Prunella vulgaris Extract Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia by Regulating Androgen Levels, Cell Proliferation, and Apoptosis
Abstract
:1. Introduction
2. Results
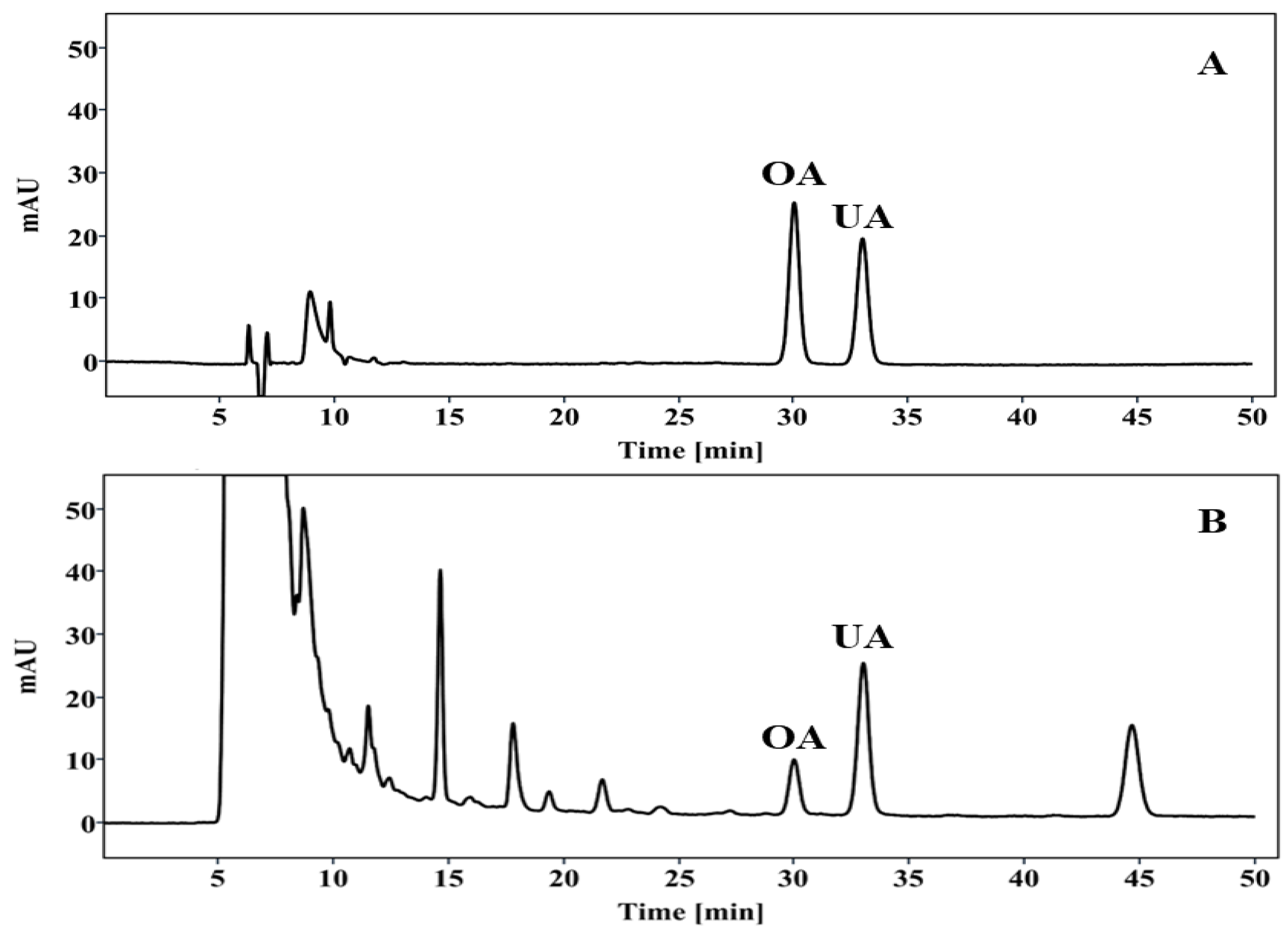
2.1. HPLC Analysis of PV Extract
2.2. PV Extract Reduces Prostate Relative Weight in Rat BPH Model
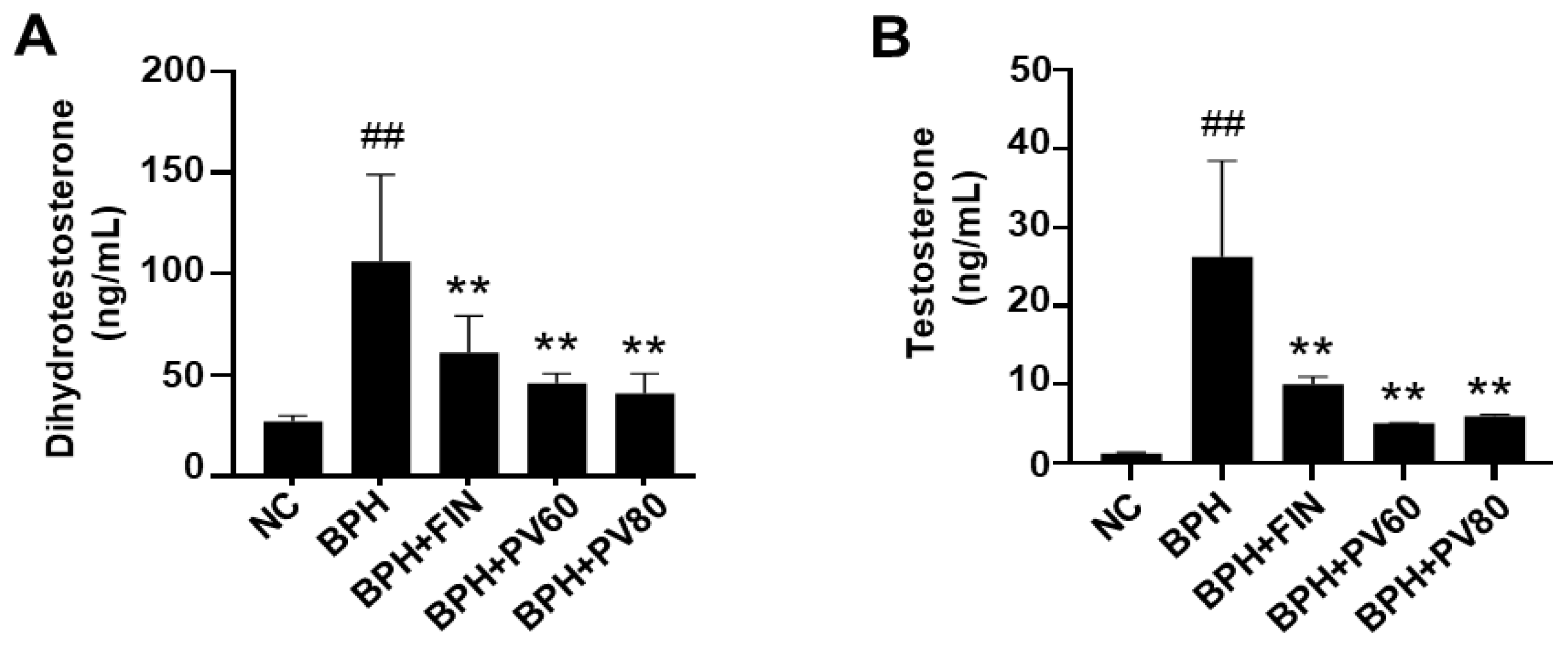
2.3. PV Extract Downregulates the Serum Levels of DHT and Testosterone in a Rat BPH Model
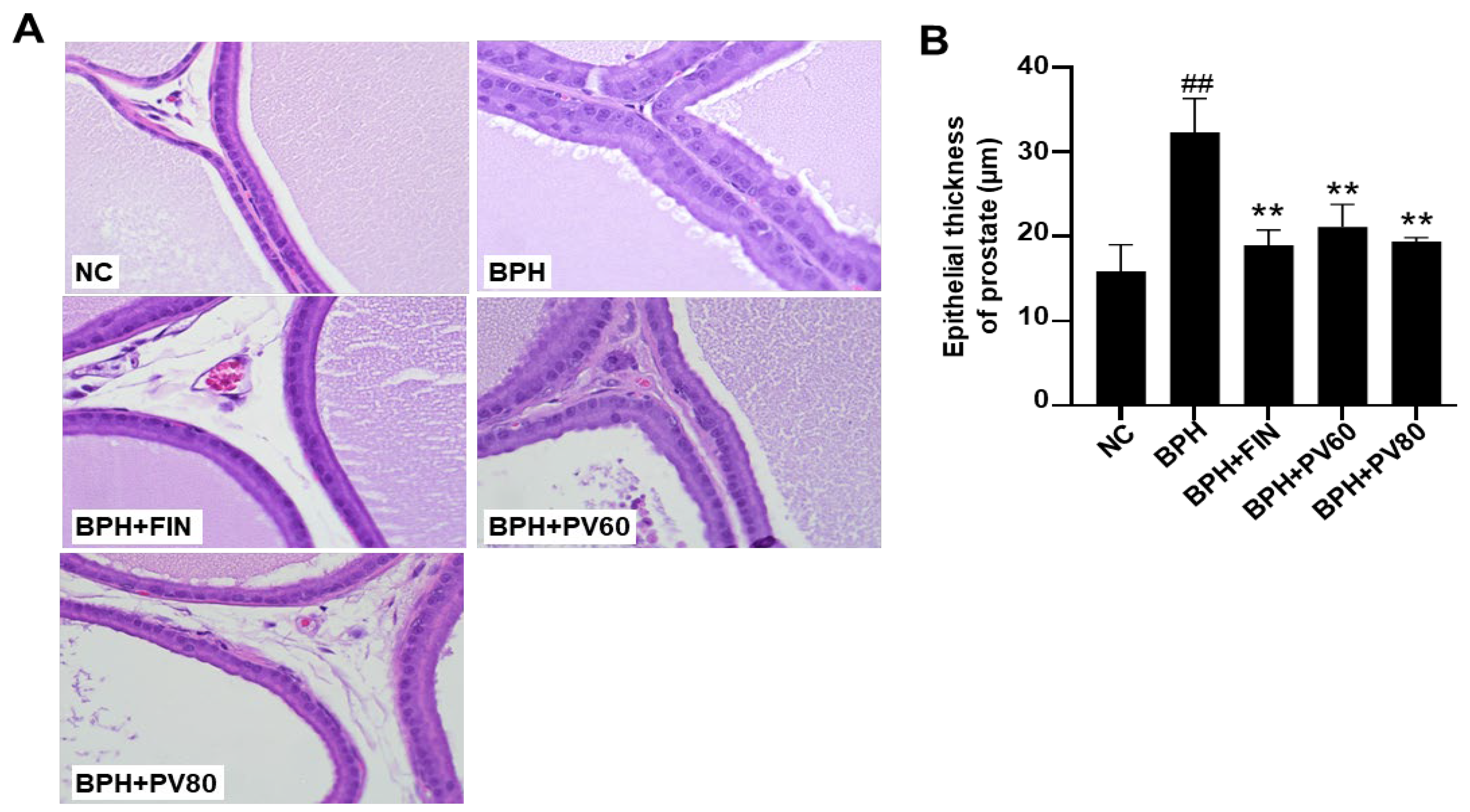
2.4. PV Extract Reduces the Epithelium Thickness of the Prostate Gland in a Rat BPH Model
2.5. PV Extract Suppresses the Proliferation of Prostate Cells in a Rat BPH Model
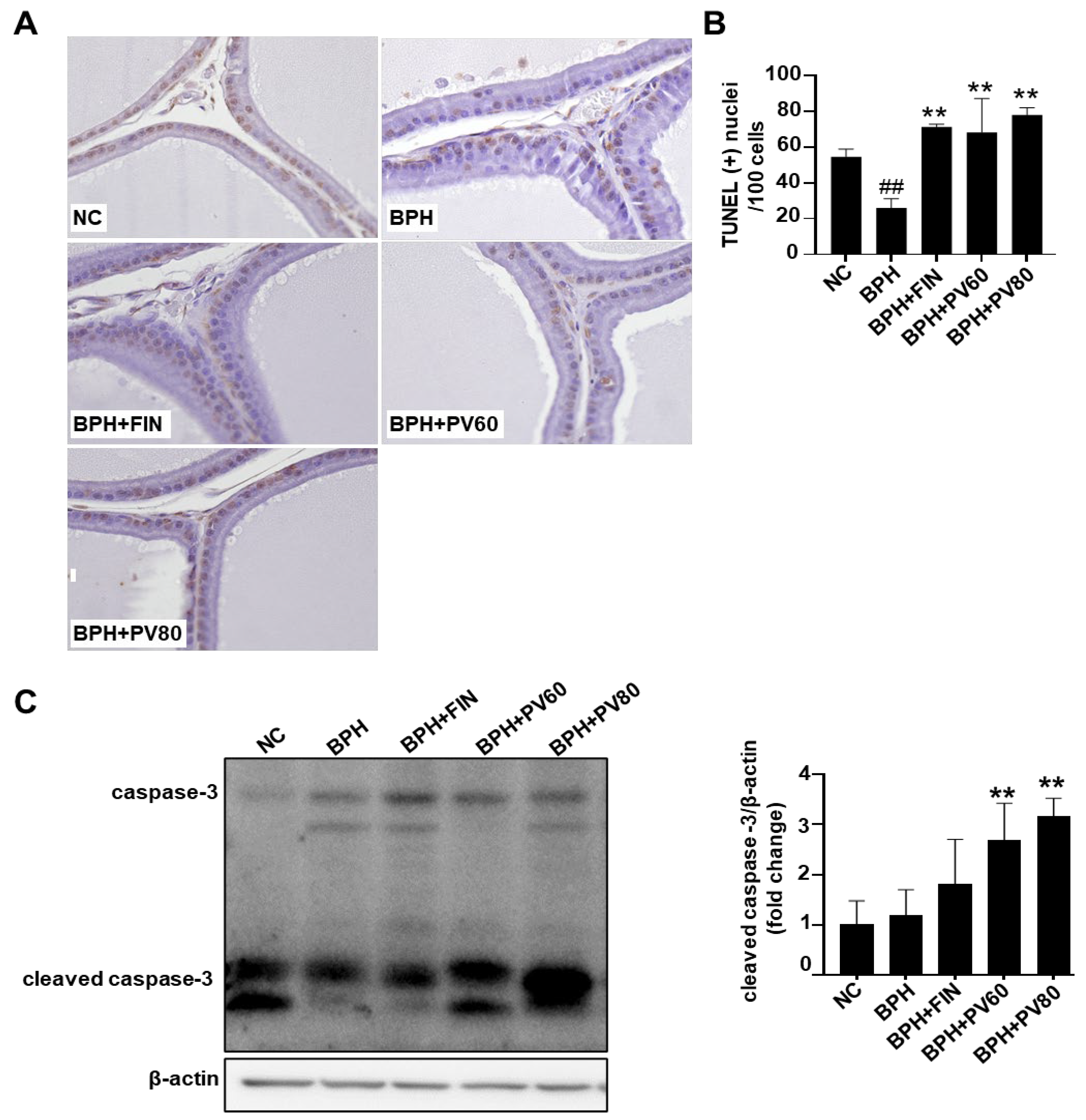
2.6. PV Extract Elevates Apoptosis of Prostatic Cells in Rat BPH Model
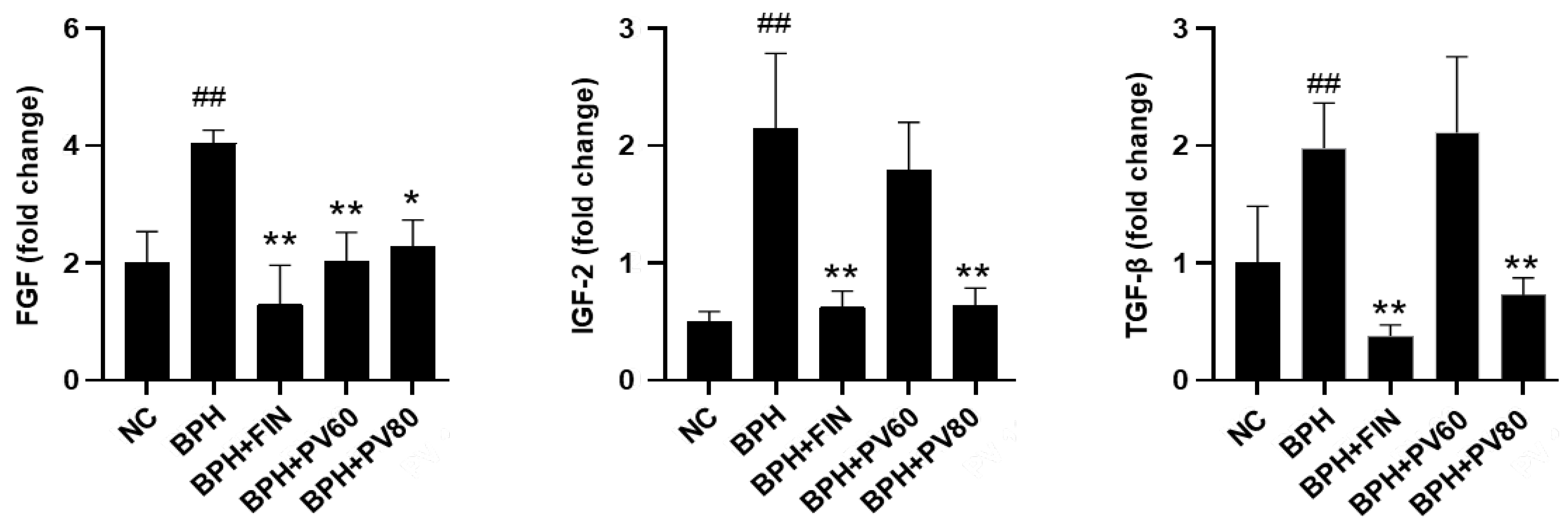
2.7. PV Extract Decreases the Expression Levels of Growth Factors in a Rat BPH Model
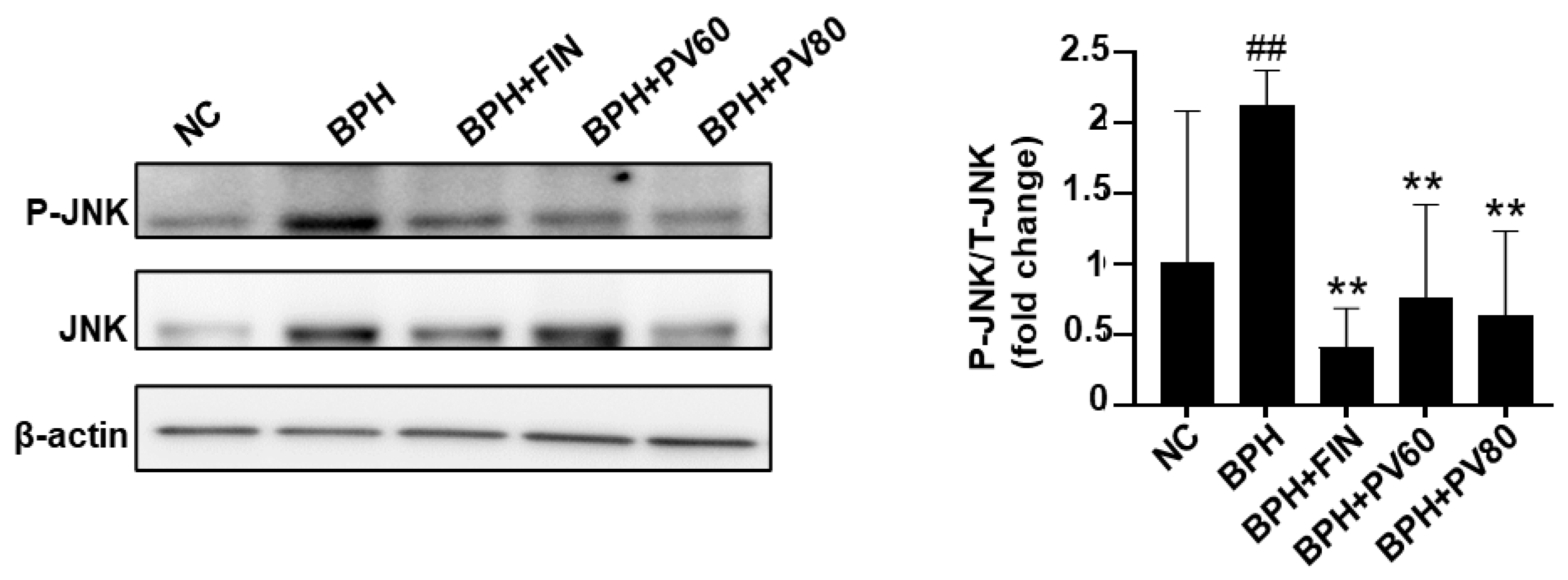
2.8. PV Extract Reduces JNK Activation in a Rat BPH Model
3. Discussion
4. Materials and Methods
4.1. Preparation of PV Extract
4.2. HPLC Analysis
4.3. Animals
4.4. Initiation of BPH in Rats and Treatment Design
4.5. Analysis Serum of DHT and Testosterone Levels
4.6. Histopathology
4.7. Immunohistochemical (IHC) Staining of Proliferative Cell Nuclear Antigen (PCNA)
4.8. TUNEL Assay
4.9. Western Blot Analysis
4.10. Quantitative Real-Time PCR (RT-qPCR)
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chughtai, B.; Forde, J.C.; Thomas, D.D.M.; Laor, L.; Hossack, T.; Woo, H.H.; Te, A.E.; Kaplan, S.A. Benign prostatic hyperplasia. Nat. Rev. Dis. Primers 2016, 2, 16031. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.J.; Coffey, D.S.; Walsh, P.C.; Ewing, L.L. The development of human benign prostatic hyperplasia with age. J. Urol. 1984, 132, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.J.; Fowler, F.J.; O’leary, M.P.; Bruskewitz, R.C.; Holtgrewe, H.L.; Mebust, W.K.; Cockett, A.T. The American Urological Association symptom index for benign prostatic hyperplasia. J. Urol. 1992, 148, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Bushman, W. Etiology, epidemiology, and natural history of benign prostatic hyperplasia. Urol. Clin. N. Am. 2009, 36, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, G.; Rittmaster, R.; Klocker, H. Dihydrotestosterone and the concept of 5α-reductase inhibition in human benign prostatic hyperplasia. World J. Urol. 2002, 19, 413–425. [Google Scholar] [CrossRef]
- Chatterjee, B. The role of the androgen receptor in the development of prostatic hyperplasia and prostate cancer. Mol. Cell. Biochem. 2003, 253, 89–101. [Google Scholar] [CrossRef]
- Lloyd, G.L.; Marks, J.M.; Ricke, W.A. Benign Prostatic Hyperplasia and Lower Urinary Tract Symptoms: What Is the Role and Significance of Inflammation? Curr. Urol. Rep. 2019, 20, 54. [Google Scholar] [CrossRef]
- McConnell, J.D. The pathophysiology of benign prostatic hyperplasia. J. Androl. 1991, 12, 356–363. [Google Scholar] [CrossRef]
- Isaacs, J.T.; Coffey, D.S. Etiology and disease process of benign prostatic hyperplasia. Prostate 1989, 15, 33–50. [Google Scholar] [CrossRef]
- Lawson, R.K. Role of growth factors in benign prostatic hyperplasia. Eur. Urol. 1997, 32 (Suppl. S1), 22–27. [Google Scholar]
- Scott Lucia, M.; Lambert, J.R. Growth factors in benign prostatic hyperplasia: Basic science implications. Curr. Urol. Rep. 2008, 9, 272–278. [Google Scholar] [CrossRef] [PubMed]
- McLaren, I.D.; Jerde, T.J.; Bushman, W. Role of interleukins, IGF and stem cells in BPH. Differentiation 2011, 82, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V.B. Various treatment options for benign prostatic hyperplasia: A current update. J. Mid-Life Health 2012, 3, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Steers, W.D. 5α-reductase activity in the prostate. Urology 2001, 58, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Blankstein, U.; BAsseldonk, V.; Elterman, D.S. BPH update: Medical versus interventional management. Can. J. Urol. 2016, 23 (Suppl. S1), 10–15. [Google Scholar] [PubMed]
- Quaresma, B.M.C.S.; Pimenta, A.R.; da Silva, A.C.S.; Pupo, A.S.; Romeiro, L.A.S.; Silva, C.L.M.; Noël, F. Revisiting the Pharmacodynamic Uroselectivity of α (1)-Adrenergic Receptor Antagonists. J. Pharmacol. Exp. Ther. 2019, 371, 106–112. [Google Scholar] [CrossRef]
- Kulig, K.; Malawska, B. Trends in the development of new drugs for treatment of benign prostatic hyperplasia. Curr. Med. Chem. 2006, 13, 3395–3416. [Google Scholar] [CrossRef]
- Facio, F.; Kashiwabuschi, R.; Nishi, Y.; Leao, R.; Mcdonnell, P.; Burnett, A. Benign prostatic hyperplasia. Clinical treatment can complicate cataract surgery. Int. Braz. J. Urol. 2010, 36, 563–570. [Google Scholar] [CrossRef]
- Wong, P.; Lawrentschuk, N.; Bolton, D.M. Phosphodiesterase 5 inhibitors in the management of benign prostatic hyperplasia and erectile dysfunction: The best of both worlds. Curr. Opin. Urol. 2009, 19, 7–12. [Google Scholar] [CrossRef]
- Laufer, M.; Shoenfeld, S. Phytotherapy and Natural Agents in Symptomatic Benign Prostatic Hyperplasia (BPH). Harefuah 2024, 163, 50–53. [Google Scholar]
- Pan, J.; Wang, H.; Chen, Y. Prunella vulgaris L.—A review of its ethnopharmacology, phytochemistry, quality control and pharmacological effects. Front. Pharmacol. 2022, 13, 903171. [Google Scholar] [CrossRef] [PubMed]
- Sukjamnong, S.; Chen, H.; Saad, S.; Santiyanont, R. Fimbristylis ovata and Artemisia vulgaris extracts inhibited AGE-mediated RAGE expression, ROS generation, and inflammation in THP-1 cells. Toxicol. Res. 2022, 38, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; Wang, X.-H.; Dai, Y.-Y.; Ma, M.-H.; Rahman, K.; Nian, H.; Zhang, H. Prunella vulgaris: A comprehensive review of chemical constituents, pharmacological effects and clinical applications. Curr. Pharm. Des. 2019, 25, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Rasool, R.; Ganai, B.A. Prunella vulgaris L.: A literature review on its therapeutic potentials. Pharmacologia 2013, 4, 441–448. [Google Scholar] [CrossRef]
- Mîrza, C.M.; Mîrza, T.-V.; Odagiu, A.C.M.; Uifălean, A.; But, A.E.; Pârvu, A.E.; Bulboacă, A.-E. Phytochemical Analysis and Antioxidant Effects of Prunella vulgaris in Experimental Acute Inflammation. Int. J. Mol. Sci. 2024, 25, 4843. [Google Scholar] [CrossRef]
- Similie, D.; Minda, D.; Bora, L.; Kroškins, V.; Lugiņina, J.; Turks, M.; Dehelean, C.A.; Danciu, C. An Update on Pentacyclic Triterpenoids Ursolic and Oleanolic Acids and Related Derivatives as Anticancer Candidates. Antioxidants 2024, 13, 952. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Palen, K.; Johnson, B.; Bui, D.; Xiong, D.; Pan, J.; Hu, M.; Wang, Y.; You, M. Cancer chemoprevention with PV-1, a novel Prunella vulgaris-containing herbal mixture that remodels the tumor immune microenvironment in mice. Front. Immunol. 2023, 14, 1196434. [Google Scholar] [CrossRef]
- Ning, N.; Nan, Y.; Chen, G.; Huang, S.; Lu, D.; Yang, Y.; Meng, F.; Yuan, L. Anti-Tumor Effects and Toxicity Reduction Mechanisms of Prunella vulgaris: A Comprehensive Review. Molecules 2024, 29, 1843. [Google Scholar] [CrossRef]
- Asiedu, B.; Anang, Y.; Nyarko, A.; Doku, D.A.; Amoah, B.Y.; Santa, S.; Ngala, R.A.; Asare, G.A. The role of sex steroid hormones in benign prostatic hyperplasia. Aging Male 2017, 20, 17–22. [Google Scholar] [CrossRef]
- Lee, G.H.; Lee, H.-Y.; Zhao, L.; Rashid, M.M.U.; Kim, M.K.; Jeong, Y.B.; Chae, H.-J.; Shin, Y.S. The Role of Reactive Oxygen Species, Inflammation, and Endoplasmic Reticulum Stress Response in the Finasteride Protective Effect against Benign Prostate Hyperplasia. World J. Mens. Health 2024, 42, 600–609. [Google Scholar] [CrossRef]
- Traish, A.M. Health Risks Associated with Long-Term Finasteride and Dutasteride Use: It’s Time to Sound the Alarm. World J. Mens. Health 2020, 38, 323–337. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Song, Y.; Kim, S.; Kong, H.; Kim, K. Alteration of Gut Microbes in Benign Prostatic Hyperplasia Model and Finasteride Treatment Model. Int. J. Mol. Sci. 2023, 24, 5904. [Google Scholar] [CrossRef] [PubMed]
- Busetto, G.M.; Del Giudice, F.; D’Agostino, D.; Romagnoli, D.; Minervini, A.; Rocco, B.; Antonelli, A.; Celia, A.; Schiavina, R.; Cindolo, L.; et al. Efficacy and safety of Finasteride (5 alpha-reductase inhibitor) monotherapy in patients with benign prostatic hyperplasia: A critical review of the literature. Arch. Ital. Urol. Androl. 2020, 91, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, M.; Taverna, G.; Grizzi, F. Is Artificial Intelligence the Key to Revolutionizing Benign Prostatic Hyperplasia Diagnosis and Management. Arch. Esp. Urol. 2023, 76, 643–656. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Genovese, T.; Cordaro, M.; Siracusa, R.; Gugliandolo, E.; Peritore, A.F.; Interdonato, L.; Crupi, R.; Cuzzocrea, S.; Di Paola, R.; et al. Palmitoylethanolamide/Baicalein Regulates the Androgen Receptor Signaling and NF-κB/Nrf2 Pathways in Benign Prostatic Hyperplasia. Antioxidants 2021, 10, 1014. [Google Scholar] [CrossRef]
- He, Y.; Franco, O.E.; Jiang, M.; Williams, K.; Love, H.D.; Coleman, I.M.; Nelson, P.S.; Hayward, S.W. Tissue-specific consequences of cyclin D1 overexpression in prostate cancer progression. Cancer Res. 2007, 67, 8188–8197. [Google Scholar] [CrossRef]
- Chen, L.M.; Hatfield, M.L.; Fu, Y.; Chai, K.X. Prostasin regulates iNOS and cyclin D1 expression by modulating protease-activated receptor-2 signaling in prostate epithelial cells. Prostate 2009, 69, 1790–1801. [Google Scholar] [CrossRef]
- Zhang, M.; Hwang, E.; Lin, P.; Gao, W.; Ngo, H.T.; Yi, T.H. Prunella vulgaris L. Exerts a Protective Effect Against Extrinsic Aging Through NF-κB, MAPKs, AP-1, and TGF-β/Smad Signaling Pathways in UVB-Aged Normal Human Dermal Fibroblasts. Rejuvenation Res. 2018, 21, 313–322. [Google Scholar] [CrossRef]
- Caggia, S.; Libra, M.; Malaponte, G.; Cardile, V. Modulation of YY1 and p53 expression by transforming growth factor-β3 in prostate cell lines. Cytokine 2011, 56, 403–410. [Google Scholar] [CrossRef]
- Gong, G.Y.; Xi, S.-Y.; Li, C.-C.; Tang, W.-L.; Fu, X.-M.; Huang, Y.-P. Bushen Tongluo formula ameliorated testosterone propionate-induced benign prostatic hyperplasia in rats. Phytomedicine 2023, 120, 155048. [Google Scholar] [CrossRef]
- Sinowatz, F.; Schams, D.; Einspanier, R.; Arnold, G.; Pfeffer, M.; Temmim-Baker, L.; Amselgruber, W.; Plendl, J. Cellular localization of fibroblast growth factor 2 (FGF-2) in benign prostatic hyperplasia. Histol. Histopathol. 2000, 15, 475–481. [Google Scholar] [PubMed]
- Correa, L.L.; Neto, L.V.; Lima, G.A.B.; Gabrich, R.; de Miranda, L.C.D.; Gadelha, M.R. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and prostate cancer: Correlation with Gleason score. Int. Braz. J. Urol. 2015, 41, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, J.; Diamandi, A.; Mistry, J.; Scorilas, A. Insulin-like growth factor I (IGF-I) and IGF-binding protein-3 in benign prostatic hyperplasia and prostate cancer. J. Clin. Endocrinol. Metab. 2001, 86, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Gnanapragasam, V.J.; Mccahy, P.; Neal, D.; Robson, C. Insulin-like growth factor II and androgen receptor expression in the prostate. BJU Int. 2000, 86, 731–735. [Google Scholar] [CrossRef]
- Ho, P.J.; Baxter, R.C. Insulin-like growth factor-binding protein-2 in patients with prostate carcinoma and benign prostatic hyperplasia. Clin. Endocrinol. 1997, 46, 145–154. [Google Scholar] [CrossRef]
- Story, M.T.; Hopp, K.A.; Molter, M. Expression of transforming growth factor beta 1 (TGF beta 1), -beta 2, and- beta 3 by cultured human prostate cells. J. Cell Physiol. 1996, 169, 97–107. [Google Scholar] [CrossRef]
- Wu, X.-Z.; Zhang, S.-X.; Zhu, C.; Chen, D. Effects of sulfated polysaccharide extracted from Prunella vulgaris on endothelial cells. J. Med. Plants Res. 2011, 5, 4218–4223. [Google Scholar]
- Fan, J.; Ren, D.; Wang, J.; Liu, X.; Zhang, H.; Wu, M.; Yang, G. Bruceine D induces lung cancer cell apoptosis and autophagy via the ROS/MAPK signaling pathway in vitro and in vivo. Cell Death Dis. 2020, 11, 126. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, H.T.; Chun, S.Y.; Chung, J.-W.; Choi, S.H.; Lee, J.N.; Kim, B.S.; Yoo, E.S.; Kwon, T.G.; Kim, T.-H.; et al. Role of the JNK Pathway in Bladder Cancer. OncoTargets Ther. 2022, 15, 963–971. [Google Scholar] [CrossRef]
- Yan, H.; He, L.; Lv, D.; Yang, J.; Yuan, Z. The Role of the Dysregulated JNK Signaling Pathway in the Pathogenesis of Human Diseases and Its Potential Therapeutic Strategies: A Comprehensive Review. Biomolecules 2024, 14, 243. [Google Scholar] [CrossRef]
- Wijerathne, C.U.; Park, H.-S.; Jeong, H.-Y.; Song, J.-W.; Moon, O.-S.; Seo, Y.-W.; Won, Y.-S.; Son, H.-Y.; Lim, J.-H.; Yeon, S.-H.; et al. Quisqualis indica improves benign prostatic hyperplasia by regulating prostate cell proliferation and apoptosis. Biol. Pharm. Bull. 2017, 40, 2125–2133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, X.; Jiang, C.; Shi, F.; Zhu, Y.; Yang, B.; Zhuo, J.; Jing, Y.; Luo, G.; Xia, S.; et al. Finasteride accelerates prostate wound healing after thulium laser resection through DHT and AR signalling. Cell Prolif. 2018, 51, e12415. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Berriguete, G.; Fraile, B.; Martínez-Onsurbe, P.; Olmedilla, G.; Paniagua, R.; Royuela, M. MAP kinases and prostate cancer. J. Signal Transduct. 2012, 2012, 169170. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, D.; Toledo Lobo, M.-V.; Baquero, P.; Ropero, S.; Angulo, J.C.; Chiloeches, A.; Lasa, M. Downregulation of Snail by DUSP1 impairs cell migration and invasion through the inactivation of JNK and ERK and is useful as a predictive factor in the prognosis of prostate cancer. Cancers 2021, 13, 1158. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gao, M.; Ha, T.; Kelley, J.; Young, A.; Breuel, K. Prunella vulgaris aqueous extract attenuates IL-1β-induced apoptosis and NF-κB activation in INS-1 cells. Exp. Ther. Med. 2012, 3, 919–924. [Google Scholar] [CrossRef]
- Dvorkin, L.; Song, K.Y. Herbs for benign prostatic hyperplasia. Ann. Pharmacother. 2002, 36, 1443–1452. [Google Scholar] [CrossRef]
- Ney, J.P.; Joseph, K.R. Neurologic uses of botulinum neurotoxin type A. Neuropsychiatr. Dis. Treat. 2007, 3, 785–798. [Google Scholar] [CrossRef]

| Group | Treatment | Body Weight (g) | Relative Prostate Weight (%) | Inhibition of Growth (%) |
|---|---|---|---|---|
| NC | Corn oil + PBS | 431.1 ± 19.3 | 0.185 ± 0.031 | - |
| BPH | TP + PBS | 393.2 ± 14.5 | 0.346 ± 0.075 ## | - |
| BPH + FIN | TP + finasteride | 450.3 ± 24.6 | 0.292 ± 0.059 * | 66.49 |
| BPH + PV60 | TP + PV 60 mg/kg | 406.7 ± 22.2 | 0.289 ± 0.064 * | 35.87 |
| BPH + PV80 | TP + PV80 mg/kg | 454.1 ± 24.2 | 0.287 ± 0.053 * | 36.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumbukgahadeniya, P.; Baek, E.-B.; Hong, E.-J.; Song, J.-Y.; Kwak, Y.-G.; Jang, M.-R.; Ji, H.-S.; Kwun, H.-J. Prunella vulgaris Extract Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia by Regulating Androgen Levels, Cell Proliferation, and Apoptosis. Pharmaceuticals 2024, 17, 1516. https://doi.org/10.3390/ph17111516
Kumbukgahadeniya P, Baek E-B, Hong E-J, Song J-Y, Kwak Y-G, Jang M-R, Ji H-S, Kwun H-J. Prunella vulgaris Extract Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia by Regulating Androgen Levels, Cell Proliferation, and Apoptosis. Pharmaceuticals. 2024; 17(11):1516. https://doi.org/10.3390/ph17111516
Chicago/Turabian StyleKumbukgahadeniya, Poornima, Eun-Bok Baek, Eun-Ju Hong, Jun-Yeop Song, Youn-Gil Kwak, Mi-Ran Jang, Hyo-Seong Ji, and Hyo-Jung Kwun. 2024. "Prunella vulgaris Extract Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia by Regulating Androgen Levels, Cell Proliferation, and Apoptosis" Pharmaceuticals 17, no. 11: 1516. https://doi.org/10.3390/ph17111516
APA StyleKumbukgahadeniya, P., Baek, E.-B., Hong, E.-J., Song, J.-Y., Kwak, Y.-G., Jang, M.-R., Ji, H.-S., & Kwun, H.-J. (2024). Prunella vulgaris Extract Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia by Regulating Androgen Levels, Cell Proliferation, and Apoptosis. Pharmaceuticals, 17(11), 1516. https://doi.org/10.3390/ph17111516








