increases Sirtuin1 Protein in Peripheral Blood Mononuclear Cell from Patients with Type 2 Diabetes: A Randomized Placebo Controlled Clinical Trial. Metab. Syndr. Relat. Disord. 2018, 16, 446–451. [Google Scholar] [CrossRef]
- Hammer, S.S.; Vieira, C.P.; McFarland, D.; Sandler, M.; Levitsky, Y.; Dorweiler, T.F.; Lydic, T.A.; Asare-Bediako, B.; Adu-Agyeiwaah, Y.; Sielski, M.S.; et al. Fasting and fasting-mimicking treatment activate SIRT1/LXRα and alleviate diabetes-induced systemic and microvascular dysfunction. Diabetologia 2021, 64, 1674–1689. [Google Scholar] [CrossRef]
- Li, X.; Wu, G.; Han, F.; Wang, K.; Bai, X.; Jia, Y.; Li, Z.; Cai, W.; Zhang, W.; Su, L.; et al. SIRT1 activation promotes angiogenesis in diabetic wounds by protecting endothelial cells against oxidative stress. Arch. Biochem. Biophys. 2019, 661, 117–124. [Google Scholar] [CrossRef]
- Potente, M.; Ghaeni, L.; Baldessari, D.; Mostoslavsky, R.; Rossig, L.; Dequiedt, F.; Haendeler, J.; Mione, M.; Dejana, E.; Alt, F.W.; et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007, 21, 2644–2658. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y.; Zullo, J.A.; Goligorsky, M.S. Endothelial sirtuin 1 inactivation enhances capillary rarefaction and fibrosis following kidney injury through Notch activation. Biochem. Biophys. Res. Commun. 2016, 478, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.; Li, S.; Zuo, B.; Zhang, X.; Wang, F.; Sun, D. Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway. Theranostics 2020, 10, 9425–9442. [Google Scholar] [CrossRef] [PubMed]
- Takiyama, Y.; Haneda, M. Hypoxia in Diabetic Kidneys. BioMed Res. Int. 2014, 2014, 837421. [Google Scholar] [CrossRef]
- Zheng, D.; Ma, J.; Yu, Y.; Li, M.; Ni, R.; Wang, G.; Chen, R.; Li, J.; Fan, G.-C.; Lacefield, J.C.; et al. Silencing of miR-195 reduces diabetic cardiomyopathy in C57BL/6 mice. Diabetologia 2015, 58, 1949–1958. [Google Scholar] [CrossRef]
- Xu, L.; Kanasaki, K.; Kitada, M.; Koya, D. Diabetic angiopathy and angiogenic defects. Fibrogenesis Tissue Repair 2012, 5, 13. [Google Scholar] [CrossRef]
- Xu, Y.; Zou, H.; Ding, Q.; Zou, Y.; Tang, C.; Lu, Y.; Xu, X. tiRNA-Val promotes angiogenesis via Sirt1-Hif-1α axis in mice with diabetic retinopathy. Biol. Res. 2022, 55, 14. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Deng, Y.; Yu, Q.; Meng, X.; Jiang, T.; Wang, Q.; Fu, Y. Effect of plasma-derived extracellular vesicles on angiogenesis and the ensuing proliferative diabetic retinopathy through a miR-30b-dependent mechanism. Diabetol. Metab. Syndr. 2022, 14, 188. [Google Scholar] [CrossRef]
- Wang, P.; Konja, D.; Singh, S.; Zhang, B.; Wang, Y. Endothelial Senescence: From Macro- to Micro-Vasculature and Its Implications on Cardiovascular Health. Int. J. Mol. Sci. 2024, 25, 1978. [Google Scholar] [CrossRef]
- Li, J.; Feng, Z.; Lu, B.; Fang, X.; Huang, D.; Wang, B. Resveratrol alleviates high glucose-induced oxidative stress and apoptosis in rat cardiac microvascular endothelial cell through AMPK/Sirt1 activation. Biochem. Biophys. Rep. 2023, 34, 101444. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Bao, M.; Chen, R.; Li, H.; Lu, B.; Chen, M.; Huang, D.; Zhang, Y.; Gao, F.; et al. Melatonin Attenuates Diabetic Myocardial Microvascular Injury through Activating the AMPK/SIRT1 Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 8882130. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wu, L.; Cao, T.; Zheng, H.M.; He, T. MiR-221/SIRT1/Nrf2 signal axis regulates high glucose induced apoptosis in human retinal microvascular endothelial cells. BMC Ophthalmol. 2020, 20, 300. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, S.; Ying, J.; Shi, T.; Wang, P. Resveratrol Prevents ROS-Induced Apoptosis in High Glucose-Treated Retinal Capillary Endothelial Cells via the Activation of AMPK/Sirt1/PGC-1α Pathway. Oxidative Med. Cell. Longev. 2017, 2017, 7584691. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, T.; Deng, X.; Ding, W.; Yue, Z.; Yang, W.; Lv, X.; Li, W. Adiponectin ameliorates lung ischemia–reperfusion injury through SIRT1-PINK1 signaling-mediated mitophagy in type 2 diabetic rats. Respir. Res. 2021, 22, 258. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, L.; Zhang, M.; Hu, J.; Wang, T.; Duan, Y.; Man, W.; Wu, B.; Feng, J.; Sun, L.; et al. Mst1 inhibits CMECs autophagy and participates in the development of diabetic coronary microvascular dysfunction. Sci. Rep. 2016, 6, 34199. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ke, L.; Zhou, J.; Ding, C.; Yang, H.; Yan, D.; Yu, C. Stachydrine Relieved the Inflammation and Promoted the Autophagy in Diabetes Retinopathy Through Activating the AMPK/SIRT1 Signaling Pathway. Diabetes Metab. Syndr. Obes. 2023, 16, 2593–2604. [Google Scholar] [CrossRef]
- Nelson, M.D.; Wei, J.; Bairey Merz, C.N. Coronary microvascular dysfunction and heart failure with preserved ejection fraction as female-pattern cardiovascular disease: The chicken or the egg? Eur. Heart J. 2018, 39, 850–852. [Google Scholar] [CrossRef]
- Dankar, R.; Wehbi, J.; Atasi, M.M.; Alam, S.; Refaat, M.M. Coronary microvascular dysfunction, arrythmias, and sudden cardiac death: A literature review. Am. Heart J. Plus 2024, 41, 100389. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; He, J.; Wang, S.; Wang, J.; Liu, J.; Wang, Y. Molecular mechanisms of endothelial dysfunction in coronary microcirculation dysfunction. J. Thromb. Thrombolysis 2023, 56, 388–397. [Google Scholar] [CrossRef]
- Wang, A.J.; Tang, Y.; Zhang, J.; Wang, B.J.; Xiao, M.; Lu, G.; Li, J.; Liu, Q.; Guo, Y.; Gu, J. Cardiac SIRT1 ameliorates doxorubicin-induced cardiotoxicity by targeting sestrin 2. Redox Biol. 2022, 52, 102310. [Google Scholar] [CrossRef]
- Lu, G.; Liu, Q.; Gao, T.; Li, J.; Zhang, J.; Chen, O.; Cao, C.; Mao, M.; Xiao, M.; Zhang, X.; et al. Resveratrol and FGF1 Synergistically Ameliorates Doxorubicin-Induced Cardiotoxicity via Activation of SIRT1-NRF2 Pathway. Nutrients 2022, 14, 4017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Tang, Y.; Huang, C. Melatonin alleviates doxorubicin-induced cardiotoxicity via inhibiting oxidative stress, pyroptosis and apoptosis by activating Sirt1/Nrf2 pathway. Biomed. Pharmacother. 2023, 162, 114591. [Google Scholar] [CrossRef] [PubMed]
- Galan-Arriola, C.; Vílchez-Tschischke, J.P.; Lobo, M.; Lopez, G.J.; de Molina-Iracheta, A.; Pérez-Martínez, C.; Villena-Gutiérrez, R.; Macías, Á.; Díaz-Rengifo, I.A.; Oliver, E.; et al. Coronary microcirculation damage in anthracycline cardiotoxicity. Cardiovasc. Res. 2022, 118, 531–541. [Google Scholar] [CrossRef]
- Gao, J.; Ren, J.; Ma, X.; Zhang, Y.; Song, L.; Liu, J.; Shi, D.; Ma, X. Ligustrazine prevents coronary microcirculation dysfunction in rats via suppression of miR-34a-5p and promotion of Sirt1. Eur. J. Pharmacol. 2022, 929, 175150. [Google Scholar] [CrossRef] [PubMed]
- Maizel, J.; Xavier, S.; Chen, J.; Lin, C.H.S.; Vasko, R.; Goligorsky, M.S. Sirtuin 1 ablation in endothelial cells is associated with impaired angiogenesis and diastolic dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1691–H1704. [Google Scholar] [CrossRef]
- Zeng, F.; Zhou, P.; Wang, M.; Xie, L.; Huang, X.; Wang, Y.; Huang, J.; Shao, X.; Yang, Y.; Liu, W.; et al. ACMSD mediated de novo NAD+ biosynthetic impairment in cardiac endothelial cells as a potential therapeutic target for diabetic cardiomyopathy. Diabetes Res. Clin. Pract. 2023, 206, 111014. [Google Scholar] [CrossRef]
- Koton, S.; Schneider, A.L.; Windham, B.G.; Mosley, T.H.; Gottesman, R.F.; Coresh, J. Microvascular Brain Disease Progression and Risk of Stroke The ARIC Study. Stroke 2020, 51, 3264–3270. [Google Scholar] [CrossRef]
- Farrall, A.J.; Wardlaw, J.M. Blood-brain barrier: Ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging 2009, 30, 337–352. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Martinez-Revollar, G.; Hu, A.; Choi, J.; Keep, R.F.; Andjelkovic, A.V. Decline in Sirtuin-1 expression and activity plays a critical role in blood-brain barrier permeability in aging. Neurobiol. Dis. 2019, 126, 105–116. [Google Scholar] [CrossRef]
- Chen, T.; Dai, S.-H.; Li, X.; Luo, P.; Zhu, J.; Wang, Y.-H.; Fei, Z.; Jiang, X.-F. Sirt1-Sirt3 axis regulates human blood-brain barrier permeability in response to ischemia. Redox Biol. 2018, 14, 229–236. [Google Scholar] [CrossRef]
- Sun, P.; Bu, F.; Min, J.; Munshi, Y.; Howe, M.D.; Liu, L.; Koellhoffer, E.C.; Qi, L.; McCullough, L.D.; Li, J. Inhibition of calcium/calmodulin-dependent protein kinase kinase (CaMKK) exacerbates impairment of endothelial cell and blood–brain barrier after stroke. Eur. J. Neurosci. 2019, 49, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, Y.; Wang, Z.; Zhang, S.; Yang, Y.; Zhu, Y.; Yang, C. LncRNA Snhg8 attenuates microglial inflammation response and blood-brain barrier damage in ischemic stroke through regulating miR-425-5p mediated SIRT1/NF-κB signaling. J. Biochem. Mol. Toxicol. 2021, 35, e22724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, X.; Peng, Z.; Liu, C.; Ren, L.; Liang, J.; Wang, P. Nicotinamide Mononucleotide Adenylyltransferase 1 Regulates Cerebral Ischemia-Induced Blood-Brain Barrier Disruption Through NAD(+)/SIRT1 Signaling Pathway. Mol. Neurobiol. 2022, 59, 4879–4891. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shan, X.; Li, L.; Dong, L.; Huang, G.; Tao, H. circHIPK3 regulates apoptosis and mitochondrial dysfunction induced by ischemic stroke in mice by sponging miR-148b-3p via CDK5R1/SIRT1. Exp. Neurol. 2022, 355, 114115. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, C.; Guan, Y.; Yang, W.; Yu, J.; Shi, H.; Ding, Z.; Zhang, Z. Long noncoding RNA KCNQ1OT1 aggravates cerebral infarction by regulating PTBT1/SIRT1 via miR-16-5p. J. Neuropathol. Exp. Neurol. 2024, 83, 276–288. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.-T.; Zhang, P.-L.; Li, Y.-C.; Sun, M.-R.; Wang, Y.-T.; Wang, S.-P.; Yang, H.; Liu, B.-L.; Wang, M.; et al. Hydroxysafflor Yellow A Blocks HIF-1α Induction of NOX2 and Protects ZO-1 Protein in Cerebral Microvascular Endothelium. Antioxidants 2022, 11, 728. [Google Scholar] [CrossRef]
- Qu, Y.; Cao, J.; Wang, D.; Wang, S.; Li, Y.; Zhu, Y. 14,15-Epoxyeicosatrienoic Acid Protect Against Glucose Deprivation and Reperfusion-Induced Cerebral Microvascular Endothelial Cells Injury by Modulating Mitochondrial Autophagy via SIRT1/FOXO3a Signaling Pathway and TSPO Protein. Front. Cell Neurosci. 2022, 16, 888836. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Fu, W.; Cheng, N.; Meng, D.; Gu, Y. Ligustrazine reduces blood-brain barrier permeability in a rat model of focal cerebral ischemia and reperfusion. Exp. Ther. Med. 2015, 9, 1757–1762. [Google Scholar] [CrossRef]
- Sun, X.; Liu, B. Donepezil ameliorates oxygen-glucose deprivation/reoxygenation-induced brain microvascular endothelial cell dysfunction via the SIRT1/FOXO3a/NF-κB pathways. Bioengineered 2022, 13, 7760–7770. [Google Scholar] [CrossRef]
- Wang, S.; Han, X.; Mao, Z.; Xin, Y.; Maharjan, S.; Zhang, B. MALAT1 lncRNA Induces Autophagy and Protects Brain Microvascular Endothelial Cells Against Oxygen–Glucose Deprivation by Binding to miR-200c-3p and Upregulating SIRT1 Expression. Neuroscience 2019, 397, 116–126. [Google Scholar] [CrossRef]
- Zhou, Z.-W.; Zheng, L.-J.; Ren, X.; Li, A.-P.; Zhou, W.-S. LncRNA NEAT1 facilitates survival and angiogenesis in oxygen-glucose deprivation (OGD)-induced brain microvascular endothelial cells (BMECs) via targeting miR-377 and upregulating SIRT1, VEGFA, and BCL-XL. Brain Res. 2019, 1707, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Zotin, M.C.Z.; Sveikata, L.; Viswanathan, A.; Yilmaz, P. Cerebral small vessel disease and vascular cognitive impairment: From diagnosis to management. Curr. Opin. Neurol. 2021, 34, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Han, F. Cerebral microvascular dysfunction and neurodegeneration in dementia. Stroke Vasc. Neurol. 2019, 4, 105–107. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Halliday, G.M.; Soh, D.; Cordato, D.J.; Kril, J.J. Impact of small vessel disease on severity of motor and cognitive impairment in Parkinson’s disease. J. Clin. Neurosci. 2018, 58, 70–74. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-M.; Park, J.; Lee, J.-H.; Song, M.K.; Kim, Y.-J. Preischemic Treadmill Exercise Ameliorates Memory Impairment and Microvasculature Damage in Rat Model of Chronic Cerebral Hypoperfusion. Int. Neurourol. J. 2021, 25 (Suppl. S2), S72–S80. [Google Scholar] [CrossRef]
- Kiss, T.; Balasubramanian, P.; Valcarcel-Ares, M.N.; Tarantini, S.; Yabluchanskiy, A.; Csipo, T.; Lipecz, A.; Reglodi, D.; Zhang, X.A.; Bari, F.; et al. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: A potential mechanism for the prevention of vascular cognitive impairment. GeroScience 2019, 41, 619–630. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Choi, J.-Y.; Mankhong, S.; Moon, S.; Kim, S.; Koh, Y.H.; Kim, J.-H.; Kang, J.-H. Sirtuin 1-dependent regulation of high mobility box 1 in hypoxia–reoxygenated brain microvascular endothelial cells: Roles in neuronal amyloidogenesis. Cell Death Dis. 2020, 11, 1072. [Google Scholar] [CrossRef]
- Wang, X.-F.; Liu, D.-X.; Liang, Y.; Xing, L.-L.; Zhao, W.-H.; Qin, X.-X.; Shang, D.-S.; Li, B.; Fang, W.-G.; Cao, L.; et al. Cystatin C Shifts APP Processing from Amyloid-β Production towards Non-Amyloidgenic Pathway in Brain Endothelial Cells. PLoS ONE 2016, 11, e0161093. [Google Scholar] [CrossRef]
- Piao, L.; Zhao, G.; Zhu, E.; Inoue, A.; Shibata, R.; Lei, Y.; Hu, L.; Yu, C.; Yang, G.; Wu, H.; et al. Chronic Psychological Stress Accelerates Vascular Senescence and Impairs Ischemia-Induced Neovascularization: The Role of Dipeptidyl Peptidase-4/Glucagon-Like Peptide-1-Adiponectin Axis. J. Am. Heart Assoc. 2017, 6, e006421. [Google Scholar] [CrossRef]
- Giuffrida, M.L.; Copani, A.; Rizzarelli, E. A promising connection between BDNF and Alzheimer’s disease. Aging 2018, 10, 1791–1792. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Chen, Y.; Wang, Y.; Ren, J.; Zhang, C. Depressive symptoms in schizophrenia patients: A possible relationship between SIRT1 and BDNF. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 95, 109673. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Im, E. Regulation of miRNAs by Natural Antioxidants in Cardiovascular Diseases: Focus on SIRT1 and eNOS. Antioxidants 2021, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Mattagajasingh, I.; Kim, C.-S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.-B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef]
- Wu, C.H.; Chiu, Y.L.; Hsieh, C.Y.; Tsung, G.S.; Wu, L.S.; Cheng, C.C.; Tsai, T.N. Cilostazol Induces eNOS and TM Expression via Activation with Sirtuin 1/Krüppel-like Factor 2 Pathway in Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 10287. [Google Scholar] [CrossRef]
- Xia, N.; Strand, S.; Schlufter, F.; Siuda, D.; Reifenberg, G.; Kleinert, H.; Förstermann, U.; Li, H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide 2013, 32, 29–35. [Google Scholar] [CrossRef]
- Ota, H.; Eto, M.; Kano, M.R.; Kahyo, T.; Setou, M.; Ogawa, S.; Iijima, K.; Akishita, M.; Ouchi, Y. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arter. Thromb. Vasc. Biol. 2010, 30, 2205–2211. [Google Scholar] [CrossRef]
- Testai, L.; Citi, V.; Martelli, A.; Brogi, S.; Calderone, V. Role of hydrogen sulfide in cardiovascular ageing. Pharmacol. Res. 2020, 160, 105125. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, B.; Deng, Q.; Cao, J.; Ding, X.; Liu, Q.; Zhang, Y.; Ye, C.; Deng, C.; Qiu, L.; et al. Nicotinamide riboside relieves the severity of experimental necrotizing enterocolitis by regulating endothelial function via eNOS deacetylation. Free. Radic. Biol. Med. 2022, 184, 218–229. [Google Scholar] [CrossRef]
- Tseng, S.-Y.; Chang, H.-Y.; Li, Y.-H.; Chao, T.-H. Effects of Cilostazol on Angiogenesis in Diabetes through Adiponectin/Adiponectin Receptors/Sirtuin1 Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 14839. [Google Scholar] [CrossRef]
- Nian, S.; Mi, Y.; Ren, K.; Wang, S.; Li, M.; Yang, D. The inhibitory effects of Dulaglutide on cellular senescence against high glucose in human retinal endothelial cells. Hum. Cell 2022, 35, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Mi, D.H.; Fang, H.J.; Zheng, G.H.; Liang, X.H.; Ding, Y.R.; Liu, X.; Liu, L.P. DPP-4 inhibitors promote proliferation and migration of rat brain microvascular endothelial cells under hypoxic/high-glucose conditions, potentially through the SIRT1/HIF-1/VEGF pathway. CNS Neurosci. Ther. 2019, 25, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.W.; Man, R.Y.; Gao, Y.; Vanhoutte, P.M.; Leung, S.W. Reduced activity of SKC a and Na-K ATPase underlies the accelerated impairment of EDH-type relaxations in mesenteric arteries of aging spontaneously hypertensive rats. Pharmacol. Res. Perspect. 2015, 3, e00150. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.W.S.; Vanhoutte, P.M. Endothelium-dependent hyperpolarization: Age, gender and blood pressure, do they matter? Acta Physiol. 2017, 219, 108–123. [Google Scholar] [CrossRef]
- Nawate, S.; Fukao, M.; Sakuma, I.; Soma, T.; Nagai, K.; Takikawa, O.; Miwa, S.; Kitabatake, A. Reciprocal changes in endothelium-derived hyperpolarizing factor- and nitric oxide-system in the mesenteric artery of adult female rats following ovariectomy. Br. J. Pharmacol. 2005, 144, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Bendale, D.S.; Karpe, P.A.; Chhabra, R.; Shete, S.P.; Shah, H.; Tikoo, K. 17-β Oestradiol prevents cardiovascular dysfunction in post-menopausal metabolic syndrome by affecting SIRT1/AMPK/H3 acetylation. Br. J. Pharmacol. 2013, 170, 779–795. [Google Scholar] [CrossRef]
- Shimabukuro, M. SIRT1 and Gender Differences in Atherosclerotic Cardiovascular Disease. J. Atheroscler. Thromb. 2020, 27, 8–10. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Lee, H.Y.; Min, K.J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef]
- Zhang, H.N.; Li, L.; Gao, P.; Chen, H.Z.; Zhang, R.; Wei, Y.S.; Liu, D.P.; Liang, C.C. Involvement of the p65/RelA subunit of NF-kappaB in TNF-alpha-induced SIRT1 expression in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2010, 397, 569–575. [Google Scholar] [CrossRef]
- Caon, I.; Bartolini, B.; Moretto, P.; Parnigoni, A.; Caravà, E.; Vitale, D.L.; Alaniz, L.; Viola, M.; Karousou, E.; De Luca, G.; et al. Sirtuin 1 reduces hyaluronan synthase 2 expression by inhibiting nuclear translocation of NF-κB and expression of the long-noncoding RNA HAS2-AS1. J. Biol. Chem. 2020, 295, 3485–3496. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Tajkhorshid, E.; Chen, L.F. Functional interplay between acetylation and methylation of the RelA subunit of NF-kappaB. Mol. Cell Biol. 2010, 30, 2170–2180. [Google Scholar] [CrossRef]
- Dai, S.H.; Chen, L.J.; Qi, W.H.; Ye, C.L.; Zou, G.W.; Liu, W.C.; Yu, B.T.; Tang, J. microRNA-145 Inhibition Upregulates SIRT1 and Attenuates Autophagy in a Mouse Model of Lung Ischemia/Reperfusion Injury via NF-κB-dependent Beclin 1. Transplantation 2021, 105, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Kowluru, R.A. Role of PARP-1 as a novel transcriptional regulator of MMP-9 in diabetic retinopathy. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1761–1769. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, D.; Yang, F.; Xia, Y.; Cai, S.; Liao, X.; Deng, W.; Wu, C. Hydrogen-rich saline upregulates the Sirt1/NF-κB signaling pathway and reduces vascular endothelial glycocalyx shedding in sepsis-induced acute kidney injury. Shock 2024, 62, 416–425. [Google Scholar] [CrossRef]
- Dragovich, M.A.; Chester, D.; Fu, B.M.; Wu, C.; Xu, Y.; Goligorsky, M.S.; Zhang, X.F. Mechanotransduction of the endothelial glycocalyx mediates nitric oxide production through activation of TRP channels. Am. J. Physiol. Cell. Physiol. 2016, 311, C846–C853. [Google Scholar] [CrossRef]
- Lipphardt, M.; Dihazi, H.; Müller, G.A.; Goligorsky, M.S. Fibrogenic Secretome of Sirtuin 1-Deficient Endothelial Cells: Wnt, Notch and Glycocalyx Rheostat. Front. Physiol. 2018, 9, 1325. [Google Scholar] [CrossRef]
- Ju, R.; Zhuang, Z.W.; Zhang, J.; Lanahan, A.A.; Kyriakides, T.; Sessa, W.C.; Simons, M. Angiopoietin-2 secretion by endothelial cell exosomes: Regulation by the phosphatidylinositol 3-kinase (PI3K)/Akt/endothelial nitric oxide synthase (eNOS) and syndecan-4/syntenin pathways. J. Biol. Chem. 2014, 289, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Wu, J. Dihydroartemisinin ameliorates cerebral I/R injury in rats via regulating VWF and autophagy-mediated SIRT1/FOXO1 pathway. Open Med. 2023, 18, 20230698. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Fozouni, P.; Evjen, G.; Chandra, V.; Jiang, J.; Lu, C.; Nicastri, M.; Bretz, C.; Winkler, J.D.; et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat. Cell Biol. 2020, 22, 1170–1179. [Google Scholar] [CrossRef]
- Li, Y.; Cui, W.; Song, B.; Ye, X.; Li, Z.; Lu, C. Autophagy-Sirtuin1(SIRT1) Alleviated the Coronary Atherosclerosis (AS) in Mice through Regulating the Proliferation and Migration of Endothelial Progenitor Cells (EPCs) via wnt/β-catenin/GSK3β Signaling Pathway. J. Nutr. Health Aging 2022, 26, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Baeken, M.W.; Schwarz, M.; Kern, A.; Moosmann, B.; Hajieva, P.; Behl, C. The selective degradation of sirtuins via macroautophagy in the MPP+ model of Parkinson’s disease is promoted by conserved oxidation sites. Cell Death Discov. 2021, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.; Wang, C.; Zhang, S.; Gao, Q.; Wang, L.; Ma, L. NaSH increases SIRT1 activity and autophagy flux through sulfhydration to protect SH-SY5Y cells induced by MPP~+. Cell Cycle 2020, 19, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Li, L.; Guo, C.; Zhang, Y.; Zhao, L.; Tao, Z.; Zhang, H.; Chen, S. MicroRNA-141-3p reduces pulmonary hypoxia/reoxygenation injury through suppression of Beclin-1-dependent autophagy. Aging 2024, 16, 1352–1373. [Google Scholar] [CrossRef]
- Mendelsohn, A.R.; Larrick, J.W. The NAD+/PARP1/SIRT1 Axis in Aging. Rejuvenation Res. 2017, 20, 244–247. [Google Scholar] [CrossRef]
- Bai, P.; Cantó, C.; Oudart, H.; Brunyánszki, A.; Cen, Y.; Thomas, C.; Yamamoto, H.; Huber, A.; Kiss, B.; Houtkooper, R.H.; et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011, 13, 461–468. [Google Scholar] [CrossRef]
- Fan, C.; Ma, Q.; Xu, M.; Qiao, Y.; Zhang, Y.; Li, P.; Bi, Y.; Tang, M. Ginsenoside Rb1 Attenuates High Glucose-Induced Oxidative Injury via the NAD-PARP-SIRT Axis in Rat Retinal Capillary Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 4936. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, H.; Li, J.; Li, T.; Zheng, B.; Zheng, Y.; Jin, H.; He, Y.; Gu, Q.; Xu, X. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes 2012, 61, 217–228. [Google Scholar] [CrossRef]
- Nemoto, S.; Fergusson, M.M.; Finkel, T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J. Biol. Chem. 2005, 280, 16456–16460. [Google Scholar] [CrossRef]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef]
- Hou, J.; Wang, S.; Shang, Y.C.; Chong, Z.Z.; Maiese, K. Erythropoietin employs cell longevity pathways of SIRT1 to foster endothelial vascular integrity during oxidant stress. Curr. Neurovascular Res. 2011, 8, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Z.; Cai, L. Diabetic cardiomyopathy and its prevention by nrf2: Current status. Diabetes Metab. J. 2014, 38, 337–345. [Google Scholar] [CrossRef]
- Holmström, K.M.; Kostov, R.V.; Dinkova-Kostova, A.T. The multifaceted role of Nrf2 in mitochondrial function. Curr. Opin. Toxicol. 2016, 1, 80–91. [Google Scholar] [CrossRef]
- Kratz, E.M.; Sołkiewicz, K.; Kubis-Kubiak, A.; Piwowar, A. Sirtuins as Important Factors in Pathological States and the Role of Their Molecular Activity Modulators. Int. J. Mol. Sci. 2021, 22, 630. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Ubaid, S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation 2020, 43, 1589–1598. [Google Scholar] [CrossRef]
- Huang, K.; Gao, X.; Wei, W. The crosstalk between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a positive feedback loop to inhibit FN and TGF-β1 expressions in rat glomerular mesangial cells. Exp. Cell Res. 2017, 361, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Baldelli, S.; Pagliei, B.; Cannata, S.M.; Rotilio, G.; Ciriolo, M.R. p53 orchestrates the PGC-1α-mediated antioxidant response upon mild redox and metabolic imbalance. Antioxidants Redox Signal. 2013, 18, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Olmos, Y.; Sanchez-Gomez, F.J.; Wild, B.; Garcia-Quintans, N.; Cabezudo, S.; Lamas, S.; Monsalve, M. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxidants Redox Signal. 2013, 19, 1507–1521. [Google Scholar] [CrossRef]
- Thounaojam, M.C.; Jadeja, R.N.; Warren, M.; Powell, F.L.; Raju, R.; Gutsaeva, D.; Khurana, S.; Martin, P.M.; Bartoli, M. MicroRNA-34a (miR-34a) Mediates Retinal Endothelial Cell Premature Senescence through Mitochondrial Dysfunction and Loss of Antioxidant Activities. Antioxidants 2019, 8, 328. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.; Guo, W.; Tan, H. FOXO6 transcription inhibition of CTRP3 promotes OGD/R-triggered cardiac microvascular endothelial barrier disruption via SIRT1/Nrf2 signalling. Folia Morphol. 2024, 83, 125–138. [Google Scholar] [CrossRef]
- Chai, D.; Zhang, L.; Xi, S.; Cheng, Y.; Jiang, H.; Hu, R. Nrf2 Activation Induced by Sirt1 Ameliorates Acute Lung Injury After Intestinal Ischemia/Reperfusion Through NOX4-Mediated Gene Regulation. Cell. Physiol. Biochem. 2018, 46, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Akil, A.; Gutiérrez-García, A.K.; Guenter, R.; Rose, J.B.; Beck, A.W.; Chen, H.; Ren, B. Notch Signaling in Vascular Endothelial Cells, Angiogenesis, and Tumor Progression: An Update and Prospective. Front. Cell Dev. Biol. 2021, 9, 642352. [Google Scholar] [CrossRef]
- Kume, T. Novel insights into the differential functions of Notch ligands in vascular formation. J. Angiogenesis Res. 2009, 1, 8. [Google Scholar] [CrossRef]
- Wang, P.; Du, H.; Zhou, C.C.; Song, J.; Liu, X.; Cao, X.; Mehta, J.L.; Shi, Y.; Su, D.F.; Miao, C.Y. Intracellular NAMPT-NAD+-SIRT1 cascade improves post-ischaemic vascular repair by modulating Notch signalling in endothelial progenitors. Cardiovasc. Res. 2021, 117, 2308. [Google Scholar] [CrossRef]
- Xi, H.; Wang, C.; Li, Q.; Ye, Q.; Zhu, Y.; Mao, Y. S-Propargyl-Cysteine Ameliorates Peripheral Nerve Injury through Microvascular Reconstruction. Antioxidants 2023, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-Inducible Factor 1 and Cardiovascular Disease. Annu. Rev. Physiol. 2014, 76, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Hou, J.; Li, Y.; Mou, S.; Wang, Z.; Horch, R.E.; Sun, J.; Yuan, Q. The pro-angiogenic role of hypoxia inducible factor stabilizer FG-4592 and its application in an in vivo tissue engineering chamber model. Sci. Rep. 2019, 9, 6035. [Google Scholar] [CrossRef]
- Lim, J.-H.; Lee, Y.-M.; Chun, Y.-S.; Chen, J.; Kim, J.-E.; Park, J.-W. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol. Cell 2010, 38, 864–878. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, L.; Sun, S.; Zhang, Z. Gliquidone improves retinal injury to relieve diabetic retinopathy via regulation of SIRT1/Notch1 pathway. BMC Ophthalmol. 2021, 21, 451. [Google Scholar] [CrossRef]
- Arunachalam, G.; Lakshmanan, A.P.; Samuel, S.M.; Triggle, C.R.; Ding, H. Molecular Interplay between microRNA-34a and Sirtuin1 in Hyperglycemia-Mediated Impaired Angiogenesis in Endothelial Cells: Effects of Metformin. J. Pharmacol. Exp. Ther. 2016, 356, 314–323. [Google Scholar] [CrossRef]
- Yuen, D.A.; Zhang, Y.; Thai, K.; Spring, C.; Chan, L.; Guo, X.; Advani, A.; Sivak, J.M.; Gilbert, R.E. Angiogenic dysfunction in bone marrow-derived early outgrowth cells from diabetic animals is attenuated by SIRT1 activation. STEM CELLS Transl. Med. 2012, 1, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Gao, Z.; Zhu, C.; Peng, Z.; Song, M.; Li, L. Overexpression of histone deacetylase SIRT1 exerts an antiangiogenic role in diabetic retinopathy via miR-20a elevation and YAP/HIF1α/VEGFA depletion. Am. J. Physiol. Metab. 2020, 319, E932–E943. [Google Scholar] [CrossRef]
- Lin, Y.; Li, L.; Liu, J.; Zhao, X.; Ye, J.; Reinach, P.S.; Qu, J.; Yan, D. SIRT1 Deletion Impairs Retinal Endothelial Cell Migration Through Downregulation of VEGF-A/VEGFR-2 and MMP14. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5431–5440. [Google Scholar] [CrossRef] [PubMed]
- Guillot, E.; Lemay, A.; Allouche, M.; Silva, S.V.; Coppola, H.; Sabatier, F.; Dignat-George, F.; Sarre, A.; Peyter, A.-C.; Simoncini, S.; et al. Resveratrol Reverses Endothelial Colony-Forming Cell Dysfunction in Adulthood in a Rat Model of Intrauterine Growth Restriction. Int. J. Mol. Sci. 2023, 24, 9747. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, G.; Wang, Y.; Gong, Y.; Wang, Y. SIRT1 activation alleviates brain microvascular endothelial dysfunction in peroxisomal disorders. Int. J. Mol. Med. 2019, 44, 995–1005. [Google Scholar] [CrossRef]
- Fu, C.; Hao, S.; Xu, X.; Zhou, J.; Liu, Z.; Lu, H.; Wang, L.; Jin, W.; Li, S. Activation of SIRT1 ameliorates LPS-induced lung injury in mice via decreasing endothelial tight junction permeability. Acta Pharmacol. Sin. 2019, 40, 630–641. [Google Scholar] [CrossRef]
- Feng, S.; Zou, L.; Wang, H.; He, R.; Liu, K.; Zhu, H. RhoA/ROCK-2 Pathway Inhibition and Tight Junction Protein Upregulation by Catalpol Suppresses Lipopolysaccaride-Induced Disruption of Blood-Brain Barrier Permeability. Molecules 2018, 23, 2371. [Google Scholar] [CrossRef]
- Bolognin, S.; Lorenzetto, E.; Diana, G.; Buffelli, M. The potential role of rho GTPases in Alzheimer’s disease pathogenesis. Mol. Neurobiol. 2014, 50, 406–422. [Google Scholar] [CrossRef]
- Bray, M.A.; Sartain, S.E.; Gollamudi, J.; Rumbaut, R.E. Microvascular thrombosis: Experimental and clinical implications. Transl. Res. 2020, 225, 105–130. [Google Scholar] [CrossRef] [PubMed]
- Vink, H.; Constantinescu, A.A.; Spaan, J.A. Oxidized lipoproteins degrade the endothelial surface layer: Implications for platelet-endothelial cell adhesion. Circulation 2000, 101, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Sato, W.; Yoshimura, A.; Zhang, L.; Kosugi, T.; Campbell-Thompson, M.; Kojima, H.; Croker, B.P.; Nakagawa, T. Endothelial von Willebrand factor release due to eNOS deficiency predisposes to thrombotic microangiopathy in mouse aging kidney. Am. J. Pathol. 2010, 176, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Eisa, M.; Fathy, M.; Abuo-Rahma, G.; Abdel-Aziz, M.; Nazmy, M.H. Anti-Proliferative and Pro-Apoptotic Activities of Synthesized 3,4,5 Tri-Methoxy Ciprofloxacin Chalcone Hybrid, through p53 Up-Regulation in HepG2 and MCF7 Cell Lines. Asian Pac. J. Cancer Prev. 2021, 22, 3393–3404. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Ali, F.; Bailey, L.; Moreno, L.; Harrington, L.S. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp. Physiol. 2008, 93, 141–147. [Google Scholar] [CrossRef]
- Ricciotti, E.; Yu, Y.; Grosser, T.; FitzGerald, G.A. COX-2, the dominant source of prostacyclin. Proc. Natl. Acad. Sci. USA 2013, 110, E183. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.; Pfeffer, M.A.; Wittes, J.; Fowler, R.; Finn, P.; Anderson, W.F.; Zauber, A.; Hawk, E.; Bertagnolli, M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 2005, 352, 1071–1080. [Google Scholar] [CrossRef]
- FitzGerald, G.A. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat. Rev. Drug Discov. 2003, 2, 879–890. [Google Scholar] [CrossRef]
- Barbieri, S.S.; Amadio, P.; Gianellini, S.; Tarantino, E.; Zacchi, E.; Veglia, F.; Howe, L.R.; Weksler, B.B.; Mussoni, L.; Tremoli, E. Cyclooxygenase-2-derived prostacyclin regulates arterial thrombus formation by suppressing tissue factor in a sirtuin-1-dependent-manner. Circulation 2012, 126, 1373–1384. [Google Scholar] [CrossRef]
- Chlopicki, S.; Swies, J.; Mogielnicki, A.; Buczko, W.; Bartus, M.; Lomnicka, M.; Adamus, J.; Gebicki, J. 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway. Br. J. Pharmacol. 2007, 152, 230–239. [Google Scholar] [CrossRef]
- Kumari, S.; Chaurasia, S.N.; Nayak, M.K.; Mallick, R.L.; Dash, D. Sirtuin Inhibition Induces Apoptosis-like Changes in Platelets and Thrombocytopenia. J. Biol. Chem. 2015, 290, 12290–12299. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Bae, J.U.; Kim, I.S.; Chang, C.L.; Oh, S.O.; Kim, C.D. SIRT1 prevents pulmonary thrombus formation induced by arachidonic acid via downregulation of PAF receptor expression in platelets. Platelets 2016, 27, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Unsworth, A.; Jones, S. BS38 SIRT1: A novel regulator of integrin αiibβ3 and actin cytoskeleton dynamics in platelets. Heart 2023, 109 (Suppl. S3), A271–A272. [Google Scholar]
- Lan, Y.; Dong, M.; Li, Y.; Diao, Y.; Chen, Z.; Li, Y. SIRT1-induced deacetylation of Akt expedites platelet phagocytosis and delays HEMEC aging. Mol. Ther. Nucleic Acids 2021, 23, 1323–1333. [Google Scholar] [CrossRef]
- van der Meer, A.J.; Scicluna, B.P.; Moerland, P.D.; Lin, J.; Jacobson, E.W.; Vlasuk, G.P.; van der Poll, T. The Selective Sirtuin 1 Activator SRT2104 Reduces Endotoxin-Induced Cytokine Release and Coagulation Activation in Humans. Crit. Care Med. 2015, 43, e199–e202. [Google Scholar] [CrossRef]
- Brown, K.; Theofanous, D.; Britton, R.G.; Aburido, G.; Pepper, C.; Undru, S.S.; Howells, L. Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities. Int. J. Mol. Sci. 2024, 25, 747. [Google Scholar] [CrossRef]
- Pollack, R.M.; Barzilai, N.; Anghel, V.; Kulkarni, A.S.; Golden, A.; O’broin, P.; Sinclair, D.A.; Bonkowski, M.S.; Coleville, A.J.; Powell, D.; et al. Resveratrol Improves Vascular Function and Mitochondrial Number but Not Glucose Metabolism in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1703–1709. [Google Scholar] [CrossRef]
- Neves, A.R.; Queiroz, J.F.; Reis, S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J. Nanobiotechnol. 2016, 14, 27. [Google Scholar] [CrossRef]
- Saleem, Z.; Rehman, K.; Akash, M.S.H. Role of Drug Delivery System in Improving the Bioavailability of Resveratrol. Curr. Pharm. Des. 2022, 28, 1632–1642. [Google Scholar] [CrossRef]
- Annabi, B.; Lord-Dufour, S.; Vézina, A.; Béliveau, R. Resveratrol Targeting of Carcinogen-Induced Brain Endothelial Cell Inflammation Biomarkers MMP-9 and COX-2 is Sirt1-Independent. Drug Target Insights 2012, 6, DTI-S9442. [Google Scholar] [CrossRef]
- Grabowska, A.D.; Wątroba, M.; Witkowska, J.; Mikulska, A.; Sepúlveda, N.; Szukiewicz, D. Interplay between Systemic Glycemia and Neuroprotective Activity of Resveratrol in Modulating Astrocyte SIRT1 Response to Neuroinflammation. Int. J. Mol. Sci. 2023, 24, 11640. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Liang, H.; Liu, X. Coumestrol mitigates retinal cell inflammation, apoptosis, and oxidative stress in a rat model of diabetic retinopathy via activation of SIRT1. Aging 2021, 13, 5342–5357. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Yu, J.; Ni, W. Wogonoside alleviates high glucose-induced dysfunction of retinal microvascular endothelial cells and diabetic retinopathy in rats by up-regulating SIRT1. Nan Fang Yi Ke Da Xue Xue Bao 2022, 42, 463–472. [Google Scholar]
- He, F.; Chen, C.; Wang, Y.; Wang, S.; Lyu, S.; Jiao, J.; Huang, G.; Yang, J. Safranal acts as a neurorestorative agent in rats with cerebral ischemic stroke via upregulating SIRT1. Exp. Ther. Med. 2024, 27, 71. [Google Scholar] [CrossRef]
- Ruan, J.; Wang, L.; Dai, J.; Li, J.; Wang, N.; Seto, S. Hydroxysafflor Yellow a Promotes Angiogenesis in Rat Brain Microvascular Endothelial Cells Injured by Oxygen-glucose Deprivation/reoxygenation(OGD/R) through SIRT1-HIF-1α-VEGFA Signaling Pathway. Curr. Neurovascular Res. 2021, 18, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.Q.; Dai, Y.Y.; Song, L.J.; Ma, D.; Liu, K.X.; Miao, Z.Y.; Wei, R.H.; Yin, J.Z.; Ma, C.G.; Huang, J.J. Tetramethylpyrazine regulates angiogenesis of endothelial cells in cerebral ischemic stroke injury via SIRT1/VEGFA signaling pathway. Zhongguo Zhong Yao Za Zhi 2024, 49, 162–174. [Google Scholar]
- Niu, W.; Wu, F.; Cao, W.; Chen, Y.; Zhang, Y.; Chen, Y.; Ding, R.; Liang, C. Salvianolic Acid B Alleviates Limb Ischemia in Mice via Promoting SIRT1/PI3K/AKT Pathway-Mediated M2 Macrophage Polarization. Evid.-Based Complement. Altern. Med. 2022, 2022, 1112394. [Google Scholar] [CrossRef]
- Hu, L.; Guo, Y.; Song, L.; Wen, H.; Sun, N.; Wang, Y.; Qi, B.; Liang, Q.; Geng, J.; Liu, X.; et al. Nicotinamide riboside promotes Mfn2-mediated mitochondrial fusion in diabetic hearts through the SIRT1-PGC1α-PPARα pathway. Free. Radic. Biol. Med. 2022, 183, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Lin, X.; Xu, W.; Zheng, F.; Cai, J.; Yang, J.; Cui, Q.; Tang, C.; Cai, J.; Xu, G.; et al. Sulfhydrated Sirtuin-1 Increasing Its Deacetylation Activity Is an Essential Epigenetics Mechanism of Anti-Atherogenesis by Hydrogen Sulfide. Antioxid. Redox Signal. 2019, 30, 184–197. [Google Scholar] [CrossRef]
- Zheng, B.; Meng, J.; Zhu, Y.; Ding, M.; Zhang, Y.; Zhou, J. Melatonin enhances SIRT1 to ameliorate mitochondrial membrane damage by activating PDK1/Akt in granulosa cells of PCOS. J. Ovarian Res. 2021, 14, 152. [Google Scholar] [CrossRef]
- Martín-Ramírez, R.; González-Fernández, R.; Hernández, J.; Martín-Vasallo, P.; Palumbo, A.; Ávila, J. Celastrol and Melatonin Modify SIRT1, SIRT6 and SIRT7 Gene Expression and Improve the Response of Human Granulosa-Lutein Cells to Oxidative Stress. Antioxidants 2021, 10, 1871. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, J.J.; Unniappan, S. Phoenixin-20 Stimulates mRNAs Encoding Hypothalamo-Pituitary-Gonadal Hormones, is Pro-Vitellogenic, and Promotes Oocyte Maturation in Zebrafish. Sci. Rep. 2020, 10, 6264. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Gu, X.; Zhang, H.; Xu, X.; Chen, L. Phoenixin 20 ameliorates pulmonary arterial hypertension via inhibiting inflammation and oxidative stress. Aging 2024, 16, 5027–5037. [Google Scholar] [CrossRef]
- Shimada, T.; Furuta, H.; Doi, A.; Ariyasu, H.; Kawashima, H.; Wakasaki, H.; Nishi, M.; Sasaki, H.; Akamizu, T. Des-acyl ghrelin protects microvascular endothelial cells from oxidative stress-induced apoptosis through sirtuin 1 signaling pathway. Metabolism 2014, 63, 469–474. [Google Scholar] [CrossRef]
- Mortuza, R.; Feng, B.; Chakrabarti, S. miR-195 regulates SIRT1-mediated changes in diabetic retinopathy. Diabetologia 2014, 57, 1037–1046. [Google Scholar] [CrossRef]
- Zeng, Y.; Cui, Z.; Liu, J.; Chen, J.; Tang, S. MicroRNA-29b-3p Promotes Human Retinal Microvascular Endothelial Cell Apoptosis via Blocking SIRT1 in Diabetic Retinopathy. Front. Physiol. 2019, 10, 1621. [Google Scholar] [CrossRef] [PubMed]
- Radmanesh, F.; Abandansari, H.S.; Ghanian, M.H.; Pahlavan, S.; Varzideh, F.; Yakhkeshi, S.; Alikhani, M.; Moradi, S.; Braun, T.; Baharvand, H. Hydrogel-mediated delivery of microRNA-92a inhibitor polyplex nanoparticles induces localized angiogenesis. Angiogenesis 2021, 24, 657–676. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, Y.; Wang, G.; Li, B.; Zhou, H.; Qiu, J.; Qin, L. miR-29a-SIRT1-Wnt/β-Catenin Axis Regulates Tumor Progression and Survival in Hepatocellular Carcinoma. Biochem. Genet. 2023, 62, 1895–1913. [Google Scholar] [CrossRef]
- Li, J.; Yang, C.; Wang, Y. miR-126 overexpression attenuates oxygen-glucose deprivation/reperfusion injury by inhibiting oxidative stress and inflammatory response via the activation of SIRT1/Nrf2 signaling pathway in human umbilical vein endothelial cells. Mol. Med. Rep. 2021, 23, 165. [Google Scholar] [CrossRef]
- Huang, A.; Zheng, H.; Wu, Z.; Chen, M.; Huang, Y. Circular RNA-protein interactions: Functions, mechanisms, and identification. Theranostics 2020, 10, 3503–3517. [Google Scholar] [CrossRef]
- Liu, C.; Ge, H.M.; Liu, B.H.; Dong, R.; Shan, K.; Chen, X.; Yao, M.D.; Li, X.M.; Yao, J.; Zhou, R.M.; et al. Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 7455–7464. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, L.; Yang, M.; Wu, C.; Lan, R.; Wang, W.; Li, Y. Circ-SIRT1 inhibits cardiac hypertrophy via activating SIRT1 to promote autophagy. Cell Death Dis. 2021, 12, 1069. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Yu, Y.; Wang, L.; Dou, Y.Q.; Zhang, X.H.; Cui, Y.; Wang, H.Y.; Yong, Y.T.; Liu, Y.B.; Hu, H.J.; et al. circ-Sirt1 controls NF-κB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res. 2019, 47, 3580–3593. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Dong, Y.; Sun, G.; Yu, Y. Circ-Sirt1 inhibits vascular smooth muscle cells proliferation via the c-Myc/cyclin B1 axis. Cell Biol. Int. 2022, 46, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Li, C.-L.; Dou, Y.-Q.; Cao, L.; Zhang, X.-Y.; Zhang, W.-D.; Bi, Z.-Q.; Peng, Z.-Y.; Yan, A.-Q.; Han, M. circ-Sirt1 Decelerates Senescence by Inhibiting p53 Activation in Vascular Smooth Muscle Cells, Ameliorating Neointima Formation. Front. Cardiovasc. Med. 2021, 8, 724592. [Google Scholar] [CrossRef]
- Liu, H.; Tu, M.; Yin, Z.; Zhang, D.; Ma, J.; He, F. Unraveling the complexity of polycystic ovary syndrome with animal models. J. Genet. Genom. 2024, 51, 144–158. [Google Scholar] [CrossRef]
- Yin, W.-G.; Huang, B.-S.; Wu, L.-X. LncRNA SNHG12 inhibits miR-199a to upregulate SIRT1 to attenuate cerebral ischemia/reperfusion injury through activating AMPK signaling pathway. Neurosci. Lett. 2019, 690, 188–195. [Google Scholar] [CrossRef]
- Saikia, M.; Hatzoglou, M. The Many Virtues of tRNA-derived Stress-induced RNAs (tiRNAs): Discovering Novel Mechanisms of Stress Response and Effect on Human Health. J. Biol. Chem. 2015, 290, 29761–29768. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, X.; Su, X.; Su, X.; Hou, C. A novel dressing seeded with embryonic artery CD133+ cells and loaded with the Sirt1 agonist SRT1720 accelerates the healing of diabetic ischemic ulcers. Exp. Ther. Med. 2018, 15, 5243–5250. [Google Scholar] [CrossRef]
- Vachharajani, V.T.; Liu, T.; Brown, C.M.; Wang, X.; Buechler, N.L.; Wells, J.D.; Yoza, B.K.; E McCall, C. SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome. J. Leukoc. Biol. 2014, 96, 785–796. [Google Scholar] [CrossRef]
- Orecchia, A.; Scarponi, C.; Di Felice, F.; Cesarini, E.; Avitabile, S.; Mai, A.; Mauro, M.L.; Sirri, V.; Zambruno, G.; Albanesi, C.; et al. Sirtinol treatment reduces inflammation in human dermal microvascular endothelial cells. PLoS ONE 2011, 6, e24307. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Wang, N.; Zheng, B.; Li, T.; Gu, Q.; Xu, X.; Zheng, Z. Fenofibrate suppresses cellular metabolic memory of high glucose in diabetic retinopathy via a sirtuin 1-dependent signalling pathway. Mol. Med. Rep. 2015, 12, 6112–6118. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, G.; Samuel, S.M.; Marei, I.; Ding, H.; Triggle, C.R. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br. J. Pharmacol. 2014, 171, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Du, J.; Dong, Y.; Wang, M.; Wang, L.; Zhao, J. Liraglutide prevents cellular senescence in human retinal endothelial cells (HRECs) mediated by SIRT1: An implication in diabetes retinopathy. Hum. Cell 2024, 37, 666–674. [Google Scholar] [CrossRef]
- Wiciński, M.; Górski, K.; Walczak, M.; Wódkiewicz, E.; Słupski, M.; Pawlak-Osińska, K.; Malinowski, B. Neuroprotective Properties of Linagliptin: Focus on Biochemical Mechanisms in Cerebral Ischemia, Vascular Dysfunction and Certain Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 4052. [Google Scholar] [CrossRef]
- Abouhish, H.; Thounaojam, M.C.; Jadeja, R.N.; Gutsaeva, D.R.; Powell, F.L.; Khriza, M.; Martin, P.M.; Bartoli, M. Inhibition of HDAC6 Attenuates Diabetes-Induced Retinal Redox Imbalance and Microangiopathy. Antioxidants 2020, 9, 599. [Google Scholar] [CrossRef]

| Natural SIRT1 Intervention | Primary Mechanisms | Reference |
|---|---|---|
| Boysenberry polyphenols | Enhanced capillarization and BAT function, increased systemic glucose tolerance, and optimized thermogenesis. | [13] |
| Lycopene | Increase skeletal muscle capillary density, prevent MEC damage | [22] |
| Stachydrine | Inducing RMEC autophagy, suppressing ROS and inflammation, | [46] |
| Ligustrazine | Promote angiogenesis of BMECs while suppressing CMD, platelet activation, inflammation, and coronary micro-embolization | [54,176] |
| 14,15 epoxyeicosatreinoic acid | Promote mitophagy | [67] |
| Hydroxysafflor Yellow A | Promote BBB integrity, angiogenesis, and survival of BMECs | [66,175] |
| Coumestrol | Suppressing inflammation, oxidative stress, and apoptosis in human RMECs | [172] |
| Wogonoside | Suppressing abnormal angiogenesis, permeability, proliferation, and migration of RMECs | [173] |
| Safranal | Promote survival, proliferation, and angiogenesis in BMECs | [174] |
| Salvianolic acid B | Promote anti-inflammatory M2 macrophage polarization, angiogenesis, muscle capillary density, and blood perfusion. | [177] |
| NAD+ Modulating Intervention | Primary Mechanisms | Reference |
|---|---|---|
| Sodium hydrosulfide+ NMN | Synergistic SIRT1 activation, promoting exercise-induced capillarization of skeletal muscle | [17] |
| Nicotinamide mononucleotide (NMN) | Promote angiogenesis and suppress ROS production in CMECs, and increase exercise-induced skeletal muscle capillary density | [17,77] |
| Recombinant human nicotinamide mononucleotide adenylyl transferase | Enhanced BBB integrity | [63] |
| Nicotinamide riboside | Prevent intestinal MEC dysfunction and reduce ROS production under inflammatory conditions | [89] |
| S-propargyl-cysteine | Promote endogenous H2S production, upregulating SIRT1 and microvascular reconstruction following peripheral nerve injury | [135] |
| MicroRNA (miR) | Effect on SIRT1 and Microcirculation | Reference |
|---|---|---|
| miR-29 | Inhibits SIRT1, but is induced by regular exercise and associated with increased antioxidant activity and reduced endothelial dysfunction. | [18,188] |
| miR-377 | Inhibit SIRT1 expression aggravates cell cycle transition, angiogenesis, migration, and inflammation in human RMECs under HG conditions. | [27] |
| miR-195 | Increased under diabetic condition. In RMECs, inhibit SIRT1 expression, increasing apoptosis and reducing proliferation. In CMECs, reduced SIRT1 impairs myocardial function, causing oxidative stress and myocardial hypertrophy | [35,185] |
| miR-30b | Negatively regulate SIRT1, promoting pathological angiogenesis in proliferative diabetic retinopathy. | [38] |
| miR-221 | Inhibit SIRT1/Nrf2 signaling in human RMECs, promoting apoptosis under HG conditions. | [42] |
| miR-34a-5p | Suppress SIRT1 in CMECs induces platelet activation, inflammation, and CMD. | [54] |
| miR-16-5p | Inhibition of miR-16-5p downregulates SIRT1, exacerbating cerebral infarction in mice. | [65] |
| miR-145 | Inhibit SIRT1, inducing NF-kB mediated inflammation, autophagy, and lung injury in a pulmonary I/R model. | [103] |
| miR-141-3p | Ameliorated lung injury by inhibit SIRT1-induced pulmonary MEC beclin-1-dependent autophagy in mice pulmonary H/R model. | [114] |
| miR-34a | Decrease SIRT1 levels, diminish mitochondrial function antioxidant capacity, and induce senescence in human RMECs under HG conditions. | [129] |
| miR-29b-3p | Downregulate SIRT1, decrease human RMEC viability, and upregulate apoptosis under HG conditions. | [186] |
| miR-92a | Inhibition of miR-92a induced SIRT1 expression and induced angiogenesis in subcutaneous tissue, elevating capillary density in a chicken chorioallantoic membrane model. | [187] |
| miR-126 | Promote SIRT1/Nrf2 signaling, and attenuate oxidative/inflammatory response to OGD/R injury in HUVECs. | [189] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Law, M.; Wang, P.-C.; Zhou, Z.-Y.; Wang, Y. From Microcirculation to Aging-Related Diseases: A Focus on Endothelial SIRT1. Pharmaceuticals 2024, 17, 1495. https://doi.org/10.3390/ph17111495
Law M, Wang P-C, Zhou Z-Y, Wang Y. From Microcirculation to Aging-Related Diseases: A Focus on Endothelial SIRT1. Pharmaceuticals. 2024; 17(11):1495. https://doi.org/10.3390/ph17111495
Chicago/Turabian StyleLaw, Martin, Pei-Chun Wang, Zhong-Yan Zhou, and Yu Wang. 2024. "From Microcirculation to Aging-Related Diseases: A Focus on Endothelial SIRT1" Pharmaceuticals 17, no. 11: 1495. https://doi.org/10.3390/ph17111495
APA StyleLaw, M., Wang, P.-C., Zhou, Z.-Y., & Wang, Y. (2024). From Microcirculation to Aging-Related Diseases: A Focus on Endothelial SIRT1. Pharmaceuticals, 17(11), 1495. https://doi.org/10.3390/ph17111495





