Rutin-Activated Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Attenuates Corneal and Heart Damage in Mice
Abstract
1. Introduction
2. Results
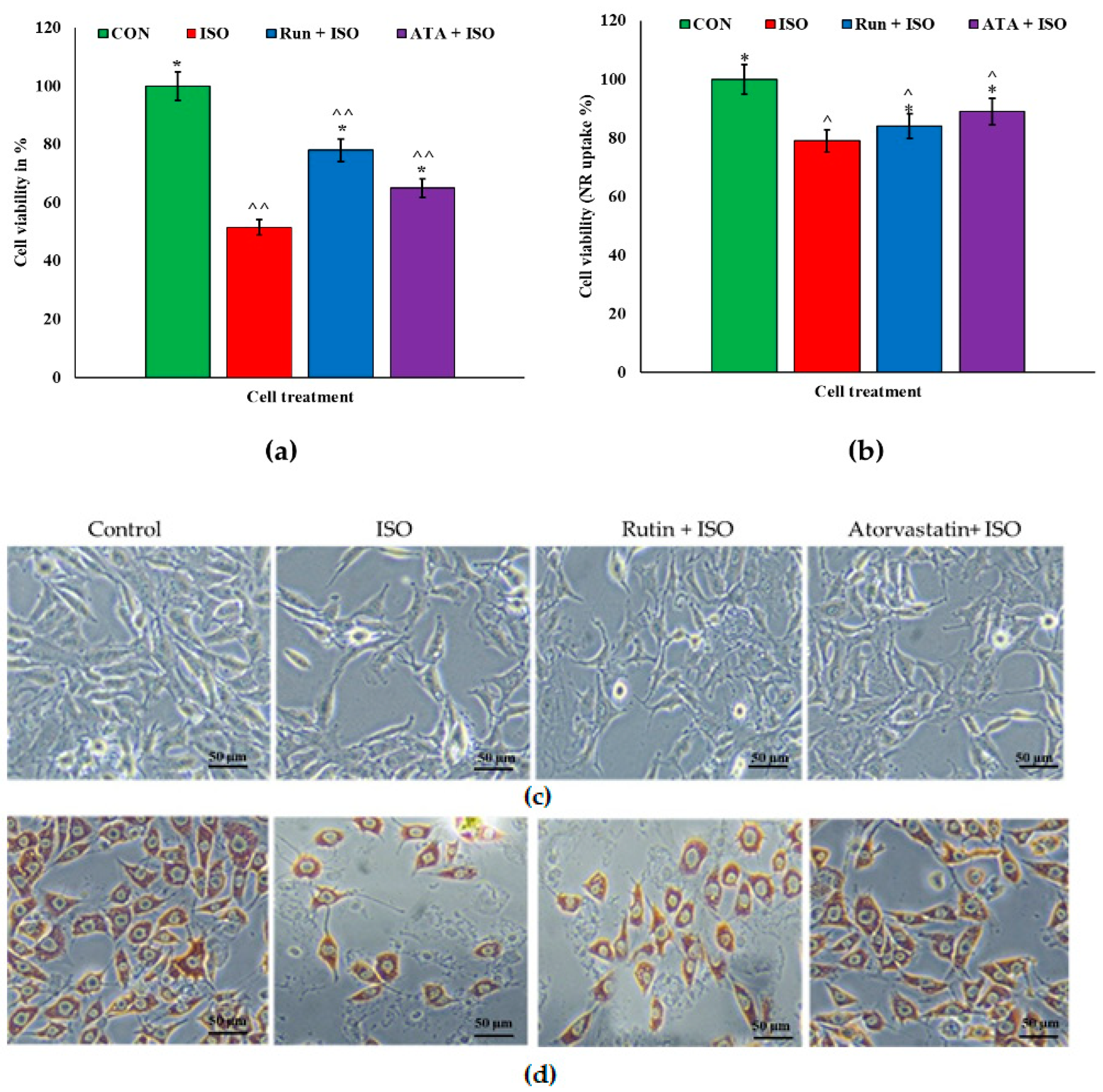
2.1. Effects of Rutin on Corneal Cells
2.2. Effects of Rutin on Isoproterenol (ISO)-Challenged Corneal Cells
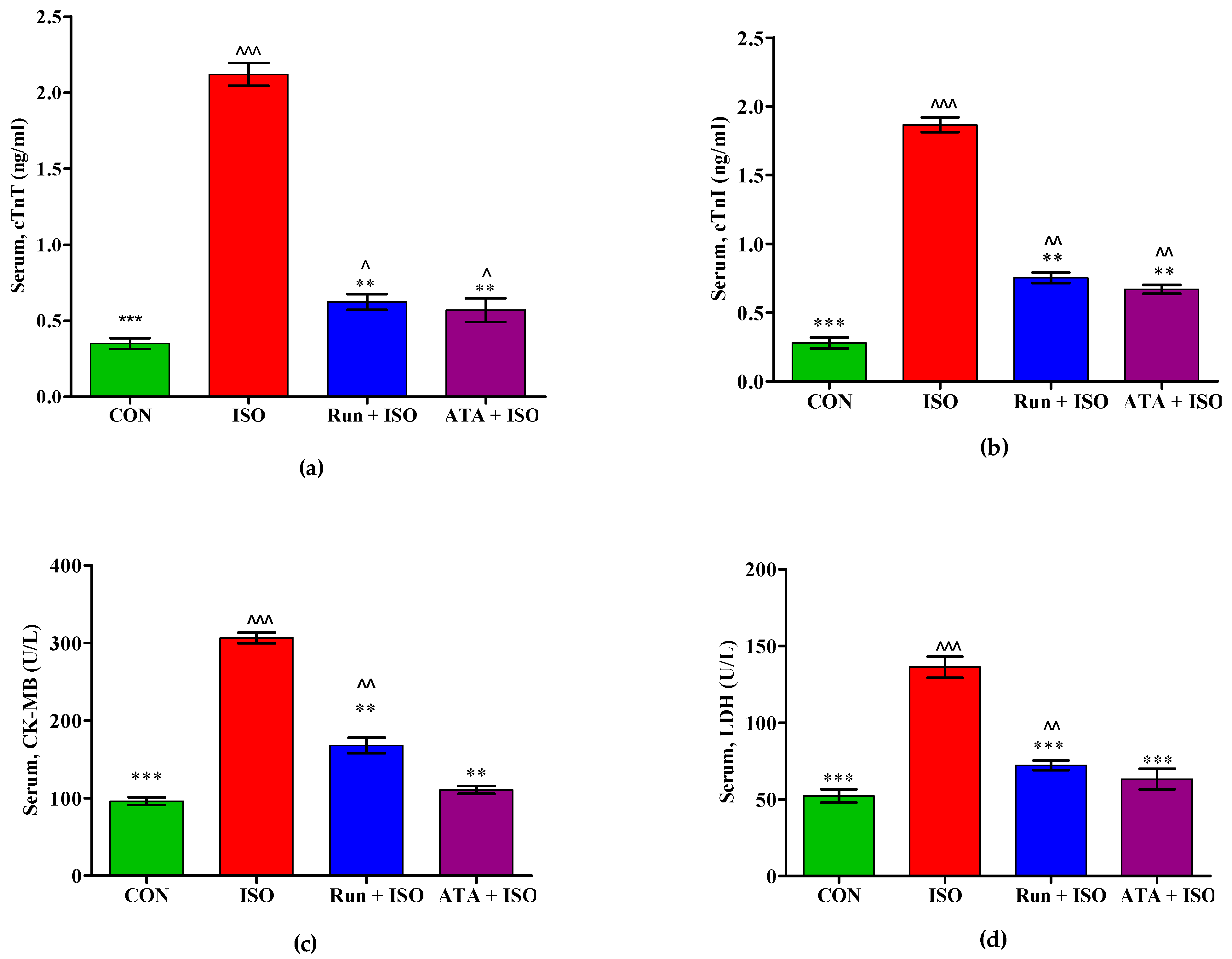
2.3. Effects of Rutin on Cardiac Biomarkers in MI in Mice
2.4. Effect of Rutin on Oxidative Stress Markers in MI in Mice
2.5. Effects of Rutin on Inflammatory Markers in ISO-Induced MI Mice
2.6. Effects of Rutin on Apoptosis Marker Expression in the Hearts of ISO-Induced Mice
2.7. Effects of Rutin on Nrf2 Signaling in ISO-Induced Mice
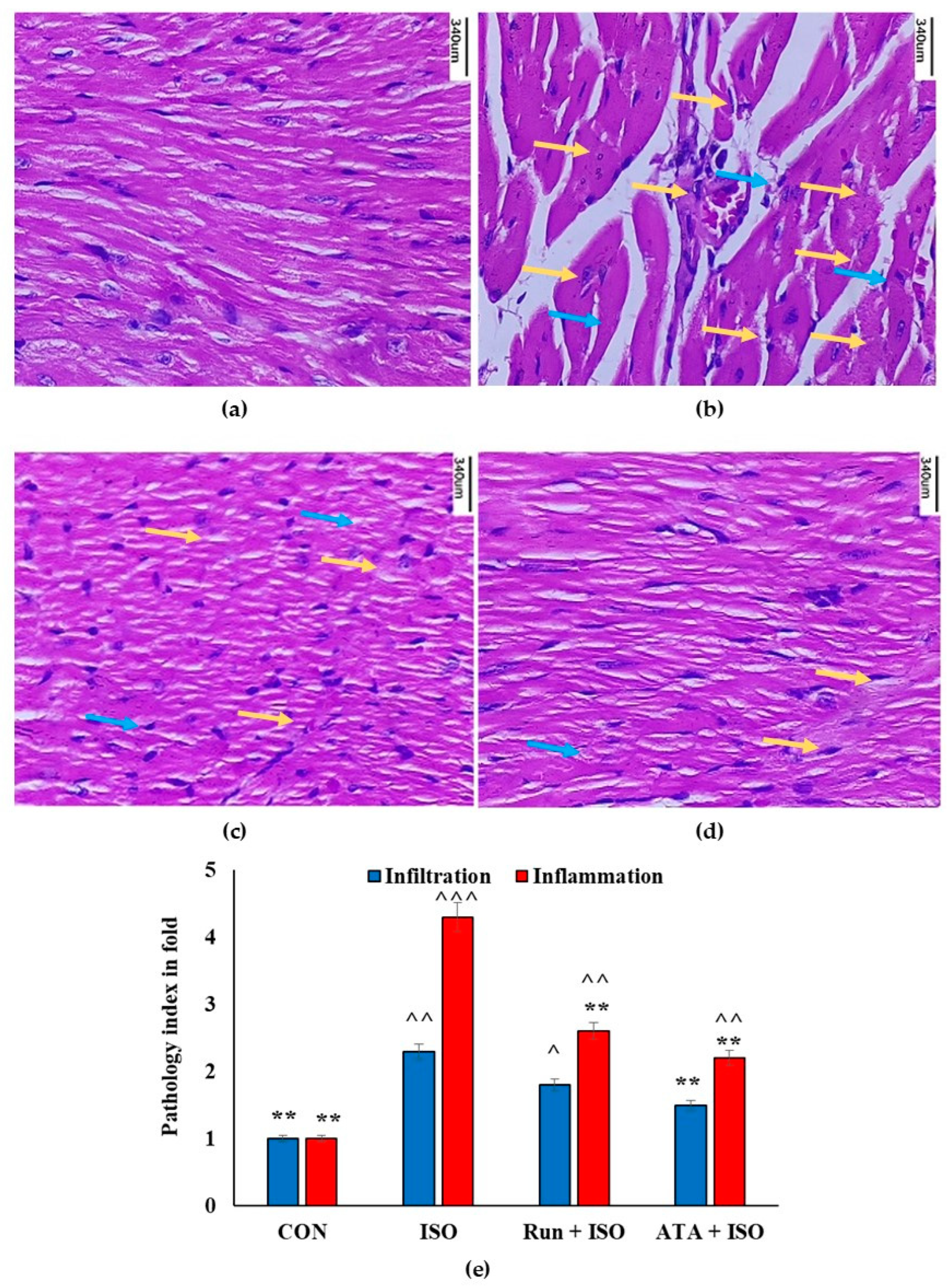
2.8. Effects of Rutin on Cardiac Histology in ISO-Induced Mice
2.9. Molecular Docking Study
3. Discussion
4. Materials and Methods
4.1. Drugs, Chemicals, and Reagents
4.2. Rabbit Corneal Cell Culture Preparation
4.3. MTT Assay
4.4. Neutral Red (NR) Uptake Assay
4.5. Nitric Oxide (NO) Measurement
4.6. Animals
4.7. Experimental Design
4.8. Measurement of cTnI, CK-MB, and LDH
4.9. Determination of the Antioxidant Level
4.10. Determination of Pro-Inflammatory Markers
4.11. Caspase 3 Assay
4.12. Quantitative Real-Time PCR
4.13. Western Blot Analysis
4.14. Histopathology of Myocardium
4.15. Molecular Docking Analysis
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alkatan, H.; Alkheraiji, N.; Alzahem; Corneal, T. Dystrophies and Degenerations. In Frontiers in Ophthalmology and Ocular Imaging; IntechOpen: London, UK, 2019. [Google Scholar]
- Kumaravel, S. Short Cases in Orthopaedics for PG Practical Examination; Jaypee Brothers Medical Publishers: New Delhi, India, 2013; ISBN 9789350900833. [Google Scholar]
- Tavakoli, M.; Kallinikos, P.A.; Efron, N.; Boulton, A.J.; Malik, R.A. Corneal Sensitivity Is Reduced and Relates to the Severity of Neuropathy in Patients with Diabetes. Diabetes Care 2007, 30, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Batliwala, S.; Xavier, C.; Liu, Y.; Wu, H.; Pang, I.-H. Involvement of Nrf2 in Ocular Diseases. Oxidative Med. Cell. Longev. 2017, 2017, 1703810. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, T.A. Reducing the Growing Burden of Cardiovascular Disease in the Developing World. Health Aff. 2007, 26, 13–24. [Google Scholar] [CrossRef]
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute myocardial infarction. Lancet 2017, 389, 197–210. [Google Scholar] [CrossRef]
- Lu, H.; Tan, Y.; Yang, L.; Dong, H.; Liao, Y.; Cao, S.; Fu, S. Cardioprotective Efficiency of Tangeretin Against Heart Failure Induced by Isoproterenol in Rats. Int. J. Pharmacol. 2018, 14, 1145–1152. [Google Scholar] [CrossRef]
- Aodah, A.H.; Devi, S.; Alkholifi, F.K.; Yusufoglu, H.S.; Foudah, A.I.; Alam, A. Effects of Taraxerol on Oxidative and Inflammatory Mediators in Isoproterenol-Induced Cardiotoxicity in an Animal Model. Molecules 2023, 28, 4089. [Google Scholar] [CrossRef]
- Kokkinaki, M.; Abu-Asab, M.; Gunawardena, N.; Ahern, G.; Javidnia, M.; Young, J.; Golestaneh, N. Klotho Regulates Retinal Pigment Epithelial Functions and Protects Against Oxidative Stress. J. Neurosci. 2013, 33, 16346–16359. [Google Scholar] [CrossRef]
- Nahar, K.; Kabir, F.; Islam, P.; Rahman, M.; Al Mamun, A.; Faruk, M.; Rahman, G.S.; Reza, H.M.; Alam, A. Cardioprotective effect of Amaranthus tricolor extract in isoprenaline induced myocardial damage in ovariectomized rats. Biomed. Pharmacother. 2018, 103, 1154–1162. [Google Scholar] [CrossRef]
- Chen, B.; Lu, Y.; Chen, Y.; Cheng, J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015, 225, R83–R99. [Google Scholar] [CrossRef]
- Gu, L.; Lin, J.; Wang, Q.; Li, C.; Peng, X.; Fan, Y.; Lu, C.; Lin, H.; Niu, Y.; Zhu, G.; et al. Dimethyl itaconate protects against fungal keratitis by activating the Nrf2/HO-1 signaling pathway. Immunol. Cell Biol. 2020, 98, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-F.; Zhou, D.-D.; Xie, T.; Malik, T.H.; Lu, C.-B.; Li, H.-J.; Wang, F.; Shu, C.; Liu, C.; Lu, C.-W.; et al. Nrf2, a Potential Therapeutic Target against Oxidative Stress in Corneal Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 2326178. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2016, 25, 149–164. [Google Scholar] [CrossRef]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2—An update. Free. Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Karthick, M.; Prince, P.S.M. Preventive effect of rutin, a bioflavonoid, on lipid peroxides and antioxidants in isoproterenol-induced myocardial infarction in rats. J. Pharm. Pharmacol. 2006, 58, 701–707. [Google Scholar] [CrossRef]
- Stanely Mainzen Prince, P.; Priya, S. Preventive Effects of Rutin on Lysosomal Enzymes in Isoproterenol Induced Cardio Toxic Rats: Biochemical, Histological and in Vitro Evidences. Eur. J. Pharmacol. 2010, 649, 229–235. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Budzynska, B.; Faggio, C.; Kruk-Slomka, M.; Samec, D.; Nabavi, S.F.; Sureda, A.; Devi, K.P.; Nabavi, S.M. Rutin as neuroprotective agent: From bench to bedside. Curr. Med. Chem. 2019, 26, 5152–5164. [Google Scholar] [CrossRef]
- Wang, M.; Ma, X.; Gao, C.; Luo, Y.; Fei, X.; Zheng, Q.; Ma, X.; Kuai, L.; Li, B.; Wang, R.; et al. Rutin attenuates inflammation by downregulating AGE-RAGE signaling pathway in psoriasis: Network pharmacology analysis and experimental evidence. Int. Immunopharmacol. 2023, 125, 111033. [Google Scholar] [CrossRef]
- Sui, C.; Wu, Y.; Zhang, R.; Zhang, T.; Zhang, Y.; Xi, J.; Ding, Y.; Wen, J.; Hu, Y. Rutin Inhibits the Progression of Osteoarthritis Through CBS-Mediated RhoA/ROCK Signaling. DNA Cell Biol. 2022, 41, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jia, K.; Zhou, Y.; Chen, L.; Wang, F.; Yi, X.; Huang, Y.; Ge, Y.; Chen, X.; Liao, D.; et al. Rutin hydrate relieves neuroinflammation in zebrafish models: Involvement of NF-κB pathway as a central network. Fish. Shellfish. Immunol. 2023, 141, 109062. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ma, Y.; Ma, Y.; Wang, Y.; Zhao, X.; Jin, D.; Xu, L.; Ge, S. Rutin prevents pyroptosis and M1 microglia via Nrf2/Mac-1/caspase-1-mediated inflammasome axis to improve POCD. Int. Immunopharmacol. 2023, 127, 111290. [Google Scholar] [CrossRef] [PubMed]
- Lupasco, T.; He, Z.; Cassagne, M.; Sagnial, T.; Brion, L.; Fournié, P.; Gain, P.; Thuret, G.; Allouche, M.; Malecaze, F.; et al. Corneal epithelium in keratoconus underexpresses active NRF2 and a subset of oxidative stress-related genes. PLoS ONE 2022, 17, e0273807. [Google Scholar] [CrossRef]
- Bayir, Y.; Cadirci, E.; Suleyman, H.; Halici, Z.; Keles, M.S. Effects of Lacidipine, Ramipril and Valsartan on Serum BNP Levels in Acute and Chronic Periods Following Isoproterenol-Induced Myocardial Infarction in Rats. Eurasian J. Med. 2009, 41, 44–48. [Google Scholar]
- Cejka, C.; Cejkova, J. Oxidative Stress to the Cornea, Changes in Corneal Optical Properties, and Advances in Treatment of Corneal Oxidative Injuries. Oxidative Med. Cell. Longev. 2015, 2015, 591530. [Google Scholar] [CrossRef]
- Zhang, X.; Seshadri, V.D.; Jiang, Q. Ameliorative Effects of Ponicidin Against the Isoproterenol-induced Acute Myocardial Infarction in Rats. Pharmacogn. Mag. 2023, 19, 427–438. [Google Scholar] [CrossRef]
- Kurtul, B.E.; Kurtul, A.; Ergashev, K. Is there a relationship between statin use and corneal specular microscopy and topography findings? Beyoglu Eye. J. 2021, 6, 280–284. [Google Scholar] [CrossRef]
- Thirunavukarasu, A.J.; Ross, A.C.; Gilbert, R.M. Vitamin A, systemic T-cells, and the eye: Focus on degenerative retinal disease. Front. Nutr. 2022, 9, 914457. [Google Scholar] [CrossRef]
- Bardag-Gorce, F.; Diaz, A.; Niihara, R.; Stark, J.; Cortez, D.; Lee, A.; Hoft, R.; Niihara, Y. Aldehyde Dehydrogenases Expression in Corneal Epithelial Cells with Limbal Stem Cell Deficiency. Int. J. Mol. Sci. 2022, 23, 4032. [Google Scholar] [CrossRef]
- Julià, P.; Farrés, J.; Parés, X. Ocular alcohol dehydrogenase in the rat: Regional distribution and kinetics of the ADH-1 isoenzyme with retinol and retinal. Exp. Eye Res. 1986, 42, 305–314. [Google Scholar] [CrossRef]
- Bertinchant, J.; Robert, E.; Polge, A.; Marty-Double, C.; Fabbro-Peray, P.; Poirey, S.; Aya, G.; Juan, J.; Ledermann, B.; de la Coussaye, J.; et al. Comparison of the diagnostic value of cardiac troponin I and T determinations for detecting early myocardial damage and the relationship with histological findings after isoprenaline-induced cardiac injury in rats. Clin. Chim. Acta 2000, 298, 13–28. [Google Scholar] [CrossRef]
- Li, H.; Song, F.; Duan, L.-R.; Sheng, J.-J.; Xie, Y.-H.; Yang, Q.; Chen, Y.; Dong, Q.-Q.; Zhang, B.-L.; Wang, S.-W. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci. Rep. 2016, 6, 23693. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, J.; Jia, S.; Feng, P.; Chang, F.; Yan, L.; Gupta, P.; Wu, H. Cardioprotective Effect of Gossypin Against Myocardial Ischemic/Reperfusion in Rats via Alteration of Oxidative Stress, Inflammation and Gut Microbiota. J. Inflamm. Res. 2022, 15, 1637–1651. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Liu, S.; Sun, Y.; Chen, C.; Yang, S.; Lin, M.; Long, J.; Yao, J.; Lin, Y.; Yi, F.; et al. Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease. J. Transl. Med. 2023, 21, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Al-Rejaie, S.S.; Aleisa, A.M.; Sayed-Ahmed, M.M.; Al-Shabanah, O.A.; Abuohashish, H.M.; Ahmed, M.M.; Al-Hosaini, K.A.; Hafez, M.M. Protective effect of rutin on the antioxidant genes expression in hypercholestrolemic male Westar rat. BMC Complement. Altern. Med. 2013, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Park, H.-R.; Lee, K.-A. A Comparative Study of Rutin and Rutin Glycoside: Antioxidant Activity, Anti-Inflammatory Effect, Effect on Platelet Aggregation and Blood Coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, D.; Sang, M.; Xu, Y.; Liu, Z.; Wu, Q. Stachydrine ameliorates isoproterenol-induced cardiac hypertrophy and fibrosis by suppressing inflammation and oxidative stress through inhibiting NF-κB and JAK/STAT signaling pathways in rats. Int. Immunopharmacol. 2017, 48, 102–109. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes. Cells 1998, 3, 697–707. [Google Scholar] [CrossRef]
- Korshunova, A.Y.; Blagonravov, M.L.; Neborak, E.V.; Syatkin, S.P.; Sklifasovskaya, A.P.; Semyatov, S.M.; Agostinelli, E. BCL2-regulated apoptotic process in myocardial ischemia-reperfusion injury (Review). Int. J. Mol. Med. 2020, 47, 23–36. [Google Scholar] [CrossRef]
- Abdelzaher, W.Y.; Ahmed, S.M.; Welson, N.N.; Alsharif, K.F.; Batiha, G.E.-S.; Labib, D.A.A. Dapsone Ameliorates Isoproterenol-Induced Myocardial Infarction via Nrf2/ HO-1; TLR4/ TNF-α Signaling Pathways and the Suppression of Oxidative Stress, Inflammation, and Apoptosis in Rats. Front. Pharmacol. 2021, 12, 669679. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, H.M.; Abdalla, A.M.; Ahmed, A.F.; Abdel-Aziz, A.M. Role of nitric oxide in mediating the cardioprotective effect of agomelatine against isoproterenol-induced myocardial injury in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1809–1823. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Prasad, S. Histoprotective effect of rutin against cisplatin-induced toxicities in tumor-bearing mice: Rutin lessens cisplatin-induced toxicities. Hum. Exp. Toxicol. 2021, 40, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Arjumand, W.; Seth, A.; Sultana, S. Rutin attenuates cisplatin induced renal inflammation and apoptosis by reducing NFκB, TNF-α and caspase-3 expression in wistar rats. Food Chem. Toxicol. 2011, 49, 2013–2021. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Li, L.-J.; Dong, Q.-X.; Zhu, J.; Huang, Y.-R.; Hou, S.-J.; Yu, X.-L.; Liu, R.-T. Rutin prevents tau pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J. Neuroinflammation 2021, 18, 131. [Google Scholar] [CrossRef]
- Wang, J.; He, X.; Lv, S. Notoginsenoside-R1 ameliorates palmitic acid-induced insulin resistance and oxidative stress in HUVEC via Nrf2/ARE pathway. Food Sci. Nutr. 2023, 11, 7791–7802. [Google Scholar] [CrossRef]
- Unni, S.; Deshmukh, P.; Krishnappa, G.; Kommu, P.; Padmanabhan, B. Structural insights into the multiple binding modes of Dimethyl Fumarate (DMF) and its analogs to the Kelch domain of Keap1. FEBS J. 2020, 288, 1599–1613. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Liu, J.; Zuo, J.; Yan, L.; Thring, R.W.; Ba, X.; Qi, D.; Wu, M.; Gao, Y.; et al. Tauroursodeoxycholic acid functions as a critical effector mediating insulin sensitization of metformin in obese mice. Redox Biol. 2022, 57, 102481. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, W.; Zhang, Z.; Zheng, Y. The Role of Nrf2-Mediated Pathway in Cardiac Remodeling and Heart Failure. Oxidative Med. Cell. Longev. 2014, 2014, 260429. [Google Scholar] [CrossRef]
- Jones, R.M.; Desai, C.; Darby, T.M.; Luo, L.; Wolfarth, A.A.; Scharer, C.D.; Ardita, C.S.; Reedy, A.R.; Keebaugh, E.S.; Neish, A.S. Lactobacilli Modulate Epithelial Cytoprotection through the Nrf2 Pathway. Cell Rep. 2015, 12, 1217–1225. [Google Scholar] [CrossRef]
- Hayashi, R.; Himori, N.; Taguchi, K.; Ishikawa, Y.; Uesugi, K.; Ito, M.; Duncan, T.; Tsujikawa, M.; Nakazawa, T.; Yamamoto, M.; et al. The role of the Nrf2-mediated defense system in corneal epithelial wound healing. Free. Radic. Biol. Med. 2013, 61, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Tabolacci, E.; Tringali, G.; Nobile, V.; Duca, S.; Pizzoferrato, M.; Bottoni, P.; Clementi, M.E. Rutin Protects Fibroblasts from UVA Radiation through Stimulation of Nrf2 Pathway. Antioxidants 2023, 12, 820. [Google Scholar] [CrossRef] [PubMed]
- Kovac, S.; Angelova, P.R.; Holmström, K.M.; Zhang, Y.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Florento, L.; Matias, R.; Tuano, E.; Santiago, K.; Cruz, F.; Tuazon, A. Comparison of Cytotoxic Activity of Anticancer Drugs against Various Human Tumor Cell Lines Using In Vitro Cell-Based Approach. Int. J. Biomed. Sci. 2012, 8, 76–80. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R., Jr. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996, 268, 237–246. [Google Scholar] [CrossRef]
- Wu, P.; Cai, M.; Liu, J.; Wang, X. Catecholamine Surges Cause Cardiomyocyte Necroptosis via a RIPK1–RIPK3-Dependent Pathway in Mice. Front. Cardiovasc. Med. 2021, 8, 740839. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gado, A.M.; Nasser, A.; Adam, I.; Aldahmash, B.A. Cardiotoxicity Induced by Cyclophosphamide in Rats: Protective Effect of Curcumin. J. Res. Environ. Sci. Toxicol. 2013, 2, 2315–5698. [Google Scholar]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar]





| Groups | Control | ISO | Rutin + ISO | ATR + ISO |
|---|---|---|---|---|
| NF-κB (pg/mL) | 24.72 ± 1.73 *** | 75.28 ± 6.45 ^^ | 39.24 ± 2.46 ^^ ** | 37.48 ± 3.78 ^^ ** |
| IL-6 (pg/mL) | 68.42 ± 5.15 ** | 127.55 ± 10.47 ^^ | 87.48 ± 6.37 ^^ *** | 79.32 ± 7.14 ^ ** |
| TNF-α (pg/mL) | 41.64 ± 4.04 ** | 75.49 ± 6.25 ^^ | 59.17 ± 4.58 ^ ** | 55.13 ± 4.47 ^ ** |
| Sl No | Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Product Size (bp) |
|---|---|---|---|---|
| 1 | bcl-2 | GTGGATGACTGAGTACCT | CCAGGAGAAATCAAACAGAG | 118 |
| 2 | Bax | CTACAGGGTTTCATCCAG | CCAGTTCATCTCCAATTCG | 133 |
| 3 | Caspase 3 | CCAACCTCAGAGAGACATTC | TTTCGGCTTTCCAGTCAGAC | 254 |
| 4 | GAPDH | GAGAAACCTGCCAAGTATG | GGAGTTGCTGTTGAAGTC | 123 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emeka, P.M.; Badger-Emeka, L.I.; Thirugnanasambantham, K.; Alatawi, A.S. Rutin-Activated Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Attenuates Corneal and Heart Damage in Mice. Pharmaceuticals 2024, 17, 1523. https://doi.org/10.3390/ph17111523
Emeka PM, Badger-Emeka LI, Thirugnanasambantham K, Alatawi AS. Rutin-Activated Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Attenuates Corneal and Heart Damage in Mice. Pharmaceuticals. 2024; 17(11):1523. https://doi.org/10.3390/ph17111523
Chicago/Turabian StyleEmeka, Promise M., Lorina I. Badger-Emeka, Krishnaraj Thirugnanasambantham, and Abdulaziz S. Alatawi. 2024. "Rutin-Activated Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Attenuates Corneal and Heart Damage in Mice" Pharmaceuticals 17, no. 11: 1523. https://doi.org/10.3390/ph17111523
APA StyleEmeka, P. M., Badger-Emeka, L. I., Thirugnanasambantham, K., & Alatawi, A. S. (2024). Rutin-Activated Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Attenuates Corneal and Heart Damage in Mice. Pharmaceuticals, 17(11), 1523. https://doi.org/10.3390/ph17111523






